Abstract
Context
Aldosterone-producing adrenocortical adenomas (APAs) are mainly composed of clear (lipid rich) and compact (eosinophilic) tumor cells. The detailed association between these histological features and somatic mutations (KCNJ5, ATP1A1, ATP2B3, and CACNA1D) in APAs is unknown.
Objective
To examine the association between histological features and individual genotypes in APAs.
Methods
Examination of 39 APAs subjected to targeted next-generation sequencing (11 KCNJ5, 10 ATP1A1, 10 ATP2B3, and 8 CACNA1D) and quantitative morphological and immunohistochemical (CYP11B2 and CYP17A1) analyses using digital imaging software.
Results
KCNJ5- and ATP2B3-mutated APAs had clear cell dominant features (KCNJ5: clear 59.8% [54.4–64.6%] vs compact 40.2% (35.4–45.6%), P = .0022; ATP2B3: clear 54.3% [48.2–62.4 %] vs compact 45.7% (37.6–51.8 %), P = .0696). ATP1A1- and CACNA1D-mutated APAs presented with marked intratumoral heterogeneity. A significantly positive correlation of immunoreactivity was detected between CYP11B2 and CYP17A1 in tumor cells of KCNJ5-mutated APAs (P = .0112; ρ = 0.7237), in contrast, significantly inverse correlation was detected in ATP1A1-mutated APAs (P = .0025; ρ = −0.8667).
Conclusion
KCNJ5-mutated APAs, coexpressing CYP11B2 and CYP17A1, were more deviated in terms of zonation-specific differentiation of adrenocortical cells than ATP1A1- and ATP2B3-mutated APAs.
Keywords: primary aldosteronism, CYP11B2, ATP1A1, ATP2B3, CACNA1D, KCNJ5
Primary aldosteronism (PA) is one of the most common forms of secondary hypertension, accounting for approximately 10% of all hypertensive patients (1–6). Aldosterone-producing adrenocortical adenoma (APA) and idiopathic hyperaldosteronism (IHA) are the 2 major subtypes of PA (1–7). In addition, APAs are well known to harbor marked intratumoral heterogeneity in terms of their morphology, genetics, and steroidogenesis (8–10). Histologically, APAs are mainly composed of 2 distinctive cell types based on their morphological features: “clear cells” and “compact cells” (8, 9). Clear cells are termed as “lipid-rich cells” or “zona fasciculata (ZF)-like cells” harboring relatively abundant lipid droplets, while “compact cells”, also termed “lipid-poor cells” or “zona glomerulosa (ZG)-like cells,” are small, spherical shaped cells with eosinophilic cytoplasm (8, 9). However, an association between the morphological and functional features of these tumor cells remains unknown.
In addition, recent studies using next-generation sequencing (NGS) revealed that the great majority of APAs harbored somatic mutations of genes encoding ion channels and ion transporters (KCNJ5 encoding the inwardly rectifying potassium channel subfamily J, member 5; ATP1A1, Na+/K+ ATPase 1; ATP2B3, Ca2+ ATPase 3; and CACNA1D, voltage-dependent, L-type calcium channel subunit 1D) (11–16). These somatic mutations were detected in approximately 90% of all APAs (14, 16, 17). Among them, somatic APA mutations in KCNJ5 were the most frequently detected in Caucasian and in Asian patients (11–16) whereas APA mutations in CACNA1D were most frequently detected in Afro-American patients (17).
Possible genotype–phenotype associations, including histological features of APAs, have been proposed especially in APAs carrying KCNJ5 mutations (9, 18, 19). KCNJ5-mutated APAs have a clear cell-dominant histology and a relatively large size. In addition, Monticone et al. reported that CYP11B2 immunoreactivity was significantly more abundant in ZG-like (n = 43) than in ZF-like (n = 28) APAs and that KCNJ5 somatic mutations were more frequently detected in the latter type (19). However, detailed histological features of KCNJ5-mutated APAs and APAs with the less frequently detected somatic mutations (ATP1A1, ATP2B3, and CACNA1D) are unknown. In addition, the majority of histological studies cited above were performed with manual analyses, which could be associated with marked inter- and intraobserver variance (10, 15, 19). We previously proposed that a quantitative histological analytical approach using digital imaging software could minimize such variance because of high reproducibility in the analysis of KCNJ5-mutated APAs (9).
Therefore, in this study, we quantitatively analyzed the morphological features and immunoreactivity of CYP11B2 and CYP17A1 in combination with targeted NGS for APA genotyping. Our objective was to apply state-of-the art and quantifiable technology to establish the correlations of histological features with the distribution of steroidogenic enzymes stratified by genotype.
