To the Editor,
The combined reflectance confocal microscopy (RCM)-optical coherence tomography (OCT) device enables simultaneous, real-time visualization of cellular features in RCM and structural features in OCT, leading to better characterization of morphologic features and improved diagnosis and depth assessment of basal cell carcinoma (BCC) 1. Amyloid and mucin deposits are established BCC histopathological features. Primary cutaneous amyloidosis has been imaged with RCM 2 and BCC peritumoral-mucin on RCM has been histopathologically verified.3 However, no prior reports have demonstrated BCC-associated amyloid on RCM or OCT, and OCT descriptions of mucin have been inferential. Herein, we report in vivo characterization of amyloid and mucin through RCM-OCT, followed by precise histopathological correlation.
In the combined RCM-OCT device, en face or horizontal RCM images (field-of-view (FOV): 750×750 µm; lateral resolution: ~1 µm; optical sectioning: ~3 µm) are centered on vertical OCT images (FOV: 1000×2000 µ m; lateral resolution: ~5 µm; optical sectioning: ~10 µ m), which are post-processed to obtain horizontal OCT. Consecutive BCCs were prospectively investigated under an IRB-approved protocol for (a) amyloid (amorphous, hyperreflective, homogenous intratumoral/peritumoral globules) and (b) mucin (intratumoral/peritumoral hyporeflective areas). Next, RCM-OCT-guided punch biopsies (2–3 mm) were obtained, followed by horizontal (for RCM) or vertical (for OCT) sectioning to permit direct histopathologic correlation using hematoxylin-eosin (H&E), Toluidine Blue (TB, mucin) and Congo Red (CR, amyloid) staining. The size and distribution of hyperreflective-globules and hyporeflective areas in RCM, OCT and histopathology were analyzed using FIJI (ImageJ, NIH).
Features of mucin and amyloid were identified in 6 patients (male:female ratio 2:1, mean age-61.5 years [range 24–78]) with 8 BCCs (1 superficial; 6 nodular; 1 nodulo-infiltrative). On histology, amyloid appeared as characteristic homogenous globules, bubble-gum pink, blue or red on H&E, TB, and CR, respectively. Mucin was an amorphous stringy substance, staining blue-purple-gray on H&E and magenta on TB. Hyperreflective globules correlated with amyloid and hyporeflective areas correlated with mucin. Mucin deposition was either intratumoral or peritumoral (Figures 1 and 2). Amyloid globules were distributed peritumorally (Figure 1) or within hyporeflective mucin pools (Figures 1 and 2). In 6/8 cases, we identified mucin and amyloid on RCM and OCT. In 2 cases, the features were only present in the deep dermis and therefore visualized only on OCT (8/8). The amyloid globule and/or cluster size measured 9–32 µm on horizontal RCM, 11–23 µm on horizontal histology (TB/CR), 26–85 µm on vertical OCT and 15–95 µm on vertical histology. The large mucin pool (Figure 1) measured 170×220 µm (RCM), 180×275 µm (OCT) and 170×270 µm (Histology).
