Abstract
Along with cognitive decline, 90% of patients with dementia experience behavioral and psychological symptoms of dementia, such as psychosis, aggression, agitation, and depression. Atypical antipsychotics are commonly prescribed off-label to manage certain symptoms, despite warnings from the regulatory agencies regarding the increased risk of mortality associated with their use in elderly patients. Moreover, these compounds display a limited clinical efficacy, mostly owing to the fact that they were developed to treat schizophrenia, a disease characterized by neurobiological deficits. Thus, to improve clinical efficacy, it has been suggested that patients with dementia should be treated with exclusively designed and developed drugs that interact with pharmacologically relevant targets. Within this context, numerous studies have suggested druggable targets that might achieve therapeutically acceptable pharmacological profiles. Based on this, several different drug candidates have been proposed that are being investigated in clinical trials for behavioral and psychological symptoms of dementia. We highlight the recent advances toward the development of therapeutic agents for dementia-related psychosis and agitation/aggression and discuss the relationship between the relevant biological targets and their etiology. In addition, we review the compounds that are in the early stage of development (discovery or preclinical phase) and those that are currently being investigated in clinical trials for dementia-related psychosis and agitation/aggression. We also discuss the mechanism of action of these compounds and their pharmacological utility in patients with dementia.
Key Points
| Current pharmacotherapy of dementia-related psychosis and agitation/aggression relies on the off-label administration of atypical antipsychotics, which have limited clinical efficacy and induce various adverse reactions. |
| Genetic studies have suggested several druggable targets that correspond with the etiology of dementia-related psychosis and agitation/aggression: serotonin 5-HT2A and 5-HT1A receptors, serotonin transporter, alpha-1 adrenoceptor, and dopamine D1 and D3 receptors. |
| Novel therapeutic approaches may benefit particularly from targeting the serotoninergic system with serotonin 5-HT2A and 5-HT1A ligands or serotonin transporter inhibitors, which are currently being investigated in phase III clinical trials. |
| Preclinical and clinical studies have suggested other relevant molecular targets that may result in therapeutically acceptable efficacy: cannabinoid receptors, metabotropic glutamate 2 receptors, muscarinic M1/M4 receptors, and glutamate N-methyl-D-aspartate receptors. |
| Blockade of M1, alpha-2 adrenergic, and histamine H1 receptors and the human ether-a-go-go-related gene channel should be avoided because elderly patients are particularly sensitive to adverse reactions induced by the drugs acting on these targets. |
Introduction
While describing the first case report of dementia, Alois Alzheimer indicated that along with memory impairment, the patient demonstrated symptoms of psychosis [1, 2]. Currently, it is widely recognized that neuropsychiatric disturbances constitute an inherent component of Alzheimer’s disease (AD) and its related dementias. These manifestations are referred to in the literature as “behavioral and psychological symptoms of dementia” (BPSD), which include psychosis, agitation, aggression, irritability, depression, and anxiety [3]. It is estimated that at least one or more behavioral symptoms will manifest in almost all patients with dementia in the course of their disease [4]. Behavioral and psychological symptoms of dementia can decrease the quality of patients’ lives and are often cited as the main reason for referring patients with dementia to nursing homes or similar institutions [5].
Currently, a specifically approved pharmacotherapy for BPSD remains elusive. The most troublesome psychiatric events such as aggression and the remaining symptoms psychosis and agitation are addressed with atypical antipsychotics administered off-label [6]. However, the clinical efficacy of these drugs is unsatisfactory because a large percentage of patients do not respond or respond partially to the drugs [7]. Moreover, atypical antipsychotics are not actually recommended for elderly patients because they pose a risk of many side effects [8]. Elderly patients seem to be particularly sensitive to severe adverse reactions induced by atypical antipsychotics such as excessive sedation, orthostatic hypotension and related complications such as falls, extrapyramidal symptoms, cognitive slowing, cardiovascular complications, and anticholinergic side effects [9]. Notably, the use of currently available antipsychotics in patients with dementia has been associated with an increased risk of death. Consequently, in April 2004, the US Food and Drug Administration (FDA) issued a black-box warning against the use of atypical antipsychotics in elderly patients [10, 11]. The American and British clinical guidelines [12–14] state that antipsychotics can be used only if the patient constitutes a threat to self or others and should be administered after evaluating the benefit/risk ratio of the treatment [15]. If the physician decides to prescribe antipsychotics, clinical guidelines recommend the exclusive usage of the following drugs: risperidone, olanzapine, quetiapine, and aripiprazole [12]. Nevertheless, several reviews in this subject emphasized that prior to treatment with antipsychotics, one should always consider that these drugs exert detrimental effects and provide limited efficacy [6, 7, 16].
The main explanation for the poor clinical performance of atypical antipsychotics in elderly patients is that these were approved specifically for the treatment of schizophrenia, which affects mostly younger adults with neurobiological deficits that are distinct from BPSD. Aging induces changes in the quality and quantity of neurotransmitters, which may account for the onset of behavioral symptoms in patients with dementia [17, 18]. Consequently, fluctuations of neurochemicals initiate changes in the expression of certain receptors that should be targeted with specific medications [19]. Thus, patients with dementia might benefit from drugs interacting with relevant molecular targets to maximize the clinical response.
In this regard, a plethora of experimental evidence has recently highlighted several druggable targets that are believed to achieve therapeutically acceptable pharmacological profiles for BPSD. Several different drug candidates are being investigated in clinical trials for BPSD, which hopefully will make the pharmacotherapy of BPSD a realistic prospect. In this review, we present the drug candidates being currently investigated in clinical trials for BPSD. We focus on the most troublesome symptoms, aggression, agitation, and psychosis, which require pharmacological intervention. We discuss their pharmacological profile in terms of privileged biological targets, and assess how they correspond with the molecular mechanisms underlying their pathology. We also provide information on the latest investigational compounds at the early stage of development (discovery or preclinical phase). (For a review of the treatment of depression in patients with dementia, the reader is referred elsewhere [20]).
Overview of Potential Druggable Targets for Pharmacological Treatment Matching the Etiology of Dementia-Related Psychosis and Agitation/Aggression
The etiology of dementia-related psychosis and agitation/aggression is very complex and is often a cluster of biological factors (anatomical and neurochemical changes) as well as psychological and social aspects (responses to stress, living arrangements [21]). Alzheimer’s disease is the most common type of dementia, and the pathophysiology of this particular disease is related to the loss of neurons. Depending on the degree of neurodegeneration, the cerebral region involved, and consequently the extent of deficits in neurotransmitters, various psychiatric symptoms may appear [22]. For instance, psychosis has been associated with neurodegeneration in the frontal and mesotemporal areas of the brain [23], while depression has been linked to the degeneration of brainstem aminergic nuclei and a decrease in serotonergic neurotransmission [24]. Additionally, neuropathological changes that cause hypofunction of the cholinergic and serotonergic systems and hyperfunction of dopaminergic and noradrenergic transmission have been linked to agitation manifested by patients with dementia [22]. Alterations in multiple neurotransmitter systems cause changes in the expression of specific receptors, which have a direct impact on the regular functioning of the central nervous system (CNS) [3, 18].
Numerous studies have revealed a close association between genetic polymorphisms and the onset of psychiatric symptoms in patients with dementia. Particularly, serotonin 5-HT2A receptors (5-HT2AR) are related to the etiopathology of dementia-related psychosis and aggression. For instance, in patients with dementia, 5-HT2AR binding is decreased [25]. Additionally, reduced density of 5-HT2AR in the prefrontal cortex [26] and the polymorphism of 5-HT2AR have been associated with an increased risk of hallucinations and aggression [26, 27]. Moreover, the type of hallucinations observed in patients with Lewy body dementia (mainly visual) suggests the involvement of serotonin 5-HT2AR [28]. In fact, these resemble the hallucinations induced by 5-HT2AR agonists lysergic acid diethylamide and mescaline rather than those found in patients with schizophrenia, which tend to be mainly auditory [29–31]. Therefore, compounds that preferentially block 5-HT2AR might be used to treat dementia-related psychosis or aggression.
Similarly, other molecular targets have been associated with the manifestation of psychiatric symptoms in patients with dementia. A genetic study showed that the polymorphism of the serotonin transporter (SERT) promoter region (L/L genotype) is correlated with aggressive behavior in patients with AD [32, 33]. Furthermore, decreased density of 5-HT1AR in the cortex has been directly linked with the onset of aggressive behavior in patients with AD [34]. Furthermore, postmortem studies in patients with AD indicated that neurodegeneration of noradrenergic neurons may account for the pathophysiology of agitation and aggression related to AD [35]. In addition, polymorphisms in dopamine D1 receptor (D1R) and D3 receptors (D3R) have been associated with dementia-related psychosis and aggression [36]. In summary, potential druggable targets that closely correspond with the pathology of dementia-related psychosis, agitation, and aggression include serotonin 5-HT2AR and 5-HT1AR, SERT, alpha-1 adrenoceptors, and D1R and D3R.
Preclinical studies disclosed another palette of interesting molecular targets that may exhibit therapeutically relevant pharmacological profiles. For instance, an experimental study showed that knockout mice deficient in the cannabinoid CB1 receptor (CB1R) displayed aggressive behavior, which could be reduced by the short-term administration of a CB1R agonist [37]. Clinical evidence showed that the cortical areas in patients with AD are characterized by reduced expression and decreased availability of CB1R [38]. Therefore, pharmacological modulation of CB1R deserves a broadened evaluation to consider it as a potential molecular target in the management of AD-related aggression [39]. Furthermore, many findings suggest that neurodegeneration induces changes in the expression and function of metabotropic glutamate receptors (mGluRs) and N-methyl-d-aspartate (NMDA) receptors [40]. Disrupted glutamatergic neurotransmission, in turn, is a well-recognized factor that contributes to the pathophysiology of neuropsychiatric disorders [41, 42]. Studies in animal models revealed that the activation of metabotropic glutamate 2 receptor (mGlu2R) resulted in antipsychotic, memory-enhancing [43], and neuroprotective activity [44], while modulation of NMDA receptors is crucial for neuroplasticity and also influences mood and behavior [40]. Therefore, while developing drugs for dementia-related psychosis in the future, those targeting mGluRs or NMDA receptors can be considered as potential pharmacological targets. Furthermore, pharmacological activation of muscarinic receptors M1/M4 (M1R/M4R) may represent a disease-modifying [45] and symptomatic treatment [46]. Stimulation of M1R/M4R mediates key effects on cognitive performance, protects neurons from beta-amyloid toxicity [45], and promotes antipsychotic activity [46]. In addition, mounting evidence shows that patients with dementia may benefit from drugs targeting serotonin 5-HT6 receptors (5-HT6R) [47]. Preclinical studies revealed that selective 5-HT6R antagonists display promising procognitive activity, as well as mood-modulating properties [48]. A summary of the potential molecular targets for the treatment of dementia-related psychosis and agitation/aggression is presented in Table 1.
Table 1.
Summary of the potential druggable targets that might be suitable for pharmacological modulation of selected behavioral and psychological symptoms of dementia (BPSD): psychosis, aggression, and agitation
| Matching with BPSD pathology | Indicated by experimental studies | ||
|---|---|---|---|
| Target | Pharmacological activity | Target | Pharmacological activity |
| Serotonin 5-HT2A receptors | Antipsychotic, antiaggressive | Muscarinic M1/M2 receptors | Antipsychotic, procognitive |
| Serotonin 5-HT1A receptors | Antiaggressive | Cannabinoid receptor CB1 | Antiaggressive |
| Serotonin transporter | Antiaggressive | Metabotrophic glutamate 2 receptor (mGlu2) | Antipsychotic |
| Dopamine D1, D2 receptors | Antipsychotic, antiaggressive | Serotonin 5-HT6 receptors | Procognitive, anxiolytic |
| Alpha-1 adrenoreceptor | Antiaggressive | ||
New Drug Candidates on the Horizon
Identification of Compounds Investigated in Preclinical and Clinical Trials
To collect data regarding the compounds being investigated in clinical trials, we searched for trials registered by the US National Institutes of Health (http://www.clinicaltrials.gov) and EudraCT (https://eudract.ema.europa.eu) using the following keywords: “dementia” or “Alzheimer’s disease” and/or “agitation,” and/or “aggression” and/or “psychosis.” We restricted the search for trials from 2014 to present to obtain the most recent overview covering the last 5 years. Additionally, we searched for literature data associated with the identified compounds, published from 2014 to the present, using the compounds’ name and other keywords such as “Alzheimer’s disease” and “dementia” or “aggression” or “psychosis.” We used PubMed, ScienceDirect, GlobalData, and Google Scholar databases. If data regarding a compound’s efficacy or the current status of its trial were not published or posted on clinical trial registries, we searched the websites of the pharmaceutical and biotech companies responsible for that compound’s development. The search was restricted to English.
The applied search resulted in the identification of several drug candidates being investigated in clinical trials. Table 2 summarizes the data extracted on the investigated drug candidates, which will be discussed in detail below (except for lithium, which has been reviewed comprehensively elsewhere [49, 50]).