Materials and Methods
APA cases
We initially retrieved cases demonstrating KCNJ5 wild type by initial sequencing after screening in 51 cases from all of the participating institutions (University of Michigan, Ludwig Maximilian University of Munich, University of Torino, and Yale University) because of the relatively small number of the cases harboring rare frequent mutations. Subsequent further sequencing by NGS validated the genotypes of those cases (KCNJ5: 11 cases, ATP1A1: 14 cases, ATP2B3: 11 cases, CACNA1D: 15 cases). We then exclusively analyzed the 10% formalin-fixed and paraffin-embedded tissue specimens that had been well prepared without any artifacts and examined by histological evaluation in hematoxylin and eosin (H&E)-stained tissue slides. We then selected by subsequent histological examination those containing the whole area of the tumor at maximum diameter. The above screening yielded the number of the cases examined in this study as follows (KCNJ5: 11 cases, ATP1A1: 10 cases, ATP2B3: 10 cases, CACNA1D: 8 cases).
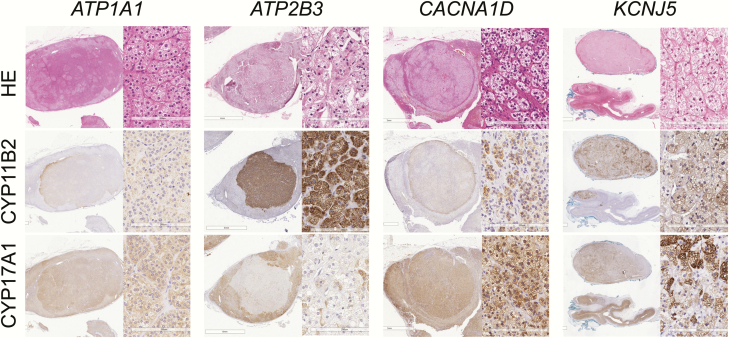
All the cases examined were clinically diagnosed according to the Endocrine Society Guidelines for PA (1). The clinicopathological variables of these cases are summarized in Table 1. All tumors were pathologically diagnosed as adrenocortical adenomas according to the criteria of Weiss (20). Immunostaining with CYP11B2 antibody was subsequently performed to confirm the histopathological diagnosis of APAs (9, 21). We first screened all available tissue sections (average 4 or 5 sections) of all the cases examined and selected a representative tissue section containing the largest area of the tumor. Whole tumor areas with maximum dimensions, which could reflect intratumoral heterogeneity (Fig. 1), were selected from all the available tissue sections of individual cases. The study protocol was approved by the Institutional Review Board of each institution.
Table 1.
Clinicopathological characteristics of aldosterone-producing adenoma (APA) cases with ATP1A1, ATP2B3, CACNA1D, and KCNJ5 mutation examined in this study.
| Mean ± SEM (25–75th percentile) | ATP1A1 | ATP2B3 | CACNA1D | KCNJ5 |
|---|---|---|---|---|
| N | 10 | 10 | 8 | 11 |
| Gender (male/female) | 9/1 | 8/2 | 5/3 | 3/8 |
| Age at adrenalectomy (years) | 50.8 ± 2.7 (41.5–58.5) | 54.9 ± 2.6 (52.0–62.0) | 47.5 ± 2.0 (42.3–53.5) | 42.2 ± 2.8 (35.0–48.0) |
| Baseline systolic blood pressure (mmHg) | 158.4 ± 6.7 (140.5–172.3) | 166.2 ± 5.4 (150.0–178.0) | 146. 9 ± 5.8 (135.0–154.5) | 140.8 ± 7.2 (125.0–153.0) |
| Baseline diastolic blood pressure (mmHg) | 90.2 ± 4.1 (84.0–97.5) | 94.6 ± 2.7 (90.0–100.0) | 92.8 ± 4.3 (85.5–100.0) | 82.6 ± 5.0 (72.0–100.0) |
| Maximal tumor Size (mm) | 13.4 ± 1.5 (9.0–15.3) | 16.3 ± 1.4 (14.0–19.0) | 11.4 ± 1.2 (8.3–14.5) | 20.7 ± 1.5 (15.0–24.0) |
| Nadir serum K+ (mmol/L) | 2.8 ± 0.14 (2.5–3.2) | 2.7 ± 0.1 (2.4–3.1) | 3.1 ± 0.1 (2.6–3.5) | 3.4 ± 0.2 (2.9–3.5) |
| Baseline plasma aldosterone concentration (PAC) (ng/dL) | 46.8 ± 9.7 (12.4–74.1) | 79.8 ± 21.0 (27.5–162.2) | 49.0 ± 14.5 (17.4–60.6) | 37.1 ± 5.8 (24.7–47.0) |
| Baseline active renin concentration (ARC) (mU/L) | 4.6 ± 1.6 (1.2–9.1) | 7.5 ± 4.7 (0.8–9.0) | 8.2 ± 1.6 (5.1–12.2) | n.d. |
| Baseline plasma renin activity (PRA) (ng/ml/h) | 0.8 ± 0.1 (0.6–1.0) | 0.6 ± 0.4 (0.15–1.4) | 0.3 ± 0.1 (0.1–0.5) | 0.2 ± 0.1 (0.1–0.2) |
