Abstract
The first workshop on Novel Optics-based approaches for Cardiac Electrophysiology (NOtiCE) was held in Florence Italy in 2018. Here, we learned how optical approaches have shaped our basic understanding of cardiac electrophysiology and how new technologies and approaches are being developed and validated to advance the field. Several technologies are being developed that may one day allow for new clinical approaches for diagnosing cardiac disorders and possibly intervening to treat human patients. In this review, we discuss several technologies and approaches to optical voltage imaging with voltage-sensitive dyes. We highlight the development and application of fluorinated and long wavelength voltage-sensitive dyes. These optical voltage sensors have now been applied and well validated in several different assays from cultured human stem cell-derived cardiomyocytes to whole hearts in-vivo. Imaging concepts such as dual wavelength ratiometric techniques, which are crucial to maximizing the information from optical sensors by increasing the useful signal and eliminating noise and artifacts, are presented. Finally, novel voltage sensors including photoacoustic voltage-sensitive dyes, their current capabilities and potential advantages, are introduced.
Keywords: voltage-sensitive dye, VSD, cardiac electrophysiology, voltage sensor, voltage imaging, ratiometric imaging
1. INTRODUCTION
At the first workshop on Novel Optics-based approaches for Cardiac Electrophysiology (NOtiCE), we learned how optical approaches have shaped our basic understanding of cardiac electrophysiology. Optical techniques are especially useful because of the inherent massively parallel recordings capable of capturing the coordinated signaling and signal propagation among large networks of cells. It is useful in a wide array of assays from cell culture to tissues and whole hearts. We learned about the continued development of new approaches and new signaling reporters that may soon provide even higher resolution data, deeper penetration into tissues, and may enable optical interventions or modulations of cardiac activity. High throughput screening in the development of new pharmaceuticals, and cardiac toxicity screening to prevent dangerous cardiac side effects of new pharmaceuticals, are also important applications of parallel recordings with optical techniques. Recent developments in voltage reporters and recording techniques are also motivated by the goal of one day bringing optical recording techniques into the clinic to diagnose and possibly treat cardiac disorders such as arrhythmias.
In this review we discuss the recent progress in voltage-sensor technology. We focus primarily on electrochromic voltage-sensitive dyes (VSDs) with response times much less than 1 millisecond, which can faithfully reproduce cardiac action potentials and accurately measure electrical signal propagation. Fluorinated voltage-sensitive dyes were developed in 2012 and promise to replace classic dyes such as Di-4-ANEPPS as the work horses of voltage-imaging. In addition to better brightness, their spectra can be tuned for specific applications. Fluorinated dyes have been put to work in a variety of studies using different assays from isolated cardiomyocytes to whole heart and even live animal studies. Two long wavelength dyes first described in 2006 have been used primarily to study signaling in blood perfused whole hearts, but also have applications in cell based assays.
Matching imaging approaches to the photophysical properties of the VSDs can maximize the information conveyed by these measurements. In particular, dual wavelength ratiometric approaches are nicely matched to electrochromic VSDs. By boosting signals and removing unwanted artifacts, due to motion during cardiac contraction and relaxation for example, ratiometric imaging has been established in many arenas from cardiac toxicity screening to whole heart optical mapping studies. In recent live animal studies, ratiometric imaging was combined with a 2D fiber array in contact with the epicardium to record action potential waveforms and conduction velocities in the presence of large movement.
Finally, we discuss a new method of voltage imaging using photoacoustic signals. Photoacoustic imaging is a hybrid approach where light is used as an excitation source that is converted to ultrasound signal outputs detectable with ultrasound transceivers. Since ultrasound experiences far less scattering compared to fluorescence, photoacoustics have been applied to extremely deep imaging applications on the order of the thickness of the heart’s walls. New dyes sensitive to voltage that produce photoacoustic signals have been demonstrated and show promise for this new combination of technologies.
2. OPTICAL VOLTAGE SENSOR TECHNOLOGIES AND APPLICATION TECHNIQUES
2.1. Fluorinated Voltage-Sensitive Dyes
Fluorinated VSDs were introduced in 2012 (Yan et al., 2012) and offer many advantages that have led to new research in the neuroscience and cardiac fields. Substituting hydrogen atoms for fluorine in dye chromophores (Fig. 1A) can reduce the probability of photobleaching (Renikuntla et al., 2004; Sun et al., 1998). This was confirmed in experiments where, in some cases, fluorinated dyes bleached 3X slower than their non-fluorinated counterparts (under 2-photon excitation, Yan et al., 2012). Reduced photobleaching probability allows the experimenter to either increase excitation intensity during short recordings or extend the duration of recordings, in either case increasing the number of emitted photons and the available signal-to-noise from optical voltage recordings. Signal-to-noise ratios as large as 23 were achieved for 95mV action potentials using 2-photon excitation and, in general, high fidelity action potentials were recorded from a variety preparations from cultured cells to whole hearts with high temporal resolution (Yan et al., 2012). Prolonged or intense light exposure of fluorescently labeled cells is known to eventually lead to cytotoxicity; importantly, the fluorinated VSDs were also less phototoxic than their unfluorinated analogs.
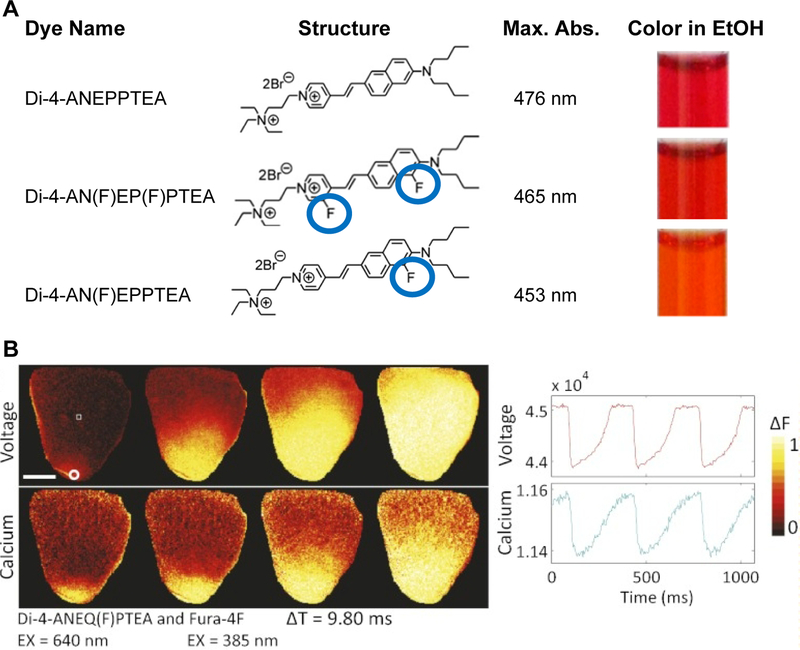
Figure 1.
