Abstract
STUDY QUESTION
Are progesterone vaginal pessaries 400 mg twice a day (bid) non-inferior to progesterone vaginal gel (90 mg) once a day (od) in the primary endpoint of clinical pregnancy rate after 38 days of luteal phase support in women undergoing in vitro fertilisation (IVF)?
SUMMARY ANSWER
Non-inferiority of progesterone vaginal pessaries 400 mg bid to progesterone 8% vaginal gel (90 mg od) was shown for clinical pregnancy rate after 38 days of luteal phase support.
WHAT IS KNOWN ALREADY
To maximise successful embryo transfer after IVF, additionally administered progesterone is used for proper endometrium transformation in the luteal phase. Vaginally administered progesterone results in adequate secretory transformation of the endometrium.
STUDY DESIGN, SIZE, DURATION
This multicentre, multinational, open, randomised, two-parallel group, non-inferiority Phase 3 clinical trial was carried out at 17 study sites in five European countries (Belgium, Bulgaria, Czech Republic, Hungary and Serbia) between October 2013 and August 2014. An interactive web response system (IWRS) was implemented for treatment allocation at the sites. Power analysis, based on the assumptions of a non-inferiority margin of −9%, a significance level of α 2.5% (one-sided), power 90%, at a reference pregnancy rate for the progesterone vaginal gel group of 30%, as well as applying a dropout rate of 10%, yielded a total number of 766 patients to be randomised.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Women aged between 18 and 40 years with a clinical indication for IVF/intracytoplasmic sperm injection (ICSI) and embryo transfer were eligible to participate. The clinical pregnancy rate was assessed by fetal heart movement measured by transvaginal ultrasound at day 38 (D38) (primary endpoint) and D70. Also assessed were biochemical pregnancy rate (assessed by serum β-hCG ≥25 IU/L), clinical implantation rates at D38, patient evaluation of vaginal bleeding and discharge (assessed by diary) and adverse event (AE) incidence, severity and relationship to study medication.
MAIN RESULTS AND THE ROLE OF CHANCE
A total of 769 female patients were randomised to progesterone 400 mg vaginal pessaries bid (n = 385, 50.1%) or progesterone 90 mg vaginal gel od (n = 384, 49.9%). Patients receiving progesterone vaginal pessaries and progesterone vaginal gel were comparable in demographics, baseline characteristics and number of retrieved oocytes. In the full analysis set (FAS; n = 369 progesterone vaginal pessaries and n = 368 progesterone vaginal gel), clinical pregnancy rates on D38 were 38.3% for progesterone vaginal pessaries and 39.9% for progesterone vaginal gel. In the per protocol analysis set (PP; n = 357 progesterone vaginal pessaries and n = 356 progesterone vaginal gel), clinical pregnancy rates on D38 were 38.1% for progesterone vaginal pessaries and 40.4% for progesterone vaginal gel. For the differences in pregnancy rates between the progesterone vaginal pessaries group and the progesterone vaginal gel, the lower limit of the 97.5% CI was −8.6 and −9.5% for the FAS and PP datasets, respectively. The original prespecified non-inferiority margin of −9% was thus met in the FAS dataset but was marginally below this in the PP dataset. However, the pregnancy rate of the comparator was higher than the anticipated rate of 30%, and a predetermined logistic regression model including treatment group, country and age group effects without interaction terms showed non-inferiority of progesterone vaginal pessaries to progesterone vaginal gel for both the FAS and PP populations, in that the lower limits of the 95% CIs were above 0.7 for both analyses. As a result of this, the relevant authorities accepted to widen the acceptable non-inferiority margin to −10%, and as such both the FAS and PP populations succeeded in showing non-inferiority. Biochemical pregnancy and clinical implantation rates were comparable for both treatments. Both treatment groups showed similar high compliance throughout the study, and the safety profiles were also comparable between the groups. Drug-related AEs occurred with frequencies of 15.1% with progesterone vaginal pessaries and 14.4% with progesterone vaginal gel.
LIMITATIONS, REASONS FOR CAUTION
Clinical pregnancy rate is a surrogate for the outcome of live birth rate.
WIDER IMPLICATIONS OF THE FINDINGS
Progesterone 400 mg pessaries bid for luteal phase support is an effective, safe and tolerable treatment option for women undergoing IVF during ART.
STUDY FUNDING/COMPETING INTEREST(S)
This work was funded by Actavis Group PTC ehf., Iceland, part of Teva Pharmaceuticals, and by L.D. Collins & Co. Ltd. Gedeon Richter plc has recently entered into a license and distribution agreement to commercialise the vaginal pessaries in the European Union (except Ireland/UK). The progesterone vaginal pessaries studied are now marketed as Cyclogest®, Amelgen®, Cyclovita®, Luteum and Cygest® throughout the EU, Asia and Middle East & North Africa. The competing interests are as follows. H.S.: employee of Gedeon Richter plc/PregLem S.A. C.K.: consultant to L.D. Collins & Co. Ltd and received consulting fees for work performed. T.D.H.: at the initiation and completion of this study, full professor at KU Leuven and Head of Leuven University Fertility Center at the University Hospital Gasthuisberg, Leuven, Belgium. In October 2015, T.D.H. became vice president of Global Medical Affairs Fertility at the pharmaceutical company Merck—marketing authorisation holder of the Progesterone vaginal gel (Crinone®)—and has remained a part-time professor at KU Leuven (Belgium) and adjunct professor at Yale University (New Haven, CT, USA). T.B.M.: at the initiation and completion of this study, employee of Actavis Group PTC ehf. I.K.: consultant to Actavis, later TEVA and received consulting fees for work performed. S.H.: at the initiation and completion of this study, employee of Actavis Group PTC ehf.