Table 2.
Compounds investigated for the treatment of agitation, aggression, and psychosis associated with dementia of various types and their current status in clinical trials from 2014 to present
| Compound | Identifier | Pharmacological action | Indication | Status | Estimated completion date |
|---|---|---|---|---|---|
| Compounds investigated in phase I | |||||
| HTL0016878 | NCT03244228 | Muscarinic M4 agonist | Compound developed for: neurobehavioral symptoms in AD | Completed | September 2019 [51] |
| Compounds investigated in phase II | |||||
| Eltoprazine |
H.134.5012 [52] GDCT0252614a |
Serotonin 5-HT1A/5-HT1B partial agonist | Aggression associated with AD | Completed | December 2015 [52] |
| Lithium | NCT02129348 | Complex | Psychosis, agitation in AD | Recruiting | January 2020 [50] |
| LY2979165 (MP-101) | NCT03044249 | mGluR2 agonist | Psychosis, aggression, and agitation in dementia | Recruiting | August 2020 [53] |
| Prazosin | NCT03710642 | Alpha-1 adrenoceptor antagonist [54] | Agitation in AD | Recruiting | December 2022 [55] |
| Pimavanserin | NCT03118947 | Serotonin 5-HT2AR inverse agonist | Agitation and aggression in AD | Completed | February 2019 [56] |
| Pimavanserin | NCT02035553 | Serotonin 5-HT2AR inverse agonist | Psychosis in AD | Completed | October 2016 [57, 58] |
| SND-51 |
GDC30016463a GDCT0310097a |
DAAO inhibitor | Dementia and psychosis | Ongoing | Date has not been disclosed |
| Compounds investigated in phase III | |||||
| Nabilone | NCT02351882 | Cannabinoid receptor (CB1) | Agitation in AD | Completed | March 2019 [59–61] |
| AXS-05 (dextromethorphan/bupropion) | NCT03226522 | Multi-receptor | Agitation in AD | Recruiting | June 2020 [62, 63] |
| Pimavanserin | NCT03325556 | Serotonin 5-HT2AR inverse agonist | Dementia-related psychosis | Recruiting | March 2020 [64] |
| Pimavanserin | 2017-004439-36 | Serotonin 5-HT2AR inverse agonist | Neuropsychiatric symptoms related to neurodegenerative disease | Ongoing | Date has not been disclosed [65] |
| Pimavanserin | 2017-002227-13 | Serotonin 5-HT2AR inverse agonist | Dementia-related psychosis | Ongoing | Date has not been disclosed [66] |
| Escitalopram | NCT03108846 | SERT inhibitor | Agitation in AD | Recruiting | August 2022 [67] |
| Brexpiprazole | NCT03724942 | Partial agonistic activity at dopamine D2 and serotonin 5-HT1AR, and antagonism of serotonin 5-HT2AR [68] | Agitation in AD | Recruiting | May 2021 [69] |
| Brexpiprazole | 2017-003940-19 | Partial agonistic activity at dopamine D2 and serotonin 5-HT1AR, and antagonism of serotonin 5-HT2AR [68] | Agitation in AD | Ongoing | Date has not been disclosed [70] |
| Brexpiprazole | 2018-002783-88 | Partial agonistic activity at dopamine D2 and serotonin 5-HT1AR, and antagonism of serotonin 5-HT2AR [68] | Agitation in AD | Ongoing | Date has not been disclosed [71] |
| Lumateperone | NCT02817906 | Antagonistic activity at the serotonin 5-HT2A receptor, partial agonistic activity at the presynaptic dopamine D2 receptor, antagonistic activity at the postsynaptic dopamine D2 receptor [72], and SERT blockade | Agitation in dementia, including AD | Terminated | December 2018 [73] |
| AVP-923 (dextromethorphan/quinidine) | NCT02446132 | Multi-receptor | Agitation in AD | Recruiting | June 2022 [74] |
| Mirtazapine | NCT03031184 | Noradrenergic and specific serotonergic antidepressant | Agitation in dementia | Recruiting |
July 2020 [75] |
| Compounds investigated in phase IV | |||||
| Gabapentin | NCT03082755 | Calcium channel inhibitor | Night-time agitation and sleep disturbance in dementia | Recruiting |
March 2022 [76] |
AD Alzheimer’s disease, DAAO D-amino acid oxidase, mGluR2 metabotropic glutamate 2 receptor, SERT serotonin transporter
aGDCT trial identifier provided by GlobalData
Drug Candidates for Dementia-Related Psychosis and Agitation/Aggression in Clinical Trials
Drugs Affecting Serotonergic Neurotransmission
Serotonin 5-HT2A Receptor Inverse Agonist: Pimavanserin
Among the various molecular targets considered as potential treatments of dementia-related psychosis or agitation/aggression, serotonin receptors have come into the limelight. Age-related decline in serotonin function has been linked with aggressive behavior in AD [77, 78]. Specifically, a reduced density and polymorphism of 5-HT2AR have been observed with the onset of aggression and psychosis in patients with dementia [77]. Therefore, modulation of the activity of 5-HT2AR seems to be a promising therapeutic strategy for reducing psychiatric symptoms that matches closely with the disease pathology.
A serotonin 5-HT2AR inverse agonist, pimavanserin, has been proposed as a suitable agent for the treatment of dementia-related psychosis and agitation/aggression [79, 80]. Pimavanserin was the first antipsychotic agent approved by the FDA (in 2016) for the treatment of psychosis in patients with Parkinson’s disease. It is a nondopaminergic, highly selective 5-HT2A inverse agonist that acts predominantly on the 5-HT2AR and, to a lesser degree, on another serotonin receptor subtype 5-HT2C (Fig. 1) [81]. Pimavanserin displays no affinity to the other G protein-coupled receptors including dopaminergic, histaminergic, muscarinic, and adrenergic receptors [82]. Thus, it is believed that this compound does not induce adverse reactions that are characteristic of atypical antipsychotics. The unique pharmacological profile of pimavanserin is characterized by high affinity and specific functional activity at the 5-HT2AR. As an inverse agonist, pimavanserin binds to the 5-HT2AR and not only blocks its natural agonistic activity but also reduces its constitutive activity. Importantly, pimavanserin does not bind to striatal D2 receptors, which indicates a high margin of safety in terms of the extrapyramidal side effects [83].
Fig. 1.
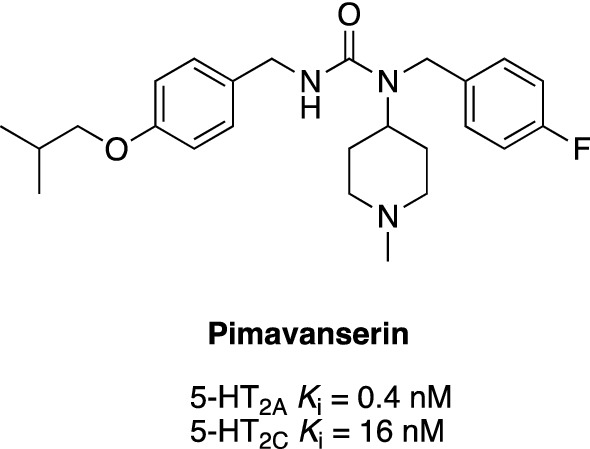
Structure and receptor profile of pimavanserin [81]
Preclinical studies revealed that pimavanserin reduced psychotic-like behavior in rodents [84, 85]. In clinical trials, pimavanserin was found to be effective in the treatment of delusions and hallucinations associated with Parkinson’s disease [86]. These promising results encouraged the evaluation of the efficacy of this agent in other psychotic disorders, including AD-related psychosis. Recently, a phase II, randomized, double-blind, placebo-controlled study evaluated the efficacy of pimavanserin in patients with AD-related psychosis (NCT02035553) [57, 58]. In the study, 181 subjects with AD were enrolled and treated with pimavanserin (17 mg twice daily) for 12 weeks (Table 3). Pimavanserin showed a statistically superior effect to placebo (change in the psychosis score on Neuropsychiatric Inventory [NPI]-Nursing Home Version scale) and an acceptable tolerability profile with no undesirable side effects on cognition [58]. The sponsor (ACADIA Pharmaceuticals) is currently running a phase III study, 52-week, open-label extension study (registered in Europe:2017-004439-36) of pimavanserin in the treatment of psychotic symptoms and agitation in 750 patients with dementia [65].
Table 3.
Summary of completed clinical trials evaluating drug candidates in psychosis, aggression, and agitation associated with dementia and Alzheimer’s disease (AD)
| Drug/indication | Trial identifier | Study design | Results | |||
|---|---|---|---|---|---|---|
| Participants | Dosing paradigm | Study design | Primary outcome measure | |||
| Pimavanserin/psychosis in AD | NCT02035553 [57] | 181 patients with AD | 34 mg/day for 12 weeks | Phase II, single-center, double-blind, placebo-controlled | Change from baseline to week 6 in the NPI-NH psychosis score | Statistically superior effect of pimavanserin compared with placebo at the primary endpoint (week 6), with an acceptable tolerability profile and no negative effect on cognition (week 12) [58] |
| Pimavanserin/agitation and aggression in AD | NCT03118947 [56] | 79 patients with AD | 20 mg/day or 34 mg/day for 52 weeks | Phase II, open-label, single-group | TEAEs, safety and tolerability of pimavanserin after 52 weeks of treatment | Results not available yet |
| Eltoprazine/aggression in AD | H.134.5012 [52] GDCT0252614a | 29 patients with SDAT or mixed SDAT/multi-infarct dementia | 5–10 mg/day for 4 weeks | Phase II, multi-center, double-blind, randomized, placebo-controlled | Social Dysfunction and Aggression Scale and the Staff Observation Scale after 4 weeks | Significant improvement of aggressive behavior within the eltoprazine-treated group compared with the placebo group [52] |
| Citalopram/agitation in AD | NCT00898807 [191] | 186 patients with AD | 10–30 mg/day for > 3 weeks (based on response and tolerability) | Phase III, randomized, multi-center, placebo-controlled, double-blind | Evaluated by NBRS-A and mADCS-CGIC at week 9 | Clinically meaningful reduction in the AD-associated agitation compared with placebo. [103] Cognitive and cardiac adverse effects associated with citalopram treatment |
| Escitalopram/psychotic symptoms and agitation in AD | NCT 01119638 [109] | 40 patients with AD | Escitalopram/risperidone 5–10 mg/day and 0.5–1.0 mg/day for 6 weeks | Phase IV, randomized, double-blind, single-center, pilot | Change in the total score on NPI (week 6) | Escitalopram and risperidone were equally effective in reducing psychotic symptoms and agitation [110] |
| Mirtazapine/agitation in AD | Pilot study [176] | 16 patients with AD | 15–30 mg/day for 12 weeks | Open-label, prospective | Changes in behavior were assessed using CMAI-SF (2, 8, and 12 weeks) | Significant reduction in CMAI-SF and CGI-S between pre- and post-treatment with mirtazapine [176]. No significant side effect and cognitive deterioration |
| Prazosin/agitation and aggression in AD | Pilot study [151] | 22 patients with AD | Mean dose: 5.7 mg/day for 8 weeks | Double-blind, placebo-controlled, randomized | Improvement on BPRS and NPI at weeks 1, 2, 4, 6, and 8 | Significant improvements in the NPI and BPRS within the prazosin-treated group compared with the placebo group [151] |
| Brexpiprazole/agitation in AD | NCT01862640 [192] | 433 patients with AD | 1 and 2 mg/day for 12 weeks | Phase III, randomized, double-blind, placebo-controlled, multi-center | Change in the CMAI total score (baseline to week 12). The secondary outcome is the change in the CGI-S score | The improvements in the primary endpoint of CMAI for brexpiprazole 2 mg were statistically better than placebo and appeared more robust than the improvements on the key secondary endpoint of CGI-S [114] |
| Brexpiprazole/agitation in AD | NCT01922258 [193] | 270 patients with AD | A flexible dose range: 0.5 mg/day, 1 mg/day, or 2 mg/day for 12 weeks | Phase III, randomized, double-blind, placebo-controlled, multi-center | Change in the CMAI total score (baseline to week 12). The secondary outcome is the change in the CGI-S score | The improvements in the primary endpoint of CMAI appeared less robust than the improvements on the key secondary endpoint of CGI-S [114] |
| Lumateperone/agitation in AD | NCT02817906 [119] | 177 patients with AD | 9 mg/day for 4 weeks | Phase III, randomized, double-blind, placebo-controlled, multi-center | CMAI-C (week 4) | Terminated (pre-specified interim analysis indicated futility) [119] |
| THC/dementia-related neuropsychiatric symptoms (agitation, aggression, or aberrant motor behavior) | NCT01608217 [194] | 50 patients diagnosed with AD, vascular dementia, or mixed dementia | 4.5 mg/day for 3 weeks | Phase II, randomized, double-blind, placebo-controlled study, multi-center | NPI assessed at baseline and after 14 and 21 days | No significant difference in reduction from baseline between THC and placebo [163] |