A. Fluorination or fluorine substitution of voltage-sensitive dyes improves photostability and also alters spectral properties. Location of fluorine atoms (circled in blue) on donor or acceptor end of the chromophore shifts absorbance spectra to the blue or red respectively and allows spectra to be tuned. Di-4-AN(F)EPPTEA has one fluorine on donor side and a peak absorbance wavelength shifted to shorter wavelengths relative to the non-fluorinated dye Di-4-ANEPPTEA. Peak absorbance and colors in ethanol are given in right columns. Di-4-AN(F)EP(F)PTEA has a fluorine on both the donor and acceptor sides, leading to an absorbance spectrum similar to the non-fluorinated dye. B. Red shifted, fluorinated dye Di-4-ANEQ(F)PTEA used with Fura-4F for simultaneous voltage and free calcium recordings in whole heart preparations (raw fluorescence changes inverted in both voltage and calcium recordings). Reprinted with modification from original (Yan et al., 2012, Table 1 and Fig. 2).
Although the primary goal of fluorinated voltage-sensitive dyes was improved brightness through reduced photobleaching, other equally important effects were studied. First, fluorination leads to shifts in dye spectra (Fig. 1A) allowing spectra to be tuned for specific experimental needs. For example, blue shifted dyes such as Di-2-AN(F)EPPTEA make high sensitivity recordings possible using 2-photon excitation around 1060nm, a wavelength routinely provided by Ti-Sapphire lasers. Without fluorination, similar sensitivity would only be obtained at longer wavelengths only accessible with more sophisticated lasers.
Spectral tuning along with excellent brightness has led to several exciting discoveries. Optical recordings using the blue-shifted fluorinated dye Di-4-AN(F)EPPTEA was used to study action potential propagation in diseased cardiomyocytes for the first time (Crocini et al., 2016a; Crocini et al., 2014; Crocini et al., 2016c; Sacconi et al., 2012). By focusing on the transverse-axial tubular system (TATS) of ventricular myocytes stained with voltage-sensitive dye, dramatic alterations in electrical propagation were observed. For a technical viewpoint on how these challenging studies were accomplished, it is important to keep in mind how high quality recordings with high temporal resolution from small, sub-cellular compartments are especially challenging owing to the limited number of photons available from limited numbers of sensor molecules. Also, overall brightness stems from the photostability properties of the sensor molecules along with their ability to be continuously replenished.
Likewise, in the neuroscience field, a similar voltage-sensitive dye (Di-2-AN(F)EPPTEA) was used to study fast electrical transients in subcellular compartments including dendritic spines (Acker et al., 2011; Acker et al., 2016; Yan et al., 2012) and neuronal axons (Rowan et al., 2014). Red shifted fluorinated dyes such as Di-4-ANEQ(F)PTEA can be combined with calcium dyes such as Fura-4F to simultaneously record voltage and calcium in whole heart preparations (Yan et al., 2012). Spectral tuning is a valuable tool for implementing voltage imaging studies on microscopes with specific laser source wavelengths, and when combining voltage imaging with other fluorescent reporters or optogenetic actuators. As detailed in the paper by Yan et al. (Yan et al., 2012), combining fluorine substitution together with extending conjugation produces a large set of hemicyanine VSDs that cover the excitation range of 420nm to 670nm with corresponding emissions from 550nm to 750nm.
Along with brightness and spectral tuning, fluorination can also lead to changes in dye sensitivity. Increased sensitivity improves signal-to-noise and, although not explained mechanistically, several cases of increased sensitivity after fluorination were observed experimentally. With 2-photon excitation in neural preparations significant increases in sensitivity were observed (~50% increase for Di-4-ANEQ(F)PTEA, Yan et al., 2012). Additionally, recordings from single dendritic spines showed typical sensitivities of 16–18%/100mV (Acker et al., 2011; Acker et al., 2016). These advantages may also be explored in cardiac tissue. Preliminary data suggest Di-4-AN(F)EP(F)PTEA is capable of high sensitivity voltage responses in human cardiac stem cells (560nm excitation, results not shown).
2.2. Long Wavelength Voltage-Sensitive Dyes
Long wavelength voltage-sensitive dyes, with emission in the near-infrared, have been shown to have several advantages over classical dyes such as Di-4 and Di-8-ANEPPS. Two long wavelength dyes are Di-4-ANBDQBS and Di-4-ANBDQPQ, which were first synthesized by Joe P. Wuskell in our lab (Wuskell et al., 2006). These two dyes are also known as JPW-6033 and JPW-6003 respectively, names that include Dr. Wuskell’s initials. They use a longer 4 carbon butadiene linker and a quinolinium acceptor instead of pyridinium, shifting the chromophores to longer wavelengths (Loew, 2015). The red shifted dyes displayed an increased sensitivity to voltage explained by numerical molecular orbital calculations. In cultured cardiomyocytes, Di-4-ANBDQBS produced highly stable, low noise action potential recordings with low toxicity (Warren et al., 2010).
Without 2-photon excitation it is difficult to perform voltage imaging in tissues or organs with dyes such as Di-4-ANEPPS that are excited by wavelengths of light (~480nm) that are highly scattered in tissue. Red shifted dyes on the other hand are excited by much longer wavelengths of light (up to 700nm excitation with emission greater than 800nm), which scatter much less in tissue. Red shifted dyes such as Di-4-ANBDQBS and Di-4-ANBDQPQ were developed for application in cardiac tissue (Matiukas et al., 2006) and allow deeper imaging penetration to study the transmural electrophysiological properties in intact hearts (Walton et al., 2010). In a study of human ex-vivo hearts from transplant patients, the reduced light scatter made it possible to detect electrical activity from deeper regions such as the sinus node (Fedorov et al., 2010). The longer wavelength also enabled whole hearts to be studied while perfusing with blood since hemoglobin does not efficiently absorb longer wavelengths of light (Matiukas et al., 2007). Additionally, compared to Di-4-ANEPPS, signal decay due to internalization of dye into the cell was found to be 5–7X slower (Matiukas et al., 2007) allowing optical voltage recordings of much longer durations.
