Abstract
Purpose:
There is limited data on cardiopulmonary exercise testing (CPX) and cardiorespiratory fitness (CRF) following open repair for a proximal thoracic aortic aneurysm or dissection. The aim was to evaluate serious adverse events (SAEs), abnormal CPX event rate, CRF (peak oxygen consumption, VO2peak), and blood pressure.
Methods:
Patients were retrospectively identified from cardiac rehabilitation participation or prospectively enrolled in a research study and grouped by phenotype: (1) bicuspid aortic valve/thoracic aortic aneurysm, (2) tricuspid aortic valve/thoracic aortic aneurysm, and (3) acute type A aortic dissection (ATAAD).
Results:
Patients (n=128) completed a CPX a median of 2.9 mo (interquartile range (IQR): 1.8, 3.5) following repair. No SAEs were reported, although 3 abnormal exercise tests (2% event rate) were observed. Eighty-one percent of CPX studies were considered peak effort (defined as respiratory exchange ratio ≥1.05). Median measured VO2peak was < 36% predicted normative values (19.2 mL.kg−1.min−1 versus 29.3 mL.kg−1.min−1, P < 0.0001); the most marked impairment in VO2peak was observed in the ATAAD group (< 40% normative values), which was significantly different from other groups (P <0.05). Peak exercise systolic and diastolic blood pressures were 160mmHg (144, 172) and 70mmHg (62, 80), with no differences noted between groups.
Conclusions:
We observed no SAEs with an abnormal CPX event rate of only 2% 3 mo following repair for a proximal thoracic aortic aneurysm or dissection. VO2peak was reduced among all patient groups, especially the ATAAD group, which may be clinically significant given the well-established prognostic importance of reduced CRF.
Keywords: thoracic aortic aneurysm, aortic dissection, exercise, cardiopulmonary exercise testing
CONDENSED ABSTRACT
There were no serious adverse events although 2% of exercise tests were abnormal 3 mo following thoracic aortic repair. Cardiorespiratory fitness (CRF) was reduced 36% among all patients, however, the dissection patients showed the most marked impairments. The prognostic importance of reduced CRF warrants further clinical investigation in this setting.
Thoracic aortic disease represents a broad range of clinical phenotypes (e.g., degenerative, congenital/structural, and genetically-mediated) predisposing to aortic aneurysm, dissection, or rupture, and typically necessitating elective or urgent repair.1 The direct adverse consequences of thoracic aortic disease, the secondary effects of repair (e.g., deconditioning), and the likely presence of known cardiovascular disease (CVD) risk factors may result in an increased CVD risk,2, 3 impaired quality of life (QoL),4 and possibly reduced cardiorespiratory fitness (CRF).
To mitigate these effects, cardiac surgery populations (e.g. valvular, coronary, transplant) are referred to cardiac rehabilitation (CR), prior to which they undergo treadmill exercise testing or cardiopulmonary exercise testing (CPX). The intent is to ensure safety, assess CRF, and guide exercise prescriptions to achieve the goals of reducing CVD-related morbidity and mortality and hospital readmission rate, as well as improve CRF and QoL.5, 6
This paradigm is not routine following repair for a thoracic aortic aneurysm or acute type A aortic dissection (ATAAD), likely due to insufficient data to support these recommendations. The Aortic Valve and Ascending Aorta Guidelines for Management and Quality Measures indicate limited data on CRF as assessed objectively by serial and formal exercise testing.7 Moreover, the American College of Cardiology/American Heart Association8 and 2014 European Society of Cardiology3 guidelines state a lack of data to support a safe and tolerable level of exercise appropriate for patients with thoracic aortic aneurysm and dissection patients.
As an initial step toward addressing these knowledge gaps, we report our experience performing CPX procedures approximately 3 months following open repair for a proximal thoracic aortic aneurysm or ATAAD. The purpose was to evaluate whether patients are able to perform CPX procedures and to assess CRF levels as there is currently no data or guidelines in this regard.
METHODS
Study Design
Patients were identified as follows: (1) retrospectively based on referral to CR between June 2010 to February 2017 prior to which they completed a CPX for exercise safety/clearance and improve the exercise prescription based upon the peak oxygen uptake (VO2peak), and (2) prospectively enrolled in a research study aiming to evaluate VO2peak utilizing CPX procedures between March 2016 and June 2017. There were no differences in the primary endpoints, including SAEs, abnormal CPX event rates, and VO2peak, and thus, data was combined for analysis (Supplemental Table 1). Other eligibility criteria included: (1) primary indication was a root and/or ascending aortic aneurysm or ATAAD, (2) completed CPX procedures utilizing the Cornell Protocol,9 (3) ≥18 yr of age, and (4) primary cardiologist or aortic surgeon approval. Connective tissue disease was defined as physician-diagnosed Marfan syndrome using the revised Ghent nosology.10 The Institutional Review Board approved all study-related procedures.
Clinical and Operative Outcomes
Investigators leveraged Society of Thoracic Surgeons data elements as previously reported.11 CPX data was extracted from the Division of Cardiovascular Medicine, Preventive Cardiology Cardiac Rehabilitation Database12 or from the research study. Electronic medical record review was conducted to confirm clinical and surgical outcomes. Phenotype groups were (1) bicuspid aortic valve (BAV)/thoracic aortic aneurysm (TAA), (2) tricuspid aortic valve (TAV)/TAA, and (3) ATAAD. Outcome differences among phenotype groups were explored since the mechanisms propagating aneurysmal formation or dissection may be unique to the designated phenotype.
