Abstract
OBJECTIVE:
Exposure to violence in childhood is associated with increased risk for multiple forms of internalizing and externalizing psychopathology. We evaluated how exposure to violence in early life influences neural responses to neutral and threat-related stimuli in childhood and adolescence, developmental variation in these associations, and whether these neural response patterns convey transdiagnostic risk for psychopathology over time.
METHOD:
Participants were 149 youth (75 female youth), ages 8 to 17 (M =12.8, SD=2.63), who experienced either physical abuse, sexual abuse, or domestic violence (n=76) or had never experienced violence (n=73). Participants underwent fMRI scanning while passively viewing fearful, neutral, and scrambled faces presented rapidly in a block design without specific attentional demands. Internalizing and externalizing psychopathology were assessed concurrently with the scan and two years later and used to compute a transdiagnostic general psychopathology factor (p-factor).
RESULTS:
Exposure to violence was associated with reduced activation in dorsal anterior cingulate cortex (dACC) and frontal pole (1985 voxels, Peak x,y,z=6,4,40) when viewing fearful (versus scrambled) faces, and reduced activation in dorsomedial prefrontal cortex and superior frontal gyrus (1970 voxels, Peak x,y,z=16,64,10) when viewing neutral faces, but not amygdala activation or connectivity. Lower dACC response to fearful faces predicted increases in the p-factor two years later (B=−0.186, p=.031) and mediated the association of violence exposure with longitudinal increases in the p-factor.
CONCLUSION:
Reduced recruitment of the dACC—a region involved in salience processing, conflict monitoring, and cognitive control—in response to threat-related cues may convey increased transdiagnostic psychopathology risk in youth exposed to violence.
Keywords: dorsal anterior cingulate cortex, fear, maltreatment, p factor, salience network
Introduction
Exposure to trauma in childhood, particularly traumatic events involving interpersonal violence, is associated with increased risk for virtually all forms of psychopathology, including mood, anxiety, disruptive behavior, substance abuse, and psychotic disorders.1 Identifying the mechanisms linking violence exposure with psychopathology transdiagnostically across childhood and adolescence is critical for informing early interventions in children exposed to violence. While potentially adaptive in dangerous environments, altered neural responses to threat-related cues, such as facial expressions of fear and anger, may be one mechanism conveying risk for psychopathology in violence-exposed youth.2 In the present study, we examined associations between childhood violence exposure, psychopathology, and neural responses to fearful faces.
Childhood violence exposure, including physical and sexual abuse and domestic violence, is associated with heightened neural reactivity to threat cues in the salience network,3–5 including the amygdala.6 Moreover, the magnitude of amygdala response to threat-related cues increases with greater severity of violence exposure5. Disruptions in prefrontal cortex regulation of the amygdala may contribute to heightened amygdala reactivity following violence exposure early in life. Indeed, childhood violence exposure is associated with reduced amygdala-PFC functional connectivity during facial emotion processing in adults.7,8 Further, because the function and connectivity of salience network regions undergo considerable developmental change across childhood and adolescence,9,10 the associations between violence exposure and neural response to threat-related cues may vary with age. Amygdala reactivity to emotional faces decreases across adolescence, corresponding with increases in mPFC activity.9 However, amygdala reactivity to fearful and angry faces increases longitudinally across adolescence in youth with high levels of exposure to stressful life events.11 Functional connectivity between the amygdala and mPFC during fearful face viewing switches from positive to negative in early adolescence.10 This pattern occurs earlier in youth exposed to deprivation and other forms of early-life stress,12,13 which may serve an adaptive function to compensate for amygdala hyperactivity, reducing anxiety risk.12 However, developmental patterns in amygdala connectivity have not been investigated in relation to childhood violence exposure specifically.
Increased salience network reactivity to threat-related cues is associated with depression, anxiety, and externalizing problems,11,14,15 especially among children and adolescents exposed to adversity3, suggesting that heightened salience network reactivity to threat-related cues may contribute to psychopathology transdiagnostically. Recent work has demonstrated the existence of a transdiagnostic psychopathology factor—termed the general factor or p-factor—that explains the co-occurrence of psychopathology symptoms across the internalizing and externalizing spectrum.16–19 The existence of “p” has been demonstrated in studies examining the structure of psychopathology symptoms in children, adolescents, and adults.16–19 Moreover, violence exposure in childhood is associated with increases in this general psychopathology factor with no residual associations with specific symptoms types after accounting for associations with “p.”18 Negative emotionality, a tendency to experience negative emotions more frequently and intensely, has been proposed as one pathway contributing to this transdiagnostic general factor20. This assertion is supported by evidence that negative emotionality predicts virtually all forms of psychopathology21 as well as comorbidity between disorders.22 We recently found that self-reported emotional reactivity and difficulties with emotion regulation mediated the longitudinal association between childhood violence exposure and “p,”23 providing preliminary evidence that emotional reactivity is a mechanism linking violence exposure with general psychopathology. To our knowledge, neural mechanisms linking childhood violence exposure with the generalized psychopathology factor have not previously been examined. In the current study, we examine neural function in response to negative emotional cues as one potential mechanism.
We investigated the influence of childhood violence exposure on neural reactivity to fearful and neutral faces. Participants were children and adolescents who experienced physical abuse, sexual abuse, or domestic violence and age- and sex-matched controls who had never experienced violence. Neural reactivity was assessed using rapid, repeated presentation of faces without explicit attentional demands. Building on prior work using tasks assessing explicit processing of facial features,4 we expected that violence exposure would be associated with greater neural activation to fearful faces in the amygdala, anterior insula, and other nodes of the salience network. Given evidence that violence-exposed children perceive neutral faces as more threatening,24 we contrasted the fearful faces with scrambled, rather than neutral, faces. We also tested whether neural reactivity to neutral relative to scrambled faces was associated with violence-exposure to determine whether differences were specific to threat cues. With regard to developmental differences, we expected heightened salience network activity to fearful faces to be more strongly associated with maltreatment among adolescent participants than among younger children. Given limited published findings on the nature of amygdala-prefrontal connectivity differences in relation to violence exposure in adolescents, we did not have directional hypotheses about differences in amygdala-mPFC connectivity as a function of violence exposure or interactions with age. Finally, we predicted that alterations in neural circuitry underlying response to threatening stimuli would be a mechanism linking violence exposure to increases in the general psychopathology factor (p-factor) over time.