More recently, long wavelength VSDs have been used to develop models of cardiac defibrillation (Caldwell et al., 2015; Crocini et al., 2016b), cardiac pacing and resynchronization (Nussinovitch and Gepstein, 2015; Scardigli et al., 2018), and to study transmural APs in rabbit hearts (Mačianskienė et al., 2015). Several of these studies incorporate the optogenetic actuator Channelrhodopsin 2 (ChR2), which absorbs blue light and can be used together with red shifted VSDs. By combining optogenetics, researchers can better understand cardiac electrical conduction physiology through controlled manipulations and measurements with optical probes. Such experiments also serve as proof of feasibility of clinic interventions in human patients.
Similar to combining optical recordings with optogenetics, the ability to select appropriate spectral properties is very important in combining optical voltage recordings with other fluorescent reporters such as calcium sensors. Although complicated by the typically broad excitation spectra of many VSDs, simultaneous optical voltage and calcium recordings are possible (Yan et al., 2012) and have led to several important discoveries. In general, coupling between electrical signaling, calcium transients, and conduction is of paramount interest in the cardiac field. On a subcellular scale, combined voltage and calcium imaging has unveiled unexpected phenomena, like calcium delay in electrically failing TATS elements, and Ca2+ sparks that have no effect on membrane voltage (Crocini et al., 2014; Sacconi et al., 2012). Long wavelength dyes facilitate the combination with other indicators for “multi-parametric” cardiac optical mapping studies including Ca2+ and even endogenous NADH, along with caged compounds for simultaneous optical stimulation or perturbation (Lee et al., 2011; Lee et al., 2012a; Lee et al., 2012b).
The fluorinated dye Di-4-ANEQ(F)PTEA is another long wavelength dye with applications including recordings from excised hearts (Yan et al., 2012, Fig. 1B). Dyes such as Di-4-ANEQ(F)PTEA with the PTEA polar head group have the added advantage of high solubility in aqueous solutions eliminating the need for co-solvents such as DMSO or vehicles. Advantages in terms of photostability (expected with fluorination), and reduced toxicity have also been reported (Lee et al., 2019).
Recently, near-infrared dyes were used for live pig, open chest, whole heart imaging (Lee et al., 2019, Fig. 2), providing a high resolution in-vivo mapping of cardiac electrophysiology in pigs even in the context of ventricular fibrillation. High quality action potential recordings were achieved in the presence of significant motion of the heart by implementing ratiometric imaging techniques (described below) and by using optical fibers lowered onto the epicardial surface. These measurements allow arrhythmias to be studied in great detail in an intact animal model closely mimicking the human heart and provide hope that the same high information recordings may one day be brought into the clinic for diagnosing and treating human patients.
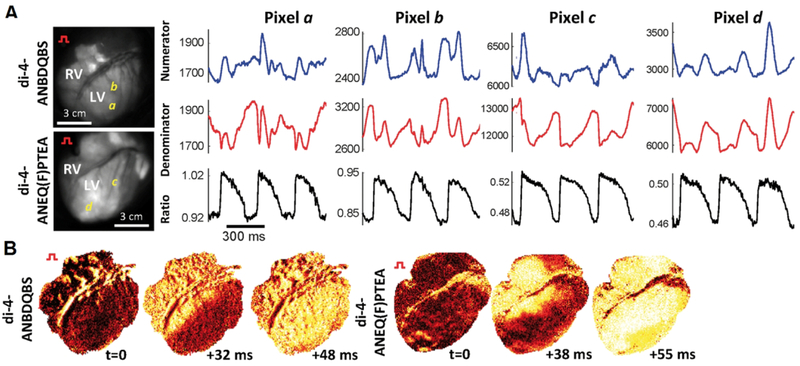
Figure 2.
A. Two long wavelength, electrochromic voltage-sensitive dyes Di-4-ANBDQBS and Di-4-ANEQ(F)PTEA are used to record electrical signal propagation from live pig hearts (open chest, anaesthetized). Two time multiplexed excitation light sources at the blue and red edge of the dyes’ excitation spectra are used to capture raw signals “Numerator” and “Denominator”, respectively. Only after taking the ratio do the true electrical signals, free of otherwise overwhelming motion artifacts, appear. B. Snapshots of normalized voltage fluorescence intensity maps following point stimulation. Reprinted with modification from original (Lee et al., 2019, Fig. 5).
2.3. Dual wavelength ratiometric imaging for mitigation of motion and other recording artifacts
All of the discussion so far has focused on voltage-sensitive dyes that change their fluorescent spectral properties in response to voltage. The voltage-sensing mechanism of these “electrochromic” dyes can be exploited to minimize imaging artifacts. Artifacts due to sample movement can be very large and are especially relevant to cardiac recordings; not only do excised hearts beat and contract but even cultured cardiac cells twitch in the dish. Without correction, even small movements can lead to highly corrupted optical recordings. However, as discussed here, the spectral shift mechanism can be exploited in several ways to minimize artifacts and allow useful information to be obtained from otherwise highly corrupted data. Stark shift electrochromic dyes such as classic dyes Di-4-ANEPPS, as well as the fluorinated and long-wavelength dyes discussed above are capable of so called dual wavelength ratiometric imaging. Two wavelength bands, either excitation or emission, can be used to produce two voltage-dependent signals with different polarities. For example, if exciting a dye on the red edge of the excitation with long wavelength light produces an inverted voltage signal, exciting at the blue edge with shorter wavelength light will produce a similar but upright or non-inverted signal. Ratiometric imaging implies that the ratio of these two signals is taken. Features such as the opposite polarity voltage signal are amplified in the ratio, while non-voltage dependent features common to both signals are normalized away. Ratio imaging can therefore compensate for uneven staining. Importantly, time dependent changes in fluorescence, such as slow photobleaching decay, and fast motion artifacts, are suppressed in dual wavelength ratio images. As demonstrated in Fig. 2, raw fluorescent signals from the blue and red sides of the fluorescence spectrum can show very large motion artifacts overwhelming the signal to be measured; these are completely removed in the ratio, revealing clear optically recorded action potentials.
Since both the excitation and emission spectra shift in response to voltage, dual wavelength ratiometry can be accomplished using either excitation wavelength selection, “excitation-based ratiometry”, or emission wavelength filtering, “emission-based ratiometry”. Emission-based ratiometry can be the easiest to implement by simply splitting the emitted fluorescence with a dichroic beam splitter and detecting fluorescence with 2 separate detectors before taking the ratio. This can lead to highly reproducible, “internally standardized”, membrane potential recordings (Montana et al., 1989). Emission-based ratiometry is used for cardiac toxicity studies (Hortigon-Vinagre et al., 2016) and is readily implemented on laser scanning systems where single pixel detectors are relatively cheap, producing highly reliable voltage recordings with excellent resolution down to ~5mV (Bullen and Saggau, 1999).