Trial registration number
EudraCT number 2013-001105-81
Trial registration date
2 July 2013
Date of first patient’s enrolment
9 October 2013
Keywords: luteal phase support, assisted reproductive technologies, ART, intracytoplasmic sperm injection, infertility
Introduction
To maximise pregnancy rates following assisted reproductive techniques (ART), luteal phase support (LPS) is now routinely provided by either progesterone, using various routes of administration, or human chorionic gonadotrophin (hCG), although hCG has been associated with higher rates of ovarian hyperstimulation syndrome (OHSS) (van der Linden et al., 2015). When progesterone is administered vaginally, high endometrial tissue progesterone levels may be achieved even if the serum levels are relatively low (Miles et al., 1994; Cicinelli et al., 2000) due to a direct vagina-to-uterus transport mechanism (Bulletti et al., 1997; Tavaniotou et al., 2000). Cyclogest® is a marketed vaginal progesterone pessary preparation registered in the EU in the past for premenstrual syndrome and postpartum depression treatment, and it has also been used for LPS in clinical practice (Russell et al., 2015).
It has previously been demonstrated in healthy female volunteers that secretory transformation of the endometrium may be achieved by 400 mg Cyclogest® pessaries twice a day (bid) as an effective alternative to 90 mg progesterone vaginal gel once a day (od) (Duijkers et al., 2018). To achieve Cyclogest® marketing authorisations in the EU for LPS, this Phase 3 study compared the clinical efficacy of the two preparations, taking an endpoint of clinical pregnancy rate defined by fetal heartbeat detection with ultrasound after 38 days of luteal phase vaginal progesterone support following in vitro fertilisation (IVF) commenced on the evening of oocyte retrieval (OR).
Materials and Methods
Study design
This multicentre, multinational, randomised, open, non-inferiority clinical trial (EudraCT number 2013-001105-81) was carried out between 9 October 2013 and 8 August 2014 at 17 study sites in five countries: Belgium (one study site), Bulgaria (three study sites), Czech Republic (six study sites), Hungary (two study sites) and Serbia (five study sites). The primary objective was to evaluate non-inferiority in the achievement of clinical pregnancy rate, i.e. fetal heart movement measured by transvaginal ultrasonography (TVUS), after 38 days (D38) of LPS 400 mg progesterone vaginal pessaries bid (test product) compared to 90 mg progesterone vaginal gel od (reference product). Secondary objectives included the clinical pregnancy rate after 10 weeks (D70) of LPS, the biochemical pregnancy rate at D18 of LPS, clinical implantation rate per number of embryos transferred at D38 of LPS, and safety, tolerability, bleeding and vaginal discharge with progesterone vaginal pessaries versus progesterone vaginal gel when used for 10 weeks of LPS.
Ethical review and competent authority approval
The study was conducted in accordance with the ethical principles that have their origins in the Declaration of Helsinki (according to its 59th WMA General Assembly, Seoul, Korea, October 2008) and in accordance with the national legal requirements as well as the principles of ICH-GCP. The study received favourable opinions from the relevant independent ethics committees and approvals from the national competent authorities in all countries based on the clinical trial protocol Version 1.0 dated 10 June 2013. All patients obtained and signed an informed consent form before any study-specific procedure was performed.
Patient population
The study population included premenopausal women with a clinical indication for IVF/intracytoplasmic sperm injection (ICSI) and embryo transfer (ET). Inclusion criteria at randomisation included women aged between 18 and 40 years; body mass index (BMI) ≥18 and ≤30 kg/m2; infertility due to tubal factor, mild endometriosis (American Society for Reproductive Medicine stage 1 to 2), male factor, unexplained infertility or ovarian ovulatory dysfunction; presence of at least one ovary; uterine cavity without significant abnormalities; first, second or third fresh cycle in the present series of ART; at least four oocytes retrieved in the current ART cycle; and serum follicle-stimulating hormone (FSH) level ≤12 IU/L. Exclusion criteria at randomisation included >2 previously failed complete ART fresh cycles; donor oocyte recipient; any contraindication for pregnancy; presence of any medical condition for which the use of progesterone is contraindicated (e.g. porphyria); vaginal abnormalities (including untreatable abnormal discharge); history of gynaecological neoplasia; uterine fibroids suspected to affect the study procedures (assessed by ultrasound or hysteroscopy/hysterosalpingogram); history of severe OHSS according to Golan (Grade IV, V or VI); use within 2 months of study start of drugs inducing or inhibiting p450 cytochrome; administration of investigational drugs within 2 months of study start; and known hypersensitivity to one of the study drugs or any of the excipients.
Study drug
The test product, progesterone vaginal pessary, was Cyclogest® 400 mg pessary (Actavis UK Ltd [ML 0142/01]), to be administered intravaginally bid for 10 weeks. The reference product, progesterone vaginal gel, was Crinone® 8% vaginal progesterone gel (90 mg) (Central Pharma [Contract Packaging] Ltd on behalf of Merck GmbH, Germany), to be administered intravaginally once daily for 10 weeks. At the time of the study, Crinone® had marketing authorisation for the LPS indication during ART treatment in the majority of European countries.