| THC/dementia-related neuropsychiatric symptoms with at least agitation or aggression | NCT01302340 [195] | 22 patients with AD, vascular dementia, or mixed dementia |
Period A: 1.5 mg/day for 6 weeks Period B: 3 mg/dayb for 6 weeks |
Phase II, repeated crossover, randomized, double-blind, placebo-controlled, multi-center | Change in NPI score (at day 3 and 10 during treatment blocks and after 1 month) |
No benefit of THC treatment (0.75 mg and 1.5 mg twice daily) on neuropsychiatric symptoms in dementia [164] |
| Dronabiol/dementia-related behavioral disturbances (aggression, agitation) | Retrospective study | 40 patients with dementia | 7.03 mg daily for 16 days | Retrospective | PAS (at day 7) | Total PAS score decreased significantly during dronabinol treatment [165] |
| Dronabiol/night-time agitation in dementia | Open-label pilot study | 6 patients diagnosed with late-stage dementia (5 with AD, 1 with vascular dementia) | 2.5 mg daily for 2 weeks | Open-label pilot | NPI, Actiwatch (at day 5) | Dronabinol led to a reduction in nocturnal motor activity [167] |
| Nabilone/agitation in AD | NCT02351882 [59] | 39 patients with AD | 1–2 mg for 14 weeksc | Phase II/III, pilot, randomized, double-blind, crossover | Change in agitation; CMAI (after 14 weeks) |
Significant reduction in agitation over 6 weeks in the nabilone group. [60, 61] Sedation occurred during treatment with nabilone |
| AVP-923 (dextromethorphan/quinidine)/agitation in AD | NCT01584440 [196] | 220 patients with AD | Dextromethorphan/quinidine at doses of 20 mg/10 mg once daily to 30 mg/10 mg twice daily for 10 weeks | Phase II randomized, multi-center, double-blind, placebo-controlled | Change from baseline in NPI Agitation/Aggression domain score (10 weeks) | Significant improvement on NPI, Agitation/Aggression score compared with placebo [128] |
BPRS Brief Psychiatric Rating Scale, CGI-S Clinical Global Impression-Severity of Illness, CMAI Cohen-Mansfield Agitation Inventory, CMAI-C Cohen-Mansfield Agitation Inventory-Community, CMAI-SF Cohen-Mansfield Agitation Inventory-Short Form, mADCS-CGIC modified Alzheimer Disease Cooperative Study-Clinical Global Impression of Change, NBRS-A Neurobehavioral Rating Scale Agitation subscale, NPI Neuropsychiatric Inventory, NPI-NH Neuropsychiatric Inventory Nursing Home Version scale, PAS Pittsburgh Agitation Scale, SDAT senile dementia of Alzheimer’s type, TEAEs treatment-emergent adverse events, THC Δ-9-tetrahydrocannabinol
aGDCT trial identifier provided by GlobalData
bPeriod A (6 weeks): 0.75 mg of THC twice daily for 3 successive days, separated by a 4-day washout. Period B: 1.5 mg of THC twice daily for 3 successive days, separated by a 4-day washout period
c14-week, randomized, double-blind, crossover trial compared nabilone to placebo (6 weeks each) with a 1-week washout between phases
As psychotic symptoms in patients with AD tend to remit and relapse [87], it has been suggested that it would be desirable to further evaluate the efficacy of pimavanserin in preventing psychotic symptoms in patients with AD. In this regard, a double-blind, placebo-controlled, phase III study (NCT03325556) is currently recruiting 356 participants to evaluate the efficacy of pimavanserin (34 mg once daily) in preventing the relapse of psychotic episodes in patients with dementia (primary outcome measure: time from randomization to relapse in the double-blind period, up to 26 weeks) [62]. In parallel, the sponsor is running a phase III study registered in Europe (2017-002227-13), which evaluates the efficacy of pimavanserin in relapse prevention of psychotic symptoms in 212 patients with dementia [66]. In addition, a phase II open-label study (NCT03118947) [64] has been completed recently. The study evaluated the safety and tolerability of pimavanserin (10 or 17 mg twice daily, primary outcome measure: treatment-emergent adverse events [Table 3]). The results have not been revealed yet.
Serotonin 5-HT1A/1B Receptor Agonist: Eltoprazine
Previous findings revealed that aggressive behaviors in patients with AD directly correlate with a reduced density of 5-HT1AR in the CNS [34]. Several clinical reports disclosed a significant reduction in agitation and aggression in patients with dementia after the administration of drugs acting via 5-HT1AR: tandospirone [88] and buspirone [89]. Therefore, it has been suggested that modulation of the activity of 5-HT1AR might be considered as a promising therapeutic strategy for the treatment of dementia-related agitation/aggression.
Eltoprazine was developed as an anti-aggressive agent [90–92]. It was reported that eltoprazine potently suppressed aggressive behavior in animal models without causing sedation [93, 94]. Eltoprazine is a 5-HT1A/5-HT1B partial agonist but also acts as a full agonist at 5-HT2CR (Fig. 2) [96]. Its anti-aggressive properties most probably result from the reduction in serotonin release due to the activation of presynaptic 5-HT1A and 5-HT1B autoreceptors [97, 98]. Moreover, mixed 5-HT1A/5-HT1B partial agonism of eltoprazine is responsible for its highly effective inhibition of aggressive behavior [98].
Fig. 2.
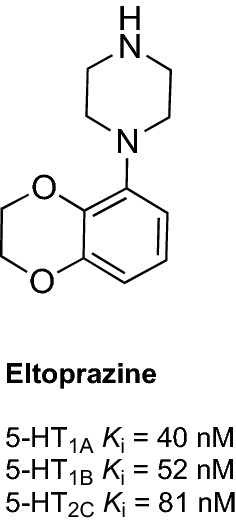
Structure and receptor profile of eltoprazine
In phase I of clinical trials, eltoprazine was found to be safe and well tolerated [97]. In a phase II study on the treatment of AD-related aggression, eltoprazine showed promising results [52]. This double-blind, randomized, placebo-controlled study found that eltoprazine (10 mg once daily for 4 weeks) significantly reduced aggressive behavior (evaluated by the Social Dysfunction and Aggression Scale and the Staff Observation Scale) in 29 subjects (Table 3). These encouraging results suggest that further phase III clinical studies should be conducted on this indication.
Selective Serotonin Reuptake Inhibitors
Genetic studies revealed that certain forms of the SERT have been linked with the onset of dementia-related psychosis and aggressive behavior [32, 33]. In addition, administration of the selective serotonin reuptake inhibitor citalopram resulted in a significant decrease in the pathological levels of amyloid-β in patients with AD [99]. Preclinical studies have shown that citalopram blocked the growth of pre-existing amyloid-β plaques and reduced the formation of new plaques by 78% [100]. Moreover, selective serotonin reuptake inhibitors increase the levels of serotonin and thus may stimulate hippocampal neurogenesis [101]. Therefore, administration of the drugs acting on SERT to mitigate behavioral symptoms in patients with dementia is a rational intervention.
One of the first studies showed the effectiveness of citalopram in a short-term in-hospital management program of dementia-related aggression and agitation in patients with dementia [102]. These preliminary data have been confirmed in a larger randomized, placebo-controlled, double-blind study (NCT00898807) that enrolled 186 patients with AD [103] (Table 3). The study showed that long-term administration of citalopram (10–30 mg/day for 3 weeks) led to a clinically meaningful reduction in the AD-associated agitation compared with placebo (primary outcome measured by the Neurobehavioral Rating Scale Agitation subscale). However, treatment with citalopram has been associated with mild cognitive decline and cardiac side effects (QT interval prolongation), which hampers its long-term clinical application. In fact, in August 2011, the FDA announced a safety warning recommending to limit the maximum dose of citalopram to 20 mg/day in elderly patients aged > 60 years because of the possible occurrence of QT prolongation [104]. Therefore, it has been suggested that lower doses (< 30 mg/day) of citalopram [103] or alternatively other non-cardiotoxic antidepressants should be evaluated in clinical trials [105].
An (S)-stereoisomer of citalopram (Fig. 3), escitalopram, seems to be safer, as the magnitude of QT prolongation observed with this compound is lower compared with citalopram and the effect is dose dependent [106–108]. A randomized, double-blind, pilot study evaluated the effectiveness of escitalopram (5–10 mg/day) in reducing psychotic symptoms and agitation in 40 patients with AD in a 6-week treatment period (primary outcome measure: change in the total score on NPI), in comparison to the antipsychotic risperidone (0.5–1.0 mg/day) [109]. This study found that escitalopram and risperidone were equally effective (Table 3) [110]. The authors did not observe any adverse event in the escitalopram group. This pilot study justified the need for a larger, more comprehensive study. Currently, a phase III, double-blind, randomized, placebo-controlled trial (NCT03108846) [67] is recruiting patients with AD-related dementia and clinically significant agitation (392 participants) to evaluate the safety and effectiveness of escitalopram at a dose of 5–15 mg/day in reducing agitation in patients with AD during 12 weeks (primary outcome measure: change in the modified Alzheimer’s Disease Cooperative Study-Clinical Global Impression of Change score after 12 weeks) [67].
Fig. 3.
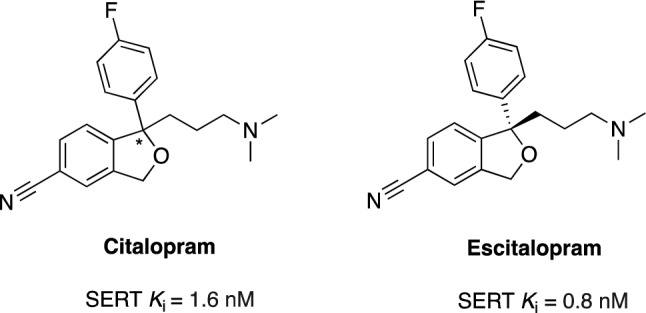
Chemical structure and binding profile of citalopram and escitalopram [107]. SERT serotonin transporter
Drugs Acting on Several Biological Targets
Novel Atypical Antipsychotics
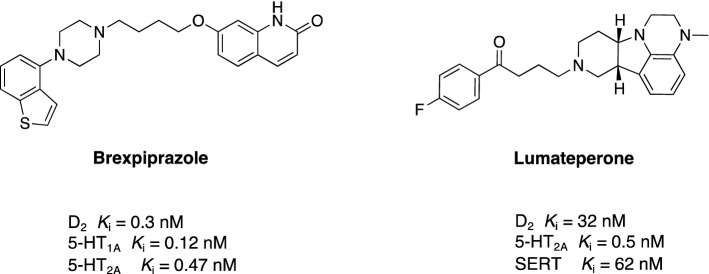
Recently, two novel atypical antipsychotic agents have been investigated in phase III clinical trials for AD-related agitation and psychosis: brexpiprazole and lumateperone. These agents target primarily the serotonin 5-HT2ARs while maintaining a relatively weaker interaction with D2R, which makes them less likely to induce unfavorable extrapyramidal side effects. Elderly patients are particularly sensitive to extrapyramidal symptoms [111] owing to an aging-related impairment of dopamine function [112]; therefore, the interaction of these agents with the D2R should be retained at the lowest level.
Brexpiprazole is a novel atypical antipsychotic approved by the FDA in 2015 for the treatment of schizophrenia and major depressive disorder as an add-on therapy [113]. Its mechanism of action is mediated via the antagonism of 5-HT2AR and partial agonism of D2R and 5-HT1AR (Fig. 4) [68]. Partial agonistic activity at the D2 receptor accounts for the reduced occurrence of extrapyramidal side effects. Lately, two phase III clinical trials have evaluated the safety and efficacy of brexpiprazole in AD-related agitation (NCT01862640 investigated two-fixed doses of 1 and 2 mg/day and NCT01922258 investigated the flexible dosing of 0.5, 1.0, or 2.0 mg/day during the 12-week treatment). These randomized, double-blind, placebo-controlled, multi-center studies enrolled 433 and 270 participants, respectively (Table 3). The sponsor announced that brexpiprazole significantly ameliorated the symptoms of agitation in comparison to placebo (evaluated by a change from baseline in the Cohen-Mansfield Agitation Inventory [CMAI] total score) [114, 115]. A corresponding trial (phase III, 12-week, multi-center, randomized, double-blind, placebo-controlled, two-arm, fixed-dose: 0.5 mg and 1 mg, 225 patients) registered in Europe (2017-003940-19) has not been accomplished yet [70]. In addition, a phase III, multi-center, active-treatment-extension trial (NCT03724942) is recruiting 250 patients to evaluate the safety and tolerability of brexpiprazole (2–3 mg/day for 12 weeks) in patients with AD-associated agitation (primary outcome data will include adverse events elicited from participants) [116]. A corresponding European active-treatment extension trial (a phase III, 12-week, multi-center study, 225 patients with dementia) is ongoing (2018-002783-88 [70]).
Fig. 4.
