Abstract
Gastric cancer remains a leading cause of cancer-related mortality. Identifying dietary and other modifiable disease determinants has important implications for risk attenuation in susceptible individuals. Our primary aim was to estimate the association between dietary and supplemental intakes of calcium and magnesium and the risk of incident gastric cancer. We conducted a prospective cohort analysis of the National Institutes of Health-American Association of Retired Persons Diet and Health Study. We used Cox proportional hazard modeling to estimate the association between calcium and magnesium intakes with risk of incident gastric adenocarcinoma (GA) overall and by anatomic location, noncardia GA (NCGA) and cardia GA (CGA). A total of 536,403 respondents (59% males, 41% females) were included for analysis, among whom 1,518 incident GAs (797 NCGA and 721 CGA) occurred. Increasing calcium intake was associated with lower risk of GA overall (p-trend = 0.05), driven primarily by the association with NCGA, where the above median calcium intakes were associated with a 23% reduction in risk compared to the lowest quartile (p-trend = 0.05). This magnitude of NCGA risk reduction was greater among nonwhite ethnic group and Hispanics (hazard ratio [HR] 0.51, 95% confidence interval [CI]: 0.24–1.07, p-trend = 0.04), current/former smokers (HR 0.58, 95% CI: 0.41–0.81), obese individuals (HR 0.54, 95% CI: 0.31–0.96) and those with high NCGA risk scores (HR 0.50, 95% CI: 0.31–0.80). Among men only, increasing magnesium intake was associated with 22–27% reduced risk of NCGA (p-trend = 0.05), while for the cohort, dietary magnesium intake in the highest vs. lowest quartile was associated with a 34% reduced risk of NCGA (HR 0.66, 95% CI: 0.48–0.90). These findings have important implications for risk factor modification. Future investigations are needed not only to confirm our results, but to define mechanisms underlying these associations.
Keywords: gastric neoplasm, epidemiology, environment and public health, digestive system neoplasm
Introduction
Worldwide, gastric cancer remains the fifth most common cancer and the second leading cause of cancer-related death worldwide, with an estimated 1 million new cases per year and over 780,000 people dying from the disease annually.1 While gastric cancer remains relatively rare in the United States, ranking 15th most common cancer overall but second most common gastrointestinal tract cancer, the incidence and mortality rates are increasing disproportionately among some groups, such as minorities and women, for unclear reasons.2–4 There are two main anatomic sites for gastric adenocarcinoma (GA)—noncardia GA (NCGA) and cardia GA (CGA)—and the pathogenesis of these subtypes are likely distinct based on epidemiologic trends and risk factor profiles.5 While the majority of gastric cancers worldwide are NCGA, the incidence gap between CGA and NCGA is narrower in the United States.6,7
Gastric cancer pathogenesis is multifactorial and represents a complex interaction of host genetic determinants, microbial virulence factors (Helicobacter pylori and non-H. pylori), as well as environmental constituents.8,9 Research to identify modifiable risk factors, such as diet, has important implications for decreasing the burden of gastric cancer. Calcium (Ca) and magnesium (Mg) are dietary elements that are essential for homeostasis and altered intake has been associated with disease risk. For example, low Mg intake has been associated with metabolic syndrome and insulin resistance, coronary artery disease, systemic inflammation and increased cancer risk.10–12 As divalent cations, Ca and Mg directly or indirectly compete for the same transporters in the intestine and kidney to maintain homeostasis.12 Given their close physiologic relationship, we have evaluated the ratio of calcium to magnesium intake (Ca:Mg ratio) as an effect modifier of the relationship between intake of the single nutrient and disease risk.13–15 There is also geographic variation in the Ca:Mg ratio patterns even when intake of the single nutrient may be similar. In general, the U.S. population has adequate to high Ca:Mg ratios and East Asian populations have adequate to low Ca:Mg ratios, although the mean intake of Mg is similar between the United States and East Asia.13 In contrast to findings from populations with high Ca:Mg ratios, among population-based cohorts from China with low Ca:Mg ratios, high Mg intake was associated with 24–66% higher all-cause mortality and cardiovascular mortality, and 120% increased risk of colorectal cancer mortality, but only when Ca:Mg ratios were low.13 We also found that in studies conducted in the United States16–18 or Ireland,12 higher intakes of Ca and Mg were associated with a decreased risk of colorectal adenomas, Barrett’s esophagus and esophageal reflux, but only when the Ca:Mg ratio was not high.
To our best knowledge, only one study has analyzed the association of Ca intake and risk of incident gastric cancer. Our study by Park et al. analyzed the association between dietary and supplemental Ca intakes or dairy intake and the risk of site-specific cancers among the National Institutes of Health-American Association of Retired Persons (NIH-AARP) Diet and Health Study cohort in an early study before follow-up was complete.19 While the authors observed an inverse association between dairy intake and gastric cancer among men and a weak inverse association among women, they reported no statistically significant association between dietary or supplemental Ca intake and gastric cancer overall; anatomic gastric cancer subtypes were not evaluated. No study has analyzed the association with Mg intake. The association of Ca and Mg with gastric cancer risk has thus been incompletely evaluated, particularly in a Western population where gastric cancer is rarer and Ca:Mg ratios are more often normal to high.
We hypothesized that higher intakes of Ca and Mg will be associated with reduced risk of GA overall, with similar directionality of effect estimates for NCGA and CGA but differential magnitude. Our aim, therefore, was to prospectively analyze the association of dietary and supplemental intakes of Ca and Mg, with risk of incident GA, stratified by anatomic location, Ca:Mg ratio and relevant clinical factors, in an updated analysis of the NIH-AARP Diet and Health Study.
Methods
Study population
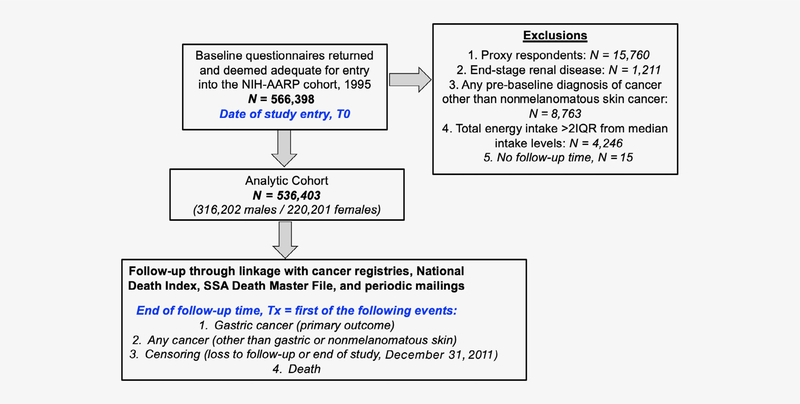
The NIH-AARP Diet and Health Study was developed at the U.S. National Cancer Institute (NCI) and is the largest prospective cohort with dietary data and cancer outcomes in the United States. Details of this cohort have been described previously.20 Briefly, from 1995 to 1996, approximately 3.5 million questionnaires were mailed to AARP members aged 50–71 who lived in 1 of 8 states. A total of 566,398 baseline questionnaires were returned and deemed adequate for entry into the NIH-AARP cohort. Date of study entry (T0) was defined as the date of receipt of the baseline questionnaire at the NIH (1995–1996). Follow-up of this cohort continued prospectively until December 2011, with record linkage to vital statistics (Social Security Administration Death Master File and National Death Index) and cancer registries within the eight states.
