Abstract
STUDY QUESTION
Have mean age at menarche or mean age at natural menopause changed from the 1939 birth cohort to the 1964 birth cohort?
SUMMARY ANSWER
We estimated a minor decrease in mean age at menarche and an increase by nearly 3 years in mean age at natural menopause.
WHAT IS KNOWN ALREADY
In the Western world, age at menarche decreased across birth cohorts from the early 1800s until the 1950s. Whether mean age at menarche has continued to decrease in birth cohorts after the 1950s remains uncertain. It is also uncertain whether mean age at natural menopause has changed across birth cohorts.
STUDY DESIGN, SIZE, DURATION
We performed a retrospective population study of 312 656 women who were born in Norway during the years 1936–1964.
PARTICIPANTS/MATERIALS, SETTING, METHODS
The data were obtained by two self-administered questionnaires from women who participated in the Norwegian breast cancer screening program (BreastScreen Norway) during the years 2006–2014. We used flexible parametric survival models with restricted cubic splines to estimate mean age at menarche, mean age at menopause and mean number of years between menarche and menopause according to the women’s year of birth. The women who were still having menstrual periods contributed with follow-up time until the time of data collection, and the women who had reported surgical removal of the uterus and/or both ovaries prior to natural menopause contributed with follow-up time until the time of surgery.
MAIN RESULTS AND THE ROLE OF CHANCE
The mean age at menarche was 13.42 years (95% CI: 13.40–13.44 years) among women born during 1936–1939, and it was 13.24 years (95% CI: 13.22–13.25 years) among women born during 1960–1964. The mean age at natural menopause increased from 50.31 years (95% CI: 50.25–50.37 years) among women born during 1936–1939 to 52.73 years (95% CI: 52.64–52.82 years) among women born during 1960–1964. The mean number of years between menarche and menopause increased from 36.83 years (95% CI: 36.77–36.89 years) to 40.22 years (95% CI: 40.11–40.34 years).
LIMITATIONS, REASONS FOR CAUTION
Information about age at menarche and age at menopause was based on self-reports.
WIDER IMPLICATIONS OF THE FINDINGS
Late menopause is associated with increased risk of breast cancer but also with increased life expectancy. Thus, higher mean age at menopause may partly explain the increase in breast cancer incidence after menopause and the increase in life expectancy in recent time. Also, a longer interval between menarche and menopause could suggest that the number of years of female fecundity has increased.
STUDY FUNDING/COMPETING INTEREST(S)
This work was funded by the South-Eastern Norway Regional Health Authority [grant number 2016112 to M.S.G.] and by the Norwegian Cancer Society [grant number 6863294-2015 to E.K.B.]. The authors declare no conflicts of interest.
Keywords: birth cohort , menarche , menopause , population study , secular trend
Introduction
Menarche and menopause are milestones in a woman’s reproductive life. Both age at menarche and age at natural menopause display considerable variation between women and may also vary across time periods (Parent et al., 2003; Dratva et al., 2009).
In the Western world, mean age at menarche decreased across birth cohorts from the early 1800s until the 1950s (Wyshak and Frisch, 1982; Rosenberg, 1991). This downward trend has been explained by improved living conditions and nutritional status among girls (Wyshak and Frisch, 1982). Some studies suggest that age at menarche has continued to decrease after 1950 (Mendoza et al., 2010; Talma et al., 2013; Lewington et al., 2014; Gentry-Maharaj et al., 2017; InterLace Study Team, 2019). However, others suggest that the downward trend has leveled off (Brundtland and Walloe, 1973; Wyshak and Frisch, 1982; Vercauteren and Susanne, 1985; Lindgren et al., 1991; Parent et al., 2003; Forman et al., 2013; Bratke et al., 2017).
Whether mean age at natural menopause has changed remains uncertain. Some studies suggest that mean age at menopause has increased across birth cohorts from the beginning of 1900 until the 1950s (Rodstrom et al., 2003; Nichols et al., 2006; Dratva et al., 2009; Lewington et al., 2014; Park et al., 2018). Few studies have investigated the trend in age at menopause across birth cohorts after 1950 (Duarte et al., 2014; Gentry-Maharaj et al., 2017; InterLace Study Team, 2019). One study found no trend in age at menopause in a multiethnic population (InterLace Study Team, 2019). The two other studies represent a large number of European postmenopausal women, and they report that age at menopause increased across birth cohorts from 1920 to 1932 (Duarte et al., 2014) and 1925 to 1944 (Gentry-Maharaj et al., 2017), respectively. Thereafter, age at menopause decreased.
If age at menarche or age at menopause changes across birth cohorts, the time interval between menarche and menopause may also change. A study from the USA reported that the number of years between menarche and menopause increased across birth cohorts from 1910 to 1939 (Nichols et al., 2006). We are not aware of any recent studies about trends in the time interval between menarche and menopause.
Age at menarche and menopause and also the time interval between these events may influence women’s fecundity and health (Cooper and Sandler, 1998; Jacobsen et al., 2003; Wu et al., 2014). Valid knowledge about temporal trends in age at menarche and menopause may therefore be important for the understanding of the disease burden in the female population. We investigated possible temporal changes in mean age at menarche, age at natural menopause and the time interval between menarche and natural menopause among 312 656 women who were born in Norway during the years 1936–1964. To avoid underestimation of age at menopause in the most recent birth cohorts, we used a time to event approach that allowed inclusion of women who still had menstrual periods.
Materials and Methods
Study design, recruitment and data collection
We performed a retrospective population study, and we aimed to include all women in Norway who were born during the years 1936–1964. The Norwegian breast cancer screening program (BreastScreen Norway) invites all women, 50–69 years of age, to biennial mammography. The BreastScreen Norway is administered by the Cancer Registry of Norway, and 84% of all women in the targeted age group have participated at least at one occasion (Sebuødegård et al., 2016).