Method
Participants
Participants were 160 children and adolescents between the ages of 8 and 17 living in the Seattle area. Youth and caregivers were recruited for participation at schools, after-school and prevention programs, adoption programs, food banks, shelters, parenting programs, medical clinics, and the general community in Seattle, WA between January 2015 and June 2017. Recruitment efforts were targeted at recruiting a sample with variation in exposure to maltreatment-related trauma. To do so, we recruited from neighborhoods with high levels of violent crime, clinics that served a predominantly low-SES catchment area, and agencies that work with families who have been victims of violence (e.g., domestic violence shelters, programs for parents mandated to receive intervention by Child Protective Services). Exposure to violence and other inclusion and exclusion criteria were assessed during the first study visit. Inclusion criteria for the violence-exposed group included exposure to physical or sexual abuse or direct witnessing of domestic violence. Children in the control group were matched to children in the violence-exposed group on age, sex, and handedness; inclusion criteria required an absence of exposure to maltreatment or other forms of significant interpersonal violence. Exclusion criteria included IQ < 80, presence of pervasive developmental disorder, active psychotic symptoms or mania, active substance abuse, and presence of safety concerns. Participants who completed the MRI visit also met standard MRI inclusion criteria (i.e., absence of braces, claustrophobia). Written informed consent was obtained from legal guardians while children provided written assent. Eleven participants were excluded from further analysis due to non-responses to a behavioral check for attention during the scan or excessive head movement, 10 of whom were violence-exposed. Excluded participants were mostly male (9/11), racial or ethnic minority (9/11), and tended to be younger, and have higher levels of psychopathology than included participants. 149 participants (76 violence-exposed, 73 unexposed) were included in whole brain and ROI analyses. 122 (59 maltreated) of the 149 participants with usable data returned for follow-up assessments of psychopathology approximately two years later (82% retention rate). See Table 1 for socio-demographic characteristics of the final MRI sample.
Table 1:
Distribution of Sociodemographic Factors and Psychopathology by Violence Exposure
| Violence-exposed (n = 76) | Unexposed (n = 73) | |||||
|---|---|---|---|---|---|---|
| % | n | % | n | χ2 | p | |
| Sex (female) | 55 | 42 | 45 | 33 | 1.13 | .288 |
| Racial/ethnic minority | 78 | 59 | 32 | 23 | 30.17 | <.001 |
| Physical Neglect | 45 | 34 | 14 | 10 | 15.78 | <.001 |
| M | SD | M | SD | t | p | |
| Age | 12.92 | 2.68 | 12.61 | 2.58 | −0.72 | 0.476 |
| Income-to-needs | 2.31 | 2.36 | 5.40 | 2.19 | −8.00 | <.001 |
| Violence severity | 7.82 | 2.97 | 1.79 | 1.70 | 15.25 | <.001 |
| p factor (baseline) | .575 | .680 | −.693 | .615 | 12.41 | <.001 |
| p factor (follow-up) | .440 | .889 | −.389 | .698 | 5.95 | <.001 |
Materials and Measures
Violence-exposure.
We used a multi-informant, multi-method approach for assessing exposure to violence. All participants completed two interviews with a trained member of our research team assessing exposure to interpersonal violence: the Childhood Experiences of Care and Abuse (CECA) Interview25 and the Violence Exposure Scale for Children-Revised (VEX-R).26 The CECA assesses caregiving experiences, including physical and sexual abuse. We modified the interview to ask parallel questions about witnessing domestic violence (i.e., directly observing violence directed at a caregiver). The VEX-R assesses the frequency of exposure to different forms of violence. Children are presented with a cartoon and caption depicting a child of the same sex witnessing a type of violence (e.g., “Chris sees a person slap another person really hard”) and experiencing that same type of violence (e.g., “A person slaps Chris really hard”). Children are then asked to report how frequently they have witnessed or experienced that type of violence (e.g., “How many times have you seen a person slap another person really hard?”; “How many times has a person slapped you really hard?”) on a Likert scale ranging from 0 (Never) to 3 (Lots of times). We added follow-up questions for each item that was endorsed to gather additional information (e.g., the perpetrator, age of onset).
Children also completed two self-report measures: the Childhood Trauma Questionnaire (CTQ)27 and the UCLA PTSD Reaction Index (PTSD-RI).28 The CTQ is a 28-item scale that assesses the frequency of maltreatment during childhood, including physical and sexual abuse. The PTSD-RI includes a trauma screen that assesses exposure to numerous traumatic events, including physical abuse, sexual abuse, and domestic violence and additionally assesses PTSD symptoms.
Caregivers completed three self-report measures: the Conflict Tactics Scale-Parent Child Version (CTS),29 the Juvenile Victimization Questionnaire (JVQ) lifetime caregiver report,30 and the caregiver version of the PTSD-RI. The CTS includes 22 items assessing caregiver responses to child disobedience or misbehavior in the past year. Caregivers indicate how frequently they have used each strategy (e.g., shook him/her) on a Likert scale ranging from 0 (This has never happened) to 6 (more than 20 times in the past year) and can also indicate if they have used the strategy in the past but not in the last year. The JVQ includes 34 items assessing exposure to crime, child maltreatment, peer and sibling victimization, sexual victimization, and witnessing and indirect. Caregivers endorsed whether their child had experienced each event in his/her lifetime. Caregivers also completed the trauma screen included in the PTSD-RI, described above. A trained interviewer followed up with the caregiver if the endorsed any form of abuse or domestic violence to gather additional information about the experience.
Children were classified as experiencing physical or sexual abuse if abuse was endorsed by the child (on the CECA interview, PTSD-RI trauma screen, or above the validated CTQ threshold) or parent (on the CTS, JVQ, or PTSD-RI trauma screen). Otherwise, they were classified as controls. A total of 69 children (46.3%) experienced physical or sexual abuse. Inter-rater reliability was fair to good for child and caregiver reports (82.0% agreement; kappa=0.62). Exposure to domestic violence (on the VEX-R interview or PTSD-RI trauma screen) was determined based on child report only. A total of 58 children (38.9%) reported witnessing domestic violence. Overall, 73 participants met criteria for violence-exposure, defined as exposure to physical abuse, sexual abuse, or domestic violence.