| Baseline PAC/ARC ratio (ng/mU) | 158.7 ± 78.5 (40.5–175.8) | 411.9 ± 116.7 (127.0–682.0) | 60.1 ± 22.5 (16.7–114.3) | n.d. |
| Baseline PAC/PRA ratio (ng/dL/ng/mL/h) | 68.6 ± 16.0 (33.4–101.6) | 152.0 ± 65.6 (40.4–285.0) | 188.4 ± 67.0 (58.4–317.5) | 270.1 ± 64.8 (133.0–333.0) |
| PAC post 240 min. saline infusion test (ng/dL) | 26.2 ± 10.8 (10.5–25.7) | 43.1 ± 19.8 (11.5–57.7) | 22.5 ± 4.5 (11.5–24.7) | 30.8 ± 10.8 (18.0–52.3) |
| Tumor cell area (%) | 80.4 ± 2.9 (71.7–89.3) | 74.8 ± 2.7 (67.2–80.1) | 70.9 ± 3.1 (60.9–77.8) | 73.5 ± 4.0 (67.9–84.4) |
| Stroma area (%) | 19.6 ± 2.9 (10.7–28.3) | 25.2 ± 2.7 (20.0–32.9) | 29.1 ± 3.1 (22.2–39.1) | 26.5 ± 4.0 (15.7–32.2) |
| Nuclear area (%) | 13.3 ± 1.7 (9.3–16.8) | 8.8 ± 0.8 (6.1–11.1) | 11.6 ± 1.4 (9.9–13.6) | 10.0 ± 1.2 (6.7–12.6) |
| Cytoplasm area (%) | 67.2 ± 3.8 (58.8–77.9) | 66.0 ± 2.2 (59.8–70.8) | 59.3 ± 3.0 (49.6–65.4) | 63.5 ± 3.5 (60.3–69.8) |
| Nuclear/Cytoplasm ratio | 0.21 ± 0.03 (0.15–0.29) | 0.13 ± 0.01 (0.09–0.16) | 0.20 ± 0.02 (0.17–0.26) | 0.16 ± 0.02 (0.11–0.21) |
| Clear | 32.9 ± 3.9 (23.9–45.8) | 35.6 ± 2.4 (31.0–39.2) | 27.4 ± 4.8 (17.8–38.2) | 38.0 ± 2.9 (32.8–45.2) |
| Compact | 34.2 ± 5.5 (21.0–40.9) | 30.4 ± 3.1 (23.1–33.7) | 31.9 ± 3.7 (26.7–40.5) | 25.5 ± 2.5 (18.5–28.8) |
| Clear/Cytoplasm | 50.3 ± 6.0 (39.0–67.4) | 54.3 ± 3.4 (48.2–62.4) | 45.1 ± 6.8 (30.2–58.9) | 59.8 ± 3.1 (54.4–64.6) |
| Compact/Cytoplasm | 49.7 ± 6.0 (32.6–61.0) | 45.7 ± 3.4 (37.6–51.8) | 54.9 ± 6.8 (41.2–69.8) | 40.2 ± 3.1 (35.4–45.6) |
| CYP11B2 positive area (%) | 34.2 ± 5.9 (12.9–52.6) | 44.7 ± 4.4 (39.0–55.2) | 34.4 ± 6.7 (10.4–53.9) | 35.5 ± 3.7 (25.9–47.1) |
| CYP11B2 H-score | 0.53 ± 0.12 (0.13–0.78) | 0.57 ± 0.08 (0.41–0.75) | 0.56 ± 0.13 (0.1–0.97) | 0.46 ± 0.06 (0.29–0.58) |
| CYP17A1 positive area (%) | 25.4 ± 6.6 (3.4–42.1) | 11.8 ± 4.2 (2.4–18.0) | 32.5 ± 6.0 (21.7–45.1) | 32.2 ± 3.2 (25.4–37.4) |
| CYP17A1 H-score | 0.27 ± 0.07 (0.04–0.43) | 0.13 ± 0.05 (0.02–0.22) | 0.39 ± 0.09 (0.23–0.54) | 0.34 ± 0.04 (0.26–0.38) |
Value: Mean ± standard error of the mean (SEM) (25–75th percentile).
Figure 1.
Representative microphotographs of ATP1A1-, ATP2B3-, CACNA1D-, and KCNJ5-mutated aldosterone-producing adrenocortical adenoma tissue sections stained with hematoxylin and eosin (H&E), and immunostained using antibodies against CYP11B2 and CYP17A1
Quantitative morphological analysis using digital imaging analysis
H&E staining was performed as reported previously (9). All H&E stained sections were digitally scanned and captured using Image Scope AT2 (Leica, Wetzlar, Germany). Digital imaging analysis was subsequently performed HALO Area Quantification ver. 1.0 software (Indica Laboratories, Corrales, NM) to minimize interobserver variance and achieve high reproducibility as reported previously (9). In brief, the whole tumor area was first classified into tumor cell and stromal areas based on architectural patterns. We classified tumor cell areas into nuclear and cytoplasm areas based on their color spectrums; cytoplasm areas within a tumor cell area were further subclassified into clear and compact cells based on the gradients of the eosinophilic color spectrum. Two observers analyzed histological parameters in an independent manner (Y.O and Y.Y).
The ratio of each histological component against the whole tumor area was then calculated. The percentage of clear and compact cell components within the tumor cell area was also calculated.