Study assessments
The primary endpoint of the study was the clinical pregnancy rate (assessed by fetal heart movement measured by TVUS) achieved after D38 of LPS. The secondary study endpoints included clinical pregnancy rate achieved after D70 (10 weeks) of LPS; clinical implantation rates per number of embryos transferred (assessed by fetal heart movement measured by TVUS) after D38 of LPS; biochemical pregnancy rate (assessed by serum β-hCG ≥25 IU/L) at D18 after OR; patient evaluation of vaginal bleeding and discharge (assessed by diary); and adverse event (AE) incidence, severity and relationship to study medication.
Safety and tolerability assessments included laboratory parameters (biochemistry and haematology); general physical and gynaecological examination (including TVUS); and vital sign measurement. Patients were asked to document their use of study medication and vaginal blood loss in a diary to be completed daily. Patient compliance was assessed based on patient diaries and on returned units of trial medication.
Conduct of the study
The ART procedure was at the discretion of the investigator, and pretreatment ART procedure details were not collected. ART procedures included routine gonadotropin-releasing hormone (GnRH) downregulation with GnRH agonist or GnRH antagonist; ovarian stimulation with FSH and/or human menopausal gonadotropin with total starting dose ranging from 100 to 300 IU per day and a maximum total dose of up to 450 IU per day; hCG 5000 or 10 000 IU trigger injection when at least three follicles with diameter ≥17 mm were observed during TVUS; OR planned for 35 to 37 h after hCG trigger injection; and ET on D2 or D3 after OR (one to three embryos according to the site’s clinical practice and national legislation, if applicable).
Patients were randomised to progesterone vaginal pessaries or progesterone vaginal gel treatment in a 1:1 ratio on the day of OR (D0). An interactive web response system (IWRS) was implemented for treatment allocation at the sites. On D0 of the treatment period, patients who met the entry criteria were sequentially assigned by the investigator to a unique random number using the IWRS. A static randomisation list was generated and implemented within the system which accounted for two strata: study site and patient age group (≤35, >35 years).
Patients started the trial medication on the evening of D0 (day of OR) and were trained by a qualified physician or clinical personnel on how to administer the trial medication. Patients self-administered the trial medication on each study day for up to 70 (±3) days (corresponding to the last scheduled visit), or until they discontinued the study in case they did not become pregnant, or until they discontinued on their own decision for other reasons, or until they were withdrawn from the study by the investigator for other reasons. To be able to compare both products in a longer treatment period, as some vaginal progesterone formulations are indicated for up to 70 days (D70), and to avoid change of medication at this critical time of pregnancy, the total duration of trial medication administration was D70 (±3 days) despite the fact that the primary endpoint of clinical pregnancy was established on D38 (+7 days). The progesterone vaginal pessaries 400 mg were self-administered twice a day, and the progesterone vaginal gel (90 mg) was self-administered once a day. The patient recorded the time and day of drug administration in her diary.
In case of a negative serum pregnancy test at visit D18 or lack of fetal heartbeat on D38, patients were recorded as having failed to achieve pregnancy and were considered evaluable for study completion.
Study design justification and statistical analyses
An open, two-parallel groups design was selected to demonstrate non-inferiority of progesterone vaginal pessaries 400 mg bid to progesterone vaginal gel (90 mg) od in the achievement of pregnancy following IVF treatment. It was not possible to blind the treatments due to their different formulations and due to the specific route (vaginal) of administration. Since a placebo-controlled trial is not justified in this indication for ethical reasons, an active control group had to be used.
The non-inferiority margin for this trial was agreed during an investigator’s meeting and confirmed as appropriate by Scientific Advice from Medicines and Healthcare Products Regulatory Agency (MHRA), UK. Sample size considerations were based on the following assumptions: non-inferiority margin for pregnancy rate (reference drug rate minus rate for test drug): −9% points; significance level α: 2.5% (one-sided); power: 90%; with a reference pregnancy rate of 30% for the progesterone vaginal gel group (Bergh and Lindenberg, 2012). An expected primary outcome (clinical pregnancy rate at D38) of 32% for progesterone vaginal pessaries was assumed due to a slightly larger secretory transformation rate of the endometrium (by 4%-points) in the PK/PD trial (Duijkers et al., 2018). Applying the formula by Farrington and Manning (Farrington and Manning, 1990), the required number of evaluable patients was 344 per treatment group (i.e. 688 patients in total). Applying a clinically justified dropout rate of 10% yielded a total number of 766 patients to be randomised. Since the total number of patients to be enrolled and randomised in the study already accounted for dropouts, patients who prematurely discontinued the study for reasons other than failed pregnancy were not replaced. Non-inferiority analysis of the clinical pregnancy rate was done on the absolute difference between proportions calculating one-sided 97.5% confidence intervals (CIs) using the Wald method.
In addition, logistic regression was used to analyse co-factors potentially influencing clinical pregnancy rate. Logistic regression examined the stratification factors of country and age group and was run with and without interaction terms for treatment by age group and treatment by country. The logistic regression was carried out using forward and backward selection examining the influence of subgroups (country, age group, number of embryos transferred, BMI group, embryo stage and type of fertilisation) on pregnancy rate. The non-inferiority margin of the logistic regression, 0.7, was generated from the absolute differences based on a pregnancy reference rate of 30% to the multiplicative (relative) scale that is the basic presumption for logistic regression models (0.7 = [30%—9%]/30%). A generalised estimating equation (GEE) analysis was carried out to test for treatment differences in clinical implantation rate.
