Abstract
Introduction:
Two common responses to stress include elevated circulating glucocorticoids and impaired luteinizing hormone (LH) secretion. We have previously shown that a chronic, stress-level of corticosterone can impair ovarian cyclicity in intact mice by preventing follicular-phase endocrine events.
Objective:
Here, we sought to investigate if corticosterone can disrupt LH pulses and whether estradiol is necessary for this inhibition.
Methods:
Our approach was to measure LH pulses prior to and following administration of chronic corticosterone or cholesterol in ovariectomized mice treated with or without estradiol, as well as assess changes in arcuate kisspeptin (Kiss1) neuronal activation, as determined by co-expression with c-Fos.
Results:
In ovariectomized mice, a chronic 48h elevation in corticosterone did not alter the pulsatile pattern of LH. In contrast, corticosterone induced a robust suppression of pulsatile LH secretion in mice treated with estradiol. This suppression represented a decrease in pulse frequency without a change in amplitude. We show that the majority of arcuate Kiss1 neurons contain glucocorticoid receptor, revealing a potential site of corticosterone action. Although arcuate Kiss1 and Tac2 gene expression did not change in response to corticosterone, arcuate Kiss1 neuronal activation was significantly reduced by chronic corticosterone, but only in mice treated with estradiol.
Conclusions:
Collectively, these data demonstrate that chronic corticosterone inhibits LH pulse frequency and reduces Kiss1 neuronal activation in female mice, both in an estradiol-dependent manner. Our findings support the possibility that enhanced sensitivity to glucocorticoids, due to ovarian steroid milieu, may contribute to reproductive impairment associated with stress or pathophysiologic conditions of elevated glucocorticoids.
Keywords: kisspeptin, glucocorticoid receptor, luteinizing hormone, arcuate nucleus, GnRH
Introduction
Stress profoundly alters physiologic function through activation of a host of chemical and hormonal pathways, including activation of the hypothalamic-pituitary-adrenal (HPA) axis and the release of glucocorticoids. An increase in circulating glucocorticoids is a common physiologic response to many stressors and has been considered a potential mediator of reproductive suppression during stress. Evidence that a glucocorticoid receptor (GR) antagonist prevents the inhibition of gonadotropin secretion during stress in intact male rats and ovariectomized (OVX) female sheep treated with estradiol, supports a mediatory role of elevated glucocorticoids [1–3]. We have previously demonstrated that a chronic elevation of a stress-like level of corticosterone, the natural glucocorticoid in rodents, disrupts the ovulatory cycle of the female mouse [4]. Female mice exposed to elevated corticosterone remain in diestrus, comparable to the early follicular phase in humans, suggesting that a suppression in luteinizing hormone (LH) secretion contributes to ovulatory cycle disruption. Indeed, increased circulating glucocorticoids, administered in the absence of stress, have been shown to impair pulsatile LH secretion during the follicular phase in women [5] or ewes [6], but the neuroendocrine mechanisms underlying reduced LH secretion remains unclear.
A large body of in vivo and in vitro work supports the hypothesis that glucocorticoids can act centrally and at the pituitary gland to inhibit LH secretion. Corticosterone can suppress pituitary responsiveness to gonadotropin-releasing hormone (GnRH) and lower LH pulse amplitude in numerous species and under a variety of gonadal steroid regimens [7–14], which suggests a pituitary mechanism. On the other hand, data from castrated rhesus monkeys demonstrates that the glucocorticoid-induced suppression of LH can be reversed by exogenous GnRH, indicating a central mechanism of suppression [15]. A hypothalamic mechanism is also supported by data in which glucocorticoids alter LH pulse frequency, which is taken as evidence for an action upon the central GnRH pulse generator. For example, elevated corticosterone decreases the frequency of both GnRH and LH pulses in ovary-intact ewes during the follicular phase of the estrous cycle or in OVX ewes treated with estradiol and progesterone to simulate a follicular phase [6, 16]. Interestingly, corticosterone does not reduce GnRH or LH pulse frequency in OVX ewes devoid of gonadal steroids, indicating that this mode of LH suppression is dependent on ovarian steroid milieu [7]. Scant evidence for the presence of either GR or ovarian steroid receptors within GnRH neurons themselves [17–19] points attention to an afferent cell type expressing kisspeptin (encoded by the Kiss1 gene). Kisspeptin neurons in the arcuate nucleus also contain neurokinin B and dynorphin and are thus termed KNDy cells [20, 21]. Based on abundant anatomical, pharmacological, and electrophysiological data in rats, goats and sheep, this neuron population was hypothesized to form the GnRH pulse generator [21–25]. More recently, arcuate kisspeptin neurons were confirmed to be the GnRH pulse generator in mice [26]. Thus, this cell population may form the pulse generator in numerous species. Importantly, KNDy cells express estradiol receptor alpha (ERα) [27, 28] and in sheep, also express GR [29]. Therefore, this cell population may be a cellular mediator of the suppressive effect of elevated glucocorticoids on GnRH and LH pulsatile secretion.
The goal of this study was to test the hypothesis that elevated corticosterone impairs ovarian cyclicity in females by inhibiting the GnRH pulse generator. For this purpose, we took advantage of our ability to monitor robust pulsatile LH secretion in female mice under OVX conditions, when the release of LH is unrestrained by gonadal steroids. As the results of our initial experiment implicated ovarian steroids as a potential factor influencing the response to corticosterone, subsequent experiments utilized a low physiologic estradiol treatment, approximating a diestrus level based on uterine weight, to test the effect of this ovarian steroid in modulating the response to chronic corticosterone. We pursued experiments to determine whether the reduction in pulsatile LH was mediated by a diminishment in gonadotrope responsiveness to GnRH or suppression of kisspeptin-containing cells within the arcuate nucleus.
Materials and Methods
Animals
Wild-type C57BL/6 (Envigo; Experiments 1–3 and 5A), Kiss1CreGFP (Experiment 4), or Kiss1hrGFP (Experiment 5B) mice were housed 2 per cage under standard conditions under a 12-h light, 12-h dark cycle with lights on at 0600 h. Kiss1CreGFP [30] transgenic mice express Cre-recombinase driven by Kiss1 regulatory elements which allows Kiss1 neurons to be identified by Cre-mediated expression of green fluorescent protein (GFP). Kiss1hrGFP [31] transgenic mice express humanized recombinant GFP (hrGFP) driven by Kiss1 regulatory elements which allows Kiss1 expression to be assessed with the GFP reporter. Animals had ad libitum access to water and Harlan irradiated chow #2920X at a University of California, San Diego vivarium. OVX was performed aseptically under isoflurane anesthesia on animals 8–14 wk old, unless otherwise stated. All surgeries and serial blood samplings were performed between 0800 h and 1200 h. All animal procedures were performed in accordance with the University of California, San Diego Institutional Animal Care and Use Committee regulations and were performed in accordance with the National Institutes of Health guidelines for the care and use of research animals.
Experimental Details
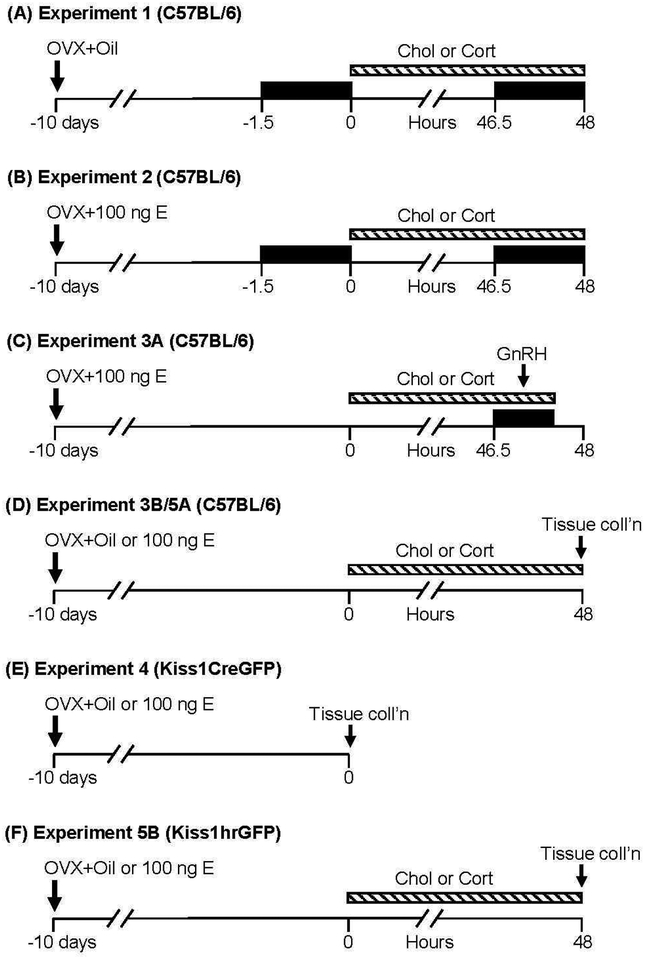
Experiment 1: Does chronic corticosterone reduce pulsatile LH secretion?
