Abstract
Tropical canopies are complex, with multiple canopy layers and pronounced gap dynamics contributing to their high species diversity and productivity. An important reason for this complexity is the large variation in shade tolerance among different tree species. At present, we lack a clear understanding of which plant traits control this variation, e.g., regarding the relative contributions of whole-plant versus leaf traits or structural versus physiological traits. We investigated a broad range of traits in six tropical montane rainforest tree species with different degrees of shade tolerance, grown under three different radiation regimes (under the open sky or beneath sparse or dense canopies). The two distinct shade-tolerant species had higher fractional biomass in leaves and branches while shade-intolerant species invested more into stems, and these differences were greater under low radiation. Leaf respiration and photosynthetic light compensation point did not vary with species shade tolerance, regardless of radiation regime. Leaf temperatures in open plots were markedly higher in shade-tolerant species due to their low transpiration rates and large leaf sizes. Our results suggest that interspecific variation in shade tolerance of tropical montane trees is controlled by species differences in whole-plant biomass allocation strategy rather than by difference in physiological leaf traits determining leaf carbon balance at low radiation.
Keywords: biomass allocation, leaf temperature, plant traits, Rwanda, shade intolerance, shade tolerance, tropical montane forest
Introduction
Tropical forests host a high diversity of species and are extremely productive ecosystems, accounting for about one-third of global terrestrial gross primary production (Dirzo and Raven 2003, Lewis 2006, Beer et al. 2010, Malhi et al. 2013). One reason for the high diversity and productivity of tropical forests is that they harbor tree species with strongly varying degrees of shade tolerance, forming complex canopies with multiple layers and pronounced gap dynamics (Osunkoya et al. 1994, Gommers et al. 2013, Valladares et al. 2016). Shade tolerance has been attributed to many different plant traits that maximize carbon gain and/or increase stress tolerance and survival rates under low light. At present, however, we lack a clear understanding of which traits are most important in controlling interspecific variation in shade tolerance (Valladares and Niinemets 2008, Valladares et al. 2016). Previous studies have often focused on either leaf physiological traits or whole-plant biomass allocation and structure (Valladares and Niinemets 2008). Furthermore, it remains unclear if there is a difference in light acclimation capacity between shade-tolerant (ST) and shade-intolerant (SI) tropical tree species, with conflicting reports in the literature (Rozendaal et al. 2006, Coste et al. 2009, 2010, Houter and Pons 2014, Dusenge et al. 2015). This is partly because, in many previous studies, ST species were not grown at high light and SI species not at low light levels (Poorter et al. 2019). Moreover, traits change with age and size (Houter and Pons 2012), complicating the use of sun- and shade-leaf data from mature trees for predicting plasticity and shade-tolerance of juvenile trees. There is thus a need for new experimental studies exploring the contributions of different plant traits (i.e., leaf vs whole-plant level, and physiological vs structural) in controlling shade tolerance of tropical trees (Valladares and Niinemets 2008, Valladares et al. 2016, Poorter et al. 2019).
Two main hypotheses on the suites of traits responsible for species shade tolerance have been proposed: the carbon gain hypothesis and the stress tolerance hypothesis (Valladares and Niinemets 2008). The carbon gain hypothesis states that shade tolerance is the consequence of high light-use efficiency resulting from traits maximizing carbon gain and minimizing carbon losses in a low-light environment. Such traits include high quantum yield of photosynthesis (QY) and low leaf respiration leading to low photosynthetic light compensation point (LCP), low leaf mass per unit area (LMA) and a high fraction of biomass invested in organs contributing to light interception in the understorey (i.e., leaves and branches; Givnish 1988, Kitajima 1994, Lusk and Pozo 2002, Niinemets 2006, Baltzer and Thomas 2007b, Luttge 2008, Valladares and Niinemets 2008). The stress tolerance hypothesis attributes shade tolerance to high investments in traits that maximize the resistance to biotic and abiotic stresses in the understorey (Kitajima 1994; Gommers et al. 2013). Such traits include high concentrations of defense metabolites and high wood density and LMA. The two hypotheses are not necessarily mutually exclusive and it is plausible that both contribute to explain shade tolerance.
The carbon gain hypothesis has been very influential for our understanding of shade tolerance, but is currently under challenge. It originally predicts that ST species should have higher relative growth rates (RGR) than SI species when growing in the shade (Givnish 1988), but in most studies lower RGR was observed in ST seedlings when growing in both high and low light conditions (Kitajima 1994, Walters and Reich 1999, Kunstler et al. 2016). The carbon gain hypothesis also suggests lower LMA for ST compared with SI species to maximize light interception (Givnish 1988, Valladares and Niinemets 2008, Poorter et al. 2009), but some studies with tropical trees found the opposite (Coste et al. 2005, Kitajima 1994, Mao et al. 2014). This suggests that the conservation of carbon is more important than the efficiency of its capture (Lusk et al. 2011, Reich et al. 2003), which is more in line with the stress tolerance hypothesis. Some studies have indeed reported that plant survival in the understorey is poorly linked to whole-plant carbon gain (Kitajima 1994, Reich et al. 2003). In addition, contrary to the carbon gain hypothesis but in agreement with the stress tolerance hypothesis, some studies have indicated that ST plants invest relatively more biomass into roots than SI species when growing in the understorey (Poorter et al. 2010, Mao et al. 2014).
Leaf physiological data also sometimes conflict with the carbon gain hypothesis. The hypothesis predicts higher QY and lower respiration and LCP for ST compared with SI species (Valladares and Niinemets 2008), but Walters and Reich (1999) could not find any significant differences in these traits between the two groups. Moreover, in a study on tropical montane rainforest tree species differing in shade tolerance, higher chlorophyll content per unit leaf area in ST compared with SI species did not translate into higher apparent QY (i.e., the initial slope of the light response of net photosynthesis; Dusenge et al. 2015). This suggests that the common observation of higher chlorophyll content in ST species (Niinemets 1997, Valladares and Niinemets 2008) does not necessarily contribute to increased leaf carbon gain. All these findings show that new and more holistic studies—including side-by-side comparisons of different types of traits across species with a broad range of shade tolerance—are needed to explore which traits and strategies are most important in controlling tropical tree shade tolerance (Valladares et al. 2016).
Tropical rainforest tree species—adapted to temporally stable climatic conditions—have been suggested to have a more narrow optimum temperature range compared with tree species from more seasonal climates (Doughty and Goulden 2008, Wright et al. 2009). It has further been proposed that they operate near their thermal optimum, above which their CO2 assimilation and growth may decline (Clark et al. 2010, Way and Oren 2010). Indeed, photosynthesis was negatively affected by warming in both branch chamber experiments (Doughty 2011) and indoor growth chamber experiments (Cheesman and Winter 2013a, Slot and Winter 2017). Moreover, daytime leaf temperatures greatly exceeded both air temperature and the optimal temperature for photosynthesis in a common garden experiment with tropical tree seedlings (Vårhammar et al. 2015). This exceedance was particularly high in sun-exposed ST species with low transpiration rates and large leaf size. Controlled experiments also indicate that tropical ST species are more sensitive to warming than SI species (Doughty 2011, Cheesman and Winter 2013b). It is therefore possible, but hitherto unexplored, that high leaf temperatures result in physiological heat stress and lower competitiveness of ST species growing in high radiation environments.
The overall aim of this study was to explore which traits were most important in controlling species shade tolerance in tropical montane trees. The specific hypotheses were as follows: (i) ST species have a whole-plant biomass allocation strategy that maximizes light interception when grown in the understorey, i.e., higher relative investment into branches and leaves. (ii) ST species have physiological leaf traits that allow for a more favorable leaf carbon balance in a low-light environment, i.e., lower respiration and LCP. In addition, a third hypothesis related to high radiation intolerance was included to explore if ST species might suffer under open sky conditions: (iii) ST species have leaf traits that cause high leaf temperature (i.e., low transpiration rates and large leaf size) and consequent physiological heat stress under high sun exposure. To address these hypotheses, we investigated a broad range of traits in seedlings of six tropical montane tree species with varying degrees of shade tolerance grown under three different radiation regimes: open sky, and beneath either sparse or dense overstorey canopies. Traits measured include structural, chemical and physiological leaf traits as well as whole-plant growth and biomass allocation.