The safety analysis set included all patients randomised to receive study medication. Non-inferiority analyses were completed for the full analysis set (FAS) and the per-protocol (PP) set. The FAS population included all patients who received at least one dose of study medication and who had TVUS assessment of fetal heart movement on visit D38, unless they showed a negative biochemical pregnancy test on D18 or were diagnosed with miscarriage before D38. The PP set included the patients in the FAS who had no major protocol deviations.
Results
Patient disposition
A total of 812 women were screened in the study and 769 were randomised to receive progesterone vaginal pessaries (n = 385, 50.1%) or progesterone vaginal gel (n = 384, 49.9%). An overview of patient disposition is given in Figure 1. A total of 44 patients failed the screening assessment, including one patient who was randomised to progesterone vaginal gel treatment but violated an inclusion/exclusion criterion (as this patient did not receive any study medication). Premature discontinuation occurred in 45 patients (n = 25 progesterone vaginal pessaries; n = 20 progesterone vaginal gel including the one patient who was randomised yet violated the inclusion/exclusion criteria). The most frequent reasons for premature termination were AEs (10 patients), lost to follow-up (7 patients) or ‘other’ (20 patients). There were 737 patients in the FAS population (369 progesterone vaginal pessaries; 368 progesterone vaginal gel), 713 patients in the PP population (357 progesterone vaginal pessaries; 356 progesterone vaginal gel) and 768 patients in the safety population (385 progesterone vaginal pessaries; 383 progesterone vaginal gel).
Figure 1.
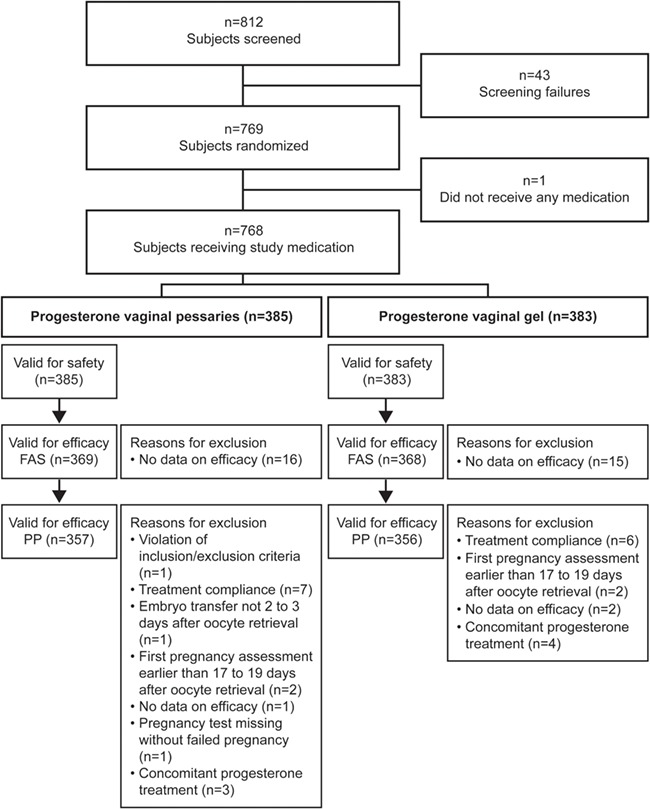
Patient disposition. FAS, full analysis set; PP, per protocol set. Patients per country (safety set) were: Belgium, n = 21; Bulgaria, n = 100; Czech Republic, n = 255; Hungary, n = 152; and Serbia, n = 240.
Patient demographics
The two treatment groups were comparable in demographic and baseline characteristics (Table I). The mean age of the patients was 33 years (range 20 to 40 years), and the majority of the patients were Caucasian (99.6%). The number of retrieved oocytes ranged from 4 to 40 and was comparable for both treatments with an overall mean (median) of 10.8 (9.0) retrieved oocytes. ICSI was performed in 79.4% of the patients. Embryo cleavage stage at ET was two to four cells in 32.9% of the patients and ≥5 cells in 63.4% of the patients (while in 3.6% of cases this information was missing). Most frequently, two embryos were transferred (51.4%), while one embryo was transferred in 29.0% of the patients and three embryos were transferred in 15.9% of the patients.
Table I.
Baseline characteristics, safety population (N = 768).
| Progesterone vaginal pessaries n = 385 | Progesterone vaginal gel n = 383 | Total N = 768 | ||||
|---|---|---|---|---|---|---|
| Age (years) | Arithmetic mean/SD (range) | 32.8/4.14 (20–40) | 33.2/3.95 (22–40) | 33.0/4.05 (20–40) | ||
| Age strata | ≤35 | 273 (70.9) | 268 (70.0) | 541 (70.4) | ||
| >35 | 112 (29.1) | 115 (30.0) | 227 (29.6) | |||
| Race | Caucasian/White | n (%) | 382 (99.2) | 383 (100) | 765 (99.6) | |
| Black | n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Asian | n (%) | 3 (0.8) | 0 (0.0) | 3 (0.4) | ||
| Hispanic | n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Other | n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Body weight (kg) | Arithmetic mean/SD (range) | 64.34/9.405 (43.0–94.0) | 64.61/9.955 (43.5–102.0) | 64.47/9.678 (43.0–102.0) | ||
| BMI (kg/m2) | Arithmetic mean/SD (range) | 22.95/3.011 (18.0–30.0) | 23.00/3.086 (18.1–30.0) | 22.97/3.047 (18.0–30.0) | ||
| Number of retrieved oocytes | Arithmetic mean/SD (range) | 11.0/5.95 (4–40) | 10.6/5.72 (4–35) | 10.8/5.84 (4–40) | ||
| Type of fertilisation | ICSI | n (%) | 305 (79.2) | 305 (79.6) | 610 (79.4) | |
| IVF | n (%) | 66 (17.1) | 64 (16.7) | 130 (16.9) | ||
| Missing | n (%) | 14 (3.6) | 14 (3.7) | 28 (3.6) | ||
| Embryo cleavage stage | 2–4 cells | n (%) | 126 (32.7) | 127 (33.2) | 253 (32.9) | |
| ≥5 cells | n (%) | 245 (63.6) | 242 (63.2) | 487 (63.4) | ||
| Missing | n (%) | 14 (3.6) | 14 (3.7) | 28 (3.6) | ||
| Number of transferred embryos | 0 | n (%) | 14 (3.6) | 14 (3.7) | 28 (3.6) | |
| 1 | n (%) | 112 (29.1) | 111 (29.0) | 223 (29.0) | ||
| 2 | n (%) | 200 (51.9) | 195 (50.9) | 395 (51.4) | ||
| 3 | n (%) | 59 (15.3) | 63 (16.4) | 122 (15.9) | ||
BMI, body mass index; ICSI, intracytoplasmic sperm injection; IVF, in vitro fertilisation; SD, standard deviation.