Chemical structures and receptor profiles of brexpiprazole and lumateperone [68, 72]. SERT serotonin transporter
Lumateperone is another atypical antipsychotic drug with a mechanism of action based on the antagonistic activity at the 5-HT2AR, partial agonistic activity at the presynaptic D2 receptors, and antagonistic activity at the postsynaptic D2R [72, 117], as well as SERT blockade (Fig. 4). Clinical studies in healthy volunteers using positron emission tomography revealed that lumateperone displayed high occupancy of the 5-HT2AR in the cortex and negligible occupancy of the D2 striatal receptors [118], suggesting that it is less likely to induce extrapyramidal side effects. Lumateperone has a unique mechanism of action, modulating synergistically multiple neurotransmitter systems, and has been suggested as a potential treatment for a range of neuropsychiatric disorders [73]. A recently completed phase III, randomized, double-blind, placebo-controlled, multi-center study (NCT02817906) has investigated the efficacy and safety of lumateperone (9 mg/day for 4 weeks; primary outcome measured using CMAI-Community Version) in 177 patients with dementia with clinically significant agitation (Table 3). The sponsor announced that the study was unlikely to meet its primary endpoint upon completion [119], and therefore, it has been terminated.
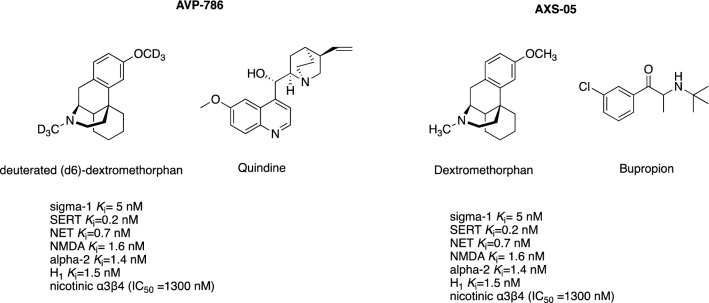
Dextromethorphan Formulations
Preclinical data revealed that a sigma-1 receptor agonist, dextromethorphan, exerts promising mood-modulating properties [121] For instance, dextromethorphan showed anti-stress activity by reducing fear stress in conditioned mice, which was suggested to involve a sigma-1-dependent stimulation of the dopaminergic system [121]. Further studies in rodents revealed that dextromethorphan displayed anti-depressive-like activity and exerted neuroprotective and antioxidant effects [122, 123]. Dextromethorphan is an FDA-approved antitussive drug that acts in the CNS [124, 125]. Dextromethorphan exhibits high affinity to several biological targets: sigma-1 receptor, SERT, norepinephrine transporter, NMDA receptor, alpha-2 adrenoceptor, histamine H1 receptor (H1R), and nicotinic α3β4 receptor (Fig. 5) [124].
Fig. 5.
Chemical structure of deuterated (d6) dextromethorphan/quinidine (AVP-786) and dextromethorphan/bupropion (AXS-05). NET noradrenaline transporter, NMDA N-methyl-d-aspartate, SERT serotonin transporter
The specific receptor profile of dextromethorphan contributes to its complex pharmacological activities, which prompted scientists to investigate its therapeutic potential in dementia-related agitation. However, dextromethorphan showed an unfavorable pharmacokinetic profile in humans, related to its rapid hepatic metabolism, which hampered the attainment of therapeutic concentrations in the brain. To reduce the first-pass effect and improve its pharmacokinetics, dextromethorphan was combined with a cytochrome P450 2D6 inhibitor, quinidine (formulation known as AVP-923). Quinidine is an anti-arrhythmic agent used in clinical practice (200–300 mg) to treat cardiac arrhythmias [124, 126]. It reversibly inhibits cytochrome P450 2D6, and has been added to formulations at subclinical doses (10 mg) to inhibit cytochrome P450 2D6 and prolong the plasma half-life of dextromethorphan [124]. AVP-923 (Neudexta) has been approved by the FDA for the treatment of pseudobulbar affect [127].
The first clinical study, a phase II, randomized, multi-center, double-blind, placebo-controlled trial (NCT01584440), evaluated the efficacy of AVP-923 (dextromethorphan/quinidine at the doses of 20/10 mg once daily to 30/10 mg twice daily for 10 weeks) in reducing agitation in 220 patients with dementia, and revealed that AVP-923 exerted statistically significant effects on agitation compared with placebo (primary outcome measure was the change from baseline in the NPI Agitation/Aggression domain score) [128] (Table 3). Meanwhile, another formulation had been developed, containing a subclinical dose of quinidine and deuterated dextromethorphan (AVP-786). Dextromethorphan (Fig. 5) was deuterated to additionally improve its pharmacokinetic profile and reduce its first-pass metabolism in the liver [126]. Using the data generated for AVP-923, the FDA agreed to initiate a follow-up, phase III clinical trial (NCT02446132), an extension study, to evaluate the long-term safety and efficacy of AVP-786 (three various doses administered twice a day over 52 weeks) in the management of dementia-associated agitation [129]. The primary outcome measure includes, inter alia, the number of participants with any treatment-emergent serious adverse event, the Mini-Mental State Examination score, and the Alzheimer’s Disease Assessment Scale-Cognitive Subscale score [74].
Based on the promising results of previous studies, experts strongly believe that AVP-786 may become the first FDA-approved deuterated compound for the treatment of dementia-related agitation [126]. However, it should be emphasized that the first dextromethorphan/quinidine formulation (AVP-923, Neudexta) label carries a warning regarding cardiac safety [127], suggesting that this compound should be avoided in patients with congenital long-QT syndrome and the QTc interval should be evaluated in the patients at risk of QTc prolongation [126, 127].
Another combination formula that includes dextromethorphan is AXS-05, which contains low doses of bupropion. Bupropion inhibits the reuptake of norepinephrine and dopamine and antagonizes nicotinic receptors. As a potent cytochrome P450 2D6 inhibitor, bupropion was added to the formulation to increase the plasma concentrations of dextromethorphan [130]. AXS-05 received a fast-track designation from the FDA for the evaluation of its efficacy in the treatment of AD-related agitation [131]. Currently, a phase II/III clinical trial (NCT03226522) [62] is evaluating the safety and efficacy of AXS-05 (one tablet daily for 5 weeks) for its use in the management of AD-related agitation (primary outcome measured using CMAI). This randomized, double-blind, placebo-controlled study enrolled 435 subjects with AD. Recently, the sponsor has announced the positive outcome of the interim analysis of the study and received recommendation for further continuation [63].
Drugs Affecting Glutamatergic Neurotransmission
Metabotropic Glutamate 2 Receptor Agonist: LY2812223
In the early 1990s, it was first proposed that dysfunction in glutamatergic neurotransmission could be implicated in mental disorders [132]. Therefore, it was postulated that modulation of glutamatergic activity may constitute a novel nondopaminergic strategy for the treatment of psychosis [133–135]. Additionally, previous studies highlighted the activation of glutamate receptors exerts neuroprotective and memory-enhancing effects in animal models, which might be particularly beneficial to patients with dementia [42]. Eli Lilly and Company discovered LY2812223 (Fig. 6), a mGluR2 agonist [136, 137]. LY2812223 was found active in an animal model of psychosis, sensitive to mGluR2 [95, 137]. Unfortunately, in the pharmacokinetic studies, LY2812223 showed a poor oral bioavailability (~ 4%). However, this did not limit the further development of the mGluR2 agonist for the treatment of mental disorders. Low bioavailability of LY2812223 was overcome by designing its alanine prodrug MP-101 (LY2979165). MP-101 is administered orally and absorbed in the gastrointestinal tract through active transport and then, it is rapidly hydrolyzed to LY281223 (Fig. 6) [95, 138].
Fig. 6.
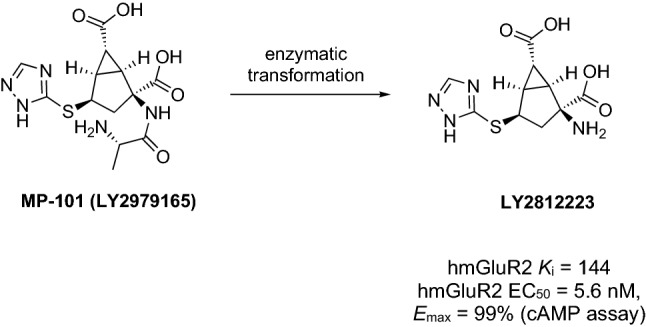
Chemical structure and metabolic activation of MP-101, a prodrug of LY281223. cAMP cyclic adenosine monophosphate, hmGluR2 human metabotropic glutamate receptor type 2
MP-101 is under development for the treatment of dementia-related psychosis and/or agitation and aggression (NCT03044249) [139]. This randomized, placebo-controlled, phase II clinical trial will assess the efficacy (measured as the change from baseline in the NPI-Psychosis subscale score), safety, and pharmacokinetic profile of MP-101 (20–60 mg/day for 10 weeks), compared to placebo. LY2979165 was previously evaluated in healthy subjects, in whom it was overall well tolerated and showed a linear pharmacokinetic profile [53, 137].
Inhibitors of D-Amino Acid Oxidase
An interesting approach for indirectly controlling the glutamatergic neurotransmission is to inhibit the activity of D-amino acid oxidase (DAAO). D-amino acid oxidase regulates the brain levels of D-amino acids, and in several psychiatric conditions, the activity and expression of DAAO are enhanced [140]. Overactivation of DAAO decreases the availability of d-serine, an endogenous agonist of the NMDA receptor. N-methyl-D-aspartate dysfunction in turn has been associated with schizophrenia and AD [141]. D-amino acid oxidase inhibitors were found to exert antipsychotic activity and improve cognitive function in AD [142, 143]. Currently, SyneuRx International Corp. is developing a competitive antagonist of DAAO, compound SND-51, for the treatment of dementia and psychosis. SND-51 is botanic in origin (chemical structure has not been disclosed), and in animal models, it elicited antipsychotic and anxiolytic activity and improved spatial memory [144]. SND-51 has now progressed to phase II clinical trials, and its efficacy in the treatment of dementia and psychosis is being evaluated [145].
Drugs Affecting Cholinergic Neurotransmission
Selective Muscarinic M4 Receptor Agonist: HTL0016878
Another promising example of compounds being developed for treating dementia-related psychosis and agitation/aggression is the selective muscarinic M4R agonist HTL0016878 (chemical structure has not been disclosed). The muscarinic receptors are abundantly expressed in the cortex and hippocampus, the areas involved in cognitive and neurobehavioral functions [146]. In animal models, nonselective M1R/M4R agonists improved psychotic behaviors and cognitive performance [46]. These findings have been confirmed in muscarinic M4R-knockout mice, and thus, it has been suggested that M4R agonists represent an attractive molecular target for the development of novel antipsychotic agents [120]. A first-in-class selective M4R agonist, HTL0016878, developed by Sosei Heptares for the treatment of neurobehavioral symptoms in patients with AD, has advanced to phase I clinical trials (NCT03244228). This randomized, double-blind, placebo-controlled study evaluated the safety, pharmacokinetics, and pharmacodynamics of this compound in 106 healthy young volunteers as well as in elderly patients [51]. The results have not been revealed yet.
Drugs Affecting Noradrenergic Neurotransmission
Alpha-1 Adrenoceptor Inhibitor: Prazosin
Several findings indicated the robust association between noradrenergic depletion and AD [35, 147, 148]. Postmortem studies revealed that the prefrontal cortex in patients with AD was characterized by a significant reduction in noradrenergic neurons [148], which may be associated with a poor cognitive performance as well as the onset of aggression [147]. Postmortem studies also showed that the compensatory upregulation of postsynaptic alpha-1 adrenoceptor is linked with AD-related aggression [149, 150]. Therefore, it has been proposed that reducing the activity of the overstimulated postsynaptic alpha-1 adrenoceptors may alleviate the aggressive behavior [151, 152].
Among dugs acting on the alpha-1 adrenoceptors, prazosin remains the only centrally acting agent that can readily pass the blood–brain barrier and block the alpha-1 adrenoceptors in the CNS (Fig. 7) [153]. The first pilot study that evaluated the effectiveness of prazosin in suppressing the dementia-related aggression and agitation was a double-blind placebo-controlled study that enrolled 22 patients with AD [151] (Table 3). Administration of prazosin (mean dose: 5.7 mg/day, 8 weeks) led to a significant reduction in aggression/agitation (improvement on the Brief Psychiatric Rating Scale and the NPI score) compared with placebo [151]. Side effects and blood pressure fluctuations were comparable between the prazosin group and the placebo group. These data provided further support to initiate a larger phase II study (NCT03710642) enrolling 186 patients with AD with agitation [55]. This double-blind, placebo-controlled, randomized study will evaluate the effectiveness of prazosin (4–6 mg/day) in reducing agitation in patients with AD during 12 weeks (primary outcome measured using Clinical Global Impression of Change in Agitation) [55].
Fig. 7.
