Abstract
The preservation of mitochondrial function is a major protective strategy for cerebral ischemic injuries. Previously our laboratory demonstrated that protein kinase C epsilon (PKCε) promotes the synthesis of mitochondrial Nicotinamide adenine dinucleotide (NAD+). NAD+ along with its reducing equivalent, NADH, is an essential co-factor needed for energy production from glycolysis and oxidative phosphorylation. Yet, NAD+/NADH are impermeable to the inner mitochondrial membrane and their import into the mitochondria requires the activity of specific shuttles. The most important neuronal NAD+/NADH shuttle is the malate-aspartate shuttle (MAS). The MAS has been implicated in synaptic function and is potentially dysregulated during cerebral ischemia. The aim of this study was to determine if metabolic changes induced by PKCε preconditioning involved regulation of the MAS. Using primary neuronal cultures, we observed that the activation of PKCε enhanced mitochondrial respiration and glycolysis in vitro. Conversely, inhibition of the MAS resulted in decreased oxidative phosphorylation and glycolytic capacity. We further demonstrated that activation of PKCε increased the phosphorylation of key components of the MAS in rat brain synaptosomal fractions. Additionally, PKCε increased the enzyme activity of Glutamic oxaloacetic transaminase 2 (GOT2), an effect that was dependent on the import of PKCε into the mitochondria and phosphorylation of GOT2. Furthermore, PKCε activation was able to rescue against decreased GOT2 activity induced by ischemia. These findings reveal novel protective targets and mechanisms against ischemic injury, which involves PKCε-mediated phosphorylation and activation of GOT2 in the MAS.
Keywords: cerebral ischemia, ischemia tolerance, ischemic preconditioning, conditioning, malate aspartate shuttle, mitochondria
Introduction
Worldwide, stroke is one of the leading causes of death and long-term disability [1]. The current FDA-approved treatments for stroke are recombinant tissue plasminogen activator and mechanical thrombectomy [1]. Although highly effective, these treatments can only benefit a small percentage of stroke patients leaving the majority of patients without treatment [1]. Preclinical studies have shown that ischemic preconditioning (IPC), which involves the induction of a sub-lethal ischemic insult prior to a lethal one, confers protection by activating intracellular adaptive mechanisms [2–4]. Our laboratory and others have previously demonstrated that preconditioning-induced neuroprotection is mediated, in part, by protein kinase C epsilon (PKCε): a serine/threonine kinase [5, 6].
Several downstream effectors of PKCε have been identified in the mitochondria. Our lab has previously shown that PKCε translocates to synaptosomal mitochondria, where it increases the phosphorylation of proteins of the mitochondrial electron transport chain and enhances mitochondrial respiration [7]. Given that the high-energy demand in the brain is primarily met by mitochondrial ATP production, the preservation of mitochondrial function is a major protective strategy for cerebral ischemic injuries [8–10].
PKCε also targets the nicotinamide adenine dinucleotide (NAD+) pathway in the mitochondria [11]. NAD+ is a necessary co-factor needed for energy production from glycolysis and oxidative phosphorylation [12]. During glycolysis, NAD+ is reduced to NADH, which is then oxidized by complex I of the mitochondrial electron transport chain to generate the proton gradient necessary for ATP production. Yet, NAD+ and NADH do not cross the inner mitochondrial membrane [13]. The reducing equivalents from the cytosol produced by glycolysis are imported into the mitochondrial matrix via the NADH shuttles [12, 14]. The malate-aspartate shuttle (MAS), which tightly controls neuronal metabolism, is considered the most important NAD+/NADH shuttle in neurons [14]. Although PKCε-mediated activation of the MAS was reported in the heart [15], it is still unclear whether PKCε regulates cellular metabolism via the MAS in neurons.
The MAS plays a prominent role in neuronal mitochondrial respiration. Knock out of the aspartate-glutamate carrier (AGC), one of several MAS components, results in a 46% drop in mitochondrial respiration in primary neurons [16]. One of the mediators of MAS activity is calcium (Ca2+). Removal of Ca2+ inhibits MAS-promoted mitochondrial respiration [16]. Given the fundamental role of calcium signaling in synaptic function, it is likely that the MAS is involved in synaptic transmission which is triggered by presynaptic Ca2+ signaling. Importantly, the MAS is selectively activated by small Ca2+ signals under physiological conditions. In pathological conditions of cerebral ischemia, excitotoxicity-induced calcium overload activates the mitochondrial Ca2+ uniporter, but not the MAS [17], implicating dysfunctional regulation of the MAS in cerebral ischemic injury.
Because of the crucial role of the MAS in neuronal metabolism and synaptic function, and its potential dysregulation during ischemia, we sought to determine if PKCε preconditioning/neuroprotection mediates cellular metabolic changes in the brain via MAS modulation. In this study, we identified proteins involved in the MAS that became phosphorylated after PKCε activation. We defined the mechanisms by which PKCε-mediated phosphorylation regulated the activity of the MAS. Furthermore, we showed that PKCε activation was capable of rescuing against the ischemia-induced decrease of MAS activity. Overall, our study reveals a novel neuroprotective mechanism of PKCε that involves increasing the activity of a component of MAS: Glutamic oxaloacetic transaminase 2 (GOT2).
Materials and Methods
Animals
Animal usage and experimentation were approved by the Institutional Animal Care and Use Committee at the University of Miami and was in accordance with the US Public Health Service’s Policy on Humane Care and Use of Laboratory Animals. Sprague-Dawley (SD) rats were obtained from Charles River (Wilmington, MA) and housed in a supervised facility of the division of veterinary resources.
Rat Primary Neuronal Cultures and Astrocyte Cultures
Primary neuronal cultures were prepared from embryonic 18–20 day old pups. Embryos were harvested, their cortical tissue was homogenized and dissociated into single cells. Cells were plated on poly-D-lysine-coated tissue culture plates in neuronal plating medium (minimum essential media, 1% Glutamax, 5% fetal bovine serum, and 15 mM glucose). Medium was replaced 2hrs later with neuronal culture medium (neurobasal plus medium supplemented with 2% B27, 0.5 mM Glutamax, Thermo Fisher Scientific, Waltham, MA). Neurons were seeded at 2,500,000 cells/well in 6-well plates or 40,000 cells/well in Seahorse Extracellular Flux Analyzer Miniplates (Agilent, Santa Clara, CA). Cultures underwent half media changes every third day for 7 to 10 days before treatment. Primary astrocyte cultures were prepared from 1–2 day old pups. Their cortical tissue was homogenized and dissociated into single cells. Cells were plated in a T-25 flask with astrocytic plating medium (Dulbecco’s Modified Eagle Medium supplemented with 10% fetal bovine serum, 1% Glutmax, 1% penicillin-streptomycin, Thermo Fisher Scientific). Astrocytes were trypsinized when confluent and seeded at 40,000 cells/well in Seahorse Extracellular Flux Analyzer Miniplates (Agilent) 1 day before experiments.
Pharmacological Treatments
For in vitro PKCε preconditioning, cultures were treated 2 days prior to experimental analysis. For PKCε activation, cells were exposed for 1 hour to 500 nM of control peptide TAT (Sigma, St. Louis, MO), specific PKCε activator TAT-conjugated ΨεRACK (CPC scientific, Sunnyvale, CA), with or without 500 nM Hsp90 inhibitor, 17-AAG (Tocris, Bristol, UK). For inhibition of the MAS, cultures were exposed to 20 µM or 80 µM of vehicle DMSO (Sigma) or GOT enzyme inhibitor, Aminooxyacetic acid (Tocris) for 48 hours. For proteomic analysis, activation of PKCε was induced by pre-incubating hippocampal synaptosomes with 1 µM TAT or TAT-conjugated ΨεRACK for 15 minutes at 4°C before isolation of mitochondria. For in vivo experiments, 12- to 14-week-old male SD rats were intraperitoneal injected with 0.2 mg/kg TAT or TAT-conjugated ΨεRACK. TAT or ΨεRACK was dissolved in saline. 17-AAG or Aminooxyacetic acid was dissolved in DMSO.
Oxygen Consumption Rate and Glycolytic Rate
Oxygen consumption rate and glycolytic rate were measured using the Seahorse Extracellular Flux Analyzer (Agilent) as described previously [18, 19] with minor modifications. To measure oxygen consumption rate, neurons or astrocytes were washed 3 times with neurobasal plus media or seahorse media (XF Base Medium-minimum Dulbecco’s Modified Eagle Medium supplemented with 20 mM glucose, 1 mM sodium pyruvate, and 2 mM glutamine), respectively. To measure glycolytic rate, neurobasal plus media or seahorse media (XF Base Medium-minimum Dulbecco’s Modified Eagle Medium supplemented with 2 mM glutamine) was used in neurons or astrocytes, respectively. Cells were then incubated in a CO2-free incubator at 37°C for 45 minutes prior to the plate run. Oxygen consumption rate and glycolytic rate were normalized to cell counts as measured by Hoechst staining.