C57BL/6 mice were handled daily for 5 wk prior to blood collection to acclimatize animals to the serial tail-bleed sampling required for pulsatile LH measurement as published previously [32, 33]. Mice were OVX and implanted subcutaneously with a silastic capsule containing oil (see Fig. 1A for experimental details). After 10 d, blood samples from the tail vein were collected with a pipette every 6 min for two 90-min intervals, pre: −1.5 – 0 h or post: 46.5 – 48 h, relative to the subcutaneous implantation of a pellet containing either cholesterol or corticosterone (n=3/group) while briefly exposed to isoflurane anesthesia. Cholesterol or corticosterone-releasing pellets were manufactured in our laboratory as described previously [4], by coating silicone-filled (Liquid Nails, Butler County, PA) Silastic tubing of 1.0 cm length (0.040 in. I.D. × 0.085 in. O.D.) with molten steroid, as per Meyer et al., 1979 [34]. Pilot studies were conducted to establish an effective corticosterone pellet composition (25 mg corticosterone:25 mg cholesterol; Sigma, St. Louis, MO) that would produce a continuous elevation in circulating corticosterone which would mimic the peak level of endogenous corticosterone we observe in response to isolation/restraint stress in female mice [35]. Note, the unchanging corticosterone level produced in the present study likely exceeds the duration of an endogenous corticosterone response to acute stress. An equivalent weight of pure cholesterol (50 mg) was used to manufacture vehicle pellets for implantation in control animals. Tail blood (15 μL) was collected 2 d following implantation at 0700 h, prior to serial sampling, for analysis of circulating corticosterone levels in OVX animals.
Fig. 1. Animal strains and experimental designs.
Time is depicted as hours relative to the implantation of corticosterone (Cort) or cholesterol (Chol), indicated by the dashed horizontal bar. Time of frequent sampling for LH analysis is identified by the black boxes. As indicated by the arrow, OVX and implantation with oil or estradiol (E) implant occurred 10 d prior to experimentation. GnRH (800 ng/kg, ip) was administered ~47 h after corticosterone or cholesterol in Experiment 3A. Time of tissue collection is indicated for Experiment 3B/5A (fresh frozen) and Experiment 4/5B (fixed).
Experiment 2: Does estradiol enable chronic corticosterone to inhibit pulsatile LH secretion?
Estradiol implants.
Silastic tubing (0.125 in. inner diameter × 0.078 in outer diameter, DOW, Midland, MI) was cut to 2 cm in length. One end of the tubing was sealed with 0.3 cm silicon allowed to cure overnight. Next, 1.4 cm of the tubing was filled with sesame oil (0 ng control group, MilliporeSigma) or 20, 40, or 100 ng of 17-β estradiol (MilliporeSigma) dissolved in sesame oil. The open end of the tubing was then sealed with 0.3 cm silicon and allowed to cure. The implants were submerged in sterile saline for 14–18 hours prior to surgery.
OVX+E treatment.
Mice were OVX and implanted subcutaneously with a silastic capsule containing 0, 20, 40 or 100 ng estradiol (n=6–17/group for 0 or 100 ng; n=4/group for 20 or 40 ng estradiol). LH pulse frequency and body weight were measured 10 d post OVX. Tail blood (15 μL) was collected at 1700 h and the following day at 0700 h (11–12 d post OVX) to evaluate the diurnal rise of corticosterone which is observed in intact females, but not in OVX mice [36]. Corticosterone values were compared to values in regularly cycling females in diestrus, as determined by vaginal lavage. Finally, mice were euthanized, and the uterus was dissected and weighed (12 d post OVX). Uterine and body weight values were compared to a diestrus control group, which received an oil capsule during a sham OVX procedure to account for surgical influences 10 d prior to assessment (animal was anesthetized and the ovaries exteriorized through lumbar laparotomy prior to routine suture and staple closure).
To test the effect of estradiol on the response to corticosterone, mice were OVX and implanted with 100 ng estradiol, hereafter referred to as OVX+E. Ten d following corticosterone or cholesterol (n=8–9/group), blood samples were collected for measurement of pulsatile LH and corticosterone, as in Experiment 1 (Fig. 1B).
Experiment 3: Does estradiol enable chronic corticosterone-induced suppression of pituitary gene expression or gonadotrope responsiveness to GnRH?
A. Pituitary responsiveness to GnRH.
Ten d after OVX+E, mice were implanted with cholesterol or corticosterone (n=8/group). Approximately 48 h later, tail blood for measurement of LH was collected every 6 min for 24 min prior to and 30 min following intraperitoneal (ip) injection with GnRH (800 ng/kg, MilliporeSigma, St. Louis, MO; Fig. 1C). Serial samples were collected to observe the LH pulse pattern prior to GnRH administration. The response to GnRH was calculated as the difference in LH concentration at time 0 compared to the peak LH value occurring within 12 min of GnRH (i.e, the higher value at either the 6- or 12-min time point).
B. Pituitary gene expression.
Ten d after OVX or OVX+E, animals received either a cholesterol or corticosterone implant (n=7–10/group). Approximately 48 h later, animals were euthanized under isoflurane via rapid decapitation. The brain and pituitary gland were immediately frozen on dry ice, and stored at −80°C for RNA extraction and gene expression analysis. Tissues were collected from one cohort of mice and analyzed in Experiment 3B (pituitary) and Experiment 5A (brain).
Experiment 4: Is GR expressed in KNDy neurons?
Ten d after OVX or OVX+E, Kiss1CreGFP mice (8–38 wk old, n=4/group) were euthanized with an overdose of pentobarbitol (Fatal Plus, MWI Animal Health, Boise, ID) and perfused by cardiac puncture with 4% paraformaldehyde in PBS. Once removed, brains were stored overnight in 4% paraformaldehyde and then transferred to 30% sucrose in phosphate buffer for at least 1 d.
Experiment 5: Does chronic corticosterone inhibit KNDy gene expression or KNDy neuron activity?
A. KNDy gene expression.
Ten d after OVX or OVX+E, mice were implanted with cholesterol corticosterone (n=6–10/group) and brains were collected 48 h later, according to Experiment 3B.
B. c-Fos in KNDy neurons.
Ten d after OVX or OVX+E, Kiss1hrGFP mice were implanted with cholesterol or corticosterone (n=5–7/group). Approximately 48 h later, animals were perfused by cardiac puncture and fixed brain tissue was collected as in Experiment 4.
RNA Isolation and Gene Expression Analysis
RNA was extracted from individual pituitaries via Trizol reagent (Life Technologies - Ambion, Waltham, MA) per manufacturer’s directions. To isolate RNA from the arcuate nucleus, brains were sectioned coronally (250 μm) on a cryostat at −10°C. Sections were collected from −1.22 to −2.54 mm from bregma, according to a mouse brain atlas [37]. Semicircular micro-punches, 2 mm in diameter, which encompassed the whole arcuate nucleus were collected and stored at −80°C. RNA was isolated from arcuate punches using the RNAqueous Micro Kit (Life Technologies - Ambion), per manufacturer’s instructions.
Following isolation, DNA contamination was removed with DNA-free DNase treatment (Life Technologies - Ambion) and RNA was quantified by nanodrop. RNA (1 μg) was reverse transcribed using the iScript Complementary DNA synthesis kit (Bio-RAD, Hercules, CA). For quantitative polymerase chain reaction (qPCR), complementary DNA and gene-specific primers were loaded according to Supplemental Table 1, along with SYBR green (Bio-RAD). Data were analyzed using the comparative cycle threshold (ΔΔCt) method using Gapdh as a reference gene, as it displayed transcriptional stability across samples [38].
Immunohistochemistry (IHC)
IH C fo r GFP and GR
Fixed tissue was sectioned (30 μm) on a cryostat in 3 series and stored in cryoprotectant solution [30% ethylene glycol, 30% sucrose, 1% polyvinyl pyrrolidone in phosphate buffer (PB)] at −20°C until processed for immunohistochemistry. The following steps were performed at room temperature with gentile agitation unless noted otherwise. Sections from one series, encompassing the entire arcuate nucleus, were processed from each animal. Tissue was rinsed in phosphate-buffer saline (PBS) with 0.1% Triton X-100 (PBST) 6 times, for 10 min each. Antigen retrieval was performed by incubating tissue in boiling Citra Buffer (Fisher Scientific, Waltham, MA) for 10 min, twice. Tissue was rinsed (4 times, 5 min each with PBST; standard rinsing step, unless noted otherwise) and incubated in a blocking solution containing 5% normal goat serum (NGS; Jackson Labs, West Grove, PA) containing 0.1% BSA in PBST, for 1 h. Tissue was then incubated in rabbit anti-GR (1:500, Thermo PA1–511A; Table 1) in blocking solution for 2 h at room temperature followed by 18 h at 4°C. The next day, tissue was rinsed, then incubated in goat anti-rabbit conjugated to Alexa 594 (1:300, Life A-11012) in blocking solution for 2 h and rinsed again. Tissue was then incubated in rabbit anti-GFP conjugated to Alexa 488 (1:1000, Life A-21311; Table 1) in blocking solution for 18 h at 4°C. Finally, tissue was rinsed, mounted on Superfrost slides (Fisher Scientific), coversliped with gelvatol [39], and stored at 4°C until microscopy. Because the antiserum raised against GFP was directly conjugated to the fluorophore (Alexa 488), and it was used after the detection of G R no cross reactivity is expected, though the primary antisera were raised in the same species. Moreover, the expected cellular distribution of these antigens and the staining pattern (GR in the nucleus and GFP throughout the cell) further demonstrates a lack of cross reactivity. This GR antiserum has been used extensively for detection of GR in mouse neural tissue [40, 41]; in our laboratory, preadsorption with recombinant GR resulted in no specific staining (Supplemental Fig. 1A). No specific staining was observed when the GFP antisera was applied to wild-type mouse neural tissue (Supplemental Fig. 1B).