Materials and methods
Study site and environmental measurements
The experiment was conducted in the Arboretum of Ruhande (‘Arboretum’ in the following), Rwanda (altitude: 1638–1737 m; latitude 2°36′S and longitude 29°44′E). The Arboretum was established in 1933 after the relocation of the population that lived and farmed in that area (Nsabimana 2009). The plantation includes 216 tree species (169 exotic and 47 native) and covers an area of 200 ha that is subdivided into around 500 plots of 50 × 50 m, most of which are monospecific (Nsabimana et al. 2008, Dusenge et al. 2015). The plants of the present study originate from seeds collected in Nyungwe National Park (‘Nyungwe’ in the following), a tropical montane rainforest that covers an area of approximately 1000 km2 and is located in southwestern Rwanda 50–100 km west of the Arboretum (2°17′−2°50′S, 29°07′−29°26′E, 1600–2950 m altitude). The Arboretum is within the altitudinal range of all six species studied (see below).
The long-term climate at the Arboretum is characterized by small variations in monthly mean air temperature and a bimodal rain pattern, with one strong dry period from mid-June to mid-August and a less distinct dry period in January and February (Nsabimana 2009). Mean daytime and 24-h air temperatures during 2013–17 were 21.1 and 19.5 °C, respectively. The difference in mean temperature between the warmest and coldest months was 1.5 °C. Mean daytime vapor pressure deficit of the air (VPD) was 1.02 kPa, mean annual rainfall 979 mm and mean daytime photosynthetic photon flux density (PPFD) 733 μmol m−2 s−1 during the same period. At a meteorological station located at 2465 m altitude in Nyungwe, mean daytime and 24-h air temperatures were 15.6 and 14.3 °C, respectively, mean daytime VPD 0.39 kPa, mean annual rainfall 1879 mm and mean daytime PPFD 633 μmol m−2 s−1 during 2007–13. More information on meteorological conditions at both Arboretum and Nyungwe was provided by Mujawamariya et al. (2018).
In addition, a weather station (VP-3, PYR and ECRN-100, Decagon Device, Inc., Pullman, WA, USA) recorded air temperature, relative humidity, solar radiation and precipitation at another open place by the Arboretum office during the last quarter of the study. These data were used for comparisons with leaf temperature measurements (see below).
Seven Tiny Tags (Model Tinytag Plus 2, Gemini Data Loggers Ltd, Chichester, UK) recorded air temperature and relative humidity at 1.8 m in plots with different radiation regimes (six plots with different overstorey canopy transmission plus one open plot; see below) during 15 successive days in March 2016. To estimate relative radiation transmission of the overstorey in each plot, below-canopy radiation at the position of each seedling was measured with a portable quantum meter (MQ-500, Apogee Instruments, Inc., Logan, Utah, USA) during 12:00–14:00 h on two sunny days, one in the wet and one in the dry season.
Plant materials and experimental design
The six montane tree species used in this species represent common ST and SI species in Nyungwe, as judged from their abundance in forest stands at different successional stages in the forest (Nyirambangutse et al. 2017 and references therein). The ST species are all among the 20 most common species in Nyungwe, together comprising 25% of the large trees (diameter at breast height >30 cm) in the forest. The ST species, which are more abundant in late-successional stands, were Carapa grandiflora (Cg; family: Meliaceae; altitudinal range in Rwanda: 1600–2500 m), Entandrophragma excelsum (Ee; family: Meliaceae; altitudinal range in Rwanda: 1500–2100 m) and Syzygium guineense spp. parvifolium (Sg; family: Myrtaceae; altitudinal range in Rwanda: 1000–2100 m). The SI species, which are more abundant in early-successional stands, were Croton megalocarpus (Cm; family: Euphorbiaceae; altitudinal range in Rwanda: 1600–2400 m), Dombeya goetzenii (Dg; family: Malvaceae; altitudinal range in Rwanda: 1300–3000 m) and Polyscias fulva (Pf; family: Araliaceae; altitudinal range in Rwanda: 1200–2400 m). Species’ altitudinal range were taken from Bloesch et al. (2009) or Fischer and Killmann (2008). Since shade tolerance varies along a continuum, classification of species into distinct successional groups is not straightforward. While Cm and Dg are typical short-lived early-successional species, trees of Pf can grow quite large and may stay around longer after canopy closure. Although all three ST species are more abundant in late-successional stands, Sg is also quite common in early-successional stand. We therefore did not apply successional group identity (ST versus SI) in the statistical analysis (see Statistical analyses section below).
The plants, grown from seeds collected in Nyungwe, were first cultivated for 3.5 months at the Arboretum’s nursery, in small pots that were irrigated daily and contained < 1 l of clayey soil from the surrounding and added organic fertilizer. In March–April 2015, they were transplanted into 10-l pots with local clayey soil. Plants were randomly distributed to nine different plots in the Arboretum, differing in overstorey leaf area index and canopy light transmittance (Table 1). These nine plots were divided into three radiation regimes: three open plots, three plots with the overstorey consisting of rather sparse canopies of early-successional species (Dg, Cm and Prunus africana), and three plots with the overstorey consisting of dense canopies of later-successional species (Cg, Sg and Magnistipula butayei). Each species had six replicate seedlings in each plot and the total number of seedlings in the experiment was thus 324 (3 radiation regimes × 3 plots in each regime × 6 species × 6 replicates).
Table 1.
Comparison of environmental conditions of the nine plots of the study. Temperature values(Tair) are means over 15 days with simultaneous measurements in all plots. Data on PPFD were recorded between 12:00 and 14:00 h on two sunny days, one in August (dry season) and one in January (wet season).
| Treatment | Plota | T air day (°C) | T air night (°C) | PPFD, dry season, mid-day (μmol m−2 s−1) | PPFD, wet season, mid-day (μmol m−2 s−1) | Light transmission (%), on dry/wet season days |
|---|---|---|---|---|---|---|
| Open field | Open 1 | 24.2b | 16.1b | 1970 | 2059 | 100/98 |
| Open 2 | 24.2b | 16.1b | 1806 | 2044 | 95/97 | |
| Open 3 | 24.2b | 16.1b | 1990 | 1979 | 98/95 | |
| Sparse canopy | Cm | 21.2 | 18.0 | 853 | 789 | 42/38 |
| Dg | 21.3 | 15.5 | 670 | 138 | 31/7 | |
| Pa | 22.1 | 15.2 | 932 | 409 | 43/19 | |
| Dense canopy | Cg | 21.6 | 17.8 | 258 | 179 | 13/9 |
| Mb | 21.6 | 17.7 | 216 | 278 | 11/13 | |
| Sg | 21.4 | 16.6 | 233 | 117 | 11/6 |
Light transmission is the plot radiation divided by the open sky radiation at a large open area 50 m outside the Arboretum of Ruhande.
aOpen 1, 2 and 3 are the open plots; Cm, Dg and Pa are the sparse canopy plots with overstorey consisting of Croton megalocarpus, Dombeya goetzenii and Prunus africana; Cg, Mb and Sg are the dense canopy plots with overstorey consisting of Carapa grandiflora, Magnistipula butayei and Syzygium guineense.
bData from the same weather sensor.
Pots were placed 0.7 m apart to minimize shading among seedlings. Plants were regularly irrigated to assure high soil water availability throughout the study. During the 3-month dry season, seedlings in open plots were watered daily while those in sparse and dense canopy plots were watered three times per week. During the rest of the year, plants were watered regularly and as needed depending on rainfall. No fertilizer was added during the shade tolerance experiment. This may have caused increased nutrient constraints in fast-growing plants in open and sparse canopy plots. However, such constraints were probably not unrealistically large since our findings of decreased mass-based but increased area-based leaf nitrogen (N) content of plants in high radiation regimes (see below) is the typical observation also in freely rooted plants (Poorter et al. 2019).
Monthly health inspections were conducted from May 2015 to April 2016. In total, 21 out of the 324 seedlings died during the experiment. All plants were harvested in May 2016.
Gas exchange measurements and calculations
Leaf gas exchange was measured in three plants per species in each plot during February–April 2016, using a portable LI-6400 leaf gas exchange instrument (LI-COR, Lincoln, NE, USA). Two leaves per plant were measured: one for net photosynthesis (An) and one for dark respiration (Rd). The selected leaves were at least 2 months old, fully developed and had comparatively healthy appearance. The An measurements to determine photosynthetic capacity (i.e., Vcmax: maximum rate of Rubisco carboxylation, and Jmax: maximum rate of photosynthetic electron transport) were done at a PPFD of 1800 μmol m−2 s−1, an air flow of 400 μmol s−1 and the block temperature of the instrument at 25 °C. In two out of three plants per species and plot, measurements were done at three different CO2 concentrations of the air entering the leaf chamber: 410, 200 and 2000 μmol mol−1. For the third plant, photosynthesis was only measured at 410 μmol mol−1. Measurements were conducted within a few minutes after clamping onto the leaf or changing CO2 concentration, without waiting for stomatal conductance to respond to respond to altered environmental conditions. Data of An in shaded plots (and the derived Vcmax and Jmax values) are thus representative for photosynthesis during sunflecks and do not reflect values where both photosynthesis and stomata have fully responded to the high measurement radiation. The stomatal conductance data point reported is that measured at 410 μmol mol−1 CO2 concentration. The photosynthesis model by Farquhar et al. (1980), with modifications of photosynthetic temperature dependencies by Bernacchi et al. (2001), was parameterized using the one-point method (De Kauwe et al. 2016). The measurements at 200 and 2000 μmol mol−1 CO2 concentrations were used to determine Vcmax and Jmax, respectively, and tree-specific Rd data described below (i.e., dark respiration) were used in both cases. The measurements at the 410 μmol mol−1 CO2 concentration were used only for determination of light-saturated An, not for Vcmax or Jmax.