Isolation of Synaptosomes from Cortices or Hippocampus
Synaptosomes from rat cortices or hippocampus were isolated according to previously published procedures [7]. Mitochondria from hippocampal synaptosomes were used for proteomic analysis and cortical synaptosomes were used for further immunoprecipitation and Western blot. In brief, rats were decapitated under isoflurane anesthesia. The cortices or hippocampi were removed immediately and immersed into cold (4°C) isolation medium, consisting of 0.32 M sucrose, 1 mg/ml BSA, 0.25 mM Dithiothereitol and 1mM EDTA. The minced tissue was homogenized in a glass Teflon homogenizer. The homogenate was centrifuged at 500 x g for 5 minutes, and the resulting supernatant was layered on a preformed Percoll gradient consisting of 23, 15, 10, and 3% (v/v) Percoll. The gradients were centrifuged at 32,500 x g for 5 minutes and the cortical synaptosomes were collected at the interface between 15–23% layers. To improve the yield, hippocampal synaptosomes were collected from 10–15 and 15–23% layers. The synaptosomes were then washed successively with isolation media and 0.32 M sucrose by centrifugation at 15,000 x g for 10 minutes. The purified synaptosomal samples were resuspended in 0.32M sucrose. All procedures were carried out at 4°C.
Phosphoprotein Staining and Mass Spectrometry
Hippocampal synaptosomes from rats were isolated as described above. The samples were then placed inside a nitrogen cell bomb and put under a pressure of 1200 psi for 7.5 min to isolate the total mitochondria [7]. The mitochondria obtained were subsequently used for proteomic analysis. Briefly, mitochondrial proteins were separated on a 4–20% acrylamide gel (Invitrogen Corporation, Carlsbad, CA). Phosphoproteins in the gradient gel were identified using Pierce Phosphoprotein Staining Kit (Thermo Fisher Scientific) as per manufacturer’s instructions and subsequently stained with Coomassie blue (Pierce Biotechnology, Rockford, IL) to confirm equal protein loading. For protein identification, gel slices were excised and digested in situ with sequencing-grade trypsin (Promega Biosciences, Inc., Madison, WI). The peptide mixtures extracted from the gel were further concentrated on the peptide trap (Cap Trap, Michrom BioResources, Auburn, CA). This was followed by a separation of the peptides using BioBasic C18 PicoFrit microcapillary column (New Objective, Woburn, MA) by a linear gradient of 7–93% acetonitrile in water and 0.1% formic acid over 45 minutes. To collect the full MS and tandem MS/MS spectra, peptides eluted from the column were analyzed by a DECA-XP Plus ion trap mass spectrometer equipped with a nano-LC electrospray ionization source (Thermo Fisher Scientific). For data analysis, BioWorks 3.2 software (Thermo Fisher Scientific), based on the SEQUEST algorithm was used to search the NCBI NR non-redundant protein databases (NCBI NR). Peptide/spectra matches were retained if the following criteria were met: XCor scores higher than 1.5 for peptides charged +1, higher than 2.0 for peptides charged +2, and higher than 2.5 for peptides charged +3 and peptide probability lower than 0.05.
Immunoprecipitation and Immunoblotting
The immunoprecipitation was carried out using the Pierce Classic IP Kit (Thermo Fisher Scientific) according to the manufacturer’s instructions. The synaptosomal fraction or cell lysate (500 µg) was immunoprecipitated with anti-phosphoserine, anti-phosphothreonine, or anti-phosphotyrosine antibodies. The resulting pellet was then subjected to immunoblotting with anti-GOT1, GOT2, or OGC. To determine the protein levels of GOT1 and GOT2, protein concentration was determined by the BioRad DC™ Protein Assay. Forty micrograms of protein was loaded into a 4–20% SDS polyacrylamide gel (BioRad, Hercules, CA). Proteins were then transferred onto a nitrocellulose membrane (Biorad) and blocked for 1 hour at RT. Incubation in primary antibody was achieved overnight at 4°C. Following addition of HRP-conjugated secondary antibodies (1:5,000, GE healthcare, Little Chalfont, UK) for 1 h, blots were developed with ECL reagents (ThermoFisher Scientific, Waltham, MA) and visualized with X-ray film (Denville Scientific, Holliston, MA). Blots were then digitalized and analyzed in Image J software (NIH) by densitometry. The following primary antibodies were used: Actin (Cell Signal, Danvers, MA, 8H10D10), GOT1 (Proteintech, Rosemont, IL, 14886-1-AP), GOT2 (Proteintech, 14800-1-AP), GOT2 (Millipore, MABS417), OGC (Santa Cruz, Dallas, TX, sc-515593), Phospho-serine (Sigma, St. Louis, MO, P3430), Phosphothreonine (Sigma, P3555), Phospho-tyrosine (Cell Signal, 9411).
Subcellular Fractionation
Cultures were fractionated into cytosolic and mitochondrial compartments as described previously [20, 21]. Briefly, cells were homogenized through a 27-gauge needle in hypotonic buffer (250 mM sucrose, 50 mM Tris-HCl, 5 mM MgCl2, 1 mM EGTA, Phosphatase and Protease inhibitor (Thermo Fisher Scientific), pH 7.4). The homogenate was then subjected to centrifugation at 800 x g for 10 minutes to remove nuclei, and 11,000 x g for 10 minutes to sediment mitochondria [21]. The mitochondria was washed twice by resuspending the pellet in suspension buffer (250 mM sucrose, 50 mM Tris-HCl, 5 mM MgCl2, pH 7.4) and repeating the 11,000 x g sedimentation. The purified fractions were then collected to measure GOT enzyme activity as described below.
Assay of GOT Activity
GOT enzyme activity was measured using Aspartate Aminotransferase Activity Colorimetric Assay Kit (Biovision, San Francisco, CA) according to the manufacturer’s instructions. Mitochondria were disrupted by four freeze-thaw cycles before measurements. Enzyme activity was normalized to protein concentrations.
Oxygen and Glucose Deprivation (OGD)
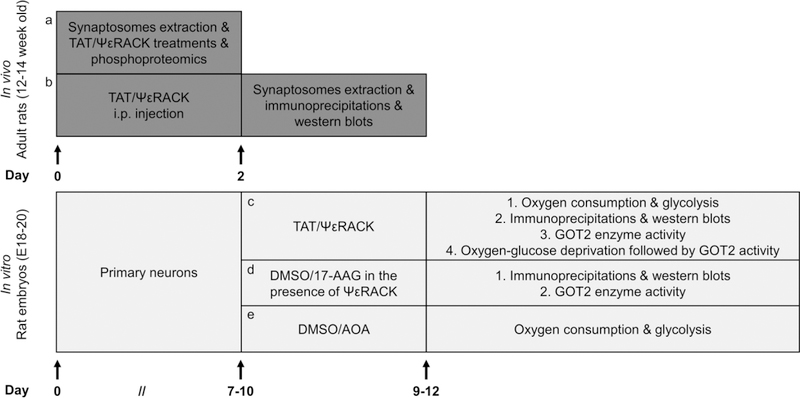
The OGD experiments were performed as our lab previously described [19]. Briefly, primary neurons were incubated in glucose-free OGD medium and exposed to OGD for 2 hours under a hypoxia chamber (Coy Lab Products, Grass Lake MI) flushed with 90% N2, 5% CO2, 5% H2 at 37 °C. The cultures were then returned to maintaining media and the normoxic incubator. The OGD media constituted 1.26 mM CaCl2, 5.37 mM KCl, 0.44 mM KH2PO3, 0.49 mM MgCl2, 0.41 mM MgSO4, 136.9 mM NaCl, 4.17 mM NaHCO3, 0.34 mM Na2HPO4, 20 mM sucrose, and 10 mM HEPES adjusted to pH 7.4. Sham OGD constitutes cells exposed to a complete media change without OGD. A schematic diagram of the experimental design is indicated in Fig. 7.
Fig. 7. Schematic diagram of the experimental design.
Briefly, (a, b) synaptosomes were extracted from adult rats. Phosphoproteomic analysis, immunoprecipitation and immunoblots were used to examine PKCε-activated phosphorylation. (c-e) Primary neurons were prepared from embryonic 18–20 day old pups. Oxygen consumption and glycolysis were evaluated by Seahorse Bioscience Technology. PKCε-mediated phosphorylation was determined by immunoprecipitation and western blots. A colorimetric assay was utilized to assess the GOT2 activity. We used oxygen-glucose deprivation as an in vitro model to evaluate the effects of ischemic injury on GOT2 activity. For pharmacological treatments and experimental details please refer to “Materials and Methods”.
Real-Time Polymerase Chain Reaction
Please refer to the online-supplement. The information about the primers used are found in Supplementary Table 1.
Statistical analysis
Rats were randomly assigned to groups throughout. Data acquisition and analyses were conducted in a blinded manner when possible. Data are presented as mean ± SEM. Statistical analysis was performed using Prism6 software (GraphPad, San Diego, CA). The respective statistical test for each experiment is indicated in the figure legends. Significance is denoted in each figure. In all cases, a P value of <0.05 was considered as statistically significant.