Inclusion and exclusion criteria
Nonproxy respondents who completed the baseline questionnaire according to quality metrics predefined by the NIH-AARP were eligible for inclusion.20 To construct the cohort for the present analysis, we subsequently excluded the following respondents: proxy respondents (n = 15,760); end-stage renal disease at or prior to study entry (n = 1,211); any diagnosis of cancer (other than nonmelanomatous skin cancer) at or prior to study entry (n = 8,763); total energy intake (kilocalories [kcal]/day) more than two interquartile ranges from the median intake level of the cohort, adjusted for gender (n = 4,246) and negative or zero follow-up time (n = 15). There were no a priori exclusions based on the length of follow-up time. However, a sensitivity analysis was performed to evaluate those participants who were diagnosed with gastric cancer within 12 months of follow-up (n = 125), given the possibility that these might represent prevalent (as opposed to incident) cancers.
After application of exclusion criteria, a total of 536,403 respondents (220,201 females, 316,202 males) comprised the analytic cohort for our primary analysis (Figure 1).
Figure 1.
Flow diagram of analytic cohort selection from the National Institutes of Health-American Association of Retired Persons Diet and Health Study cohort. [Color figure can be viewed at wileyonlinelibrary.com]
Ethical oversight
The study was approved by the Special Studies Institutional Review Board of the U.S. NCI. Informed consent was obtained by the NIH-AARP investigators at the study inception.
Nutritional intakes (exposure assessment)
The baseline questionnaire included a validated, comprehensive self-administered 124-item semiquantitative food frequency questionnaire (FFQ), which was developed at the NCI.21 Respondents were asked to estimate their average daily intakes of foods and beverages over the preceding 12 months using 10 frequency categories (range: “never” to “6 or more times per day” for beverages; “never” to “two or more times per day” for solid foods) and three portion size categories. The food items, standardized frequency and portion categories and the nutrient database were adapted from the U.S. Department of Agriculture’s (USDA) 1994–1996 Continuing Survey of Food Intake by Individuals.22 This FFQ has been validated for the NIH-AARP cohort using two nonconsecutive 24-hr recalls in nearly 2,000 participants.21 The Healthy Eating Index (HEI) score was also derived from the FFQ data. The HEI score includes 13 components that overall measure compliance with the Dietary Guidelines for Americans, with 100 being the highest score (perfect compliance).23,24
Dietary (nonsupplemental), supplemental and total Ca and Mg intakes were reported in milligrams according to responses from the validated 124-item comprehensive FFQ. For each food item, the frequency (how many times a participant ate the food per day) was recorded and the amount in grams was calculated using the DietAARP food database. Total intake represented the sum of dietary and supplemental intakes. Supplemental intakes of Ca and Mg represented supplemental intakes from Ca- or Mg-containing multivitamins, supplements, or other nondietary sources. In the NIH-AARP Diet and Health Study, other than dairy vs. nondairy intake of Ca, dietary and supplemental Ca and Mg intakes are available only as composite sums; accordingly, individual components, such as percentage of supplemental intake from Ca- or Mg-containing multivitamins are unable to be determined. Ca:Mg ratio was calculated as the ratio of total Ca intake (milligrams) to total Mg intake (milligrams). Based on our prior studies in colorectal adenomas and cancer,16,17 we used the following previously determined Ca:Mg ratio cutoffs: low Ca:Mg (<1.7), normal Ca:Mg (1.7–2.6), high Ca:Mg (>2.6) ratios.
Cancer ascertainment
Incident cancer cases were identified through linkage to state cancer registries. In addition to the original eight state cancer registries, starting in 2003 cancer linkage also occurred from three additional states (Arizona, Nevada and Texas). Thus, if respondents from the 1995–1996 baseline questionnaire moved to Arizona, Nevada, or Texas and were diagnosed with cancer, this would be captured. During the study period, each cancer registry was certified by the North American Association of Central Cancer Registries as being at least 90% complete within 2 years of cancer diagnosis. Linkage to cancer registries is excellent in the NIH-AARP, with approximately 90% accuracy.25
Cancer sites were identified by anatomic site and histologic code of the International Classification of Disease for Oncology (ICD-O, third edition). CGA and NCGA cases were identified as ICD-O codes C16.0 and C16.1–16.9, respectively.
Study outcomes and follow-up
The primary outcomes were incident diagnosis of any GA, NCGA and CGA. Vital status and cancer diagnosis were ascertained by periodic linkage to the Social Security Administration Death Master file, follow-up searches of the National Death Index, linkage with cancer registries as described below and other mailings.
The follow-up time was defined as the time from the baseline questionnaire to the earliest of the following events: gastric cancer diagnosis, any cancer diagnosis other than gastric or nonmelanomatous skin cancer, censoring, or death. Individuals were censored at loss to follow-up due to respondent relocation outside of the cancer registry coverage area or the end of the follow-up period for the study (December 31, 2011). People who moved out of the coverage area but then returned remain included in the analysis if they otherwise meet the aforementioned inclusion criteria. Because the time periods are nonoverlapping and the endpoint occurs only once, statistical independence is preserved. Over a total of 6,421,022 person-years of follow-up time, 1,518 incident cases of GA occurred (797 NCGA, 721 CGA).
Statistical analysis
Ca and Mg intakes were categorized into quantiles using the distribution among the analytic cohort. We analyzed both gender-specific and common quantiles. Because there was no substantive difference in findings between these, we elected to use common quantiles throughout the analysis.
Baseline demographic and lifestyle variables were presented according to the above vs. below median Ca and Mg intakes for the cohort, with nutritional intake values additionally adjusted for age, gender and energy intake using generalized linear regression modeling. Student’s t-test and Chi2 tests were used for between-group comparisons of means and frequencies for continuous and categorical variables, respectively.
Minimally adjusted (age, gender, ethnicity and energy intake) and fully adjusted Cox proportional hazard models were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for risk of incident GA overall and stratified by anatomic location. Schoenfeld residuals were used to test the proportional hazards assumption. The underlying timescale was follow-up years (person-years).
Factors previously identified or hypothesized to be associated with GA or the exposures were evaluated as potential confounders or effect modifiers. These included age (continuous) gender (categorical, dichotomous: male, female), ethnicity (categorical: non-Hispanic white, non-Hispanic black, Hispanic, Asian, Pacific Islander/Alaskan natives/Native Americans/other), obesity (categorical, dichotomous: body mass index (body mass index [BMI, kg/m2] ≥ 30, BMI < 30), highest level of education achieved (categorical: less than high school, completed high school, some posthigh school training, or completed college or any postgraduate training), smoking status (categorical: never smoker, former smoker, current smoker), self-reported health status (categorical: excellent or very good, good, fair or poor) and alcohol intake by drinks per day (categorical: none, up to 1 drink/day, up to 2 drinks/day, 2–3 drinks/day, >3 drinks/day) and according to gender-specific standards of moderate/heavy alcohol consumption (categorical, dichotomous: yes, no). Although respondents were asked about family history of cancer, data on family history of gastric cancer specifically were not available. Each covariate was adjusted for age, gender and total daily energy intake (calories) and individually tested for an association between the primary exposure variables (Ca, Mg) and GA (overall, NCGA and CGA). Covariates that were associated with both the exposure and outcome were included in the multivariable model due to their potential as confounders. These covariates included: ethnicity (collapsed categorization as non-Hispanic white vs. nonwhite, which included non-Hispanic black, Hispanic, Asian and Pacific Islander/Alaskan natives/Native Americans/other); obesity; smoking status (collapsed categorization as never smoker vs. ever smoker, which included current or former smokers); highest level of education achieved; and self-reported health status (collapsed categorization as excellent/very good/good vs. fair/poor). For models with Ca as the primary exposure, Mg was included as a continuous covariate; for models with Mg as the primary exposure, Ca was included as a continuous covariate. We adjusted for total daily energy intake using the standard multivariate model method.26 Comparison to other common statistical methods for energy adjustment including the energy density model and the residual method yielded no significant differences in final conclusions. Linear dose–response was evaluated by including the quantile as a continuous variable in the model. The reference group for all analyses was the lowest quantile of intake.