A violence severity score was created by summing the total number of types of maltreatment and violence experienced by the child, including physical abuse, sexual abuse, domestic violence, and exposure to violence in the broader community. Each type of maltreatment or violence exposure was coded as present or absent, and these indicators were summed to create a severity score.
General Psychopathology.
As described in detail previously23 and in Supplement 1, available online, symptoms of child psychopathology were assessed by both child and parent report at baseline and about two years later at a follow-up visit. The Children’s Depression Inventory-2 was used to measure depressive symptoms.31 Anxiety symptoms were assessed with the Screen for Child Anxiety Related Emotional Disorders.32 Attention problems, rule breaking behaviors, and aggressive behavior were assessed on the Youth Self-Report and Child Behavior Checklist.33 Post-traumatic stress disorder symptoms were assessed with the UCLA PTSD Reaction Index (PTSD-RI).28 Following prior work,17,19 we performed confirmatory factor analysis (CFA) to test two standard models: a correlated-factors model specifying Internalizing and Externalizing latent factors and a bi-factor model specifying both a General Psychopathology latent factor (“p”) and residual Internalizing and Externalizing factors (Figure 1). In order to ensure that our latent factors were not being driven by one or more indicators simply because of measurement differences across psychopathology instruments (i.e., different number of items, scoring, etc.), we binned scores on each indicator into deciles prior to CFA analyses. All CFA analyses were performed in MPlus version 8.1. Given that our observed indicator variables were slightly skewed and kurtotic, we used the robust maximum likelihood estimator (MLR), which employs a sandwich estimator to arrive at standard errors robust to non-normality of observations. As assessed by relative fit indices and factor loadings, both models fit the data well at the baseline assessment, with a relatively better fit for the bi-factor model (See Table S1, available online). In the present analyses, we used the bi-factor model because it is the most commonly reported general factor model of psychopathology in the existing literature.16–19
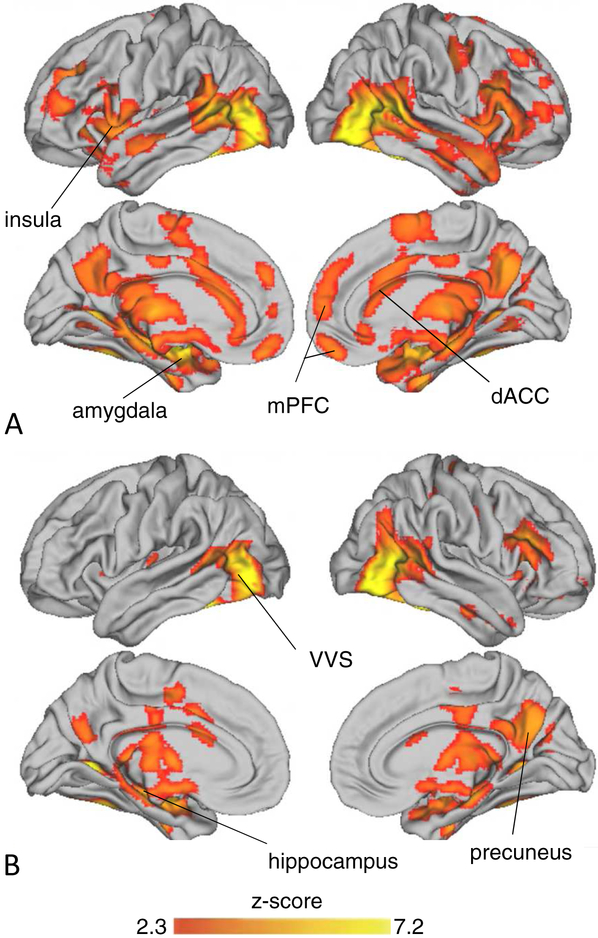
Figure 1: Neural Activation During Emotional Face Viewing.
Note: Depicts significant activation in the left lateral (top) and medial (bottom) surfaces of the brain when participants viewed A) fearful vs. scrambled and B) neutral vs. scrambled faces. dACC = dorsal anterior cingulate cortex: mPFC = medial prefrontal cortex; VVS = ventral visual stream.
Emotional Face Task.
The emotional face task consisted of 2 runs of 9 18-second blocks, during which participants passively viewed emotional face stimuli. Faces were drawn from the NimStim stimulus set.34 The “calm” faces from this dataset were used as neutral expressions, as these expressions are potentially less emotionally evocative than neutral faces,35 which are perceived as negatively valenced.35 Each run consisted of 3 blocks of calm, fearful, and scrambled faces and 3 ITI blocks displayed in a pseudo-random order that ensured that no block type was displayed twice in a row. During each block, 36 faces of different actors expressing the same emotion were displayed for 300 ms each, with 200 ms between each face, based on prior face processing tasks.36 Once every run, a cartoon character appeared on the screen, and respondents were asked to push a button to ensure they were paying attention.
fMRI data acquisition and preprocessing
Details on MRI acquisition are described in Supplement 1, available online. Preprocessing and statistical analysis of fMRI data was performed in a pipeline using Gnu Make. The following preprocessing steps were applied: 1) motion correction followed by slice-time correction in FSL; 2) skull-stripping using FSL’s bet tool; 3) despiking using AFNI’s 3dDespike tool; and 4) smoothing with a 6mm full-width half-max kernel using SUSAN in FSL. Outlier volumes in which framewise displacement exceeded 1mm, the derivative of variance in BOLD signal across the brain (DVARS) exceeded the upper fence (above 75th percentile + 1.5 × inter-quartile range), or signal intensity was more than 3 SD from the mean were regressed out of person-level models. Six rigid-body motion regressors and the time-series extracted from white matter and ventricles were included in person-level models to reduce noise associated with motion and physiological fluctuations. Person- and group-level models were estimated in FSL. Following estimation of person-level models, the resulting contrast images were normalized into standard space, and anatomical co-registration of the functional data with each participant’s T1-weighted image was performed using Advanced Normalization Tools (ANTs) software.
fMRI Analysis
FMRI data processing was performed using FEAT (FMRI Expert Analysis Tool) Version 6.00, part of FSL. Regressors were created by convolving a boxcar function of phase duration with the standard double-gamma hemodynamic response function for each phase of the task (fearful, neutral, and scrambled faces). A general linear model was constructed for each participant. Higher level analysis was carried out using FLAME1. We applied cluster-level correction in FSL (z > 2.3, p < .01) to our models run in FSL FLAME. We examined differences in BOLD response during contrasts of interest as a function of violence exposure in whole-brain analysis.