Quantitative analysis of CYP11B2 and CYP17A1 immunoreactivity using digital imaging analysis
IHC analysis was performed using antibodies against CYP11B2 (mouse monoclonal) (22) and CYP17A1 (rabbit polyclonal) (23) as reported previously (24) All IHC sections were scanned and captured as above (9). The modified H-score system was adopted in this study to evaluate immunoreactivity of CYP11B2 and CYP17A1 in a quantitative fashion (9, 22, 24). The gradient of relative immunointensity was tentatively defined as follows: negative as “0,” weak as “+1,” moderate as “+2,” and marked as “+3.” Thresholds for score 1+ and 3+ were determined based on the gradient of the color spectrum in individual cases and the threshold of score 2+ was set as the midpoint between score 1+ and 3+. H-score of the unit area (mm2) was calculated as follows: Σ (Area of the individual gradients in positive cells × score 1+, 2+ and 3+)/tumor area [the “cytoplasm”area]) (9, 22, 24, 25).
Somatic mutation analysis in APAs by next-generation sequencing
Surgically resected PA adrenals were fixed in 10% neutral-buffered formalin and embedded in paraffin (formalin-fixed paraffin-embedded, FFPE) to prepare 5-µm serial sections. Tissue samples were isolated from 6 unstained sections by dissecting areas corresponding to serial sections of CYP11B2 IHC as previously reported (9, 10, 21, 26, 27). Genomic DNA (gDNA) was extracted using AllPrep DNA/RNA FFPE kit (QIAGEN) as previously reported (10, 21, 26, 27). In each case, 20 ng of isolated gDNA was used to generate a barcoded library by multiplexed PCR using a custom Ion AmpliSeq Panel and the Ion AmpliSeq Library kit 2.0 (Life Technologies) according to the manufacturer’s instructions. The custom Ion AmpliSeq Panel was designed to target the genes previously reported to be mutated in APA or other adrenal diseases (APA_v2 Panel). The APA_v2 Panel includes 499 independent primer pairs targeting the entire coding regions of genes reported to be somatically mutated in APAs (KCNJ5, ATP1A1, ATP2B3, and CACNA1D). Template preparation and sequencing of multiplexed templates were performed as previously reported (10, 21, 26, 27) using Ion PI Chip on the Ion Torrent Proton sequencer (Life Technologies, Carlsbad, CA).
Statistical analysis
Multicomparison analyses were performed for the comparison of histological factors among all genotypes of APAs examined (KCNJ5, ATP1A1, ATP2B3 and CACNA1D) using Kruskal-Wallis test. The correlation between the proportion of the area of tumor cell subtypes and H-SCORE of CYP11B2 and CYP17A1 was evaluated using Spearman’s correlation coefficient. P < .05 was considered statistically significant in this study. The software of JMP Pro ver.14.2.0 was used for statistical analysis.
Results
Comparison of histological features among APAs with different somatic mutations
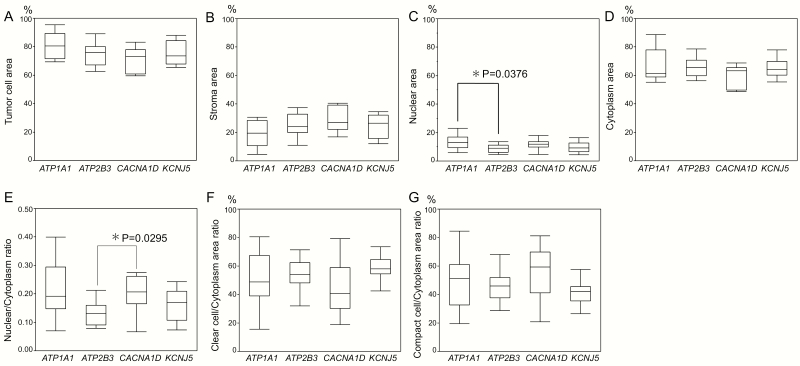
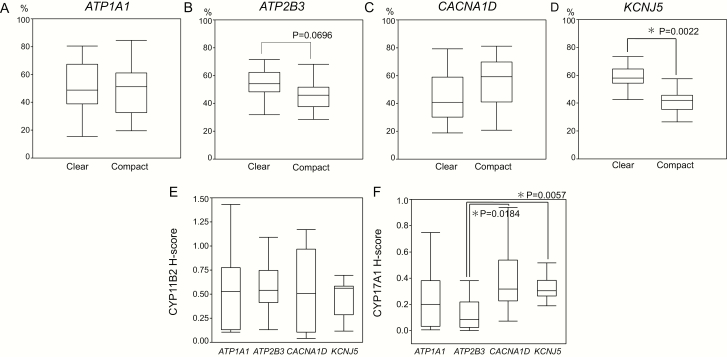
The proportions of tumor and stromal areas were not significantly different among APAs with different genotypes. The proportion of the nuclear area in ATP1A1-mutated APAs was significantly higher than that in ATP2B3-mutated APAs (ATP1A1-mutated: 13.3% [9.3–16.8 %] vs ATP2B3-mutated: 8.8% [6.1–11.1 %], P = .0376). CACNA1D-mutated APAs had a significantly higher nuclear/cytoplasm ratio than ATP2B3-mutated APAs (0.20 [0.17–0.26] vs 0.13 [0.09–0.16], P = .0295) although the proportion of cytoplasm was not significantly different among the different genotypes examined (Table 1 and Fig. 2). The proportion of the clear tumor cell component was significantly higher than that of the compact one in KCNJ5-mutated APAs (59.8% [54.4%–64.6 %] vs 40.2% [35.4%–45.6%], P = .0022) but not significantly higher in ATP2B3-mutated APAs (54.3% [48.2–62.4%] vs 45.7% [37.6–51.8%], P = .0696) (Table 1, Fig. 3). Both ATP1A1- and CACNA1D-mutated APAs harbored more marked histological intratumoral heterogeneity in terms of clear and compact tumor cell distribution, but there was no significant correlation between the proportion of clear or compact tumor cells and specific genotypes of APAs.
