Abstract
The aim of this study was to investigate the test-retest reliability of quantitative sensory testing (QST) and mechanical sensitivity mapping of the periauricular skin. Twenty volunteers (10 men, 10 women) participated in two sessions at intervals of one week. Cold and warm detection threshold (CDT&WDT), cold and heat pain threshold (CPT&HPT), mechanical detection and pain threshold (MDT&MPT), pressure pain threshold (PPT) and two-point discrimination (2PD) were measured at five sites: bilateral subauricular and postauricular sites (LA, RA, LB, RB) and the dorsum of left hand (control). Pressure stimulation was applied at each of the four periauricular test sites. The test-retest reliability of the QST data implied fair to excellent agreement as evaluated by the intra-class correlation coefficients (ICC; all >0.4) for different days. There was no difference between each side in the QST parameters and mechanical sensitivity mapping (P ≥ 0.057). Significant differences between subauricular and postauricular sites were shown for WDT and PPT (P ≤ 0.028). NRS scores of mechanical sensitivity mapping showed significant effects of gender, site and point (P ≤ 0.040). QST and mechanical sensitivity mapping can be considered to be a reliable technique to assess somatosensory function of the periauricular skin.
Subject terms: Saliva, Oral diseases
Introduction
The great auricular nerve (GAN) is a superficial sensory branch of the cervical plexus arising from the second and third cervical spinal nerves. It emerges from the posterior border of the sternocleidomastoid muscle (SCM) and courses anteriorly over the belly of that muscle1,2. Then the GAN courses superiorly and divides into 2 branches, anterior and posterior, which are both cutaneous nerves. The anterior branch supplies the skin over the parotid gland and lower preauricular region, and the posterior branch provides sensory innervation for the skin over the dorsal lower third of the auricle and mastoid process3,4.
Quantitative sensory testing (QST) is a reliable, noninvasive psychophysical test which allows to determine a comprehensive somatosensory profile5. By giving the participant quantitative somatosensory stimuli, for example sensory and pain thresholds can be measured accurately. The German Research Network on Neuropathic Pain (DFNS) has developed a standardized QST protocol which has been demonstrated to have good reliability in the orofacial region and upper and lower limbs5,6. Since then, more and more studies have confirmed the test-retest reliability of QST of the oral and maxillofacial region and analyzed the characteristics of the sensory and pain thresholds of the inter-oral and extra-oral regions7,8. However, the QST technique is limited by the need for elaboration and quantitative stimulators, professional training of operators and specialized software support, which unfortunately reduces the applicability in clinical settings. As a consequence, there remains multiple and clinically important questions related to orofacial pain and changes in somatosensory function.
Methodological advancements over the last decade have allowed new mapping techniques to be implemented in a comprehensive QST evaluation, e.g. with the repeated use of a pressure algometer for measurement of pressure pain threshold (PPT) at multiple test sites9–11. Compared with many of the other QST instruments, the pressure algometer is easy to use and convenient to carry, but the item it measures is relatively simple. Compared with manual palpation, pressure algometers and PPTs may consume more time, but it can provide standardized palpation pressure which is more reliable than manual palpation12. Recently, a study has confirmed that the technique for mechanical sensitivity mapping in the masseter muscle region and temporomandibular joint (TMJ) region with a new quantitative palpometer has an excellent reliability and can assess the spatial aspects of mechanical sensitivity in a specific anatomical region13.
Surgical injury and primary neurological disorders may cause GAN disturbance, so it can become necessary to perform a somatosensory examination of the periauricular skin innervated by the GAN14–16. However, there is so far no information on QST or mechanical sensitivity mapping of the periauricular skin. Therefore, it is not known whether the previous methods of the QST and mechanical sensitivity mapping can be applied to provide representative characteristics of the periauricular skin sensitivity.
This study aimed to investigate the test-retest reliability of QST of the periauricular skin and the surface of left hand (control), and the mechanical sensitivity mapping of the periauricular skin. An additional aim was to test for gender and site-to-site differences in the QST and mechanical sensitivity mapping of the periauricular skin.
Materials and Methods
Study participants
Twenty healthy young adults participated in the study. According to the gender, they were divided into two groups: 10 women (mean age ± SD: 23.2 ± 0.8 years; range: 22–25 years) and 10 men (mean age ± SD: 23.1 ± 1.1 years; range: 21–25 years). All the participants were healthy without orofacial pain complaints or symptoms of pain in head, face, and neck regions. Exclusion criteria were: history of trauma in the orofacial area that interfered with normal somatosensory function, any acute or chronic orofacial diseases (for example, burning mouth syndrome, trigeminal neuralgia, chronic headache, systemic musculoskeletal pain disorders such as fibromyalgia, or symptoms of rheumatoid arthritis, etc.), use of medication such as muscle relaxants, anticonvulsants, antidepressants, or anxiolytics within the last month, and severe systematic diseases or mental disorders. The study was conducted in accordance with the guidelines set forth in the Declaration of Helsinki II. Declaration and informed consent were obtained from all participants prior to participation. The study was approved by the Nanjing Medical University Research Ethics Committee with NO: PJ 2018-040-001.
Experimental protocol
The experiment was performed in a quiet room with the temperature controlled between 21–25 degree Celsius. At the beginning of the experiment, all participants were informed about the purpose, content and method of this experiment, and confirmed that they fully understood. All tests were performed by the same tester using the same instrument and the tests were repeated by the same tester after one week.
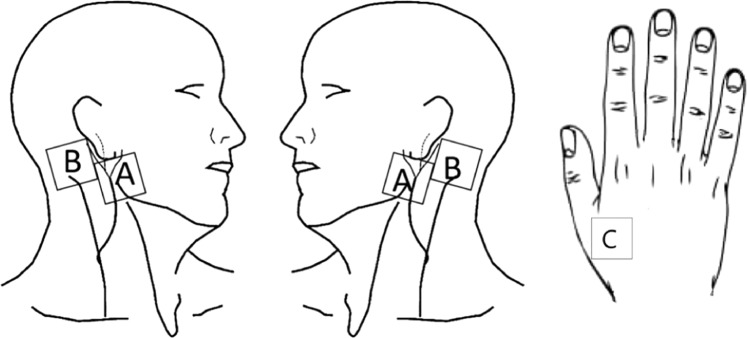
The following QST parameters were tested by a method of limits: cold detection threshold (CDT), warm detection threshold (WDT), cold pain threshold (CPT), heat pain threshold (HPT), mechanical detection threshold (MDT), mechanical pain threshold (MPT), pressure pain threshold (PPT) and two-point discrimination (2PD). All parameters were measured at four sites innervated by the great auricular nerve (GAN): bilateral subauricular sites (LA, RA), bilateral postauricular sites (LB, RB) and one site innervated by the radial nerve: the dorsum of the left hand (control) (Fig. 1)17. In addition, a standardized palpometer (PalpeterR Sunstar Suisse) with a 0.5 kg force was used to apply pressure stimulation at 9 bilateral points at each of the subauricular and postauricular sites.
Figure 1.
Test sites for QST: bilateral subauricular sites (A), bilateral postauricular sites (B), the surface of left hand (C).
Thermal detection and thermal pain thresholds
Thermal quantitative sensory tests were performed with the use of a computerized thermal stimulator (MEDOC TSA-2001 apparatus, Medoc Ltd, Ramat-Yishai, Israel)18,19. The contact area of the thermode was 30 × 30 mm. CDT, WDT were measured first, followed by CPT, HPT. The temperature of the thermode started at a baseline of 32 °C and cooled down or heated up at a rate of 1 °C/s to the lower limit of 0 °C or upper limit of 55 °C. Participants were instructed to press a button on the computer mouse as soon as they perceived the thermal sensation of cold, warm, cold pain, or heat pain. Then, the procedure ended, and the temperature returned to baseline. The mean thresholds of three consecutive measurements were calculated. The verbal instructions given to the participants were in accordance with the QST guidelines as specified by the German Neuropathic Pain Network (DFNS)5.