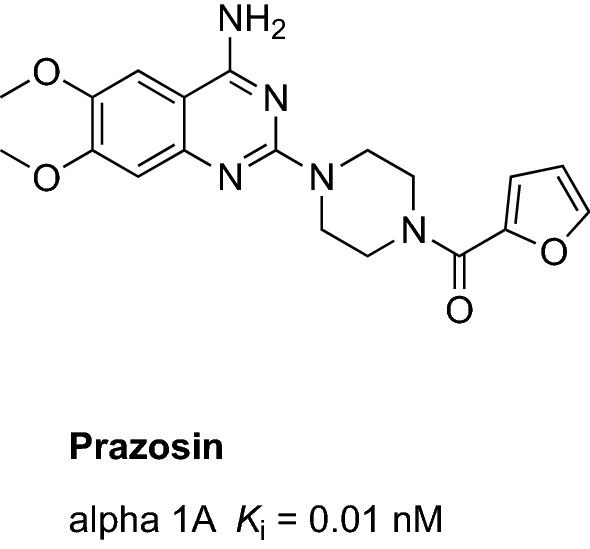
Chemical structure and binding profile of prazosin [154, 155]
Drugs Targeting the Endocannabinoid System
Targeting cannabinoid receptors has acquired paramount importance as it offers a perspective to treat the symptomatology and pathology of AD [156]. Activation of cannabinoid receptors by agonists at non-psychoactive doses induces neuroprotective effects [157, 158] and can influence mood and behavior [156]. Preclinical studies have shown that cannabinoid receptor agonists decrease amyloid-β levels and reduce neuroinflammation [159, 160]. Further preclinical studies showed that a cannabinoid receptor agonist reduced aggressive behaviors [37, 161, 162]. These data suggest that cannabinoid receptor agonists may serve as a potential treatment for managing AD-related aggression.
Among the various cannabinoid receptor agonists, Δ-9-tetrahydrocannabinol (THC), dronabinol, and nabilone (Fig. 8) [synthetic analogs of THC] have been the most widely investigated in clinical trials [156]. A randomized, double-blind, placebo-controlled study that investigated the effect of THC (1.5 mg administered three times daily for 3 weeks) in 50 patients with dementia agitation or aggression found no significant differences between the treatment group and the placebo group (primary outcome measured using the NPI) [163]. Similarly, another trial showed no efficacy for THC in reducing agitation or aggression [164] (Table 3).
Fig. 8.
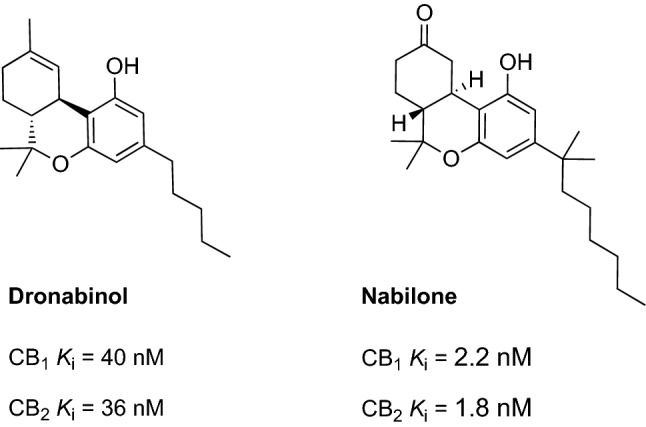
Chemical structure and receptor affinity of dronabinol and nabilone [160, 161]. CB cannabinoid
In contrast, a synthetic analog of THC, dronabinol, was found to be effective in reducing agitation in patients with dementia [156]. A retrospective study conducted on 40 acutely hospitalized patients with severe dementia showed that dronabinol (7 mg/day for 2 weeks) significantly reduced motor agitation and aggressiveness (a significant decrease in all domains of the Pittsburgh Agitation Scale) [165]. However, some authors reported the occurrence of adverse effects associated with dronabinol use, with a particular emphasis on sedation [165, 166]. Other studies found that dronabinol reduced night-time agitation [165, 167–169] in patients with dementia. An open-label pilot study of six patients with dementia with night-time agitation treated with dronabinol (2.5 mg/day for 2 weeks) reported a significant reduction in nocturnal motor activity [167] (assessed using the NPI scale) (Table 3). These encouraging results led to further interest in this class of compounds and the initiation of a clinical investigation of another synthetic cannabinoid, nabilone. First, the efficacy of nabilone has been only described in two case reports, which described the improvement of behavioral disturbances (agitation and psychosis) in patients with dementia after treatment with nabilone [170, 171]. Further, a randomized, double-blind, placebo-controlled cross-over trial (NCT02351882) [59] evaluated the safety and effectiveness of nabilone (1–2 mg) for 14 weeks (6-week treatment with a 1-week washout between phases) in the management of agitation in 39 patients with AD (primary outcome measured using CMAI) (Table 3). The authors reported a statistically significant reduction in agitation within the nabilone group [60, 61]. During the treatment, the patients treated with nabilone experienced sedation. The authors concluded that nabilone may constitute a promising treatment for agitation associated with AD; however, the obtained data should be confirmed in a longer and larger study and side effects such as sedation and possibly cognitive decline should be monitored [61]. Nevertheless, nabilone seems to have a greater effect in reducing agitation, compared with other cannabinoids such as THC and dronabinol [61] and offered the most favorable pharmacokinetic profile among all the studied cannabinoids [172]. Further clinical studies are warranted to confirm its efficacy.
Drugs Improving Sleep Quality Displaying Various Mechanisms of Action
Noradrenergic and Specific Serotonergic Antidepressant: Mirtazapine
Nocturnal sleep quality may contribute to the onset of behavioral problems in patients with dementia [173]. Clinical observations suggested that agitative behavior in patients with dementia might be caused by sleep disturbances [174, 175]. In this regard, it has been proposed that both conditions could be effectively addressed by a drug that displays anxiolytic as well as sleep-promoting activity [176].
Mirtazapine is an antidepressant with sedative properties and an ability to promote sleep [177, 178]. It interacts with several types and subtypes of receptors including serotonin 5-HT2AR and 5-HT3R, alpha-2 adrenoceptors, and H1R (Fig. 9) [179]. The unique pattern of receptor modulation by mirtazapine not only ameliorates psychiatric symptoms but also helps to improve sleep quality [178]. The first pilot study evaluating the effect of mirtazapine (15–30 mg/day) on AD-related agitation was a 12-week open-label, prospective study that included 16 patients who had clinically significant agitation [176] (Table 3). The authors observed a significant reduction in agitation in the patients treated with mirtazapine compared with pre-treatment with mirtazapine (changes in behavior were assessed using CMAI-Short Form) [176]. These encouraging data prompted the initiation of a larger phase III, pragmatic, multi-center, double-blind, placebo-controlled, randomized study (NCT03031184), which is currently recruiting 222 patients to evaluate the safety and effectiveness of mirtazapine (15–30 mg/day for 12 weeks) in reducing AD-related agitation (primary outcome measure: CMAI score at 12 weeks) [75].
Fig. 9.
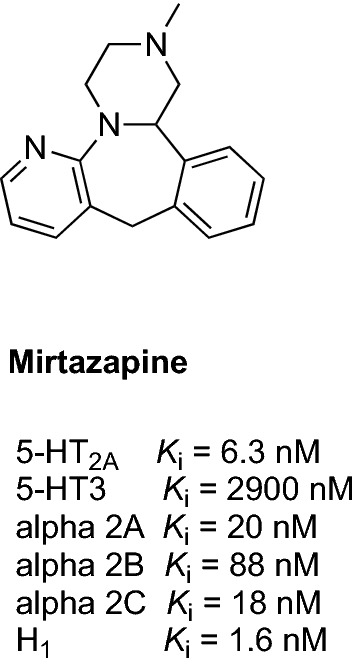
Chemical structure and binding profile of mirtazapine [180]
α2δ Subunit-Containing Voltage-Dependent Calcium Channel Blocker: Gabapentin
In a large percentage of patients with dementia, agitation and wandering manifest during the night-time [173]. The nocturnal episodes of behavioral disturbances have a negative influence on daily functioning and additionally induce aggression during the day [173]. This vicious circle might be broken by treatment with a sedative agent that can help to decrease agitation and improve sleep quality during the night.
In 2011, a case report described the effectiveness of gabapentin in reducing the dementia-associated nocturnal agitation [181]. Gabapentin is an FDA-approved drug for the treatment of epilepsy [182]. Gabapentin blocks the α2δ subunit-containing voltage-dependent calcium channels (Fig. 10), which are associated with the release of neurotransmitters [183, 184]. Gabapentin displays a versatile pharmacological profile, including anticonvulsant, sedating, and anxiolytic activities [185]. In addition, the positive effects of gabapentin on the sleep quality and architecture have been well documented [186]. A series of case reports have described the treatment of patients with dementia with agitation using gabapentin and showed that the patients responded to the treatment favorably [187, 188]. Currently, gabapentin is being investigated in a phase IV study (NCT03082755) to test its effect (300–600 mg/day) on night-time agitation (primary outcome measure: night-time agitation measured using CMAI) [119]. During the 8-week treatment, this double-blind, placebo-controlled, randomized clinical trial will enroll 136 participants [76]. The remaining options available for the treatment of sleep disturbances and nocturnal agitation such as benzodiazepines are not recommended in vulnerable elderly individuals because these agents are well documented to cause harmful effects such as cognitive worsening and increase the risk of fall-related injuries [189].
Fig. 10.
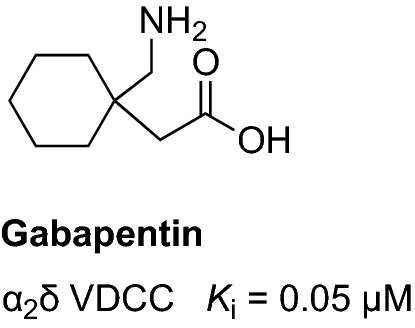
Chemical structure and binding profile of gabapentin [190]. VDCC voltage-dependent calcium channel
Compounds in the Early Stage of Development (Discovery or Preclinical Phase)
We additionally searched for literature data associated with the identified compounds in the early stage of development (discovery or preclinical phase), published from 2014 to the present day to obtain the most recent overview covering the last 5 years, using the compounds’ name and other keywords such as “Alzheimer’s disease” and “dementia” or “aggression” or “psychosis.” We used PubMed, ScienceDirect, GlobalData, and Google Scholar databases for our search (papers written in English). If data regarding the identified compounds were not published, we searched the websites of pharmaceutical and biotech companies responsible for the compounds’ development. Several molecules that matched the selection criteria were identified, which are described in detail below.
Multi-Target-Directed Ligand Concept
It has been suggested that parallel modulation of several molecular targets may result in a balanced, and thus superior, pharmacological action, compared to a selectively acting drug. Considering this, a novel approach in drug discovery involves designing a single chemical entity that can simultaneously modulate the activity of several biological targets. Such compounds are described in the literature as “multi-target-directed ligands” [197]. The multi-target-directed ligand concept intended to design “clean” ligands that bind to selected molecular targets while sparing off targets, and thus reduce the risk of side effects [198]. The multi-target-directed ligand concept is relatively new; however, it has recently been widely explored in the literature and several experimental compounds have emerged from this strategy.
Ligands Targeting Several Monoaminergic Receptors
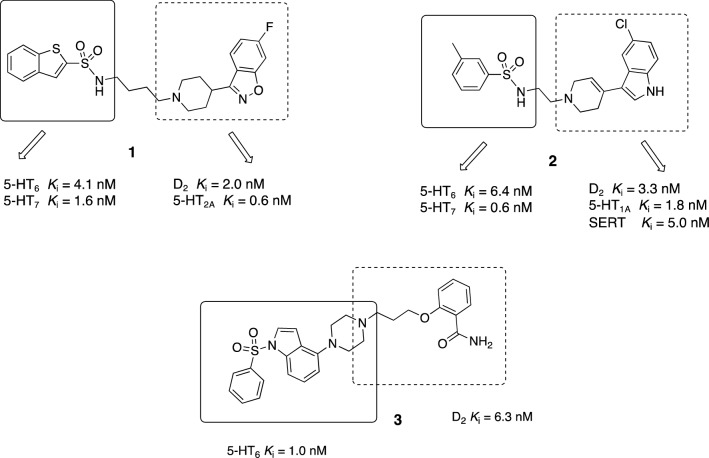
A combination of chemical scaffolds responsible for interaction with 5-HT6R/5-HT7R and 5-HT2AR/D2R yielded a hybrid molecule 1, which elicited favorable psychotropic effects (Fig. 11). In pharmacological studies, the selected multi-modal ligand 1 showed antidepressant and antipsychotic activity and did not affect cognition, in contrast to the eight antipsychotic drugs tested in the same experimental setting [199].
Fig. 11.
Examples of multi-target-directed ligands obtained by combining the scaffolds responsible for interactions with 5-HT6R, 5-HT7R, 5-HT2AR, and D2R (discovery stage). SERT serotonin transporter
Similarly, a series of multi-modal ligands exhibiting high affinity to SERT, D2R, 5-HT1AR, 5-HT6R, and 5-HT7R have been identified (Fig. 11). It has been shown that tetrahydropyridin-4-yl‐1H‐indole is a privileged scaffold, which accounts for an interaction with SERT, D2R and 5-HT1AR, and its combination with the aryl sulfonamide moiety provides additional interaction with 5-HT6R and 5-HT7R. The selected compound 2 was tested in vivo and found to exhibit antipsychotic and antidepressant activity, as well as exert memory-enhancing effects [200].