Results
PKCε preconditioning increases the oxygen consumption and glycolytic rate in neurons
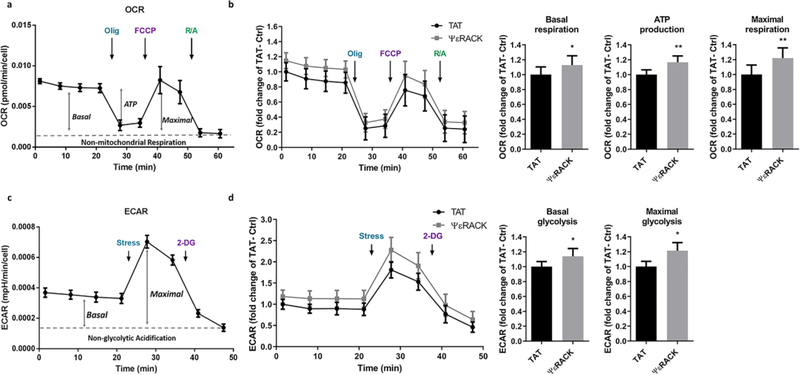
PKCε is a crucial mediator of preconditioning-induced ischemic tolerance [5, 6, 22, 23]. Previously, our laboratory demonstrated that transient activation of PKCε for 1 hour induced an increase in the concentration of mitochondrial NAD+ in neuronal-glial cortical cultures 48 hours later [11]. Increases in NAD+ can lead to the activation of important signaling pathways, but its primary function is metabolic. To specify whether preconditioning with a specific PKCε activator, ΨεRACK, induced metabolic changes, we performed live cell bioenergetics analysis in primary neuronal cultures using the Seahorse Bioscience Technology. The measurements of oxidative phosphorylation and glycolytic rate obtained from this assay are illustrated in Fig. 1a and Fig. 1c, respectively. In primary neurons, PKCε preconditioning through the specific PKCε activator, ΨεRACK (500 nM), induced a modest increase in the basal oxygen consumption rate (13%, P<0.05) and oxygen consumption linked to ATP production (17%, P<0.05) compared to the control neurons treated with TAT carrier peptide. PKCε activation also increased maximal oxygen consumption rate (22%, P<0.05) in response to energetic stress (Fig. 1b) compared to the TAT control. Similarly, we observed increased basal (14%, P<0.05) and maximal glycolytic rate (21%, P<0.05) in the PKCε preconditioned neurons compared to TAT-treated control (Fig. 1d). Together, these data show that PKCε preconditioning increases oxidative phosphorylation and glycolysis in neurons.
Fig. 1. Activation of PKCε increases the respiratory and glycolytic rate in primary neurons.
(a) Representative profile of the oxygen consumption rate (OCR) showing the sequential injection of metabolic inhibitors: complex V inhibition by oligomycin (Olig), mitochondrial uncoupling by FCCP, complex I and complex III inhibition by rotenone and antimycin (R/A); arrow indicates application. Basal: Basal respiration rate; ATP: oxygen consumption rate linked to ATP production; Maximal: maximal uncoupled respiration rate. (b) Primary neurons were exposed to control -TAT or specific PKCε activator-ΨεRACK (500 nM) for 1 hour. OCR was measured 48 hours later using the Seahorse Analyzer. Values were normalized to cell counts and represented as fold change of TAT control (n=4, mean ± SEM, *P<0.05, Two-tailed paired Student’s t-test). (c) Representative profile of the extracellular acidification rate (ECAR) showing the sequential injection of metabolic inhibitors. Energetic stress cocktail: complex V inhibition by oligomycin and mitochondrial uncoupling by FCCP; glycolysis inhibition by 2-Deoxyglucose (2-DG); arrow indicates application. Basal: Basal glycolysis rate; Maximal: maximal glycolytic rate. (d) Primary neurons were treated with TAT or ΨεRACK (500 nM) for 1 hour. ECAR was measured 48 hours later using the Seahorse Analyzer. Values were normalized to cell counts and represented as fold change of TAT control (n=4, mean ± SEM, * P <0.05, Two-tailed paired Student’s t-test).
PKCε activation enhances phosphorylation of proteins in the malate aspartate shuttle in the brain
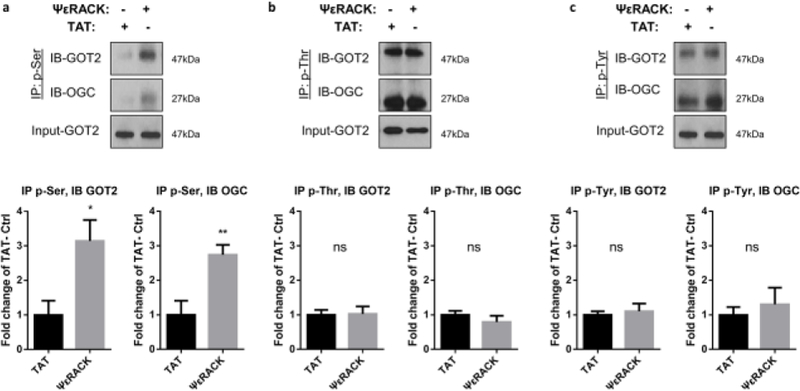
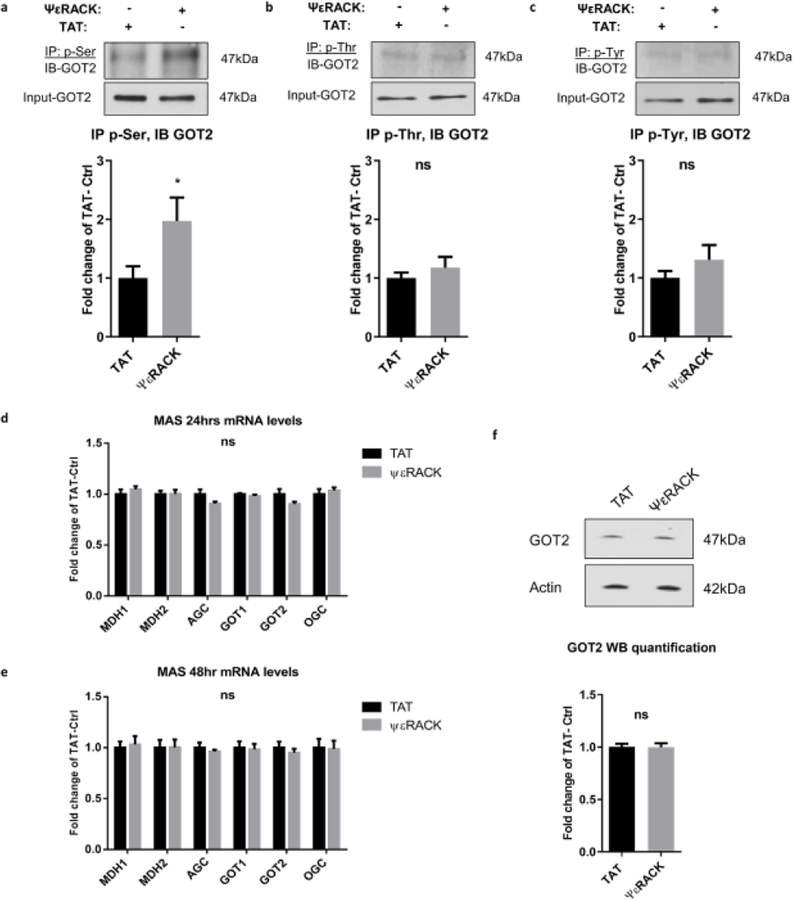
To determine the potential substrates of PKCε upon activation, phospho-proteins were identified in mitochondria isolated from rat hippocampal synaptosomes treated with TAT or ΨεRACK (1 µM) for 15 mins. A protein band of 37 kDa size that demonstrated increased phosphorylation by 23% with ΨεRACK treatment (n = 3 for TAT and ΨεRACK each, p = 0.064, data not shown) was subjected to proteomic analysis. Protein identification was performed using MS/MS analysis, which identified the presence of GOT2, Glutamic oxaloacetic transaminase 1 (GOT1) and Oxoglutarate carrier (OGC) in the band (Table 1). These proteins are essential components of the malate aspartate shuttle (MAS), which is involved in transferring reducing equivalents (NADH) generated by glycolysis across the mitochondrial inner membrane, thus, tightly controlling neuronal metabolism (illustrated in Fig. 6b). Since the proteomic data suggested increases in GOT phosphorylation, we proceeded to determine the phosphorylation by Western blot. The levels of GOT2 and OGC phosphorylation were determined in cortical synaptosomes from rats 48 hours after treatment with TAT or ΨεRACK (0.2 mg/kg, i.p.). After PKCε activation, we observed a significant increase in the serine phosphorylation of GOT2 (214%, P<0.05) and OGC (174%, P<0.01) (Fig. 2a–c) compared to the TAT control. We did not observe significant changes in threonine and tyrosine phosphorylation. Together, the phosphoproteomic analysis and Western blot analysis reveal that PKCε activation increases phosphorylation of proteins in the MAS in the brain. These data, along with our previous observations of enhanced respiration and glycolysis in neurons, suggest that PKCε phosphorylates proteins in the MAS and increases activity of the MAS.
Table 1. Proteomic analysis suggested proteins in the malate-aspartate shuttle phosphorylated by PKCε.