Serious Adverse Events (SAEs) were defined as any occurrence of syncope, sustained ventricular tachycardia (VT), acute coronary syndrome, external defibrillation or implantable cardioverter-defibrillator discharge, provision of cardiac life support medications, direct admission to the emergency room, or death.13 An abnormal exercise test was defined as: (1) significant ischemic changes in ECG during exercise or recovery (≥1 mm horizontal or downsloping ST depression or ST elevation ≥1 mm in leads without diagnostic Q waves) in the absence of ECG/echocardiographic left ventricular (LV) hypertrophy (LVH), (2) development of an exercise-induced arrhythmias including sustained and nonsustained VT ≥ 4 beats [sustained VT is defined as an arrhythmia originating from the ventricles at a rate >100 bpm lasting >30 sec. Nonsustained VT is defined as an arrhythmia originating from the ventricles at a rate >100 bpm lasting <30 sec but ≥ 4 beats] and atrioventricular block, (3) hypotension [decrease in systolic blood pressure (BP) of > 20 mmHg or > 10 mmHg drop in systolic BP with signs and symptoms].5, 14 Echocardiographic and clinical outcomes associated with abnormal CPX were independently reviewed to identify potential abnormalities not identifiable on the ECG. Readmission events were assessed by reviewing electronic medical records within seven-days following CPX procedures.
CPX and Echocardiographic Outcomes
CRF (as measured by VO2peak) was evaluated using an electronic/motorized treadmill test performed by two advanced cardiovascular life support (ACLS) and American College of Sports Medicine certified clinical exercise physiologists under the supervision of the interpreting cardiologist.15 Expired gases were analyzed continuously by a metabolic stress test system (MGC Diagnostics, Ultima CPX™). After stable resting values had been achieved, patients were tested according to the Cornell protocol until patient request to stop, general/leg fatigue, clinical decision to terminate, or a VO2peak was achieved/maximal effort.5, 14 The Cornell protocol increases the treadmill speed and grade every 2 min with standardized metabolic equivalent (MET) increases per stage. VO2peak was defined as the greatest VO2 mL.kg−1.min−1 value for a given 30-sec interval; anaerobic threshold was calculated using standard methods.16 Percent-predicted VO2peak was calculated according to the FRIEND normative values for VO2 (referred to as normative valves from this point onward).17, 18 A peak CPX criterion was defined as a respiratory exchange ratio (RER) ≥1.05.19–22 Heart rate (HR) response as evaluated utilizing continuous 12-lead ECG monitoring (Mac ® 5000, GE Healthcare) and BP response was measured manually by auscultatory sphygmomanometer every 2 minutes during CPX procedures. BP termination criteria for the research study cohort was ≥ 180 mmHg systolic over ≥ 90 mmHg diastolic BP.22 Although, BP exceeded above these thresholds due to the time interval between BP measurements. Exercise termination BP criteria5, 14 in the CR cohort was standard at > 240mmHg systolic or > 110mmHg diastolic. Patients were not asked to discontinue medications prior to exercise testing. The criteria utilized to determine an abnormal HR recovery was ≤ 12 bpm−1 (max HR – 1-minute HR recovery) following a walking protocol.23 Echocardiography outcomes including LV ejection fraction (LVEF) and aortic valve insufficiency were obtained from the 3 mo post-operative transthoracic or intra-operative transesophageal echocardiogram.
Statistical Analysis
The initial analysis provided descriptive information on demographic, clinical, and surgical outcomes. Results are presented as median (interquartile range (IQR): 25% and 75%) for continuous data and n (%) for categorical data. Chi-square tests were performed for categorical variables. Wilcoxon rank sum tests were performed for continuous variables to test the significance among groups. The pairwise comparisons for continuous variables were performed using analysis of variance tests with the Tukey-Kramer method. Pearson correlation was used to evaluate the association between estimated and objectively measured VO2peak. All statistical calculations used SAS 9.4 (SAS Institute) and were considered significant at P < 0.05.
RESULTS
Study Design and Clinical Outcomes
Patients were either retrospectively identified based on participation in CR evaluation (n=67, 55%) or prospectively enrolled in a research study (n=61, 45%). Symptom-limited CPX was performed a median of 2.9 mo (1.8, 3.5) following open repair. Patients were grouped by phenotype as follows: (1) BAV/TAA (n=49, 38%), (2) TAV/TAA (n=51, 40%), and (3) ATAAD (n=28, 22%). All patients underwent proximal thoracic aortic repair (including the aortic root, ascending, or arch aorta) through sternotomy for a root and/or ascending aortic aneurysm or an ATAAD. Details regarding demographics and clinical characteristics are provided in Table 1.
Table 1.