Multiple imputation was planned a priori for covariates with >10% missing data. Because all covariates had only <5% missing data, including smoking (3.7% missing data) and ethnicity (1.3% missing data), missing or unknown values for covariates were excluded from the analysis.
We conducted stratified analyses to evaluate effect modification including a priori selection of the Ca:Mg ratio, gender, ethnicity, obese status, smoking status and risk for NCGA or CGA based on risk factor scoring (see below). We tested for statistical interactions using the −2 log (likelihood) test comparing models with and without the interaction term. We additionally evaluated the association of Ca and Mg intakes with risk of NCGA and CGA risk according to a quantitative risk score calculated based on known demographic and clinical risk factors for these cancers. For NCGA, one point was given for each of the following risk factors: male, nonwhite ethnic group (Hispanic, black, Asian and other), age > 65 and ever smoker (maximum 4 points); while for CGA, 1 point was given for the same factors, except that non-Hispanic white ethnic group instead of nonwhite ethnic group received 1 point. These scores were tested for their association with NCGA and CGA within the NIH-AARP cohort. For NCGA, having a moderate (2 risk factors) or high (3–4 risk factors) risk score was associated with a statistically significant 2.1- or 4-fold higher risk of NCGA, respectively, compared to the lowest risk score (0–1 risk factors). For CGA, moderate (3 risk factors) or high (4 risk factors) risk scores were associated with a 3.5- or 6.3-fold higher risk of CGA, respectively, compared to the lowest risk score (0–2 risk factors). Additional stratified analyses included Ca intake source (dietary vs. supplemental; dairy vs. nondairy), as well as dietary Mg intake by quartiles. Supplemental Mg intake was 100 mg for the majority (71.2%) of participants; thus, supplemental Mg intake was not separately evaluated. Dairy vs. nondairy sources of Mg were not available.
A sensitivity analysis excluding respondents with less than 12 months of follow-up time (i.e., cancer diagnosis, relocation outside of study coverage area, death) was planned a priori.
All analyses were conducted in Stata version 15 (StataCorp, College Station, TX). Statistical significance was set at α of 0.05 for all analyses.
Data availability
The data supporting the findings of our study are available from the NIH AARP Diet and Health Study. Restrictions apply to the availability of these data, which were used under license for our study. Data are available with the permission of the NIH AARP.
Results
Cohort characteristics by intake levels of Ca and Mg
The median total Ca intake was 881.3 mg and Mg intake was 358.6 mg. The baseline characteristics of study participants stratified by above vs. below median intakes of Ca or Mg are detailed in Table 1. In general, with the exception of gender, the absolute difference in mean value or distribution for each characteristic was similar irrespective of Ca or Mg intake although these differences were statistically significant due to the very large sample size. The mean age was 62 years. Most participants were non-Hispanic white, former or current smokers and high school graduates or above. In comparison to participants with above median Ca or Mg intakes, participants with below median intakes were more likely to be non-Hispanic whites, ever smokers (low Ca), never smokers (low Mg), moderate/heavy alcohol drinkers (low Ca), to self-report lower overall health, to have lower educational attainment and to have a higher HEI. The majority of participants in the NIH-AARP cohort overall reported moderate-heavy alcohol use by gender-specific standards.
Table 1.
Cohort characteristics stratified by median calcium and magnesium total intakes, NIH-AARP Diet and Health Study 1995–2011
| Characteristics | Calcium intake (median: 881.3 mg) |
p-value | Magnesium intake (median: 358.6 mg) |
p-value | ||
|---|---|---|---|---|---|---|
| Below median (N = 268,211) | Above median (N = 268,192) | Below median(N = 268,252) | Above median (N = 268,151) | |||
| Age at entry (mean, SD) (years) | 61.6 (5.4) | 61.7 (5.4) | <0.001 | 61.7 (5.4) | 61.6 (5.4) | <0.001 |
| Male (%) | 63.9% | 54.0% | <0.001 | 52.0% | 65.9% | <0.001 |
| Ethnicity (%) | <0.001 | <0.001 | ||||
| Non-Hispanic white | 91.7% | 93.6% | 92.0% | 93.2% | ||
| Non-Hispanic black | 4.6% | 3.1% | 4.4% | 3.4% | ||
| Hispanic | 1.9% | 1.9% | 1.9% | 1.9% | ||
| Asian | 1.4% | 1.0% | 1.4% | 1.1% | ||
| Pacific Islanders/Alaskan/ Native American/Other | 0.4% | 0.4% | 0.4% | 0.4% | ||
| BMI (mean, SD) (m/kg2) | 27.2 (5.0) | 27.0 (5.2) | <0.001 | 27.1 (5.2) | 27.1 (5.0) | <0.001 |
| Obese status (BMI > 30) (%) | 24.3% | 23.6% | <0.001 | 24.3% | 23.6% | <0.001 |
| Smoking status (%) | <0.001 | <0.001 | ||||
| Never smoker | 34.5% | 38.0% | 38.0% | 34.5% | ||
| Former smoker | 52.2% | 50.5% | 49.4% | 53.3% | ||
| Current smoker | 13.3% | 11.5% | 12.6% | 12.2% | ||
| Alcohol use (%) | <0.001 | <0.001 | ||||
| None | 23.8% | 25.3% | 26.0% | 23.1% | ||
| Up to 1 drink/day (but not 0 g/day) | 18.7% | 19.9% | 21.9% | 16.7% | ||
| Up to 2 drinks/day | 8.8% | 8.5% | 9.2% | 8.1% | ||
| 2–3 drinks/day | 6.4% | 6.3% | 6.6% | 6.1% | ||
| >3 drinks/day | 42.2% | 40.1% | 36.2% | 46.1% | ||
| Moderate/heavy alcohol use, gender-specific standards (%) | 51.6% | 50.2% | <0.001 | 46.8% | 54.9% | <0.001 |
| Highest education achieved (%) | <0.001 | <0.001 | ||||
| Less than high school | 27.3% | 25.3% | 28.1% | 24.5% | ||
| Completed high school | 10.2% | 10.1% | 10.2% | 10.0% | ||
| Some posthigh school training | 23.8% | 24.0% | 24.3% | 23.5% | ||
| Completed college and/or postgraduate | 38.7% | 40.7% | 37.4% | 42.0% | ||
| Self-reported health (%) | <0.001 | <0.001 | ||||
| Excellent or very good | 51.0% | 53.1% | 50.6% | 53.4% | ||
| Good | 35.8% | 34.1% | 35.9% | 34.0% | ||
| Fair or poor | 13.2% | 12.8% | 13.5% | 12.6% | ||
| Daily energy intake1 (mean, SD) (kcal/day) | 1869.2 (222.4) | 1824.1(230.9) | <0.001 | 1815.0(230.3) | 1878.4(220.8) | <0.001 |
| Healthy Eating Index score2 (mean, SD) | 68.1 (1.6) | 67.2 (2.1) | <0.001 | 68.5 (1.4) | 66.7 (2.0) | <0.001 |
| Total calcium intake2 (mean, SD) (mg) | 899.1 (218.5) | 1,139.7 (305.8) | <0.001 | 877.1(193.5) | 1,161.8(303.8) | <0.001 |
| Dietary, total (mean, SD) (mg) | 668.3 (205.7) | 887.3 (320.1) | 618.2 (165.6) | 937.5 (300.5) | ||
| Dairy source (mean, SD) (mg) | 369.6 (116.0) | 494(180.0) | 342.0(93.7) | 521.6(169.5) | ||
| Nondairy source (mean, SD) (mg) | 298.8(89.9) | 393.2(140.2) | 276.2(72.2) | 415.9(131.2) | ||
| Supplemental intake (mean, SD) (mg) | 545.9 (37.2) | 699.8(74.1) | 570.1 (55.3) | 536.4 (58.6) | ||
| Calcium supplement (yes) (%) | 30.2% | 71.9% | <0.001 | 54.8% | 55.6% | <0.001 |
| Total magnesium intake2 (mean, SD) (mg) | 335.8 (82.3) | 421.3(128.3) | <0.001 | 314.7(66.1) | 442.5(119.8) | <0.001 |
| Dietary, total (mean, SD) (mg) | 291.7 (84.4) | |||||
| Supplemental intake (mean, SD) (mg) | 93.2 (1.5) | 93.7 (1.9) | 93.0 (1.4) | 93.8 (2.0) | ||
| Magnesium supplement (yes) (%) | 40.3% | 65.0% | <0.001 | 36.4% | 69.0% | <0.001 |
Adjusted for age, gender.