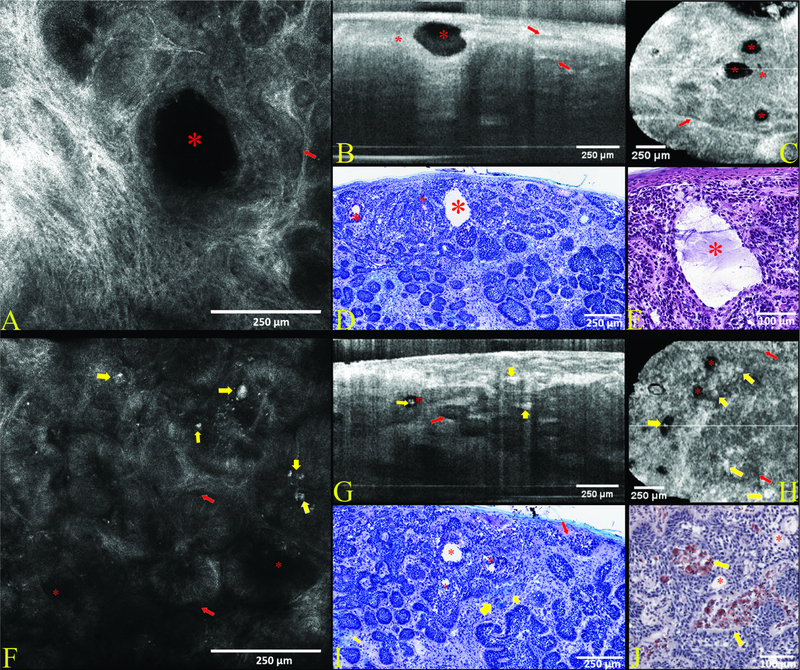
Figure 1. Case 1—Nodular basal cell carcinoma (BCC). Morphologic patterns of intra and peritumoral mucin and stromal amyloid deposits in nodular BCC.
Case 1 features a nodular BCC characterized by small to medium-sized branching tumor lobules within the dermis. On reflectance confocal microscopy (RCM) in (A) peripheral palisading helps confirm the diagnosis along with dark peritumoral rims of mucin (red arrows) which are also seen on vertical (B) and horizontal optical coherence tomography (OCT) in (C) and toluidine blue (TB) stained histopathology (D). A large pool of mucin (red asterisk) appears as a dark oval intratumoral structure seen on RCM (A) and OCT (B-C), and a white cavity on toluidine blue (D) where the mucin was washed out during processing but is clearly visualized as stringy purple gray mucin on hematoxylin & eosin (H&E) stained histopathology (E). Additional smaller mucin pools (red asterisks) are present in the plane of sectioning in vertical (B) and horizontal OCT (C), and low power histopathology (D). Another area (F-I) in the same tumor shows amyloid deposits (yellow arrows) in the superficial dermis, seen as hyperreflective globules within peritumoral stroma on RCM (F) and OCT (G-H), blue peritumoral globules on TB stained histopathology (I) and red peritumoral globules on CR stained histopathology (J). Peritumoral mucin (red arrows) is subtle but recognized as dark peritumoral rims on RCM (F) and OCT (G) as well as white peritumoral clefts (from mucin washout during processing) on histopathology (I).
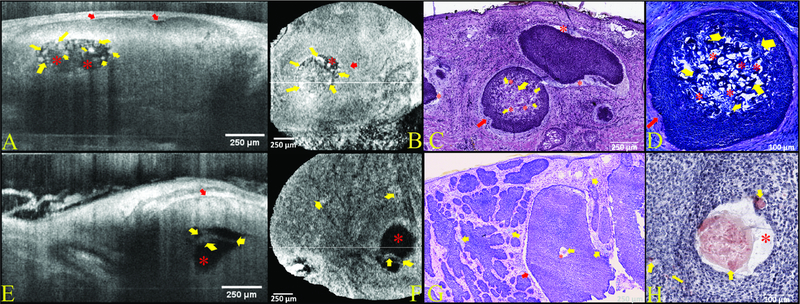
Figure 2. Cases 2&3—Nodular basal cell carcinomas (BCC). Morphological patterns of intratumoral amyloid deposits as well as intra and peritumoral mucin deposits in nodular BCCs confirmed on optical coherence tomography (OCT).