Another notable approach of identifying novel MTLDs relies on combining D2R partial agonism with 5-HT6R antagonism. The partial D2 agonism concept is based on the modulation of dopaminergic neurotransmission at a low sufficient level that simultaneously prevents the occurrence of extrapyramidal side effects by preventing excessive D2R blockade in the striatum [201]. This strategy resulted in the development of a series of hybrid molecules, combining the 5-HT6 and D2 pharmacophores (Fig. 11). The selected molecule 3 displayed antidepressant and anxiolytic activity in aged animals, as well as promising procognitive effects [201]. The abovementioned compounds (2 and 3) are currently at an early stage of development by Adamed Pharma [202, 203].
Molecules Targeting Muscarinic M1 and M4 Receptors
Modulation of muscarinic receptors activity may improve both cognitive and psychosis-related symptoms. Targeting the centrally located M1R and M4R proved to enhance the memory function and reduce psychosis both in animal models and in clinics [204, 205]. Additionally, stimulation of muscarinic receptors controls the increase of amyloid-β levels and prevents memory impairments [206]. This evidence encouraged the development of dual ligands acting on M1R/M4R. Sosei Heptares, jointly with Allergan, is developing a series of dual M1/M4 agonists for the treatment of memory deficits and comorbid psychosis in patients with AD. The joint project has progressed to an advanced preclinical development stage [207]. Further data have not been revealed.
Cholinesterase and Monoamine Oxidase Inhibitor: Ladostigil
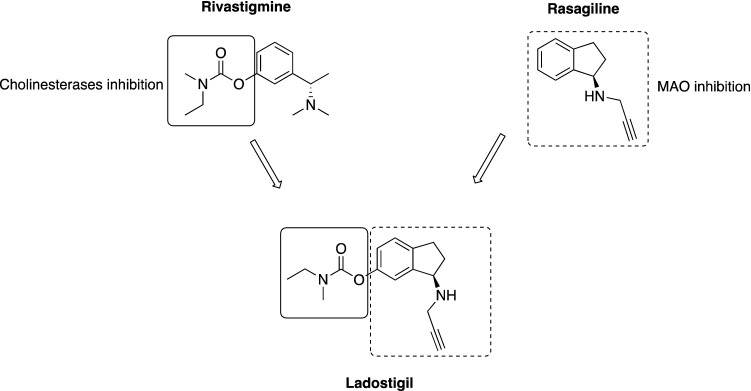
Degeneration of cholinergic cortical neurons is considered as a key pathology that accounts for the cognitive deficit in patients with AD. Drugs that act by inhibiting cholinesterase, and as a result, increasing cholinergic transmission in the affected cortical areas are expected to improve memory function. Interestingly, a cholinesterase inhibitor rasagiline reduced aggression and delusions in patients with AD [208]. In a dual-acting drug, ladostigil, the carbamate fragment of rivastigmine, which accounts for the inhibition of cholinesterases, was merged with the N-(prop-2-yn-1-yl)-2,3-dihydro-1H-inden-1-amine fragment of rasagiline, which blocks the activity of monoamine oxidases A and B (Fig. 12) [209]. Ladostigil is a multi-modal agent developed by Avraham Pharmaceuticals to treat patients with mild cognitive impairment with concomitant behavioral symptoms. Preclinical studies showed that ladostigil improves memory deficits and exerts anxiolytic and antidepressant-like activity [210, 211]. Therefore, ladostigil has been suggested for use in patients with dementia with comorbid depression; however, no clinical data are available so far [210].
Fig. 12.
Chemical structure of ladostigil. Ladostigil is composed of two fragments: the carbamate moiety of rivastigmine, which accounts for the inhibition of cholinesterases, merged with the N-(prop-2-yn-1-yl)-2,3-dihydro-1H-inden-1-amine fragment of rasagiline, which blocks the monoamine oxidases (MAO) A and B [209]
Ligands Targeting Serotonin 5-HT6 and 5-HT2A Receptors
Serotonin 5-HT6 receptors are localized in the cortical and limbic areas, and ligands modulating the activity of 5-HT6 receptors exert memory-enhancing, anxiolytic, and antidepressant effects [47, 48]. Importantly, several selective 5-HT6 antagonists entered clinical trials and have been regarded as potential drug candidates for the treatment of cognitive impairment associated with AD [48, 212].
Similarly, serotonin 5-HT2A receptor has emerged as a promising target for the treatment of AD, owing to its strong relationship with the pathology of dementia-related psychosis (see Sect. 2). Therefore, merging 5-HT2AR and 5-HT6R into a single chemical entity, having the pharmacological activities of both the receptors, may be beneficial in the treatment of dementia-related psychosis. Small molecules targeting 5-HT6R and 5-HT2AR are currently under development (discovery phase/advanced preclinical development stage) for the treatment of dementia-related psychosis by Adamed Pharma [213]. The chemical structure and preclinical data have not been disclosed.
Safety Issues Regarding Pharmacotherapy of Dementia-Related Psychosis and Agitation/Aggression: Summary of Off Targets
Previous studies indicated that patients with dementia were found to be particularly sensitive to side effects induced by atypical antipsychotics [214]. Patients with dementia seem to be at a greater risk of developing antipsychotic-associated stroke events [215]. It should be emphasized that atypical antipsychotics used so far in clinical settings (risperidone, olanzapine, quetiapine, and aripiprazole) interact with multiple receptors, including “off targets,” which induce serious side effects (Table 4). For instance, similar to the first generation of antihistamines, antipsychotic agents tend to induce excessive daytime sleepiness owing to their high affinity to H1R present in the brain [216]. Interaction with alpha-adrenoceptors (risperidone and quetiapine) causes orthostatic hypotension, and as a result, may increase the risk of falls and hip fractures [217]. Moreover, a large clinical study found a correlation between the increased risk of stroke and treatment with antipsychotics that exhibit high binding affinity to alpha-2 adrenoceptor and M1R. A possible mechanism underlying this relationship is that blockade of alpha-2 adrenoceptor and M1 may induce orthostatic hypotension and tachycardia, which prompts unstable hemodynamic conditions and increases the risk of stroke [214, 215]. In addition, blockade of the muscarinic receptors intensifies the cholinergic deficit and worsens cognitive functioning in patients with dementia, who already have memory impairment. Blockade of M1R may induce additional uncomfortable symptoms, such as severe constipation and urine retention [218]. Furthermore, previous studies showed that inhibition of hERG (human ether-a-go–go-related gene) potassium channel has been considered as the most common mechanism of drug-induced cardiotoxicity (prolongation of QT interval). Therefore, evaluation of the potential inhibitory effects on the hERG channel should become an obligatory safety practice in the early stages of drug discovery for dementia-related psychosis and agitation/aggression [219, 220].
Table 4.
Simplified representation of an off-target receptor profile
| Receptor | Pharmacological activity |
|---|---|
| Side-effect receptor action | |
| 5-HT2C | Weight gain |
| Alpha-2 | Orthostatic hypotension |
| H1 | Excessive sedation, weight gain |
| M1 | Memory deficits, anticholinergic side effect |
| hERG channel | QT prolongation, arrhythmia |
hERG human ether-a-go–go-related gene
Considering the safety issues regarding elderly patients and their particular sensitiveness to adverse reactions, future therapeutic agents designed for dementia-related psychosis and agitation/aggression should not antagonize the muscarinic, adrenergic, and histaminergic receptors or the hERG channel [112].
Conclusions and Future Perspectives
Treatments currently used for dementia-related psychosis and agitation/aggression pose a great challenge to clinicians, and specifically targeted pharmacotherapies are lacking. Despite known harms and modest clinical efficacy, antipsychotics still are often administered off-label to control some symptoms [221]. Several clinical experts have agreed that to optimize the clinical response, patients with dementia should be treated with specifically developed medications that interact with pharmacologically relevant targets [16, 222, 223]. Within this context, preclinical and clinical studies have pointed out various biological targets that might constitute potential therapeutics for dementia-related psychosis and agitation/aggression.
Considering that the polymorphism of serotonin 5-HT2AR and its disrupted functioning has been associated with the onset of psychosis and aggression in AD, it seems justifiable that the ligands modulating the activity of 5-HT2AR could be attractive therapeutic agents for dementia-related psychosis and aggression [26, 27, 82]. A selective inverse agonist of 5-HT2AR (pimavanserin) and novel atypical antipsychotics acting as 5-HT2A antagonists (lumateperone, brexpiprazole) have advanced to phase III clinical trials after showing promising efficacy in phase II studies in the management of dementia-related psychosis and agitation [58, 73, 114]. A phase III study has reported promising results for brexpiprazole, which significantly reduced agitation in patients with dementia, and its long-term efficacy will be further evaluated in a treatment-extension study [114]. In contrast, a phase III clinical trial that investigated the efficacy of lumateperone in reducing dementia-related agitation has been terminated because of a lack of efficacy [119]. Moreover, several compounds targeting 5-HT2AR are in the early stage of the drug discovery process [199, 213]. In addition, the role of 5-HT1AR is well elucidated in the onset of neuropsychiatric symptoms [34]. A 5-HT1AR/5-HT1BR agonist eltoprazine has also completed phase II clinical trials, showing positive results in suppressing AD-related aggression. Similarly, pathological changes in the function of alpha-1 adrenoceptor have been linked with the onset of aggressive behavior in patients with AD [148]. A centrally acting alpha-1 adrenergic antagonist prazosin is currently being investigated in a phase II study in the treatment of dementia-related aggression.
Several groups of patients with dementia with comorbid conditions may require specific adjusted pharmacological treatment. The episodes of nocturnal agitation in patients with dementia seem to require an agent displaying sedative properties and adjunctive sleep-promoting activity [174]. Within this context, two generic drugs, mirtazapine and gabapentin, are currently being investigated in phase III and phase IV trials, respectively. Both agents exhibit sedative properties and the ability to improve sleep quality, which may be suitable for this subgroup of patients.
Molecules acting via the inhibition of SERT appear to constitute another class of promising therapeutics [32, 33]. Polymorphism of the SERT has been associated with AD-related aggression. Significant clinical efficacy of a selective SERT inhibitor, citalopram, has been observed in a phase III clinical trial. However, citalopram induced cardiac side effects, and therefore, its long-term clinical application remains dubious. Currently, a phase III clinical trial is recruiting patients to evaluate the safety and efficacy of the S-enantiomer of citalopram, escitalopram, in reducing AD-related aggression. Escitalopram seems to be a safer alternative and does not tend to induce risky cardiac reactions [106]. It is worth nothing that dextromethorphan displays high affinity to SERT, which further supports the evaluation of its combined formulations (AVP-786 and AXS-05) in clinical trials. Both formulations are currently being investigated in ongoing phase III studies. The interim analysis of the study with AXS-05 showed promising outcomes and reported no safety issues [63].
Although certain drug candidates presented here act on the molecular targets that do not meticulously match the pathology of dementia-related psychosis and agitation/aggression, their promising pharmacological profile suggests their potential therapeutic utility. Favorable pharmacological properties have been reported for mGluR2, CB1, NMDA, M4, and M1/M4 agonists. A CB1R agonist, nabilone, showed efficacy in reducing aggression in patients with dementia in a recently completed phase II/III clinical trial. An mGluR2 agonist, LY2979165, has advanced to phase II clinical trials that will investigate its safety and efficacy in the treatment of dementia-related psychosis and agitation/aggression. Similarly, an indirect NMDA agonist compound SND-51 has advanced to phase II clinical trials that will evaluate its efficacy in the treatment of dementia and psychosis. A selective M4 receptor agonist, HTL0016878, has progressed to phase I clinical trials. In addition, various compounds acting on the pharmacologically relevant targets (5-HT6R and M1R/M4R) have progressed to an advanced preclinical development stage. To sum up, future drugs targeting mGluR, NMDA receptors, CB1R, M1/M4R, and 5-HT6R also offer the possibility to tackle dementia-related psychosis and agitation/aggression.
Additionally, the key success of a clinically effective agent will likely depend on its safety and lack of harmful side effects [8, 9, 11]. Considering that previous clinical observations have shown that patients with dementia are at a higher risk of stroke [112], future drug candidates for patients with dementia should consistently avoid interactions and further blockade of alpha-2 adrenoceptors, M1R, H1R, and the hERG channel to avoid cardiotoxicity, stroke, excessive sedation, and memory impairments.
Nevertheless, the future pharmacotherapy of dementia-related psychosis, agitation/aggression will likely depend on the successful compilation of phase III clinical trials and further approvals. So far, the most promising results have been observed for compounds modulating serotoninergic signaling. Another set of molecules displaying favorable pharmacological activity are at an early stage of development.