Adjusted for age, gender, energy intake.
Abbreviations: NIH-AARP, National Institutes of Health-American Association of Retired Persons; SD, standard deviation.
When adjusted for age and gender, participants with below median Ca intake had slightly higher average daily caloric intake (1869 vs. 1824 kcal/day, p < 0.001) and participants with below median Mg intake had slightly lower (1815 vs. 1878, p < 0.001) average caloric daily intakes compared to participants with above median Ca and Mg intakes, respectively. Only a minority of men and women achieved the age-and gender-specific Recommended Dietary Allowance (RDA) for Ca (11.2% women and 24.7% men) and Mg (34.7% women and 25.2% men) in the cohort (data not shown in table). Regarding supplemental Ca intake, 73.7% of women and 38.9% of men overall reported ever taking Ca-containing supplements; of these individuals, 67% of women and 53% of men reported taking supplemental Ca daily. Regarding supplemental Mg intake, 52.7% of all participants (49.6% of all male participants, 57.1% of all female participants) reported taking Mg-containing supplements; of these individuals, 71.2% reported taking 100 mg of supplemental Mg daily (data not shown in table).
Association of Ca and Mg intakes with gastric cancer
Table 2 shows the associations of total (dietary and supplemental) Ca and Mg intakes with risk of total incident GA, NCGA and CGA. Increasing Ca was inversely associated with GA in minimally and fully adjusted models (p-trend = 0.04 and p = 0.05, respectively) (Table 2). There was no significant association between Mg intake and GA. Compared to the lowest quartile of Ca intake, higher intakes of Ca were inversely associated with risk of GA (p-trend = 0.05) and strongest for quartile 3 (HR 0.82, 95% CI: 0.69–0.97). A similar pattern of association was observed for NCGA (p-trend, fully adjusted model = 0.05) with Ca intakes in quartile 3 associated with the lowest risk of NCGA (HR 0.77, 95% CI: 0.61–0.98). While increasing Mg was inversely associated with risk of NCGA in the minimally adjusted model (p-trend =0.04), this association was no longer significant after adjusting for relevant confounders (p-trend = 0.14). There were no significant associations between total Ca and Mg intakes and CGA. In sensitivity analyses, excluding respondents with less than 12 months follow-up time did not significantly affect the findings overall (Table S1).
Table 2.
Associations between calcium and magnesium total intakes and risk of gastric cancer (total GA, NCGA, CGA), NIH-AARP Diet and Health Study 1995–2011
| Total follow-up time: 6,421,022 person-years | Quartiles of calcium or magnesium intake |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Quartile 1 (low): Ca, mean (SD): 440 (111) mg, mean (SD): 215 (45) mg |
Quartile 2: Ca, mean (SD): 737 (80) mg; Mg, mean (SD): 318 (24) mg |
Quartile 3: Ca, mean (SD): 1068 (119) mg; Mg, mean (SD): 404 (29) mg |
Quartile 4 (high): Ca, mean (SD): 1833 (515) mg; Mg, mean (SD): 578 (124) mg |
||||||
| Cases/person-Year | HR, 95% CI | Cases | HR, 95% CI | Cases | HR, 95% CI | Cases | HR, 95% CI | p-Trend | |
| GA, total (1,518 cases1) | 427 | 404 | 359 | 328 | |||||
| Calcium | |||||||||
| Minimally adjusted2 | 1.00 (ref) | 0.91 (0.78–1.05) | 0.83 (0.71–0.98) | 0.84 (0.69–1.02) | 0.04 | ||||
| Fully adjusted2,3 | 1.00 (ref) | 0.94 (0.81–1.09) | 0.82 (0.69–0.97) | 0.85 (0.70–1.04) | 0.05 | ||||
| Magnesium | 355 | 391 | 384 | 388 | |||||
| Minimally adjusted2 | 1.00 (ref) | 1.03 (0.89–1.20) | 0.96 (0.82–1.13) | 0.88 (0.71–1.08) | 0.06 | ||||
| Fully adjusted2,3 | 1.00 (ref) | 1.06 (0.90–1.24) | 1.03 (0.87–1.22) | 0.97 (0.78–1.21) | 0.82 | ||||
| Noncardia (797 cases) | 230 | 203 | 189 | 175 | |||||
| Calcium | |||||||||
| Minimally adjusted2 | 1.00 (ref) | 0.86 (0.70–1.05) | 0.81 (0.65–1.01) | 0.79 (0.60–1.02) | 0.06 | ||||
| Fully adjusted3 | 1.00 (ref) | 0.84 (0.68–1.04) | 0.77 (0.61–0.98) | 0.77 (0.56–1.02) | 0.05 | ||||
| Magnesium | 198 | 211 | 186 | 202 | |||||
| Minimally adjusted2 | 1.00 (ref) | 1.01 (0.82–1.24) | 0.85 (0.68–1.06) | 0.76 (0.57–1.02) | 0.04 | ||||
| Fully adjusted2,3 | 1.00 (ref) | 1.01 (0.82–1.25) | 0.89 (0.70–1.13) | 0.81 (0.60–1.10) | 0.14 | ||||
| Cardia (721 cases) | 197 | 201 | 170 | 153 | |||||
| Calcium | |||||||||
| Minimally adjusted2 | 1.00 (ref) | 0.96 (0.78–1.19) | 0.86 (0.68–1.09) | 0.91 (0.69–1.21) | 0.37 | ||||
| Fully adjusted2,3 | 1.00 (ref) | 1.05 (0.85–1.31) | 0.88 (0.69–1.14) | 0.97 (0.72–1.30) | 0.51 | ||||
| Magnesium | 157 | 180 | 198 | 186 | |||||
| Minimally adjusted2 | 1.00 (ref) | 1.06 (0.85–1.32) | 1.10 (0.87–1.32) | 1.01 (0.74–1.36) | 0.82 | ||||
| Fully adjusted2,3 | 1.00 (ref) | 1.11 (0.87–1.41) | 1.22 (0.95–1.56) | 1.16 (0.84–1.60) | 0.25 | ||||
Eighteen cases of gastric cancer (13 NCGA, 5 CGA) had missing ethnicity data and were excluded from the analysis.
Adjusted for age, gender, ethnicity, energy intake.