Mechanical detection and mechanical pain thresholds
MDT was measured with the use of standardized Semmes–Weinstein monofilaments with 20 different diameters (North Coast Medical, Canada). The number of each filament (1.65–6.65) corresponds to a logarithmic function of the equivalent forces of 0.008–300 g. To detect the MPT, weighted pinprick stimuli delivered with a custom-made set of seven pinprick stimulators (Aalborg University, Denmark) were used. Each stimulator had a flat contact surface of 0.2 mm that exerted forces of 8–512 mN20. MDT and MPT were determined by the method of limits, and defined as the geometric mean of 3 series of descending and ascending stimulus intensities5.
Pressure pain threshold
A handheld pressure algometer (Algometer, MEDOC, Israel) with a probe diameter of 1.0 cm was used to test the pain sensitivity to stimuli applied to test sites by a qualified dentist who was specifically trained according to the DFNS examination protocol5. The algometer was applied vertically to the test sites and the applied pressure was linearly increased according to the computer prompt (30 kPa/sec). The participant was instructed to press a hold switch connected to the computer as soon as the sensation of non-painful pressure changed to a sensation of pain. The PPT value was then determined from the display. Three trials were made in random order at each test sites. There was of 1 min interval between each measurement to minimize sensitization and/or habituation to the stimulus. The mean threshold of three measurements was calculated.
Two-point discrimination (2PD)
A vernier caliper was used to vertically contact the two tips with a distance of 15 mm to the test site, the participant was asked to judge if the stimulus was perceived as one or two points. If two points was reported, the distance between the tips was reduced by 1 mm each time until the participant reported only one point, then the threshold (distance between tips) was recorded. Three threshold measurements were made and the mean was used for further statistical analysis.
Mechanical sensitivity mapping
A quantitative mechanical palpometer (PalpeterR, Sunstar Suisse SA company was used for standardized palpation in the bilateral subauricular and postauricular sites. Each of the subauricular and postauricular sites was divided into 3 × 3 grids. The palpometer with 0.5 kg force was applied to the 9 grids in randomized order13. All test points were stimulated for approximately 2 seconds during each measurement. After each measurement, there was a 10-second interval for the participant to rate the perceived intensity of the stimulus on a 0-50-100 numerical rating scale (NRS), in which 0 means no sensation, 50 means just barely painful, 100 means most pain imaginable21. In one session, both sides of the subauricular and postauricular sites were tested three times and the average NRS score of the three stimuli was calculated for each measurement point.
In order to make sure that the same test points were measured, a standard template was fabricated to identify the test sites prior to the test, consisting of 2 squares (3*3 cm) (A、B). Each of the 2 squares was divided into 9 squares, the side of which was 10 mm (Fig. 2a). The center of the square A is on the line between the otobasion inferius (Obi) and the gonion (Go), and the Obi is at the midpoint of the superior edge of the square A (Fig. 2b). The 18 points were marked on the skin of subauricular and postauricular sites with a marker pen in accordance with the standard template (Fig. 2c).
Figure 2.
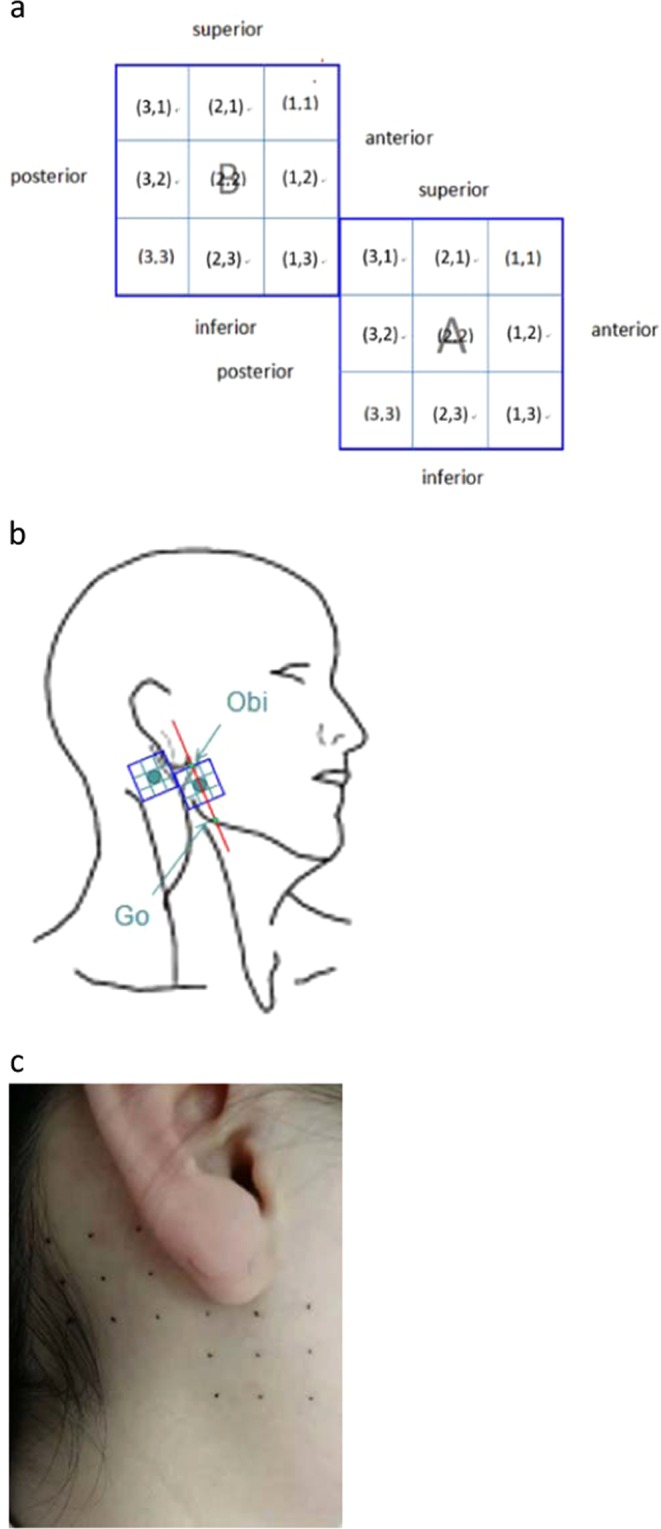
Test points for mechanical sensitivity mapping. (a) A standard template was fabricated to identify the test sites prior to the test, consisting of 2 squares (3*3 cm) (A,B). Each of the 2 squares was divided into 9 squares, the side of which is 10 mm. (b) Each of the subauricular and postauricular sites was divided into 3 × 3 grids. The center of the square A is on the line between the otobasion inferius (Obs) and the gonion (Go) and the Obi is at the midpoint of the superior edge of the square A. (c) The 18 points were marked on the skin of subauricular and postauricular sites with a marker pen in accordance with the standard template.