On the same leaf measured for An at different CO2 concentrations, the initial light response of An was also measured at PPFD of 25 and 75 μmol m−2 s−1, the CO2 concentration at 410 μmol mol−1 and the block temperature of the instrument at 25 °C. These data were originally planned to be used for the determination of apparent QY and LCP. However, subsequent tests with measurements at 25, 50 and 75 μmol m−2 s−1 showed that the light response was not linear in the entire PPFD range of 25–75 μmol m−2 s−1. We therefore do not report any QY results, while LCP was instead calculated by linear intrapolation between the An measurement at a PPFD of 25 μmol m−2 s−1 and Rd measured on a neighboring leaf on the same plant (see below). The method does not account for the Kok effect (Kok 1948), which is difficult to determine for large numbers of plants in the field since it requires several data points around the LCP. Although not ideal, this use of two data points from two neighboring leaves to derive LCP is not likely to cause significant systematic bias with respect to species or radiation treatments.
Before Rd measurements, leaves were dark acclimated by covering them with tinfoil for at least 30 min. This measurement was done with an air flow of 250 μmol s−1, CO2 concentration of 410 μmol m−2 s−1 and leaf chamber block temperature of 25 °C. After the measurement of each plant, an empty chamber measurement was done to allow for corrections of possible leaks and gasket diffusion.
Other leaf trait measurements
Leaf temperature was measured on all plants in all plots at 12:00–15:00 h on three sunny days, using three infrared thermometers (Model: BP 10, Trotec, Heinsberg, Germany). Three people measured simultaneously, one in each radiation regime (open sky, sparse canopy and dense canopy) and then rotating such that each person measured one plot of each regime. The infrared thermometer had a 6:1 ratio of the distance to the surface compared with the diameter of the surface area being measured. It was held about 6 cm from the leaf surface and pointed towards the leaf without shading it, aiming at the central position on one of the leaf halves for large leaves and at the middle for small leaves. The leaf was horizontally positioned (= 0°) and the angle of the infrared thermometer was held at 45° relative to the leaf.
On all leaves measured for gas exchange, leaf discs were sampled (three discs with 18 mm diameter for large leaves or five discs with 10 mm diameter for smaller leaves) for LMA determination and subsequent nutrient and stable isotope analyses. Leaf samples were oven-dried at 70 °C for at least 72 h. After determining LMA, leaf discs and remaining leaf material was ground into a fine powder with a ball mill grinder (MM 301, MM 200, Retsch, Germany). In total, 160 samples were sent for analyses of leaf N and stable carbon isotope composition using a continuous flow isotope ratio mass spectrometer (UC Davis Stable Isotope Facility, Davis, CA, USA). Carbon isotopes were used to estimate plant intrinsic WUE (iWUE). Analysis of 37 essential non-N elements was done for one combined sample per species (except Dg where there was too little leaf sample material) and plot, using inductively coupled plasma mass spectrometry (ACME Analytical Laboratories, Vancouver, Canada). Out of these non-N elements, only data for phosphorus are reported. The iWUE determined based on carbon isotope data was calculated as the difference between the ambient CO2 concentration and intracellular CO2 concentration times 1.6 to account for the difference in stomatal conductance of water vapor compared with CO2. Ambient CO2 was assumed to be 402 μmol mol−1, and intercellular CO2 was calculated using equations in Farquhar et al. (1989).
Biomass harvest
In the harvest at the end of experiment, we first measured stem diameter and height and counted the number of attached leaves in each living plant. Leaf angles of five leaves per plant at randomly selected canopy positions were also measured and leaf discs were taken from the same leaves for LMA determination and chemical analyses. A SPAD-502 meter (Konica Minolta Sensing, Inc., Ltd, Osaka, Japan) was used to measure the SPAD value (a proxy of area-based leaf chlorophyll content) of one fully developed, healthy and at least 2-month-old leaf per plant. Stem base diameter and height were measured using a ruler and digital caliper, respectively. The seedlings were then harvested and divided into roots, main stem, branches, petioles and leaves. Plant parts were oven-dried at 70 °C until constant mass. Wood density of dry wood was determined based on measurements of mass and volume on a piece of a dry stem without bark from each plant.
In addition, eight plants per species which were not included in the main experiment were harvested in April 2015 to determine the initial biomass of different plant parts at the beginning of the experiment. The mean total initial dry mass ranged from 5 g to 14 g in the six species (Table S1 available as Supplementary Data at Tree Physiology Online).
Statistical analyses
Data were statistically analyzed using analysis of variance (ANOVA) with radiation regime as a fixed factor and plot and species as random factors. The linear model of the experiment was the following:
y = μ + Ri + Sj + P(R)k(i) + RSij + SP(R)jk(i) + el(ijk),
where y is the response variable, μ is the intercept, R is radiation regime, S is species identity, P is plot, e is individual random variation and the subscripts indicate the levels of each factor. Plots were nested within radiation regimes and plant replication for each species in a plot was six. With the experimental design used, F ratios for the effects of Sj, P(R)k(i) and RSij were calculated using SP(R)jk(i) as the error term, while the F ratio for SP(R)jk(i) was calculated using el(ijk) as the error term (Underwood 1997). Significant species by radiation interactions were found for most variables, and main effects of radiation were tested by simple one-way ANOVA within each species individually. Effects were considered statistically significant at P ≤ 0.05.
Results
Environmental conditions
Daytime temperatures at 1.8 m above ground were 2–3 °C lower in both sparse and dense canopy plots compared with open plots (Table 1). Nighttime temperatures were on average 1.1–1.3 °C higher in dense canopy plots compared with open or sparse canopy plots. Nighttime temperature was lowest in two of the sparse canopy plots as a result of their lower position in the Arboretum area, just at the end of a downhill slope. On average, across two measurement days, dense canopy plots had 10% midday canopy transmission while sparse canopy plots had 30%. Daytime measurements made during the dry season in August showed marked differences in canopy transmission between all sparse versus dense canopy plots. In the wetter season in January, however, one of the sparse canopy plots (with Dg as overstorey species) had canopy transmission comparable to that in dense canopy plots. This difference between August and January was a result of high seasonality in both leaf shedding and leaf production in Dg. On average across the two measurement days, however, the Dg sparse canopy plot had higher canopy transmission than those in all three dense canopy plots.
Biomass, allocation and health inspections
All tree biomass and structure parameters exhibited significant (i.e., P ≤ 0.05) or, in two cases, near-significant (0.050 < P ≤ 0.068) species by radiation interactions (Table 2), meaning that effects of shade were species-dependent. The radiation regime had a significant influence on total tree biomass at harvest (Figure 1a) and RGR (Figure 1b) in all species. However, the reduction in RGR in dense canopy compared with open plots was smallest in the ST species Ee (34%) and greatest in the SI species Cm (95%). In the other four species, the RGR reductions were intermediate but somewhat larger in the SI species Pf (59%) and Dg (52%) than in the ST species Sg (49%) and Cg (46%).
Table 2.
