Abstract
Background:
Despite guideline recommendations, rates of concomitant tricuspid valve repair are suboptimal, possibly due to fear of complications. We reviewed morbidity, mortality, recurrent tricuspid regurgitation, and right ventricular remodeling after guideline-directed concomitant tricuspid valve repair.
Methods:
We performed guideline-directed concomitant tricuspid valve repair on 171 consecutive patients who underwent left-sided valve surgery (degenerative mitral surgery or aortic valve replacement) between May 2012–March 2016. Exclusion criteria included functional mitral regurgitation, rheumatic disease, active endocarditis, and concomitant coronary artery bypass grafting or complex aortic surgery.
Results:
Mean age was 68±12 years and 47% (81/171) were female. Preoperative atrial fibrillation was present in 57% (98/171) and preoperative tricuspid regurgitation was moderate or higher in 64% (108/171). Rate of de novo pacemaker placement was 4.1% (7/171) and 30-day mortality rate was 0.6% (1/171). Estimated survival was 95±4% at 1 year and 92±5% at 5 years. Freedom from moderate or worse residual/recurrent tricuspid regurgitation was 93±6% at 6 months and 89±8% at 3 years. On quantitative echocardiography, there was no significant increase in right ventricular dimensions or area at 1-year in subgroup analysis. Mean echocardiographic follow-up was 14.1 months, while mean clinical follow-up was 33.9 months.
Conclusions:
Guideline-directed concomitant tricuspid valve repair resulted in excellent safety end-points and survival. At 14 months, freedom from moderate or worse tricuspid regurgitation was high and right ventricular performance did not worsen, while pacemaker rate was comparable to rates after isolated mitral repair. Given these findings, adherence to current guidelines regarding functional tricuspid regurgitation should be encouraged.
Keywords: Heart valves, multiple; Heart valve repair; Tricuspid valve
Functional tricuspid regurgitation (TR) is common in the setting of left-sided valvular disease, with a reported prevalence between 25% to 30% [1]. Although TR rarely may originate as a primary functional problem, secondary intrinsic anatomical abnormalities of the tricuspid valve (TV) apparatus (i.e. annular dilation) most commonly occur. This is an important consideration since correction of the primary left-sided disorder may not lead to resolution of secondary, functional TR [2,3].
Conservative, nonoperative strategies have historically been recommended for patients with functional TR [4]. However, untreated TR in an inpatient and outpatient non-surgical population of veterans [5] and in patients with isolated TR [6] have been shown to carry substantial morbidity and mortality. Furthermore, untreated TR has been found to confer increased mortality and re-hospitalization among patients undergoing transcatheter aortic valve replacement (AVR) [7], as well as transcatheter mitral valve repair (MVr) [8]. Increasing evidence supports concomitant surgical tricuspid valve repair (TVr) for patients with functional TR [9–12]. Consequently, the 2014 American College of Cardiology (ACC)/American Heart Association (AHA) guidelines mirrored the 2006 guidelines in giving concomitant TVr a class I indication for patients with severe TR and upgraded concomitant TVr from a class IIb to IIa recommendation for patients with mild to moderate TR and a dilated annulus (≥40mm) [13,14]. However, surgical repair remains underutilized, as a review of national contemporary practice revealed that only 79% of patients with severe TR and only 39% of patients with moderate TR undergo concomitant TVr at the time of mitral surgery (Society of Thoracic Surgeons Adult Cardiac Surgery Database [STS ACSD], version 2.73, 2011 to 2013). Several fears may be responsible for the gap between guideline recommendations and current practice. These include safety concerns of increased morbidity (e.g. increased pacemaker rates) and higher mortality with an added procedure, as well as the possibility of recurrent TR [15–17].
Given these concerns, we sought to describe the morbidity and mortality following guideline-directed concomitant TVr in patients undergoing left-sided valve surgery at our institution. Additionally, we examined short-term freedom from recurrent TR and effect of TVr on right ventricular (RV) geometry and function.