Overview of Clinical Characteristics
| All Patients N = 128 (100%) |
BAV/TAA N = 49 (38%) |
TAV/TAA N = 51 (40%) |
ATAAD N = 28 (22%) |
P valuea | |
|---|---|---|---|---|---|
| Repair to CPX, mo | 2.9 (1.8,3.5) | 2.2 (1.7,3.2) | 2.5 (1.7,3.4) | 3.6 (2.8,4.4) | 0.001c |
| Age, yr | 59 (48,66) | 57 (47,65) | 64 (55,72) | 52 (44,60) | 0.001b,d |
| Male | 113 (88) | 46 (94) | 43 (84) | 24 (86) | 0.29 |
| Aortic valve indications | |||||
| Aortic insufficiency, mod-to-severe | 59 (47) | 22 (45) | 26 (51) | 11 (41) | 0.66 |
| Aortic stenosis, mod-to-severe | 27 (21) | 24 (49) | 3 (6) | 0 | <0.0001b,c |
| Bicuspid aortic valve | 50 (39) | 49 (38) | 0 | 1 (4) | <0.0001b,c |
| Calcification | 30 (23) | 19 (39) | 11 (22) | 0 | 0.0005c,d |
| Thoracic aortic aneurysm | |||||
| Root | 86 (67) | 26 (53) | 41 (80) | 19 (68) | 0.01b |
| Ascending | 112 (88) | 47 (96) | 41 (80) | 24 (86) | 0.06 |
| Arch | 46 (36) | 11 (23) | 14 (28) | 21 (75) | <0.0001b,d |
| Descending | 16 (13) | 0 | 3 (6) | 13 (46) | <0.0001b,d |
| Max diameter, cm | 5.2 (4.7,5.5) | 5.1 (4.6,5.4) | 5.3 (4.9,5.6) | 5.1 (4.5,5.5) | 0.15 |
| Risk Factors | |||||
| HTN | 89 (70) | 32 (65) | 34 (67) | 23 (82) | 0.25 |
| Dyslipidemia | 61 (48) | 30(61) | 23 (45) | 8 (29) | 0.02c |
| Peripheral vascular disease | 12 (9) | 1 (2) | 5 (10) | 6 (21) | 0.02c |
| Smoking history (former or current) | 55 (43) | 20 (43) | 21 (43) | 14 (50) | 0.79 |
| Chronic lung disease | 13 (10) | 6 (12) | 6 (12) | 1 (4) | 0.52 |
| Chronic kidney disease | 9 (7.0) | 3 (6.1) | 3 (5.9) | 3 (11) | 0.74 |
| Coronary artery disease | 26 (20) | 9(18) | 16(31) | 1(3.6) | 0.012d |
| Previous cardiac intervention e | 2 (18) | 12 (25) | 8 (16) | 3 (11) | 0.27 |
| Medications | |||||
| ACE-I | 31 (27) | 8 (16) | 12 (24) | 11 (39) | 0.08 |
| Calcium channel blocker | 17 (13) | 1 (2) | 6 (12) | 10 (36) | 0.0001c,d |
| ARB | 12 (10) | 2 (4) | 4 (8) | 6 (21) | 0.06 |
| ß-Blocker | 118 (92) | 45 (92) | 46 (90) | 27 (96) | 0.64 |
| Diuretic | 20 (16) | 9 (18) | 8 (16) | 3 (11) | 0.67 |
| Any anti-HTN | 123 (96) | 47 (96) | 48 (94) | 28 (100) | 0.63 |
| Number of HTN | 1 (1,2) | 1 (1,2) | 1 (1,2) | 2 (1,3) | 0.0009c,d |
Data are reported as median (interquartile range) or n (%).
Overall p value,
BAV/TAA and TAV/TAA are significantly different,
BAV/TAA and ATAAD are significantly different,
TAV/TAA and ATAAD are significantly different
Include coronary artery bypass grafting, cardiac valve repair/replacement, other cardiac procedure, etc.
Abbreviations: ATAAD, acute type A aortic dissection; ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BAV, bicuspid aortic valve; CPX, cardiopulmonary exercise test; HTN, hypertension; TAA, thoracic aortic aneurysm; TAV, tricuspid aortic valve.
Intra- and Post-operative Outcomes
Intra- and post-operative outcomes are provided in Table 2. There were no significant differences between groups for ascending aortic replacement or concomitant surgery, defined as a concurrent intervention including coronary bypass grafting and mitral/tricuspid valve repair/replacement. The ATAAD group had significantly more aortic root repairs and arch replacements compared to the BAV/TAA and TAV/TAA groups (P <0.05). The ATAAD group suffered more post-operative complications including higher rates of postoperative stroke, pneumonia, and new-onset renal failure requiring dialysis as well as longer time ventilated and intubated with longer length of stays compared to BAV/TAA and TAV/TAA groups (P <0.05).
Table 2.
Operative Outcomes
| All Patients N=128 (100%) |
BAV/TAA N=49 (38%) |
TAV/TAA N=51 (40%) |
ATAAD N=28 (22%) |
P valuea | |
|---|---|---|---|---|---|
| Intraoperative | |||||
| Aortic valve/root procedure | 120 (94) | 48 (98) | 46 (90) | 26 (93) | |
| Ascending replacement | 11 (87) | 44 (90) | 41 (80) | 26 (93) | 0.21 |
| Arch replacement | 51 (40) | 12 (25) | 12 (23) | 27 (96) | <0.0001c,d |
| Concomitant surgerye | 34 (27) | 16 (33) | 13 (26) | 5 (18) | 0.36 |
| CPB time, minutes | 198 (163, 240) | 186.5 (159, 228) | 200 (159, 243) | 215 (183, 268) | 0.16 |
| Cross clamp time, minutes | 145 (116, 183) | 143.5(115, 171) | 146 (129, 187) | 150.5 (105, 195) | 0.61 |
| Hypothermic circulatory arrest | 50 (39) | 12 (25) | 12 (24) | 26 (93) | <0.0001c,d |
| Cerebral Perfusion | <0.0001c,d | ||||
| Antegrade/Retrograde | 49 (38) | 12 (24) | 11 (22) | 26 (93) | |
| Postoperative | |||||
| Cerebrovascular accident | 3 (2.3) | 0 | 0 | 3 (11) | 0.0096c,d |
| Atrial fibrillation | 44 (34) | 17 (35) | 20 (39) | 7 (25) | 0.44 |
| Pneumonia | 3 (2) | 0 | 0 | 3(11) | 0.0096c,d |
| New-onset renal failure | 9 (7) | 2 (4) | 1 (2) | 6 (21) | 0.005c,d |
| Requiring dialysis | 3 (2.3) | 0 | 0 | 3 (11) | 0.0096c,d |
| Prolonged ventilation | 13 (10) | 3 (6.3) | 1 (2.0) | 9 (32) | 0.0002c,d |
| Hours intubated | 16 (12.5, 20) | 15 (12,19) | 15 (11,17) | 19 (16.5 ,44) | <0.0001c,d |
| Reintubation | 1 (0.8) | 0 | 0 | 1 (3.6) | 0.22 |
| Postoperative length of stay, d | 6 (5,9) | 6 (5,8) | 6 (5,8) | 8.5 (5.5, 12.5) | 0.0056c,d |
| Total length of stay, d | 7 (5,10) | 6 (5,9) | 6 (5,8) | 10 (6, 13.5) | 0.0071c,d |
Data are reported as median (interquartile range) or n (%).