Compliance with Ethical Standards
Funding
This work was supported by the National Science Center of Poland (Grant DEC-2014/15/D/NZ7/01789) and National Center for Research and Development (Grant POIR.01.01.01-00-0108/17). The open access has been sponsored by Jagiellonian University, Kraków, Poland.
Conflict of interest
Marcin Kołaczkowski and Joanna Śniecikowska are employees of Adamed Pharma S.A. Monika Marcinkowska, Nikola Fajkis, Paweł Paśko, and Weronika Franczyk have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in this article apart from those disclosed. No writing assistance was utilized in the production of this article.
References
- 1.Jeste DV, Finkel SI. Psychosis of Alzheimer’s disease and related dementias: diagnostic criteria for a distinct syndrome. Am J Geriatr Psychiatry. 2000;8:29–34. doi: 10.1097/00019442-200002000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Alzforum. https://www.alzforum.org/papers/uber-eine-eigenartige-erkrankung-der-hirnrinde/en. Accessed 16 Dec 2019.
- 3.Müller-Spahn F. Behavioral disturbances in dementia. Dialog Clin Neurosci. 2003;5:49–59. doi: 10.31887/DCNS.2003.5.1/fmuellerspahn. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cerejeira J, Lagarto L, Mukaetova-Ladinska EB. Behavioral and psychological symptoms of dementia. Front Neurol. 2012;3:73. doi: 10.3389/fneur.2012.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toot S, Swinson T, Devine M, Challis D, Orrell M. Causes of nursing home placement for older people with dementia: a systematic review and meta-analysis. Int Psychogeriatr. 2017;29:195–208. doi: 10.1017/S1041610216001654. [DOI] [PubMed] [Google Scholar]
- 6.Gerlach LB, Kales HC. Managing behavioral and psychological symptoms of dementia. Psychiatr Clin North Am. 2018;41:127–139. doi: 10.1016/j.psc.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 7.Tampi RR, Tampi DJ, Balachandran S, Srinivasan S. Antipsychotic use in dementia: a systematic review of benefits and risks from meta-analyses. Ther Adv Chronic Dis. 2016;7:229–245. doi: 10.1177/2040622316658463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farlow MR, Shamliyan TA. Benefits and harms of atypical antipsychotics for agitation in adults with dementia. Eur Neuropsychopharmacol. 2017;27:217–231. doi: 10.1016/j.euroneuro.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Parker C, Coupland C, Hippisley-Cox J. Antipsychotic drugs and risk of venous thromboembolism: nested case-control study. BMJ. 2010;341:42–45. doi: 10.1136/bmj.c4245. [DOI] [PubMed] [Google Scholar]
- 10.FDA Public Health Advisory. Deaths with antipsychotics in elderly patients with behavioral disturbances. http://psychrights.org/drugs/FDAantipsychotics4elderlywarning.htm. Accessed 16 Dec 2019.
- 11.Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA. 2005;294:1934–1943. doi: 10.1001/jama.294.15.1934. [DOI] [PubMed] [Google Scholar]
- 12.Reus VI, Fochtmann LJ, Eyler AE, Hilty DM, Horvitz-Lennon M, Jibson MD, et al. The American Psychiatric Association practice guideline on the use of antipsychotics to treat agitation or psychosis in patients with dementia. Am J Psychiatry. 2016;173:543–546. doi: 10.1176/appi.ajp.2015.173501. [DOI] [PubMed] [Google Scholar]
- 13.Dupuis DS, Mannoury la Cour C, Chaput C, Verrièle L, Lavielle G, Millan MJ. Actions of novel agonists, antagonists and antipsychotic agents at recombinant rat 5-HT6 receptors: a comparative study of coupling to G alpha s. Eur J Pharmacol. 2008;588:170–7. [DOI] [PubMed]
- 14.NICE. Dementia: supporting people with dementia and their carers in health and social care. https://www.nice.org.uk/guidance/cg42. Accessed 16 Dec 2019.
- 15.Hewer W, Thomas C. Treatment with psychotropic agents in patients with dementia and delirium: gap between guideline recommendations and treatment practice. Z Gerontol Geriatr. 2017;50:106–114. doi: 10.1007/s00391-016-1176-0. [DOI] [PubMed] [Google Scholar]
- 16.Kales HC, Lyketsos CG, Miller EM, Ballard C. Management of behavioral and psychological symptoms in people with Alzheimer’s disease: an international Delphi consensus. Int Psychogeriatr. 2019;31:83–90. doi: 10.1017/S1041610218000534. [DOI] [PubMed] [Google Scholar]
- 17.VanGuilder HD, Yan H, Farley JA, Sonntag WE, Freeman WM. Aging alters the expression of neurotransmission-regulating proteins in the hippocampal synaptoproteome. J Neurochem. 2010;113:1577–1588. doi: 10.1111/j.1471-4159.2010.06719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choudhury A, Sahu T, Ramanujam PL, Banerjee AK, Chakraborty I, Kumar AR, et al. Neurochemicals, behaviours and psychiatric perspectives of neurological diseases. Neuropsychiatry. 2018;8:395–424. [Google Scholar]
- 19.Vogt I, Prinz J, Campillos M. Molecularly and clinically related drugs and diseases are enriched in phenotypically similar drug-disease pairs. Genome Med. 2014;6:52. doi: 10.1186/s13073-014-0052-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ford AH, Almeida OP. Management of depression in patients with dementia: is pharmacological treatment justified? Drugs Aging. 2017;34:89–95. doi: 10.1007/s40266-016-0434-6. [DOI] [PubMed] [Google Scholar]
- 21.Hersch EC, Falzgraf S. Management of the behavioral and psychological symptoms of dementia. Clin Interv Aging. 2007;2:611–621. doi: 10.2147/CIA.S1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murley AG, Rowe JB. Neurotransmitter deficits from frontotemporal lobar degeneration. Brain. 2018;141:1263–1285. doi: 10.1093/brain/awx327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bondareff W. Neuropathology of psychotic symptoms in Alzheimer’s disease. Int Psychogeriatr. 1996;8(Suppl. 3):233–7 (discussion 269–72). [DOI] [PubMed]
- 24.Zubenko GS. Clinicopathologic and neurochemical correlates of major depression and psychosis in primary dementia. Int Psychogeriatr. 1996;83:219–223. doi: 10.1017/s1041610297003384. [DOI] [PubMed] [Google Scholar]
- 25.Meltzer CC, Smith G, DeKosky ST, Pollock BG, Mathis CA, Moore RY, et al. Serotonin in aging, late-life depression, and Alzheimer’s disease: the emerging role of functional imaging. Neuropsychopharmacology. 1998;18:407–430. doi: 10.1016/S0893-133X(97)00194-2. [DOI] [PubMed] [Google Scholar]
- 26.Lorke DE, Lu G, Cho E, Yew DT. Serotonin 5-HT2A and 5-HT6 receptors in the prefrontal cortex of Alzheimer and normal aging patients. BMC Neurosci. 2006;7:36. doi: 10.1186/1471-2202-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holmes C, Arranz MJ, Powell JF, Collier DA, Lovestone S. 5-HT2A and 5-HT2C receptor polymorphisms and psychopathology in late onset Alzheimer’s disease. Hum Mol Genet. 1998;7:1507–1509. doi: 10.1093/hmg/7.9.1507. [DOI] [PubMed] [Google Scholar]
- 28.Gomperts SN. Lewy body dementias: dementia with Lewy bodies and Parkinson disease dementia. Contin Minneap Minn. 2016;22:435–463. doi: 10.1212/CON.0000000000000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsuang D, Larson EB, Bolen E, Thompson ML, Peskind E, Bowen J, et al. Visual hallucinations in dementia: a prospective community-based study with autopsy. Am J Geriatr Psychiatry. 2009;17:317–323. doi: 10.1097/JGP.0b013e3181953b9a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sinforiani E, Terzaghi M, Pasotti C, Zucchella C, Zambrelli E, Manni R. Hallucinations and sleep-wake cycle in Alzheimer’s disease: a questionnaire-based study in 218 patients. Neurol Sci. 2007;28:96–99. doi: 10.1007/s10072-007-0794-0. [DOI] [PubMed] [Google Scholar]
- 31.Brodaty H, Ames D, Snowdon J, Woodward M, Kirwan J, Clarnette R, et al. Risperidone for psychosis of Alzheimer’s disease and mixed dementia: results of a double-blind, placebo-controlled trial. Int J Geriatr Psychiatry. 2005;20:1153–1157. doi: 10.1002/gps.1409. [DOI] [PubMed] [Google Scholar]
- 32.Sukonick DL, Pollock BG, Sweet RA, Mulsant BH, Rosen J, Klunk WE, et al. The 5-HTTPR*S/*L polymorphism and aggressive behavior in Alzheimer disease. Arch Neurol. 2001;58:1425–1428. doi: 10.1001/archneur.58.9.1425. [DOI] [PubMed] [Google Scholar]
- 33.Sweet RA, Pollock BG, Sukonick DL, Mulsant BH, Rosen J, Klunk WE, et al. The 5-HTTPR polymorphism confers liability to a combined phenotype of psychotic and aggressive behavior in Alzheimer disease. Int Psychogeriatr. 2001;13:401–409. doi: 10.1017/S1041610201007827. [DOI] [PubMed] [Google Scholar]
- 34.Lai MKP, Tsang SWY, Francis PT, Esiri MM, Keene J, Hope T, et al. Reduced serotonin 5-HT1A receptor binding in the temporal cortex correlates with aggressive behavior in Alzheimer disease. Brain Res. 2003;974:82–87. doi: 10.1016/S0006-8993(03)02554-X. [DOI] [PubMed] [Google Scholar]
- 35.Gannon M, Che P, Chen Y, Jiao K, Roberson ED, Wang Q. Noradrenergic dysfunction in Alzheimer’s disease. Front Neurosci. 2015;9:220. doi: 10.3389/fnins.2015.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sweet RA, Nimgaonkar VL, Kamboh MI, Lopez OL, Zhang F, DeKosky ST. Dopamine receptor genetic variation, psychosis, and aggression in Alzheimer disease. Arch Neurol. 1998;55:1335–1340. doi: 10.1001/archneur.55.10.1335. [DOI] [PubMed] [Google Scholar]
- 37.Rodriguez-Arias M, Navarrete F, Daza-Losada M, Navarro D, Aguilar MA, Berbel P, et al. CB1 cannabinoid receptor-mediated aggressive behavior. Neuropharmacology. 2013;75:172–180. doi: 10.1016/j.neuropharm.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 38.Ahmad R, Goffin K, Van den Stock J, De Winter F-L, Cleeren E, Bormans G, et al. In vivo type 1 cannabinoid receptor availability in Alzheimer’s disease. Eur Neuropsychopharmacol. 2014;24:242–250. doi: 10.1016/j.euroneuro.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 39.Mulder J, Zilberter M, Pasquaré SJ, Alpár A, Schulte G, Ferreira SG, et al. Molecular reorganization of endocannabinoid signalling in Alzheimer’s disease. Brain J Neurol. 2011;134:1041–1060. doi: 10.1093/brain/awr046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang R, Reddy PH. Role of glutamate and NMDA receptors in Alzheimer’s disease. J Alzheimers Dis. 2017;57:1041–1048. doi: 10.3233/JAD-160763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Niciu MJ, Kelmendi B, Sanacora G. Overview of glutamatergic neurotransmission in the nervous system. Pharmacol Biochem Behav. 2012;100:656–664. doi: 10.1016/j.pbb.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crupi R, Impellizzeri D, Cuzzocrea S. Role of metabotropic glutamate receptors in neurological disorders. Front Mol Neurosci. 2019;12:20. doi: 10.3389/fnmol.2019.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hovelsø N, Sotty F, Montezinho LP, Pinheiro PS, Herrik KF, Mørk A. Therapeutic potential of metabotropic glutamate receptor modulators. Curr Neuropharmacol. 2012;10:12–48. doi: 10.2174/157015912799362805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bratek E, Ziembowicz A, Bronisz A, Salinska E. The activation of group II metabotropic glutamate receptors protects neonatal rat brains from oxidative stress injury after hypoxia-ischemia. PLoS One. 2018;13(7). [DOI] [PMC free article] [PubMed]
- 45.Farías GG, Godoy JA, Hernández F, Avila J, Fisher A, Inestrosa NC. M1 muscarinic receptor activation protects neurons from beta-amyloid toxicity: a role for Wnt signaling pathway. Neurobiol Dis. 2004;17:337–348. doi: 10.1016/j.nbd.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 46.Mirza NR, Peters D, Sparks RG. Xanomeline and the antipsychotic potential of muscarinic receptor subtype selective agonists. CNS Drug Rev. 2003;9:159–186. doi: 10.1111/j.1527-3458.2003.tb00247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ferrero H, Solas M, Francis PT, Ramirez MJ. Serotonin 5-HT6 receptor antagonists in Alzheimer’s disease: therapeutic rationale and current development status. CNS Drugs. 2017;31:19–32. doi: 10.1007/s40263-016-0399-3. [DOI] [PubMed] [Google Scholar]
- 48.Marcos B, Gil-Bea FJ, Hirst WD, García-Alloza M, Ramírez MJ. Lack of localization of 5-HT6 receptors on cholinergic neurons: implication of multiple neurotransmitter systems in 5-HT6 receptor-mediated acetylcholine release. Eur J Neurosci. 2006;24:1299–1306. doi: 10.1111/j.1460-9568.2006.05003.x. [DOI] [PubMed] [Google Scholar]
- 49.Cummings J, Lee G, Ritter A, Zhong K. Alzheimer’s disease drug development pipeline: 2018. Alzheimers Dement Transl Res Clin Interv. 2018;4:195–214. doi: 10.1016/j.trci.2018.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Devanand DP, Strickler JG, Huey ED, Crocco E, Forester BP, Husain MM, et al. Lithium treatment for agitation in Alzheimer’s disease (Lit-AD): clinical rationale and study design. Contemp Clin Trials. 2018;71:33–39. doi: 10.1016/j.cct.2018.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.ClinicalTrials.gov. A two part study to assess safety, PK, PD, and food effect of oral HTL0016878. https://clinicaltrials.gov/ct2/show/NCT03244228. Accessed 20 Aug 2019.