To investigate study hypotheses, we first conducted whole-brain analyses comparing youths with and without violence exposure for the contrasts of fear vs. scrambled and neutral vs. scrambled faces. Associations of violence severity with neural activation within the violence-exposed group were examined using linear regression. We next tested whether age interacted with violence exposure or severity to predict neural activation for each contrast. Region of significance analyses37 were conducted to identify the values of the moderator (age) at which the association between violence and neural function were significant. Mean z-scores were extracted from the functional masks of regions where significant effects were detected. Finally, we examined the association of average neural activity within brain regions that were significantly associated with violence exposure as a predictor of the p-factor at baseline, controlling for violence exposure, and the p-factor at the follow-up assessment, controlling for p-factor at baseline and violence exposure.
Participant race/ethnicity and income-to-needs ratio varied as a function of violence exposure and were included as covariates in all group comparisons. Age and sex were matched between groups, but were also included as covariates in all regressions within the violence-exposed group. To evaluate whether the pattern of findings was specific to violence exposure, we conducted sensitivity analyses controlling for exposure to neglect, as measured on the CTQ Physical Neglect subscale, instead of income-to-needs ratio. The pattern of results was unchanged (see Supplement 1, Table S2, available online).
To test whether neural activity mediated the association between violence exposure and p-factor at follow-up, controlling for p-factor at baseline, mediation models with bootstrapped confidence intervals (10,000 iterations) were tested using version 2.13 of the PROCESS macro in SPSS38.
Amygdala region of interest (ROI)
A bilateral amygdala ROI was extracted based on the Harvard Oxford subcortical probabilistic structural atlas, thresholded at 50% probability and warped back into each subjects’ native space. Mean z-scores were extracted from the bilateral amygdala for each contrast for each participant. We examined differences based on violence exposure and severity, and interactions with age, and associations with psychopathology using the same steps outlined above implemented in R version 3.5.1. To account for multiple testing, we used a significance threshold of p < .01 for analyses that were run at both the whole-brain level and using a bilateral amygdala ROI and with violence exposure as a dichotomous variable and severity of exposure as a continuous variable (4 tests).
Functional Connectivity Analyses
We used Psychophysiological Interaction (PPI) analyses to examine functional connectivity of the amygdala with other regions while viewing fearful and neutral faces relative to scrambled faces. We found no differences in amygdala functional connectivity as a function of violence exposure or severity and no age by violence interactions. See Supplement 1, available online for details on PPI analysis and results.
Results
Descriptive Statistics
Descriptive statistics and distributions of sociodemographic and psychopathology variables by violence exposure are summarized in Table 1.
Neural activation during emotional face viewing
Compared to scrambled faces, both neutral and fearful faces elicited widespread activation throughout the brain, including throughout the ventral visual stream, along with the superior temporal sulcus, amygdala, hippocampus, bilateral insula, anterior cingulate cortex (ACC), and lateral and mPFC in whole brain analyses (Figure 1, Table S3, available online).
Violence-related differences in neural activation
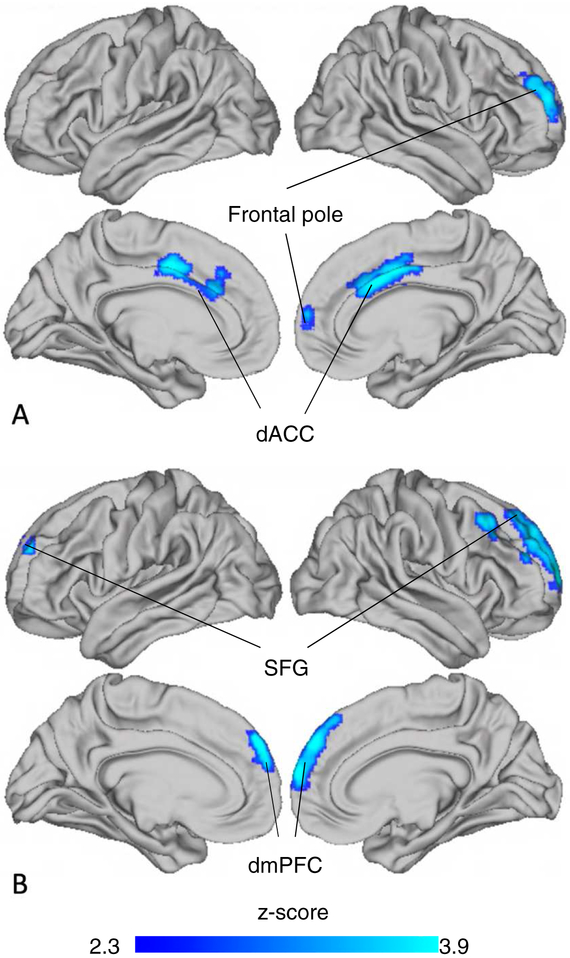
Violence exposure was associated with decreased activation to fearful vs. scrambled faces in the dorsal ACC (dACC, Table 2, Figure 2A) and increased activation in the lateral occipital cortex (Table 2) in whole brain analyses. This effect was specific to threat cues, as violence exposure was not associated with dACC activation to neutral vs. scrambled faces. However, violence exposure was associated with decreased activation to neutral vs. scrambled faces in a cluster spanning dorsomedial PFC and frontal pole (Table 2, Figure 2B).