Efficacy outcomes
Primary efficacy outcome
Clinical pregnancy rates in the FAS on D38 were 38.3% in the progesterone vaginal pessaries group and 39.9% in the progesterone vaginal gel group, respectively. The corresponding results for the PP group were 38.1 and 40.4% in the progesterone vaginal pessaries and progesterone vaginal gel groups, respectively (Fig. 2, Table II). For the difference in pregnancy rates between the progesterone vaginal pessaries group and the progesterone vaginal gel group, the lower limit of the 97.5% CI was −8.6 and −9.5%, for the FAS and PP datasets, respectively.
Figure 2.
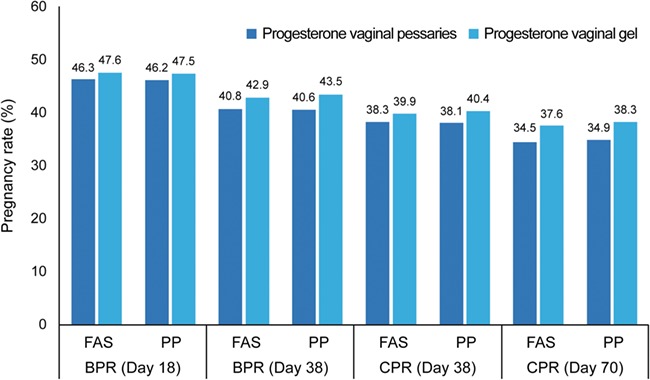
Pregnancy rates (biochemical and clinical), Days 18, 38 and 70 in the FAS (N = 737). BPR, biochemical pregnancy rate; CPR, clinical pregnancy rate; FAS, full analysis set; PP, per protocol set. Three patients were included in the FAS but did not have Day 38 pregnancy information.
Table II.
Comparison of clinical efficacy of progesterone vaginal pessaries vs progesterone vaginal gel.
| Clinical endpoint | Population | Progesterone vaginal pessaries % (n) | Progesterone vaginal gel % (n) | Difference |
|---|---|---|---|---|
| Biochemical pregnancy rate (Day 18) | FAS* | 46.3% (171/369) | 47.6% (175/368) | −1.2% |
| PP | 46.2% (165/357) | 47.5% (169/356) | −1.3% | |
| Biochemical pregnancy rate (Day 38) | FAS* | 40.8% (150/368) | 42.9% (157/366) | −2.1% |
| PP | 40.6% (145/357) | 43.5% (155/356) | −2.9% | |
| Clinical pregnancy rate (Day 38)** | FAS* | 38.3% (141/368) | 39.9% (146/366) | −1.6% |
| PP | 38.1% (136/357) | 40.4% (144/356) | −2.4% | |
| Clinical pregnancy rate (Day 70) | FAS* | 34.5% (126/365) | 37.6% (137/364) | −3.1% |
| PP | 34.9% (124/355) | 38.3% (136/355) | −3.4% | |
| Clinical implantation rate (Day 38) | FAS* | 26.3% | 27.8% | −1.5% |
| PP | 26.5% | 28.4% | −1.9% | |
| Clinical implantation rate (Day 70) | FAS* | 24.6% | 25.9% | −1.3% |
| PP | 24.8% | 26.5% | −1.7% |
FAS, full analysis set; PP, per-protocol set.
*Three patients were included in FAS but did not have Day 38 pregnancy information.
**Primary endpoint of trial: for the difference in pregnancy rates between the progesterone vaginal pessaries group and the progesterone vaginal gel group, the lower limit of the 97.5% CI was −8.6 and −9.5%, for the FAS and PP datasets, respectively.
The predefined logistic regression model examining treatment group and stratification factors, country and age group, as well as the interaction terms for treatment group by country and treatment group by age group, on clinical pregnancy rate showed non-significant interaction terms for both the FAS and PP analyses (P values >0.1). The logistic regression model including treatment group, country and age group effects without interaction terms showed non-inferiority of progesterone vaginal pessaries to progesterone vaginal gel for both FAS and PP populations in that the lower limits of the 95% CIs were above 0.7 for both analyses. Estimated pregnancy rates for progesterone vaginal pessaries versus progesterone vaginal gel were 32.9 versus 34.3% (FAS) and 33.4 versus 35.7% (PP), respectively, with relative rates (RR) of 0.957 (95% CI of RR, 0.776–1.158) for the FAS and 0.937 (95% CI of RR, 0.759–1.134) for the PP analysis.