For widefield fluorescence imaging, emission-based ratiometry can be more difficult and expensive to implement since two high speed cameras are needed instead of one and must have their fields of view carefully aligned. Specialized dual camera systems are available from high speed camera maker RedShirt Imaging. Additionally, image splitter hardware options available from companies such as Hamamatsu and Cairn Research, are an alternative to using two cameras. These optomechanical devices project the same image with different filters to separate areas of the camera’s detector.
In contrast to emission-based ratiometry, dual-excitation ratiometric imaging uses two excitation wavelengths and a single emission band and detector. It can be performed with VSDs as well as electrochromic free calcium indicators such as fura-2 in whole hearts (Lee et al., 2011). This requires only one camera but requires synchronized high speed time multiplexing (switching back and forth) of two excitation wavelengths, typically using LEDs. This technique has been implemented in cardiac preparations with classic electrochromic VSDs and shown to reduce motion artifacts (Bachtel et al., 2011). Four wavelengths of light can even be combined to perform simultaneous ratiometric voltage and calcium imaging using a single camera detection system (Lee et al., 2012b). This has been the modality of choice for recent whole heart and, most recently, live animal cardiac imaging (Lee et al., 2019).
SEER (shifted emission excitation ratioing) imaging combines both emission and excitation-based ratiometry (Manno et al., 2013). By combining the excitation wavelengths with emission bands that produce additive effects before taking the ratio, SEER can boost sensitivity to 2 or even 3 times the sensitivity available from non-ratiometric imaging and can be implemented on commercial confocal scanning microscopes.
2.4. Optical voltage-imaging for drug screening, and cardiac toxicity screening
Voltage-sensitive dyes (VSDs) are compatible with human induced pluripotent stem cell-derived cardiomyocytes (iPSC-CMs), which are used to test for side effects of developmental drugs, such as QT prolongation (see CiPA project, Fermini et al., 2016). They can be used to stably track changes in AP waveform and duration (Lopez-Izquierdo et al., 2014). Recording optical action potentials in human iPSC-CMs with VSDs can be used to predict “the cardiac arrhythmogenic liability” (Lu et al., 2017) of developmental drugs to remove potentially harmful compounds from the development process. At the same time, compounds such as Verapamil, which block hERG potassium channels but may still be safe, never causing arrhythmias thanks to concomitant effects on different channel populations (Zhang et al., 1999), may be studied further without being unnecessarily removed from the development process.
Voltage-sensitive dyes can be combined with optogenetic tools for optical stimulation of cell populations and optical readout of electrical signaling (Klimas et al., 2016). Emission ratiometric imaging has been implemented for iPSC-CM recordings in order to remove motion artifacts of the iPSC-CM, which twitch in the dish (Hortigon-Vinagre et al., 2016, Fig. 3). This approach has been extensively validated using a library of drugs with known effects on cardiomyocytes including QT prolongation and known hazards to human patients (Blinova et al., 2017). Cardiac toxicity can even be performed in live pig hearts as demonstrated using long wavelength and fluorinated dyes with excitation-based ratiometry to measure APD and CV (Lee et al., 2019).
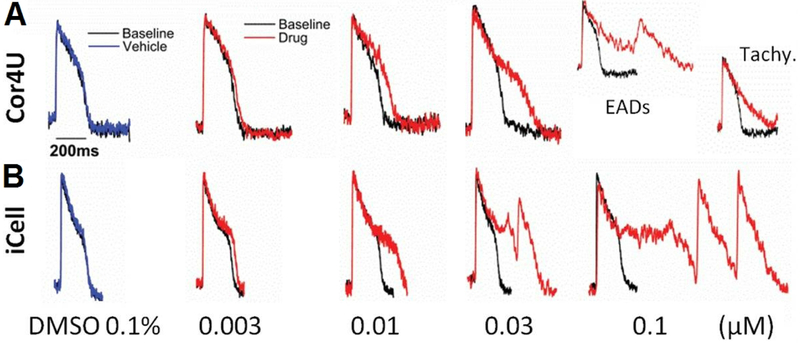
Figure 3.
Electrochromic voltage-sensitive dye Di-4-ANEPPS applied to hiPSC-CMs from 2 commercial sources exposed to increasing concentrations of an inhibitor of the repolarizing hERG potassium channel. Clear measurement of increases in AP duration and early after depolarizations (EADs) is possible with the voltage-sensitive dye. “Ratiometric” optical recordings show ratio of two emission wavelength bands from the dye. A. Cor4U hiPSC-CMs. B. iCell hiPSC-CMs. Reprinted with modification from original (Hortigon-Vinagre et al., 2016, Fig. 7).
2.5. Voltage imaging with Stark-shift electrochromic dyes: wavelength and power considerations
Success of voltage-sensitive dye imaging is tied closely to the imaging approach used, more so than typical calcium imaging for example. Imaging parameters must be chosen based on the specific sensor and voltage-dependent mechanism exploited to produce voltage-dependent changes in fluorescence, for example. Electrochromic dyes that sense voltage via a Stark-shift of their excitation and emission spectra (Loew, 2015) include classic dyes such as Di-4-ANEPPS (Fluhler et al., 1985; Loew et al., 1992). When inserted into the plasma membrane of a cell with proper alignment the energies for photon absorbance and emission are influenced by the membrane potential and small shifts in spectra are observed. Relative changes in absorbance and emission efficiencies as a function of voltage are greatest near the edges of the spectra and zero near the peaks. As a result, optimal (for voltage sensitivity) excitation wavelengths, for example, may be well away from the excitation spectrum’s peak. A common concern/misconception is that exciting at these non-optimal wavelengths (for excitation/absorbance) leads to dim, noisy signals. In reality, this can usually be compensated for with little or no penalty by increasing the excitation intensity, assuming of course that the experimenter’s excitation source has sufficient power. Excitation near the edges of the spectra with increased power effectively increases the brightness and signal-to-noise for wavelengths that are not optimal for excitation but are optimal for voltage-sensitivity. On the red side of the excitation spectrum, using longer wavelengths of light can have additional benefits; even using 2-photon imaging, the excitation light intensities necessary cause minimal bleaching and are not otherwise damaging (Acker et al., 2011).