During the years 2006–2014, all women who participated in the BreastScreen Norway were asked to answer two self-administered questionnaires at their first screening examination (Tsuruda et al., 2018). The questionnaires were sent by post along with the invitation to the screening examination, and they were returned at the screening site. The first questionnaire included questions about demographics, reproductive factors and lifestyles prior to the age of 50. The second questionnaire included questions about current health, menstruation and surgery on the uterus or ovaries. A total of 387 273 women, born during the years 1936–1964, completed both questionnaires and were eligible to participate in our study.
Study sample
Of the 387 273 women, we excluded women who reported that menstruation had never occurred (n = 155) and women with missing information or outlying values (<5 and > 25 years) on age at menarche (n = 25 275) (Supplementary Fig. SI). Thereafter, we excluded women with missing information or outlying values (<15 and >71 years) on age at menopause (n = 28 404). We also excluded women with missing information or outlying values on age at hysterectomy and/or bilateral oophorectomy (n = 1101). Since mean age at menarche and menopause may vary between countries (Kaplowitz, 2006; Dratva et al., 2009), we excluded women who were not born in Norway or had missing information about country of birth (n = 19 682). Thus, a total of 312 656 women who were born in Norway during the years 1936–1964 could be included in our data analyses.
Study factors
Our main exposure variable was the woman’s year of birth (as a continuous variable). In additional analyses, we grouped the woman’s birth year into five-year intervals: 1936–1939 (reference), 1940–1944, 1945–1949, 1950–1954, 1955–1959 and 1960–1964.
Our outcome variables were age at menarche, age at natural menopause and number of years between menarche and menopause. Age at menarche was based on the following question: ‘At what age (years old) did you have your first menstrual period?’ Age at menopause was based on the following two questions: ‘Are you still having menstrual periods?’ (yes/yes, but unregularly/no) and ‘If you no longer have menstrual periods, how old were you at your last menstrual period?’ For descriptive purposes, we also categorized age at menopause into menopause before the age of 45 (early menopause, yes/no), and menopause before the age of 40 (primary ovarian insufficiency, yes/no).
Information about surgery on the uterus was based on the following questions: ‘Have you had your uterus removed?’ (no/yes/don’t know), and ‘If yes, how old were you at the time of surgery?’ The questions about surgery on the ovaries were as follows: ‘Have you had both your ovaries removed?’ (no/no, I have only had one ovary removed/yes/don’t know), ‘If yes, how old were you at the time of surgery?’
Statistical methods
To avoid underestimation of age at natural menopause in the most recent birth cohorts, we applied time to event analyses. In the analyses of reported age at menopause according to birth year, the time to event was from birth until attained age at menopause. The women who were still having menstrual periods (16.6%) or had irregular menstrual periods (7.7%) contributed with follow-up time until their attained age at data collection (censoring). The women who reported hysterectomy (6.3%), bilateral oophorectomy (0.6%) or both surgeries (3.0%) prior to menopause contributed with follow-up time until their attained age at surgery. We used the same approach when estimating the trend in the number of years between menarche and natural menopause, but in these analyses the time to event was from menarche until menopause or censoring (time of data collection or surgery). When estimating age at menarche according to birth year, the time to event was from birth until menarche, and all women contributed with follow-up time until menarche.
We estimated restricted mean age at menarche, natural menopause and number of years between menarche and natural menopause according to birth year (as a continuous variable and in five-year intervals) by applying flexible parametric survival models (the stpm2 command in Stata) (Crowther and Lambert, 2014). In our analyses, age at menopause was restricted by the highest reported age at menopause in the cohort (71 years). Thus, the restricted mean time to menopause can be interpreted as mean time to menopause. For the younger birth cohorts, the calculations of restricted mean age at menopause were performed assuming fixed baseline hazard and proportional hazards. The proportional hazards assumption was evaluated by the Schoenfeld residuals, and by inspection of the log–log plots. By using restricted cubic splines with four degrees of freedom (five knots), we allowed for possible non-linear trends (Crowther and Lambert, 2014).
In additional analyses, we estimated the association of birth year with age at menarche, age at natural menopause and number of years between menarche and menopause as crude hazard ratios by applying flexible parametric survival models. We calculated 95% CI for the estimated restricted means and for the hazard ratios. All data analyses were performed by using Stata/SE version 14.2 (StataCorp, College Station, TX, USA).
The participation rate in the Breast Screen Norway was lowest among women with low education (Le et al., 2018), and low education has been associated with late menarche (Deardorff et al., 2014) and early menopause (Gold et al., 2013). In supplementary analyses, we therefore studied the mean age in menarche, mean age at menopause and mean number of years between menarche and menopause according to birth cohort within levels of completed education (less than high school, high school, college/university). It may be argued that menopause before the age at 40 and after the age at 60 years is not part of the normal distribution of age at menopause, but rather a consequence of disease, treatment or errors in reporting. Thus, we performed supplementary analyses after exclusion of women with menopause before the age of 40 years and after the age of 60 years (n = 11 628).
Ethical considerations
This study was approved by the Regional Committee for Medical and Health Research Ethics (reference no. 2014/1711 REK South-East D). All women received written information. By returning the questionnaires the women agreed to participate.
Results
Mean age at data collection was 56.8 years (SD 5.8 years, range 48–71 years), mean reported age at menarche was 13.23 years (95% CI: 13.22–13.24 years) and mean reported age at natural menopause was 51.10 years (95% CI: 51.07–51.11 years). Of all women, 65.8% had reached natural menopause (Table I). The proportion was highest among women born in 1936–1939 (88.7%) and lowest among women born in 1960–1964 (31.1%).