Overall P value,
BAV/TAA and TAV/TAA are significantly different,
BAV/TAA and ATAAD are significantly different,
TAV/TAA and ATAAD are significantly different
Included coronary artery bypass grafting, mitral valve repair/replacement, tricuspid valve repair/replacement.
Abbreviations: ATAAD, acute type A aortic dissection; BAV, bicuspid aortic valve; CPB, cardiopulmonary bypass; HCA, hypothermic circulatory arrest; TAA, thoracic aortic aneurysm; TAV, tricuspid aortic valve.
SAEs and Abnormal Exercise Test Event Rates
There were no SAEs observed during or within 7 d of CPX procedures. Initially, 5 CPX were interpreted as abnormal, however, 2 CPX demonstrating ST segment depression or T wave inversion were determined to be “false positive” given echocardiographic findings of LVH not observed on the resting ECG. Three CPX were abnormal representing a 2% event rate with 1 event observed per group as follows: (1) 67-yr-old male post-op BAV/TAA had baseline 1st degree AV block and occasional sinus pauses on metoprolol for whom exercise testing was stopped due to a sudden transient Mobitz type I 2nd degree AV block at a VO2peak of 14.8 mL.kg−1.min−1 and RER 1.22; (2) 70-yr-old male post-op TAV/TAA with a LVEF of 50% and mild global hypokinesis on losartan, metoprolol, and furosemide with recent endorsements of positional lightheadedness who during CPX experienced a symptomatic decrease in BP from baseline 120/80 to 90/60 mmHg with rapid normalization upon sitting; CPX was stopped due to hypotension at a VO2peak of mL.kg−1.min−1 and RER 0.94; and (3) 57-yr-old male post-op ATAAD had LVH, normal LVEF, and a normal functioning dual chamber pacemaker (DDD mode) who experienced a symptomatic decrease in systolic BP of 30 mmHg related to upper tracking rate behavior at greater than 130 bpm with 2:1 conduction which normalized as the atrial rate slowed; CPX was stopped at VO2peak of 15.8 mL.kg−1.min−1 and RER 1.13.
CPX Procedure Outcomes
Details regarding CPX, HR, BP, and echocardiographic outcomes are provided in Table 3 and Figures 1 and 2. The median VO2peak was 36% below normative values (19.2 mL.kg−1.min−1 versus 29.5 mL.kg−1.min−1, P < 0.0001). When compared to normative values, the most significant decrement was observed in the ATAAD group (40% below normative values) compared to the BAV/TAA (32% below, p<0.05) and TAV/TAA groups (33% below, p<0.05). Eighty-one percent of studies were considered “peak” as defined by a RER ≥1.05. Primary reasons for CPX termination included general/leg fatigue (n=74), BP response (total n=21), maximal effort reported (n=13), technican termination (n=8), shortness of breath/lightheadedness (n=6), and patient request due to mouth piece or CPX discomfort (n=6). Only 2 patients whose CPX were terminated due to BP experienced a hypotensive response and met criteria for an abnormal CPX. The highest peak exercise BP was 210 mmHg, occurring in 2 patients within the BAV/TAA (n=1) and ATAAD (n=1) groups. Systolic BP was ≥180 mmHg in 26 patients (range 180–210 mmHg) occurring in BAV/TAA (n=10), TAV/TAA (n=7), and ATAAD (n=9) groups. The highest peak diastolic BP was 100 mmHg, occurring in 2 patients within the TAV/TAA (n=1) and ATAAD (n=1) groups. Diastolic BP was ≥90 in 14 patients (range 90–100) occurring in in BAV/TAA (n=7), TAV/TAA (n=4), and ATAAD (n=3) groups. Systolic and diastolic BPs were ≥ 180 mmHg / ≥ 90 mmHg in eight patients. The median 1-minute HR recovery was 12 bpm−1 (IQR: 7, 16) and 52% (66/128) of patients had an abnormal 1-minute HR recovery response; there were no significant differences between groups.
Table 3.