Table 1:
Primary antibodies
| Peptide/protein target | Antigen Sequence (if known) | Name of Antibody | Manufacturer, Catalog # and/or name of individual providing the antibody | Species raised in; monoclonal or polyclonal | Dilution used | RRID |
|---|---|---|---|---|---|---|
| Glucocorticoid Receptor | Synthetic sequence from human GR: D(346) Q K P I F N V I P P I P V G S E N W N R C(367) | Glucocorticoid Receptor Polyclonal Antibody | Thermo Fisher Scientific Cat# PA1–511A | Rabbit; polyclonal | 1:500 | AB_2236340 |
| Green Fluorescent Protein | GFP from jellyfish (Aequorea Victoria). | GFP Polyclonal Antibody, Alexa Fluor 488 | Thermo Fisher Scientific Cat# A-21311 | Rabbit; polyclonal | 1:1000 | AB_221477 |
| c-Fos | N-terminus c-Fos | Anti-c-Fos Antibody | MilliporeSigma, Cat# ABE457 | Rabbit; polyclonal | 1:15,000 | AB_2631318 |
IHC for GFP and c-Fos
One series of 30 μm sections, encompassing the entire arcuate nucleus, was processed for each animal. The following steps were performed at room temperature with gentile agitation, unless noted otherwise. Tissue was rinsed in PB, 12 times for 15 minutes, and incubated overnight at 4°C. The next day, tissue was rinsed 6 times in PB and then 6 times in PBS, for 5 min each (the following rinsing steps were performed in PBS). Antigen retrieval was performed by incubating tissue in boiling Citra Buffer for 10 min, twice. Tissue was rinsed and incubated in 0.3% hydrogen peroxide in PBS for 10 minutes. Next, tissue was rinsed and then incubated in blocking solution, containing PBS with 0.4% Triton X-100 and 4% NGS, for 1 hr. Tissue was then incubated in rabbit anti-c-Fos (1:15,000, MilliporeSigma, ABE457; Table 1) in blocking solution for 18 h at 4°C. The next day tissue was rinsed and then incubated with biotinylated goat anti-rabbit antisera in blocking solution (1:500, Vector Laboratories, BA1000, Burlingame, CA) for 1 h. Next, tissue was rinsed and then signal was amplified with Vectastain Elite ABC Kit (1:500, Vector Laboratories, PK-6100), in PBS for 1 h. Tissue was rinsed and then incubated with biotinylated tyramine (1:250, Perkin Elmer Inc, SAT70001EA, Shelton, CT) in PBS containing 0.003% hydrogen peroxide for 10 minutes. Tissue was rinsed and incubated with streptavidin conjugated to Alexa 647 (1:100, Life S32357) in PBS. Tissue was rinsed and incubated in blocking solution for 1 h and incubated in rabbit anti-GFP conjugated to Alexa 488 (1:1000, Life A-21311) in blocking solution for 18 h at 4°C. Finally, tissue was rinsed, mounted on Superfrost slides, coversliped with gelvatol [39], and stored at 4°C until microscopy. Omission of the c-Fos antisera resulted in no specific staining in the arcuate or any other region we examined (Supplemental Fig. 1C). As described in the previous section, the anti-GFP antisera was conjugated directly to the fluorophore (Alexa 488) which obviates the need for a secondary antiserum which could have cross-reacted with the other primary antibody. Additionally, the c-Fos antiserum was used at a sufficiently low concentration that omission of the tyramide step prevented detection of the antigen (Supplemental Fig. 1D).
Imaging
Imaging was performed with a Nikon Ti2-E inverted microscope with DS-Qi2 monochrome CMOS camera controlled with NIS-elements. At least two hemi-sections from each of three arcuate regions were selected for analysis (i.e. rostral, middle, and caudal). Within each region of the arcuate, the total number of GFP cells, and the percentage of GFP cells that contained GR (Experiment 4) or the percentage of GFP cells that contained c-Fos (Experiment 5), were determined in each hemi-section. The mean ± SEM number of GFP cells per hemisection or mean percentage of GFP cells that contained either GR (Experiment 4) or c-Fos (Experiment 5) is reported. Comparisons were then made between treatment groups (within region) with animal as the experimental unit. All cell counting was done by an observer blinded to treatment group using ImageJ software [42].
Ultrasensitive Mouse LH Enzyme-Linked Immunosorbent Assay
Whole blood (3 μL) samples for measurement of LH were immediately diluted (1:20) into 57 μL of assay buffer, mixed, and placed on ice until storage at −20°C [33]. LH was measured in singleton by in-house enzyme-linked immunosorbent assay at the University of Virginia Ligand Assay Core, based on a method and reagents published in Steyn et al. [43]. The limit of quantitation (functional sensitivity) is defined as the lowest concentration that demonstrates accuracy within 20% of expected values and intraassay coefficient of variation (%CV) <20% and was determined by serial dilutions of a defined sample pool. Functional sensitivity was 0.320 ng/mL. Intra- and interassay %CVs were 2.3% and 7.0%, respectively.
Corticosterone Enzyme-Link Immunosorbent Assay
Blood was centrifuged at 5000 rpm for 15 min and serum was isolated and stored at −20°C until assayed. Serum (4 μL) was diluted 1:100–1:400 and assayed using DetectX Corticosterone EIA kit (K014; Arbor Assays, Ann Arbor, MI), per Luo et al. [4]. Intra-assay and interassay %CVs were 1.8% and 4.4%, respectively. Assay sensitivity was 18.6 pg/mL.
Data Analysis
LH pulse analysis.
Detection of an LH pulse was based on three criteria, proposed by Goodman and Karsch [44]: 1) the pulse peak must be within 3 consecutive samples (i.e. 18 min) from the preceding nadir, 2) the amplitude must be greater than the sensitivity of the assay, and 3) the amplitude must also be 2 standard deviations above the variability of the assay. Average values for pulse frequency, mean LH, and pulse amplitude were calculated across the pre and post periods. Pulse frequency was defined as the number of pulses in each 90-min period. Mean LH was calculated by averaging all LH values in each sampling period. Pulse amplitude was calculated as the difference from the pulse peak to the preceding nadir, and an average pulse amplitude value for each animal during the pre and post sampling period was determined. Note, 2 of 8 animals did not demonstrate detectable pulses following corticosterone and were excluded from the assessment of pulse amplitude.
Two-way analysis of variance (ANOVA) followed by Tukey’s honestly significant difference test was used to determine significant differences across time (pre vs. post, AM vs. PM) or treatments or groups (oil vs. estradiol, cholesterol vs. corticosterone), and to identify significant interactions between variables: LH characteristics, mean corticosterone, ΔΔCt relative quantity, and colocalization of c-Fos/Kiss1 values. One-way ANOVA followed by Tukey’s honestly significant difference test was used to determine significant differences in estradiol response measures: uterine weight, weight gain, or pulse frequency. Values for c-Fos/Kiss1 were square-root transformed prior to analysis to conform with the assumptions for ANOVA, as the non-transformed values and were not normally distributed and the standard deviations not homogeneous. Student’s t-test was used to determine differences in mean corticosterone, LH response to GnRH, or colocalization of GR/Kiss1. Values are expressed as mean ± SEM. All statistical analyses were performed using JMP 10.0.0 (SAS Institute, Cary, NC) and statistical significance was defined as p<0.05.
Results
Experiment 1: Does chronic corticosterone reduce pulsatile LH secretion?
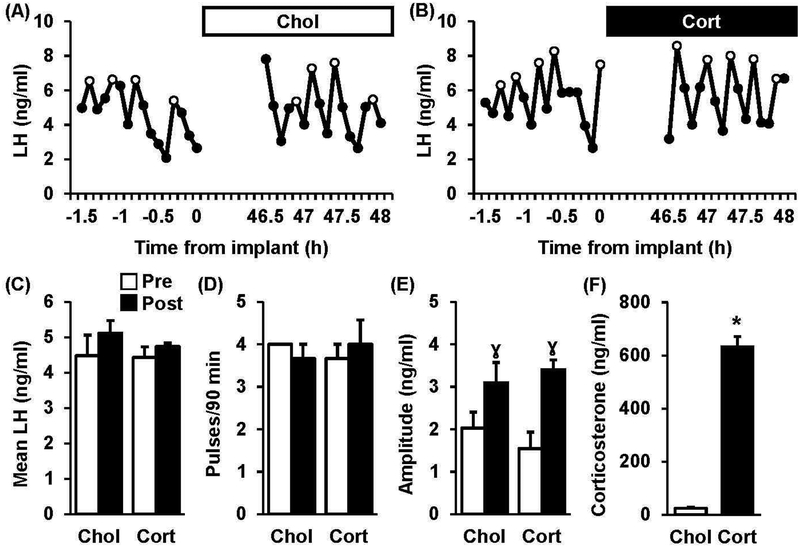
Representative LH pulse profiles collected from OVX mice prior to (−1.5–0 h, pre) and 48 h following (46.5–48 h, post) implantation with cholesterol (A, chol) or corticosterone (B, cort), are shown in Figure 2. During the pre-implant period in both groups, mean LH averaged 4.5 ng/ml and LH pulse frequency averaged 4 pulses/90 minutes (Fig. 2C–D). Regardless of treatment, values for mean LH and LH pulse frequency did not significantly differ across pre and post periods. Although LH pulse amplitude did not significantly differ between groups in the pre period, amplitude was significantly increased in the post period in both groups (Fig. 2E, pre vs. post, p<0.05), likely due to increased time from OVX. Circulating levels of corticosterone were significantly elevated in animals treated with corticosterone compared to controls (Fig. 2F, 23 ± 3 ng/ml vs. 632 ± 38 ng/ml, chol vs. cort, p<0.05).