PATIENTS AND METHODS
Patient Population
We analyzed 171 consecutive patients who underwent left-sided valve surgery, degenerative MVr and/or AVR, with concomitant TVr between May 2012 and March 2016. We excluded patients with functional MR, rheumatic disease, or active endocarditis, and those undergoing concomitant CABG or complex aortic surgery (any ascending aortic or arch procedure). This resultant cohort therefore all met either class I or class IIa/IIb 2006 and 2014 ACC/AHA guideline indications for concomitant tricuspid repair underwent TVr [13,14]. As stated in the guidelines, patients undergoing left-sided valve surgery (e.g. mitral and/or aortic valve surgery) were included. The study was given the status of “not regula ted” by the Institutional Review Board of the University of Michigan (HUM00113976).
Indications and Operative Technique
Indications for concomitant TVr included: 1) the presence of severe TR or 2) the presence of mild to moderate TR and annular dilation of at least 40mm at end-diastolic diameter as viewed in the standard four-chamber transesophageal echocardiographical view. All tricuspid repairs were performed using the Tri-Ad® tricuspid annuloplasty ring using sizes 26 mm or 28 mm. Techniques for repair of the tricuspid valve have previously been described [18]. At our institution, the tricuspid annuloplasty ring was implanted using ten interrupted mattress sutures from the 10 o’clock to the 6 o’clock position, avoiding the atrioventricular node.
Clinical and Echocardiographic Outcomes
Primary safety end-points included in-hospital death or death within 30 days of surgery and permanent pacemaker implantation. Postoperative echocardiographic data was available in 100% (171/171) of patients and included postoperative TR grade for 98% (168/171). A subgroup of patients underwent additional follow up with pre- and postoperative echocardiography for RV quantitation to assess for the presence of RV remodeling and function. Grade of TR from the most recent follow-up echocardiogram was used to analyze short-term repair success according to the following scale: none, mild, moderate, and severe. Moderate or worse TR on echocardiogram was considered residual/recurrent TR. A subset analysis was performed comparing survival and freedom from residual/recurrent moderate or worse TR between patients with and without preoperative atrial fibrillation. Clinical follow-up was a mean 33.9 months (95% confidence interval, 30.6–37. 3 months).
Statistical Analysis
Data are presented as means with standard deviation and frequencies as appropriate. Pre- and postoperative echocardiographic data were analyzed using Wilcoxon signed rank test p-value for continuous variables. Time-to-event analyses for overall survival and freedom from recurrent TR (moderate or worse) were evaluated by Kaplan-Meier estimates. Follow-up time was calculated from the date of surgery until the date of death/date of echocardiogram with moderate or worse TR, or date of the latest clinical encounter. All analyses were performed using STATA version 15.0 (StataCorp, College Station, TX). A p-value of 0.05 or less indicated statistical significance.
RESULTS
Patient Characteristics
Preoperative patient characteristics are listed in Table 1. The mean age of patients was 68±12 years (range, 27 to 88), 47% were female (81/171) and 93% were white (159/171). Preoperative atrial fibrillation was present in 57% of patients (98/171). Fifty-nine percent (101/171) had a prior diagnosis of heart failure and 46% (78/171) had undergone a prior cardiovascular intervention. Preoperative echocardiographic characteristics are summarized in Table 2. Moderate TR was present in 66 patients (39%) and severe TR in 42 patients (25%). Mean LV ejection fraction was 55±11% and the mean pulmonary artery systolic pressure was 47±17 mmHg.
Table 1.
Patient Characteristics
| Variable | Mean ± standard deviation or n (%) |
|---|---|
| Age, years | 68±12 |
| Female | 81 (47) |
| Race | |
| White | 159 (93) |
| Black | 7 (4) |
| Diabetes | 32 (19) |
| Cerebrovascular disease | 11 (6) |
| Liver disease | 3 (2) |
| Preoperative creatinine | 1.23 ± 1.11 |
| Preoperative atrial fibrillation | 98 (57) |
| Previous myocardial infarction | 21 (12) |
| Prior heart failure | 101 (59) |
| Previous cardiovascular intervention | 78 (46) |
Table 2.