2.6. Photo-acoustic voltage-sensitive dyes – a promising new modality for deep tissue measurements.
We recently described an alternative modality for recording membrane potential with voltage sensitive dyes using photoacoustic (PA) detection (Zhang et al., 2017a). In the PA technique, an intense short laser pulse excites chromophores to the excited state and the subsequent thermal relaxation to the ground state produces a shock wave that can be detected with an ultrasound transducer. We will first describe the general concept of how fluorescence quenching relates to photoacoustic (PA) signals. The key conceptual point is that the excited sate can lose its energy either as a photon of fluorescence or as non-radiative decay in the form of heat (phosphorescence can be neglected for most organic fluorophores). If a sufficient number of molecules are brought into the excited state concurrently, the resultant thermal relaxation can produce a shock wave detectable as sound. When the excitation is concentrated at the focus of a laser and ultrasound imaging technology is employed, it is possible to use the approach for deep tissue imaging. (Wang and Gao, 2014; Wang and Hu, 2012). But the efficiency of PA signal production is inversely related to the efficiency of radiative decay, i.e. fluorescence. This can be best understood via simple relationships:
QEF and QEth describe the probability for fluorescence and photoacoustics, respectively, where kr and kt are the radiative and thermal decay rates of the excited chromophore. Our work has shown that VSDs using a mechanism with voltage-dependent fluorescence quenching would produce a voltage dependent PA enhancement (Zhang et al., 2017a; Zhang et al., 2017b).
The best fluorophores for this purpose currently are long wavelength membrane permeant cyanines. These compounds have delocalized positive charges and can pass through cell membranes; their distribution across the membrane depends on voltage according to the Nernst equations. When they accumulate inside polarized cells their fluorescence is quenched and their PA signals are enhanced. Figure 4 provides the principle behind the design of PA-VSDs. Since PA signals experience almost no scattering compared to visible or even IR fluorescence, this hybrid imaging modality may be advantageous for deep tissue, whole heart or in-vivo cardiac optical recordings.
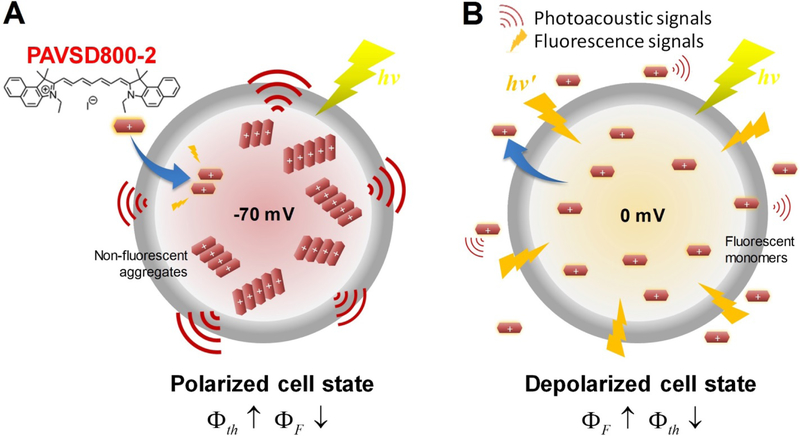
Figure 4.
Concept behind the reciprocal relationship between fluorescence and PA in a voltage-dependent self-quenching cyanine dye PAVSD800–2. A. Cartoon depiction of hyperpolarized cell state with −70mV transmembrane potential with strong photoacoustic output signals and weak fluorescence output from dye, shown mostly in aggregates. Structure of the cyanine dye is shown in the upper left and is represented as a red rectangle in the diagram. B. Depolarized cell state with 0mV transmembrane potential (or higher) with weak photoacoustic output signals and strong fluorescence output from lower concentration, fully dissolved dye inside the cell. φF and φth correspond the fluorescence and thermal quantum efficiencies of the cyanine chromophore. Reproduced from Zhang et al. 2017 under the CC BY 4.0 license: http://creativecommons.org/licenses/by/4.0/legalcode.
3. DISCUSSION
Dual wavelength ratiometric imaging has proven itself as an invaluable tool for voltage imaging in cardiac preparations owing to its ability to amplify useful signals and suppress artifacts that would otherwise overwhelm the signals being recorded. Even cardiomyocytes in a dish contract and twitch leading to large motion artifacts that must be contended with. Alternatives to the simple ratio for motion artifact reduction exist (Tai et al., 2004), but still require the same class of electrochromic voltage-sensitive dye. In our experience, excitation-based ratiometry produces larger voltage-dependent signals than emission-based ratiometry. At the same time both techniques separately, or when brought together with SEER, bring penalties of additional noise compared to non-ratiometric techniques. Splitting fluorescence emission reduces numbers of photons and time multiplexing also reduces the numbers of photons available per ms, reducing signal-to-noise. Even though the additional noise is usually much smaller than the artifacts removed by ratiometry, these tradeoffs should still be studied in more detail. Although SEER voltage imaging is perhaps the ultimate approach to ratiometric imaging, implementations are limited. Examples of synchronized, camera-based SEER should be compared to laser scanning confocal, spinning disk versions of SEER to determine the best possible imaging modality, which could depend on the specific cardiac preparation, the desired temporal and spatial resolutions, etc.
Although voltage sensor technologies have been steadily improving over the past few decades, there is still a great deal to be done and recently there has even been a resurgence of new approaches to voltage sensing. Fluorinated Stark shift electrochromic dyes provided several important benefits such as the ability to tune spectra (Fig. 1A) for greater flexibility in designing experiments including, thick samples and tissues, and inclusion of other fluorescent indicators. Still, more needs to be done to identify VSDs that are optimal for specific preparations, including the human stem-cell derived iPSC-CMs, and to identify the optimal imaging parameters to maximize the information and reliability of the optical recordings. New voltage indicators based on a different voltage-sensing mechanism, photo-induced electron transfer (PeT), were recently introduced (Miller et al., 2012; Woodford et al., 2015). As with the electrochromic dyes, these dyes are extremely fast, easily able to record action potentials in detail. However, the PeT mechanism does not lend itself to dual wavelength ratiometric measurements and to our knowledge dyes of this class have not been used ratiometrically.