Fig. 2. Chronic Corticosterone does not disrupt LH pulses in OVX mice.
Pattern of pulsatile LH secretion in a representative OVX mouse treated with either cholesterol (A, Chol) or corticosterone (B, Cort) via a subcutaneous implant as depicted by the bar at the top of each panel. LH pulses are identified by open circles. Values (mean ± SEM) for mean LH (C), pulse frequency (D), and pulse amplitude (E) were calculated across time (Pre, white bars vs. Post, black bars) in each treatment group and analyzed (n=3 mice/group). Note, no significant time × treatment interactions were identified. γ, effect of time (p<0.05). (F) Serum corticosterone concentrations (mean ± SEM) in mice prior to the serial sampling which occurred 2 d following implantation (n=4–5 per group). *, effect of treatment (p<0.05).
Experiment 2: Does estradiol enable chronic corticosterone to inhibit pulsatile LH secretion?
OVX+E treatment.
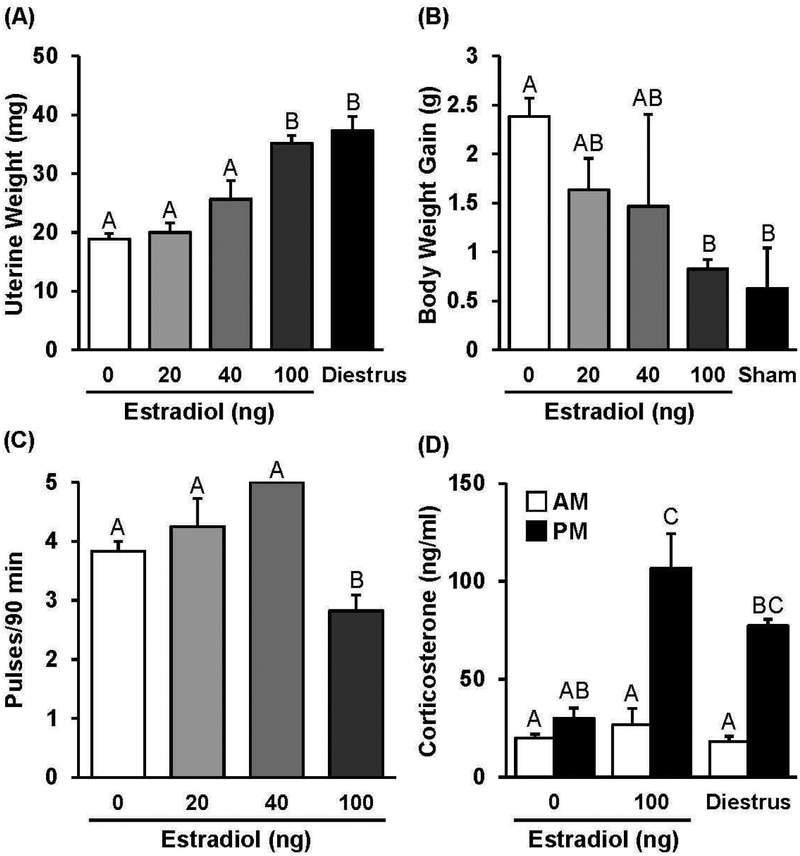
Mice were OVX and implanted subcutaneously with either an implant containing 0 ng estradiol (oil control) or an implant containing 20, 40, or 100 ng estradiol. Approximately 10 d after OVX and estradiol replacement, uterine weight, weight gain following OVX, and LH pulse frequency were examined to document and determine the effectiveness of each estradiol treatment. In the absence of estradiol replacement, average uterine weight was 18.9 ± 1.0 mg, which did not significantly differ from mice implanted with 20 ng estradiol or 40 ng estradiol (Fig. 3A, p>0.05). In contrast, 100 ng estradiol significantly increased uterine weight compared to values in oil control animals (Fig. 3A, 35 ± 1.3 mg, 0 vs. 100 ng estradiol, p<0.05), and values did not significantly differ from uterine weight values obtained from sham OVX controls sampled in diestrus (Fig. 3A, 37.3 ± 2.5 mg, p>0.05). Although all animals gained weight during the 10 d period from OVX, the body weight change in animals treated with 100 ng estradiol was not different from the increase in body weight in sham OVX controls, indicating treatment with 100 ng estradiol was sufficient to prevent the weight gain that resulted from OVX (Fig. 3B, 2.4 ± 0.2 g vs. 0.8 ± 0.1 g, 0 ng vs. 100 ng E2, p<0.05). LH pulse frequency in response to 20 or 40 ng estradiol did not differ from the frequency in control animals (Fig. 3C, p>0.05); yet, replacement with 100 ng estradiol significantly slowed LH pulse frequency (Fig. 3C, 0 vs. 100 ng E2, p<0.05). Although not significant, treatment with 100 ng estradiol lowered mean LH and elicited an increase in pulse amplitude (0 ng vs. 100 ng E2, p<0.1; Supplemental Table 2). Interestingly, treatment with 100 ng estradiol also restored an aspect of HPA axis activity that dissipates when estradiol is removed, as indicated by elevated evening corticosterone that was not significantly different from diestrus control females (Fig. 3D).
Fig. 3. Assessment of OVX+E model.
Mean (± SEM) uterine weight (A) or weight gain (B) in mice OVX and replaced with an implant containing 0, 20, 40, or 100 ng of estradiol, compared to a diestrus control group or a control group that were sham OVX and replaced with an oil implant. (C) Mean (± SEM) LH pulse frequency measured in OVX mice replaced with an implant containing 0, 20, 40, or 100 ng of estradiol. (D) Mean (± SEM) serum corticosterone concentrations measured at 0700 h (AM) and 1700 h (PM) following OVX plus 0 ng estradiol (OVX) or 100 ng estradiol (OVX+E) and compared to diestrus controls. Unique letters signify significant differences between values (p<0.05).
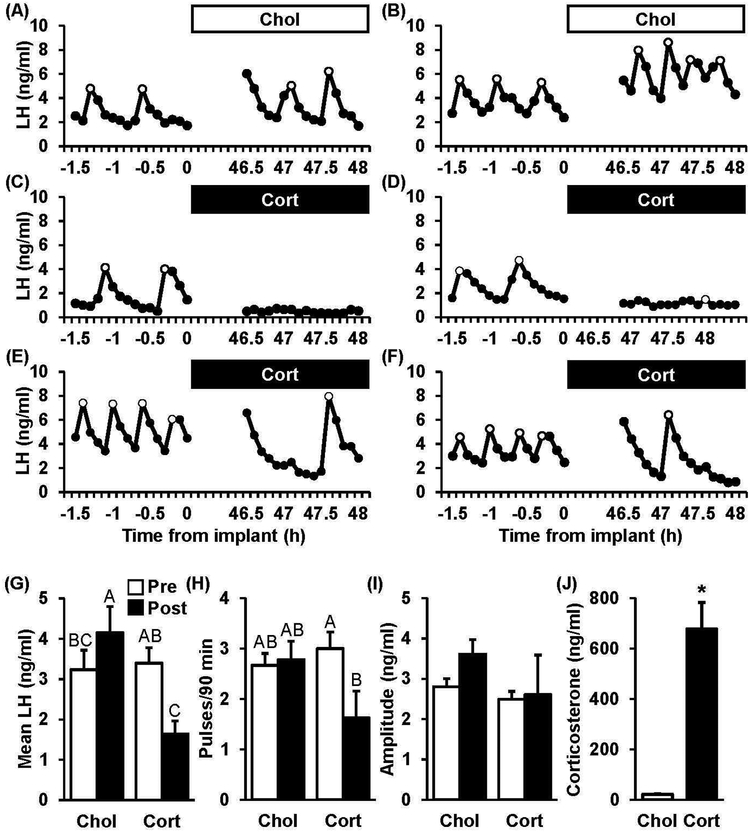
Figure 4 shows representative LH pulse profiles during the two periods of frequent tail-tip blood sampling collected from OVX+E mice prior to and 48 h following implantation with cholesterol (Fig. 4 A–B) or corticosterone (Fig. 4 C–F). In animals treated with cholesterol, mean LH averaged 3.2 ng/ml, pulse frequency averaged 2.7 pulses/90 minutes, and pulse amplitude averaged 2.8 ng/ml in the pre-implant period; values for each LH characteristic did not significantly differ during the pre period between corticosterone- and cholesterol-treated controls (Fig. 4 G–I, chol pre vs. cort pre, p>0.05). During the post-implant period in animals treated with cholesterol, mean LH values were significantly elevated while frequency and amplitude were both unchanged compared to pre values. In contrast, corticosterone significantly reduced mean LH by 51.7% and decreased LH pulse frequency by 45.8% across the two sampling windows (Fig. 4G–H, cort, pre vs. post, p<0.05). Notably, the individual LH responses during treatment with corticosterone were variable: in two animals, no pulses were identified (Fig. 4C): in four animals, low amplitude pulses were identified (0.4–1.7 ng/ml, Fig. 4D); and in two animals, high amplitude pulses were identified (5.1–6.6 ng/ml, Fig. 4E–F). Again, corticosterone levels were significantly elevated by the corticosterone treatment (Fig. 4J, chol vs. cort, p<0.05).