Secondary efficacy outcomes
Clinical pregnancy rate at D70, biochemical pregnancy rate at D18 and D38 and clinical implantation rate at D38 and D70 were assessed in the FAS and PP set (Fig. 2, Table II). Clinical pregnancy rates for both treatments were lower on D70 compared to D38, as could be expected due to the natural course of pregnancies (e.g. occurrence of miscarriage). Observed pregnancy rates on D70 were 34.5% (FAS) and 34.9% (PP) for progesterone vaginal pessaries and 37.6% (FAS) and 38.3% (PP) for progesterone vaginal gel. Progesterone vaginal pessaries and progesterone vaginal gel groups were comparable for biochemical pregnancy rates on D18 (FAS, 46.3 and 47.6%, respectively) and D38 (FAS, 40.8 and 42.9%, respectively) and for clinical implantation rates on D38 (FAS, 26.3 and 27.8%, respectively) and D70 (FAS, 24.6 and 25.9%, respectively) (Table II). Applying the GEE model, no statistically significant difference in implantation rates was observed between the progesterone vaginal pessaries and progesterone vaginal gel groups for both the FAS and PP analyses (P values >0.4).
Safety and tolerability
Adverse events
Frequencies of patients reporting AEs (Table III) were comparable for both treatments (progesterone vaginal pessaries 43.6%; progesterone vaginal gel 44.6%). AEs of mild, moderate and severe intensity were reported by 33.7, 9.9 and 0.5% of the patients, respectively. There were 19 patients (2.5%) who had serious AEs, including six patients treated with progesterone vaginal pessaries (1.6%) and 13 with progesterone vaginal gel (3.4%). Two patients treated with progesterone vaginal pessaries and three patients treated with progesterone vaginal gel discontinued the study due to serious AEs (OHSS, ectopic pregnancy, Bartholin’s cyst removal). Serious AEs observed during the study were in all cases unrelated to the study drug and in most cases could be explained as consequences of ART procedures (e.g. OHSS) or were due to abnormal pregnancy courses (e.g. ectopic pregnancy). Drug-related AEs occurred with frequencies of 15.1% with progesterone vaginal pessaries and 14.4% with progesterone vaginal gel and were explained by the hormonal action of progesterone. Overall, 31 patients (4.0%) discontinued the study due to AEs, of whom 19 were treated with progesterone vaginal pessaries (4.9%) and 12 with progesterone vaginal gel (3.1%). In six of these cases, the event leading to discontinuation was miscarriage (i.e. failure to maintain pregnancy). AEs regarded as possibly related to study drug and leading to premature study termination were reported for six patients treated with progesterone vaginal pessaries and one patient treated with progesterone vaginal gel. Abnormal laboratory parameters (increased transaminases) were reported as AEs in three (0.4%) patients, and no relevant abnormalities of vital signs were reported.
Table III.
Overview of treatment-emergent adverse events (AEs) (safety population N = 768).
| Progesterone vaginal pessaries | Progesterone vaginal gel | Total | |
|---|---|---|---|
| N = 385 | N = 383 | N = 768 | |
| Number of patients with any AE | 168 (43.6%) | 171 (44.6%) | 339 (44.1%) |
| Number of patients with any serious AE | 6 (1.6%) | 13 (3.4%) | 19 (2.5%) |
| Number of patients with drug-related AEs | 58 (15.1%) | 55 (14.4%) | 113 (14.7%) |
| Number of patients with drug-related serious AEs | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Number of patients who discontinued due to AE | 19 (4.9%) | 12 (3.1%) | 31 (4.0%) |
| Intensity | |||
| Number of patients with mild AEs | 126 (32.7%) | 133 (34.7%) | 259 (33.7%) |
| Number of patients with moderate AEs | 40 (10.4%) | 36 (9.4%) | 76 (9.9%) |
| Number of patients with serious AEs | 2 (0.5%) | 2 (0.5%) | 4 (0.5%) |
| Related to study drug | 58 (15.1%) | 55 (14.4%) | 113 (14.7%) |
| Number of patients with mild AEs | 40 (10.4%) | 42 (11.0%) | 82 (10.7%) |
| Number of patients with moderate AEs | 17 (4.4%) | 13 (3.4%) | 30 (3.9%) |
| Number of patients with serious AEs | 1 (0.3%) | 0 (0.0%) | 1 (0.1%) |
Blood loss
Significant vaginal blood loss, defined as requiring sanitary protection, was comparable between treatment groups throughout the study: at least 1 day of blood loss that required sanitary protection was reported by 33.2% of progesterone vaginal pessaries patients and 41.0% of progesterone vaginal gel patients from D0 to D18, 41.5% of progesterone vaginal pessaries patients and 44.4% of progesterone vaginal gel patients through D38, and 43.8% of progesterone vaginal pessaries patients and 44.6% of progesterone vaginal gel patients through D70. In pregnant women, the mean percentage of days with blood loss was comparably low in both treatment groups, at about 1% of the days.
Compliance
There were no relevant differences in compliance between patients treated with progesterone vaginal pessaries or progesterone vaginal gel throughout the study. Mean compliance ranged from 97 to 100% for both treatments and was similar in both groups with 96.5% of the patients treated with progesterone vaginal pessaries having a compliance of at least 90% until D38 compared to 94.9% of the patients treated with progesterone vaginal gel. Thus, patient compliance with the twice-daily application regimen for progesterone vaginal pessaries was as high as for the once daily progesterone vaginal gel application.