Table 2:
Brain Regions With Significant Differences in Activation Based on Violence Exposure
| Voxels | Peak (x, y, z) | Region | BA | Peak voxel z-score |
|---|---|---|---|---|
| Fear > Scrambled, V− > V+ | ||||
| 1985 | 6, 4, 40 | Anterior Cingulate Cortex | 24 | 4.61 |
| 16, 60, 8 | Frontal Pole | 9 | 3.85 | |
| Fear > Scrambled, V+ > V− | ||||
| 1501 | −26, −78, 16 | Lateral Occipital Cortex | 31 | 4.05 |
| −8, −76, −12 | Lingual Gyrus | 18 | 3.02 | |
| Calm > Scrambled, V− > V+ | ||||
| 1970 | 14, 64, 10 | Frontal Pole | 9 | 4.54 |
| 6, 54, 32 | Superior Frontal Gyrus | 6 | 3.82 | |
| 44, 24, 44 | Middle Frontal Gyrus | 8 | 3.64 |
Note: BA = Brodmann’s area; Peak (x, y, z) = MNI coordinates for the voxels with the highest coefficients within each cluster as well as subcluster local maxima; V+ = violence exposure, V− = no violence exposure.
Figure 2: Violence-exposure related differences in neural activation to emotional faces.
Note: Youth exposed to violence had less activation than control youth in distinct regions of medial prefrontal cortex in response to A) fearful vs. scrambled and B) neutral vs. scrambled faces. dACC = dorsal anterior cingulate cortex; dmPFC = dorsomedial prefrontal cortex; SFG = superior frontal gyrus.
Amygdala activation to fearful (B= −0.022, S.E.=0.210, t=−0.11, p=.916) and neutral faces (B= 0.068, S.E.=0.195, t=0.35, p=.730) relative to scrambled faces did not differ between violence-exposed and control children. Within the violence-exposed group, the severity of violence exposure was associated with decreased amygdala reactivity to fearful vs. scrambled faces (B= −0.082, S.E.=0.041, t=−2.01, p=.049) but not neutral vs. scrambled faces (B= −0.015, S.E.=0.039, t=−0.39, p=.696). However, the association between violence exposure severity and amygdala reactivity was not significant after correcting for multiple comparisons.
Age-related variation in associations of violence with neural activation
Neural activation to fearful vs. scrambled faces was positively associated with age in whole brain analyses in a cluster centered in bilateral hippocampus (4,374 voxels; Peak x,y,z=−26, −32, −6, z=4.46) and occupying portions of bilateral thalamus, lingual gyrus, and precuneus. Age was not associated with neural activation to neutral vs. scrambled faces. Amygdala response to fearful vs. scrambled faces was unrelated to age (B=0.043, p=.178) and increased with age to neutral vs. scrambled faces (B=0.061, p=.037). However, the association between amygdala reactivity and age was not significant after multiple comparison correction. No age x violence interactions were observed in whole-brain analyses or in the amygdala ROI.
Neural function and psychopathology
Neural activation to fearful vs. scrambled faces in the dACC cluster associated with violence exposure was not related to p-factor at baseline, controlling for violence exposure (B=0.0138, p=.847), but did significantly predict “p” at the longitudinal follow-up, controlling for “p” at baseline and violence exposure (B=−0.186, p=.031).
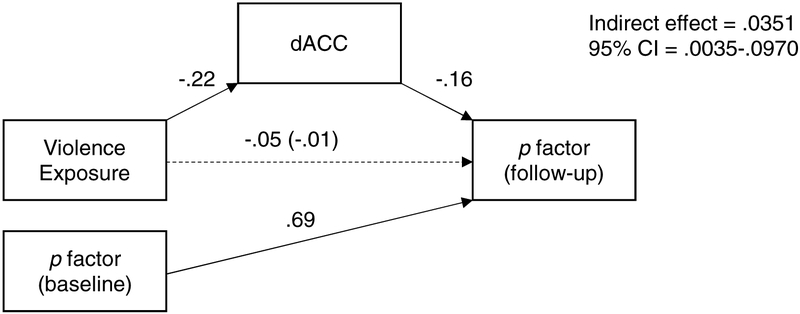
In mediation analysis, there was a significant indirect effect of violence exposure on p-factor at follow-up, via lower dACC activation, controlling for p-factor at baseline (b = .0351, 95% CI = .0035–.0970), suggesting that lower dACC activation mediates the association between violence exposure and increases in psychopathology transdiagnostically (Figure 3). Neural activation to neutral vs. scrambled faces in the dmPFC cluster associated with violence exposure was not related to p-factor at baseline (B=0.0170, p=.766) or follow-up (B=0.0459, p=.496).
Figure 3: Mediation of the Effect of Violence Exposure on p factor by Lower Dorsal Anterior Cingulate Cortex (dACC) Activation.
Note: Model used to test the indirect effects of violence-exposure on psychopathology (p factor) at follow-up, via lower dACC activation, controlling for baseline psychopathology. Standardized coefficients are shown. Solid lines indicate significant paths. Dotted lines indicate nonsignificant paths.
Discussion
This study examined neural activation to fearful and neutral faces among youth exposed to violence and age- and sex-matched controls. Youth exposed to violence exhibited lower dACC activation when viewing fearful and neutral faces than those who had not experienced violence. Lower dACC activation when viewing fearful faces predicted increases in the general psychopathology (“p”) factor two years later and mediated the association between violence exposure and increases in “p”. Contrary to hypotheses, neither amygdala reactivity, nor amygdala-mPFC connectivity differed between youth with and without violence exposure. In fact, violence severity was associated with lower amygdala reactivity among violence-exposed youth, although this association was not significant after accounting for multiple comparisons. Overall, findings suggest that violence exposure influences the function and development of key nodes within the salience network, with implications for the emergence of psychopathology across adolescence.
Reduced dACC reactivity to faces was observed in violence-exposed adolescents. This brain region is a key node in the salience network6 and has been theorized to play a role in action selection in emotional contexts.39 Trauma is associated with greater engagement of this region during explicit processing of angry faces, suggesting a heightened salience response.40 Conversely, greater disengagement of dACC occurs when attention is directed away from threat-related cues, as has been demonstrated during a dot probe task.41 Reduced engagement of dACC during emotion processing among violence-exposed youth when attention is not constrained may therefore reflect greater attentional avoidance of threat-related cues. While this type of attentional avoidance may reduce distress in the short term, it may contribute to fear generalization over time.42 However, these interpretations remain speculative given the absence of behavioral data on attentional mechanisms in our study.