Intratumoral deposits were present deep to the maximal imaging depth of reflectance confocal microscopy (RCM) and therefore only visualized by OCT. Case 2 shows a large intratumoral round-oval dark structure filled with hyperreflective globules on vertical (A) and horizontal (B) OCT corresponding to a round collection of mucin (red asterisks) intermixed with amyloid globules (yellow arrows) within the center of tumor nodule. Amyloid appeared characteristic bubble gum pink on H&E (C) and blue on TB (D). Peritumoral mucin (red arrows) is identified as a dark peritumoral rim on OCT (A-B) and corresponds to stringy amorphous material which is purple-gray on H&E (C) and magenta TB (D). Case 3 harbors a large tumor nodule with a central pool of mucin (red asterisk), which is dark on OCT (E-F) and amorphous stringy purple-gray material on H&E (G) and CR (H). Hyperreflective aggregated globules (yellow arrows) noted within the mucin pool on OCT (E-F) are partially obscured by mucin on TB but confirmed to be amyloid (yellow arrows) on CR-stained histopathology (H). Also noted is a characteristic peritumoral rim of mucin (red arrows), which appears dark on OCT (E) and white due to mucin washout on H&E (G) histopathology.
Amyloid and intratumoral mucin deposition are frequently observed in less-aggressive nodular BCCs (up to 85% positive for amyloid).4 Our findings confirmed presence of amyloid and mucin in all cases; of these 87.5% were non-aggressive. Thus, non-invasive visualization of prominent amyloid and mucin features may be clinically relevant for diagnosis of less-aggressive BCCs, which in turn can help streamline management. Furthermore, given the anticipated increased use of RCM-OCT CPT codes,5 these features should be identified by physicians performing RCM and/or OCT.
Acknowledgments
Funding: This research was funded by the NIH/NCI SBIR Grant R44CA162561, the NIH/NIBIB Grant R01EB020029, and, partly supported by NIH/NCI Cancer Center Support Grant P30 CA008748.
IRB: This study was performed under the Memorial Sloan Kettering Cancer Center IRB protocol 99–099.
Conflicts of interest: Melissa Gill serves as a consultant to the Dermatology Service, Department of Medicine, MSKCC, New York, NY, USA and is also a consult investigator for an investigator-initiated study funded by DBV Technologies. Christi Alessi-Fox is employed by and owns equity in Caliber Imaging and Diagnostics, Inc. (formerly Lucid, Inc), the company that manufactures and sells the VivaScope confocal microscope. Nicusor Iftimia reports a patent (US9655521 B2) that is assigned to Physical Sciences Inc. (PSI) and Memorial Sloan Kettering Cancer Center (MSKCC), which is related to the technology described in this study. Milind Rajadhyaksha is a former employee of and owns equity in Caliber Imaging and Diagnostics. The VivaScope is the commercial version of an original laboratory prototype that he had developed at Massachusetts General Hospital, Harvard Medical School. Chih-Shan J. Chen is the principal investigator of a research project sponsored by Apollo Medical Optics, Inc. (AMO). The other authors have no disclosures or conflicts of interest to report. This research was completed without financial support from either or Caliber I.D., MSKCC, PSI or AMO (see Funding Acknowledgement), and data were acquired and processed from patients by co-authors unaffiliated with any commercial entity.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Sahu A, Yélamos O, Iftimia N et al. Evaluation of a Combined Reflectance Confocal Microscopy–Optical Coherence Tomography Device for Detection and Depth Assessment of Basal Cell Carcinoma. JAMA dermatology 2018;154:1175–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lei W, Ai-e X. Reflectance confocal microscopy for the characterization of primary cutaneous amyloidosis: a pilot study. Skin Research and Technology 2017;23:441–3. [DOI] [PubMed] [Google Scholar]
- 3.Ulrich M, Roewert-Huber J, González S et al. Peritumoral clefting in basal cell carcinoma: correlation of in vivo reflectance confocal microscopy and routine histology. Journal of cutaneous pathology 2011;38:190–5. [DOI] [PubMed] [Google Scholar]
- 4.Cohen PR. Basal Cell Carcinoma Associated with Secondary Localized Cutaneous Amyloid Deposits: Case Report and Review. Cureus 2019;11. [DOI] [PMC free article] [PubMed]
- 5.Current Procedural Terminology, Professional Edition Chicago IL: American Medical Association; 2016. [Google Scholar]