An alternative approach to voltage-sensitive dyes is the application of genetically encoded voltage indicators or GEVIs. GEVIs carry the distinct advantage of targetability since their expression can be directed to specific cell types using appropriate promoters. VSFP2.3, a FRET (Förster resonance energy transfer)-based voltage sensor including cyan and yellow fluorescent proteins (CFP and YFP), was expressed in cardiomyocytes of transgenic mice, making optical mapping studies possible (Chang Liao et al., 2015). No toxicity was detected compared to wild type hearts even though transgenic mice expressing a calcium sensor did show signs of toxicity. FRET-based sensors are especially attractive for cardiac recordings because they are usually amenable to dual wavelength ratiometric techniques, which, as discussed, are very useful for motion artifact mitigation. Unfortunately, some of the latest GEVIs for neuroscience research use a non-fluorescent quencher and are not good candidates for dual wavelength ratiometric techniques even though they were designed for improved brightness and have extremely fast response kinetics (Abdelfattah et al. bioRxiv 436840; doi: https://doi.org/10.1101/436840, Gong et al., 2015; Piatkevich et al., 2018; Xu et al., 2018). Still, GEVI technology and techniques are advancing rapidly (Xu et al., 2017) and are being used in several cardiac research arenas including drug screening and cardiac toxicity assays in cultured cell preparations (Streit and Kleinlogel, 2018).
Although, voltage-sensitive dyes alone cannot be targeted to specific cell types, strategies do exist to achieve targeting. Genetic approaches can be used to label cells of interest with a highly specific “hook” that interacts with a chemical marker added to the dye. Existing examples include voltage dye molecules that can be freed from non-fluorescent forms (Liu et al., 2017) or can be altered to bind to labeled cells (Grenier et al., 2019; Hinner et al., 2006), thereby lighting up cells of interest with bright voltage sensitive dye molecules.
At the first workshop on Novel Optics-based approaches for Cardiac Electrophysiology (NOtiCE, 2018) new optical approaches under development were discussed along with more established methods. For example, photoacoustic (PA) imaging is a unique hybrid imaging approach using light for excitation and ultrasound for signal detection. Intended for deep tissue or whole organ recordings where detection of fluorescence is not possible, this technique has many biomedical imaging applications (Wang and Hu, 2012), and has recently been demonstrated with photoacoustic voltage-sensitive dyes (PA-VSDs, Zhang et al., 2017b). Additionally, a new voltage sensor technology to consider is the tethered bichromophoric fluorophore quencher (TBFQ) voltage-sensitive dyes (Yan et al., 2018). These new dyes were developed with the primary goal of maximizing sensitivity in order to make voltage imaging much more routine and widely adopted; the first examples of this new technology showed sensitivities much higher than any previously described voltage sensor that we know of. While work on this new platform is ongoing, it is also worth mentioning that TBFQ dyes, which include a quencher, are also potential targets for photoacoustics and can be developed for this modality as well as for fluorescence imaging. The authors are optimistic that further advances and validation of these novel technologies will be available to present and discuss at the second NOtiCE conference being planned.
Acknowledgment:
This work from our lab that is reviewed in this paper was supported by the National Institutes of Health via grant No. EB001963.
Footnotes
Declaration of Interest: Corey Acker, Ping Yan, Leslie Loew are owners of Potentiometric Probes LLC. Corey Acker and Ping Yan are part time employees of Potentiometric Probes.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Acker C, Yan P and Loew L, 2011. Single-Voxel Recording of Voltage Transients in Dendritic Spines, Biophysical Journal 101, L11–L13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acker CD, Hoyos E and Loew LM, 2016. EPSPs Measured in Proximal Dendritic Spines of Cortical Pyramidal Neurons, eNeuro 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachtel AD, Gray RA, Stohlman JM, Bourgeois EB, Pollard AE and Rogers JM, 2011. A novel approach to dual excitation ratiometric optical mapping of cardiac action potentials with di-4-ANEPPS using pulsed LED excitation, IEEE Trans Biomed Eng 58, 2120–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blinova K, Stohlman J, Vicente J, Chan D, Johannesen L, Hortigon-Vinagre MP, Zamora V, Smith G, Crumb WJ, Pang L, Lyn-Cook B, Ross J, Brock M, Chvatal S, Millard D, Galeotti L, Stockbridge N and Strauss DG, 2017. Comprehensive Translational Assessment of Human-Induced Pluripotent Stem Cell Derived Cardiomyocytes for Evaluating Drug-Induced Arrhythmias, Toxicol Sci 155, 234–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullen A and Saggau P, 1999. High-speed, random-access fluorescence microscopy: II. Fast quantitative measurements with voltage-sensitive dyes, Biophys J 76, 2272–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell BJ, Trew ML and Pertsov AM, 2015. Cardiac response to low-energy field pacing challenges the standard theory of defibrillation, Circ Arrhythm Electrophysiol 8, 685–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Liao ML, de Boer TP, Mutoh H, Raad N, Richter C, Wagner E, Downie BR, Unsöld B, Arooj I, Streckfuss-Bömeke K, Döker S, Luther S, Guan K, Wagner S, Lehnart SE, Maier LS, Stühmer W, Wettwer E, van Veen T, Morlock MM, Knöpfel T and Zimmermann WH, 2015. Sensing Cardiac Electrical Activity With a Cardiac Myocyte--Targeted Optogenetic Voltage Indicator, Circ Res 117, 401–12. [DOI] [PubMed] [Google Scholar]