Although patterns of neural function have been argued to contribute to the generalized psychopathology factor (or p-factor),20 few empirical studies have examined this issue. Here, we provide novel evidence that reduced dACC activation in response to threat cues predicts increases in p-factor over time during adolescence, and mediates the association of violence exposure with increases in p. Reduced dACC activation during emotional processing has previously been reported in post-traumatic stress disorder44 and externalizing problems.45 If reduced engagement of dACC in response to fearful faces among violence-exposed youth indeed reflects greater attentional avoidance of threat-related cues, this may contribute to increased risk for transdiagnostic psychopathology given that avoidance, as an emotion regulation strategy, is associated with increased risk for psychopathology transdiagnostically.43
Amygdala reactivity did not vary as a function of exposure to violence. Further, within the violence-exposed group, violence severity was negatively associated with amygdala reactivity, although this association was not significant after correction for multiple comparisons. This is surprising given that amygdala hyper-reactivity is typically observed among violence-exposed youth.3,4 In fact, in this same sample, elevated amygdala reactivity was observed during a task involving passive viewing of negatively-valenced images over a longer presentation interval (6–10 seconds).46 It is possible that the divergent findings in this case are attributable to task design. Emotional face paradigms employed in most studies of adversity constrain attention either implicitly by requiring participants to attend to the gender of the face or explicitly by requiring participants to attend to the emotion of a face presented for several seconds. The present study’s paradigm did not constrain attention due to evidence that attentional constraints due to task demands produce lower amygdala activation,47 and faces were displayed for only 300 ms. A prior study found that adults exposed to childhood adversity had greater amygdala reactivity to fearful and angry faces when attention was constrained, but lower amygdala reactivity when it was not.48 This suggests that when not forced by task demands to engage with emotional cues, individuals exposed to adversity may avoid the processing of emotional information by redirecting attention elsewhere. Indeed, attention bias away from threat cues is associated with abuse severity among maltreated children.49 Moreover, in a recent paper from this sample, violence-exposure and severity were associated with attention bias toward threat in children that shifted to an attention bias away from threat in adolescents.23 It may be that an increasing capacity to regulate reactivity to threat through top-down inhibition and attention modulation allows adolescents to inhibit processing of emotional information as an adaptation to severe violence exposure.
This study had several strengths, including a well-powered sample recruited to ensure sufficient variability in age and violence exposure, and integration of multiple measures of violence exposure and psychopathology from both youth and parents. However, some limitations constrain interpretation and suggest directions for future research. Because this sample was recruited for high levels of violence exposure, and because violence exposure and neglect frequently co-occur, experiences of neglect were considerably more common in the violence-exposed group, with very few youth experiencing neglect but not violence-exposure. This diminishes our ability to determine the specificity of violence exposure. However, results were largely unchanged when we controlled for neglect. Moreover, violence exposure in the home environment occurs within a broader ecological context. The nature of that context is not the same for all children exposed to violence, nor would the same environment be experienced in the same way by violence-exposed and unexposed youth. A more complete understanding of how childhood adversity influences children’s development and functioning requires a more complex ecological transactional approach.50 Next, although a general check for attention was included within the task, how adolescents directed their attention during the task was unclear, and may have been informative in interpreting the unexpected findings. Future work utilizing eye-tracking are necessary to support our interpretation of attentional avoidance in the violence exposed group in the presence of emotionally evocative stimuli. Finally, while psychopathology was measured longitudinally, and we found that dACC activity predicted “p” at follow-up, developmental patterns in neural function could only be inferred through cross-sectional associations between neural function and age. However, longitudinal follow-up of neural reactivity to faces and psychopathology in this sample is currently underway.
During passive viewing of rapidly presented emotional faces, youths exposed to violence exhibited reduced activity in the dACC relative to those without a history of violence exposure, a pattern associated with longitudinal increases in transdiagnostic psychopathology. Elevated amygdala activity was not observed among youths exposed to violence. These results suggest that the nature of the relation between violence exposure and the neural processing of emotionally salient cues may vary based on attentional constraints. When attention is not constrained, reduced engagement of dACC, a region involved in directing attention in response to emotionally salient cues, may be a mechanism linking violence exposure with increased risk for transdiagnostic psychopathology over time.
Supplementary Material
Acknowledgements
This research was funded by the National Institute of Mental Health (R01-MH103291 to McLaughlin; K01-MH116325 to Miller; F32-MH114317 to Colich; K23-MH112872 to Jenness), an Early Career Research Fellowship from the Jacobs Foundation (McLaughlin), and a OneMind Institute Rising Star Award (McLaughlin), and Behavior Research Foundation NARSAD Young Investigator Grant (Jenness).
We are grateful to Debbie Bitran, BA, of the University of Washington and Pittsburgh University, Andrea Duys, BA, of the University of Washington, and Azure Reid-Russell, BA, of Harvard University, for help with participant recruitment and testing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
Dr. David G. Weissman reports no biomedical financial interests or potential conflicts of interest.
Dr. Jessica L. Jenness reports no biomedical financial interests or potential conflicts of interest.
Dr. Natalie L. Colich reports no biomedical financial interests or potential conflicts of interest.
Dr. Adam Bryant Miller reports no biomedical financial interests or potential conflicts of interest.
Ms. Kelly A. Sambrook reports no biomedical financial interests or potential conflicts of interest.
Dr. Margaret A. Sheridan reports no biomedical financial interests or potential conflicts of interest.
Dr. Katie A. McLaughlin reports no biomedical financial interests or potential conflicts of interest.