Preoperative Echocardiographic Characteristics
| Variable | Mean ± SD or n(%) |
|---|---|
| MR grade | |
| None/Trace/Trivial | 5 (3) |
| Mild | 15 (9) |
| Moderate | 26 (15) |
| Severe | 125 (73) |
| TR grade | |
| None/Trace/Trivial | 20 (12) |
| Mild | 43 (25) |
| Moderate | 66 (39) |
| Severe | 42 (25) |
| Pulmonary artery systolic pressure (mmHg) | 47±17 |
| LV ejection fraction (%) | 55±11 |
| LV end systolic diameter (mm) | 36±9 |
| LV end diastolic diameter (mm) | 53±9 |
LV = left ventricle; MR = mitral regurgitation; SD = standard deviation; TR = tricuspid regurgitation.
Operative Characteristics
A total of 150 patients (88%) underwent a mitral valve operation as the primary procedure (Table 3). Of these patients, 134 (89%) underwent MVr and 16 (11%) required mitral valve replacement. Surgical ablation was performed in 51% of patients (88/171) undergoing TVr, which was 90% (88/98) of patients with a diagnosis of preoperative atrial fibrillation. Mean cardiopulmonary bypass and cross-clamp times were 109±51 and 87±44 minutes, respecti vely.
Table 3.
Operative Characteristics
| Variable | Mean ± SD or n (%) |
|---|---|
| Concomitant left-sided procedure | |
| Mitral valve repair | 134 (78) |
| Mitral valve replacement | 16 (9) |
| Aortic valve replacement | 9 (5) |
| Mitral repair + aortic valve replacement | 8 (5) |
| Mitral replacement + aortic valve replacement | 4 (2) |
| Surgical ablation procedure | 88 (51) |
| Cardiopulmonary bypass time, minutes | 109 ± 51 |
| Cross-clamp time, minutes | 87±44 |
Clinical Outcomes
Seven patients (4.1%) required de novo pacemaker due to complete heart block postoperatively (Table 4). The 30-day mortality rate was 1% (1/171) and the 30-day readmission rate was 8% (14/171). Estimated survival was 95±4% at 1 year and 92±5% at 5 years (Figure 1). Freedom from recurrent TR (moderate or greater) was 93±6% at 6 months and 89± 8% at 3 years (Figure 2). No patients required TV reoperation. There was no documented clinically significant tricuspid stenosis. No patient was found to have annuloplasty ring dehiscence and there were no known episodes of tricuspid valve endocarditis.
Table 4.
Clinical and Echocardiographic Outcomes
| Variable | Mean ± SD or n (%) |
|---|---|
| Intensive care unit length of stay, hours | 85 ± 126 |
| De novo pacemaker | 7 (4) |
| 30-day mortality | 1 (1) |
| 30-day readmission | 14 (8) |
| Clinical follow-up time, mean (95% CI) months | 33.9 (30.6– 37.3) |
| Patients with postoperative echocardiogram | 171 (100) |
| Postoperative echocardiogram follow-up time, mean (95% CI) months | 14.1 (11.3– 16.9) |
| Postoperative TR grade (n=168) | |
| None | 119 (71) |
| Mild | 37 (22) |
| Moderate | 9 (5) |
| Severe | 3 (2) |
| Tricuspid valve gradient (mmHg) | 2.02 ± 1.17 |
CI = confidence interval; SD = standard deviation; TR = tricuspid regurgitation
Figure 1.
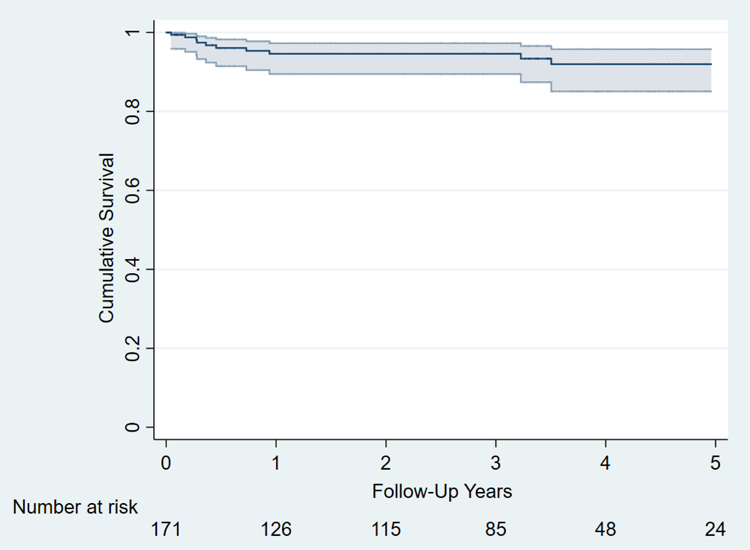
Kaplan-Meier survival curve following concomitant tricuspid valve repair.