Phospho-proteins were identified in mitochondria isolated from rat hippocampal synaptosomes treated with TAT or ΨεRACK (1 µM) for 15mins. A protein band of 37 kDa size that demonstrated increased phosphorylation by 23% with ΨεRACK treatment (n = 3 for TAT and ΨεRACK each, p = 0.064, data not shown) was subjected to proteomic analysis, which identified phosphoproteins as shown in the table above. GOT1: Glutamic oxaloacetic transaminase 1; GOT2: Glutamic oxaloacetic transaminase 2; OGC: Oxoglutarate carrier. An illustration of MAS components is shown in Fig. 6b.
| Accession NCBI NR | Protein | MW KDa | Peptides identified | Score Xcalibur |
|---|---|---|---|---|
| 6980972 | Glutamic oxaloacetic transaminase 2 (GOT2) | 47 | 11 | 120.22 |
| 6980970 | Glutamic oxaloacetic transaminase 1 (GOT1) | 46 | 3 | 40.22 |
| 21312994 | Oxoglutarate carrier (OGC) | 34 | 6 | 52.23 |
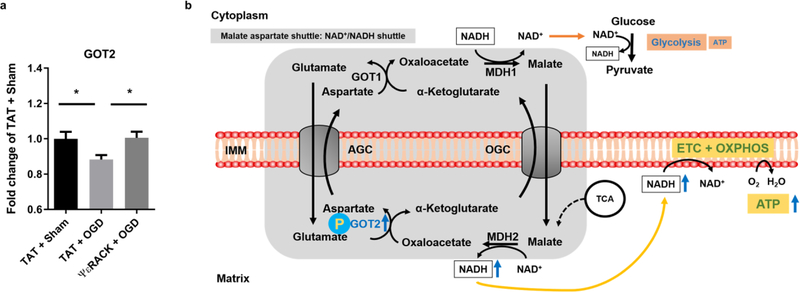
Fig. 6. PKCε rescues decreased GOT2 activity after ischemia reperfusion injury.
Primary neurons exposed to TAT or ΨεRACK (500 nM) were subjected to oxygen and glucose deprivation (OGD) for 2 hours. Activity of GOT2 was assessed 12 hours following OGD using Biovision AST colorimetric assay and normalized to protein concentration. Values are represented as fold change of control (TAT + sham). (a) In TAT-treated neurons, GOT2 activity was decreased after 2 hours of OGD compared to Sham OGD. In neurons subjected to OGD, PKCε preconditioning rescued the decreased activity of GOT2 compared to TAT treated group (n=10, mean ± SEM, * P <0.05, One-way ANOVA, post-hoc Bonferroni). (b) Schematic diagram of the proposed effects of PKCε on GOT2. Our results suggest that PKCε activation increases the phosphorylation and enzyme activity of GOT2. This leads to increased activity of the NAD+/NADH shuttle, and enhanced respiratory and glycolytic rates. Ischemic injury reduces the enzyme activity of GOT2 that gives rise to the impaired respiratory and glycolytic rates. GOT1: Glutamic oxaloacetic transaminase 1; GOT2: Glutamic oxaloacetic transaminase 2; AGC: Aspartate glutamate carrier; OGC: Oxoglutarate carrier; MDH1: Malate dehydrogenase 1; MDH2: Malate dehydrogenase 2; NAD+/NADH: Nicotinamide adenine dinucleotide; ATP: Adenosine triphosphate; IMM: Inner mitochondrial membrane; ETC: Electron transport chain; OXPHOS: Oxidative phosphorylation; TCA: Tricarboxylic acid cycle.
Fig. 2. Activation of PKCε increases serine phosphorylation of the MAS components GOT2 and OGC in synaptosomes.
Synaptosomes from rat cortices were isolated 48 hours after TAT or ΨεRACK (0.2 mg/kg i.p.) treatment. Proteins were immunoprecipitated with (a) phospho-serine, (b) phospho-threonine, (c) phospho-tyrosine, and immunoblotted for GOT2 and OGC. Input-GOT2 acts as a loading control. Quantification fold change of TAT control and representative blot as shown above (n=4–5, mean ± SEM, * P <0.05, ** P <0.01, ns = non-significant, Two-tailed unpaired Student’s t-test). GOT2: Glutamic oxaloacetic transaminase 2; OGC: Oxoglutarate carrier.
Effects of PKCε activation on the phosphorylation levels of GOT2 in vitro
Given the increased levels of cellular respiration and glycolysis, together with enhanced phosphorylation of MAS proteins, we hypothesized that PKCε-mediated phosphorylation of GOT increases its enzyme activity. Because the MAS is found predominantly in neurons [14], we used an in vitro model of primary neuronal cultures. To test the hypothesis, we firstly determined the levels of protein phosphorylation of GOT2 in neurons 48 hours after PKCε preconditioning. Similar to our data in synaptosomes (Fig. 2), we observed a significant increase in the serine phosphorylation of GOT2 in PKCε treated neurons (197%, P<0.05) (Fig. 3a) compared to the TAT treated control neurons. The threonine and tyrosine phosphorylation of GOT2 were not significantly altered by activation of PKCε (Fig. 3b, c). The protein levels of tyrosine phosphorylation in GOT2 were very low requiring higher primary antibody concentration and longer exposure time. These data demonstrate that PKCε activation induces an increase in the serine-phosphorylation of GOT2 in primary neurons.
Fig. 3. Effects of PKCε activation on the phosphorylation levels of GOT2 in primary neurons.
(a-c) Neurons were exposed to TAT or ΨεRACK (500 nM) for 1 hour. Forty-eight hours after treatment, protein from cells was immunoprecipitated with (a) phospho-serine, (b) phospho-threonine, (c) phospho-tyrosine, and immunoblotted for GOT2. Input-GOT2 represents as a loading control. Quantification fold change of TAT control and representative blot as shown above. (d-f) PKCε activation does not alter the expression of MAS components in neurons. Real-time qPCR was performed (d) 24 hours and (e) 48 hours following the 1-hour treatment of TAT or ΨεRACK (500 nM) in primary neurons. mRNA levels were normalized to GAPDH and shown as fold change of TAT control. (f) Western blots of GOT2 48 hours following the 1-hour treatment with TAT or ΨεRACK (500 nM) in neurons. Actin acts as a loading control. Quantification fold change of TAT control and representative blot as shown above (n=5–7, mean ± SEM, * P <0.05, ns = non-significant, Two-tailed unpaired Student’s t-test). MDH1: Malate dehydrogenase 1; MDH2: Malate dehydrogenase 2; AGC: Aspartate glutamate carrier; GOT1: Glutamic oxaloacetic transaminase 1; GOT2: Glutamic oxaloacetic transaminase 2; OGC: Oxoglutarate carrier.
PKCε activation does not alter the mRNA and protein levels of GOT2 in neurons
It has been reported that transgenic mice overexpressing PKCε exhibit increased levels of GOT1 and malate dehydrogenase 1 (MDH1) in the heart [24]. To determine if PKCε preconditioning, in addition to phosphorylating MAS components, also alters the expression of the MAS components in neurons, we measured the mRNA levels of MDH1, MDH2, AGC, GOT1, GOT2, and OGC using real-time qPCR analysis. Expression of the MAS components did not differ between the control-TAT and specific PKCε-activator ΨεRACK group at 24 hours (Fig. 3d) and 48 hours (Fig. 3e) following the 1-hour treatment. Additionally, we did not observe significant changes in the protein levels of GOT1 (Supplementary Fig. 1) and GOT2 (Fig. 3f) after PKCε activation, which is consistent with our data of non-altered mRNA expression of GOT1 and GOT2. These data suggest that PKCε preconditioning does not alter expression levels of the MAS components in neurons.
PKCε activation increases the enzyme activity of GOT2 via phosphorylation
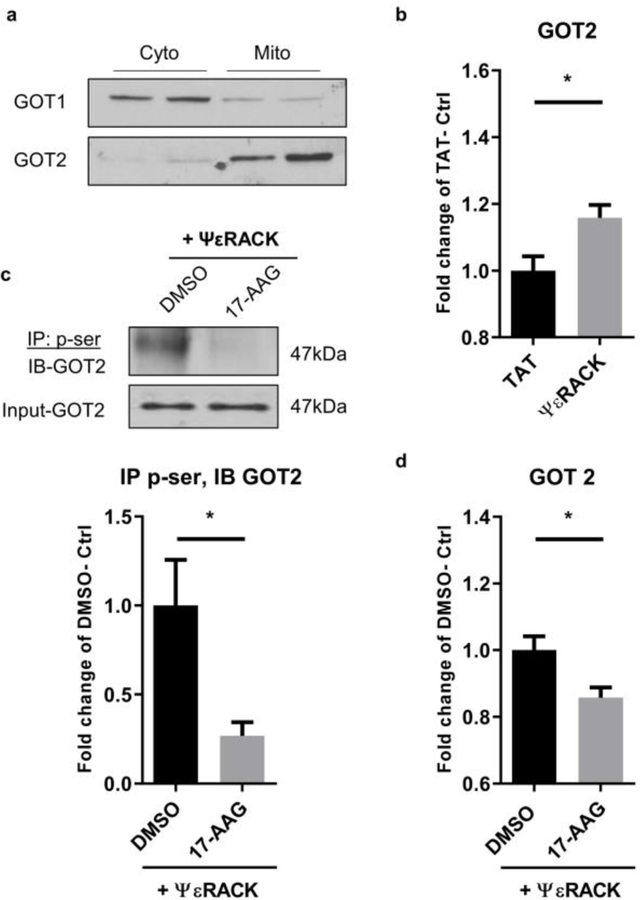
To determine if PKCε-mediated phosphorylation of GOT increases its enzyme activity, primary neurons were treated with TAT or ΨεRACK (500 nM), and then fractionated 48 hours later to allow specific investigation of GOT1 or GOT2. The enrichment of GOT1 and GOT2 is shown by Western blot from cytosolic or mitochondrial fractions (Fig. 4a). We observed that PKCε preconditioning induced a significant increase in the enzyme activity of GOT2 (GOT in the mitochondrial fraction, 15.8%, P <0.05) (Fig. 4b). Next, we sought to determine if PKCε-mediated phosphorylation of GOT2 is required for the increased enzyme activity. Previously, our group and others reported that PKCε translocates to mitochondria and mediates mitochondrial protection [7, 25, 26]. Although PKCε lacks a mitochondrial targeting sequence, it is imported into the mitochondria through the cytoprotective protein chaperone, heat shock protein 90 (Hsp 90) [25, 26]. Our laboratory has demonstrated that inhibition of the activity of Hsp90, using 17-AAG, blocks the import of PKCε into the mitochondria and inhibits its role in regulating mitochondrial proteins [26]. Therefore, neurons were treated with vehicle (DMSO) or Hsp90 inhibitor (17-AAG, 500 nM) in the presence of ΨεRACK (500 nM). Inhibition of Hsp90 resulted in a significant decrease in PKCε-mediated serine-phosphorylation of GOT2 (73.1%, P <0.05) (Fig. 4c). In addition, we observed decreased activity of GOT2 (14.2%, P <0.05) (Fig. 4d). Together, these results suggest that PKCε increases the enzyme activity of GOT2, an effect that is dependent on PKCε import into the mitochondria and phosphorylation of the enzyme.