Fig. 4. Chronic Corticosterone suppresses LH pulse frequency only in OVX+E mice.
Patterns of pulsatile LH secretion in representative OVX+E mice, either implanted with cholesterol (A-B, Chol) or corticosterone (C-F, Cort). LH pulses are identified by open circles. Values (mean ± SEM) for mean LH(G), pulse frequency (H), and pulse amplitude (I) were compared across time (Pre, white bars vs. Post, black bars) in each treatment group (n=8–9 mice/group). Unique letters signify significant differences between values (p<0.05). (J) Serum corticosterone concentrations (mean ± SEM) in mice prior to the serial sampling which occurred 2 d following implantation (n=13/group). *, effect of treatment (p<0.05).
Experiment 3: Does estradiol enable chronic corticosterone-induced suppression of pituitary gene expression or gonadotrope responsiveness to GnRH?
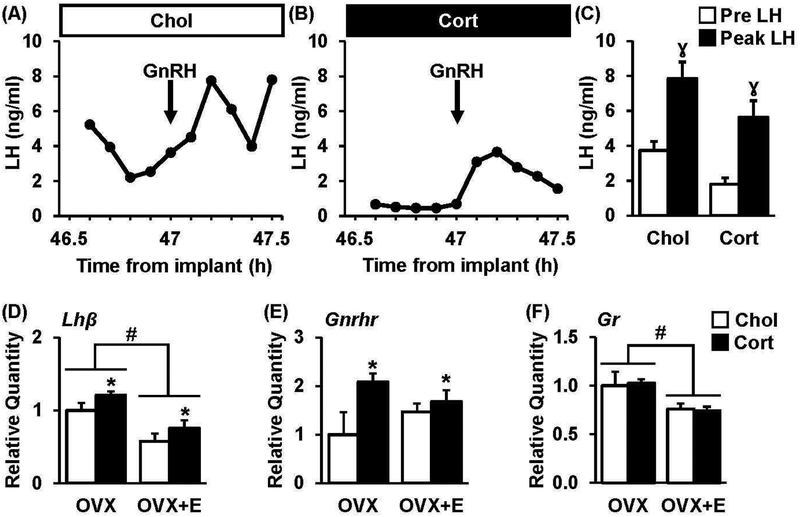
We tested the hypothesis that a suppression in gonadotrope cell activity contributes to decreased LH during chronic corticosterone. Representative profiles of LH in tail-tip blood, collected prior to and following an injection of GnRH, from OVX+E mice either implanted with cholesterol or corticosterone are shown (Fig. 5A–B). Prior to injection, LH levels averaged 3.7 ng/ml in the control group and 1.8 ng/ml in the corticosterone group (Fig. 5C, pre). In response to GnRH, peak LH levels reached an average value of 7.9 ng/ml in mice treated with cholesterol compared to 5.6 ng/ml in mice treated with corticosterone (Fig. 5C, p>0.05); the amplitude of the LH response to GnRH did not significantly differ between treatments (4.1 ± 0.6 vs. 3.8 ± 0.9 ng/ml, chol vs. cort, p>0.05).
Fig. 5. Chronic corticosterone does not impair pituitary responsiveness.
Representative LH responses to GnRH in OVX+E mice treated with cholesterol (A, Chol) or corticosterone (B, Cort) and 800 ng/kg of GnRH (ip) at time 0. (C) Mean (± SEM) LH at time 0 (Pre LH) and in response to GnRH (Peak LH). γ, p<0.05, effect of time. Analysis of (D) Lhβ, (E) Gnrhr, and (F) Gr expression in individual pituitary glands collected from OVX or OVX+E mice treated with cholesterol (white bars) or corticosterone (black bars). Note, no significant estradiol × treatment interactions were identified. #, effect of estradiol; *, effect of corticosterone, p<0.05.
Pituitaries were collected from animals treated with chronic corticosterone or cholesterol in the absence or presence of estradiol. Although significant effects of corticosterone or estradiol treatment were identified, no significant interactions between these two factors were observed. For example, Lhp was suppressed by estradiol in animals treated with or without corticosterone and increased by corticosterone regardless of estradiol treatment (Fig. 5D, p<0.05). Gnrhr also showed a significant increase in response to corticosterone regardless of estradiol treatment (Fig. 5E). Gr expression was suppressed by estradiol in both groups (Fig. 5F).
Experiment 4: Is GR expressed in KNDy neurons?
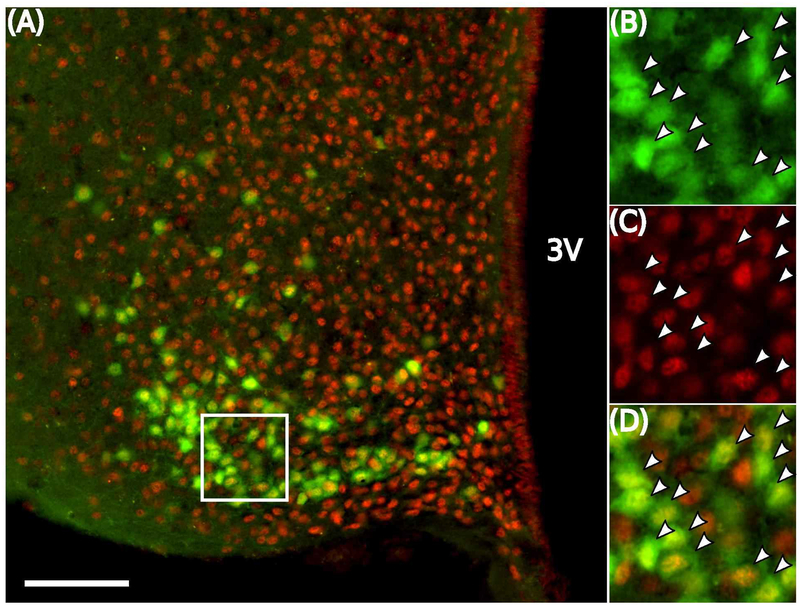
To determine if corticosterone can act directly on KNDy cells, we performed immunohistochemistry for GR using brain tissue from Kiss1CreGFP females treated with or without estradiol. The majority of GFP-labeled Kiss1 neurons throughout the arcuate hypothalamus, in either OVX or OVX+E mice, contained clear nuclear labeling for GR (Fig. 6). Estradiol treatment did not significantly alter the percentage of Kiss1 neurons that contained GR, except for a modest increase in the rostral arcuate (Table 2).
Fig. 6. GR is expressed in Kiss1 cells of the mediobasal hypothalamus.
Representative photomicrograph of dual-labeled Kiss1CreGFP and GR cells in the middle arcuate (A). White box indicates location of zoomed panels depicting staining for GFP (B, green), GR (C, red), and the merged image (D). White arrowheads indicate dual-labeled Kiss1CreGFP-GR cells. Scale bar equals 40 μm.
Table 2:
IHC for GFP and GR
| OVX | OVX+E | |
|---|---|---|
| # GFP Cells/hemi-section | ||
| rARC | 26.4 ± 8.0 | 24.2 ± 9.2 |
| mARC | 69.4 ± 9.5 | 54.9 ± 8.0 |
| cARC | 54.1 ± 9.4 | 74.2± 14.8 |
| % GFP Cells with GR | ||
| rARC | 86.0 ± 1.3A | 97.4 ± 1.3B |
| mARC | 93.3 ± 0.7 | 92.7 ± 3.1 |
| cARC | 96.2 ± 2.2 | 89.3 ± 4.0 |
Values are mean ± SEM.
Regions of the arcuate hypothalamus are referred to as: rostral (r), middle (m), and caudal (c).
Values with unique superscripts are significantly different between OVX and OVX+E (p<0.05).
Experiment 5: Does chronic corticosterone inhibit or KNDy gene expression or KNDy neuron activity?
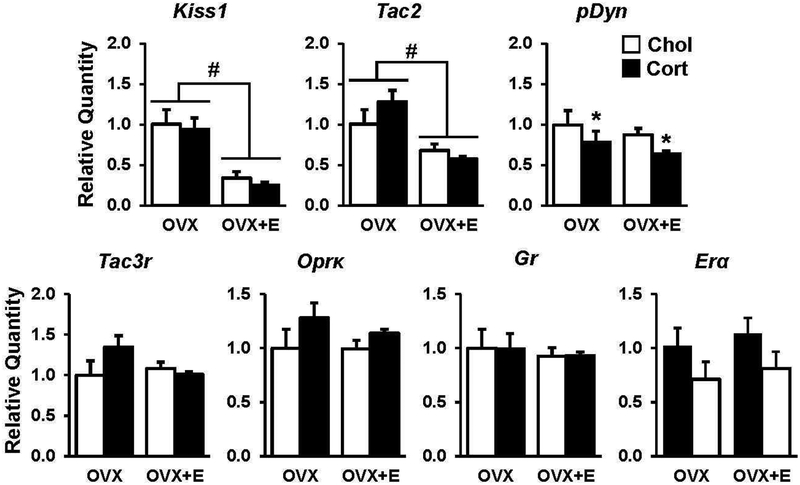
Brains were collected from OVX or OVX+E mice following treatment with chronic corticosterone or cholesterol and gene expression for KNDy peptides and receptors were analyzed in micropunches of the arcuate hypothalamus (Fig. 7). Both Kiss1 and Tac2 were downregulated by estradiol (p<0.05), confirming the effectiveness of the estradiol treatment; however, neither Kiss1 nor Tac2 were further suppressed by corticosterone in OVX+E mice. In contrast, corticosterone decreased pDyn, the gene encoding dynorphin, regardless of estradiol treatment. (Fig. 7, p<0.05). Neither the mRNA expression of receptors for neurokinin B or dynorphin, Tac3r and Oprk, respectively, nor Gr or E ra were altered by corticosterone or estradiol (Fig. 7, p>0.05).