Figure 2.
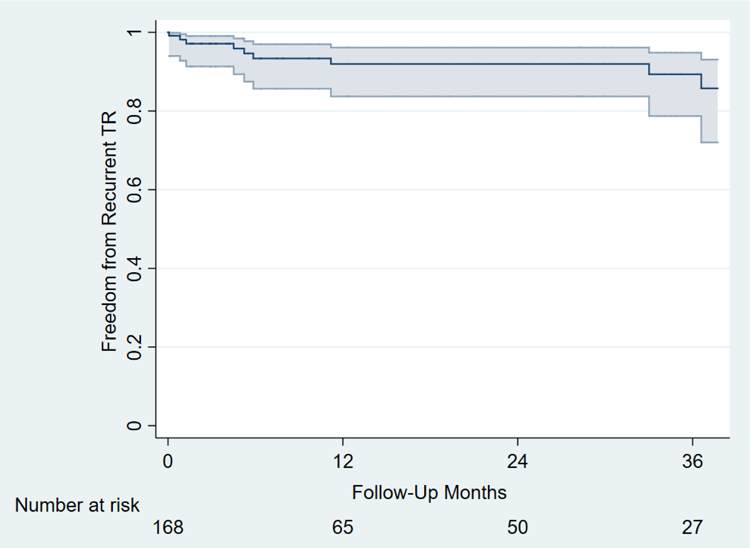
Kaplan-Meier analysis showing freedom from recurrent severe tricuspid regurgitation. Patients without tricuspid regurgitation grade on follow-up echocardiograms have been excluded (n = 3).
In subset analysis, overall survival estimates among patients with preoperative atrial fibrillation (1-year: 93±6%; 5-year: 90±8%) and those without pr eoperative atrial fibrillation (1-year: 97±5%; 5-year: 94±9%) did not differ (log-rank p=0.38). Freedom from residual/recurrent moderate or worse TR among those with atrial fibrillation (6-month: 90±8%; 3-year: 86±11%) and those without atrial fibrillation (6-month: 100%; 3-year: 96±11%) also did not differ (log-rank p=0.59).
Echocardiographic Follow-Up
Postoperative echocardiograms were performed in all patients (100%, 171/171). Mean time to echocardiographic follow-up was 14.1 months (95% CI, 11.3–16.9; range, 0 to 78). Moderate residual/recurrent TR was present in 5% of patients (9/168), severe TR in 2% (3/168), and mean TV gradient was 2.0±1.2 mmHg. There was no decrement in RV function and no significant enlargement in RV dimensions at 1 year (Table 5). There were no significant differences in the RV basal diameter (43mm vs 45mm, p=0.51), or RV area during systole (1397mm2 vs 150mm2, p=0.54) or diastole (2192mm2 vs 2217mm2, p=0.59). Furthermore, there were no differences in the tethering height (6.5mm vs 7.1mm, p=0.34) or coaptation length (5.5mm vs 5.6mm, p=0.93) of the TV.
Table 5.
Echocardiographic Right Ventricular Quantitation
| Measurements | Preoperative | 1-year postoperative | p-value |
|---|---|---|---|
| RV basal diameter (mm) | 43 ± 8 | 45 ± 9 | 0.51 |
| RV area in systole (mm2) | 1397 ± 576 | 1503 ± 662 | 0.54 |
| RV area in diastole (mm2) | 2192 ± 672 | 2217 ± 850 | 0.59 |
| Tethering height (mm) | 6.5 ± 3.7 | 7.1 ± 2.4 | 0.34 |
| Coaptation length (mm) | 5.5 ± 2.2 | 5.6 ± 1.5 | 0.93 |
RV = right ventricular; mm = millimeter
COMMENT
We evaluated clinical and echocardiographic safety end-points and outcomes of patients undergoing guideline-directed concomitant TVr during left-sided valve surgery. We show that concomitant TVr for patients with TR and/or a dilated annulus resulted in acceptable rates of morbidity, mortality, and freedom from recurrent moderate or severe TR. Patients undergoing RV quantitation echocardiography did not worsen in RV geometry or function.