Figure 2.
Comparison of histological features of ATP1A1-, ATP2B3-, CACNA1D-, and KCNJ5-mutated aldosterone-producing adrenocortical adenomas (APAs) (A–G). The proportion of nuclear area was significantly higher in ATP1A1-mutated APAs than in ATP2B3-mutated APAs [ATP1A1: 13.3% (9.3–16.8%) versus ATP2B3: 8.8% (6.1–12.0%), P < .05]. The nuclear to cytoplasm ratio was significantly higher in CACNA1D-mutated APAs than in ATP2B3-mutated APAs (0.20 [0.17–0.26] versus 0.13 [0.09– 0.16]; P < .05).
Figure 3.
Comparison of clear and compact tumor cell ratios in ATP1A1-, ATP2B3-, CACNA1D-, and KCNJ5-mutated aldosterone-producing adrenocortical adenomas (APAs) (A–D). The ratio of the clear cell component tended to be more abundant than the compact cell component in ATP2B3-mutated APAs (54.3% [48.2–62.4%]; versus 45.7% [37.6–51.8%]; P = .0696). In KCNJ5-mutated APAs, the clear cell component was significantly much higher than the compact cell component [59.8% (54.4%–64.6%) versus 40.2% (35.4%–45.6%); P = .0022]. Comparison of the H-score of CYP11B2 and CYP17A1 among ATP1A1-, ATP2B3-, CACNA1D-, and KCNJ5-mutated APAs (E,F). The status of CYP17A immunoreactivity was significant different between KCNJ5 and ATP2B3 (P = .0057), as well as between ATP2B3- and CACNA1D-mutated APAs (P = .0184).
Comparison of CYP11B2 and CYP17A1 immunoreactivity among APAs with different somatic mutations
The status of CYP11B2 immunoreactivity (CYP11B2 H score/mm2) was not significantly different among ATP1A1-, ATP2B3-, CACNA1D-, and KCNJ5-mutated APAs (ATP1A1: 0.53 [0.13–0.78], ATP2B3: 0.57 [0.41–0.75], CACNA1D: 0.56 [0.10–0.97], and KCNJ5-mutated APA: 0.46 [0.29–0.58]). However, CYP17A1 immunoreactivity (CYP17A1 H score/mm2) was significantly higher in KCNJ5- than in ATP2B3-mutated APAs (0.34 [0.26–0.38] vs 0.13 [0.02–0.22], P = .0057) and in CACNA1D- than in ATP2B3-mutated APAs (0.39 [80.23–0.54] vs 0.13 [0.02–0.22], P = .0184) (Table 1 and Fig. 3).
Correlation between histological features and immunoreactivity of CYP11B2 and CYP17A1 in individual genotypes of APAs
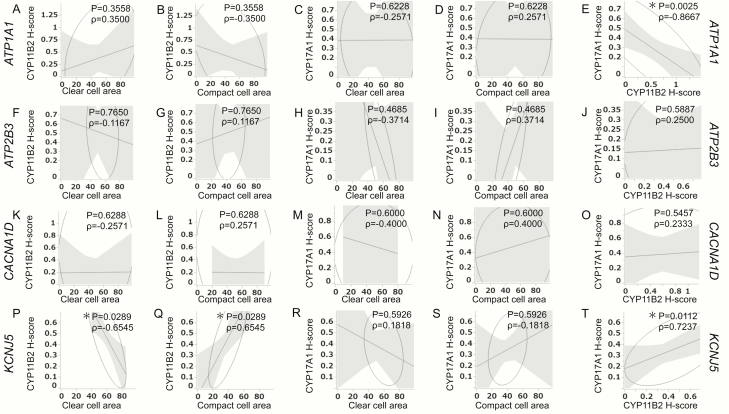
In KCNJ5-mutated APAs, the status of CYP11B2 immunoreactivity (CYP11B2 H score/mm2) was significantly inversely correlated with the proportion of the clear tumor cell component (P = .00289; ρ = −0.6545) but positively with that of compact cells (P = .00289; ρ = 0.6545). There were, however, no significant correlations between CYP11B2 immunoreactivity and clear/compact tumor cell component in ATP1A1-, ATP2B3-, and CACNA1D-mutated APAs as well as between the proportion of clear/compact tumor cell component and the status of CYP17A1 immunoreactivity (CYP17A1 H score/mm2) in APAs, regardless of their somatic mutations. Of particular interest, CYP11B2 and CYP17A1 were significantly positively correlated in KCNJ5-mutated APAs (P = .0112; ρ = 0.7237) but inversely in ATP1A1-mutated APAs (P = .0025; ρ = −0.8667). However, there were no significant correlations between CYP11B2 and CYP17A1 immunoreactivity in both ATP2B3- and CACNA1D-mutated APAs (Fig. 4).