Table I. Study characteristics according to year of birth among 312 656 women in the BreastScreen Norway (2006–2014).
|
Total
N (%) |
1936–1939
N (%) |
1940–1944
N (%) |
1945–1949
N (%) |
1950–1954
N (%) |
1955–1959
N (%) |
1960–1964
N (%) |
|
|---|---|---|---|---|---|---|---|
| Number of women | 312 656 (100.0) | 17 409 (5.6) | 45 831 (14.7) | 63 716 (20.4) | 70 507 (22.6) | 72 789 (23.3) | 42 404 (13.6) |
| Natural menopause | 205 731 (65.8) | 15 442 (88.7) | 40 036 (87.4) | 55 719 (87.5) | 51 617 (73.2) | 29 729 (40.8) | 13 188 (31.1) |
| <45 years at menopausea | 19 333 (6.5) | 1456 (8.9) | 3428 (7.9) | 4427 (7.3) | 4121 (6.1) | 3865 (5.5) | 2036 (5.0) |
| <40 years at menopauseb | 4062 (1.3) | 269 (1.6) | 673 (1.5) | 905 (1.5) | 825 (1.2) | 851 (1.2) | 539 (1.3) |
| Surgery on uterus and ovaries prior to menopause | |||||||
| Hysterectomy | 19 607 (6.3) | 1097 (6.3) | 3153 (6.9) | 4012 (6.3) | 4418 (6.3) | 4452 (6.1) | 2475 (5.8) |
| Bilateral oophorectomy | 1908 (0.6) | 132 (0.8) | 358 (0.8) | 445 (0.7) | 459 (0.7) | 348 (0.5) | 166 (0.4) |
| Hysterectomy and bilateral oophorectomy | 9390 (3.0) | 694 (4.0) | 2060 (4.5) | 2716 (4.3) | 2114 (3.0) | 1276 (1.8) | 530 (1.3) |
| Oral contraceptive usec | |||||||
| Yes | 166 408 (55.0) | 3774 (22.7) | 15 610 (35.5) | 28 151 (45.9) | 37 852 (55.4) | 47 580 (67.0) | 33 441 (80.6) |
| No | 136 282 (45.0) | 12 877 (77.3) | 28 384 (64.5) | 33 150 (54.1) | 30 425 (44.6) | 23 387 (33.0) | 8059 (19.4) |
| Educational leveld | |||||||
| Less than high school | 78 707 (25.5) | 8497 (50.0) | 19 196 (42.6) | 21 364 (33.9) | 16 031 (23.0) | 10 237 (14.2) | 3382 (8.1) |
| High school | 129 694 (42.0) | 5304 (31.2) | 15 267 (33.9) | 24 657 (39.2) | 29 992 (42.9) | 34 243 (47.5) | 20 231 (48.2) |
| College/university | 100 595 (32.6) | 3198 (18.8) | 10 578 (23.5) | 16 919 (26.9) | 23 829 (42.1) | 27 690 (38.4) | 18 381 (43.8) |
aExclusion of women with hysterectomy and/or bilateral oophorectomy before the age of 45 (N = 15 329).
bExclusion of women with hysterectomy and/or bilateral oophorectomy before the age of 40 (N = 7241).
c N = 302 690 women due to missing information about oral contraceptives.
d N = 308 996 women due to missing information about educational level.
The mean age at menarche displayed a weak u-shaped pattern across birth cohorts (Fig. 1A). Thus, mean age at menarche was 13.42 years (95% CI: 13.40–13.44 years) among women born during 1936–1939 and decreased to 13.18 years (95% CI: 13.17–13.19 years) among women born during 1955–1959. Among women born during 1960–1964, mean age at menarche was 13.24 years (95% CI: 13.22–13.25 years) (Table II).
Figure 1. Temporal trends in age at menarche, age at menopause and the number of years between menarche and menopause among 312 656 women born in Norway, during the years 1936–1964. Flexible parametric survival models were used to estimate (A) mean age at menarche, (B) mean age at menopause and (C) mean number of years between menarche and menopause.
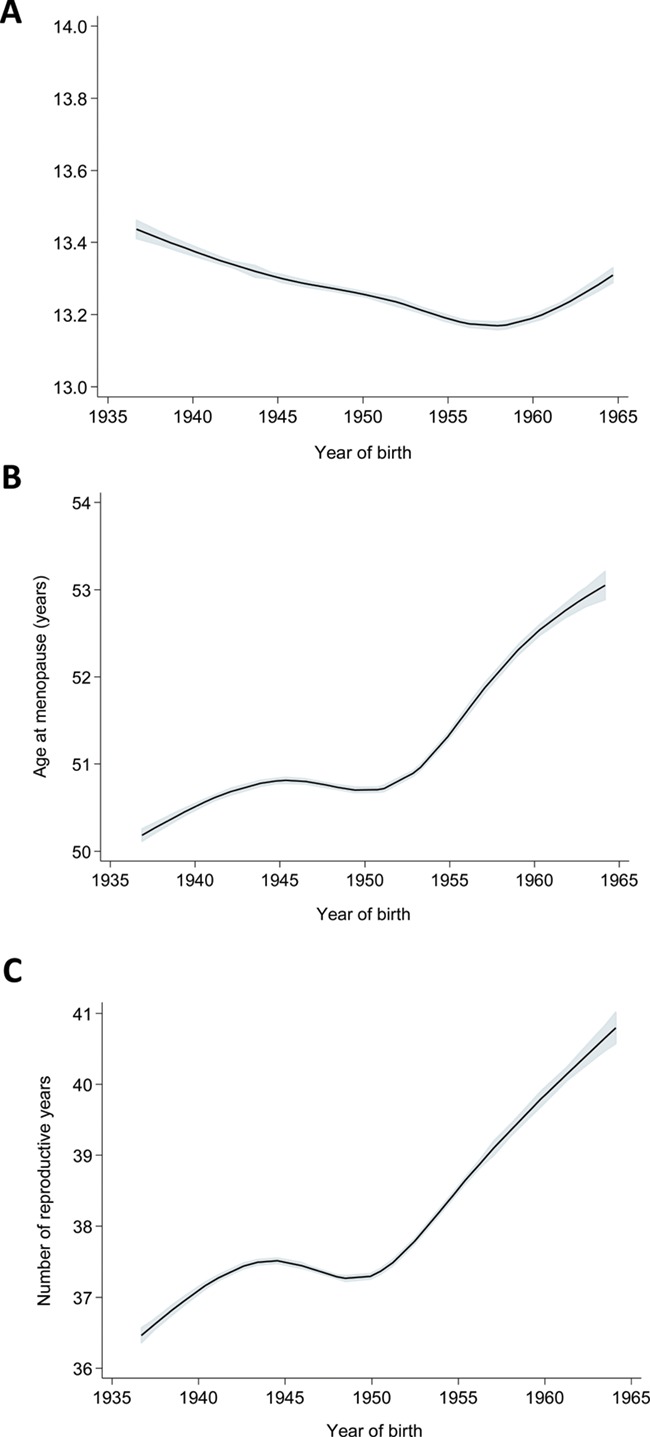
Table II. Temporal trends in age at menarche, age at menopause and number of years between menarche and menopause among 312 656 women in the BreastScreen Norway, born during the years 1936–1964.