Additionally adjusted for obesity, smoking, alcohol intake, self-reported health, educational status and calcium (or magnesium) intake.
Abbreviations: CGA, cardia gastric adenocarcinoma; CI, confidence interval; GA, gastric adenocarcinoma; HR, hazard ratio; NCGA, noncardia gastric adenocarcinoma; NIH-AARP, National Institutes of Health-American Association of Retired Persons; SD, standard deviation.
Stratified analyses
The Ca:Mg ratio did not strongly modify the association between Ca and Mg intakes and risk of GA, NCGA, or CGA (Table 3). There was a reduced risk of NCGA with moderate (Tertile 2) vs. lowest Ca intake when the Ca:Mg ratio was high (HR 0.57, 95% CI: 0.30–1.06), but this was not statistically significant. Among those with low Ca:Mg ratio, intakes of Mg in the highest vs. lowest quartile was inversely associated with risk of NCGA (HR 0.69, 95% CI: 0.43–1.09) and intakes of Ca in the highest vs. lowest quartile was inversely associated with risk of CGA (HR 0.33, 95% CI: 0.11–0.99), albeit neither with statistically significant linear trends overall.
Table 3.
Associations between calcium and magnesium total intakes and risk of gastric cancer stratified by Ca:Mg ratio,1 NIH-AARP Diet and Health Study 1995–2011
| Tertiles of calcium or magnesium intake | |||||||
|---|---|---|---|---|---|---|---|
| Total follow-up time: 6,421,022 person-years | Tertile 1 (low): Ca, mean (SD): 491.1 (132) mg; Mg, mean (SD): 233.5 (51) mg |
Tertile 2: Ca, mean (SD): 890.2 (125) mg; Mg, mean (SD): 359.5 (33) mg |
Tertile 3 (high): Ca, mean (SD): 1676.7 (521) mg; Mg, mean (SD): 542.6 (124) mg |
p-trend | |||
| Cases/person-year | HR, 95% CI | Cases | HR, 95% CI | Cases | HR, 95% CI | ||
| GA, total (1,518 cases) | 568 | 510 | 440 | ||||
| Calcium | |||||||
| Overall2 | 1.00 (ref) | 0.85 (0.74–0.97) | 0.84 (0.70–0.99) | 0.04 | |||
| Ca:Mg < 1.7 (293) | 1.00 (ref) | 0.89 (0.69–1.15) | 0.58 (0.30–1.10) | 0.19 | |||
| Ca:Mg ≥ 1.7, <2.6 (698) | 1.00 (ref) | 0.99 (0.75–1.30) | 0.93 (0.58–1.49) | 0.80 | |||
| Ca:Mg ≥ 2.6 (527) | 1.00 (ref) | 0.68 (0.41–1.12) | 0.90 (0.54–1.49) | 0.46 | |||
| Magnesium | 494 | 514 | 510 | ||||
| Overall2 | 1.00 (ref) | 0.98 (0.85–1.13) | 0.91 (0.76–1.10) | 0.35 | |||
| Ca:Mg < 1.7 (293) | 1.00 (ref) | 1.04 (0.83–1.31) | 0.82 (0.58–1.17) | 0.38 | |||
| Ca:Mg ≥ 1.7, <2.6 (698) | 1.00 (ref) | 0.88 (0.67–1.14) | 0.88 (0.60–1.30) | 0.49 | |||
| Ca:Mg ≥ 2.6 (527) | 1.00 (ref) | 0.98 (0.74–1.31) | 0.95 (0.66–1.38) | 0.80 | |||
| Noncardia (797 cases) | 299 | 260 | 238 | ||||
| Calcium | |||||||
| Overall2 | 1.00 (ref) | 0.81 (0.67–0.98) | 0.82 (0.64–1.04) | 0.09 | |||
| Ca:Mg < 1.7 (152) | 1.00 (ref) | 0.92 (0.64–1.31) | 0.82 (0.35–1.86) | 0.60 | |||
| Ca:Mg ≥ 1.7, <2.6 (359) | 1.00 (ref) | 1.05 (0.71–1.55) | 1.15 (0.60–2.22) | 0.69 | |||
| Ca:Mg ≥ 2.6 (286) | 1.00 (ref) | 0.57 (0.30–1.06) | 0.82 (0.43–1.56) | 0.62 | |||
| Magnesium | 271 | 262 | 264 | ||||
| Overall2 | 1.00 (ref) | 0.90 (0.74–1.10) | 0.82 (0.64–1.06) | 0.12 | |||
| Ca:Mg < 1.7 (152) | 1.00 (ref) | 0.80 (0.59–1.10) | 0.69 (0.43–1.09) | 0.23 | |||
| Ca:Mg ≥ 1.7, <2.6 (359) | 1.00 (ref) | 0.86 (0.60–1.24) | 0.72 (0.42–1.23) | 0.71 | |||
| Ca:Mg ≥ 2.6 (286) | 1.00 (ref) | 1.02 (0.70–1.50) | 0.90 (0.54–1.49) | 0.70 | |||
| Cardia (721 cases) | 269 | 250 | 202 | ||||
| Calcium | |||||||
| Overall2 | 1.00 (ref) | 0.89 (0.73–1.09) | 0.86 (0.67–1.10) | 0.22 | |||
| Ca:Mg < 1.7 (141) | 1.00 (ref) | 0.86 (0.59–1.25) | 0.33 (0.11–0.99) | 0.18 | |||
| Ca:Mg ≥ 1.7, <2.6 (339) | 1.00 (ref) | 0.93 (0.63–1.37) | 0.75 (0.38–1.47) | 0.44 | |||
| Ca:Mg ≥ 2.6 (241) | 1.00 (ref) | 0.91 (0.40–2.07) | 1.08 (0.47–2.50) | 0.56 | |||
| Magnesium | 223 | 252 | 246 | ||||
| Overall2 | 1.00 (ref) | 1.08 (0.88–1.32) | 1.02 (1.00–1.00) | 0.83 | |||
| Ca:Mg < 1.7 (141) | 1.00 (ref) | 1.42 (1.00–2.00) | 1.07 (0.63–1.81) | 0.59 | |||
| Ca:Mg ≥ 1.7, <2.6 (339) | 1.00 (ref) | 0.90 (0.61–1.33) | 1.11 (0.64–1.94) | 0.78 | |||
| Ca:Mg ≥ 2.6 (241) | 1.00 (ref) | 0.94 (0.61–1.45) | 1.03 (0.60–1.75) | 0.93 | |||
Tertiles instead of quartiles were analyzed to preserve sample size across strata, which represent predetermined cutoffs for low, normal and high Ca: Mg (see text).
Models adjusted for age, gender, ethnicity, energy intake, smoking, obesity, alcohol intake, self-reported health, educational status and calcium (or magnesium) intake.
Abbreviations: CI, confidence interval; GA, gastric adenocarcinoma; HR, hazard ratio; NIH-AARP, National Institutes of Health-American Association of Retired Persons; SD, standard deviation.
We also conducted stratified analyses by gender, ethnicity, obesity status, smoking status, NCGA or CGA risk score (Tables S2–S6). There was a stronger inverse association between Ca and Mg intakes and NCGA risk among males compared to females, Ca intake and NCGA risk among non-Hispanic whites (quartile 3 HR 0.54, 95% CI: 0.30–0.97) compared to Hispanics and nonwhites, among obese (HR 0.54, 95% CI: 0.31–0.96) compared to nonobese individuals, among ever smokers (HR 0.58, 95% CI: 0.41–0.81) compared to never smokers and among those categorized as high risk based on NCGA risk score (HR 0.50, 95% CI: 0.31–0.80) compared to moderate or low risk scores. Among participants categorized as moderate risk for NCGA, there was an inverse association between Mg and NCGA risk in the highest vs. lowest quartile of Mg intake (HR 0.61, 95% CI: 0.38–0.99), with a suggestive linear trend. Among participants with a high or moderate CGA risk score compared to a low CGA risk score, above median Ca intakes were inversely associated with risk of CGA and above median Mg intakes were positively associated with CGA risk. Notably, there was no statistically significant interaction of any covariates on the association between Ca and Mg intakes and GA, NCGA, or CGA (all p-interactions >0.05).