Fig. 4. PKCε activation increases the enzyme activity of GOT2 via phosphorylation.
(a) Western blot reveals enriched GOT1 in the cytosolic fraction and GOT2 in the mitochondrial fraction. (b) Primary neurons were exposed to TAT or ΨεRACK (500 nM) for 1 hour. Forty-eight hours later, activity of GOT2 (GOT in the mitochondrial fraction) was measured using Biovision AST colorimetric assay kit and normalized to protein concentration. Values are shown as fold-change of TAT control. (c, d) Neurons were treated with 500 nM vehicle (DMSO) or Hsp90 inhibitor (17-AAG) in the presence of ΨεRACK (500 nM). (c) Representative Western blot for serine-phosphorylation of GOT2. Input-GOT2 acts as a loading control. Quantification fold change of TAT control as shown above. (d) Activity of GOT2 is represented as fold change of control (n=5–7, mean ± SEM, * P <0.05, ns = non-significant, Two-tailed unpaired Student’s t-test).
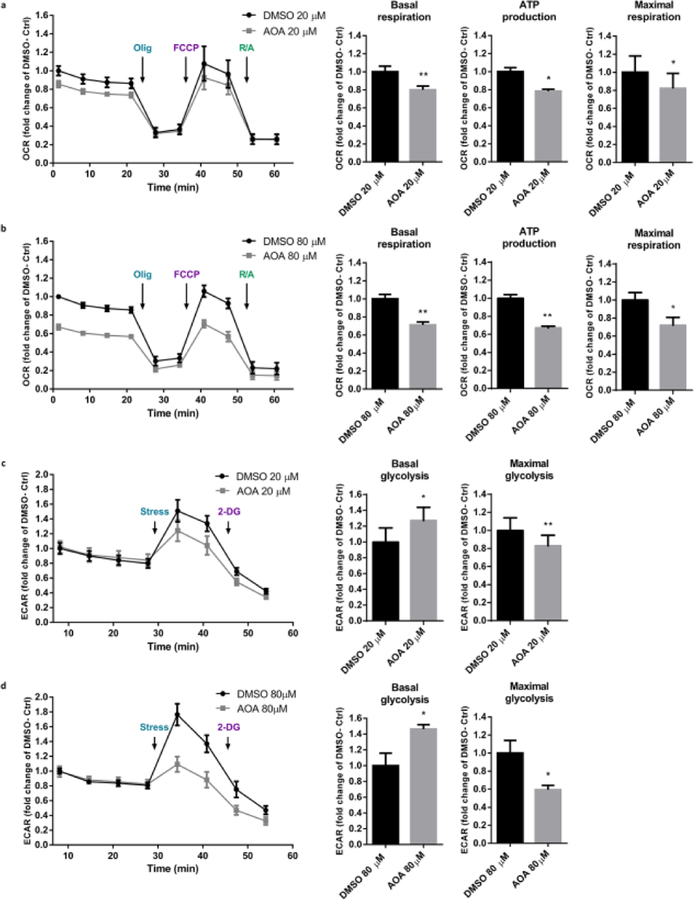
Inhibition of MAS activity alters mitochondrial respiration and glycolysis in neurons
Since PKCε activation increases the enzyme activity of GOT2 and increases cellular metabolic rates in neurons, we postulated that inhibition of GOT activity results in suppressed respiration and glycolysis. To this end, we used aminooxyacetic acid (AOA), a non-specific inhibitor of amino acid transaminase that inhibits GOT in the MAS [27, 28] and assessed live cell bioenergetics analysis. As predicted, AOA (20 µM or 80 µM) -mediated inhibition of GOT induced a significant reduction in basal oxygen consumption rate (20 µM--20%, 80 µM--29%, P<0.01), oxygen consumption linked to ATP production (20µM--22%, P<0.05; 80µM--33%, P<0.01), and maximal oxygen consumption rate (20 µM--18%, 80 µm--28%, P<0.05) (Fig. 5a, b) compared to the DMSO control. Interestingly, the inhibitor increased the basal glycolytic rate (20 µM--27%, 80 µM--46%, P<0.05) (Fig. 5c, d), which may be an adaptive metabolic change in response to the constrained mitochondrial respiration. However, the maximal glycolytic rate that corresponds to a stressor is significantly decreased with the inhibition (20 µM--17%, P<0.01; 80µM--41%, P<0.05) compared to the DMSO treated control neurons. Together, these data suggest that inhibition of GOT, and subsequent disruption of MAS activity, decreases mitochondrial respiration and, under energetic stress, suppresses glycolytic capacity as well.
Fig. 5. Respiratory and glycolytic rates are impaired with the inhibition of GOT in neurons.
Primary neurons were treated with vehicle (DMSO) or GOT enzyme inhibitor, Aminooxyacetic acid (AOA), for 48 hours. OCR and ECAR were assessed using the Seahorse Biosciences Technology. Values were normalized to cell counts and represented as fold change of DMSO control. (a, b) Measurements and quantifications of OCR with (a) 20 µM and (b) 80 µM DMSO or AOA. (c, d) Measurements and quantifications of ECAR with (c) 20 µM, and (d) 80 µM DMSO or AOA (n=4–5, mean ± SEM, * P <0.05, ** P <0.01, Two-tailed paired Student’s t-test).
PKCε preconditioning restores the decreased activity of GOT2 following ischemia reperfusion injury
Given that interruption of the MAS results in impaired cellular metabolism, we sought to elucidate the effects of ischemic injury on GOT2 activity and determine if enzymatic activity in this context is modulated by PKCε. Primary neurons exposed to TAT or ΨεRACK (500 nM) were subjected to oxygen and glucose deprivation (OGD) or Sham OGD for 2 hours. Activity of GOT2 was assessed 12 hours following this treatment. The results revealed decreased activity of GOT2 (11.7%, P<0.05) in the TAT-treated OGD group compared to the TAT-treated Sham OGD group. PKCε preconditioning was able to rescue the decreased GOT2 activity. PKCε-treated OGD neurons exhibited higher GOT2 activity (12.26%, P<0.05) (Fig. 6a) compared to the TAT-treated OGD neurons and was statistically similar to sham OGD, reflecting the protection induced by PKCε activation.
Discussion
Our study identifies a novel protective mechanism of PKCε-mediated regulation of the MAS. PKCε preconditioning promotes cellular metabolism by increasing mitochondrial respiration and anaerobic glycolysis. This is likely through the activation of the MAS as reflected by increased phosphorylation and enzyme activity of some of the MAS component(s). Specifically, PKCε activation increased the phosphorylation and activity of GOT2. Finally, PKCε preconditioning restored the decreased activity of GOT2 caused by ischemia-reperfusion injury.
Cerebral ischemia induces profound disturbances in metabolic pathways. Sustained ischemia leads to the deprivation of intracellular glucose and oxygen, which results in the inhibition of glycolysis and mitochondrial respiration. One of the many protective roles of preconditioning is to prevent ATP depletion following ischemic injury. Indeed, resveratrol, a polyphenolic compound that mimics ischemic preconditioning, was shown to increase the glycolytic rate and protect against ischemia-induced ATP depletion [18, 19]. In addition, our lab previously reported that PKCε increases the phosphorylation of proteins of the mitochondrial electron transport chain and enhances mitochondrial respiration in synaptosomes from preconditioned animals [7], suggesting preconditioning improves the capability of oxidative phosphorylation. Consistent with these findings, we observed increased oxygen consumption and glycolysis after PKCε preconditioning in primary neurons. In addition, PKCε preconditioning increased mitochondrial respiration linked to ATP production. This regulation is likely crucial for protection against cerebral ischemia given the susceptibility of the brain to energy derangements.
Our phosphoproteomic analysis and Western blot results show increased serine phosphorylation, with no significant changes in the threonine and tyrosine phosphorylation of MAS components. The increased serine phosphorylation may result from direct phosphorylation by PKCε, or indirect phosphorylation via PKCε-induced activation of other kinases. For example, serine/threonine kinase mitogen-activated protein kinase (MAPK) is one of the downstream transducers of PKCε preconditioning. It was shown that preconditioning induced protection is at least, partly, mediated by PKCε dependent regulation of MAPK/ERK [29, 30], MAPK/JNK [31–33] and p38 MAPK [31–33]. Tyrosine kinase Lck, a member of the Src family, is another downstream component of PKCε signaling [34–36]. In cardiac cells, PKCε phosphorylates, activates, and forms a complex with Lck, which is required for the cardiac protection induced by preconditioning [35]. Similarly, in primary neurons and a mouse model of cerebral focal ischemia, it was shown that the PKCε-Lck axis is a key mediator of ischemic preconditioning induced neuroprotection [36].