| Age at menarche (years) | Age at menopause (years) | Interval a (years) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. women | Mean | 95% CI | HR | 95% CI | Mean | 95% CI | HR | 95% CI | Mean | 95% CI | HR | 95% CI | ||
| Birth cohort | ||||||||||||||
| 1936–1939 | 17 409 | 13.42 | 13.40–13.44 | Reference | 50.31 | 50.25–50.37 | Reference | 36.83 | 36.77–36.89 | Reference | ||||
| 1940–1944 | 45 831 | 13.32 | 13.30–13.33 | 1.08 | 1.06–1.10 | 50.80 | 50.76–50.83 | 0.88 | 0.86–0.90 | 37.44 | 37.39–37.48 | 0.86 | 0.85–0.88 | |
| 1945–1949 | 63 716 | 13.29 | 13.28–13.30 | 1.10 | 1.08–1.12 | 50.70 | 50.66–50.73 | 0.90 | 0.89–0.92 | 37.35 | 37.31–37.38 | 0.88 | 0.86–0.90 | |
| 1950–1954 | 70 507 | 13.21 | 13.20–13.22 | 1.17 | 1.15–1.19 | 50.93 | 50.90–50.97 | 0.85 | 0.83–0.86 | 37.80 | 37.76–37.84 | 0.79 | 0.78–0.81 | |
| 1955–1959 | 72 789 | 13.18 | 13.17–13.19 | 1.20 | 1.18–1.22 | 51.86 | 51.80–51.91 | 0.68 | 0.67–0.70 | 39.18 | 39.11–39.25 | 0.59 | 0.58–0.60 | |
| 1960–1964 | 42 404 | 13.24 | 13.22–13.25 | 1.15 | 1.13–1.17 | 52.73 | 52.64–52.82 | 0.57 | 0.55–0.58 | 40.22 | 40.11–40.34 | 0.49 | 0.48–0.50 | |
The associations of birth cohort with age at menarche, age at menopause and number of years between menarche and menopause are estimated as crude HRs with 95% CI by applying flexible parametric models.
aInterval = number of years between menarche and menopause.
HR = hazard ratio.
The mean age at menopause increased by almost 3 years across birth cohorts, from 50.31 years (95% CI: 50.25–50.37 years) among women born during 1936–1939 to 52.73 years (95% CI: 52.64–52.82 years) among women born during 1960–1964 (Table II). The women who were born during 1945–1949, deviated from the increasing trend (Fig. 1B) and reached menopause earlier (mean 50.70 years, 95% CI: 50.66–50.73 years) than the women born during 1940–1944 (mean 50.80 years, 95% CI: 50.76–50.83 years) (Table II).
In total, 6.5% of the women reported menopause before the age of 45, and 1.3% reported menopause before the age of 40 (Table I). The proportion of women with menopause before the age of 45 or before the age of 40 decreased across birth cohorts from 1936 until 1964.
The decrease in mean age at menarche along with the increase in mean age at menopause across birth cohorts resulted in an increase in the number of years between menarche and menopause (Fig. 1C). The estimated mean number of years increased from 36.83 years (95% CI: 36.77–36.89 years) among women born during 1936–1939 to 40.22 years (95% CI: 40.11–40.34 years) among women born during 1960–1964 (Table II). The women born during 1945–1949 deviated from an almost linear increase in the time interval between menarche and menopause (Fig. 1C, Table II).
We found similar results, independent of the women’s level of education (Supplementary Table SI). Also after exclusion of women with menopause before the age of 40 years and after the age of 60 years, we found similar increase in mean age at menopause across birth cohorts as in our main analyses (Supplementary Table SII).
Discussion
Summary of findings
In this population study of 312 656 women who were born in Norway during the years 1936–1964, age at menarche did not change substantially across birth cohorts. However, age at natural menopause and the time interval between menarche and menopause increased by ~3 years.
Strengths and limitations
To our knowledge, this is the largest study yet to apply a time to event approach for investigation of temporal trends in age at menopause and the interval between menarche and menopause. By using such a data analytic approach, we could also include women who still had menstrual periods and the biases caused by oversampling of women with early menopause were minimized. More than 50% of the women in the most recent birth cohorts still had menstrual periods.
We used data from the BreastScreen Norway, which invites all women in Norway at the age of 50–69 years to participate. Among the women who participated, 63% answered both questionnaires in our study and could thereby be included in the data analyses (Tsuruda et al., 2018). Women with low education are underrepresented in the BreastScreen Norway (Le et al., 2018), and low education has been associated with late menarche (Deardorff et al., 2014) and early menopause (Gold et al., 2013). However, in women with high and low education we found similar trends in age at menarche and menopause across birth cohorts.