Figure 4.
Correlation between histological components and steroidogenic enzymes in ATP1A1- (A–E), ATP2B3- (F-J), CACNA1D- (K–O), and KCNJ5- (P–T) mutated APAs. Correlation between CYP11B2 immunoreactivity and proportion of clear cell area (A,F,K,P). Correlation between CYP11B2 immunoreactivity and proportion of compact cell area (B,G,L,Q). Correlation between the proportion of clear cell area and CYP17A1 immunoreactivity (C,H,M,R). Correlation between the proportions of compact cell area and CYP17A1 immunoreactivity (D,I,N,S). Correlation between the immunoreactivity of CYP11B2 and CYP17A1 (E,J,O,T). E, Both CYP11B2 and CYP17A1 showed a significant inverse correlation in ATP1A1-mutated APAs (P = .0025; ρ = −0.8667). (P) CYP11B2 immunoreactivity also showed a significant inverse correlation with the proportion of clear cell area in KCNJ5-mutated APAs (P = .0289; ρ = −0.6545). (Q) CYP11B2 immunoreactivity showed a significant correlation with the proportion of compact cell area in KCNJ5-mutated APAs (P = .0289; ρ = 0.6545). (T) Both CYP11B2 and CYP17A1 showed a significant correlation (P = .0112; ρ = 0.7237) in KCNJ5-mutated APAs.
Discussion
This is the first study demonstrating detailed quantitative morphological characteristics of APAs with different somatic mutations identified by targeted NGS and including the relatively rare ATP1A1, ATP2B3 and CACNA1D somatic mutations.
Histological differentiation between clear and compact tumor cells can be occasionally difficult in APAs (9). In addition, the previously proposed histological classification of APAs as “ZG” or “ZF” did not sufficiently represent the biological or functional features of tumor cells (9). Therefore, in this study, we focused on the histological characterization of tumor cells in APAs including those with relatively rare somatic mutations (ATP1A1, ATP2B3, and CACNA1D) based on their morphological and biological or functional features.
The results of our present study revealed that clear tumor cells were indeed predominant in KCNJ5-mutated APAs but not in ATP1A1-, ATP2B3-, and CACNA1D-mutated APAs, all of which demonstrated marked intratumoral morphological heterogeneity. These findings were consistent with previously reported manual analyses (16, 19, 28–31). ATP2B3-mutated APAs had relatively smaller nuclei than ATP1A1-mutated APAs and lower nuclear to cytoplasm ratios than CACNA1D-mutated APAs, indicating that ATP2B3-mutated APAs had smaller nuclei but relatively more abundant cytoplasm containing lipid droplets than APAs with other genotypes. Thus, it has become important to explore the functional significance of these histological differences among different mutated APAs. The status of CYP11B2 immunoreactivity was not significantly different among KCNJ5-, ATP1A1-, ATP2B3-, and CACNA1D-mutated APAs. These findings did indicate that there were no significant differences concerning intratumoral aldosterone biosynthesis among APAs with different somatic mutations. However, the status of CYP17A1 immunoreactivity in tumor cells was indeed significantly lower in ATP2B3-mutated APAs than in CACNA1D- and KCNJ5-mutated APAs. These findings all demonstrated that ATP2B3-mutated APAs could have relatively lower capability of neoplastic aberrant cortisol and aldosterone biosynthesis than KCNJ5- and CACNA1D-mutated APAs. However, further studies including the analysis of cosecretion of cortisol or other glucocorticoids possibly demonstrated by the dexamethasone suppression test and of secretion of hybrid steroids such as 18-oxocortisol in order to explore the biological significance of the findings above.
KCNJ5-mutated APAs are larger and more abundant than clear cell dominant tumors with a much higher frequency of neoplastic aldosterone and cortisol cosecretion than non-KCNJ5-mutated genotypes (32–34). In this study, both CYP11B2 and CYP17A in tumor cells of KCNJ5-mutated APAs were significantly more abundant than in those of APAs of other genotypes. Hybrid tumor cells which coexpressed CYP11B1+/CYP11B2+ and/or CYP17A+/CYP11B2+, and even triple positive hybrid cells (CYP17A+/CYP11B1+/CYP11B2+) have been reported in APAs (33, 34). These hybrid cells were also reported to be specific for APAs, as they were not detected in normal or hyperplastic aldosterone producing cortical cells (31, 33). Tezuka et al., also recently reported that these hybrid cells were significantly more abundant and synthesized increased amounts of hybrid steroids such as 18-oxocortisol in KCNJ5-mutated APAs compared with non KCNJ5-mutated APAs (34). These finding also indicated that KCNJ5-mutated APAs could represent more deviated features from zonation-based differentiation of normal adrenocortical cells.