We conducted stratified analyses to evaluate whether there were differences in association by source of Ca or Mg intakes (Tables S7 and S8). Intakes of Ca or Mg by source of intake were not associated with risks of GA or CGA. Unlike total Ca, intake of Ca from dietary or supplemental was not associated with risk of NCGA. However, increasing amounts of dietary Mg intake were inversely associated with risk of NCGA (p-trend 0.02), with the highest vs. lowest quartile of intake associated with a 34% reduced risk (HR 0.66, 95% CI: 0.48–0.90). There was a suggestive trend of increasing Ca from dairy sources and decreased risk of GA overall (p-trend = 0.05), but there was otherwise no difference between Ca from dairy vs. nondairy sources and risk of NCGA or CGA.
Discussion
We conducted a comprehensive analysis of the associations between Ca and Mg intakes and the risk of incident GA, NCGA and CGA in a large U.S. prospective cohort study. As hypothesized, we found that increasing levels of Ca intake are associated with a lower risk of GA overall, which appears to be driven mostly by the inverse association of Ca with a reduced risk of NCGA, even after adjusting for relevant confounders. This association was more pronounced for nonwhite ethnic group and Hispanic ethnicity, current or former smokers, obese participants, or individuals with high NCGA risk scores. There was a suggestive trend for increasing total Mg intake and an inverse association with NCGA, with a more pronounced inverse association among males and dietary sources of Mg intake. The relationships between Ca and Mg intakes and GA risk may vary by the Ca:Mg ratio particularly for low vs. normal/high Ca:Mg ratios, but these were not statistically significant in the present analysis. The associations did not vary based on dietary vs. supplemental sources of Ca, nor dairy vs. nondairy sources of Ca for NCGA and CGA. Associations between Ca and Mg intake and risk of incident CGA were otherwise generally null in the NIH-AARP cohort.
The present study represents the first prospective cohort study analyzing the association between Ca and Mg intake and risk of incident GA and by anatomic subsite. An earlier study of the NIH-AARP cohort with follow-up only through 2003 (512 GA cases total) instead of 2011 reported a null association between Ca intake and GA overall, which could reflect insufficient power or potentially a diluted effect, because neither anatomic location of GA nor Mg intake were considered.19 While the authors did report a modest inverse association between dairy intake and digestive tract cancers including gastric cancer, they acknowledged that they could not rule out confounding related to other health benefits of dairy products. We similarly identified a suggestive inverse association between increasing Ca from dairy sources and risk of GA overall, but there was no association when stratified by anatomic location. The sparse epidemiologic data that exist are otherwise limited to the association of Ca and Mg with gastric cancer mortality in a single East Asian population from the prospective Shanghai Women’s and Men’s Health Studies.13 Among Chinese women, higher Ca intake was associated with gastric cancer-related mortality, but only when the Ca: Mg ratio was above 1.7. The Swedish AMORIS cohort study, which analyzed serum calcium and the association with gastrointestinal cancers, found no strong association between albumin adjusted-serum Ca and incident gastric cancer, although there was a suggestive inverse association in the highest vs. lowest quartiles of serum Ca.27 Among other limitations of our study, serum calcium intake is not reflective of dietary intake or total body stores and is influenced by many other factors including albumin level; dietary intake, on the other hand, is the major determinant of total body calcium balance.27,28 Gastric cancer-related mortality was not reported among Chinese men, nor was the risk of incident gastric cancer evaluated. Results for NCGA and CGA were also not presented, which is relevant given their epidemiologic and biologic differences, as well as differences in clinical management and prognosis. To this end, stratification by anatomic location in our study unmasked possible differences in the associations between Ca and Mg intakes and the risk of NCGA or CGA. Other limited previous data include ecologic studies (e.g. Ca and Mg concentrations in hard water and gastric cancer mortality in East Asia)29,30 as well as a handful of older case-control studies from Europe and Asia, which overall report mixed results and often reported on dairy products as opposed to calcium intake specifically.31–40 It is worth noting that gastric cancer mortality is not necessarily an appropriate surrogate for incidence, particularly in countries participating in early detection programs. The introduction of structured gastric cancer screening and surveillance programs in some East Asian countries has led to earlier detection of gastric neoplasia with the opportunity for curative resection. This has led to both decreased gastric cancer incidence and mortality. By contrast, in countries such as the United States where screening and surveillance do not routinely occur, gastric cancer is most often diagnosed in the advanced stages when symptoms present and when there are limited if any treatment options.
While the Western diet is energy dense, key nutrient deficiencies are increasingly recognized. A comprehensive analysis of the National Health and Nutrition Examination Survey data found that only a minority of men and women achieved adequate recommended intakes of Ca, even when supplemental Ca was considered. Indeed, <10% of women 51 years or older met adequate dietary Ca intakes.41 These data were confirmed more recently by the USDA42 and are also consistent with our findings among the NIH-AARP cohort. Total Mg intake is similarly inadequate, with only 21% of U.S. adults meeting the RDA for Mg.43 The adverse health consequences of low dietary intakes of Ca and Mg could be broad and encompass nonmalignant and malignant diseases. Ca and Mg homeostasis are intimately related and both directly or indirectly compete for reabsorption in the kidney and intestine to maintain homeostasis.12 We found that Ca:Mg ratio may be a clinically relevant effect modifier of the association between Ca intake and gastric cancer risk. This finding provides support for the more systemic effect of the Ca:Mg ratio that has been increasingly suggested by the literature. Studies to date have shown that changes in dietary Ca:Mg ratio modulate systemic inflammation, and play a role in insulin resistance and cardiovascular disease, not to mention several downstream pathways that, if aberrant, promote oncogenesis (e.g. DNA repair, cell differentiation, apoptosis, angiogenesis).44–46 The mechanisms underlying how the Ca: Mg ratio may modulate gastric carcinogenesis related to Ca intake are likely complex, but defining these mechanisms, as well as the mechanisms underlying other modifying factors such as ethnicity, smoking and obesity would have important translational impact with respect to gastric cancer prevention, risk attenuation and potentially treatment. Notably, the potential benefit of increasing Ca intake was observed for total Ca intake as opposed to dietary or supplemental intakes alone. Whether there is a difference in risk associated with dairy vs. nondairy sources of Ca remains to be clarified, particularly because dairy and nondairy products have other related health consequences depending on the product (e.g. soy products); the NIH-AARP Diet and Health Study was not designed to discriminate other components of dairy vs. nondairy products with the level of granularity needed to inform these observations more completely.