Fig. 7. Chronic corticosterone does not suppress arcuate KNDy-associated gene expression.
qPCR analysis of Kiss1, Tac2, pDyn, Tac3r, OprK, Gr, and Erα gene expression in arcuate micropunches collected from OVX or OVX+E mice and treated with cholesterol (white bars, Chol) or corticosterone (black bars, Cort). Note, no significant estradiol × treatment interactions were identified. #, effect of estradiol; *, effect of corticosterone, p<0.05. All data represented as mean ± SEM.
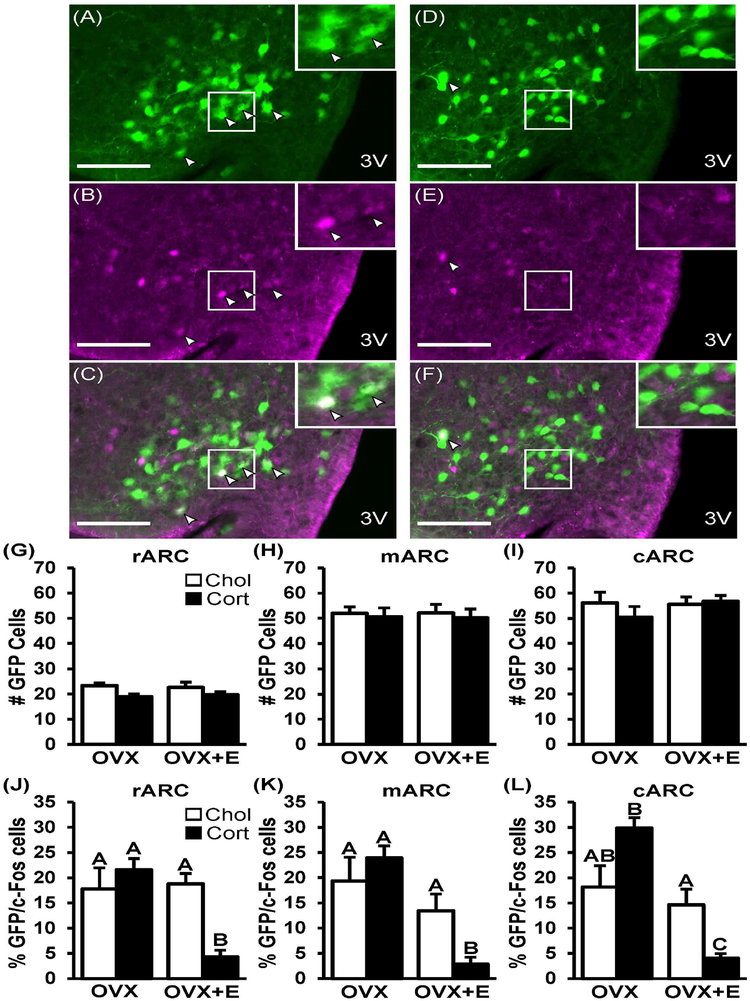
Using Kiss1hrGFP females, we investigated whether corticosterone-induced inhibition of pulsatile LH was associated with changes in Kiss1 neuronal activity. Figure 8 shows representative photomicrographs of nuclear c-Fos within GFP-labeled Kiss 1 cells in a Kiss1hrGFP OVX+E female treated with either cholesterol (Fig. 8A–C) or corticosterone (Fig. 8D–F). A robust population of Kiss1 cells were identified in the rostral, middle, and caudal regions of the arcuate nucleus using this mouse model, in which GFP expression is under the control of the Kiss1 promoter. Although treatment with estradiol did not alter the total number of Kiss1hrGFP cells detected (Fig. 8G–I) according to our methods, we did observe a 70% suppression in GFP intensity/cell in OVX+E females compared to OVX animals across all three regions of the arcuate hypothalamus. This may indicate estradiol-dependent regulation of the Kiss1 population in Kiss1hrGFP mice. Using this mouse model, we determined that the number of Kiss1hrGFP cells detected was not changed by chronic corticosterone in either OVX or OVX+E females (Fig. 8G–I); under these conditions, GFP intensity/cell was not altered by chronic corticosterone. Approximately 17–20% GFP-labeled Kiss1 cells contained c-Fos in OVX mice treated with cholesterol; in OVX mice, corticosterone did not significantly change co-expression in any region (Fig. 8J–L). In contrast, corticosterone induced a robust suppression in the percent of Kiss1 cells expressing c-Fos in OVX+E females compared to controls (p<0.05, 72–78% suppression); the suppression was present in all three regions of the arcuate nucleus.
Fig. 8. Corticosterone-induced suppression of Kiss1 neuronal activation requires estradiol.
Representative photomicrographs depicting staining for Kiss1hrGFP (A and D, green), c-Fos (B and E, purple), and dual-labeled Kiss1/c-Fos cells (C and F) in the middle arcuate of OVX+E mice treated with cholesterol (A-C) or corticosterone (D-F). White box indicates location of zoomed panels. White arrowheads indicate dual labeled Kiss1/c-Fos cells. Scale bar equals 40 μm. Number of Kissl cells (G-I) and percent of Kiss1 cells with c-Fos (J-L) per hemi-section of OVX or OVX+E mice treated with cholesterol (white bars, Chol) or corticosterone (black bars, Cort). Regions of the arcuate hypothalamus are referred to as: rostral (r), middle (m), and caudal (c). Back-transformed mean (± SEM) values are displayed. Unique letters signify significant differences between values (p<0.05).
Discussion/Conclusion
The goal of the present study was to test the hypothesis that a chronic elevation in glucocorticoids can disrupt pulsatile LH secretion in female mice. This investigation was based on our prior finding that a chronic elevation of corticosterone impairs ovarian cyclicity in mice by preventing progression through the follicular phase [4]. We demonstrate that chronic corticosterone has no effect on LH secretion in OVX mice; however, in the presence of a physiologic level of estradiol, corticosterone is able to reduce LH secretion by slowing LH pulse frequency. The suppression in LH is not associated with reduced Lhβ or Gnrhr expression or impaired pituitary responsiveness to GnRH, suggesting corticosterone is acting upstream of the gonadotrope cell. As for a potential neuronal cell type mediating the suppression of pulsatile LH secretion, this study shows that a majority of arcuate Kiss1 neurons in the female mouse contain GR and that corticosterone decreases neuronal activation of this population of Kiss1 cells, as measured by c-Fos, in females treated with estradiol. Taken together, these data demonstrate that estradiol is necessary for corticosterone to impair the frequency of LH pulses in female mice, potentially via a direct action upon arcuate kisspeptin neurons.
A pertinent question raised by these findings is whether chronic corticosterone is acting directly or indirectly on arcuate kisspeptin cells to inhibit LH. Arcuate kisspeptin cells contain ERα [28], and the current study reveals that ~90% of arcuate Kiss1 neurons contain GR. Together, these observations identify the KNDy cell as a potential site whereby estradiol could enable corticosterone to impair pulsatile LH secretion. Our results in the mouse are consistent with studies in sheep. Considering that nearly all arcuate dynorphin cells coexpress kisspeptin in ewes [20], and that 80% of dynorphin cells are colocalized with GR [29], we can infer that a majority of arcuate kisspeptin neurons in the sheep contain GR. Interestingly, the female rat may demonstrate a species difference in both GR colocalization and sensitivity to corticosterone-induced LH suppression. Evidence that less than 3% of arcuate kisspeptin neurons express GR in diestrus female rats [45] offers a potential explanation for why OVX+E female rats did not demonstrate a disruption in LH pulsatile secretion following either acute or chronic corticosterone [46]. Takumi et al. did observe that a majority of kisspeptin-containing cells within the anteroventral periventricular nucleus and periventricular nucleus continuum were found to express GR in female rats administered colchicine. Although interesting to consider a potential species difference, a direct comparison cannot be made as the gonad-status of the animals and GR antibody employed were also different. These findings do clearly show that corticosterone has the potential to act on KNDy cells, at least in the mouse, and provide the foundation for future studies utilizing neuronal specific GR knockouts to elucidate whether chronic corticosterone is acting directly on Kiss1 neurons or via afferent projections to this cell population.
Another question raised by these findings is the cellular mechanism whereby estradiol enables the suppression of pulse frequency by chronic corticosterone. A role for estradiol was demonstrated by previous work in female sheep which showed that elevated glucocorticoids decreased LH pulse frequency, also in an ovarian steroid-dependent manner, via a reduction in the frequency of GnRH pulses [6, 16]. We extend this observation by demonstrating that chronic corticosterone markedly impairs Kiss1 neuronal activity throughout the arcuate nucleus in an estradiol-dependent manner. Based on our current understanding that these cells function as the GnRH pulse generator, we hypothesized that both estradiol and corticosterone could induce changes directly within this population of cells to influence pulse frequency. We assessed levels of GR and determined that estradiol does not upregulate Gr expression nor increase GR colocalization in kisspeptin cells. In lieu of a change in GR expression, we explored transcriptional regulation by estradiol and corticosterone. Estradiol caused a robust reduction in Tac2 and Kiss1, lowering levels by 40% and 70%, respectively, in OVX+E females; however, levels were not further reduced by corticosterone treatment.