P values for effects on different plant traits according to the ANOVA. Brackets indicate that the preceding factor is nested inside the factor within brackets; × indicate interacting effects of the preceding and following factors.
| Plant trait | P values for different sources of variation | |||
|---|---|---|---|---|
| Species | Plot (radiation) | Species × radiation | Species × plot (radiation) | |
| Tree size and structure | ||||
| Total | 0.001 | 0.199 | 0.008 | 0.0001 |
| Leaves | <0.0001 | 0.008 | 0.002 | 0.001 |
| Branches + petioles | <0.001 | 0.232 | 0.002 | 0.253 |
| Stem | 0.268 | 0.489 | 0.006 | 0.001 |
| Roots | 0.023 | 0.005 | 0.015 | <0.0001 |
| RGR | <0.0001 | 0.183 | 0.052 | <0.0001 |
| % leaves | <0.0001 | 0.004 | 0.068 | <0.0001 |
| % branches + petioles | 0.076 | 0.014 | 0.029 | 0.127 |
| % stem | <0.0001 | 0.355 | 0.003 | <0.0001 |
| % roots | 0.002 | 0.0001 | 0.007 | 0.029 |
| Root:shoot ratio | 0.002 | 0.001 | 0.012 | 0.014 |
| Stem diameter | <0.0001 | 0.158 | 0.030 | 0.014 |
| Stem height | <0.0001 | 0.087 | 0.020 | 0.0002 |
| Stem height:diameter ratio | <0.0001 | 0.084 | 0.048 | <0.0001 |
| Height increment | <0.0001 | 0.036 | 0.001 | 0.001 |
| Canopy leaf number | <0.001 | 0.006 | 0.0001 | 0.002 |
| Wood density | <0.0001 | 0.251 | 0.003 | 0.351 |
| Leaf structure | ||||
| LMA | <0.001 | <0.001 | <0.001 | 0.007 |
| Leaf mass | <0.001 | 0.140 | 0.0004 | 0.174 |
| Leaf area | <0.001 | 0.0004 | 0.0002 | 0.006 |
| Leaf length | <0.001 | 0.032 | 0.176 | 0.082 |
| Leaf width | <0.001 | 0.317 | 0.003 | 0.447 |
| Leaf thickness | <0.001 | <0.001 | 0.503 | 0.599 |
| Leaf angle | <0.001 | 0.063 | 0.002 | 0.050 |
| Leaf physiology | ||||
| An, light-saturated | <0.001 | <0.001 | 0.002 | 0.872 |
| Vcmax | 0.006 | 0.086 | 0.006 | 0.720 |
| Jmax | 0.198 | 0.616 | 0.075 | 0.037 |
| Jmax:Vcmax ratio | 0.004 | 0.010 | 0.262 | 0.165 |
| Rd, area-based | 0.400 | 0.003 | 0.795 | 0.115 |
| Rd, mass-based | <0.001 | 0.006 | 0.750 | 0.142 |
| LCP | 0.593 | 0.041 | 0.704 | 0.374 |
| gs | <0.001 | <0.001 | 0.115 | 0.490 |
| iWUE | <0.001 | <0.001 | 0.015 | 0.082 |
| Leaf chemistry | ||||
| Na | <0.001 | 0.784 | 0.332 | 0.022 |
| Nm | <0.001 | 0.090 | 0.551 | 0.005 |
| Pa | 0.0002 | 0.163 | 0.070 | <0.001 |
| Pm | 0.0003 | 0.101 | 0.040 | <0.001 |
| SPAD | <0.001 | 0.002 | 0.005 | 0.105 |
Figures in bold indicate significant P values.
V cmax: maximum rates of photosynthetic carboxylation, Jmax: maximum rates of electron transport, Rd: leaf dark respiration, LCP: light compensation point, gs: stomatal conductance, An: light-saturated net CO2 assimilation, Pa: leaf phosphorus content per unit area, Pm: leaf phosphorus content per unit mass, Na: leaf nitrogen content per unit area, Nm: leaf nitrogen content per unit mass, LMA: leaf mass per unit leaf area, SPAD: proxy for leaf chlorophyll content, RGR: relative growth rate.
Figure 1.
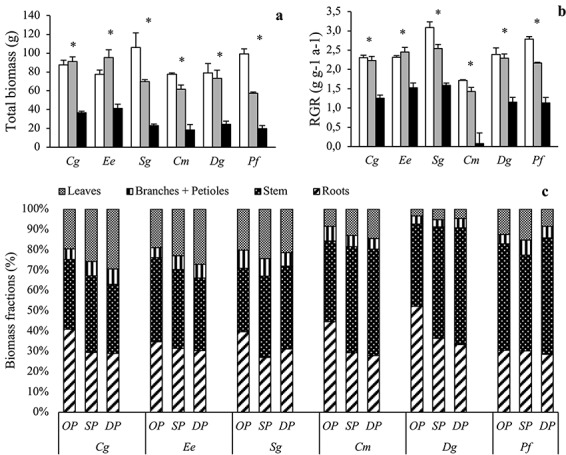
(a) Total biomass (g), (b) relative growth rate (RGR) and (c) biomass allocation (%) of Carapa grandiflora (Cg), Entandrophragma excelsum (Ee), Syzygium guineense (Sg), Croton megalocarpus (Cm), Dombeya goetzenii (Dg) and Polyscias fulva (Pf) planted in open (white in a, b; OP in c), sparse canopy (gray in a, b; SP in c) and dense canopy (black in a, b; DP in c) plots. Species to the left (Cg, Ee, Sg) are shade tolerant and species to the right (Cm, Dg, Pf) shade-intolerant. Leaf biomass data represent leaves attached at the time of harvest. The error bars represent standard errors (n = 3). The symbol * in (a) and (b) indicates significant variation among radiation regimes within a species. Overall statistics results are provided in Table 2.
The ST species all exhibited lower stem biomass fractions but higher fractions of leaves and branches plus petioles compared with the SI species (Figure 1c). The ST species Cg and Ee responded to shading by increasing the fractions of leaves, branches and petioles, a strategy not observed in the other species. The SI species plus Sg instead responded to shading by increasing their stem biomass fractions. Both ST and SI species had similar fractional investments into roots and all except Ee and Pf responded to shading by decreasing this fraction.
Monthly health inspections showed that some seedlings suffered from different kinds of health problems at some point during the experiment, including insect infection (4 plants) and herbivory (8 plants), partial dehydration (22 plants) and mammal herbivory (41 plants). Insect infection or herbivory caused no significant reduction in total biomass at harvest. Partially dehydrated plants either recovered well or subsequently died and were excluded from the biomass analysis. Seedlings affected by mammal herbivory had 21% lower total biomass at the final harvest, and these were therefore removed from the biomass analysis. A total of 21 out of 324 trees died throughout the experiment (Figure S1 Supplementary data for this article are available at Tree Physiology Online). Twelve of these trees were in the dense canopy plots and 14 belonged to SI species (i.e., Cm, Dg, Pf), but differences among species or treatments were not statistically significant.
Dry wood density was lowest in the SI species Dg and Pf and generally decreased with shading (Table S2 available as Supplementary Data at Tree Physiology Online).
Leaf structural, chemical and physiological traits
Values of LMA were highest in Sg, lowest in Cm and Dg, and quite similar in the remaining three species (Figure 2a). It declined with shading in all species. Mean leaf angles (horizontal = 0°) were below or about 30° in all plants growing beneath sparse or dense canopies (Figure 2b). They were considerably steeper in open plots in all species and the highest values were found for Cg (66°) followed by Pf (56°) and Cm (52°). Neither mean leaf angles nor their sun-shade plasticity differed in a systematic way between ST and SI species. SPAD values were higher in the ST compared with the SI species (Figure 2c). Moreover, there was a significant species by radiation interaction on SPAD (Table 2), with all species except the SI species Dg and Pf exhibiting increases in SPAD under low radiation conditions. The ST species thus had both intrinsically high chlorophyll content and an ability to increase these levels further upon shading.
Figure 2.
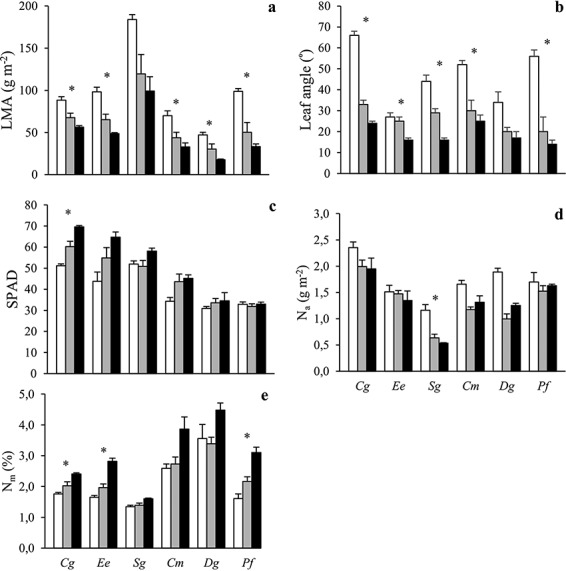
The (a) leaf mass per unit leaf area (LMA), (b) leaf angle, (c) SPAD, (d) nitrogen content per unit leaf area (Na) and (e) nitrogen content per unit leaf mass (Nm) of six tropical tree species grown in open (white), sparse canopy (gray) and dense canopy (black) plots. Species to the left (Cg, Ee, Sg) are shade-tolerant and species to the right (Cm, Dg, Pf) shade-intolerant. The error bars represent standard errors (n = 3). The symbol * indicates significant variation among radiation regimes within a species. Species: Carapa grandiflora (Cg), Entandrophragma excelsum (Ee), Syzygium guineense (Sg), Croton megalocarpus (Cm), Dombeya goetzenii (Dg) and Polyscias fulva (Pf).