Information about age at menarche and menopause was based on self-report. Previous studies have found moderate to high agreement of age at menarche as reported in adulthood with the true age at menarche (Cooper et al., 2006; Must et al., 2002). The agreement between self-reported and true age at menopause is also found to be high (Rodstrom et al., 2005).
Age at menopause is typically defined retrospectively as 12 months without menstrual periods (Soules et al., 2001). In our study, the time since the last menstrual period was not reported. Menstrual cycles could possibly reoccur for women who reported their last menstrual period close to the data collection, and some women may have been misclassified according to menopausal status. Such error in reporting would most likely have occurred among the youngest women in our study, and age at menopause may have been underestimated in the youngest birth cohorts. In accordance with a previous study (Hahn et al., 1997), we also observed a digit preference for menopause ages ending in 0, 2 and 5 (not shown). Digit preference may represent errors in the reporting of age at menopause, but there is no reason to believe that such erroneous reporting can explain our findings.
Errors in reporting may have been most common among older women, since the time from menarche and menopause to study participation was longer for older than for younger women. However, there is little reason to believe that the older women systematically reported a later age at menarche and earlier age at menopause than the younger women. Unsystematic errors in reporting would rather have underestimated than overestimated the association of birth year with age at menarche and age at menopause in our study (Clarke et al., 1999).
Comparison with other studies
In many Western countries, mean age at menarche has decreased from 15–17 years among women born in the early 1800s to 13–13.5 years among women born in the 1950s (Tanner, 1973; Wyshak and Frisch, 1982; Rosenberg, 1991). Some studies suggest that age at menarche has continued to decrease in birth cohorts after 1950 (Kaplowitz, 2006; Mendoza et al., 2010; Talma et al., 2013; Lewington et al., 2014; Gentry-Maharaj et al., 2017; Meng et al., 2017; InterLace Study team, 2019). Other studies, however, suggest that the decreasing trend in age at menarche has leveled off after the 1950 birth cohort (Brundtland and Walloe, 1973; Wyshak and Frisch, 1982; Vercauteren and Susanne, 1985; Lindgren et al., 1991; Parent et al., 2003; Forman et al., 2013; Bratke et al., 2017), and such a finding is in agreement with our results.
Age at natural menopause has been reported to increase across birth cohorts from the beginning of 1900 until the 1950s (Rodstrom et al., 2003; Nichols et al., 2006; Dratva et al., 2009; Lewington et al., 2014; Park et al., 2018). A study from the USA supports such an increase and reports that age at menopause increased from 49.1 years in the 1915 birth cohort to 50.5 years in the 1939 birth cohort. This study also reports that the number of years between menarche and menopause increased from 36.9 to 37.7 years across the birth cohorts (Nichols et al., 2006). However, the evidence of an increase in age at menopause across birth cohorts before 1950 is inconsistent (Dratva et al., 2009; Gentry-Maharaj et al., 2017; InterLace Study Team, 2019). Also, it is not known whether age at menopause has continued to increase among women born after 1950. Recent analyses of 172 125 women born during 1900–1959 in 10 different countries did not support a change in age at menopause across birth cohorts (InterLace Study Team, 2019). However, an increase in age at menopause was reported among 5288 European women born during 1940–1973 (Dratva et al., 2009). In another study of ~200 000 postmenopausal women in the UK, age at menopause increased across the birth cohorts from 1925 to 1944, but decreased across the birth cohorts from 1945 to 1955 (Gentry-Maharaj et al., 2017). A similar reversed trend in age at menopause was reported among postmenopausal Portuguese women born during the years 1900–1963, and the decrease was observed after the 1932 birth cohort (Duarte et al., 2014). These two European studies had excluded women who were still having menstrual periods. Thus, women with early menopause may have been overrepresented, particularly in the most recent birth cohorts. Such overrepresentation of women with early menopause may have resulted in an underestimation of mean age at menopause that may explain the reversed trend in these studies.
Interpretations
Our results suggest that mean age at menarche remained almost unchanged across birth cohorts in Norway from 1936 to 1964. The previously reported decrease in age at menarche during the 1800s until the 1950s has been explained by improved nutritional status and health among women (Rees, 1993). However, it is possible that there is a biological lower limit of mean age at menarche in a population and that this limit is around 13 years. Such biological lower limit of the mean age at menarche may explain the minimal changes in our study.
We found that mean age at menopause increased from 50.31 years among women born during 1936–1939 to 52.73 years among women born during 1960–1964. Although it is out of our scope to explain the temporal trends, such an increase could possibly be a result of changes in women’s lifestyles. High body mass index is associated with late menopause (Zhu et al., 2018), and mean body mass index has increased among Norwegian women during the 1900s (Midthjell et al., 1999).
Menopause is estimated to occur when less than 1000 ovarian follicles remain in the ovaries (Faddy et al., 1992). The ovaries are fully developed by the 20th week of fetal life, and atresia of the ovarian follicles follows thereafter (Wallace and Kelsey, 2010). Growth restriction during fetal life may possibly impair ovarian development, and poor nutritional status during early life could increase the rate of follicle atresia and thereby decrease age at menopause (Mishra et al., 2009; Bjelland et al., 2019). Therefore, the increase in birthweight across birth cohorts (Fudvoye and Parent, 2017) could possibly explain part of the increase in age at menopause.