Dynorphin is another interesting candidate that could mediate corticosterone-induced suppression of KNDy activity. Indeed, blocking dynorphin action with a kappa-opioid receptor antagonist elicits an increase in LH pulse frequency [47, 48] which is associated with increased multi-unit activity in the mediobasal hypothalamus [48]. We evaluated dynorphin expression in animals treated with chronic corticosterone and estradiol, based on observations that ER and GR can alter transcriptional activity via crosstalk at promoter response elements [49, 50], including at the dynorphin promoter leading to increased dynorphin expression [51]. However, the current study showed a decrease in dynorphin mRNA expression in response to corticosterone, suggesting upregulation of this inhibitory peptide is not likely to underlie the suppression in LH here. Interestingly, a few pieces of evidence support an alternate mechanism, whereby estradiol enhances the inhibitory action of dynorphin on the GnRH pulse generator. Electrophysiological recordings of KNDy cells in male mice showed that in vivo treatment with estradiol enhanced the inhibitory effect of dynorphin on KNDy neuron firing [52]. Furthermore, work in the female rat demonstrates that central delivery of a kappa-opioid receptor antagonist has no effect on the pulse generator in OVX animals, but prevents the negative feedback effect of estradiol on LH pulses in OVX+E females [53]. Future work is needed to test the possibility that estradiol enables chronic corticosterone to inhibit KNDy cells via a dynorphin-dependent pathway.
A couple of comments are warranted regarding the estradiol and corticosterone treatments employed in the current study. First, the estradiol replacement dose utilized here recapitulates a host of normal physiologic responses. For example, this dose restores peak serum corticosterone and prevents increased weight gain induced by OVX. Based on uterine weight, which is a simple and effective correlate of circulating estradiol [54], this estradiol dose mimics a diestrus level. Importantly for our studies, while this level of estradiol slows pulse frequency, the frequency and amplitude of LH pulsatile secretion is robust, and the detection of pulses is reliable as compared to published reports of LH profiles in diestrus mice that may be slower and lower in amplitude [55]. Therefore, we utilized this OVX+E model, which normalized estradiol levels across animals and removed other factors influenced by the ovarian cycle, to directly test the effect of estradiol on the LH response during a chronic elevation of corticosterone. Although the magnitude of the increment of corticosterone is physiologic [4, 35], the duration of this elevation exceeds that of an acute stress-induced increase, which would be lowered over time due to negative feedback. Whether an acute rise in corticosterone can also inhibit LH via a suppression in frequency in the female mouse remains to be tested. However, findings in OVX sheep that an acute increase in cortisol inhibits LH pulse amplitude via a pituitary mechanism without altering frequency [7] points to a potential role of glucocorticoid duration underlying LH suppression, in addition to a role of estradiol. Interestingly, we have shown that acute psychosocial restraint stress dramatically reduces LH pulse frequency in OVX mice, demonstrating that estradiol is not necessary for the suppression in frequency in response to this stress type [33]. Therefore, acute stressors may act via pathways independent of corticosterone or corticosterone may not require estradiol to inhibit LH pulses when administered acutely.
In summary, we utilized a female mouse model that allows for reliable detection of LH pulses to demonstrate that estradiol is necessary for chronic corticosterone to impair the frequency of LH pulses. We show that arcuate kisspeptin cells contain GR and demonstrate reduced kisspeptin neuronal activation in response to chronic corticosterone via an estradiol-dependent mechanism. Future studies are warranted to investigate acute elevations in glucocorticoids and whether a direct action by this stress hormone upon arcuate kisspeptin neurons mediates altered gonadotropin secretion commonly associated with stress [56, 57].
Supplementary Material
Acknowledgements
The authors wish to thank Dr. Carol Elias (University of Michigan) for her generous gift of the Kiss1CreGFP and Kiss1hrGFP mouse lines. We thank the Nikon Imaging Center at UC San Diego for access to microscopes and technical assistance with imaging.
Funding Sources
This work was supported by NIH grant R01 HD086100, NSF grant IOS-1457226 and the UCSD Health Sciences Senate. R.B.M. was supported by NIH grant F32 HD096811 and T32 HD007203. K.T. was partially supported by an Endocrine Society Summer Research Fellowship; K.T. and C.I.S. were partially supported by UCSD Eureka! Undergraduate Research Scholarships. Serum hormone assays were performed by The University of Virginia Ligand Assay Core Laboratory, which is supported through National Institute of Child Health and Human Development Grant P50 HD028934.
Footnotes
Statement of Ethics: The authors have no ethical conflicts to disclose.
Disclosure Statement: The authors have no conflicts of interest to declare.
References
- 1.Briski KP, Vogel KL: Role of endogenous opioid peptides in central glucocorticoid receptor (GR)-induced decreases in circulating LH in the male rat. Neuropeptides 1995;28:175–181. [DOI] [PubMed] [Google Scholar]
- 2.Breen KM, Oakley AE, Pytiak AV, Tilbrook AJ, Wagenmaker ER, Karsch FJ: Does cortisol acting via the type II glucocorticoid receptor mediate suppression of pulsatile luteinizing hormone secretion in response to psychosocial stress? Endocrinology 2007;148:1882–1890. [DOI] [PubMed] [Google Scholar]
- 3.Briski KP, Sylvester PW: Antagonism of type II, but not type I glucocorticoid receptors results in elevated basal luteinizing hormone release in male rats. Neuroendocrinology 1994;60:601–608. [DOI] [PubMed] [Google Scholar]
- 4.Luo E, Stephens SB, Chaing S, Munaganuru N, Kauffman AS, Breen KM: Corticosterone Blocks Ovarian Cyclicity and the LH Surge via Decreased Kisspeptin Neuron Activation in Female Mice. Endocrinology 2016;157:1187–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saketos M, Sharma N, Santoro NF: Suppression of the hypothalamic-pituitary-ovarian axis in normal women by glucocorticoids. Biol Reprod 1993;49:1270–1276. [DOI] [PubMed] [Google Scholar]
- 6.Breen KM, Billings HJ, Wagenmaker ER, Wessinger EW, Karsch FJ: Endocrine basis for disruptive effects of cortisol on preovulatory events. Endocrinology 2005;146:2107–2115. [DOI] [PubMed] [Google Scholar]
- 7.Breen KM, Karsch FJ: Does cortisol inhibit pulsatile luteinizing hormone secretion at the hypothalamic or pituitary level? Endocrinology 2004;145:692–698. [DOI] [PubMed] [Google Scholar]
- 8.Chantaraprateep P, Thibier M: Effects of dexamethasone on the responses of luteinizing hormone and testosterone to two injections of luteinizing hormone releasing hormone in young postpubertal bulls. The Journal of endocrinology 1978;77:389–395. [DOI] [PubMed] [Google Scholar]
- 9.Melis GB, Mais V, Gambacciani M, Paoletti AM, Antinori D, Fioretti P: Dexamethasone reduces the postcastration gonadotropin rise in women. The Journal of clinical endocrinology and metabolism 1987;65:237–241. [DOI] [PubMed] [Google Scholar]
- 10.Pearce GP, Paterson AM, Hughes PE: Effect of short-term elevations in plasma cortisol concentration on LH secretion in prepubertal gilts. Journal of reproduction and fertility 1988;83:413–418. [DOI] [PubMed] [Google Scholar]
- 11.Li PS, Wagner WC: In vivo and in vitro studies on the effect of adrenocorticotropic hormone or cortisol on the pituitary response to gonadotropin releasing hormone. Biology of reproduction 1983;29:25–37. [DOI] [PubMed] [Google Scholar]
- 12.Suter DE, Orosz G: Effect of treatment with cortisol in vivo on secretion of gonadotropins in vitro. Biology of reproduction 1989;41:1091–1096. [DOI] [PubMed] [Google Scholar]
- 13.Suter DE, Schwartz NB, Ringstrom SJ: Dual role of glucocorticoids in regulation of pituitary content and secretion of gonadotropins. Am J Physiol 1988;254:E595–600. [DOI] [PubMed] [Google Scholar]
- 14.Ringstrom SJ, Schwartz NB: Cortisol suppresses the LH, but not the FSH, response to gonadotropin-releasing hormone after orchidectomy. Endocrinology 1985;116:472–474. [DOI] [PubMed] [Google Scholar]
- 15.Dubey AK, Plant TM: A suppression of gonadotropin secretion by cortisol in castrated male rhesus monkeys (Macaca mulatta) mediated by the interruption of hypothalamic gonadotropin-releasing hormone release. Biol Reprod 1985;33:423–431. [DOI] [PubMed] [Google Scholar]
- 16.Oakley AE, Breen KM, Clarke IJ, Karsch FJ, Wagenmaker ER, Tilbrook AJ: Cortisol reduces gonadotropin-releasing hormone pulse frequency in follicular phase ewes: influence of ovarian steroids. Endocrinology 2009;150:341–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burger LL, Vanacker C, Phumsatitpong C, Wagenmaker ER, Wang L, Olson DP, et al. : Identification of Genes Enriched in GnRH Neurons by Translating Ribosome Affinity Purification and RNAseq in Mice. Endocrinology 2018;159:1922–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dufourny L, Skinner DC: Type II glucocorticoid receptors in the ovine hypothalamus: distribution, influence of estrogen and absence of co-localization with GnRH. Brain Res 2002;946:79–86. [DOI] [PubMed] [Google Scholar]