- Crocini C, Coppini R, Ferrantini C, Yan P, Loew LM, Poggesi C, Cerbai E, Pavone FS and Sacconi L, 2016a. T-Tubular Electrical Defects Contribute to Blunted β-Adrenergic Response in Heart Failure, Int J Mol Sci 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocini C, Coppini R, Ferrantini C, Yan P, Loew LM, Tesi C, Cerbai E, Poggesi C, Pavone FS and Sacconi L, 2014. Defects in T-tubular electrical activity underlie local alterations of calcium release in heart failure, Proc Natl Acad Sci U S A 111, 15196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocini C, Ferrantini C, Coppini R, Scardigli M, Yan P, Loew LM, Smith G, Cerbai E, Poggesi C, Pavone FS and Sacconi L, 2016b. Optogenetics design of mechanistically-based stimulation patterns for cardiac defibrillation, Sci Rep 6, 35628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocini C, Ferrantini C, Scardigli M, Coppini R, Mazzoni L, Lazzeri E, Pioner JM, Scellini B, Guo A, Song LS, Yan P, Loew LM, Tardiff J, Tesi C, Vanzi F, Cerbai E, Pavone FS, Sacconi L and Poggesi C, 2016c. Novel insights on the relationship between T-tubular defects and contractile dysfunction in a mouse model of hypertrophic cardiomyopathy, J Mol Cell Cardiol 91, 42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorov VV, Glukhov AV, Chang R, Kostecki G, Aferol H, Hucker WJ, Wuskell JP, Loew LM, Schuessler RB, Moazami N and Efimov IR, 2010. Optical mapping of the isolated coronary-perfused human sinus node, J Am Coll Cardiol 56, 1386–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fermini B, Hancox JC, Abi-Gerges N, Bridgland-Taylor M, Chaudhary KW, Colatsky T, Correll K, Crumb W, Damiano B, Erdemli G, Gintant G, Imredy J, Koerner J, Kramer J, Levesque P, Li Z, Lindqvist A, Obejero-Paz CA, Rampe D, Sawada K, Strauss DG and Vandenberg JI, 2016. A New Perspective in the Field of Cardiac Safety Testing through the Comprehensive In Vitro Proarrhythmia Assay Paradigm, J Biomol Screen 21, 1–11. [DOI] [PubMed] [Google Scholar]
- Fluhler E, Burnham VG and Loew LM, 1985. Spectra, membrane binding, and potentiometric responses of new charge shift probes, Biochemistry 24, 5749–55. [DOI] [PubMed] [Google Scholar]
- Gong Y, Huang C, Li JZ, Grewe BF, Zhang Y, Eismann S and Schnitzer MJ, 2015. High-speed recording of neural spikes in awake mice and flies with a fluorescent voltage sensor, Science 350, 1361–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenier V, Daws BR, Liu P and Miller EW, 2019. Spying on Neuronal Membrane Potential with Genetically Targetable Voltage Indicators, J Am Chem Soc 141, 1349–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinner MJ, Hübener G and Fromherz P, 2006. Genetic targeting of individual cells with a voltage-sensitive dye through enzymatic activation of membrane binding, Chembiochem 7, 495–505. [DOI] [PubMed] [Google Scholar]
- Hortigon-Vinagre MP, Zamora V, Burton FL, Green J, Gintant GA and Smith GL, 2016. The Use of Ratiometric Fluorescence Measurements of the Voltage Sensitive Dye Di-4-ANEPPS to Examine Action Potential Characteristics and Drug Effects on Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes, Toxicol Sci 154, 320–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimas A, Ambrosi CM, Yu J, Williams JC, Bien H and Entcheva E, 2016. OptoDyCE as an automated system for high-throughput all-optical dynamic cardiac electrophysiology, Nat Commun 7, 11542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P, Bollensdorff C, Quinn TA, Wuskell JP, Loew LM and Kohl P, 2011. Single-sensor system for spatially resolved, continuous, and multiparametric optical mapping of cardiac tissue, Heart Rhythm 8, 1482–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P, Quintanilla JG, Alfonso-Almazan JM, Galan-Arriola C, Yan P, Sanchez-Gonzalez J, Perez-Castellano N, Perez-Villacastin J, Ibanez B, Loew LM and Filgueiras-Rama D, 2019. In-Vivo Ratiometric Optical Mapping Enables High-Resolution Cardiac Electrophysiology in Pig Models, Cardiovasc Res [DOI] [PMC free article] [PubMed]
- Lee P, Taghavi F, Yan P, Ewart P, Ashley EA, Loew LM, Kohl P, Bollensdorff C and Woods CE, 2012a. In situ optical mapping of voltage and calcium in the heart, PLoS One 7, e42562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P, Yan P, Ewart P, Kohl P, Loew LM and Bollensdorff C, 2012b. Simultaneous measurement and modulation of multiple physiological parameters in the isolated heart using optical techniques, Pflugers Arch 464, 403–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Grenier V, Hong W, Muller VR and Miller EW, 2017. Fluorogenic Targeting of Voltage-Sensitive Dyes to Neurons, J Am Chem Soc 139, 17334–17340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loew LM, 2015. Design and Use of Organic Voltage Sensitive Dyes, Adv Exp Med Biol 859, 27–53. [DOI] [PubMed] [Google Scholar]
- Loew LM, Cohen LB, Dix J, Fluhler EN, Montana V, Salama G and Wu JY, 1992. A naphthyl analog of the aminostyryl pyridinium class of potentiometric membrane dyes shows consistent sensitivity in a variety of tissue, cell, and model membrane preparations, J Membr Biol 130, 1–10. [DOI] [PubMed] [Google Scholar]
- Lopez-Izquierdo A, Warren M, Riedel M, Cho S, Lai S, Lux RL, Spitzer KW, Benjamin IJ, Tristani-Firouzi M and Jou CJ, 2014. A near-infrared fluorescent voltage-sensitive dye allows for moderate-throughput electrophysiological analyses of human induced pluripotent stem cell-derived cardiomyocytes, Am J Physiol Heart Circ Physiol 307, H1370–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu HR, Hortigon-Vinagre MP, Zamora V, Kopljar I, De Bondt A, Gallacher DJ and Smith G, 2017. Application of optical action potentials in human induced pluripotent stem cells-derived cardiomyocytes to predict drug-induced cardiac arrhythmias, J Pharmacol Toxicol Methods 87, 53–67. [DOI] [PubMed] [Google Scholar]
- Manno C, Figueroa L, Fitts R and Ríos E, 2013. Confocal imaging of transmembrane voltage by SEER of di-8-ANEPPS, J Gen Physiol 141, 371–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matiukas A, Mitrea BG, Pertsov AM, Wuskell JP, Wei MD, Watras J, Millard AC and Loew LM, 2006. New near-infrared optical probes of cardiac electrical activity, Am J Physiol Heart Circ Physiol 290, H2633–43. [DOI] [PubMed] [Google Scholar]