In our study, the increasing trend in age at menopause halted among women who were born at the end and immediately after the Second World War. This finding could possibly be explained by stress or insufficient supply of nutrients during their fetal life (Sadrzadeh et al., 2018). It is known that daughters of pregnant women who were exposed to the Dutch Hunger Winter in 1944–1945, reached menopause early (Elias et al., 2003). Our findings do not suggest a change in age at menarche among women born during or immediately after the Second World War. Some studies have, however, observed a delay in age at menarche among girls who were approaching puberty during war times (van Noord and Kaaks, 1991; Prebeg and Bralic, 2000). The oldest women in our study were born in 1936, and they did not approach puberty until after the Second World War.
Many childbirths have been associated with high age at menopause (Dorjgochoo et al., 2008; Gold et al., 2013), and changes in reproductive patterns could influence mean age at menopause in the population. However, the mean number of childbirths remained unchanged across the birth cohorts after 1944 in our study (not shown). Thus, changes in number of childbirths cannot explain the changes in age at menopause.
It has also been suggested that the use of hormonal contraceptives may delay menopause (van Noord et al., 1997; Gold et al., 2013). Oral contraceptives became generally available in Norway in the mid-1960s, and the use of oral contraceptives increased across birth cohorts in our study (Table I). Thus, increased use of oral contraceptives is consistent with an increase in age at menopause.
Prolonged exposure and high levels of estrogens when not counterbalanced by progesterone may increase the risk of estrogen sensitive breast cancers (Key and Pike, 1988), and endometrial cancer (Pettersson et al., 1986). Late menopause is associated with increased risk of breast cancer (Collaborative Group on Hormonal Factors in Breast Cancer, 1997; Ellingjord-Dale et al., 2017) and endometrial cancer (Xu et al., 2004). Thus, the increased age at menopause and increased number of years between menarche and menopause may have resulted in increased cumulative exposure to endogenous estrogen. It is therefore possible that part of the increased incidence in postmenopausal breast cancer and endometrial cancer during recent years could be attributed to an overall higher age at menopause in the population.
As opposed to breast cancer, the risk of cardiovascular disease, dementia and osteoporosis seem to be decreased in women with late menopause (Ossewaarde et al., 2005; Gallagher, 2007; Gilsanz et al., 2019). Late menopause has also been associated with decreased all-cause mortality (Jacobsen et al., 2003; Ossewaarde et al., 2005). Thus, it is conceivable that the increase in life expectancy for women in Norway during the last decades (Leon, 2011) in part could be explained by the increased age at menopause.
Our results suggest that the mean number of years between menarche and menopause has increased by ~3 years. This finding should encourage studies of possible changes in fecundity, particularly at advanced reproductive age.
Conclusions
Our population study of 312 656 women born in Norway suggest minor changes in age at menarche across birth cohorts from 1936 to 1964. However, age at menopause and also the time interval between menarche and menopause have increased by ~3 years.
Authors’ roles
A.E. and E.K.B. had the original idea for this study. M.S.G., A.E., J.M.G. and E.K.B. discussed the design and planned the data analytic approaches. M.S.G. and E.K.B. performed the data analyses. M.S.G., A.E. and E.K.B. interpreted the results and wrote the manuscript. J.M.G. and S.H. contributed with interpretation of the results, critically revised the article. E.K.B. is the guarantor of the study. All authors had full access to the data and can take responsibility for the integrity of the data and the accuracy of the data analyses. All authors have approved the submitted version of the manuscript.
Funding
South-Eastern Norway Regional Health Authority (2016112 to M.S.G.); Norwegian Cancer Society ( 6863294-2015 to E.K.B.).
Conflict of interest
None to declare.
Supplementary Material
References
- Bjelland EK, Gran JM, Hofvind S, Eskild A. The association of birthweight with age at natural menopause: a population study of women in Norway. Int J Epidemiol 2019; [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratke H, Bruserud IS, Brannsether B, Aßmus J, Bjerknes R, Roelants M, Júlíusson PB. Timing of menarche in Norwegian girls: associations with body mass index, waist circumference and skinfold thickness. BMC Pediatr 2017;17:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundtland GH, Walloe L. Menarchal age in Norway: halt in the trend towards earlier maturation. Nature 1973;241:478–479. [DOI] [PubMed] [Google Scholar]
- Clarke R, Shipley M, Lewington S, Youngman L, Collins R, Marmot M, Peto R. Underestimation of risk associations due to regression dilution in long-term follow-up of prospective studies. Am J Epidemiol 1999;150:341–353. [DOI] [PubMed] [Google Scholar]
- Cooper GS, Sandler DP. Age at natural menopause and mortality. Ann Epidemiol 1998;8:229–235. [DOI] [PubMed] [Google Scholar]
- Cooper R, Blell M, Hardy R, Black S, Pollard TM, Wadsworth ME. Validity of age at menarche self-reported in adulthood. J Epidemiol Community Health 2006;60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collaborative Group on Hormonal Factors in Breast Cancer Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 women without breast cancer. Collaborative Group on Hormonal Factors in Breast Cancer. Lancet 1997;350:1047–1059. [PubMed] [Google Scholar]