- 19.Lehman MN, Karsch FJ: Do gonadotropin-releasing hormone, tyrosine hydroxylase-, and betaendorphin-immunoreactive neurons contain estrogen receptors? A double-label immunocytochemical study in the Suffolk ewe. Endocrinology 1993;133:887–895. [DOI] [PubMed] [Google Scholar]
- 20.Goodman RL, Lehman MN, Smith JT, Coolen LM, de Oliveira CV, Jafarzadehshirazi MR, et al. : Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology 2007;148:5752–5760. [DOI] [PubMed] [Google Scholar]
- 21.Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA: Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci 2009;29:11859–11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohkura S, Takase K, Matsuyama S, Mogi K, Ichimaru T, Wakabayashi Y, et al. : Gonadotrophinreleasing hormone pulse generator activity in the hypothalamus of the goat. J Neuroendocrinol 2009;21:813–821. [DOI] [PubMed] [Google Scholar]
- 23.Wakabayashi Y, Nakada T, Murata K, Ohkura S, Mogi K, Navarro VM, et al. : Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci 2010;30:3124–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lehman MN, Coolen LM, Goodman RL: Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology 2010;151:3479–3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rance NE, Krajewski SJ, Smith MA, Cholanian M, Dacks PA: Neurokinin B and the hypothalamic regulation of reproduction. Brain Res 2010;1364:116–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clarkson J, Han SY, Piet R, McLennan T, Kane GM, Ng J, et al. : Definition of the hypothalamic GnRH pulse generator in mice. Proc Natl Acad Sci U S A 2017;114:E10216–E10223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Franceschini I, Lomet D, Cateau M, Delsol G, Tillet Y, Caraty A: Kisspeptin immunoreactive cells of the ovine preoptic area and arcuate nucleus co-express estrogen receptor alpha. Neurosci Lett 2006;401:225–230. [DOI] [PubMed] [Google Scholar]
- 28.Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA: Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology 2005;146:3686–3692. [DOI] [PubMed] [Google Scholar]
- 29.Oakely AE: Central Inhibitory Effects of Glucocorticoids on Reproductive Function: Permissive Role of Estradiol (Doctor of Philosophy): University of Michigan, 2008, Doctor of Philosophy Dissertation, [Google Scholar]
- 30.Cravo RM, Margatho LO, Osborne-Lawrence S, Donato J Jr., Atkin S, Bookout AL, et al. : Characterization of Kiss1 neurons using transgenic mouse models. Neuroscience 2011;173:37–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cravo RM, Frazao R, Perello M, Osborne-Lawrence S, Williams KW, Zigman JM, et al. : Leptin signaling in Kiss1 neurons arises after pubertal development. PLoS One 2013;8:e58698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCosh RB, Kreisman MJ, Breen KM: Frequent Tail-tip Blood Sampling in Mice for the Assessment of Pulsatile Luteinizing Hormone Secretion. Journal of visualized experiments : JoVE 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang JA, Song CI, Hughes JK, Kreisman MJ, Parra RA, Haisenleder DJ, et al. : Acute Psychosocial Stress Inhibits LH Pulsatility and Kiss1 Neuronal Activation in Female Mice. Endocrinology 2017;158:3716–3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meyer JS, Micco DJ, Stephenson BS, Krey LC, McEwen BS: Subcutaneous implantation method for chronic glucocorticoid replacement therapy. Physiology & behavior 1979;22:867–870. [DOI] [PubMed] [Google Scholar]
- 35.Breen KM, Thackray VG, Hsu T, Mak-McCully RA, Coss D, Mellon PL: Stress levels of glucocorticoids inhibit LHbeta-subunit gene expression in gonadotrope cells. Molecular endocrinology (Baltimore, Md) 2012;26:1716–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen A, Zorrilla E, Smith S, Rousso D, Levy C, Vaughan J, et al. : Urocortin 2-deficient mice exhibit gender-specific alterations in circadian hypothalamus-pituitary-adrenal axis and depressive-like behavior. J Neurosci 2006;26:5500–5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Franklin KBJ, Paxinos G: Paxinos and Franklin’s The mouse brain in stereotaxic coordinates, ed Fourth edition Amsterdam, Academic Press, an imprint of Elsevier, 2013. [Google Scholar]
- 38.Livak KJ, Schmittgen TD: Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001;25:402–408. [DOI] [PubMed] [Google Scholar]
- 39.Harlow E, Lane D: Mounting samples in gelvatol or mowiol. CSH Protoc 2006;2006 [DOI] [PubMed] [Google Scholar]
- 40.Lerch JK, Alexander JK, Madalena KM, Motti D, Quach T, Dhamija A, et al. : Stress Increases Peripheral Axon Growth and Regeneration through Glucocorticoid Receptor-Dependent Transcriptional Programs. eNeuro 2017;4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Navailles S, Zimnisky R, Schmauss C: Expression of glucocorticoid receptor and early growth response gene 1 during postnatal development of two inbred strains of mice exposed to early life stress. Dev Neurosci 2010;32:139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al. : Fiji: an open-source platform for biological-image analysis. Nat Methods 2012;9:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Steyn FJ, Wan Y, Clarkson J, Veldhuis JD, Herbison AE, Chen C: Development of a methodology for and assessment of pulsatile luteinizing hormone secretion in juvenile and adult male mice. Endocrinology 2013;154:4939–4945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goodman RL, Karsch FJ: Pulsatile secretion of luteinizing hormone: differential suppression by ovarian steroids. Endocrinology 1980;107:1286–1290. [DOI] [PubMed] [Google Scholar]
- 45.Takumi K, Iijima N, Higo S, Ozawa H: Immunohistochemical analysis of the colocalization of corticotropin-releasing hormone receptor and glucocorticoid receptor in kisspeptin neurons in the hypothalamus of female rats. Neuroscience letters 2012;531:40–45. [DOI] [PubMed] [Google Scholar]
- 46.Kinsey-Jones JS, Li XF, Knox AM, Wilkinson ES, Zhu XL, Chaudhary AA, et al. : Downregulation of hypothalamic kisspeptin and its receptor, Kiss1r, mRNA expression is associated with stressinduced suppression of luteinising hormone secretion in the female rat. J Neuroendocrinol 2009;21:20–29. [DOI] [PubMed] [Google Scholar]
- 47.Goodman RL, Hileman SM, Nestor CC, Porter KL, Connors JM, Hardy SL, et al. : Kisspeptin, neurokinin B, and dynorphin act in the arcuate nucleus to control activity of the GnRH pulse generator in ewes. Endocrinology 2013;154:4259–4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wakabayashi Y, Nakada T, Murata K, Ohkura S, Mogi K, Navarro VM, et al. : Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci 2010;30:3124–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vahrenkamp JM, Yang CH, Rodriguez AC, Almomen A, Berrett KC, Trujillo AN, et al. : Clinical and Genomic Crosstalk between Glucocorticoid Receptor and Estrogen Receptor alpha In Endometrial Cancer. Cell Rep 2018;22:2995–3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Karmakar S, Jin Y, Nagaich AK: Interaction of glucocorticoid receptor (GR) with estrogen receptor (ER) alpha and activator protein 1 (AP1) in dexamethasone-mediated interference of ERalpha activity. J Biol Chem 2013;288:24020–24034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ayrout M, Le Billan F, Grange-Messent V, Mhaouty-Kodja S, Lombes M, Chauvin S: Glucocorticoids stimulate hypothalamic dynorphin expression accounting for stress-induced impairment of GnRH secretion during preovulatory period. Psychoneuroendocrinology 2019;99:47–56. [DOI] [PubMed] [Google Scholar]
- 52.Ruka KA, Burger LL, Moenter SM: Both Estrogen and Androgen Modify the Response to Activation of Neurokinin-3 and kappa-Opioid Receptors in Arcuate Kisspeptin Neurons From Male Mice. Endocrinology 2016;157:752–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mostari P, Ieda N, Deura C, Minabe S, Yamada S, Uenoyama Y, et al. : dynorphin-kappa opioid receptor signaling partly mediates estrogen negative feedback effect on LH pulses in female rats. J Reprod Dev 2013;59:266–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shim WS, Conaway M, Masamura S, Yue W, Wang JP, Kmar R, et al. : Estradiol hypersensitivity and mitogen-activated protein kinase expression in long-term estrogen deprived human breast cancer cells in vivo. Endocrinology 2000;141:396–405. [DOI] [PubMed] [Google Scholar]
- 55.Czieselsky K, Prescott M, Porteous R, Campos P, Clarkson J, Steyn FJ, et al. : Pulse and Surge Profiles of Luteinizing Hormone Secretion in the Mouse. Endocrinology 2016;157:4794–4802. [DOI] [PubMed] [Google Scholar]
- 56.Fourman LT, Fazeli PK: Neuroendocrine causes of amenorrhea--an update. J Clin Endocrinol Metab 2015;100:812–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Akhter S, Marcus M, Kerber RA, Kong M, Taylor KC: The impact of periconceptional maternal stress on fecundability. Ann Epidemiol 2016;26:710–716 e717. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.