Functional TR leads to excess mortality and decreased quality of life if left untreated.
Prior studies have shown that increasing grades of TR in a non-surgical population of veterans was associated with increased mortality, regardless of pulmonary hypertension or ejection fraction [5], while isolated moderate or severe TR carries an added mortality risk, independent of cardiovascular or comorbid conditions [6]. Furthermore, untreated TR has been found to confer increased mortality among patients undergoing left-sided transcatheter valve procedures, with an up to 2-fold increased risk of mortality for patients with significant untreated TR and severe AS undergoing transcatheter aortic valve replacement (TAVR) [7] and moderate to severe TR independently predicting death and re-hospitalization at 12 months among MitraClip patients [8].The exact mechanism by which TR leads to decreased survival remains unknown, but likely relates to decreased RV function. In addition to survival, it has also been shown that TR may negatively affect functional status, as untreated moderate or greater TR has been identified as a risk factor for lower midterm survival and higher NYHA class compared to De Vega TVr in a propensity-matched analysis [19].
Functional TR is a progressive disease.
Historically, it was believed that functional TR would resolve after successful mitral surgery [4]. In contrast, TR is often progressive and may worsen if left uncorrected at the time of initial surgery. In fact, Dreyfus et al. found that patients who underwent isolated MVr had a higher incidence of late TR when compared to patients undergoing concomitant TVr [2]. After a mean follow-up of 4.8 years, patients undergoing isolated MVr had higher reported NYHA functional class (1.6 vs 1.1; p<0.0001) and worse TR grade (2.07 vs 0.36; p=0.001). Similarly, Gursoy et al. found that over half of patients with mild to moderate TR during mitral operations showed progression to moderate to severe TR in the mean 8-year follow-up period [20]. A meta-analysis of 2,488 patients from 10 studies showed that the rate of progression from mild to moderate to moderate to severe TR was 22.6% among patients who had no tricuspid intervention at the time of mitral surgery [21].
Current guidelines for functional TR support concomitant tricuspid repair.
Both the 2006 and 2014 ACC/AHA guidelines give concomitant TVr a class I indication in the setting of severe TR and a class IIb (2006) or IIa (2014) recommendation for mild or moderate TR with a dilated annulus (≥40mm) [13,14]. In the present study, we show that guideline-directed concomitant TVr is associated with favorable rates of postoperative pacemaker implantation, 30-day mortality and overall survival at 39 months, as well as initial freedom from recurrent moderate or severe TR at 14 months, indicating that the current guidelines should therefore be followed with reasonable safety end-points and short-term outcomes.
Concomitant tricuspid repair does not necessarily increase operative mortality.
It has been suggested that additional valvular procedures carry increased risk and operative mortality [15–17]. However, prior studies have found no added morbidity or mortality for patients undergoing mitral valve surgery and concomitant TVr [9]. This finding was supported by a recent analysis of the STS ACSD, which showed no increase in risk-adjusted operative mortality for TVr at all grades of TR [22], suggesting concomitant TVr may not confer added mortality risk. Conversely, operative risk for patients requiring a subsequent reoperation for residual/recurrent TR remains as high as 35% [23]. Thus, concomitant TVr at the initial operation should be strongly considered.
Tricuspid annuloplasty does not negatively impact RV function.
In subgroup analysis, we found no adverse effect of concomitant TVr on RV geometry or function (Table 5). One prior analysis found concomitant TVr to be the main independent positive predictor of late RV recovery among patients with pre-discharge RV dysfunction, despite up to 70% of patients undergoing concomitant TVr initially exhibiting TV dysfunction prior to discharge [9]. Likewise, Bertrand et al. showed that adding tricuspid valve annuloplasty to mitral valve surgery leads to favorable changes in RV geometry and prevents postoperative dilation [24], an effect most pronounced in patients with more than moderate TR at baseline.
Controversy remains surrounding potential increased pacemaker rate due to concomitant TVr.