Cardiopulmonary Exercise Test Outcomes
| All Patients N=128 (100%) |
BAV/TAA N=49 (38%) |
TAV/TAA N=51 (40%) |
ATAAD N=28 (22%) |
P valuea | |
|---|---|---|---|---|---|
| Weight, kg | 90 (80,104) | 93 (84,106) | 89 (78,101) | 85 (83,96) | 0.17 |
| Body mass index, kg.m−2 | 28.0 (26.2,31.6) | 29.9 (26.3,33.6) | 28.0 (26.0,31.3) | 27.4 (26.3,29.9) | 0.25 |
| Resting, supine | |||||
| HR, bpm | 65 (59,73) | 67 (60,76) | 64 (57,71) | 63 (59,70) | 0.21 |
| Systolic BP, mmHg | 124 (116,138) | 123 (116,132) | 122 (112,134) | 134 (120,145) | 0.03c,d |
| Diastolic BP, mmHg | 77 (70,82) | 77 (68,82) | 76 (72,80) | 77 (70,82) | 0.92 |
| Anaerobic Threshold (AT) | |||||
| VO2 at AT, mL.kg−1.min−1 | 13.1 (11.4,15.2) | 13.6 (12.0,15.8) | 13.0 (11.1,14.9) | 13.1 (11.0,15.4) | 0.44 |
| VE/VCO2 slope at ATe,f | 25.9 (22.2,29.7) | 24.4 (22.1,28.0) | 27.4 (23.8,33.1) | 23.7 (20.8,26.9) | 0.03b,d |
| Peak | |||||
| HR, bpm | 123 (108,142) | 134(113,151) | 118(107,142) | 113(102,132) | 0.01c |
| % predicted, HR | 77 (68,87) | 81 (73,90) | 77 (67,89) | 71(64,77) | 0.002c,d |
| Systolic BP, mmHg | 160 (144,172) | 163 (151,175) | 156 (140,170) | 156 (140,180) | 0.23 |
| Diastolic BP, mmHg | 70 (62,80) | 70 (62,80) | 71 (64,80) | 70 (62,80) | 0.95 |
| VO2peak, mL.kg−1min−1 | 19.2 (15.9,22.9) | 20.3 (16.2,24.0) | 18.9 (15.9,22.5) | 18.7 (16.1,20.4) | 0.43 |
| Predicted VO2peak-FRIEND | 29.5 (26.4, 34.8) | 29.3 (27.3, 33.2) | 27.8 (23.2, 30.9) | 33.9 (27.7, 38.5) | 0.008d |
| % predicted, VO2peak-FRIEND | 64 (55,76) | 68 (57, 79) | 67 (58, 83) | 60 (47, 65) | 0.006c,d |
| Oxygen pulse | 14.5 (12.0,16.6) | 14.5 (12.8,17.0) | 14.0 (11.9,16.6) | 15.0 (11.9,16.5) | 0.55 |
| VE/VCO2 slopef | 28.3 (24.2,32.8) | 26.6 (24.0, 30.5) | 31.6 (27.2,34.5) | 25.0 (22.9,31.4) | 0.0008b,d |
| METs | 5.5 (4.5,6.5) | 5.8 (4.6,6.8) | 5.4 (4.5,6.4) | 5.3 (4.6,5.8) | 0.43 |
| Respiratory exchange ratio | 1.16 (1.09,1.24) | 1.19 (1.09,1.26) | 1.17 (1.10,1.25) | 1.12 (1.01,1.21) | 0.07 |
| Exercise test duration, sec | 600 (480,720) | 606 (540,720) | 600 (480,720) | 540 (427,685) | 0.13 |
| Recovery | |||||
| 1 min-HR, bpm | 12 (7,16) | 12(8,15) | 10(7,15) | 13(8,17) | 0.37 |
| % abnormal, 1 min-HR | 66 (52) | 25 (51) | 31 (63) | 10 (36) | 0.09 |
| Aortic insufficiency, mod-to-severe | 2 (2) | 0 | 1 (1.9) | 1 (3.6) | 0.23 |
| LVEF, % | 50 (50,60) | 50 (50,60) | 50 (50,60) | 60 (50,60) | 0.03c |
Data are reported as median (interquartile range) or n (%).
Overall p value,
BAV/TAA and TAV/TAA are significantly different,
BAV/TAA and ATAAD are significantly different,
TAV/TAA and ATAAD are significantly different
Slope from the start of exercise through AT
Slope was calculated using all exercise data (start through VO2peak)
Abbreviations: ATAAD, acute type A aortic dissection; BAV, bicuspid aortic valve; BP, bloods pressure; FRIEND, fitness registry and the importance of exercise national database; HR, heart rate; LVEF, left ventricular ejection fraction; MET, metabolic equivalent; TAA, thoracic aortic aneurysm; TAV, tricuspid aortic valve; VO2peak, peak oxygen consumption.
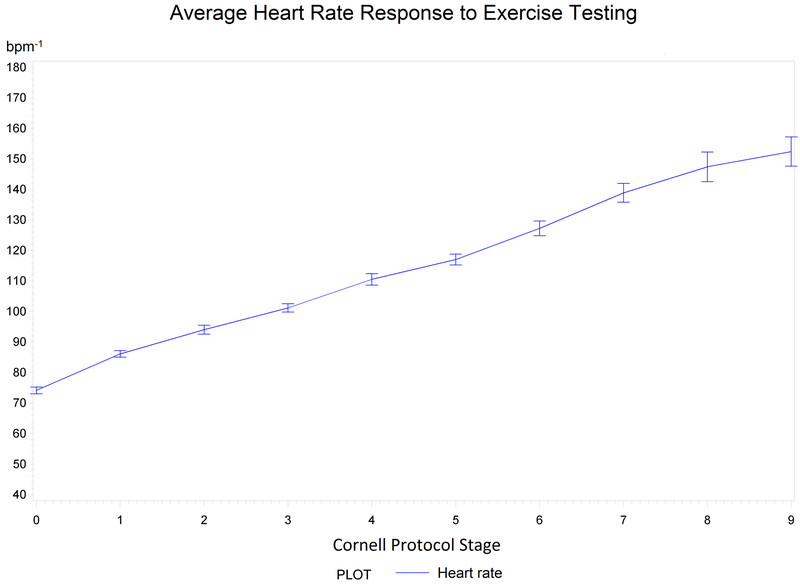
Figure 1.
Heart Rate Response to Exercise Testing. Median heart rate (bpm−1) with standard errors of means (combined all groups) per stage according to the Cornell Protocol.
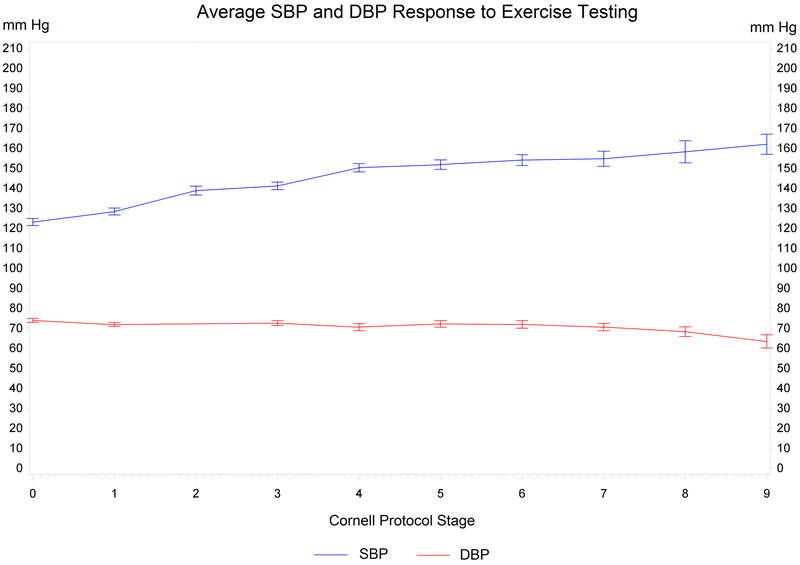
Figure 2.