- Matiukas A, Mitrea BG, Qin M, Pertsov AM, Shvedko AG, Warren MD, Zaitsev AV, Wuskell JP, Wei MD, Watras J and Loew LM, 2007. Near-infrared voltage-sensitive fluorescent dyes optimized for optical mapping in blood-perfused myocardium, Heart Rhythm 4, 1441–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mačianskienė R, Martišienė I, Navalinskas A, Vosyliūtė R, Treinys R, Vaidelytė B, Benetis R and Jurevičius J, 2015. Evaluation of excitation propagation in the rabbit heart: optical mapping and transmural microelectrode recordings, PLoS One 10, e0123050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EW, Lin JY, Frady EP, Steinbach PA, Kristan WB and Tsien RY, 2012. Optically monitoring voltage in neurons by photo-induced electron transfer through molecular wires, Proc Natl Acad Sci U S A 109, 2114–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montana V, Farkas DL and Loew LM, 1989. Dual-wavelength ratiometric fluorescence measurements of membrane potential, Biochemistry 28, 4536–9. [DOI] [PubMed] [Google Scholar]
- Nussinovitch U and Gepstein L, 2015. Optogenetics for in vivo cardiac pacing and resynchronization therapies, Nat Biotechnol 33, 750–4. [DOI] [PubMed] [Google Scholar]
- Piatkevich KD, Jung EE, Straub C, Linghu C, Park D, Suk HJ, Hochbaum DR, Goodwin D, Pnevmatikakis E, Pak N, Kawashima T, Yang CT, Rhoades JL, Shemesh O, Asano S, Yoon YG, Freifeld L, Saulnier JL, Riegler C, Engert F, Hughes T, Drobizhev M, Szabo B, Ahrens MB, Flavell SW, Sabatini BL and Boyden ES, 2018. A robotic multidimensional directed evolution approach applied to fluorescent voltage reporters, Nat Chem Biol 14, 352–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renikuntla BR, Rose HC, Eldo J, Waggoner AS and Armitage BA, 2004. Improved photostability and fluorescence properties through polyfluorination of a cyanine dye, Org Lett 6, 909–12. [DOI] [PubMed] [Google Scholar]
- Rowan MJ, Tranquil E and Christie JM, 2014. Distinct kv channel subtypes contribute to differences in spike signaling properties in the axon initial segment and presynaptic boutons of cerebellar interneurons, J Neurosci 34, 6611–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacconi L, Ferrantini C, Lotti J, Coppini R, Yan P, Loew LM, Tesi C, Cerbai E, Poggesi C and Pavone FS, 2012. Action potential propagation in transverse-axial tubular system is impaired in heart failure, Proc Natl Acad Sci U S A 109, 5815–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scardigli M, Müllenbroich C, Margoni E, Cannazzaro S, Crocini C, Ferrantini C, Coppini R, Yan P, Loew LM, Campione M, Bocchi L, Giulietti D, Cerbai E, Poggesi C, Bub G, Pavone FS and Sacconi L, 2018. Real-time optical manipulation of cardiac conduction in intact hearts, J Physiol 596, 3841–3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit J and Kleinlogel S, 2018. Dynamic all-optical drug screening on cardiac voltage-gated ion channels, Sci Rep 8, 1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W-C, Gee KR and Haugland RP, 1998. Synthesis of novel fluorinated coumarins: Excellent UV-light excitable fluorescent dyes, Bioorganic & Medicinal Chemistry Letters 8, 3107–3110. [DOI] [PubMed] [Google Scholar]
- Tai DC, Caldwell BJ, LeGrice IJ, Hooks DA, Pullan AJ and Smaill BH, 2004. Correction of motion artifact in transmembrane voltage-sensitive fluorescent dye emission in hearts, Am J Physiol Heart Circ Physiol 287, H985–93. [DOI] [PubMed] [Google Scholar]
- Walton RD, Benoist D, Hyatt CJ, Gilbert SH, White E and Bernus O, 2010. Dual excitation wavelength epifluorescence imaging of transmural electrophysiological properties in intact hearts, Heart Rhythm 7, 1843–9. [DOI] [PubMed] [Google Scholar]
- Wang LV and Gao L, 2014. Photoacoustic microscopy and computed tomography: from bench to bedside, Annu Rev Biomed Eng 16, 155–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LV and Hu S, 2012. Photoacoustic tomography: in vivo imaging from organelles to organs, Science 335, 1458–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren M, Spitzer KW, Steadman BW, Rees TD, Venable P, Taylor T, Shibayama J, Yan P, Wuskell JP, Loew LM and Zaitsev AV, 2010. High-precision recording of the action potential in isolated cardiomyocytes using the near-infrared fluorescent dye di-4-ANBDQBS, Am J Physiol Heart Circ Physiol 299, H1271–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodford CR, Frady EP, Smith RS, Morey B, Canzi G, Palida SF, Araneda RC, Kristan WB, Kubiak CP, Miller EW and Tsien RY, 2015. Improved PeT molecules for optically sensing voltage in neurons, J Am Chem Soc 137, 1817–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuskell JP, Boudreau D, Wei MD, Jin L, Engl R, Chebolu R, Bullen A, Hoffacker KD, Kerimo J, Cohen LB, Zochowski MR and Loew LM, 2006. Synthesis, spectra, delivery and potentiometric responses of new styryl dyes with extended spectral ranges, J Neurosci Methods 151, 200–15. [DOI] [PubMed] [Google Scholar]
- Xu Y, Peng L, Wang S, Wang A, Ma R, Zhou Y, Yang J, Sun DE, Lin W, Chen X and Zou P, 2018. Hybrid Indicators for Fast and Sensitive Voltage Imaging, Angew Chem Int Ed Engl 57, 3949–3953. [DOI] [PubMed] [Google Scholar]
- Xu Y, Zou P and Cohen AE, 2017. Voltage imaging with genetically encoded indicators, Curr Opin Chem Biol 39, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan P, Acker C and Loew LM, 2018. Tethered Bichromophoric Fluorophore Quencher Voltage Sensitive Dyes, ACS Sens [DOI] [PMC free article] [PubMed]
- Yan P, Acker CD, Zhou WL, Lee P, Bollensdorff C, Negrean A, Lotti J, Sacconi L, Antic SD, Kohl P, Mansvelder HD, Pavone FS and Loew LM, 2012. Palette of fluorinated voltage-sensitive hemicyanine dyes, Proceedings of the National Academy of Sciences of the United States of America 109, 20443–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HK, Kang J, Yan P, Abou DS, Le HND, Thorek DLJ, Kang JU, Gjedde A, Rahmim A, Wong DF, Loew LM and Boctor EM, 2017a. Recording membrane potential changes through photoacoustic voltage sensitive dye, in: Editor (Ed.)^(Eds.), Book Recording membrane potential changes through photoacoustic voltage sensitive dye City, pp. 1006407–1006407–10. [DOI] [PMC free article] [PubMed]
- Zhang HK, Yan P, Kang J, Abou DS, Le HN, Jha AK, Thorek DL, Kang JU, Rahmim A, Wong DF, Boctor EM and Loew LM, 2017b. Listening to membrane potential: photoacoustic voltage-sensitive dye recording, J Biomed Opt 22, 45006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Zhou Z, Gong Q, Makielski JC and January CT, 1999. Mechanism of block and identification of the verapamil binding domain to HERG potassium channels, Circ Res 84, 989–98. [DOI] [PubMed] [Google Scholar]