ATP2B3-mutated APAs demonstrated relative clear cell dominant histology but CYP11B2 and CYP17A in tumor cells did not necessarily show a positive correlation. ATP1A1-mutated APAs had more compact or eosinophilic tumor cells than other genotypes despite a more pronounced intratumoral morphological heterogeneity. Of particular interest, CYP11B2 and CYP17A in tumor cells showed an inverse correlation in ATP1A1-mutated APAs. These findings all indicated that ATP1A1- and ATP2B3-mutated APAs displayed a more zonation-based or organized differentiation than KCNJ5-mutated APAs. In addition, aldosterone biosynthesis in these tumors was more similar to that in normal or hyperplastic zona glomerulosa. There were no significant correlations in CACNA1D-mutated APAs in contrast to KCNJ5-, ATP1A1-, and ATP2B3-mutated APAs. Therefore, further investigations are required to clarify the mechanistic aspects of the correlation between individual somatic mutations and the phenotypes revealed by our present study to achieve a better understanding of neoplastic aldosterone overproduction in APAs.
Acknowledgments
Financial Support: M. Reincke is supported by the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement No. 694913); F. Beuschlein, M. Reincke and T.A. Williams are supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) Projektnummer: 314061271-TRR 205. T. Else is sponsored by NHLBI (5R01HL130106-04). This study was also supported by the Friedrich Baur Stiftung (F-B-S), grant number 46/16 “Genetics and Tissue-based Metabolomics of Aldosterone Producing Adenoma” awarded to Y. Rhayem. F. Satoh and H. Sasano have received grants from the Ministry of Health, Labor, and Welfare, Japan (No. H29-Nanji-Ippan-046).
Glossary
Abbreviations
- APA
aldosterone-producing adrenocortical adenoma
- H&E
hematoxylin and eosin
- IHA
idiopathic hyperaldosteronism
- PA
primary aldosteronism
- NGS
next-generation sequencing
- ZF
zona fasciculata
- ZG
zona glomerulosa
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability: The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Funder JW, Carey RM, Mantero F, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(5):1889–1916. [DOI] [PubMed] [Google Scholar]
- 2. Mulatero P, Stowasser M, Loh KC, et al. Increased diagnosis of primary aldosteronism, including surgically correctable forms, in centers from five continents. J Clin Endocrinol Metab. 2004;89(3):1045–1050. [DOI] [PubMed] [Google Scholar]
- 3. Rossi GP, Bernini G, Caliumi C, et al. ; PAPY Study Investigators A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J Am Coll Cardiol. 2006;48(11):2293–2300. [DOI] [PubMed] [Google Scholar]
- 4. Stowasser M, Taylor PJ, Pimenta E, Ahmed AH, Gordon RD. Laboratory investigation of primary aldosteronism. Clin Biochem Rev. 2010;31(2):39–56. [PMC free article] [PubMed] [Google Scholar]
- 5. Hannemann A, Bidlingmaier M, Friedrich N, et al. Screening for primary aldosteronism in hypertensive subjects: results from two German epidemiological studies. Eur J Endocrinol. 2012;167(1):7–15. [DOI] [PubMed] [Google Scholar]
- 6. Calhoun DA. Hyperaldosteronism as a common cause of resistant hypertension. Annu Rev Med. 2013;64:233–247. [DOI] [PubMed] [Google Scholar]
- 7. Satoh F, Abe T, Tanemoto M, et al. Localization of aldosterone-producing adrenocortical adenomas: significance of adrenal venous sampling. Hypertens Res. 2007;30(11):1083–1095. [DOI] [PubMed] [Google Scholar]
- 8. Neville AM, O’Hare MJ.. The Human Adrenal Cortex: Pathology and Biology-An Integrated Approach. Berlin, Germany: Springer-Verlag; 1982. [Google Scholar]
- 9. Yamazaki Y, Omata K, Tezuka Y, et al. Tumor cell subtypes based on the intracellular hormonal activity in KCNJ5-mutated aldosterone-producing adenoma. Hypertension. 2018;72(3):632–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nanba K, Chen AX, Omata K, et al. Molecular heterogeneity in aldosterone-producing adenomas. J Clin Endocrinol Metab. 2016;101(3):999–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Choi M, Scholl UI, Yue P, et al. K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension. Science. 2011;331(6018):768–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Beuschlein F, Boulkroun S, Osswald A, et al. Somatic mutations in ATP1A1 and ATP2B3 lead to aldosterone-producing adenomas and secondary hypertension. Nat Genet. 2013;45(4):440–444, 444e1. [DOI] [PubMed] [Google Scholar]
- 13. Åkerström T, Maharjan R, Sven Willenberg H, et al. Activating mutations in CTNNB1 in aldosterone producing adenomas. Sci Rep. 2016;6:19546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Berthon A, Drelon C, Ragazzon B, et al. WNT/β-catenin signalling is activated in aldosterone-producing adenomas and controls aldosterone production. Hum Mol Genet. 2014;23(4):889–905. [DOI] [PubMed] [Google Scholar]