Blood Pressure Response to Exercise Testing. Median systolic and diastolic blood pressures (mm Hg) with standard errors of means (combined all groups) per stage according to the Cornell Protocol.
Echocardiography Outcomes
There were no significant differences between groups for post-surgical aortic insufficiency, although LVEF was significantly higher in the ATAAD group compared to the BAV/TAA group (60% versus 50%, p<0.05) (Table 3).
DISCUSSION
To our knowledge, this is the first study to perform CPX procedures three months following repair for a proximal thoracic aortic aneurysm or an ATAAD. We report that the risk of a major CPX event appears to be low, as there were no SAEs and an abnormal CPX event rate of 2%. Second, the median VO2peak was 19.2 mL.kg−1.min−1 corresponding to 36% below normative values, indicating moderately reduced overall CRF among these patients. Third, we observed normal HR and BP responses to CPX for patients on anti-hypertensive medications.
First, this study provides evidence that the risk of a major event occurring during an acute bout of exercise appears to be low (no SAEs). A distinct minority (2%) of patients experienced unexpected but explainable abnormal ECG or BP responses to CPX. Our findings are similar to Delsart et. al.,24 who reported no SAEs and one abnormal exercise test. Test procedures were terminated due to VT resolving without clinical consequence, representing a <1% event rate among 105 type A and B aortic dissection patients 22 mo following hospitalization (not necessarily surgical repair). In contrast, our patients were evaluated 3 mo following surgery, which aligns with recommendations for referral to CR, in which an exercise test is typically conducted to ensure exercise safety/clearance and to guide exercise prescriptions.25, 26, 27 The present study extends upon prior work by including both thoracic aortic aneurysm and ATAAD phenotypes, which is important considering the pathophysiology of these presentations can vary. Additionally, ATAAD patients typically undergo more complex operations involving the aortic arch and hypothermic circulatory arrest, are at a greater risk for post-operative complications, and have prolonged hospitalizations (i.e., length of stay).
Second, VO2peak was 36% (median, 19.2 mL.kg−1.min−1) below normative values 3 mo following repair, which is moderately reduced compared to a mean VO2peak of 23.5 mL.kg−1.min−1 among 10 ATAAD patients 3 mo following repair. Delsart et. al.,24 reported a mean VO2peak of 19.2 mL.kg−1.min−1 among 105 type A and B aortic dissection patients; however, CPX was evaluated 22 ± 30 mo following hospitalization. Modest differences between studies may be attributable to sample size, timeframe of repair or hospitalization to CPX, and CPX modality (cycle ergometry versus treadmill).28 Nonetheless, VO2peak is reduced following repair and it may not may not recover years following repair or hospitalization. We contend that addressing this important knowledge gap is critical considering the established data highlighting the association between reduced VO2peak (1 MET or 3.5 mL.kg−1.min−1) and a 12–17% increased risk of CVD-related mortality.29, 30
Third, we observed normal HR and systolic BP (n=128, 124 mmHg at rest to 160 mmHg at peak) and diastolic BP (77 mmHg at rest to 70 mmHg at peak) responses to CPX for patients on anti-hypertensive medications (Table 3, Figures 1 and 2).31 The BP trends are similar to findings reported by Fuglsang et al. [systolic BP of 143 mmHg at rest to 200 mmHg at peak) and (diastolic BP of 80 mmHg at rest to 95 mmHg at peak)] with similar BP termination criteria, although BP was only reported for 10 ATAAD patients.22 Per the AHA Scientific Statement14 on standards for exercise testing, it is a relative indication to terminate exercise testing if BP is > 250 mmHg/115 mmHg. An exaggerated systolic BP response to exercise testing has been defined as a peak systolic BP of >210 mmHg for men and >190 mmHg for women. There is a need for more evidence to support the utilization of the above criteria when performing exercise testing in this high-risk population. Overall, it is reassuring that no one, male or female, demonstrated exaggerated BP responses as defined by the above systolic BP threshold during CPX procedures.32
Blood pressure responses to exercise may be the single most important factor guiding moderate-intensity physical activity recommendations in this patient population. International Registry of Acute Aortic Dissection (IRAD) data showed physical inactivity significantly increased seven years after discharge following an aortic dissection.4 Reduced VO2peak and exercise behavior may be the result of both clinicians being hesitant to promote exercise33 and patients being fearful4 of exercise given that it is physiologically plausible that acute elevations in BP, induced by vigorous-intensity physical activity (i.e., weight lifting34, 35), may increase the risk of aortic dissection, rupture, and/or death.8 Although, moderate-intensity exercise should be safe given that it confers only small increases in systolic BP and diastolic BP remains stable.36 IRAD data showed that systolic BP was significantly lower among post-dissection patients engaging in ≥ 2 sessions/wk of aerobic exercise 36 mo following discharge.4 Initial evidence provided by Corone et al. and Fuglsang et al. support the beneficial effects of moderate-intensity aerobic exercise on CRF22,37 and quality of life22 following surgical repair for an ATAAD.