In an analysis of over 88,000 patients in the STS ACSD, patients undergoing concomitant TVr had a 14.5% pacemaker rate compared to 6.2% in those who did not undergo concomitant TVr (p<0.0001) [22]. Furthermore, concomitant TVr was associated with an increase in major morbidity (OR 1.36, 95% CI: 1.24–1.48). However, Chikwe et al. report a pacemak er rate of only 2.4% among patients undergoing concomitant TVr, no different from their rate for isolated MVr [9]. Similarly, our reported pacemaker rate of 4.1% in this series is lower than other studies examining concomitant tricuspid surgery and is comparable to observed pacemaker rates after isolated mitral valve surgery [25].
There are several limitations to this study. First, this study was performed at a single institution and our results may not be generalizable. Second, we purposely did not have a comparison or control group, since we treated every patient consecutively with guideline-directed concomitant TVr and every patient who met guideline indication underwent TVr. Third, our mean echocardiographic follow-up time is relatively short at 14.1 months and longer follow-up is necessary to determine long-term durability of tricuspid valve repair. Whereas this study was intended to demonstrate safety end-points of concomitant TVr, such as mortality and pacemaker rate, we feel long-term durability and outcomes of concomitant TVr, including its subgroups, will be best answered by the ongoing CTSNet randomized, controlled trial addressing this topic [26].
In this series, guideline-directed concomitant tricuspid valve repair resulted in low rates of morbidity and mortality at 39 months of clinical and 14 months of echocardiographic follow-up. Furthermore, freedom from tricuspid regurgitation was acceptable and right ventricular performance did not worsen in the follow-up period. Given these findings, adherence to current guidelines regarding functional TR should be encouraged. A randomized, controlled trial to validate guideline-directed therapy has been initiated with results expected in the next few years [26].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Raja SG, Dreyfus GD. Basis for intervention on functional tricuspid regurgitation. Semin Thorac Cardiovasc Surg 2010;22:79–83. [DOI] [PubMed] [Google Scholar]
- 2.Dreyfus GD, Corbi PJ, Chan KM, Bahrami T. Secondary tricuspid regurgitation or dilatation: which should be the criteria for surgical repair? Ann Thorac Surg 2005;79:127–32. [DOI] [PubMed] [Google Scholar]
- 3.Goldstone AB, Howard JL, Cohen JE, et al. Natural history of coexistent tricuspid regurgitation in patients with degenerative mitral valve disease: implications for future guidelines. J Thorac Cardiovasc Surg 2014;148:2802–9. [DOI] [PubMed] [Google Scholar]
- 4.Braunwald NS, Ross J Jr., Morrow AG. Conservative management of tricuspid regurgitation in patients undergoing mitral valve replacement. Circulation 1967;35:I63–9. [DOI] [PubMed] [Google Scholar]
- 5.Nath J, Foster E, Heidenreich PA. Impact of tricuspid regurgitation on long-term survival. J Am Coll Cardiol 2004;43:405–9. [DOI] [PubMed] [Google Scholar]
- 6.Topilsky Y, Nkomo VT, Vatury O, et al. Clinical outcome of isolated tricuspid regurgitation. JACC Cardiovasc Imaging 2014;7:1185–94. [DOI] [PubMed] [Google Scholar]
- 7.Prasitlumkum N, Kewcharoen J, Kittipibul V, et al. BASELINE SIGNIFICANT TRICUSPID REGURGITATION INCREASES RISK OF MORTALITY IN POST TRANSCATHETER AORTIC VALVE REPLACEMENT: SYSTEMIC REVIEW AND META-ANALYSIS OF MULTIVARIABLE ADJUSTED OBSERVATIONAL STUDIES. J Am Coll Cardiol 2019:73(9 Supplement 1), 1198. [Google Scholar]
- 8.Ohno Y, Attizzani GF, Capodanno D, et al. Association of tricuspid regurgitation with clinical and echocardiographic outcomes after percutaneous mitral valve repair with the MitraClip System: 30-day and 12-month follow-up from the GRASP Registry. Eur Heart J Cardiovasc Imaging 2014;15(11):1246–55. [DOI] [PubMed] [Google Scholar]