Statistical analysis
All data were tested for normality distribution before further statistical analysis and log converted if not normally distributed. Intra-class correlation coefficients (ICC) and a 95% confidence interval (CI) were presented to analyze the consistency of the thresholds and NRS values over days. The value of ICC is between 0 and 1. An ICC less than 0.4 represents a poor agreement, an ICC of 0.4–0.59 is regarded fair agreement, an ICC of 0.6–0.75 is considered good agreement, and an ICC more than 0.75 indicates an excellent agreement22. Besides, the standard error of measurement (SEM) and its 95% confidence interval, the smallest real difference (SRD) were calculated (standard error of measurement = intra-individual standard deviation * √(1-ICC), SRD = 1.96* standard error of measurement *√2). The standard error of measurement indicates the expected error between two measurements conducted under the same circumstances in the same subject over a defined period of time. The lower the standard error of measurement, the better the test-retest-reliability23.
One-way analysis of variation (ANOVA) was used to test for differences in the thresholds and NRS scores over days with a significance level of 5%. Two-way ANOVA was used to test differences in the thresholds with the following factors: gender (2 levels), test sites (4 levels). Three-way ANOVA was used to test differences in NRS scores with the following factors: gender (2 levels), test sites (4 levels) and test points (9 levels).
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Results
The study was carried out from November 17, 2018 to March 18, 2019. All enrolled participants completed the experiment. All data were reported as mean and standard deviation (M ± SD).
Reliability findings
ICC values and 95% CI of all values are listed in Tables 1 and 2 for different days. All ICC values of QST values were above 0.4 between different days (ICCCDT: 0.507–0.913; ICCWDT: 0.689–0.940; ICCCPT: 0.644–0.971; ICCHPT: 0.652–0.957; ICCMDT: 0.669–0.945; ICCMPT: 0.775–0.935; ICCPPT: 0.625–0.869; ICC2PD: 0.693–0.927) which meant the test-retest reliability of the data implied fair to excellent agreement (Table 1). Figure 3 presents the percentages of the ICC values of each of the two groups according to their magnitude distribution (Poor women: 2.5%; Good women: 17.5%; Excellent women: 80%; Poor men: 5%; Good men: 25%; Excellent men: 70%). All ICC values of NRS scores were above 0.75 between different days (ICCLA: 0.750–0.946; ICCLB: 0.776–0.947; ICCRA: 0.784–0.976; ICCRB: 0.774–0.934) which meant the test-retest reliability of the data implied excellent agreement (Table 2).
Table 1.
The reliability scores of the QST values between different days in different sites and genders (n = 20).
| item | gender | LA | LB | RA | RB | C | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ICC (95%CI) | SEM (SRD) | ICC (95%CI) | SEM (SRD) | ICC (95%CI) | SEM (SRD) | ICC (95%CI) | SEM (SRD) | ICC (95%CI) | SEM (SRD) | ||
| CDT (°C) | women | 0.761 (0.323,0.934) | 0.379 (1.051) | 0.913 (0.706,0.977) | 0.221 (0.612) | 0.874 (0.596,0.967) | 0.248 (0.688) | 0.893 (0.647,0.972) | 0.274 (0.761) | 0.507 (−0.106,0.847) | 0.457 (1.268) |
| men | 0.834 (0.491,0.955) | 0.334 (0.927) | 0.550 (−0.046,0.864) | 0.385 (1.066) | 0.849 (0.528,0.960) | 0.294 (0.814) | 0.751 (0.302,0.931) | 0.436 (1.208) | 0.531 (−0.073,0.856) | 0.364 (1.008) | |
| WDT (°C) | women | 0.940 (0.791,0.985) | 0.177 (0.490) | 0.855 (0.544,0.961) | 0.502 (1.390) | 0.899 (0.665,0.974) | 0.382 (1.060) | 0.827 (0.473,0.953) | 0.530 (1.468) | 0.850 (0.531,0.960) | 0.197 (0.545) |
| men | 0.689 (0.179,0.911) | 0.301 (0.834) | 0.815 (0.443,0.950) | 0.406 (1.124) | 0.785 (0.373,0.941) | 0.286 (0.792) | 0.896 (0.657,0.973) | 0.348 (0.963) | 0.859 (0.555,0.962) | 0.256 (0.710) | |
| CPT (°C) | women | 0.848 (0.525,0.959) | 1.191 (3.301) | 0.718 (0.234,0.920) | 1.397 (3.873) | 0.854 (0.543,0.961) | 1.050 (2.910) | 0.851 (0.533,0.960) | 1.016 (2,817) | 0.830 (0.480,0.954) | 1.055 (2.925) |
| men | 0.644 (0.101,0.897) | 0.776 (2.152) | 0.971 (0.895,0.993) | 1.208 (3.350) | 0.833 (0.488,0.955) | 1.360 (3.770) | 0.935 (0.776,0.983) | 1.769 (4.902) | 0.848 (0.527,0.959) | 0.954 (2.643) | |
| HPT (°C) | women | 0.781 (0.365,0.940) | 0.830 (2.300) | 0.876 (0.601,0.967) | 1.149 (3.186) | 0.912 (0.703,0.977) | 0.917 (2.543) | 0.788 (0.381,0.942) | 1.114 (3.087) | 0.929 (0.755,0.982) | 0.656 (1.818) |
| men | 0.668 (0.141,0.904) | 0.937 (2.596) | 0.652 (0.113,0.899) | 0.580 (1.608) | 0.957 (0.848,0.989) | 0.548 (1.519) | 0.949 (0.820,0.987) | 0.530 (1.470) | 0.916 (0.715,0.978) | 0.683 (1.892) | |
| MDT (mN) | women | 0.945 (0.806,0.986) | 0.010 (0.027) | 0.703 (0.206,0.916) | 0.017 (0.048) | 0.869 (0.580,0.965) | 0.014 (0.038) | 0.669 (0.144,0.905) | 0.022 (0.061) | 0.890 (0.640,0.971) | 0.017 (0.047) |
| men | 0.849 (0.529,0.960) | 0.020 (0.054) | 0.881 (0.613,0.968) | 0.014 (0.039) | 0.781 (0.365,0.940) | 0.013 (0.037) | 0.688 (0.177,0.911) | 0.016 (0.044) | 0.824 (0.465,0.952) | 0.065 (0.180) | |
| MPT (mN) | women | 0.885 (0.626,0.970) | 0.482 (1.336) | 0.917 (0.720,0.978) | 0.332 (0.921) | 0.893 (0.648,0.972) | 0.414 (1.149) | 0.935 (0.774,0.983) | 0.328 (0.910) | 0.848 (0.527,0.959) | 0.437 (1.211) |
| men | 0.788 (0.38,0.942) | 0.135 (0.374) | 0.866 (0.572,0.964) | 0.411 (1.139) | 0.818 (0.452,0.951) | 0.368 (1.019) | 0.775 (0.352,0.938) | 0.377 (1.045) | 0.857 (0.549,0.962) | 0.350 (0.971) | |
| PPT (kPa) | women | 0.661 (0.129,0.902) | 8.972 (24.870) | 0.796 (0.399,0.944) | 11.621 (32.211) | 0.699 (0.198,0.914) | 12.320 (34.149) | 0.699 (0.197,0.914) | 13.512 (37.455) | 0.712 (0.223,0.919) | 15.056 (41.732) |
| men | 0.625 (0.068,0.890) | 12.664 (35.102) | 0.682 (0.167,0.909) | 12.856 (35.635) | 0.869 (0.582,0.965) | 8.767 (24.300) | 0.820 (0.455,0.951) | 10.950 (30.352) | 0.827 (0.474,0.953) | 17.933 (49.708) | |
| 2PD (mm) | women | 0.807 (0.425,0.947) | 0.941 (2.609) | 0.918 (0.721,0.979) | 0.510 (1.414) | 0.809 (0.430,0.948) | 0.942 (2.611) | 0.927 (0.748,0.981) | 0.406 (1.126) | 0.790 (0.386,0.943) | 1.295 (3.590) |
| men | 0.778 (0.358,0.939) | 0.323 (0.895) | 0.708 (0.215,0.917) | 0.475 (1.316) | 0.693 (0.187,0.913) | 0.377 (1.045) | 0.883 (0.620,0.969) | 0.229 (0.636) | 0.712 (0.223,0.919) | 0.680 (1.885) | |
Cold detection threshold (CDT), warm detection threshold (WDT), cold pain threshold (CPT), heat pain threshold (HPT), mechanical detection threshold (MDT), mechanical pain threshold (MPT), pain-pressure threshold (PPT) and two-point discrimination (2PD). LA: left subauricular site, LB: left postauricular site, RA: right subauricular site, RB: right postauricular site, C: the surface of left hand. Values are shown by ICC (95%CI), SEM and SRD.