- Crowther MJ, Lambert PC. A general framework for parametric survival analysis. Stat Med 2014;33:5280–5297. [DOI] [PubMed] [Google Scholar]
- Deardorff J, Abrams B, Ekwaru JP, Rehkopf DH. Socioeconomic status and age at menarche: an examination of multiple indicators in an ethnically diverse cohort. Ann Epidemiol 2014;24:727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorjgochoo T, Kallianpur A, Gao YT, Cai H, Yang G, Li H, Zheng W, Shu XO. Dietary and lifestyle predictors of age at natural menopause and reproductive span in the Shanghai Women’s Health Study. Menopause 2008;15:924–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dratva J, Gomez Real F, Schindler C, Ackermann-Liebrich U, Gerbase MW, Probst-Hensch NM, Svanes C, Omenaas ER, Neukirch F, Wjst M et al. . Is age at menopause increasing across Europe? Results on age at menopause and determinants from two population-based studies. Menopause 2009;16:385–394. [DOI] [PubMed] [Google Scholar]
- Duarte E, de Sousa B, Cadarso-Suarez C, Rodrigues V, Kneib T. Structured additive regression modeling of age of menarche and menopause in a breast cancer screening program. Biometrical journal Biometrische Zeitschrift 2014;56:416–427. [DOI] [PubMed] [Google Scholar]
- Elias SG, van Noord PA, Peeters PH, den Tonkelaar I, Grobbee DE. Caloric restriction reduces age at menopause: the effect of the 1944-1945 Dutch famine. Menopause 2003;10:399–405. [DOI] [PubMed] [Google Scholar]
- Ellingjord-Dale M, Vos L, Tretli S, Hofvind S, Dos-Santos-Silva I, Ursin G. Parity, hormones and breast cancer subtypes - results from a large nested case-control study in a national screening program. Breast Cancer Res 2017;19:10–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faddy MJ, Gosden RG, Gougeon A, Richardson SJ, Nelson JF. Accelerated disappearance of ovarian follicles in mid-life: implications for forecasting menopause. Hum Reprod 1992;7:1342–1346. [DOI] [PubMed] [Google Scholar]
- Forman MR, Mangini LD, Thelus-Jean R, Hayward MD. Life-course origins of the ages at menarche and menopause. Adolesc Health Med Ther 2013;4:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fudvoye J, Parent AS. Secular trends in growth. Ann Endocrinol 2017;78:88–91. [DOI] [PubMed] [Google Scholar]
- Gallagher JC. Effect of early menopause on bone mineral density and fractures. Menopause 2007;14:567–571. [DOI] [PubMed] [Google Scholar]
- Gentry-Maharaj A, Glazer C, Burnell M, Ryan A, Berry H, Kalsi J, Woolas R, Skates SJ, Campbell S, Parmar M et al. . Changing trends in reproductive/lifestyle factors in UK women: descriptive study within the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS). BMJ Open 2017;7:e011822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilsanz P, Lee C, Corrada MM, Kawas CH, Quesenberry CP Jr, Whitmer RA. Reproductive period and risk of dementia in a diverse cohort of health care members. Neurology 2019;92:e2005–e2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold EB, Crawford SL, Avis NE, Crandall CJ, Matthews KA, Waetjen LE, Lee JS, Thurston R, Vuga M, Harlow SD. Factors related to age at natural menopause: longitudinal analyses from SWAN. Am J Epidemiol 2013;178:70–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn RA, Eaker E, Rolka H. Reliability of reported age at menopause. Am J Epidemiol 1997;146:771–775. [DOI] [PubMed] [Google Scholar]
- InterLace Study Team Variations in reproductive events across life: a pooled analysis of data from 505 147 women across 10 countries. Hum Reprod 2019; dez015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen BK, Heuch I, Kvale G. Age at natural menopause and all-cause mortality: a 37-year follow-up of 19,731 Norwegian women. Am J Epidemiol 2003;157:923–929. [DOI] [PubMed] [Google Scholar]
- Kaplowitz P. Pubertal development in girls: secular trends. Curr Opin Obstet Gynecol 2006;18:487–491. [DOI] [PubMed] [Google Scholar]
- Key TJ, Pike MC. The dose-effect relationship between ‘unopposed’ oestrogens and endometrial mitotic rate: its central role in explaining and predicting endometrial cancer risk. Br J Cancer 1988;57:205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le M, Hofvind S, Tsuruda K, Braaten T, Bhargava S. Lower attendance rates in BreastScreen Norway among immigrants across all levels of socio-demographic factors: a population-based study. J Public Health 2018;2:229–2240. [Google Scholar]
- Leon DA. Trends in European life expectancy: a salutary view. Int J Epidemiol 2011;40:271–277. [DOI] [PubMed] [Google Scholar]
- Lewington S, Li L, Murugasen S, Hong LS, Yang L, Guo Y, Bian Z, Collins R, Chen J, He H et al. . Temporal trends of main reproductive characteristics in ten urban and rural regions of China: the China Kadoorie biobank study of 300 000 women. Int J Epidemiol 2014;43:1252–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren GW, Degerfors IL, Fredriksson A, Loukili A, Mannerfeldt R, Nordin M, Palm K, Petterson M, Sundstrand G, Sylvan E. Menarche 1990 in Stockholm schoolgirls. Acta Paediatr Scand 1991;80:953–955. [DOI] [PubMed] [Google Scholar]
- Mendoza N, Galliano D, Salamanca A, Castro JE, Mozas J, Sanchez-Borrego R, Quereda F, Vazquez F, Martinez-Astorquiza T. Lowering the age at menarche and risk of early menarche in a population of Spanish postmenopausal women during the past two decades. Menopause Int 2010;16:111–114. [DOI] [PubMed] [Google Scholar]