Mass-based leaf N concentration was generally higher under shading while the opposite was sometimes true for area-based leaf N content (Figure 2d and e). There were no species by radiation interaction on area- or mass-based leaf N (Table 2). Area-based leaf N was weakly positively correlated with both photosynthetic capacity parameters: Vcmax and Jmax (Figure S2 available as Supplementary Data at Tree Physiology Online). For leaf phosphorus (P), mass-based concentrations were highest in Ee trees growing in sparse canopy plots and area-based P content was generally lowest in dense canopy plots (Figure S3 available as Supplementary Data at Tree Physiology Online). Species by radiation interactions had P values of 0.040 and 0.070 for mass- and area-based leaf P, respectively (Table 2).
In open plots, light-saturated An, Vcmax and Jmax were higher in the three SI species (i.e., Cm, Dg, Pf) and Sg than in Cg and Ee (Figure 3a–c). In dense canopy plots, however, values were mostly similar among species. In the ST species Cg and Ee, photosynthetic parameters were mostly equal in the three radiation regimes while in the other species they decreased with shading. These interspecific differences in shading responses resulted in significant (An, Vcmax) or nearly significant (Jmax, P = 0.075) species by radiation interactions (Table 2). The Jmax:Vcmax ratios also differed among species but not in a pattern consistent with species shade tolerance (Figure 3d). It was generally higher under shading and there was no significant difference in radiation responses among species (P = 0.26; Table 2). Stomatal conductance exhibited similar patterns to An except that SI species had higher values than ST species also in the dense canopy plots (Figure 3e).
Figure 3.
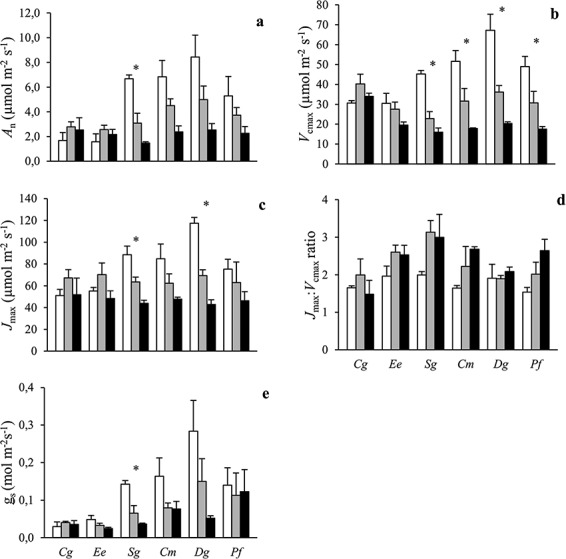
The (a) light-saturated net CO2 assimilation (An), (b) maximum rates of photosynthetic carboxylation (Vcmax) and (c) electron transport (Jmax), (d) Jmax:Vcmax ratio and (e) stomatal conductance (gs) of six tropical tree species grown in open (white), sparse canopy (gray) and dense canopy (black) plots. Species to the left (Cg, Ee, Sg) are shade tolerant and species to the right (Cm, Dg, Pf) shade-intolerant. The error bars represent standard errors (n = 3). The symbol * indicates significant variation among radiation regimes within a species. Species: Carapa grandiflora (Cg), Entandrophragma excelsum (Ee), Syzygium guineense (Sg), Croton megalocarpus (Cm), Dombeya goetzenii (Dg) and Polyscias fulva (Pf).
Dark respiration did not vary much among species and was generally lower under shading (Figure 4a). There was no significant species by radiation interaction on Rd (P = 0.80; Table 2). The LCP was lower under shading (Figure 4b). There was no overall significant species by radiation interaction for this trait (P = 0.70), and LCP was 5–7 μmol mol−1 for all species in dense canopy plots.
Figure 4.
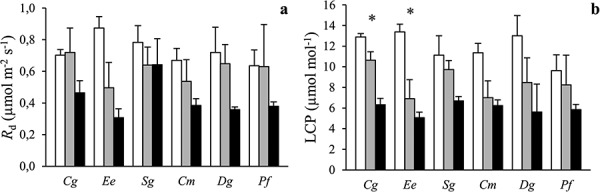
The (a) leaf dark respiration (Rd) and (b) photosynthetic light compensation point (LCP) of six tropical tree species grown in open (white), sparse canopy (gray) and dense canopy (black) plots. Species to the left (Cg, Ee, Sg) are shade tolerant and species to the right (Cm, Dg, Pf) shade-intolerant. The error bars represent standard errors (n = 3). The symbol * indicates significant variation among radiation regimes within a species. Species: Carapa grandiflora (Cg), Entandrophragma excelsum (Ee), Syzygium guineense (Sg), Croton megalocarpus (Cm), Dombeya goetzenii (Dg) and Polyscias fulva (Pf).
Intrinsic WUE was considerably higher in open plots, and in dense canopy plots it was highest in the ST species Cg and Ee (Figure 5). There was a significant species by radiation interaction, reflecting a smaller shade-induced reduction in iWUE in ST compared with SI species (P = 0.015; Table 2).
Figure 5.
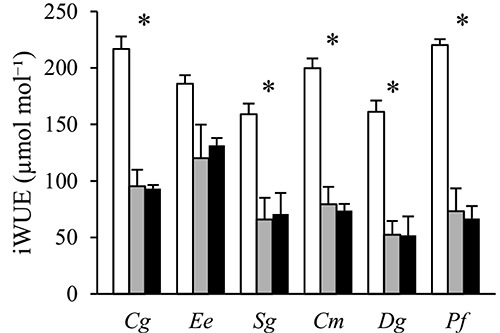
The intrinsic water-use efficiency (iWUE) determined based on leaf stable carbon isotope data from six tropical tree species grown in open (white), sparse canopy (gray) and dense canopy (black) plots. Species to the left (Cg, Ee, Sg) are shade-tolerant and species to the right (Cm, Dg, Pf) shade-intolerant. The error bars represent standard errors (n = 3). The symbol * indicates significant variation among radiation regimes within a species. Species: Carapa grandiflora (Cg), Entandrophragma excelsum (Ee), Syzygium guineense (Sg), Croton megalocarpus (Cm), Dombeya goetzenii (Dg) and Polyscias fulva (Pf).
Leaf temperature
Plant species in open plots experienced higher leaf temperatures (Tleaf) compared with their counterparts under sparse and dense canopies (Figure 6). In open plots, Tleaf was always considerably higher than the prevailing air temperature measured at the local Arboretum weather station. This exceedance was 10–12 °C in the ST species Cg and Ee, while it was 5–8 °C in the other species. In dense canopy plots, Tleaf was rather similar across species, around 5 °C below the air temperature recorded by the weather station. These low Tleaf values likely resulted from a combination of low radiation, lower daytime air temperature under dense canopies compared with open plots (Table 1) and transpiratory cooling.
Figure 6.
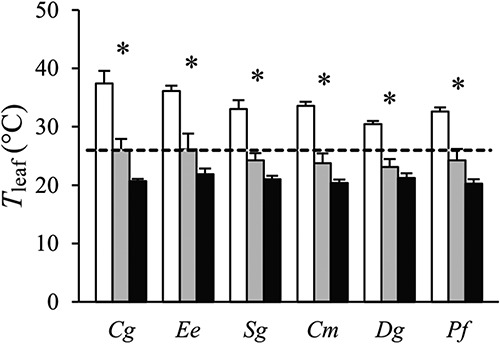
Leaf temperature (Tleaf) of six tropical tree species grown in open (white), sparse canopy (gray) and dense canopy (black) plots. The dashed line indicates the Arboretum weather station air temperature (25.9 °C) during the period of the leaf temperature measurements. Species to the left (Cg, Ee, Sg) are shade-tolerant and species to the right (Cm, Dg, Pf) shade-intolerant. The error bars represent standard errors (n = 3). The symbol * indicates significant variation among radiation regimes within a species. Species: Carapa grandiflora (Cg), Entandrophragma excelsum (Ee), Syzygium guineense (Sg), Croton megalocarpus (Cm), Dombeya goetzenii (Dg) and Polyscias fulva (Pf).
The species with the highest Tleaf in open plots, Cg and Ee, also had the largest leaf sizes (60–64 cm2 compared with 10–42 cm2 for the other species, Table S2 available as Supplementary Data at Tree Physiology Online) and the lowest stomatal conductance (Figure 3e). Their large air temperature exceedances can thus be explained by a combination of poor heat dissipation (i.e., low leaf boundary layer conductance) and low transpiratory cooling.