Table 2.
The reliability scores of the NRS scores between days in different sites and genders (n = 20).
| point | gender | LA | LB | RA | RB | ||||
|---|---|---|---|---|---|---|---|---|---|
| ICC (95%CI) | SEM (SRD) | ICC (95%CI) | SEM (SRD) | ICC (95%CI) | SEM (SRD) | ICC (95%CI) | SEM (SRD) | ||
| (1,1) | women | 0.946 (0.811,0.986) | 3.523 (9.764) | 0.924 (0.740,0.980) | 2.648 (7.341) | 0.930 (0.760,0.982) | 3.837 (10.635) | 0.916 (0.716,0.978) | 2.741 (7.598) |
| men | 0.750 (0.298,0.930) | 4.258 (11.802) | 0.786 (0.378,0.941) | 2.359 (6.538) | 0.883 (0.618,0.969) | 2.671 (7.403) | 0.807 (0.424,0.947) | 2.815 (7.804) | |
| (1,2) | women | 0.836 (0.495,0.956) | 5.990 (16.603) | 0.928 (0.754,0.981) | 2.436 (6.752) | 0.925 (0.744,0.981) | 4.272 (11.841) | 0.931 (0.763,0.982) | 3.073 (8.518) |
| men | 0.782 (0.368,0.940) | 4.034 (11.181) | 0.792 (0.390,0.943) | 2.490 (6.901) | 0.789 (0.385,0.942) | 3.505 (9.715) | 0.854 (0.540,0.961) | 2.695 (7.469) | |
| (1,3) | women | 0.844 (0.516,0.958) | 6.262 (17.358) | 0.828 (0.475,0.954) | 3.192 (8.847) | 0.976 (0.912,0.994) | 2.953 (8.185) | 0.906 (0.685,0.975) | 3.225 (8.94) |
| men | 0.811 (0.434,0.949) | 2.961 (8.208) | 0.790 (0.385,0.942) | 2.584 (7.162) | 0.791 (0.387,0.943) | 4.193 (11.621) | 0.868 (0.577,0.965) | 2.452 (6.797) | |
| (2,1) | women | 0.867 (0.575,0.965) | 4.006 (11.104) | 0.906 (0.685,0.975) | 3.761 (10.426) | 0.945 (0.808,0.986) | 2.964 (8.216) | 0.875 (0.599,0.967) | 3.957 (10.967) |
| men | 0.761 (0.323,0.934) | 2.952 (8.182) | 0.816 (0.445,0.950) | 2.893 (8.020) | 0.819 (0.453,0.951) | 2.747 (7.616) | 0.847 (0.524,0.959) | 2.625 (7.276) | |
| (2,2) | women | 0.916 (0.715,0.978) | 4.535 (12.570) | 0.917 (0.719,0.978) | 2.819 (7.813) | 0.903 (0.677,0.975) | 4.587 (12.715) | 0.918 (0.722,0.979) | 3.019 (8.367) |
| men | 0.778 (0.359,0.939) | 3.425 (9.495) | 0.814 (0.440,0.949) | 2.144 (5.944) | 0.784 (0.371,0.941) | 3.384 (9.381) | 0.863 (0.564,0.963) | 3.006 (8.332) | |
| (2,3) | women | 0.898 (0.662,0.973) | 4.836 (13.405) | 0.947 (0.814,0.986) | 2.393 (6.633) | 0.945 (0.806,0.986) | 4.103 (11.374) | 0.925 (0.742,0.980) | 2.945 (8.163) |
| men | 0.817 (0.448,0.950) | 4.269 (11.832) | 0.821 (0.458,0.952) | 1.968 (5.454) | 0.803 (0.414,0.946) | 4.219 (11.695) | 0.881 (0.615,0.969) | 2.664 (7.385) | |
| (3,1) | women | 0.927 (0.750,0.981) | 2.915 (8.081) | 0.917 (0.718,0.978) | 3.161 (8.762) | 0.899 (0.667,0.974) | 3.984 (11.043) | 0.838 (0.499,0.956) | 4.263 (11.816) |
| men | 0.852 (0.537,0.961) | 2.438 (6.758) | 0.848 (0.526,0.959) | 1.631 (4.520) | 0.795 (0.398,0.944) | 2.997 (8.306) | 0.774 (0.351,0.938) | 4.193 (11.622) | |
| (3,2) | women | 0.904 (0.681,0.975) | 4.539 (12.582) | 0.883 (0.621,0.969) | 3.859 (10.697) | 0.933 (0.769,0.983) | 3.738 (10.363) | 0.825 (0.468,0.953) | 4.426 (12.269) |
| men | 0.880 (0.610,0.968) | 2.490 (6.901) | 0.796 (0.400,0.944) | 2.353 (6.521) | 0.843 (0.512,0.958) | 1.891 (5.24) | 0.862 (0.561,0.963) | 3.209 (8.895) | |
| (3,3) | women | 0.893 (0.647,0.972) | 4.191 (11.617) | 0.906 (0.686,0.975) | 3.065 (8.497) | 0.953 (0.833,0.988) | 3.422 (9.486) | 0.934 (0.771,0.983) | 2.535 (7.025) |
| men | 0.808 (0.428,0.948) | 4.189 (11.612) | 0.776 (0.355,0.938) | 2.167 (6.006) | 0.827 (0.474,0.953) | 4.409 (12.221) | 0.809 (0.431,0.948) | 2.871 (7.957) | |
LA: left subauricular site, LB: left postauricular site, RA: right subauricular site, RB: right postauricular site. Values are shown by ICC (95%CI), SEM and SRD.
Figure 3.
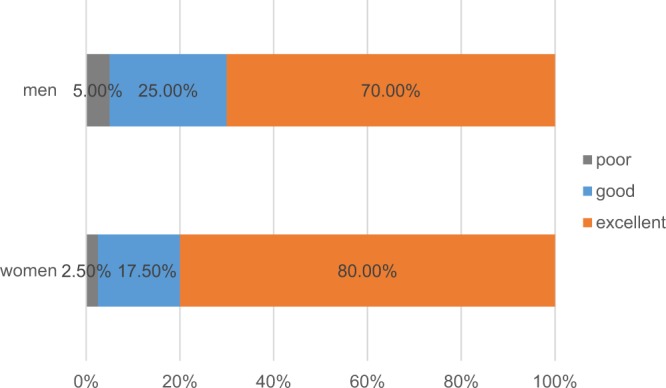
The percentages of the ICC values of each of the two groups according to their magnitude distribution.
The ANOVA of the values showed no significant main effects of days (P ≥ 0.132) (Table 3).
Table 3.