References
- 1.McLaughlin KA, Green JG, Gruber MJ, Sampson NA, Zaslavsky AM, Kessler RC. Childhood adversities and first onset of psychiatric disorders in a national sample of US adolescents. Archives of General Psychiatry. 2012. doi: 10.1001/archgenpsychiatry.2011.2277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McLaughlin KA, Lambert HK. Child trauma exposure and psychopathology: mechanisms of risk and resilience. Current Opinion in Psychology. 2017. doi: 10.1016/j.copsyc.2016.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hein TC, Monk CS. Research Review: Neural response to threat in children, adolescents, and adults after child maltreatment – a quantitative meta-analysis. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2017. doi: 10.1111/jcpp.12651 [DOI] [PubMed] [Google Scholar]
- 4.McCrory EJ, De Brito SA, Sebastian CL, et al. Heightened neural reactivity to threat in child victims of family violence. Current Biology. 2011. doi: 10.1016/j.cub.2011.10.015 [DOI] [PubMed] [Google Scholar]
- 5.McLaughlin KA, Peverill M, Gold AL, Alves S, Sheridan MA. Child Maltreatment and Neural Systems Underlying Emotion Regulation. Journal of the American Academy of Child and Adolescent Psychiatry. 2015;54(9):753–762. doi: 10.1016/j.jaac.2015.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Menon V Large-scale brain networks and psychopathology: A unifying triple network model. Trends in Cognitive Sciences. 2011;15(10):483–506. doi: 10.1016/j.tics.2011.08.003 [DOI] [PubMed] [Google Scholar]
- 7.Jedd K, Hunt RH, Cicchetti D, et al. Long-term consequences of childhood maltreatment: Altered amygdala functional connectivity. Development and Psychopathology. 2015. doi: 10.1017/S0954579415000954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fonzo GA, Flagan TM, Sullivan S, et al. Neural functional and structural correlates of childhood maltreatment in women with intimate-partner violence-related posttraumatic stress disorder. Psychiatry Research - Neuroimaging. 2013. doi: 10.1016/j.pscychresns.2012.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silvers JA, Insel C, Powers A, et al. The transition from childhood to adolescence is marked by a general decrease in amygdala reactivity and an affect-specific ventral-to-dorsal shift in medial prefrontal recruitment. Developmental Cognitive Neuroscience. 2017;25:128–137. doi: 10.1016/j.dcn.2016.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gee DG, Humphreys KL, Flannery J, et al. A Developmental Shift from Positive to Negative Connectivity in Human Amygdala-Prefrontal Circuitry. Journal of Neuroscience. 2013;33(10):4584–4593. doi: 10.1523/JNEUROSCI.3446-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swartz JR, Williamson DE, Hariri AR. Developmental change in amygdala reactivity during adolescence: Effects of family history of depression and stressful life events. American Journal of Psychiatry. 2015;172(3):276–283. doi: 10.1176/appi.ajp.2014.14020195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gee DG, Gabard-Durnam LJ, Flannery J, et al. Early developmental emergence of human amygdala–prefrontal connectivity after maternal deprivation. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(39):15638–15643. doi: 10.1073/pnas.1307893110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colich NL, Williams ES, Ho TC, et al. The association between early life stress and prefrontal cortex activation during implicit emotion regulation is moderated by sex in early adolescence. Development and Psychopathology. 2017. doi: 10.1017/S0954579417001444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pagliaccio D, Luby JL, Luking KR, Belden AC, Barch DM. Brain-behavior relationships in the experience and regulation of negative emotion in healthy children: Implications for risk for childhood depression. Development and Psychopathology. 2014. doi: 10.1017/S0954579414001035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coccaro EF, McCloskey MS, Fitzgerald DA, Phan KL. Amygdala and Orbitofrontal Reactivity to Social Threat in Individuals with Impulsive Aggression. Biological Psychiatry. 2007. doi: 10.1016/j.biopsych.2006.08.024 [DOI] [PubMed] [Google Scholar]
- 16.Schaefer JD, Moffitt TE, Arseneault L, et al. Adolescent Victimization and Early-Adult Psychopathology: Approaching Causal Inference Using a Longitudinal Twin Study to Rule Out Noncausal Explanations. Clinical Psychological Science. 2018. doi: 10.1177/2167702617741381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lahey BB, Applegate B, Hakes JK, Zald DH, Hariri AR, Rathouz PJ. Is There a general factor of prevalent psychopathology during adulthood? Journal of Abnormal Psychology. 2012. doi: 10.1037/a0028355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keyes KM, Eaton NR, Krueger RF, et al. Childhood maltreatment and the structure of common psychiatric disorders. British Journal of Psychiatry. 2012. doi: 10.1192/bjp.bp.111.093062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caspi A, Houts RM, Belsky DW, et al. The p factor: One general psychopathology factor in the structure of psychiatric disorders? Clinical Psychological Science. 2014. doi: 10.1177/2167702613497473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lahey BB, Krueger RF, Rathouz PJ, Waldman ID, Zald DH. A hierarchical causal taxonomy of psychopathology across the life span. Psychological Bulletin. 2017. doi: 10.1037/bul0000069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Graaf R, Bijl RV., Ten Have M, Beekman ATF, Vollebergh WAM. Rapid onset of comorbidity of common mental disorders: Findings from the Netherlands Mental Health Survey and Incidence Study (NEMESIS). Acta Psychiatrica Scandinavica. 2004. doi: 10.1046/j.0001-690X.2003.00222.x [DOI] [PubMed] [Google Scholar]
- 22.Khan AA, Jacobson KC, Gardner CO, Prescott CA, Kendler KS. Personality and comorbidity of common psychiatric disorders. British Journal of Psychiatry. 2005. doi: 10.1192/bjp.186.3.190 [DOI] [PubMed] [Google Scholar]
- 23.Weissman DG, Bitran D, Miller AB, Schaefer JD, Sheridan MA, McLaughlin KA. Difficulties with emotion regulation as a transdiagnostic mechanism linking child maltreatment with the emergence of psychopathology. Development and Psychopathology. April 2019:1–17. doi: 10.1017/S0954579419000348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pollak SD, Cicchetti D, Hornung K, Reed A. Recognizing emotion in faces: developmental effects of child abuse and neglect. Developmental psychology. 2000. doi: 10.1037/0012-1649.36.5.679 [DOI] [PubMed] [Google Scholar]
- 25.Bifulco A, Brown GW, Harris TO. Childhood Experience of Care and Abuse (CECA): A Retrospective Interview Measure. Journal of Child Psychology and Psychiatry. 1994;35(8):1419–1435. doi: 10.1111/j.1469-7610.1994.tb01284.x [DOI] [PubMed] [Google Scholar]
- 26.Raviv A, Erel O, Fox NA, et al. Individual measurement of exposure to everyday violence among elementary schoolchildren across various settings. Journal of Community Psychology. 2001. doi: [DOI] [Google Scholar]
- 27.Bernstein DP, Ahluvalia T, Pogge D, Handelsman L. Validity of the childhood trauma questionnaire in an adolescent psychiatric population. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36(3):340–348. doi: 10.1097/00004583-199703000-00012 [DOI] [PubMed] [Google Scholar]
- 28.Steinberg AM, Brymer MJ, Decker KB, Pynoos RS. The University of California at Los Angeles Post-Traumatic Stress Disorder Reaction Index. Vol 6 Current Medicine Group; 2004:96–100. doi: 10.1007/s11920-004-0048-2 [DOI] [PubMed] [Google Scholar]
- 29.Straus MA, Hamby SL, Finkelhor D, Moore DW, Runyan D. Identification of child maltreatment with the parent-child Conflict Tactics Scales: Development and psychometric data for a national sample of American parents. Child Abuse and Neglect. 1998. doi: 10.1016/S0145-2134(97)00174-9 [DOI] [PubMed] [Google Scholar]
- 30.Finkelhor D, Hamby SL, Ormrod R, Turner H. The Juvenile Victimization Questionnaire: Reliability, validity, and national norms. Child Abuse and Neglect. 2005. doi: 10.1016/j.chiabu.2004.11.001 [DOI] [PubMed] [Google Scholar]
- 31.Kovacs M Children’s Depression Inventory (CDI2). North Tonawanda NY: Multi-Health Systems Inc.; 2011. [Google Scholar]
- 32.Birmaher B, Khetarpal S, Brent D, et al. The Screen for Child Anxiety Related Emotional Disorders (SCARED): Scale construction and psychometric characteristics. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36(4):545–553. doi: 10.1097/00004583-199704000-00018 [DOI] [PubMed] [Google Scholar]
- 33.Achenbach TM. Manual for the Child Behavior Checklist/4–18 and 1991 Profile.; 1991.