- Meng X, Li S, Duan W, Sun Y, Jia C. Secular trend of age at menarche in Chinese adolescents born from 1973 to 2004. Pediatrics 2017;2:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midthjell K, Kruger O, Holmen J, Tverdal A, Claudi T, Bjorndal A, Magnus P. Rapid changes in the prevalence of obesity and known diabetes in an adult Norwegian population. The Nord-Trondelag Health Surveys: 1984-1986 and 1995-1997. Diabetes Care 1999;22:1813–1820. [DOI] [PubMed] [Google Scholar]
- Mishra GD, Cooper R, Tom SE, Kuh D. Early life circumstances and their impact on menarche and menopause. Womens Health 2009;5:175–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Must A, Phillips SM, Naumova EN, Blum M, Harris S, Dawson-Hughes B. Recall of early menstrual history and menarcheal body size: after 30 years, how well do women remember? Am J Epidemiol 2002;155. [DOI] [PubMed] [Google Scholar]
- Nichols HB, Trentham-Dietz A, Hampton JM, Titus-Ernstoff L, Egan KM, Willett WC, Newcomb PA. From menarche to menopause: trends among US women born from 1912 to 1969. Am J Epidemiol 2006;164:1003–1011. [DOI] [PubMed] [Google Scholar]
- Ossewaarde ME, Bots ML, Verbeek AL, Peeters PH, van der Graaf Y, Grobbee DE, van der Schouw YT. Age at menopause, cause-specific mortality and total life expectancy. Epidemiology 2005;16:556–562. [DOI] [PubMed] [Google Scholar]
- Parent A-S, Teilmann G, Juul A, Skakkebaek NE, Toppari J, Bourguignon J-P. The timing of normal puberty and the age limits of sexual precocity: variations around the world, secular trends, and changes after migration. Endocr Rev 2003;24:668–693. [DOI] [PubMed] [Google Scholar]
- Park CY, Lim JY, Park HY. Age at natural menopause in Koreans: secular trends and influences thereon. Menopause 2018;25:423–429. [DOI] [PubMed] [Google Scholar]
- Pettersson B, Bergström R, Johansson EDB. Serum estrogens and androgens in women with endometrial carcinoma. Gynecol Oncol 1986;25:223–233. [DOI] [PubMed] [Google Scholar]
- Prebeg Z, Bralic I. Changes in menarcheal age in girls exposed to war conditions. Am J Hum Biol 2000;12:503–508. [DOI] [PubMed] [Google Scholar]
- Rees M. Menarche when and why? Lancet 1993;342:1375–1376. [DOI] [PubMed] [Google Scholar]
- Rodstrom K, Bengtsson C, Lissner L, Bjorkelund C. Reproducibility of self-reported menopause age at the 24-year follow-up of a population study of women in Goteborg, Sweden. Menopause 2005;12:275–280. [DOI] [PubMed] [Google Scholar]
- Rodstrom K, Bengtsson C, Milsom I, Lissner L, Sundh V, Bjourkelund C. Evidence for a secular trend in menopausal age: a population study of women in Gothenburg. Menopause 2003;10:538–543. [DOI] [PubMed] [Google Scholar]
- Rosenberg M. Menarcheal age for Norwegian women born 1830-1960. Ann Hum Biol 1991;18:207–219. [DOI] [PubMed] [Google Scholar]
- Sadrzadeh S, Verschuuren M, Schoonmade LJ, Lambalk CB, Painter RC. The effect of adverse intrauterine conditions, early childhood growth and famine exposure on age at menopause: a systematic review. J Dev Orig Health Dis 2018;2:127–136. [DOI] [PubMed] [Google Scholar]
- Sebuødegård S, Sagstad S, Hofvind S. Oppmøte i Mammografiprogrammet. [In Norwegian]. Tidsskr Nor Legeforen 2016;136:1448–1451. [DOI] [PubMed] [Google Scholar]
- Soules MR, Sherman S, Parrott E, Rebar R, Santoro N, Utian W, Woods N. Executive summary: stages of reproductive Aging Workshop (STRAW), Park City, Utah, July, 2001. Menopause 2001;8:402–407. [DOI] [PubMed] [Google Scholar]
- Talma H, Schonbeck Y, van Dommelen P, Bakker B, van Buuren S, Hirasing RA. Trends in menarcheal age between 1955 and 2009 in the Netherlands. PLoS One 2013;8:e60056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner JM. Trend towards earlier menarche in London, Olso, Copenhagen, the Netherlands and Hungary. Nature 1973;243:95–96. [DOI] [PubMed] [Google Scholar]
- Tsuruda KM, Sagstad S, Sebuodegard S, Hofvind S. Validity and reliability of self-reported health indicators among women attending organized mammographic screening. Scand J Public Health 2018;46:744–751. [DOI] [PubMed] [Google Scholar]
- van Noord PA, Dubas JS, Dorland M, Boersma H, te Velde E. Age at natural menopause in a population-based screening cohort: the role of menarche, fecundity, and lifestyle factors. Fertil Steril 1997;68:95–102. [DOI] [PubMed] [Google Scholar]
- van Noord PA, Kaaks R. The effect of wartime conditions and the 1944-45 ‘Dutch famine’ on recalled menarcheal age in participants of the DOM breast cancer screening project. Ann Hum Biol 1991;18:57–70. [DOI] [PubMed] [Google Scholar]
- Vercauteren M, Susanne C. The secular trend of height and menarche in Belgium: are there any signs of a future stop? Eur J Pediatr 1985;144:306–309. [DOI] [PubMed] [Google Scholar]
- Wallace WH, Kelsey TW. Human ovarian reserve from conception to the menopause. PLoS One 2010;5:e8772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Cai H, Kallianpur A, Gao YT, Yang G, Chow WH, Li HL, Zheng W, Shu XO. Age at menarche and natural menopause and number of reproductive years in association with mortality: results from a median follow-up of 11.2 years among 31,955 naturally menopausal Chinese women. PLoS One 2014;9:e103673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyshak G, Frisch RE. Evidence for a secular trend in age of menarche. N Engl J Med 1982;306:1033–1035. [DOI] [PubMed] [Google Scholar]
- Zhu D, Chung HF, Pandeya N, Dobson AJ, Kuh D, Crawford SL, Gold EB, Avis NE, Giles GG, Bruinsma F et al. . Body mass index and age at natural menopause: an international pooled analysis of 11 prospective studies. Eur J Epidemiol 2018;33:699–710. [DOI] [PubMed] [Google Scholar]
- Xu WH, Xiang YB, Ruan ZX, Zheng W, Cheng JR, Dai Q, Gao YT, Shu XO. Menstrual and reproductive factors and endometrial cancer risk: results from a population-based case-control study in urban Shanghai. Int J Cancer 2004;108:613–619. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