Our study has several strengths. Rigorous investigations of dietary intake and disease risk are difficult to execute due to several logistic limitations including response rate to validated questionnaires, duration of follow-up needed to observe outcomes of interest, reliably capturing participants’ outcomes over time and cost. That said, well-designed nutritional intake studies yield valuable information as diet is one of the few modifiable disease determinants and is particularly relevant for digestive tract cancers. Gastric cancer is an uncommon diagnosis among the general U.S. population. Our use of the NIH-AARP Diet and Health Study cohort with complete follow-up data through 2011 ensured a large number of GA cases could be captured to investigate our hypotheses with rigor, given the high response rate to the validated diet and lifestyle questionnaires and the over 15-year median follow-up duration for the cohort with over 6.4 million person-years of follow-up time, coupled with the excellent linkage to cancer registry data and death determination. The prospective design eliminates the potential for recall bias or reverse causality— that is, gastric cancer diagnosis modifying nutritional intakes of Ca and Mg—in the present study, both critical limitations of case-control studies. Because gastric cancer was the first primary cancer for all cases in our study, there was also no risk of detection bias which might result from increased endoscopic surveillance from prior head and neck or esophageal cancer diagnosis, for example. By performing a sensitivity analysis excluding participants with less than 12 months of follow-up, we minimized the potential for reverse causality that might result from altered dietary intake due to clinical manifestations of an already prevalent gastric cancer. Furthermore, because we had near-complete data for all included covariates, with less than 5% missing data, we were able to robustly evaluate relevant confounders. While our analysis was comprehensive and included statistical tests for confounders and interactions, there are some limitations that must be highlighted. Generalizability is one consideration. Reflective of the demography of the AARP, the NIH-AARP Diet and Health Study cohort is predominantly non-Hispanic whites, who are overall well educated. We were not able to rigorously evaluate differences by ethnicity due to low case numbers, although there was certainly a strong suggestion of an association for NCGA in particular. A majority of the cohort also reported moderate to heavy alcohol use based on gender-specific standards. Only a minority of our population achieved the RDA for Ca and Mg intake, which is consistent with other population-based studies in the United States.42,43 It is unclear how our findings would differ among a more diverse population with more conservative alcohol intake and who consistently achieve RDA intakes. Geographic and cultural differences might also limit generalizability, as we have shown previously that Ca:Mg ratio might differ according to geography and likely reflects a combination of cultural differences and dietary preferences, but perhaps also differences in genetics and gene–environment interactions. Specific information on Ca or Mg intake levels from antacids was not available in this analysis. Thus, we cannot comment on whether supplemental Ca or Mg derived from antacids specifically is associated with differential risk of gastric cancer vs. that derived from other supplemental sources, nor can we comment on whether antacids, perhaps by altering gastric pH, might obscure relevant associations. Because the majority of Mg supplement users reported fixed doses (100 mg), we were not able to evaluate linear trends for supplemental Mg intake. Lastly, although the study used a validated FFQ,21 it is impossible to discern whether a single measurement of diet adequately reflects intakes over the study period. There was a limited sample size for some analyses; for the Ca:Mg ratio stratified analysis, for example, it was necessary to collapse quartiles of intakes to tertiles.
The public health implications of our study are several, given that dietary determinants are modifiable risk factors. In addition to validating our findings among other populations, future studies should specifically focus on the high-risk groups we identified, for whom the risk reduction was most pronounced, as well as more completely analyzing the association between Ca and Mg from dairy vs. nondairy products and risk of gastric cancer. These high-risk groups will plausibly benefit most from personalized interventions and counseling on risk factor modification such as increasing dietary calcium intake. The composite NCGA and CGA risk score that we constructed for our study relies on readily available clinical data, including age, gender, ethnicity and smoking status.
In conclusion, we conducted a comprehensive prospective cohort analysis evaluating the association between Ca and Mg intakes and the risk of incident GA, NCGA and CGA. Diet plays an important role in modulating risk for gastrointestinal neoplasia and, generally speaking, dietary modifications are noninvasive and safe interventions. Gastric cancer remains a leading cause of cancer-related mortality worldwide with rates rising among some populations in the United States. In this context, our findings warrant additional clinical investigation, including validation in other large cohorts, as well as experimental studies defining biological mechanisms underlying these observations.
Supplementary Material
What’s new?
Some dietary factors may modulate gastric cancer risk. The impact of calcium and magnesium dietary and supplemental intakes on gastric cancer risk remains understudied, however. Here, the authors analyzed the NIH-AARP Diet and Health Study cohort and found that higher total calcium intakes and, among men, higher magnesium intakes were inversely associated with non-cardia gastric cancer risk. The associations were stronger in certain groups; for example, among ever-smokers, there was a 42% reduced risk of non-cardia gastric cancer associated with the highest versus lowest quartile of calcium intake. These findings have important implications for risk factor modification.
Acknowledgments
Research reported in this publication was supported by the Agency for Healthcare Research and Quality (AHRQ) and Patient-Centered Outcomes Research Institute (PCORI) under award number K12 HS026395, awarded to SCS. The content is solely the responsibility of the listed authors and does not necessarily represent the official views of AHRQ or PCORI. No additional writing assistance was used for this article.
Grant sponsor: Agency for Healthcare Research and Quality; Grant number: K12 HS026395; Grant sponsor: Patient-Centered Outcomes Research Institute
Abbreviations:
- BMI
body mass index
- CGA
cardia gastric adenocarcinoma
- CIs
confidence intervals
- FFQ
food frequency questionnaire
- GA
gastric adenocarcinoma
- HEI
Healthy Eating Index
- HRs
hazard ratios
- ICD-O
International Classification of Disease for Oncology
- NCGA
noncardia gastric adenocarcinoma
- NCI
National Cancer Institute
- NIH-AARP
National Institutes of Health-American Association of Retired Persons
- RDA
Recommended Dietary Allowance
Footnotes
Additional Supporting Information may be found in the online version of this article.