Discussion
This paper presents a Phase 3 non-inferiority study to support registration in Europe of Cyclogest® for LPS following IVF. The original prespecified non-inferiority margin of −9% was thus met in the FAS dataset but was marginally below this in the PP dataset. However, a reference pregnancy rate of 30% was used to derive the non-inferiority margin of −9%, whereas at the actual reference pregnancy rate of 40%, a non-inferiority margin of up to 12% may be more appropriate (FDA, 2007; Heijnen et al., 2007; Devroey et al., 2012). The EMEA 2005 guideline recognises such situations and states: ‘If the performance of the reference product in a trial is very different from what was assumed when defining the non-inferiority margin then the chosen margin may no longer be appropriate’ (European Medicines Agency, 2005). Furthermore, a predetermined logistic regression model showed non-inferiority of progesterone vaginal pessaries to progesterone vaginal gel for both FAS and PP populations. Thus, the MHRA accepted to widen the non-inferiority margin to −10% and, following completion of a decentralised procedure, all EU member states agreed to grant marketing authorisations for LPS use of Cyclogest® in January 2017.
It is recognised that the variation in ART treatment between centres might have impacted whether a patient achieved pregnancy following ART. However, this study demonstrated equal efficacy of both tested products despite this heterogeneity, indicating that the results of this study are applicable to different stimulation protocols.
The progesterone vaginal pessaries and the progesterone vaginal gel treatment groups showed high compliance throughout the study, which is likely supported by the high motivation of patients undergoing IVF (Murto et al., 2017). High compliance rates were similar in both treatment groups despite progesterone vaginal pessaries being administered twice daily compared with the once-daily progesterone vaginal gel. Both vaginal progesterone treatments were safe and well tolerated by patients in this study, and progesterone vaginal pessaries and progesterone vaginal gel had similar results with respect to AEs. Serious AEs observed during the study were unrelated to the study drug and could, in most of the cases, be explained as consequences of ART procedures (e.g. OHSS) or were due to abnormal pregnancy courses (e.g. ectopic pregnancy). Drug-related AEs were explained by the hormonal action of the drugs. As expected, pregnant patients reported fewer days with blood loss compared to non-pregnant patients, and no differences in blood loss were found between progesterone vaginal pessaries and progesterone vaginal gel in pregnant patients throughout the study period.
Conclusion
This study demonstrated non-inferiority for progesterone vaginal pessaries (400 mg bid) versus progesterone vaginal gel (90 mg od) in clinical pregnancy rate after 38 days of LPS (primary outcome), clinical pregnancy rate after 70 days, biochemical pregnancy rate after 18 days and implantation rate after 38 days, with no significant effect of country or age group, indicating that the results are applicable to a broad range of women in European countries. Both medications were safe and well tolerated by patients, and women in both groups demonstrated similarly high levels of compliance to the self-administered treatments. Serious AEs observed during the study were unrelated to study medication and could be attributed to ART procedures or expected deviations from a normal pregnancy course in this specific patient population. Drug-related AEs occurred as could be expected due to the hormonal action and were in accordance with the respective product information. The use of vaginal progesterone as LPS to maximise pregnancy rates following ART is well established, and the results of this study suggest that progesterone 400 mg pessaries bid provides an effective, safe and tolerable treatment option in women undergoing IVF.
Acknowledgements
This manuscript was prepared according to the International Society for Medical Publication Professionals’ ‘Good Publication Practice for Communicating Company-Sponsored Medical Research: the GPP3 Guidelines’. The authors would like to thank all clinical investigators for their participation in this trial and Dr Julian Jenkins, Repromed Sàrl, for his input into the medical writing of this paper. All parties involved, i.e. L.D. Collins, Gedeon Richter/PregLem S.A., Actavis and Pharmaplex, provided a full review of the article.
Authors represent the Vaginal Progesterone Luteal Phase Support Post IVF Study Group: Belgium: Prof. Dr Thomas M. D'Hooghe; Bulgaria: Dr Georgi Stamenov, Dr Ljubomir Boichev, Dr Tanya Temiva; Czech Republic: Dr Barbora Kuřecová, Dr Karel Řežabek, Dr Jíři Štěpán, Dr Jaroslav Hulvert, Dr Katerina Veselá, Dr David Rumpík; Hungary: Prof. János Urbancsek, Dr János Konc; Serbia: Prof. Aleksandar Ljubić, Prof. Ivan Tulić, Dr Jasmina Popović, Dr Eliana Garalejić, Dr Zorica Crnogorac.
Country Coordinator.
Authors’ roles
H.S. was significantly involved in the data interpretation and in the writing, review and approval of this manuscript. C.K. participated in the planning and design of the study and review of the manuscript. T.D.H. is the coordinating investigator for this multicentre study and was significantly involved in the development of the study proposal and protocol, as well as in data interpretation and manuscript writing. T.B.M. participated in the planning and design of the study and in data analysis and interpretation. I.K. coordinated the design and protocol development, the performance of the clinical trial and the evaluation and reporting of the trial and contributed to the manuscript. S.H. participated in the planning and design of the study, study supervision, data analysis and interpretation as well as the manuscript review.
Funding
The study was funded by Actavis Group PTC ehf., Iceland, part of Teva Pharmaceuticals, and by L.D. Collins & Co. Ltd. Medical writing assistance was provided by Gedeon Richter/PregLem S.A. Gedeon Richter plc has recently entered into a license and distribution agreement to commercialise the vaginal pessaries in the European Union (except Ireland/UK). The progesterone vaginal pessaries studied are now marketed as Cyclogest®, Amelgen®, Cyclovita®, Luteum and Cygest® throughout the EU, Asia and Middle East & North Africa.