There are important limitations that need to be considered when interpreting the findings. First, the sample size is small, limiting the statistical power to appropriately assess SAEs, abnormal CPX event rates, and VO2peak. Larger longitudinal studies are necessary to determine the safety of CPX following repair for a root and/or ascending aneurysm or ATAAD. Secondly, it can be argued that VO2peak was reduced compared to normative values since patients attaining a RER <1.05 were included. To address this point, we evaluated only patients achieving a RER >1.05, and VO2peak remained 34% (overall, 104/128) below normative values compared to 36% for all patients. We acknowledge the potential for selection bias as the patients were referred to CR or enrolled a research study. To address this limitation, we examined outcomes between the CR group (n=68) and the research study group (n=61). Given no differences in the primary endpoints, including SAEs, abnormal CPX event rates, and VO2peak, the data was combined for analysis (Supplemental Table 1). Finally, readmission events were examined via electronic medical record review within a 7 d timeframe following CPX procedures. We acknowledge that events may have occurred, as it is possible that patients could have been admitted elsewhere.
In conclusion, we provide preliminary evidence to clinicians that under proper conditions and physician supervision, CPX procedures can be conducted and a peak effort can be attained while maintaining an appropriate BP response to exercise testing. VO2peak was reduced among all patients (36% below normative values), especially ATAAD (40% below normative values), which may affect long-term survival. As such, prospective studies are warranted considering the well-established prognostic importance of reduced VO2peak among other clinical and healthy populations. Finally, the risks versus benefits of moderate-intensity aerobic exercise in this patient population are not known and future studies aiming to improve VO2peak are likely warranted. These studies have the potential to inform clinical practice by providing precision-based evidence to support the need for CR.
Supplementary Material
Acknowledgements
We thank all patients of the University of Michigan, Michigan Medicine for their contributions. We appreciate the contribution of cases from the aortic surgeons at the University of Michigan including George M. Deeb, Karen Kim, Bo Yang, and Himanshu Patel. We appreciate the valuable efforts from the exercise physiologists from the Preventive Cardiology Department at the University of Michigan (Jacob Sitzmann, Patrick Walden, Steven Walsh, Samantha Fink, and Joseph Bryant). We appreciate the Aikens Fund for Aortic Research (CJW, BY, WEH, SS). We also acknowledge the contributions of Anisa Driscoll and Linda Farhat.
External Financial Support
Bo Yang is supported by the National Institutes of Health (K08HL130614 and R01HL141891) and The Phil Jenkins and Darlene & Stephen J. Szatmari Funds
Cristen J. Willer is supported by the National Institutes of Health (R01-HL127564, R35-HL135824, and R01-HL142023)
Himanshu Patel is supported by The Joe D. Morris Collegiate Professorship, the David Hamilton Fund, and the Phil Jenkins Breakthrough Fund in Cardiac Surgery
Kim A. Eagle is consultant for NHLBI and has a research grant from Gore
Lee W Jones is supported by the National Cancer Institute, AKTIV Against Cancer, and the Kavli Trust
Sara Saberi is the site principal investigator for two clinical trials funded by Myokardia and receives financial support from Myokardia.
Internal Financial Support
Whitney E. Hornsby, Bo Yang, Cristen J. Willer, and Sara Saberi are supported by The University of Michigan, Michigan Medicine, Frankel Cardiovascular Center, Aikens Fund for Aortic Research
Footnotes
Conflicts of Interest
Himanshu J. Patel is a consultant for WL gore Edwards and Medtronic.
REFERENCES
- 1.Olsson C, Thelin S, Stahle E, Ekbom A, Granath F. Thoracic aortic aneurysm and dissection: increasing prevalence and improved outcomes reported in a nationwide population-based study of more than 14,000 cases from 1987 to 2002. Circulation. 2006;114(24):2611–2618. [DOI] [PubMed] [Google Scholar]
- 2.Ramanath VS, Oh JK, Sundt TM3rd, Eagle KA. Acute aortic syndromes and thoracic aortic aneurysm. Mayo Clin Proc. 2009;84(5):465–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Erbel R, Aboyans V, Boileau C, et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J. 2014;35(41):2873–2926. [DOI] [PubMed] [Google Scholar]
- 4.Chaddha A, Kline-Rogers E, Braverman AC, et al. Survivors of Aortic Dissection: Activity, Mental Health, and Sexual Function. Clin Cardiol. 2015;38(11):652–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.ACSM’s Guidelines for Exercise Testing and Prescription 10th ed: Wolters Kluwer; 2017. [Google Scholar]
- 6.Anderson L, Oldridge N, Thompson DR, et al. Exercise-Based Cardiac Rehabilitation for Coronary Heart Disease: Cochrane Systematic Review and Meta-Analysis. J Am Coll Cardiol. 2016;67(1):1–12. [DOI] [PubMed] [Google Scholar]
- 7.Svensson LG, Adams DH, Bonow RO, et al. Aortic valve and ascending aorta guidelines for management and quality measures. Ann Thorac Surg. 2013;95(6 Suppl):S1–66. [DOI] [PubMed] [Google Scholar]
- 8.Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: executive summary. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Catheter Cardiovasc Interv. 2010;76(2):E43–86. [DOI] [PubMed] [Google Scholar]
- 9.Hollenberg M, Ngo LH, Turner D, Tager IB. Treadmill exercise testing in an epidemiologic study of elderly subjects. J Gerontol A Biol Sci Med Sci. 1998;53(4):B259–267. [DOI] [PubMed] [Google Scholar]
- 10.Loeys BL, Dietz HC, Braverman AC, et al. The revised Ghent nosology for the Marfan syndrome. J Med Genet. 2010;47(7):476–485. [DOI] [PubMed] [Google Scholar]