Conflict of interest: The authors have no potential conflicts (financial, professional, nor personal) that are relevant to this article.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Fedewa SA, Miller KD, et al. Cancer statistics for Hispanics/Latinos, 2015. CA Cancer J Clin 2015;65:457–80. [DOI] [PubMed] [Google Scholar]
- 3.Merchant SJ, Kim J, Choi AH, et al. A rising trend in the incidence of advanced gastric cancer in young Hispanic men. Gastric Cancer 2017;20: 226–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson WF, Rabkin CS, Turner N, et al. The changing face of noncardia gastric cancer incidence among US non-Hispanic whites. J Natl Cancer Inst 2018;110:608–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamangar F, Dawsey SM, Blaser MJ, et al. Opposing risks of gastric cardia and noncardia gastric adenocarcinomas associated with Helicobacter pylori seropositivity. J Natl Cancer Inst 2006;98: 1445–52. [DOI] [PubMed] [Google Scholar]
- 6.Corley DA, Kubo A. Influence of site classification on cancer incidence rates: an analysis of gastric cardia carcinomas. J Natl Cancer Inst 2004;96: 1383–7. [DOI] [PubMed] [Google Scholar]
- 7.Devesa SS, Blot WJ, Fraumeni JF. Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer 1998;83: 2049–53. [PubMed] [Google Scholar]
- 8.Polk DB, Peek RM. Helicobacter pylori: gastric cancer and beyond. Nat Rev Cancer 2010;10: 403–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wroblewski LE, Peek RM. Helicobacter pylori in gastric carcinogenesis: mechanisms. Gastroenterol Clin North Am 2013;42:285–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dong J-Y, Xun P, He K, et al. Magnesium intake and risk of type 2 diabetes: meta-analysis of prospective cohort studies. Diabetes Care 2011;34: 2116–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Champagne CM. Magnesium in hypertension, cardiovascular disease, metabolic syndrome, and other conditions: a review. Nutr Clin Pract 2008; 23:142–51. [DOI] [PubMed] [Google Scholar]
- 12.Dai Q, Cantwell MM, Murray LJ, et al. Dietary magnesium, calcium:magnesium ratio and risk of reflux oesophagitis, Barrett’s oesophagus and oesophageal adenocarcinoma: a population-based case-control study. Br J Nutr 2016;115:342–50. [DOI] [PubMed] [Google Scholar]
- 13.Dai Q, Shu X-O, Deng X, et al. Modifying effect of calcium/magnesium intake ratio and mortality: a population-based cohort study. BMJ Open 2013; 3:e002111. 10.1136/bmjopen-2012-002111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wark PA, Lau R, Norat T, et al. Magnesium intake and colorectal tumor risk: a case-control study and meta-analysis. Am J Clin Nutr 2012;96: 622–31. [DOI] [PubMed] [Google Scholar]
- 15.Dai Q, Motley SS, Smith JA, et al. Blood magnesium, and the interaction with calcium, on the risk of high-grade prostate cancer. PLoS ONE 2011;6:e18237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dai Q, Shrubsole MJ, Ness RM, et al. The relation of magnesium and calcium intakes and a genetic polymorphism in the magnesium transporter to colorectal neoplasia risk. Am J Clin Nutr 2007;86: 743–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dai Q, Sandler R, Barry E, et al. Calcium, magnesium, and colorectal cancer. Epidemiology 2012; 23:504–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu X, Shrubsole MJ, Ness RM, et al. Calcium/magnesium intake ratio, but not magnesium intake, interacts with genetic polymorphism in relation to colorectal neoplasia in a two-phase study. Mol Carcinog 2016;55:1449–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park Y, Leitzmann MF, Subar AF, et al. Dairy food, calcium, and risk of cancer in the NIH-AARP Diet and Health Study. Arch Intern Med 2009;169:391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schatzkin A, Subar AF, Thompson FE, et al. Design and serendipity in establishing a large cohort with wide dietary intake distributions: The National Institutes of Health-American Association of Retired Persons Diet and Health Study. Am J Epidemiol 2001;154:1119–25. [DOI] [PubMed] [Google Scholar]
- 21.Thompson FE, Kipnis V, Midthune D, et al. Performance of a food-frequency questionnaire in the US NIH-AARP (National Institutes of Health-American Association of Retired Persons) Diet and Health Study. Public Health Nutr 2008;11: 183–95. [DOI] [PubMed] [Google Scholar]
- 22.Agricultural Research Service, Design and Operation: The Continuing Survey of Food Intakes by Individuals and the Diet and Health Knowledge Survey, 1994–96 NFS Report No. 96–1, United States: Department of Agriculture, 1997. https://www.ars.usda.gov/ARSUserFiles/80400530/pdf/Dor9496.pdf. [Google Scholar]
- 23.Healthy Eating Index (HEI)/Center for Nutrition Policy and Promotion. https://www.cnpp.usda.gov/healthyeatingindex (accessed March 27, 2019).
- 24.Li W-Q, Park Y, Wu JW, et al. Index-based dietary patterns and risk of esophageal and gastric cancer in a large cohort study. Clin Gastroenterol Hepatol 2013;11:1130–1136.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Michaud D, Midthune D, Hermansen S, et al. Comparison of cancer registry case ascertainment with SEER estimates and self-reporting in a subset of the NIH-AARP. Diet and Health Study 2005;32 ((2):70–5. [Google Scholar]
- 26.Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol 1986;124:17–27. [DOI] [PubMed] [Google Scholar]
- 27.Wulaningsih W, Michaelsson K, Garmo H, et al. Serum calcium and risk of gastrointestinal cancer in the Swedish AMORIS study. BMC Public Health 2013;13:663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peacock M. Calcium metabolism in health and disease. Clin J Am Soc Nephrol 2010;5(Suppl 1): S23–30. [DOI] [PubMed] [Google Scholar]
- 29.Yang CY, Cheng MF, Tsai SS, et al. Calcium, magnesium, and nitrate in drinking water and gastric cancer mortality. Jpn J Cancer Res 1998;89: 124–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sakamoto N, Shimizu M, Wakabayashi I, et al. Relationship between mortality rate of stomach cancer and cerebrovascular disease and concentrations of magnesium and calcium in well water in Hyogo prefecture. Magnes Res 1997;10:215–23. [PubMed] [Google Scholar]
- 31.Cornée J, Pobel D, Riboli E, et al. A case-control study of gastric cancer and nutritional factors in Marseille, France. Eur J Epidemiol 1995;11:55–65. [DOI] [PubMed] [Google Scholar]
- 32.Pelucchi C, Tramacere I, Bertuccio P, et al. Dietary intake of selected micronutrients and gastric cancer risk: an Italian case-control study. Ann Oncol 2009;20:160–5. [DOI] [PubMed] [Google Scholar]
- 33.Bertuccio P, Edefonti V, Bravi F, et al. Nutrient dietary patterns and gastric cancer risk in Italy. Cancer Epidemiol Biomarkers Prev 2009;18: 2882–6. [DOI] [PubMed] [Google Scholar]
- 34.van der Pols JC, Bain C, Gunnell D, et al. Childhood dairy intake and adult cancer risk: 65-y follow-up of the Boyd Orr cohort. Am J Clin Nutr 2007;86:1722–9. [DOI] [PubMed] [Google Scholar]
- 35.Guo Y, Shan Z, Ren H, et al. Dairy consumption and gastric cancer risk: a meta-analysis of epidemiological studies. Nutr Cancer 2015;67: 555–68. [DOI] [PubMed] [Google Scholar]
- 36.Tian S, Yu J, Kang W, et al. Association between dairy intake and gastric cancer: a meta-analysis of observational studies. PLoS ONE 2014;9:e101728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun Y, Lin L-J, Sang L-X, et al. Dairy product consumption and gastric cancer risk: a meta-analysis. World J Gastroenterol 2014;20: 15879–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.You WC, Blot WJ, Chang YS, et al. Diet and high risk of stomach cancer in Shandong, China. Cancer Res 1988;48:3518–23. [PubMed] [Google Scholar]
- 39.Buiatti E, Palli D, Decarli A, et al. A case-control study of gastric cancer and diet in Italy: II. Association with nutrients. Int J Cancer 1990; 45:896–901. [DOI] [PubMed] [Google Scholar]
- 40.La Vecchia C, Ferraroni M, D’Avanzo B, et al. Selected micronutrient intake and the risk of gastric cancer. Cancer Epidemiol Biomarkers Prev 1994;3:393–8. [PubMed] [Google Scholar]
- 41.Bailey RL, Dodd KW, Goldman JA, et al. Estimation of total usual calcium and vitamin D intakes in the United States. J Nutr 2010;140:817–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dietary Guidelines for Americans, 2015–2020, 8th ed. https://health.gov/dietaryguidelines/2015/guidelines/
- 43.Ford ES, Mokdad AH. Dietary magnesium intake in a national sample of US adults. J Nutr 2003; 133:2879–82. [DOI] [PubMed] [Google Scholar]
- 44.Wolf FI, Maier JAM, Nasulewicz A, et al. Magnesium and neoplasia: from carcinogenesis to tumor growth and progression or treatment. Arch Biochem Biophys 2007;458:24–32. [DOI] [PubMed] [Google Scholar]
- 45.Iseri LT, French JH. Magnesium: nature’s physiologic calcium blocker. Am Heart J 1984;108:188–93. [DOI] [PubMed] [Google Scholar]
- 46.Bussière FI, Gueux E, Rock E, et al. Protective effect of calcium deficiency on the inflammatory response in magnesium-deficient rats. Eur J Nutr 2002;41:197–202. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of our study are available from the NIH AARP Diet and Health Study. Restrictions apply to the availability of these data, which were used under license for our study. Data are available with the permission of the NIH AARP.