Conflict of interest
H.S.: employee of Gedeon Richter plc/PregLem S.A. C.K.: consultant to L.D. Collins & Co. Ltd and received consulting fees for work performed. T.D.H.: at the initiation and completion of this study, full professor at KU Leuven and Head of Leuven University Fertility Center at the University Hospital Gasthuisberg, Leuven, Belgium. In October 2015, T.D.H. became vice president of Global Medical Affairs Fertility at the pharmaceutical company Merck—marketing authorisation holder of the Progesterone vaginal gel (Crinone®)—and has remained a part-time professor at KU Leuven (Belgium) and adjunct professor at Yale University (New Haven, CT, USA). T.B.M.: at the initiation and completion of this study, employee of Actavis Group PTC ehf. I.K.: consultant to Actavis, later TEVA, and received consulting fees for work performed. S.H.: at the initiation and completion of this study, employee of Actavis Group PTC ehf.
References
- Bergh C, Lindenberg S. A prospective randomised multicentre study comparing vaginal progesterone gel and vaginal micronised progesterone tablets for luteal support after in vitro fertilisation/intracytoplasmic sperm injection. Hum Reprod 2012;27:3467–3473. [DOI] [PubMed] [Google Scholar]
- Bulletti C, de Ziegler D, Flamigni C, Giacomucci E, Polli V, Bolelli G, Franceschetti F. Targeted drug delivery in gynaecology: the first uterine pass effect. Hum Reprod 1997;12:1073–1079. [DOI] [PubMed] [Google Scholar]
- Cicinelli E, de Ziegler D, Bulletti C, Matteo MG, Schonauer LM, Galantino P. Direct transport of progesterone from vagina to uterus. Obstet Gynaecol 2000;95:403–406. [DOI] [PubMed] [Google Scholar]
- Devroey P, Pellicer A, Nyboe Andersen A, Arce J-C. On behalf of the Menopur in GnRH Antagonist Cycles with Single Embryo Transfer (MEGASET) Trial Group. A randomised assessor-blind trial comparing highly purified hMG and recombinant FSH in a GnRH antagonist cycle with compulsory single-blastocyst transfer. Fertil Steril 2012;97:561–571. [DOI] [PubMed] [Google Scholar]
- Duijkers IJM, Klingmann I, Prinz R, Wargenau M, Hrafnsdottir S, Magnusdottir TB, Klipping C. Effect on endometrial histology and pharmacokinetics of different dose regimens of progesterone vaginal pessaries, in comparison with progesterone vaginal gel and placebo. Hum Reprod 2018;33:2131–2140. [DOI] [PubMed] [Google Scholar]
- European Medicines Agency. Committee for Medicinal Products for Human Use (CHMP) Guideline on the choice of the non-inferiority margin. EMEA/CPMP/EWP/2158/99. London: 2005. Available at: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-choice-non-inferiority-margin_en.pdf (Accessed 16 July 2019).
- Farrington CP, Manning G. Test statistics and sample size formulae for comparative binomial trials with null hypothesis of non-zero risk difference or non-unity relative risk. Stat Med 1990;9:1447–1454. [DOI] [PubMed] [Google Scholar]
- FDA, U.S. Food & Drug Administration Drug Approval Package, Endometrin (progesterone) Vaginal Insert, 100 mg. NDA 022057. FDA; 2007. Available at:https://www.accessdata.fda.gov/drugsatfda_docs/nda/2007/022057_endometrin_toc.cfm.
- Heijnen EM, Eijkemans MJ, De Klerk C, Polinder S, Beckers NG, Klinkert ER, Broekmans FJ, Passchier J, Te Velde ER, Macklon NS et al. A mild treatment strategy for in-vitro fertilisation: a randomised non-inferiority trial. Lancet 2007;369:743–749. [DOI] [PubMed] [Google Scholar]
- Miles RA, Paulson RJ, Lobo RA, Press MF, Dahmoush L, Sauer MV. Pharmacokinetics and endometrial tissue levels of progesterone after administration by intramuscular and vaginal routes: a comparative study. Fertil Steril 1994;62:485–490. [DOI] [PubMed] [Google Scholar]
- Murto T, Yngve A, Skoog Svanberg A, Altmae S, Salumets A, Wanggren K, Stavreus-Evers A. Compliance to the recommended use of folic acid supplements for women in Sweden is higher among those under treatment for infertility than among fertile controls and is also related to socioeconomic status and lifestyle. Food Nutr Res 2017;61:1334483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell R, Kingsland C, Alfirevic Z, Gazvani R. Duration of luteal support after IVF is important, so why is there no consistency in practice? The results of a dynamic survey of practice in the United Kingdom. Hum Fertil (Camb) 2015;18:43–47. [DOI] [PubMed] [Google Scholar]
- Tavaniotou A, Smitz J, Bourgain C, Devroey P. Comparison between different routes of progesterone administration as luteal phase support in infertility treatments. Hum Reprod Update 2000;6:139–148. [DOI] [PubMed] [Google Scholar]
- van der Linden M, Buckingham K, Farquhar C, Kremer JA, Metwally M. Luteal phase support for assisted reproduction cycles. Cochrane Database Syst Rev 2015; Cd009154. [DOI] [PMC free article] [PubMed] [Google Scholar]