Results of ANOVA analysis (F-estimates and P-values) for the QST values and NRS scores to mechanical stimuli in different sites over different days (n = 20).
| gender | LA | LB | RA | RB | |||||
|---|---|---|---|---|---|---|---|---|---|
| F | P | F | P | F | P | F | P | ||
| CDT (°C) | women | 1.027 | 0.324 | 0.124 | 0.729 | 0.024 | 0.879 | 0.006 | 0.939 |
| men | 0.054 | 0.818 | 2.493 | 0.132 | 0.003 | 0.956 | 0.149 | 0.704 | |
| WDT (°C) | women | 0.307 | 0.586 | 0.045 | 0.834 | 0.074 | 0.788 | 0.014 | 0.907 |
| men | 0.340 | 0.567 | 0.206 | 0.655 | 0.225 | 0.641 | 0.026 | 0.873 | |
| CPT (°C) | women | 0.491 | 0.492 | 1.205 | 0.287 | 0.006 | 0.939 | 0.111 | 0.743 |
| men | 0.004 | 0.952 | 0.073 | 0.791 | 0.677 | 0.421 | 0.002 | 0.963 | |
| HPT (°C) | women | 0.000 | 0.991 | 0.361 | 0.556 | 0.010 | 0.922 | 0.324 | 0.576 |
| men | 0.640 | 0.434 | 0.026 | 0.874 | 0.125 | 0.728 | 0.025 | 0.875 | |
| MDT (mN) | women | 0.073 | 0.791 | 0.559 | 0.464 | 0.287 | 0.599 | 1.354 | 0.260 |
| men | 0.361 | 0.555 | 0.084 | 0.775 | 0.778 | 0.389 | 0.251 | 0.622 | |
| MPT (mN) | women | 0.000 | 0.988 | 0.322 | 0.577 | 0.095 | 0.762 | 0.225 | 0.641 |
| men | 0.182 | 0.675 | 0.000 | 0.985 | 0.420 | 0.525 | 0.006 | 0.938 | |
| PPT (kPa) | women | 0.338 | 0.568 | 0.185 | 0.672 | 0.012 | 0.914 | 0.485 | 0.495 |
| men | 0.038 | 0.848 | 0.024 | 0.878 | 0.277 | 0.605 | 0.157 | 0.697 | |
| 2PD (mm) | women | 0.000 | 1.000 | 0.055 | 0.818 | 0.010 | 0.922 | 0.024 | 0.878 |
| men | 0.450 | 0.511 | 0.194 | 0.665 | 0.101 | 0.754 | 0.117 | 0.736 | |
| point | |||||||||
| (1,1) | women | 0.172 | 0.684 | 0.070 | 0.794 | 0.159 | 0.694 | 0.155 | 0.698 |
| men | 0.016 | 0.902 | 0.182 | 0.675 | 0.070 | 0.795 | 0.016 | 0.902 | |
| (1,2) | women | 0.114 | 0.740 | 0.059 | 0.811 | 0.126 | 0.727 | 0.065 | 0.801 |
| men | 0.565 | 0.462 | 0.693 | 0.416 | 0.125 | 0.728 | 0.222 | 0.643 | |
| (1,3) | women | 0.004 | 0.948 | 0.216 | 0.648 | 0.048 | 0.829 | 0.000 | 0.995 |
| men | 0.200 | 0.660 | 1.102 | 0.308 | 0.308 | 0.586 | 0.299 | 0.591 | |
| (2,1) | women | 0.130 | 0.723 | 0.302 | 0.590 | 0.012 | 0.914 | 0.001 | 0.973 |
| men | 0.000 | 0.994 | 0.090 | 0.767 | 0.096 | 0.760 | 0.721 | 0.407 | |
| (2,2) | women | 0.048 | 0.829 | 0.000 | 0.984 | 0.289 | 0.598 | 0.030 | 0.865 |
| men | 0.205 | 0.656 | 0.194 | 0.664 | 0.206 | 0.655 | 0.259 | 0.617 | |
| (2,3) | women | 0.056 | 0.815 | 0.075 | 0.787 | 0.004 | 0.950 | 0.095 | 0.761 |
| men | 0.030 | 0.865 | 0.004 | 0.948 | 0.301 | 0.590 | 0.094 | 0.763 | |
| (3,1) | women | 0.002 | 0.966 | 0.137 | 0.716 | 0.321 | 0.578 | 0.023 | 0.880 |
| men | 0.587 | 0.454 | 0.363 | 0.554 | 0.186 | 0.671 | 0.051 | 0.824 | |
| (3,2) | women | 0.368 | 0.552 | 0.054 | 0.819 | 0.092 | 0.765 | 0.005 | 0.943 |
| men | 0.113 | 0.741 | 0.162 | 0.692 | 0.401 | 0.535 | 0.008 | 0.932 | |
| (3,3) | women | 0.079 | 0.782 | 0.005 | 0.947 | 0.062 | 0.806 | 0.083 | 0.777 |
| men | 0.149 | 0.704 | 0.014 | 0.907 | 0.197 | 0.662 | 0.183 | 0.674 | |
Cold and warm detection threshold (CDT&WDT), cold and heat pain threshold (CPT&HPT), mechanical detection and pain threshold (MDT&MPT), pain-pressure threshold (PPT) and two-point discrimination (2PD). LA: left subauricular site, LB: left postauricular site, RA: right subauricular site, RB: right postauricular site.
Effect of gender and site
Results of ANOVA analysis (F-estimates and P-values) of gender and site effects for the QST values are listed in Table 4. Most of the QST parameters at the periauricular skin site were not gender-related except 2PD. The 2PD was significantly lower in women than in men (P = 0.048). There were no significant main effects of side in any of the QST parameters (P ≥ 0.057). Left and right sides showed corresponding values for all QST parameters and at all sites. Significant differences between the subauricular and postauricular sites were shown for WDT and PPT (P ≤ 0.028). The WDT and PPT at the postauricular site were significantly higher than at the subauricular site (P ≤ 0.028).
Table 4.
Results of ANOVA analysis (F-estimates and P-values) of gender and site effects for the QST values.
| gender | site | LA-LB | LA-RA | LB-RB | RA-RB | |||
|---|---|---|---|---|---|---|---|---|
| F | P | F | P | p | p | p | p | |
| CDT (°C) | 1.126 | 0.292 | 0.388 | 0.762 | 0.609 | 0.500 | 0.437 | 0.348 |
| WDT (°C) | 1.315 | 0.255 | 11.430 | 0.000*** | 0.001** | 0.288 | 0.057 | <0.001*** |
| CPT (°C) | 1.113 | 0.295 | 0.118 | 0.949 | 0.802 | 0.782 | 0.970 | 0.625 |
| HPT (°C) | 0.060 | 0.808 | 1.357 | 0.263 | 0.126 | 0.369 | 0.751 | 0.339 |
| MDT (mN) | 0.290 | 0.592 | 0.404 | 0.750 | 0.937 | 0.343 | 0.987 | 0.394 |
| MPT (mN) | 0.544 | 0.463 | 0.202 | 0.894 | 0.507 | 0.718 | 0.516 | 0.729 |
| PPT (kPa) | 3.635 | 0.061 | 3.900 | 0.012* | 0.028 * | 0.967 | 0.778 | 0.012* |
| 2PD (mm) | 4.034 | 0.048* | 0.390 | 0.761 | 0.663 | 0.913 | 0.587 | 0.385 |
Cold detection threshold (CDT), warm detection threshold (WDT), cold pain threshold (CPT), heat pain threshold (HPT), mechanical detection threshold (MDT), mechanical pain threshold (MPT), pain-pressure threshold (PPT) and two-point discrimination (2PD). LA: left subauricular site, LB: left postauricular site, RA: right subauricular site, RB: right postauricular site. *P < 0.05, **P < 0.01, ***P < 0.001.