- 9.Chikwe J, Itagaki S, Anyanwu A, Adams DH. Impact of concomitant tricuspid annuloplasty on tricuspid regurgitation, right ventricular function, and pulmonary artery hypertension after repair of mitral valve prolapse. J Am Coll Cardiol 2015;65:1931–8. [DOI] [PubMed] [Google Scholar]
- 10.Desai RR, Vargas Abello LM, Klein AL, et al. Tricuspid regurgitation and right ventricular function after mitral valve surgery with or without concomitant tricuspid valve procedure. J Thorac Cardiovasc Surg 2013;146:1126–32.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benedetto U, Melina G, Angeloni E, et al. Prophylactic tricuspid annuloplasty in patients with dilated tricuspid annulus undergoing mitral valve surgery. J Thorac Cardiovasc Surg 2012;143:632–8. [DOI] [PubMed] [Google Scholar]
- 12.Matsuyama K, Matsumoto M, Sugita T, Nishizawa J, Tokuda Y, Matsuo T. Predictors of residual tricuspid regurgitation after mitral valve surgery. Ann Thorac Surg 2003;75:1826–8. [DOI] [PubMed] [Google Scholar]
- 13.Bonow RO, Carabello BA, Kanu C, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation 2006;114(5):e84–231. [DOI] [PubMed] [Google Scholar]
- 14.Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:2438–88. [DOI] [PubMed] [Google Scholar]
- 15.Rankin JS, Hammill BG, Ferguson TB Jr., et al. Determinants of operative mortality in valvular heart surgery. J Thorac Cardiovasc Surg 2006;131:547–57. [DOI] [PubMed] [Google Scholar]
- 16.Lee R, Li S, Rankin JS, et al. Fifteen-year outcome trends for valve surgery in North America. Ann Thorac Surg 2011;91:677–84. [DOI] [PubMed] [Google Scholar]
- 17.Vassileva CM, Li S, Thourani VH, et al. Outcome characteristics of multiple-valve surgery: comparison with single-valve procedures. Innovations 2014;9:27–32. [DOI] [PubMed] [Google Scholar]
- 18.Carpentier A, Adams DH, Filsoufi F. Carpentier’s Reconstructive Valve Surgery Philadelphia, PA: Saunders Elsevier, 2010:194–200. [Google Scholar]
- 19.Calafiore AM, Gallina S, Iaco AL, et al. Mitral valve surgery for functional mitral regurgitation: should moderate-or-more tricuspid regurgitation be treated? a propensity score analysis. Ann Thorac Surg 2009;87:698–703. [DOI] [PubMed] [Google Scholar]
- 20.Gursoy M, Bakuy V, Hatemi AC, et al. Long-term prognosis of mild functional tricuspid regurgitation after mitral valve replacement. Anadolu Kardiyol Derg 2014;14:34–9. [DOI] [PubMed] [Google Scholar]
- 21.Kara I, Koksal C, Erkin A, et al. Outcomes of mild to moderate functional tricuspid regurgitation in patients undergoing mitral valve operations: A meta-analysis of 2,488 patients. Ann Thorac Surg 2015;100:2398–407. [DOI] [PubMed] [Google Scholar]
- 22.Badhwar V, Rankin JS, He M, et al. Performing concomitant tricuspid valve repair at the time of mitral valve operations is not associated with increased operative mortality. Ann Thorac Surg 2017;103:587–93. [DOI] [PubMed] [Google Scholar]
- 23.Bernal JM, Morales D, Revuelta C, Llorca J, Gutierrez-Morlote J, Revuelta JM. Reoperations after tricuspid valve repair. J Thorac Cardiovasc Surg 2005;130:498–503. [DOI] [PubMed] [Google Scholar]
- 24.Bertrand PB, Koppers G, Verbrugge FH, et al. Tricuspid annuloplasty concomitant with mitral valve surgery: effects on right ventricular remodeling. J Thorac Cardiovasc Surg 2014;147:1256–64. [DOI] [PubMed] [Google Scholar]
- 25.Mestres CA, Suri RM. Pacemaker risk associated with prophylactic tricuspid annuloplasty: Balancing beneficence and nonmaleficence. J Thorac Cardiovasc Surg 2016;15:104–5. [DOI] [PubMed] [Google Scholar]
- 26.Cardiothoracic Surgical Trials Network. Evaluating the Benefit of Concurrent Tricuspid Valve Repair During Mitral Surgery Available from: https://www.clinicaltrials.gov/ct2/show/NCT02675244. ClinicalTrials.gov Identifier: NCT02675244. Accessed December 9, 2018.