Interestingly, MAS is highly expressed in neurons, with relatively low expression in astrocytes [14]. The loss of the aspartate glutamate carrier (AGC, a MAS component) results in a significant decrease in oxygen consumption in neurons [16]. In contrast, AGC-deficient astrocytes display no differences in mitochondrial respiration [37], indicating the low activity of the MAS in astrocytes. In line with these findings, we did not observe metabolic alterations regarding oxygen consumption and glycolytic rate in primary astrocytes (Supplementary Fig. 2). This may be due to cell-specific effects mediated by PKCε that elicit different mechanisms of protection in neurons and astrocytes [38]. We did not observe significant changes in mRNA expression of the MAS components in neurons. In the heart of transgenic mice overexpressing PKCε, there is increased protein expression of MAS components (MDH1 and GOT1) [24], suggesting PKCε may increase MDH1 and GOT1 at the transcriptional level in the heart. This discrepancy may reflect tissue specific regulation of PKCε on the MAS or a difference between the constitutive activation of PKCε by transgenic overexpression and a transient preconditioning treatment as implemented in our study. Still, these findings support a regulatory effect on the MAS by the PKCε signaling pathway.
To characterize the contributions of the MAS in cellular metabolism, we used a pharmacological inhibitor, AOA. Although AOA is a non-specific inhibitor of amino acid transaminases, it has been shown to inhibit the MAS [27, 28] and has been used for inhibition of the MAS in neurons [39, 40], as well as in different types of cells [41–44]. Our data show that inhibition of the MAS decreases oxygen consumption and glycolysis, demonstrating the MAS plays a pivotal role in cellular metabolism. Importantly, deficiency of the MAS was reported in ischemic stroke. In post-mortem brain tissue from patients who suffered from ischemia, proteins that participate in the MAS were downregulated in the infarcted samples compared to the controls [45]. Therefore, dysregulation of the MAS may account for the failure of energy metabolism following cerebral ischemia.
Consistent with previous findings of MAS deficiency following ischemic stroke, we also observed reduced activity of GOT2 following ischemic injury in primary neurons. As an essential component of the MAS, GOT2 is also implicated in cerebral ischemia. As mentioned above, GOT2 was decreased in infarcted brain tissue compared to non-infarcted controls [45]. On the contrary, overexpression or activation of GOT in mice improved the anatomical and functional outcomes after stroke [46, 47]. Here, we found that PKCε preconditioning increased the activity of GOT2, an effect that was lost by the blockade of PKCε-mediated phosphorylation of the enzyme. Furthermore, PKCε preconditioning alleviated the decreased activity of GOT2 after ischemic injury, suggesting PKCε-mediates post-ischemic protective mechanisms against the dysfunctional MAS. This is likely to restore cellular ATP production and protect against the energy crisis following ischemic injury. Indeed, previous findings highlighted an essential role of the MAS during the reperfusion phase. Inhibition of the MAS during reperfusion was shown to disrupt recovery and block preconditioning-induced protection in heart [48]. Whether restoration of MAS activity after ischemic injury is related to altered cerebral blood flow (CBF) remains undefined. Previous studies suggest that PKCε preconditioning may play different roles in the regulation of CBF in reperfusion. Our lab previously showed that, in rats undergoing global cerebral ischemia, PKCε preconditioning resulted in a significant reduction in CBF during the reperfusion phase [49]. However, in a mouse model of focal cerebral ischemia, we observed that PKCε preconditioning did not change the CBF during reperfusion [50]. Consistent with the latter finding, another study showed that there were no significant differences in CBF between PKCε-null mice and control mice during the reperfusion phase [51]. These discrepancies may derive from the disparities of cerebral ischemic models used in these experiments.
Although PKCε preconditioned neurons exhibit an increased trend in the phosphorylation of GOT1 (Supplementary Fig. 3), we did not observe a significant change in the enzyme activity (Supplementary Fig. 4). This may reflect differential regulation of GOT1 and GOT2 that has been reported previously. Posttraumatic injury of the hippocampus gave rise to a significant decrease in the expression of both GOT1 and GOT2 in the dentate gyrus [56]. However, in the CA1 region, only protein expression of GOT2 was significantly reduced [56]. In addition, the enzyme activity of GOT2, but not GOT1, was significantly decreased in ischemic and hypoxic rat retinas [57]. In contrast, hyperoxia induced a significant increase in the expression of GOT1 in the brain while GOT2 remained unaltered [58]. Since previous studies reported an aspartate-depleting role of GOT1 and an aspartate-producing role of GOT2 [59, 60], our results may indicate increased biosynthesis of aspartate, a metabolite that has been shown to affect mitochondrial function [61]. Whether PKCε preconditioning increases aspartate production warrants future investigation. It is also noteworthy that the MAS is composed of multiple proteins including cytosolic and mitochondrial enzymes as well as mitochondrial carriers. The shuttle shares substrates with glycolysis in the cytosol and the tricarboxylic acid (TCA) cycle in the mitochondria. Therefore, it is difficult to dissect out the regulation of the MAS comprehensively. Future techniques that allow live assessments of specific changes in cytosolic and mitochondrial metabolites may allow for better characterization of the dynamic flux of the MAS.
In the synaptosomes from rat cortices, we observed increased phosphorylation of OGC after PKCε activation. Whether PKCε-induced phosphorylation of OGC is functionally important and whether OGC is essential in PKCε-mediated protection remain to be determined. OGC is a mitochondrial carrier protein that exchanges malate and α-ketoglutarate across the inner mitochondrial membrane [52]. In addition to its important roles in the MAS, it is also part of the oxoglutarate/isocitrate shuttle, which transports the reducing equivalents of NADPH from mitochondria into the cytosol [53]. Considering that OGC is involved in the transports of these important equivalents of NAD and NADP, it is tempting to speculate that OGC plays important roles in ischemic preconditioning. Future studies from our lab will address these potential mechanisms.
A caveat in our study was that we utilized an in vitro model of ischemic injury (OGD). This approach enables us to control external factors and study direct effects on the MAS by PKCε activation. In addition, the in vitro culture of primary neurons allows us to determine cell-specific roles of PKCε and further dissect out specific mechanisms. However, we are cognizant that in vitro models do not fully recapitulate the structure and network complexity that exist in vivo, which may involve several different mechanisms. Therefore, in vivo studies in animal models are needed to facilitate a better understanding of PKCε-mediated regulation of the MAS.
A final note about clinical relevance and the clinical translation of ischemic preconditioning, which continues to be a promising therapeutic strategy. A meta-analysis of randomized controlled trials showed that remote ischemic conditioning (RIC) significantly reduced the incidence of stroke and improved post-stroke patient outcomes [62]. In addition, in patients undergoing carotid artery surgeries, the volume and incidence of post-surgery brain lesions was significantly decreased in the RIC group [63]. Future studies will need to evaluate the possibility of using PKCε peptide agonists (i.e., ΨεRACK), as the one used in our study, as a potential therapeutic strategy against stroke. Furthermore, the clinical efficacy of other direct modulators of GOT2 remains to be evaluated. Previous studies in mice showed that GOT overexpression reduced ischemic stroke lesion volumes and improved poststroke sensorimotor functions [46]. Thus, agents like ΨεRACK or any other direct activator of GOT2 may prove to be a novel therapeutic agent against stroke. Finally, although GOT 2 is a promising target, more basic research is needed to further characterize other MAS components in order to elucidate its translational application.
In summary, our study illustrates a novel neuroprotective mechanism whereby PKCε increases the activity of GOT2 in the MAS. We show that PKCε phosphorylates proteins in the MAS, which is crucial for cellular metabolism. These findings lend support for protective mechanisms of the MAS, the NAD+/NADH shuttle, as a potential therapeutic intervention to protect against energy failure from cerebral ischemia. More broadly, our study supports future investigation into the MAS in other neurological disorders such as cerebrovascular and neurodegenerative diseases, in which cellular metabolic stress is commonly involved in the pathogenesis.
Supplementary Material
Acknowledgments
We would like to thank Clemer Abad for his assistance with the real-time PCR experiments.
Funding
This work was supported by the National Institutes of Health (NIH)/ National Institute of Neurological Disorders and Stroke (NINDS) grants NS45676, NS097658, and NS34773 (to M.A.P.P.), and the American Heart Association (AHA) predoctoral award 19PRE34400074 (to J.X.).
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Compliance with Ethics Requirements
All institutional and national guidelines for the care and use of laboratory animals were followed. Animal usage and experimentation were approved by the Institutional Animal Care and Use Committee at the University of Miami and was in accordance with the US Public Health Service’s Policy on Humane Care and Use of Laboratory Animals.
Conflict of Interest
The authors declare that they have no conflict of interest.
The authors declare no potential conflicts of interest.