The ANOVA of the NRS scores showed that there were significant main effects of gender, site and point (Table 5). However, there were no significant main effects of side (P ≥ 0.333). Women were significantly more sensitive than men (P < 0.05). The NRS scores at the subauricular site were significantly higher than those at the postauricular site. At the subauricular site, the women reported the lowest mean NRS value (lowest sensitivity) corresponding to 33.0 at point (x,y) = (1,2) and the highest mean NRS value (highest sensitivity) 43.0 at point (x,y) = (3,2). The men reported the lowest mean NRS value as 24.9 at point (x,y) = (1,3) and the highest mean NRS value 34.1 at point (x,y) = (3,2). At the postauricular site, the women reported the lowest mean NRS value as 23.9 at point (x,y) = (1,1) and the highest mean NRS value as 31.6 at point (x,y) = (1,3). The men reported the lowest mean NRS value as 20.3 at point (x,y) = (1,1) and the highest mean NRS value as 26.1 at point (x,y) = (1,3).Two-dimensional illustrations of the mean NRS values of each of the two groups at the 18 points at the subauricular and postauricular sites are presented in Fig. 4 to show the considerable point-to-point differences in mechanical sensitivity.
Table 5.
Results of ANOVA analysis (F-estimates and P-values) of gender, site and point effects for 0-50-100 NRS scores to mechanical stimuli.
| F | P | |
|---|---|---|
| gender | 31.252 | <0.001*** |
| site | 70.879 | <0.001*** |
| point | 2.039 | 0.040* |
| LA-LB | <0.001*** | |
| LA-RA | 0.333 | |
| LB-RB | 0.956 | |
| RA-RB | <0.001*** |
LA: left subauricular site, LB: left postauricular site, RA: right subauricular site, RB: right postauricular site. *P < 0.05, **P < 0.01, ***P < 0.001.
Figure 4.
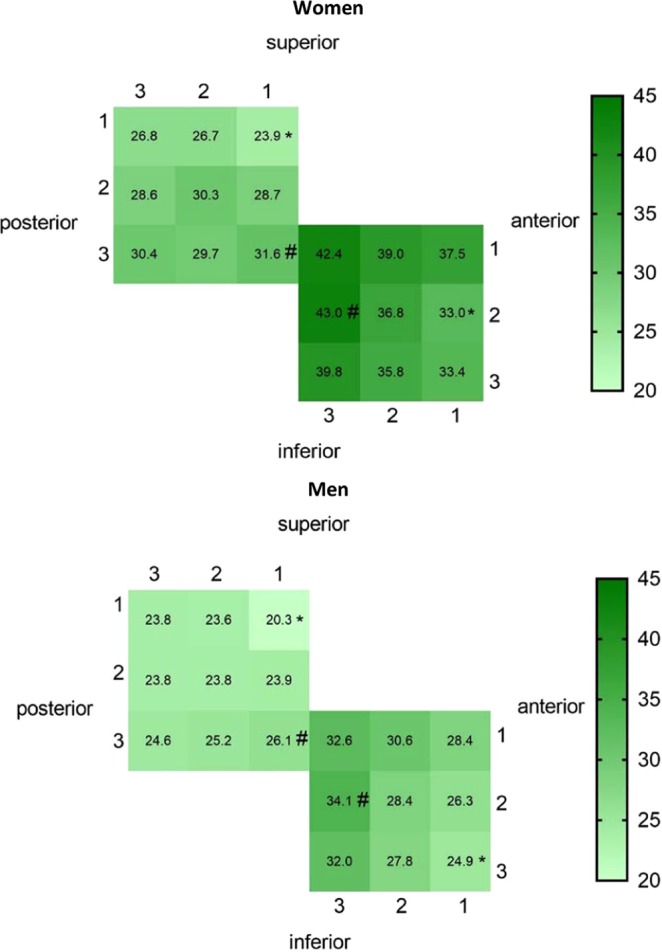
Two-dimensional illustrations of the mean NRS scores of each group at 18 points in the subauricular and postauricular sites. The more sensitive the area, the deeper the color represented. *The least sensitive point in the site. #The most sensitive point in the site.
Discussion
The present study was devoted to the development of standardized quantitative tests to assess the somatosensory function at the periauricular skin in healthy individuals. The mechanical sensitivity mapping was for the first time applied to this particular area. The results of this study showed that QST and mechanical sensitivity mapping of the periauricular skin in healthy adults are stable and with a sufficient test-retest reliability to allow clinical implementation. It was noted that, some QST parameters and mechanical sensitivity mapping were affected by gender and site and therefore needs to be taken into account in future research studies.
When the main trunk of the GAN is damaged, different degrees and different ranges of somatosensory abnormalities may appear in the skin of the lower two-thirds of the ear and periauricular region14,15. The somatosensory function of other sites around the external ear are dominated by three other nerves, such as the auricular branch of the vagus nerve (ABVN), the auriculotemporal nerve (ATN), and the lesser occipital nerve (LON) which were not the focus of the present study. In addition, the temporomandibular joint (TMJ) is located in the preauricular site and painful TMJ disorders may influence the somatosensory function24,25. Many previous studies have analyzed the QST and mechanical sensitivity mapping of the TMJ region13,26, so the preauricular site was not described again in this study. Though the tail of the helix and the earlobe are dominated by the GAN, these sites are too small and difficult to assess with current QST techniques. The subauricular and postauricular sites are large enough, well-positioned, and representative of the GAN innervation territory. The results of these two sites may therefore reflect the somatosensory function of the GAN to a large extent. Therefore, these two sites were assessed in this experiment.
In previous studies, the evaluation of the somatosensory function of the periauricular skin was mainly based on the stimulation with low-tech instruments such as cotton wool, a writing brush, a latex glove full of iced water, a warmed steel spoon and so on27–29. Such studies have used different scoring standards in order to quantify the perceived intensity of the stimulus. However, it seems difficult to ensure that the same stimulus would be applied to the same position every time, moreover, the expectation of the examiner and the psychological state of the participant may decrease the accuracy and test-retest reliability. Currently, QST is the only technology that can be used to examine the function of small nerve fibers30, mainly by assessment of sensory and pain thresholds. Modern physiology studies have identified three subtypes of sensory nerve fibers: Aβ, Aδ, and C fibers. A related study with the use of nerve blocks showed that CDT and MPT is transmitted by myelinated fine Aδ fibers31. The WDT and MDT reflect functions of unmyelinated C fibers and large, myelinated Aβ fibers, respectively31. The CPT is transmitted by C fibers and Aδ fibers, and the HPT is transmitted by C fibers and part of Aβ fibers31. Therefore, the sensory thresholds reflect dysfunctions of the peripheral nerve. In addition to the objective factors such as the method of examination, the pressure of the probe, the contact area between the probe and the skin, and the frequency of stimulation changes, the results of QST are affected by the cooperation and understanding of the participants32. Simultaneously, the participant´s attention and drowsiness will affect the reaction time. In general, protective measures can be taken such as indifferent stimulus (blank stimuli) and threshold changes to prevent this decline in participant-related factors.
Furthermore, a map of mechanical sensitivity was constructed in the present study with the use of a standardized palpometer in order to get more spatial details of the somatosensory function in healthy volunteers. Mapping is considered a pivotal approach in a comprehensive survey of the somatosensory system pathology31 and can provide an overall description of changes in somatosensory function with time or sensitivity variations among test sites21.