Discussion
The overall aim of this study—to explore which traits were most important in controlling species shade tolerance in tropical montane trees—could be fulfilled by taking a controlled experimental approach in which we investigated a broad range of structural, chemical and physiological traits in species with different successional strategies grown under three different radiation regimes. The dense canopy plots transmitted more radiation (Table 1; 10% at noon, corresponding to about 7–8% for the daylight period if assuming 75% direct radiation and 25% diffuse radiation and spherical leaf angle distribution) than multi-layered canopies of many lowland rainforests (1–2%, Montgomery and Chazdon 2001). However, this radiation regime is representative for many late-successional stands of the highly topographical and steeply sloped montane forest from which our species originate and obviously dense enough to induce strong structural (Figure 1) and physiological (Figures 3–5) responses to shading. Our results suggest that interspecific variation in shade tolerance of tropical montane trees is controlled by species differences in whole-plant biomass allocation strategy (Figure 1) rather than by difference in physiological leaf traits determining leaf carbon balance at low radiation (Figure 4). In the shade, two of the ST species (Cg and Ee) had high fractional biomass investments in laterally light-intercepting organs (i.e., leaves, petioles and branches) and comparatively small RGR reductions below dense canopies (Figure 1). At the other end of the shade tolerance spectrum, the SI species (Cm, Dg and Pf) had high fractional investments in stems and larger growth reductions when shaded. The sixth species (Sg), which is most common in late-successional stands but also abundant in early-successional stands in Nyungwe (Nyirambangutse et al. 2017), was intermediate with respect to both RGR reductions and biomass allocation. Species with contrasting shade tolerance did not differ in leaf physiological traits controlling leaf carbon balance at low radiation (i.e., Rd and LCP; Figure 4).
Growth and biomass allocation
Biomass allocation results were in line with our first hypothesis and provided partial support for both the carbon gain and stress tolerance hypotheses. The ST species invested relatively more into plant organs maximizing light interception (i.e., leaves and branches) than SI species (Figure 1c). In the two typical ST species (Cg and Ee), this difference was strongest under low radiation, showing that it is the result of both species adaptations and acclimation responses. The allocation strategy of these two ST species was successful with respect to total growth, as judged by the smaller growth declines under low radiation in ST compared with SI species (Figure 1a and b). Leaf morphology, however, did not vary in agreement with the carbon gain hypothesis since ST species had higher LMA than SI species in the shade (Figure 2a), in line with several previous studies on tropical tree species (Kitajima 1994, Coste et al. 2005, Mao et al. 2014). A successful ST species thus seems to need both a whole-plant architecture that is favorable for light interception (in agreement with the carbon gain hypothesis) and leaves that are strong enough to endure biotic and abiotic stress in the understorey (in agreement with the stress tolerance hypothesis; Valladares and Niinemets 2008, Valladares et al. 2016). Even though high LMA does not maximize short-term light interception and carbon gain, it might increase the carbon gain over the entire leaf lifespan thanks to better physical protection against herbivores and mechanical stresses (Kitajima and Poorter 2010, Mao et al. 2014) and longer leaf lifespan (Coste et al. 2011, Gommers et al. 2013, Reich 2014).
Shade-intolerant species grown under dense canopies had high investments in stem biomass and exhibited low RGR (Figure 1). This represents a strategy to try to escape from the shade of neighbors by rapid vertical growth (Montgomery 2004, Grubb 2015, Poorter et al. 2018). However, unless stem elongation results in increased light interception this strategy will not be successful in the long run (Henry and Aarssen 1997, Valladares et al. 2016). In this 1-year study, SI seedlings prioritizing stem growth were not rewarded by reaching higher radiation since overstorey canopies were several meters above. There was an indication that the shade-intolerant growth strategy was linked to increased mortality since seedlings that died were predominantly SI species growing below dense canopies (Figure S1 available as Supplementary Data at Tree Physiology Online). However, differences in mortality rates between species or radiation regimes were not statistically significant and the total number of trees that died during the experiment was rather low (21 out of 324 trees).
Leaf physiology
Contrary to our second hypothesis, seedlings of ST and SI species did not significantly differ in Rd or LCP, two key physiological traits controlling leaf carbon balance at low radiation (Figure 4). However, light-saturated An, photosynthetic capacity (i.e., Vcmax and Jmax) and stomatal conductance were generally lower in ST compared with SI species grown in open plots (Figure 3). These results indicate that light-use efficiency under low radiation is not higher in ST compared with SI species while carbon gain in open plots is clearly superior in SI species. The higher leaf-level iWUE in the ST species Cg and Ee in dense canopy plots (Figure 5) is not necessarily indicating better drought tolerance since these species also had relatively more transpiring leaf biomass and less root biomass compared with the other species (Figure 1c).
Both Rd and LCP were markedly lower in dense canopy plots, demonstrating typical sun-shade acclimation of leaf physiology (Figure 4). However, neither the degree of radiation acclimation nor the magnitude of Rd or LCP in dense canopy plots differed among species, indicating that these traits are poor predictors of species shade tolerance in tropical montane forests. This study therefore gives little support to the carbon gain hypothesis at the leaf physiological level. A previous study with tropical seedlings indicated leaf Rd to be the best predictor of shade tolerance–better than photosynthetic capacity or leaf N content (Baltzer and Thomas 2007a). However, that study as well as many previous investigations (Valladares et al. 2008, Coste et al. 2010, Cheng et al. 2013, Grubb 2015) have measured leaf gas exchange of understorey plants where they naturally grow, with the obvious risk of cofounding effects of acclimation and adaptation. The design of the present study allowed for the separation of acclimation and adaptation. Additional experimental studies are needed to determine if our results are valid across a broader range of species and conditions.
For Rd and LCP, acclimation to different radiation regimes was similar in ST and SI species (Figure 4) while the responses of photosynthetic capacity (i.e., Vcmax and Jmax) to growth radiation differed markedly between the two groups (Figure 3b and c). These findings thus only partially support previous studies on tropical trees which have indicated that sun–shade leaf plasticity is similar in ST and SI species (Rozendaal et al. 2006, Coste et al. 2009, 2010, Dusenge et al. 2015). They are rather in line with another study showing that ST species are more plastic in some traits while SI species are more plastic in others (Houter and Pons 2014). Our results indicate that interspecific variation in sun–shade leaf plasticity may markedly differ among physiological traits, but that shade tolerance is not the consequence of higher acclimation capacity of traits linked to leaf carbon balance at low radiation (i.e., Rd and LCP).
Photosynthetic capacity was lower in ST compared with SI species in open plots (Figure 3) and SPAD values (a proxy for chlorophyll content; Figure 2c) higher in ST species generally. This reflects a shift from investments in structures and compounds maximizing photosynthetic capacity towards higher investments for light harvesting in ST compared with SI species (Anderson et al. 1995, Dusenge et al. 2015). However, the link between area-based leaf N content and photosynthetic capacity appears weak since the latter but not the former differed markedly between ST and SI species (Figures 2 and 3). This result adds to a growing number of studies on tropical tree species challenging the view that leaf nutrient content is a good predictor of interspecific variation in photosynthesis (Coste et al. 2005, van de Weg et al. 2012, Houter and Pons 2014, Dusenge et al. 2015, Bahar et al. 2016, Hasper et al. 2017).
Leaf temperature
The leaf temperatures under sunny conditions in open plots were highest in the ST species Cg and Ee, in line with our third hypothesis (Figure 6). In these species and under these conditions, leaf temperatures were on average 36–38 °C. These values clearly exceed the biochemical optimal Tleaf for light-saturated An (at a common intercellular CO2 concentration of 272 μmol mol−1) of these species, which are at 25–30 °C (Vårhammar et al. 2015). There were indeed indications that ST trees growing in open plots suffered in our experiment. The ST species Cg and Ee had markedly lower values of Vcmax and Jmax than SI species in open plots but not in lower radiation regimes (Figure 3), which may indicate negative heat effects on photosynthetic enzymes and the electron transport chain (Sage and Kubien 2007).
It should be noted, however, that the standardized measurement of Tleaf (around noon in leaves held in horizontal position) may have caused some overestimation for leaves with steep leaf angles in open plots. The Tleaf of a horizontal leaf at noon may be comparable to that of a sun-facing leaf at 45° at 15:00h, since 14:00–15:00h is the hottest hour of the day at the Arboretum and solar radiation is then still high at an angle perpendicular to the sun. However, Cg in open plots had a mean leaf angle of 66° and the Tleaf measured for this species in open plots is thus an overestimation for most leaves at their natural angles (but may occur in some individual leaves with angles at or below 45 or even 30°, which also occurred).