- 34.Tottenham N, Borscheid A, Ellertsen K, Marcus DJ, Nelson CA. Categorization of facial expressions in children and adults: Establishing a larger stimulus set. Journal of Cognitive Neuroscience. 2002:74. [Google Scholar]
- 35.Tottenham N, Tanaka JW, Leon AC, et al. The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Research. 2009;168(3):242–249. doi: 10.1016/J.PSYCHRES.2008.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Somerville LH, Kim H, Johnstone T, Alexander AL, Whalen PJ. Human amygdala responses during presentation of happy and neutral faces: Correlations with state anxiety. Biological Psychiatry. 2004. doi: 10.1016/j.biopsych.2004.01.007 [DOI] [PubMed] [Google Scholar]
- 37.Preacher KJ, Curran PJ, Bauer DJ. Computational Tools for Probing Interactions in Multiple Linear Regression, Multilevel Modeling, and Latent Curve Analysis. Journal of Educational and Behavioral Statistics. 2006. doi: 10.3102/10769986031004437 [DOI] [Google Scholar]
- 38.Hayes AF. Introduction to Mediation, Moderation, and Conditional Process Analysis.; 2013. doi:978-1-60918-230-4 [DOI] [PubMed]
- 39.Dixon ML, Thiruchselvam R, Todd R, Christoff K. Emotion and the prefrontal cortex: An integrative review. Psychological Bulletin. 2017. doi: 10.1037/bul0000096 [DOI] [PubMed] [Google Scholar]
- 40.Herringa RJ, Phillips ML, Fournier JC, Kronhaus DM, Germain A. Childhood and adult trauma both correlate with dorsal anterior cingulate activation to threat in combat veterans. Psychological Medicine. 2013. doi: 10.1017/S0033291712002310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Price RB, Siegle GJ, Silk JS, et al. Looking under the hood of the dot-probe task: An fmri study in anxious youth. Depression and Anxiety. 2014. doi: 10.1002/da.22255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Waters AM, Craske MG. Towards a cognitive-learning formulation of youth anxiety: A narrative review of theory and evidence and implications for treatment. Clinical Psychology Review. 2016. doi: 10.1016/j.cpr.2016.09.008 [DOI] [PubMed] [Google Scholar]
- 43.Aldao A, Nolen-Hoeksema S, Schweizer S. Emotion-regulation strategies across psychopathology: A meta-analytic review. Clinical Psychology Review. 2010. doi: 10.1016/j.cpr.2009.11.004 [DOI] [PubMed] [Google Scholar]
- 44.Etkin A, Wager TD. Functional neuroimaging of anxiety: A meta-ana lysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. American Journal of Psychiatry. 2007. doi: 10.1176/appi.ajp.2007.07030504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baker RH, Clanton RL, Rogers JC, De Brito SA. Neuroimaging findings in disruptive behavior disorders. CNS Spectrums. 2014. doi: 10.1017/S1092852914000789 [DOI] [PubMed] [Google Scholar]
- 46.Jenness JL, Peverill M, Miller AB, et al. Alterations in neural circuits underlying emotion regulation following child maltreatment emerge across adolescence: A transdiagnostic mechanism underlying trauma-related psychopathology. [DOI] [PMC free article] [PubMed]
- 47.Costafreda SG, Brammer MJ, David AS, Fu CHY. Predictors of amygdala activation during the processing of emotional stimuli: A meta-analysis of 385 PET and fMRI studies. Brain Research Reviews. 2008. doi: 10.1016/j.brainresrev.2007.10.012 [DOI] [PubMed] [Google Scholar]
- 48.Taylor SE, Eisenberger NI, Saxbe D, Lehman BJ, Lieberman MD. Neural Responses to Emotional Stimuli Are Associated with Childhood Family Stress. Biological Psychiatry. 2006;60(3):296–301. doi: 10.1016/J.BIOPSYCH.2005.09.027 [DOI] [PubMed] [Google Scholar]
- 49.Pine DS, Mogg K, Bradley BP, et al. Attention bias to threat in maltreated children: Implications for vulnerability to stress-related psychopathology. American Journal of Psychiatry. 2005. doi: 10.1176/appi.ajp.162.2.291 [DOI] [PubMed] [Google Scholar]
- 50.Lynch M, Cicchetti D. An ecological-transactional analysis of children and contexts: the longitudinal interplay among child maltreatment, community violence, and children’s symptomatology. Dev Psychopathol. 1998;10(2):235–257. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.