The test-retest reliability or stability of the experiment is an extremely important indicator for the evaluation and interpretation of the results which is directly related to the sensitivity and specificity of the outcome measures. In this study, a comprehensive battery included the CDT, WDT, CPT, HPT, MDT, MPT, 2PD, PPT and mechanical sensitivity mapping on the skin of left hand, bilateral subauricular sites and bilateral postauricular sites of 20 healthy Chinese in two sessions (one week apart). All ICC values of QST values were above 0.4, even 80% in the female group and 70% in the male group were above 0.75, and all ICC values of NRS scores were above 0.75, which meant the test-retest reliability of the data implied fair to excellent agreement. According to the ICC values, we believe that QST and mechanical sensitivity mapping can be used as a novel tool to evaluate the somatosensory function of the periauricular skin.
In the present study, most of the QST parameters at the periauricular skin site were not gender-related except 2PD. Women were more sensitive than men only in this particular parameter. This result is at odds with previous research. Riley et al.33 reported that women were more sensitive than men in the most of the QST parameters. The study by Komiyama34 shows that in the terms of QST on the cheek skin, women are much more sensitive than men. This novel finding may be caused by the different test sites, or it may be due to the relatively small sample size of this study affecting the results. In terms of the mechanical sensitivity mapping, the mean NRS score from the female group was significantly higher than that of the male group, which is consistent with previous experimental results33,35. Currently, the mechanism of gender-related pain threshold difference has not been fully understood. The possible factors are hormonal differences, the difference in resting blood pressure, psychological influences and the effects of dopamine and central serotonin36,37. In addition, when subjected to different types of external stimuli, genders will have different skin/muscle structure and thickness, anatomical characteristics, biochemical composition and different density of innervation38. Further research will be needed to better understand gender differences in somatosensory function.
For both QST or mechanical sensitivity mapping, there was no significant difference between the left and right sides. This suggests similar sensory thresholds on both the left and right sides, which is important information in clinical practice and future studies of patients with unilateral GAN damage.
Significant differences between the subauricular and postauricular sites were shown for WDT and PPT, which may be related to the difference in the density of nerve distribution of the branches of GAN, and may also be related to the difference in muscle and bone composition under the skin. Speaking of mechanical sensitivity mapping, the mean NRS score at the subauricular site was significantly higher than at the postauricular site. Moreover, the mechanical sensitivity was unevenly distributed over the two sites. The most sensitive part of the subauricular site was located at the midpoint of the trailing edge, and the most sensitive part of the postauricular site was at the lowest point of the leading edge. These two points are relatively close in the distance. The mean NRS value at the subauricular site showed an anterior to posterior increased inclination which may reflected in differences in somatosensory function related to the underlying parotid gland and mandible. The mean NRS value at the periauricular site showed a superior to inferior increased inclination approximately, which meant that the sensitivity of the mastoid area is lower than that of the sternocleidomastoid muscle. It could be conjectured that differences in anatomical structure lead to differences in mechanical sensitivity, which is consistent with the existing research results13.
The participants of the present study were 20 young healthy adults, which is a relatively small sample size, and the age effect to the QST and mechanical sensitivity was not considered. Due to the large number of the measurement parameters, it took a long time to complete the experiment, almost about 45 min, which would affect the attention and cooperation of the participants and clinical implementation. It should be considered whether it is necessary to streamline items in future measurement. Furthermore, the short-term stability of QST and mechanical sensitivity mapping was evaluated in the present study, however, its long-term stability is still not known.
Conclusion
Both QST and mechanical sensitivity mapping are sufficiently reliable to assess the somatosensory function of the periauricular skin. The description of somatosensory function at the periauricular skin in healthy adults is a prerequisite to understand the normal range of values and to help establish a diagnosis of somatosensory dysfunction in patients undergoing surgery or following trauma to the region. In future studies, the proposed QST protocol and mechanical sensitivity mapping can be applied to patients with abnormal sensations of periauricular skin, so that it will be interesting to study further details in favor of diagnosis, treatment and prognosis in the clinic.
Acknowledgements
This work was supported by A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD, 2018-87), The Project of Invigorating Health Care through Science, Technology and Education (Jiangsu Provincial Medical Youth Talent QNRC2016852), Jiangsu Provincial Medical Innovation Team (CXTDA2017036) and sponsored by Qing Lan Project.
Author contributions
H.Y. and L.W. conceived and designed the experiments. F.Z., W.L. and L.Y. performed the experiment. W.L., F.Z. and H.Y. analyzed the data. W.L., F.Z. and H.Y. wrote the manuscript, P.S. and K.W. reviewed the manuscript.
Data availability
A statistical package for social sciences version 19 (SPSS, IBM, Armonk, NY, USA) was used in this statistical procedure. The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
W.L. declares that she has no conflict of interest. F.Z. declares that she has no conflict of interest. L.Y. declares that she has no conflict of interest. L.W. declares that he has no conflict of interest. H.Y. declares that he has no conflict of interest. K.W. declares that he has no conflict of interest. P.S. declares that he has no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Wen Lin and Fan Zhou.
References
- 1.McKinney P, Gottlieb J. The relationship of the great auricular nerve to the superficial musculoaponeurotic system. Annals of plastic surgery. 1985;14:310–314. doi: 10.1097/00000637-198504000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Tubbs RS, Salter EG, Wellons JC, Blount JP, Oakes WJ. Landmarks for the identification of the cutaneous nerves of the occiput and nuchal regions. Clinical anatomy (New York, N.Y.) 2007;20:235–238. doi: 10.1002/ca.20297. [DOI] [PubMed] [Google Scholar]
- 3.Peuker ET, Filler TJ. The nerve supply of the human auricle. Clinical anatomy (New York, N.Y.) 2002;15:35–37. doi: 10.1002/ca.1089. [DOI] [PubMed] [Google Scholar]
- 4.Ginsberg LE, Eicher SA. Great auricular nerve: anatomy and imaging in a case of perineural tumor spread. AJNR. American journal of neuroradiology. 2000;21:568–571. [PMC free article] [PubMed] [Google Scholar]
- 5.Rolke R, et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values. Pain. 2006;123:231–243. doi: 10.1016/j.pain.2006.01.041. [DOI] [PubMed] [Google Scholar]
- 6.Rolke R, et al. Quantitative sensory testing: a comprehensive protocol for clinical trials. European journal of pain (London, England) 2006;10:77–88. doi: 10.1016/j.ejpain.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Yekta SS, Smeets R, Stein JM, Ellrich J. Assessment of trigeminal nerve functions by quantitative sensory testing in patients and healthy volunteers. Journal of oral and maxillofacial surgery: official journal of the American Association of Oral and Maxillofacial Surgeons. 2010;68:2437–2451. doi: 10.1016/j.joms.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 8.Pigg M, Baad-Hansen L, Svensson P, Drangsholt M, List T. Reliability of intraoral quantitative sensory testing (QST) Pain. 2010;148:220–226. doi: 10.1016/j.pain.2009.10.024. [DOI] [PubMed] [Google Scholar]