Reference
- 1.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation. 2019;139(10):e56–e66. 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 2.Jackson CW, Escobar I, Xu J, Perez-Pinzon MA. Effects of ischemic preconditioning on mitochondrial and metabolic neruoprotection: 5’ adenosine monophosphate-activated protein kinase and sirtuins. Brain Circ. 2018;4(2):54–61. 10.4103/bc.bc_7_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kristin V. Ischemic conditioning in organ transplantation. Cond Med. 2018;1(4):212–9. [Google Scholar]
- 4.Cuomo Ornella, Vinciguerra Antonio, Cepparulo Pasquale, Anzilotti Serenella, Brancaccio Paola, Formisano Luigi et al. Differences and similarities in neuroprotective molecular pathways activated by distinct preconditioning inducers. Cond Med. 2018;1(4):187–203. [Google Scholar]
- 5.Raval AP, Dave KR, Mochly-Rosen D, Sick TJ, Perez-Pinzon MA. Epsilon PKC is required for the induction of tolerance by ischemic and NMDA-mediated preconditioning in the organotypic hippocampal slice. J Neurosci. 2003;23(2):384–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li J, Niu C, Han S, Zu P, Li H, Xu Q et al. Identification of protein kinase C isoforms involved in cerebral hypoxic preconditioning of mice. Brain Res. 2005;1060(1–2):62–72. 10.1016/j.brainres.2005.08.047. [DOI] [PubMed] [Google Scholar]
- 7.Dave KR, DeFazio RA, Raval AP, Torraco A, Saul I, Barrientos A et al. Ischemic preconditioning targets the respiration of synaptic mitochondria via protein kinase C epsilon. J Neurosci. 2008;28(16):4172–82. 10.1523/JNEUROSCI.5471-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Selvaraji S, Poh L, Natarajan V, Mallilankaraman K, Arumugam TV. Negative conditioning of mitochondrial dysfunction in age-related neurodegenerative diseases. Cond Med. 2019;2(1):30–9. [PMC free article] [PubMed] [Google Scholar]
- 9.Bastian C, Politano S, Day J, McCray A, Brunet S, Baltan S. Mitochondrial dynamics and preconditioning in white matter. Cond Med. 2018;1(2):64–72. [PMC free article] [PubMed] [Google Scholar]
- 10.Owens K, Park JH, Schuh R, Kristian T. Mitochondrial dysfunction and NAD(+) metabolism alterations in the pathophysiology of acute brain injury. Transl Stroke Res. 2013;4(6):618–34. 10.1007/s12975-013-0278-x. [DOI] [PubMed] [Google Scholar]
- 11.Morris-Blanco KC, Cohan CH, Neumann JT, Sick TJ, Perez-Pinzon MA. Protein kinase C epsilon regulates mitochondrial pools of Nampt and NAD following resveratrol and ischemic preconditioning in the rat cortex. J Cereb Blood Flow Metab. 2014;34(6):1024–32. 10.1038/jcbfm.2014.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khoury N, Koronowski KB, Young JI, Perez-Pinzon MA. The NAD(+)-Dependent Family of Sirtuins in Cerebral Ischemia and Preconditioning. Antioxid Redox Signal. 2018;28(8):691–710. 10.1089/ars.2017.7258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lehninger AL. Phosphorylation coupled to oxidation of dihydrodiphosphopyridine nucleotide. J Biol Chem. 1951;190(1):345–59. [PubMed] [Google Scholar]
- 14.McKenna MC, Waagepetersen HS, Schousboe A, Sonnewald U. Neuronal and astrocytic shuttle mechanisms for cytosolic-mitochondrial transfer of reducing equivalents: current evidence and pharmacological tools. Biochem Pharmacol. 2006;71(4):399–407. 10.1016/j.bcp.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 15.Lee CF, Chavez JD, Garcia-Menendez L, Choi Y, Roe ND, Chiao YA et al. Normalization of NAD+ Redox Balance as a Therapy for Heart Failure. Circulation. 2016;134(12):883–94. 10.1161/CIRCULATIONAHA.116.022495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Llorente-Folch I, Rueda CB, Amigo I, del Arco A, Saheki T, Pardo B et al. Calcium-regulation of mitochondrial respiration maintains ATP homeostasis and requires ARALAR/AGC1-malate aspartate shuttle in intact cortical neurons. J Neurosci. 2013;33(35):13957–71, 10.1523/JNEUROSCI.0929-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pardo B, Contreras L, Serrano A, Ramos M, Kobayashi K, Iijima M et al. Essential role of aralar in the transduction of small Ca2+ signals to neuronal mitochondria. J Biol Chem. 2006;281(2):1039–47. 10.1074/jbc.M507270200. [DOI] [PubMed] [Google Scholar]
- 18.Koronowski KB, Khoury N, Saul I, Loris ZB, Cohan CH, Stradecki-Cohan HM et al. Neuronal SIRT1 (Silent Information Regulator 2 Homologue 1) Regulates Glycolysis and Mediates Resveratrol-Induced Ischemic Tolerance. Stroke. 2017;48(11):3117–25. 10.1161/STROKEAHA.117.018562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khoury N, Xu J, Stegelmann SD, Jackson CW, Koronowski KB, Dave KR et al. Resveratrol Preconditioning Induces Genomic and Metabolic Adaptations within the Long-Term Window of Cerebral Ischemic Tolerance Leading to Bioenergetic Efficiency. Mol Neurobiol. 2018. 10.1007/s12035-018-1380-6. [DOI] [PMC free article] [PubMed]
- 20.Koronowski KB, Khoury N, Morris-Blanco KC, Stradecki-Cohan HM, Garrett TJ, Perez-Pinzon MA. Metabolomics Based Identification of SIRT5 and Protein Kinase C Epsilon Regulated Pathways in Brain. Front Neurosci. 2018;12:32 10.3389/fnins.2018.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dimauro I, Pearson T, Caporossi D, Jackson MJ. A simple protocol for the subcellular fractionation of skeletal muscle cells and tissue. BMC Res Notes. 2012;5:513 10.1186/1756-0500-5-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inagaki K, Churchill E, Mochly-Rosen D. Epsilon protein kinase C as a potential therapeutic target for the ischemic heart. Cardiovasc Res. 2006;70(2):222–30. 10.1016/j.cardiores.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 23.Bright R, Mochly-Rosen D. The role of protein kinase C in cerebral ischemic and reperfusion injury. Stroke. 2005;36(12):2781–90. 10.1161/01.STR.0000189996.71237.f7. [DOI] [PubMed] [Google Scholar]
- 24.Mayr M, Liem D, Zhang J, Li X, Avliyakulov NK, Yang JI et al. Proteomic and metabolomic analysis of cardioprotection: Interplay between protein kinase C epsilon and delta in regulating glucose metabolism of murine hearts. J Mol Cell Cardiol. 2009;46(2):268–77. 10.1016/j.yjmcc.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Budas GR, Churchill EN, Disatnik MH, Sun L, Mochly-Rosen D. Mitochondrial import of PKCepsilon is mediated by HSP90: a role in cardioprotection from ischaemia and reperfusion injury. Cardiovasc Res. 2010;88(1):83–92. 10.1093/cvr/cvq154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thompson JW, Dave KR, Saul I, Narayanan SV, Perez-Pinzon MA. Epsilon PKC increases brain mitochondrial SIRT1 protein levels via heat shock protein 90 following ischemic preconditioning in rats. PLoS One. 2013;8(9):e75753 10.1371/journal.pone.0075753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bunger R, Glanert S, Sommer O, Gerlach E. Inhibition by (aminooxy)acetate of the malate-aspartate cycle in the isolated working guinea pig heart. Hoppe Seylers Z Physiol Chem. 1980;361(6):907–14. [DOI] [PubMed] [Google Scholar]
- 28.Kauppinen RA, Sihra TS, Nicholls DG. Aminooxyacetic acid inhibits the malate-aspartate shuttle in isolated nerve terminals and prevents the mitochondria from utilizing glycolytic substrates. Biochim Biophys Acta. 1987;930(2):173–8. [DOI] [PubMed] [Google Scholar]
- 29.Kim EJ, Raval AP, Hirsch N, Perez-Pinzon MA. Ischemic preconditioning mediates cyclooxygenase-2 expression via nuclear factor-kappa B activation in mixed cortical neuronal cultures. Transl Stroke Res. 2010;1(1):40–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lange-Asschenfeldt C, Raval AP, Dave KR, Mochly-Rosen D, Sick TJ, Perez-Pinzon MA. Epsilon protein kinase C mediated ischemic tolerance requires activation of the extracellular regulated kinase pathway in the organotypic hippocampal slice. J Cereb Blood Flow Metab. 2004;24(6):636–45. 10.1097/01.WCB.0000121235.42748.BF. [DOI] [PubMed] [Google Scholar]
- 31.Baines CP, Zhang J, Wang GW, Zheng YT, Xiu JX, Cardwell EM et al. Mitochondrial PKCepsilon and MAPK form signaling modules in the murine heart: enhanced mitochondrial PKCepsilon-MAPK interactions and differential MAPK activation in PKCepsilon-induced cardioprotection. Circ Res. 2002;90(4):390–7. 10.1161/01.res.0000012702.90501.8d. [DOI] [PubMed] [Google Scholar]
- 32.Jung YS, Jung YS, Kim MY, Kim E. Identification of caspase-independent PKCepsilon-JNK/p38 MAPK signaling module in response to metabolic inhibition in H9c2 cells. Jpn J Physiol. 2004;54(1):23–9. [DOI] [PubMed] [Google Scholar]