- 15. Scholl UI, Healy JM, Thiel A, et al. Novel somatic mutations in primary hyperaldosteronism are related to the clinical, radiological and pathological phenotype. Clin Endocrinol. 2015;83(6):779–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fernandes-Rosa FL, Williams TA, Riester A, et al. Genetic spectrum and clinical correlates of somatic mutations in aldosterone-producing adenoma. Hypertension. 2014;64(2):354–361. [DOI] [PubMed] [Google Scholar]
- 17. Nanba K, Omata K, Gomez-Sanchez CE, et al. Genetic characteristics of aldosterone-producing adenomas in blacks. Hypertension. 2019;73(4):885–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lenzini L, Rossitto G, Maiolino G, Letizia C, Funder JW, Rossi GP. A meta-analysis of somatic KCNJ5 K(+) channel mutations in 1636 patients with an aldosterone-producing adenoma. J Clin Endocrinol Metab. 2015;100(8):E1089–E1095. [DOI] [PubMed] [Google Scholar]
- 19. Monticone S, Castellano I, Versace K, et al. Immunohistochemical, genetic and clinical characterization of sporadic aldosterone-producing adenomas. Mol Cell Endocrinol. 2015;411:146–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Weiss LM. Comparative histologic study of 43 metastasizing and nonmetastasizing adrenocortical tumors. Am J Surg Pathol. 1984;8(3):163–169. [DOI] [PubMed] [Google Scholar]
- 21. Yamazaki Y, Nakamura Y, Omata K, et al. Histopathological classification of cross-sectional image-negative hyperaldosteronism. J Clin Endocrinol Metab. 2017;102(4):1182–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gomez-Sanchez CE, Qi X, Velarde-Miranda C, et al. Development of monoclonal antibodies against human CYP11B1 and CYP11B2. Mol Cell Endocrinol. 2014;383(1-2):111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nakamura Y, Kurotaki Y, Ise K, Felizola SJ, McNamara KM, Sasano H. GATA6, SF1, NGFIB and DAX1 in the remodeled subcapsular zones in primary aldosteronism. Endocr J. 2014;61(4):393–401. [DOI] [PubMed] [Google Scholar]
- 24. Konosu-Fukaya S, Nakamura Y, Satoh F, et al. 3β-Hydroxysteroid dehydrogenase isoforms in human aldosterone-producing adenoma. Mol Cell Endocrinol. 2015;408:205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McCarty KS Jr, Miller LS, Cox EB, Konrath J, McCarty KS Sr. Estrogen receptor analyses. Correlation of biochemical and immunohistochemical methods using monoclonal antireceptor antibodies. Arch Pathol Lab Med. 1985;109(8):716–721. [PubMed] [Google Scholar]
- 26. Omata K, Yamazaki Y, Nakamura Y, et al. Genetic and histopathologic intertumor heterogeneity in primary aldosteronism. J Clin Endocrinol Metab. 2017;102(6):1792–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Omata K, Anand SK, Hovelson DH, et al. Aldosterone-producing cell clusters frequently harbor somatic mutations and accumulate with age in normal adrenals. J Endocr Soc. 2017;1(7):787–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Azizan EA, Poulsen H, Tuluc P, et al. Somatic mutations in ATP1A1 and CACNA1D underlie a common subtype of adrenal hypertension. Nat Genet. 2013;45(9):1055–1060. [DOI] [PubMed] [Google Scholar]
- 29. Kitamoto T, Suematsu S, Yamazaki Y, et al. Clinical and steroidogenic characteristics of aldosterone-producing adenomas with ATPase or CACNA1D gene mutations. J Clin Endocrinol Metab. 2016;101(2):494–503. [DOI] [PubMed] [Google Scholar]
- 30. Scholl UI, Healy JM, Thiel A, et al. Novel somatic mutations in primary hyperaldosteronism are related to the clinical, radiological and pathological phenotype. Clin Endocrinol. 2015;83(6):779–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nakamura Y, Maekawa T, Felizola SJ, et al. Adrenal CYP11B1/2 expression in primary aldosteronism: immunohistochemical analysis using novel monoclonal antibodies. Mol Cell Endocrinol. 2014;392(1–2):73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ono Y, Nakamura Y, Maekawa T, et al. Different expression of 11β-hydroxylase and aldosterone synthase between aldosterone-producing microadenomas and macroadenomas. Hypertension. 2014;64(2):438–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nakamura Y, Kitada M, Satoh F, et al. Intratumoral heterogeneity of steroidogenesis in aldosterone-producing adenoma revealed by intensive double- and triple-immunostaining for CYP11B2/B1 and CYP17. Mol Cell Endocrinol. 2016;422:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tezuka Y, Yamazaki Y, Kitada M, et al. 18-Oxocortisol synthesis in aldosterone-producing adrenocortical adenoma and significance of KCNJ5 mutation status. Hypertension. 2019;73(6):1283–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]