- 9.Knapstad MK, et al. Measuring pressure pain threshold in the cervical region of dizzy patients-The reliability of a pressure algometer. Physiotherapy research international: the journal for researchers and clinicians in physical therapy. 2018;23:e1736. doi: 10.1002/pri.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Leeuwen RJ, Szadek K, de Vet H, Zuurmond W, Perez R. Pain Pressure Threshold in the Region of the Sacroiliac Joint in Patients Diagnosed with Sacroiliac Joint Pain. Pain physician. 2016;19:147–154. [PubMed] [Google Scholar]
- 11.Ettlin DA, et al. Quantitative sensory testing of intraoral open wounds. International journal of oral and maxillofacial surgery. 2013;42:401–405. doi: 10.1016/j.ijom.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 12.Futarmal S, Kothari M, Ayesh E, Baad-Hansen L, Svensson P. New palpometer with implications for assessment of deep pain sensitivity. Journal of dental research. 2011;90:918–922. doi: 10.1177/0022034511402997. [DOI] [PubMed] [Google Scholar]
- 13.Tang Z, et al. Reliability of Mechanical Sensitivity Mapping in the Orofacial Region of Healthy Chinese Individuals: Towards Standardized Assessment of Somatosensory Function. Journal of oral & facial pain and headache. 2018;32:400–408. doi: 10.11607/ofph.2137. [DOI] [PubMed] [Google Scholar]
- 14.Grosheva M, et al. Sensation loss after superficial parotidectomy: A prospective controlled multicenter trial. Head & neck. 2017;39:520–526. doi: 10.1002/hed.24647. [DOI] [PubMed] [Google Scholar]
- 15.Rozeboom AVJ, Dubois L, Bos RRM, Spijker R, de Lange J. Open treatment of condylar fractures via extraoral approaches: A review of complications. Journal of cranio-maxillo-facial surgery: official publication of the European Association for Cranio-Maxillo-Facial Surgery. 2018;46:1232–1240. doi: 10.1016/j.jcms.2018.04.020. [DOI] [PubMed] [Google Scholar]
- 16.Park SW, Choi JY, Jung KJ. Management of Great Auricular Neuralgia Confirmed by Electrophysiologic Examination: A Case Report. Journal of oral & facial pain and headache. 2018;32:e53–e56. doi: 10.11607/ofph.2144. [DOI] [PubMed] [Google Scholar]
- 17.Mowlavi, A., Meldrum, D. G., Wilhelmi, B. J., Ghavami, A. & Zook, E. G. The aesthetic earlobe: classification of lobule ptosis on the basis of a survey of North American Caucasians. Plastic and reconstructive surgery112, 266–272; discussion 273–264, 10.1097/01.Prs.0000066368.07829.9b (2003). [DOI] [PubMed]
- 18.Yarnitsky D, Sprecher E, Zaslansky R, Hemli JA. Heat pain thresholds: normative data and repeatability. Pain. 1995;60:329–332. doi: 10.1016/0304-3959(94)00132-x. [DOI] [PubMed] [Google Scholar]
- 19.Fruhstorfer H, Lindblom U, Schmidt WC. Method for quantitative estimation of thermal thresholds in patients. Journal of neurology, neurosurgery, and psychiatry. 1976;39:1071–1075. doi: 10.1136/jnnp.39.11.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan AW, MacFarlane IA, Bowsher D, Campbell JA. Weighted needle pinprick sensory thresholds: a simple test of sensory function in diabetic peripheral neuropathy. Journal of neurology, neurosurgery, and psychiatry. 1992;55:56–59. doi: 10.1136/jnnp.55.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thygesen TH, Norholt SE, Jensen J, Svensson P. Spatial and temporal assessment of orofacial somatosensory sensitivity: a methodological study. Journal of orofacial pain. 2007;21:19–28. [PubMed] [Google Scholar]
- 22.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychological bulletin. 1979;86:420–428. doi: 10.1037/0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 23.Weir JP. Quantifying test-retest reliability using the intraclass correlation coefficient and the SEM. Journal of strength and conditioning research. 2005;19:231–240. doi: 10.1519/15184.1. [DOI] [PubMed] [Google Scholar]
- 24.Kothari SF, Baad-Hansen L, Oono Y, Svensson P. Somatosensory assessment and conditioned pain modulation in temporomandibular disorders pain patients. Pain. 2015;156:2545–2555. doi: 10.1097/j.pain.0000000000000325. [DOI] [PubMed] [Google Scholar]
- 25.Ayesh EE, Jensen TS, Svensson P. Hypersensitivity to mechanical and intra-articular electrical stimuli in persons with painful temporomandibular joints. Journal of dental research. 2007;86:1187–1192. doi: 10.1177/154405910708601209. [DOI] [PubMed] [Google Scholar]
- 26.Costa YM, et al. Reproducibility of quantitative sensory testing applied to musculoskeletal orofacial region: Site and sex differences. European journal of pain (London, England) 2019;23:81–90. doi: 10.1002/ejp.1287. [DOI] [PubMed] [Google Scholar]
- 27.Grammatica A, et al. Great auricular nerve preservation in parotid gland surgery: Long-term outcomes. The Laryngoscope. 2015;125:1107–1112. doi: 10.1002/lary.25025. [DOI] [PubMed] [Google Scholar]
- 28.Hu J, Ye W, Zheng J, Zhu H, Zhang Z. The feasibility and significance of preservation of the lobular branch of the great auricular nerve in parotidectomy. International journal of oral and maxillofacial surgery. 2010;39:684–689. doi: 10.1016/j.ijom.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 29.Vieira MB, Maia AF, Ribeiro JC. Randomized prospective study of the validity of the great auricular nerve preservation in parotidectomy. Archives of otolaryngology–head & neck surgery. 2002;128:1191–1195. doi: 10.1001/archotol.128.10.1191. [DOI] [PubMed] [Google Scholar]
- 30.Kelly KG, Cook T, Backonja MM. Pain ratings at the thresholds are necessary for interpretation of quantitative sensory testing. Muscle & nerve. 2005;32:179–184. doi: 10.1002/mus.20355. [DOI] [PubMed] [Google Scholar]
- 31.Hansson P, Backonja M, Bouhassira D. Usefulness and limitations of quantitative sensory testing: clinical and research application in neuropathic pain states. Pain. 2007;129:256–259. doi: 10.1016/j.pain.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 32.McArthur JC, Stocks EA, Hauer P, Cornblath DR, Griffin JW. Epidermal nerve fiber density: normative reference range and diagnostic efficiency. Archives of neurology. 1998;55:1513–1520. doi: 10.1001/archneur.55.12.1513. [DOI] [PubMed] [Google Scholar]
- 33.Riley JL, 3rd, Robinson ME, Wise EA, Myers CD, Fillingim RB. Sex differences in the perception of noxious experimental stimuli: a meta-analysis. Pain. 1998;74:181–187. doi: 10.1016/s0304-3959(97)00199-1. [DOI] [PubMed] [Google Scholar]
- 34.Komiyama O, De Laat A. Tactile and pain thresholds in the intra- and extra-oral regions of symptom-free subjects. Pain. 2005;115:308–315. doi: 10.1016/j.pain.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 35.Fillingim RB. Sex, gender, and pain: women and men really are different. Current review of pain. 2000;4:24–30. doi: 10.1007/s11916-000-0006-6. [DOI] [PubMed] [Google Scholar]
- 36.Wang R, et al. Reliability study of thermal quantitative sensory testing in healthy Chinese. Somatosensory & motor research. 2014;31:198–203. doi: 10.3109/08990220.2014.914485. [DOI] [PubMed] [Google Scholar]
- 37.Aggarwal VR, McBeth J, Zakrzewska JM, Lunt M, Macfarlane GJ. The epidemiology of chronic syndromes that are frequently unexplained: do they have common associated factors? International journal of epidemiology. 2006;35:468–476. doi: 10.1093/ije/dyi265. [DOI] [PubMed] [Google Scholar]
- 38.List T, Leijon G, Svensson P. Somatosensory abnormalities in atypical odontalgia: A case-control study. Pain. 2008;139:333–341. doi: 10.1016/j.pain.2008.05.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
A statistical package for social sciences version 19 (SPSS, IBM, Armonk, NY, USA) was used in this statistical procedure. The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.