The magnitude of leaf to air temperatures exceedance among species was linked to interspecific variation in leaf size and stomatal conductance, as also found in a previous common garden experiment with tropical ST tree seedlings (Vårhammar et al. 2015). Species with large leaf size and low stomatal conductance have poor heat dissipation and low transpiratory cooling capacity, leading to high leaf temperatures. Large leaf size and low stomatal conductance are common traits of ST species (Valladares and Niinemets 2008) and may offer partial explanation of why ST species are more negatively affected by warming than SI species in controlled experiments (Doughty 2011, Cheesman and Winter 2013b).
These results are in agreement with the emerging poly-tolerance concept which suggests that shade tolerance should be evaluated together with plant tolerance to other stressors, such as drought and waterlogging (Laanisto and Niinemets 2015, Grubb 2015, Kunstler et al. 2016, Valladares et al. 2016). Our study indicates that species growing in the understorey are not only shade-tolerant, but may in fact also be sun-intolerant at the seedling stage due to negative effects of high leaf temperature on stomatal conductance and photosynthetic biochemistry.
Conclusions
Results from this study suggest that the large interspecific variation in shade tolerance among tropical montane trees is controlled by whole-plant biomass allocation strategy rather than by variation in physiological leaf traits determining leaf carbon balance at low radiation. Species with varying shade tolerance had distinctly different biomass allocation patterns (Figure 1): the two distinct ST species (Cg and Ee) invested relatively more into plant organs maximizing light interception in the understorey (i.e., leaves and branches) while SI species invested more into stems. These differences in fractional biomass investments were more pronounced under dense canopies. This shows that ST and SI species in our tropical montane forest have two fundamentally different strategies to deal with low-light conditions: ST species maximize light-use efficiency in the shade while SI species try to escape the deep shade through vertical stem growth. Contrary to our expectations, however, ST and SI species did not differ in leaf Rd or LCP, i.e., traits controlling leaf carbon balance at low radiation (Figure 4).
Our results highlight the importance of a whole-plant perspective for understanding tree shade tolerance. Based on leaf level physiological data only, we found little support for the carbon gain hypothesis (stating that shade tolerance is the consequence of high light use-efficiency resulting from traits maximizing carbon gain and minimizing carbon losses in a low-light environment). However, based on both leaf physiological and whole-plant structural data we can conclude that the carbon gain hypothesis is relevant, but that carbon gain of our two distinct ST species is increased by biomass allocation strategy rather than by leaf level physiological traits.
Supplementary Material
Acknowledgments
We are grateful to Mrs Valentine Mukanyabyenda for assistance in irrigating the plants and to Mr Gélase Kayindo and Mrs Valentine Mukanyabyenda for field assistance at the final harvest. We also thank Rwanda Agriculture and Animal Resources Development Board for authorizing data collection in the Arboretum of Ruhande.
Funding
The study was supported by Formas, the Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning (grant number 942-2015-1458), and the Swedish strategic research area ‘Biodiversity and Ecosystem services in a Changing Climate’ (BECC; http://www.becc.lu.se/).
References
- Anderson JM, Chow WS, Park YI (1995) The grand design of photosynthesis: acclimation of the photosynthetic apparatus to environmental cues. Photosynth Res 46:129–139. [DOI] [PubMed] [Google Scholar]
- Bahar NHA, Ishida FY, Weerasinghe LK, Guerrieri R, Sullivan OSO, Bloomfield KJ, Atkin OK (2016) Leaf-level photosynthetic capacity in lowland Amazonian and high-elevation Andean tropical moist forests of Peru. New Phytol 214:1002–1018. [DOI] [PubMed] [Google Scholar]
- Baltzer JL, Thomas SC (2007a) Physiological and morphological correlates of whole-plant light compensation point in temperate deciduous tree seedlings. Oecologia 153:209–223. [DOI] [PubMed] [Google Scholar]
- Baltzer JL, Thomas SC (2007b) Determinants of whole-plant light requirements in Bornean rain forest tree saplings. J Ecol 95:1208–1221. [Google Scholar]
- Beer C, Reichstein M, Tomelleri E, Ciais P, Jung M, Carvalhais N, Dario P (2010) Terrestrial gross carbon dioxide uptake: global distribution and covariation with climate. Science 329:834–838. [DOI] [PubMed] [Google Scholar]
- Bloesch U, Troupin G, Derungs N (2009) Les plantes ligneuses du Rwanda Flore, ecologie et usages. Shaker, Aachen.
- Cheesman AW, Winter K (2013a) Elevated night-time temperatures increase growth in seedlings of two tropical pioneer tree species. New Phytol 197:1185–1192. [DOI] [PubMed] [Google Scholar]
- Cheesman AW, Winter K (2013b) Growth response and acclimation of CO2 exchange characteristics to elevated temperatures in tropical tree seedlings. J Exp Bot 64:3817–3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng AX, Yu M, Wang GG, Wu T, Zhang C (2013) Growth, morphology and biomass allocation in response to light gradient in five subtropical evergreen broadleaved tree seedlings. J Trop For Sci 25:537–546. [Google Scholar]
- Clark DB, Clark DA, Oberbauer SF (2010) Annual wood production in a tropical rain forest in NE Costa Rica linked to climatic variation but not to increasing CO2. Glob Chang Biol 16:747–759. [Google Scholar]
- Coste S, Roggy JC, Imbert P, Born C, Bonal D, Dreyer E (2005) Leaf photosynthetic traits of 14 tropical rain forest species in relation to leaf nitrogen concentration and shade tolerance. Tree Physiol 25:1127–1137. [DOI] [PubMed] [Google Scholar]
- Coste S, Roggy JC, Garraud L, Heuret P, Nicolini E, Dreyer E (2009) Does ontogeny modulate irradiance-elicited plasticity of leaf traits in saplings of rain-forest tree species? A test with Dicorynia guianensis and Tachigali melinonii (Fabaceae, Caesalpinioideae). Ann For Sci 66:709–709. [Google Scholar]
- Coste S, Roggy JC, Sonnier G, Dreyer E (2010) Similar irradiance-elicited plasticity of leaf traits in saplings of 12 tropical rainforest tree species with highly different leaf mass to area ratio. Funct Plant Biol 37:342–355. [Google Scholar]
- Coste S, Roggy JC, Schimann H, Epron D, Dreyer E (2011) A cost-benefit analysis of acclimation to low irradiance in tropical rainforest tree seedlings: leaf life span and payback time for leaf deployment. J Exp Bot 62:3941–3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Kauwe MG, Lin YS, Wright IJ, Medlyn BE, Crous KY, Ellsworth DS, Domingues TF (2016) A test of the “one-point method” for estimating maximum carboxylation capacity from field-measured, light-saturated photosynthesis. New Phytol 210:1130–1144. [DOI] [PubMed] [Google Scholar]
- Dirzo R, Raven PH (2003) Global state of biodiversity and loss. Annu Rev Env Resour 28:137–167. [Google Scholar]
- Doughty CE. (2011) An in situ leaf and branch warming experiment in the Amazon. Biotropica 43:658–665. [Google Scholar]
- Doughty CE, Goulden ML (2008) Are tropical forests near a high temperature threshold? J Geophys Res 113:1–12. [Google Scholar]
- Dusenge ME, Göran W, Gårdesten J, Niyonzima F, Adolfsson L, Nsabimana D, Uddling J (2015) Photosynthetic capacity of tropical montane tree species in relation to leaf nutrients, successional strategy and growth temperature. Oecologia 177:1183–1194. [DOI] [PubMed] [Google Scholar]
- Farquhar GD, Caemmerer SV, Berry JA (1980) A biochemical model of photosynthetic CO2 assimilation. Planta 90:78–90. [DOI] [PubMed] [Google Scholar]
- Farquhar GD, Ehleringer JR, Hubick KT (1989) Carbon isotope discrimination and photosynthesis. Annu Rev Plant Physiol Plant Mol Biol 40:503–537. [Google Scholar]
- Fischer E, Killmann D (2008) Illustrated field guide to the plants of Nyungwe National Park Rwanda. Koblenz Geographical Colloquia Series Biogeographical Monographs 1 Universität Koblenz-Landau, Mainz, Germany. [Google Scholar]
- Givnish TJ. (1988) Adaptation to sun and shade: A whole-plant perspective. Aust J of Plant Physiol 15:63–92. [Google Scholar]
- Gommers CMM, Visser EJW, Onge KRS, Voesenek LAC, Pierik R (2013) Shade tolerance: when growing tall is not an option. Trends Plant Sci 18:65–71. [DOI] [PubMed] [Google Scholar]