- 11.Yang B, DeBenedictus C, Watt T, et al. The impact of concomitant pulmonary hypertension on early and late outcomes following surgery for mitral stenosis. J Thorac Cardiovasc Surg. 2016;152(2):394–400 e391. [DOI] [PubMed] [Google Scholar]
- 12.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skalski J, Allison TG, Miller TD. The safety of cardiopulmonary exercise testing in a population with high-risk cardiovascular diseases. Circulation. 2012;126(21):2465–2472. [DOI] [PubMed] [Google Scholar]
- 14.Fletcher GF, Ades PA, Kligfield P, et al. Exercise standards for testing and training: a scientific statement from the American Heart Association. Circulation. 2013;128(8):873–934. [DOI] [PubMed] [Google Scholar]
- 15.Balady GJ, Arena R, Sietsema K, et al. Clinician’s Guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation. 2010;122(2):191–225. [DOI] [PubMed] [Google Scholar]
- 16.ATS/ACCP Statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003;167(2):211–277. [DOI] [PubMed] [Google Scholar]
- 17.Myers J, Kaminsky LA, Lima R, Christle JW, Ashley E, Arena R. A Reference Equation for Normal Standards for VO2 Max: Analysis from the Fitness Registry and the Importance of Exercise National Database (FRIEND Registry). Prog Cardiovasc Dis. 2017;60(1):21–29. [DOI] [PubMed] [Google Scholar]
- 18.Kaminsky LA, Arena R, Myers J. Reference Standards for Cardiorespiratory Fitness Measured With Cardiopulmonary Exercise Testing: Data From the Fitness Registry and the Importance of Exercise National Database. Mayo Clin Proc. 2015;90(11):1515–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saberi S, Wheeler M, Bragg-Gresham J, et al. Effect of Moderate-Intensity Exercise Training on Peak Oxygen Consumption in Patients With Hypertrophic Cardiomyopathy: A Randomized Clinical Trial. Jama. 2017;317(13):1349–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keteyian SJ, Brawner CA, Ehrman JK, Ivanhoe R, Boehmer JP, Abraham WT. Reproducibility of peak oxygen uptake and other cardiopulmonary exercise parameters: implications for clinical trials and clinical practice. Chest. 2010;138(4):950–955. [DOI] [PubMed] [Google Scholar]
- 21.Guazzi M, Adams V, Conraads V, et al. EACPR/AHA Scientific Statement. Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation. 2012;126(18):2261–2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fuglsang S, Heiberg J, Hjortdal VE, Laustsen S. Exercise-based cardiac rehabilitation in surgically treated type-A aortic dissection patients. Scand Cardiovasc J. 2017;51(2):99–105. [DOI] [PubMed] [Google Scholar]
- 23.Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS. Heart-rate recovery immediately after exercise as a predictor of mortality. N Engl J Med. 1999;341(18):1351–1357. [DOI] [PubMed] [Google Scholar]
- 24.Delsart P, Maldonado-Kauffmann P, Bic M, et al. Post aortic dissection: Gap between activity recommendation and real life patients aerobic capacities. Int J Cardiol. 2016;219:271–276. [DOI] [PubMed] [Google Scholar]
- 25.Dafoe W, Arthur H, Stokes H, Morrin L, Beaton L, Canadian Cardiovascular Society Access to Care Working Group on Cardiac R. Universal access: but when? Treating the right patient at the right time: access to cardiac rehabilitation. Can J Cardiol. 2006;22(11):905–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pack QR, Lahr BD, Squires RW, et al. Survey Reported Participation in Cardiac Rehabilitation and Survival After Mitral or Aortic Valve Surgery . Am J Cardiol. 2016;117(12):1985–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Price KJ, Gordon BA, Bird SR, Benson AC. A review of guidelines for cardiac rehabilitation exercise programmes: Is there an international consensus? Eur J Prev Cardiol. 2016;23(16):1715–1733. [DOI] [PubMed] [Google Scholar]
- 28.Miyamura M, Honda Y. Oxygen intake and cardiac output during maximal treadmill and bicycle exercise. J Appl Physiol. 1972;32(2):185–188. [PubMed] [Google Scholar]
- 29.Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346(11):793–801. [DOI] [PubMed] [Google Scholar]
- 30.Gulati M, Pandey DK, Arnsdorf MF, et al. Exercise capacity and the risk of death in women: the St James Women Take Heart Project. Circulation. 2003;108(13):1554–1559. [DOI] [PubMed] [Google Scholar]
- 31.Fletcher GF, Balady G, Froelicher VF, Hartley LH, Haskell WL, Pollock ML. Exercise standards. A statement for healthcare professionals from the American Heart Association. Writing Group. Circulation. 1995;91(2):580–615. [DOI] [PubMed] [Google Scholar]
- 32.Le VV, Mitiku T, Sungar G, Myers J, Froelicher V. The blood pressure response to dynamic exercise testing: a systematic review. Prog Cardiovasc Dis. 2008;51(2):135–160. [DOI] [PubMed] [Google Scholar]
- 33.Chaddha A, Eagle KA, Braverman AC, et al. Exercise and Physical Activity for the Post-Aortic Dissection Patient: The Clinician’s Conundrum. Clin Cardiol. 2015;38(11):647–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elefteriades JA, Hatzaras I, Tranquilli MA, et al. Weight lifting and rupture of silent aortic aneurysms. JAMA. 2003;290(21):2803. [DOI] [PubMed] [Google Scholar]
- 35.Hatzaras I, Tranquilli M, Coady M, Barrett PM, Bible J, Elefteriades JA. Weight lifting and aortic dissection: more evidence for a connection. Cardiology. 2007;107(2):103–106. [DOI] [PubMed] [Google Scholar]
- 36.Williams MA, Haskell WL, Ades PA, et al. Resistance exercise in individuals with and without cardiovascular disease: 2007 update: a scientific statement from the American Heart Association Council on Clinical Cardiology and Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2007;116(5):572–584. [DOI] [PubMed] [Google Scholar]
- 37.Corone S, Iliou MC, Pierre B, et al. French registry of cases of type I acute aortic dissection admitted to a cardiac rehabilitation center after surgery. Eur J Cardiovasc Prev Rehabil. 2009;16(1):91–95. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.