- 33.Ping P, Zhang J, Huang S, Cao X, Tang XL, Li RC et al. PKC-dependent activation of p46/p54 JNKs during ischemic preconditioning in conscious rabbits. Am J Physiol. 1999;277(5):H1771–85. 10.1152/ajpheart.1999.277.5.H1771. [DOI] [PubMed] [Google Scholar]
- 34.Ping P, Zhang J, Zheng YT, Li RC, Dawn B, Tang XL et al. Demonstration of selective protein kinase C-dependent activation of Src and Lck tyrosine kinases during ischemic preconditioning in conscious rabbits. Circ Res. 1999;85(6):542–50. 10.1161/01.res.85.6.542. [DOI] [PubMed] [Google Scholar]
- 35.Ping P, Song C, Zhang J, Guo Y, Cao X, Li RC et al. Formation of protein kinase C(epsilon)-Lck signaling modules confers cardioprotection. J Clin Invest. 2002;109(4):499–507. 10.1172/JCI13200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bae ON, Rajanikant K, Min J, Smith J, Baek SH, Serfozo K et al. Lymphocyte cell kinase activation mediates neuroprotection during ischemic preconditioning. J Neurosci. 2012;32(21):7278–86. 10.1523/JNEUROSCI.6273-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Juaristi I, Garcia-Martin ML, Rodrigues TB, Satrustegui J, Llorente-Folch I, Pardo B. ARALAR/AGC1 deficiency, a neurodevelopmental disorder with severe impairment of neuronal mitochondrial respiration, does not produce a primary increase in brain lactate. J Neurochem. 2017;142(1):132–9. 10.1111/jnc.14047. [DOI] [PubMed] [Google Scholar]
- 38.Wang J, Bright R, Mochly-Rosen D, Giffard RG. Cell-specific role for epsilon- and betaI-protein kinase C isozymes in protecting cortical neurons and astrocytes from ischemia-like injury. Neuropharmacology. 2004;47(1):136–45. 10.1016/j.neuropharm.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 39.Mali Y, Zisapel N. VEGF up-regulation by G93A superoxide dismutase and the role of malate-aspartate shuttle inhibition. Neurobiol Dis. 2010;37(3):673–81. 10.1016/j.nbd.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 40.McKenna MC, Tildon JT, Stevenson JH, Boatright R, Huang S. Regulation of energy metabolism in synaptic terminals and cultured rat brain astrocytes: differences revealed using aminooxyacetate. Dev Neurosci. 1993;15(3–5):320–9. 10.1159/000111351. [DOI] [PubMed] [Google Scholar]
- 41.Chouchani ET, Pell VR, Gaude E, Aksentijevic D, Sundier SY, Robb EL et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature. 2014;515(7527):431–5. 10.1038/nature13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stottrup NB, Lofgren B, Birkler RD, Nielsen JM, Wang L, Caldarone CA et al. Inhibition of the malate-aspartate shuttle by pre-ischaemic aminooxyacetate loading of the heart induces cardioprotection. Cardiovasc Res. 2010;88(2):257–66. 10.1093/cvr/cvq205. [DOI] [PubMed] [Google Scholar]
- 43.Dalgas C, Povlsen JA, Lofgren B, Erichsen SB, Botker HE. Effects of fatty acids on cardioprotection by pre-ischaemic inhibition of the malate-aspartate shuttle. Clin Exp Pharmacol Physiol. 2012;39(10):878–85. 10.1111/j.1440-1681.2012.05749.x. [DOI] [PubMed] [Google Scholar]
- 44.Mitchell M, Cashman KS, Gardner DK, Thompson JG, Lane M. Disruption of mitochondrial malate-aspartate shuttle activity in mouse blastocysts impairs viability and fetal growth. Biol Reprod. 2009;80(2):295–301. 10.1095/biolreprod.108.069864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Datta A, Akatsu H, Heese K, Sze SK. Quantitative clinical proteomic study of autopsied human infarcted brain specimens to elucidate the deregulated pathways in ischemic stroke pathology. J Proteomics. 2013;91:556–68. 10.1016/j.jprot.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 46.Rink C, Gnyawali S, Stewart R, Teplitsky S, Harris H, Roy S et al. Glutamate oxaloacetate transaminase enables anaplerotic refilling of TCA cycle intermediates in stroke-affected brain. FASEB J. 2017;31(4):1709–18. 10.1096/fj.201601033R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Campos F, Sobrino T, Ramos-Cabrer P, Argibay B, Agulla J, Perez-Mato M et al. Neuroprotection by glutamate oxaloacetate transaminase in ischemic stroke: an experimental study. J Cereb Blood Flow Metab. 2011;31(6):1378–86. 10.1038/jcbfm.2011.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lofgren B, Povlsen JA, Rasmussen LE, Stottrup NB, Solskov L, Krarup PM et al. Amino acid transamination is crucial for ischaemic cardioprotection in normal and preconditioned isolated rat hearts--focus on L-glutamate. Exp Physiol. 2010;95(1):140–52. 10.1113/expphysiol.2009.049452. [DOI] [PubMed] [Google Scholar]
- 49.Della-Morte D, Raval AP, Dave KR, Lin HW, Perez-Pinzon MA. Post-ischemic activation of protein kinase C epsilon protects the hippocampus from cerebral ischemic injury via alterations in cerebral blood flow. Neurosci Lett. 2011;487(2):158–62. 10.1016/j.neulet.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morris-Blanco KC, Dave KR, Saul I, Koronowski KB, Stradecki HM, Perez-Pinzon MA. Protein Kinase C Epsilon Promotes Cerebral Ischemic Tolerance Via Modulation of Mitochondrial Sirt5. Sci Rep. 2016;6:29790 10.1038/srep29790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kumar V, Weng YC, Wu YC, Huang YT, Liu TH, Kristian T et al. Genetic inhibition of PKCepsilon attenuates neurodegeneration after global cerebral ischemia in male mice. J Neurosci Res. 2019;97(4):444–55. 10.1002/jnr.24362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fiermonte G, Walker JE, Palmieri F. Abundant bacterial expression and reconstitution of an intrinsic membrane-transport protein from bovine mitochondria. Biochem J. 1993;294 ( Pt 1):293–9. 10.1042/bj2940293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Monne M, Miniero DV, Iacobazzi V, Bisaccia F, Fiermonte G. The mitochondrial oxoglutarate carrier: from identification to mechanism. J Bioenerg Biomembr. 2013;45(1–2):1–13. 10.1007/s10863-012-9475-7. [DOI] [PubMed] [Google Scholar]
- 54.Rathore R, Zheng YM, Niu CF, Liu QH, Korde A, Ho YS et al. Hypoxia activates NADPH oxidase to increase [ROS]i and [Ca2+]i through the mitochondrial ROS-PKCepsilon signaling axis in pulmonary artery smooth muscle cells. Free Radic Biol Med. 2008;45(9):1223–31. 10.1016/j.freeradbiomed.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bell RM, Cave AC, Johar S, Hearse DJ, Shah AM, Shattock MJ. Pivotal role of NOX-2-containing NADPH oxidase in early ischemic preconditioning. FASEB J. 2005;19(14):2037–9. 10.1096/fj.04-2774fje. [DOI] [PubMed] [Google Scholar]
- 56.Cole JT, Mitala CM, Kundu S, Verma A, Elkind JA, Nissim I et al. Dietary branched chain amino acids ameliorate injury-induced cognitive impairment. Proc Natl Acad Sci U S A. 2010;107(1):366–71. 10.1073/pnas.0910280107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Endo S, Ishiguro S, Tamai M. Possible mechanism for the decrease of mitochondrial aspartate aminotransferase activity in ischemic and hypoxic rat retinas. Biochim Biophys Acta. 1999;1450(3):385–96. [DOI] [PubMed] [Google Scholar]
- 58.Hinkelbein J, Feldmann RE Jr., Kalenka A. Time-dependent alterations of cerebral proteins following short-term normobaric hyperoxia. Mol Cell Biochem. 2010;339(1–2):9–21. 10.1007/s11010-009-0365-1. [DOI] [PubMed] [Google Scholar]
- 59.Allen EL, Ulanet DB, Pirman D, Mahoney CE, Coco J, Si Y et al. Differential Aspartate Usage Identifies a Subset of Cancer Cells Particularly Dependent on OGDH. Cell Rep. 2016;17(3):876–90. 10.1016/j.celrep.2016.09.052. [DOI] [PubMed] [Google Scholar]
- 60.Son J, Lyssiotis CA, Ying H, Wang X, Hua S, Ligorio M et al. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature. 2013;496(7443):101–5. 10.1038/nature12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Birsoy K, Wang T, Chen WW, Freinkman E, Abu-Remaileh M, Sabatini DM. An Essential Role of the Mitochondrial Electron Transport Chain in Cell Proliferation Is to Enable Aspartate Synthesis. Cell. 2015;162(3):540–51. 10.1016/j.cell.2015.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao JJ, Xiao H, Zhao WB, Zhang XP, Xiang Y, Ye ZJ et al. Remote Ischemic Postconditioning for Ischemic Stroke: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Chin Med J (Engl). 2018;131(8):956–65. 10.4103/0366-6999.229892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhao W, Meng R, Ma C, Hou B, Jiao L, Zhu F et al. Safety and Efficacy of Remote Ischemic Preconditioning in Patients With Severe Carotid Artery Stenosis Before Carotid Artery Stenting: A Proof-of-Concept, Randomized Controlled Trial. Circulation. 2017;135(14):1325–35. 10.1161/CIRCULATIONAHA.116.024807. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.