- Grubb PJ. (2015) Trade-offs in interspecific comparisons in plant ecology and how plants overcome proposed constraints. Plant Ecol Divers 9:3–33. [Google Scholar]
- Hasper TB, Dusenge ME, Breuer F, Uwizeye FK, Wallin G, Uddling J (2017) Stomatal CO2 responsiveness and photosynthetic capacity of tropical woody species in relation to taxonomy and functional traits. Oecologia 184:43–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry HAL, Aarssen LW (1997) On the relationship between shade tolerance and shade avoidance strategies in woodland. Oikos 80:575–582. [Google Scholar]
- Houter NC, Pons TL (2012) Ontogenetic changes in leaf traits of tropical rainforest trees differing in juvenile light requirement. Oecologia 169:33–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houter NC, Pons TL (2014) Gap effects on leaf traits of tropical rainforest trees differing in juvenile light requirement. Oecologia 175:37–50. [DOI] [PubMed] [Google Scholar]
- Kitajima K. (1994) Relative importance of photosynthetic traits and allocation patterns as correlates of seedling shade tolerance of 13 tropical trees. Oecologia 98:419–428. [DOI] [PubMed] [Google Scholar]
- Kitajima K, Poorter L (2010) Tissue-level leaf toughness, but not lamina thickness, predicts sapling leaf lifespan and shade tolerance of tropical tree species. New Phytol 186:708–721. [DOI] [PubMed] [Google Scholar]
- Kok B. (1948) A critical consideration of the quantum yield of chlorella-photosynthesis. Enzymologia 13:1–56. [Google Scholar]
- Kunstler G, Falster D, Coomes DA, Hui F, Kooyman RM, Laughlin DC, Westoby M (2016) Plant functional traits have globally consistent effects on competition. Nature 529:204–207. [DOI] [PubMed] [Google Scholar]
- Laanisto L, Niinemets Ü (2015) Polytolerance to abiotic stresses: how universal is the shade-drought tolerance trade-off in woody species? Glob Ecol Biogeogr 24:571–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis SL. (2006) Tropical forests and the changing earth system. Phil Trans R Soc B 261:195–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusk CH, Pozo AD (2002) Survival and growth of seedlings of 12 Chilean rainforest trees in two light environments: gas exchange and biomass distribution correlates. Austral Ecol 27:173–182. [Google Scholar]
- Lusk CH, Saldan A, Piper FI, Matı M (2011) Ontogeny, understorey light interception and simulated carbon gain of juvenile rainforest evergreens differing in shade tolerance. Ann Bot 108:419–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttge U. (2008) Physiological ecology of tropical plants. Springer-Verlag, Berlin Heidelberg. [Google Scholar]
- Malhi Y, Adu-bredu S, Asare RA, Lewis SL, Mayaux P (2013) African rainforests: past, present and future. Phil Trans R Soc B 368:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao P, Zang R, Shao H, Yu J (2014) Functional trait trade-offs for the tropical montane rain forest species responding to light from simulating experiments. Sci World J 2014:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery R. (2004) Relative importance of photosynthetic physiology and biomass allocation for tree seedling growth across a broad light gradient. Tree Physiol 24:155–167. [DOI] [PubMed] [Google Scholar]
- Montgomery RA, Chazdon RL (2001) Forest structure, canopy architecture, and light transmittance in tropical wet forests. Ecology 82:2707–2718. [Google Scholar]
- Mujawamariya M, Manishimwe A, Ntirugulirwa B, Bahati EN, Nyirambangutse B, Nsabimana D, Wallin G, Uddling J (2018) Climate sensitivity of tropical trees along an elevation gradient in Rwanda. Forests 647:1–19. [Google Scholar]
- Niinemets Ü. (1997) Role of foliar nitrogen in light harvesting and shade tolerance of four temperate deciduous woody species. Funct Ecol 11:518–531. [Google Scholar]
- Niinemets Ü. (2006) The controversy over traits conferring shade-tolerance in trees: ontogenetic changes revisited. J Ecol 94:464–470. [Google Scholar]
- Nsabimana D. (2009) Carbon stock and fluxes in Nyungwe forest and Ruhande Arboretum in Rwanda PhD thesis. Gothenburg University, Gothenburg. [Google Scholar]
- Nsabimana D, Klemedtson L, Kaplin BA, Wallin G (2008) Soil carbon and nutrient accumulation under forest plantations in southern Rwanda. Afr J Environ Sci Technol 2:142–149. [Google Scholar]
- Nyirambangutse B, Zibera E, Uwizeye FK, Nsabimana D, Bizuru E, Pleijel H (2017) Carbon stocks and dynamics at different successional stages in an Afromontane tropical forest. Biogeosciences 14:1285–1303. [Google Scholar]
- Osunkoya OO, Ash JE, Hopkins MS, Graham AW, Ash E, Hopkins MS (1994) Influence of seed size and seedling ecological attributes on shade-tolerance of rain-forest tree species in northern Queensland. J Ecol 82:149–163. [Google Scholar]
- Poorter H, Niinemets Ü, Poorter L, Wright IJ, Villar R, Niinemets U, Villar R (2009) Causes and consequences of variation in leaf mass per area (LMA): a meta-analysis. New Phytol 182:565–588. [DOI] [PubMed] [Google Scholar]
- Poorter H, Niinemets Ü, Ntagkas N, Siebenkäs A, Mäenpää M, Matsubara S, Pons TL (2019) A meta-analysis of plant responses to light intensity for 70 traits ranging from molecules to whole plant performance. New Phytol 223:1073–1105. [DOI] [PubMed] [Google Scholar]
- Poorter L, Castilho CV, Schietti J, Oliveira RS, Costa FRC (2018) Can traits predict individual growth performance? A test in a hyperdiverse tropical forest. New Phytol 219:109–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poorter L, Kitajima K, Mercado P, Chubina J, Melgar I, Prins HHT (2010) Resprouting as a persistence strategy of tropical forest trees: relations with carbohydrate storage and shade tolerance. Ecology 91:2613–2627. [DOI] [PubMed] [Google Scholar]
- Reich PB. (2014) The world-wide “fast-slow” plant economics spectrum: a traits manifesto. J Ecol 102:275–301. [Google Scholar]
- Reich PB, Wright IJ, Bares JC, Craine JM, Oleksyn J, Westoby M (2003) The evolution of plant functional variation: traits, spectra, and strategies. Int J Plant Sci 164:143–164. [Google Scholar]
- Rozendaal DM, Hurtado VH, Poorter L (2006) Plasticity in leaf traits of 38 tropical tree species in response to light. Funct Ecol 20:207–216. [Google Scholar]
- Sage RF, Kubien DS (2007) The temperature response of C3 and C4 photosynthesis. Plant Cell Environ 30:1086–1106. [DOI] [PubMed] [Google Scholar]
- Slot M, Winter K(2017) Photosynthetic acclimation to warming in tropical forest tree seedlings. J Exp Bot, 68: 2275–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood AJ. (1997) Experiments in ecology: their logical design and interpretation using analysis of variance. Cambridge University Press, Cambridge, UK. [Google Scholar]
- Valladares F, Zaragoza-Castells J, Sánchez-Gómez D, Matesanz S, Alonso B, Portsmuth A, Delgado A, Atkin OK (2008) Is shade beneficial for Mediterranean shrubs experiencing periods of extreme drought and late-winter frosts?. Annals of Botany 102:923–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valladares F, Niinemets Ü (2008) Shade tolerance, a key plant feature of complex nature and consequences. Annu Rev Ecol Evol Syst 39:237–257. [Google Scholar]
- Valladares F, Laanisto L, Niinemets Ü, Zavala MA (2016) Shedding light on shade: ecological perspectives of understorey plant life. Plant Ecol Divers 9:237–251. [Google Scholar]
- Van de Weg MJ, Meir P, Grace J, Ramos GD (2012) Photosynthetic parameters, dark respiration and leaf traits in the canopy of a Peruvian tropical montane cloud forest. Oecologia 168:23–34. [DOI] [PubMed] [Google Scholar]
- Vårhammar A, Mclean CM, Dusenge ME, Medlyn BE, Hasper TB, Nsabimana D, Uddling J (2015) Photosynthetic temperature responses of tree species in Rwanda: evidence of pronounced negative effects of high temperature in montane rainforest climax species. New Phytol 206:1000–1012. [DOI] [PubMed] [Google Scholar]
- Walters MB, Reich PB (1999) Low-light carbon balance and shade tolerance in the seedlings of woody plants: do winter deciduous and broad-leaved evergreen species differ? New Phytol 143:143–154. [Google Scholar]
- Way DA, Oren R (2010) Differential responses to changes in growth temperature between trees from different functional groups and biomes: a review and synthesis of data. Tree Physiol 30:669–688. [DOI] [PubMed] [Google Scholar]
- Wright SJ, Muller-Landau HC, Schipper J (2009) The future of tropical species on a warmer planet. Conserv Biol 23:1418–1426. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