- 52.Amarantus announces positive phase 2 data for eltoprazine in Alzheimer’s aggression. Available from: https://www.amarantus.com/news/press-releases/detail/2033/amarantus-announces-positive-phase-2-data-for-eltoprazine. Accessed 20 Aug 2019.
- 53.ClinicalTrials.gov. A study of MP-101 in dementia-related psychosis and/or agitation and aggression. https://clinicaltrials.gov/ct2/show/NCT03044249. Accessed 20 Aug 2019.
- 54.Davey MJ. The pharmacology of prazosin, an alpha 1-adrenoceptor antagonist and the basis for its use in the treatment of essential hypertension. Clin Exp Hypertens A. 1982;4(1–2):47–59. doi: 10.3109/10641968209061575. [DOI] [PubMed] [Google Scholar]
- 55.ClinicalTrials.gov. Prazosin for agitation in Alzheimer’s disease. https://clinicaltrials.gov/ct2/show/NCT03710642. Accessed 20 Aug 2019.
- 56.ClinicalTrials.gov. A study of pimavanserin for the treatment of agitation and aggression in subjects with Alzheimer’s disease. https://clinicaltrials.gov/ct2/show/NCT03118947. Accessed 20 Aug 2019.
- 57.ClinicalTrials.gov. A study of the safety and efficacy of pimavanserin in patients with Alzheimer’s disease psychosis. https://clinicaltrials.gov/ct2/show/NCT02035553. Accessed 20 Aug 2019.
- 58.Ballard C, Banister C, Khan Z, Cummings J, Demos G, Coate B, et al. Evaluation of the safety, tolerability, and efficacy of pimavanserin versus placebo in patients with Alzheimer’s disease psychosis: a phase 2, randomised, placebo-controlled, double-blind study. Lancet Neurol. 2018;17:213–222. doi: 10.1016/S1474-4422(18)30039-5. [DOI] [PubMed] [Google Scholar]
- 59.ClinicalTrials.gov. Safety and efficacy of nabilone in Alzheimer’s disease. https://clinicaltrials.gov/ct2/show/NCT02351882. Accessed 20 Aug 2019.
- 60.Lanctot KL, Ruthirakuhan M, Gallagher D, Sherman C, Abraham EH, Verhoeff NPLG, et al. Nabilone significantly improves agitation/adression in patients with moderate-to-severe AD: preliminary results of a placebo-controlled, double-blind, cross-over trial. Alzheimers Dement. 2018;14:1385. doi: 10.1016/j.jalz.2018.06.2871. [DOI] [Google Scholar]
- 61.Herrmann N, Ruthirakuhan M, Gallagher D, Verhoeff NPLG, Kiss A, Black SE, et al. Randomized placebo-controlled trial of nabilone for agitation in Alzheimer’s disease. Am J Geriatr Psychiatry. 2019;27(11):1161–1173. doi: 10.1016/j.jagp.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 62.ClinicalTrials.gov. Addressing dementia via agitation-centered evaluation. https://clinicaltrials.gov/ct2/show/NCT03226522. Accessed 20 Aug 2019.
- 63.Axsome Therapeutics, Inc. Axsome Therapeutics announces positive outcome of interim analysis of ADVANCE-1 phase 2/3 trial of AXS-05 in Alzheimer’s disease agitation. GlobeNewswire News Room. 2018. http://www.globenewswire.com/news-release/2018/12/10/1664251/0/en/Axsome-Therapeutics-Announces-Positive-Outcome-of-Interim-Analysis-of-ADVANCE-1-Phase-2-3-Trial-of-AXS-05-in-Alzheimer-s-Disease-Agitation.html. Accessed 20 Aug 2019.
- 64.ClinicalTrials.gov. Relapse prevention study of pimavanserin in dementia-related psychosis. https://clinicaltrials.gov/ct2/show/NCT03325556. Accessed 20 Aug 2019.
- 65.Clinical Trials Register. Search for 2017-004439-36. https://www.clinicaltrialsregister.eu/ctr-search/search?query=2017-004439-36. Accessed 16 Dec 2019.
- 66.Clinical Trials Register. Search for 2017-002227-13. https://www.clinicaltrialsregister.eu/ctr-search/search?query=2017-002227-13. Accessed 16 Dec 2019.
- 67.ClinicalTrials.gov. Escitalopram for agitation in Alzheimer’s disease. https://clinicaltrials.gov/ct2/show/NCT03108846. Accessed 20 Aug 2019.
- 68.Maeda K, Sugino H, Akazawa H, Amada N, Shimada J, Futamura T, et al. Brexpiprazole I: in vitro and in vivo characterization of a novel serotonin-dopamine activity modulator. J Pharmacol Exp Ther. 2014;350:589–604. doi: 10.1124/jpet.114.213793. [DOI] [PubMed] [Google Scholar]
- 69.ClinicalTrials.gov. Brexpiprazole for the long-term treatment of patients with agitation associated with dementia of the Alzheimer’s type. https://clinicaltrials.gov/ct2/show/NCT03724942. Accessed 20 Aug 2019.
- 70.Clinical Trials Register. Search for 2017-003940-19. https://www.clinicaltrialsregister.eu/ctr-search/search?query=2017-003940-19. Accessed 16 Dec 2019.
- 71.Clinical Trials Register. https://www.clinicaltrialsregister.eu/ctr-search/trial/2018-002783-88/BG. Accessed 16 Dec 2019.
- 72.Zhang L, Hendrick JP. The presynaptic D2 partial agonist lumateperone acts as a postsynaptic D2 antagonist. Matters. 2018;4:201712000006. [Google Scholar]
- 73.Kumar B, Kuhad A, Kuhad A. Lumateperone: a new treatment approach for neuropsychiatric disorders. Drugs Today Barc Spain. 1998;2018(54):713–719. doi: 10.1358/dot.2018.54.12.2899443. [DOI] [PubMed] [Google Scholar]
- 74.ClinicalTrials.gov. Long term, extension study of the safety and efficacy of AVP-786 for the treatment of agitation in patients with dementia of the Alzheimer’s type. https://clinicaltrials.gov/ct2/show/NCT02446132. Accessed 20 Aug 2019.
- 75.ClinicalTrials.gov. Study of mirtazapine for agitation in dementia. https://clinicaltrials.gov/ct2/show/NCT03031184. Accessed 20 Aug 2019.
- 76.ClinicalTrials.gov. Nighttime agitation and restless legs syndrome in people with Alzheimer’s disease. https://clinicaltrials.gov/ct2/show/NCT03082755. Accessed 20 Aug 2019.
- 77.Serretti A, Drago A, De Ronchi D. HTR2A gene variants and psychiatric disorders: a review of current literature and selection of SNPs for future studies. Curr Med Chem. 2007;14:2053–2069. doi: 10.2174/092986707781368450. [DOI] [PubMed] [Google Scholar]
- 78.Fidalgo S, Ivanov DK, Wood SH. Serotonin: from top to bottom. Biogerontology. 2013;14(1):21–45. doi: 10.1007/s10522-012-9406-3. [DOI] [PubMed] [Google Scholar]
- 79.ACADIA Pharmaceuticals announces positive top-line results from phase II study of pimavanserin for Alzheimer’s disease psychosis. 2016. https://www.businesswire.com/news/home/20161220005379/en/ACADIA-Pharmaceuticals-Announces-Positive-Top-Line-Results-Phase. Accessed 16 Dec 2019.
- 80.Business Wire. ACADIA Pharmaceuticals initiates phase III study of pimavanserin in dementia-related psychosis. https://www.businesswire.com/news/home/20171004006297/en/ACADIA-Pharmaceuticals-Initiates-Phase-III-Study-Pimavanserin. Accessed 16 Dec 2019.
- 81.Hacksell U, Burstein ES, McFarland K, Mills RG, Williams H. On the discovery and development of pimavanserin: a novel drug candidate for Parkinson’s psychosis. Neurochem Res. 2014;39:2008–2017. doi: 10.1007/s11064-014-1293-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Weiner DM, Burstein ES, Nash N, Croston GE, Currier EA, Vanover KE, et al. 5-Hydroxytryptamine2A receptor inverse agonists as antipsychotics. J Pharmacol Exp Ther. 2001;299:268–276. [PubMed] [Google Scholar]
- 83.Nordstrom AL, Mansson M, Jovanovic H, Karlsson P, Halldin C, et al. PET analysis of the 5-HT2A receptor inverse agonist ACP-103 in human brain. Int J Neuropsychopharmacol. 2008;11(2):163–171. doi: 10.1017/S1461145707007869. [DOI] [PubMed] [Google Scholar]
- 84.Vanover KE, Weiner DM, Makhay M, Veinbergs I, Gardell LR, Lameh J, et al. Pharmacological and behavioral profile of N-(4-fluorophenylmethyl)-N-(1-methylpiperidin-4-yl)-N′-(4-(2-methylpropyloxy)phenylmethyl) carbamide (2R,3R)-dihydroxybutanedioate (2:1) (ACP-103), a novel 5-hydroxytryptamine(2A) receptor inverse agonist. J Pharmacol Exp Ther. 2006;317:910–918. doi: 10.1124/jpet.105.097006. [DOI] [PubMed] [Google Scholar]
- 85.Price DL, Bonhaus DW, McFarland K. Pimavanserin, a 5-HT2A receptor inverse agonist, reverses psychosis-like behaviors in a rodent model of Alzheimer’s disease. Behav Pharmacol. 2012;23:426–433. doi: 10.1097/FBP.0b013e3283566082. [DOI] [PubMed] [Google Scholar]
- 86.Cummings J, Isaacson S, Mills R, Williams H, Chi-Burris K, Corbett A, et al. Pimavanserin for patients with Parkinson’s disease psychosis: a randomised, placebo-controlled phase 3 trial. Lancet. 2014;383:533–540. doi: 10.1016/S0140-6736(13)62106-6. [DOI] [PubMed] [Google Scholar]
- 87.Ballard CG, Saad K, Patel A, Gahir M, Solis M, Coope B, et al. The prevalence and phenomenology of psychotic symptoms in dementia sufferers. Int J Geriatr Psychiatry. 1995;10:477–485. doi: 10.1002/gps.930100607. [DOI] [Google Scholar]
- 88.Sato S, Mizukami K, Asada T. A preliminary open-label study of 5-HT1A partial agonist tandospirone for behavioural and psychological symptoms associated with dementia. Int J Neuropsychopharmacol. 2007;10:281–283. doi: 10.1017/S1461145706007000. [DOI] [PubMed] [Google Scholar]
- 89.Cantillon M, Brunswick R, Molina D, Bahro M. Buspirone vs. haloperidol: a double-blind trial for agitation in a nursing home population with Alzheimer’s disease. Am J Geriatr Psychiatry. 1996;4:263–7. [DOI] [PubMed]
- 90.Tiihonen J, Hakola P, Paanila J, Turtiainen M. Eltoprazine for aggression in schizophrenia and mental retardation. Lancet. 1993;341:307. doi: 10.1016/0140-6736(93)92660-L. [DOI] [PubMed] [Google Scholar]
- 91.Amarantus BioScience Holdings, Inc. (AMBS). Eltoprazine. https://www.amarantus.com/therapeutics-pipeline/therapeutics/eltoprazine. Accessed 20 Aug 2019.
- 92.ClinicalTrials.gov. A study of efficacy and safety of eltoprazine HCl for treating levodopa-induced dyskinesia in Parkinson’s disease patients. https://clinicaltrials.gov/ct2/show/NCT02439125. Accessed 20 Aug 2019.
- 93.Raghoebar M, Mak M, Cournot A, et al. Pharmacokinetics of eltoprazine inhealthy male subjects after single dose oral and intravenous administration. Br J Clin Pharmacol. 1990;30:879–883. doi: 10.1111/j.1365-2125.1990.tb05454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mos J, Olivier B, Poth M, van Aken H. The effects of intraventricular administration of eltoprazine, 1-(3-trifluoromethylphenyl)piperazine hydrochloride and 8-hydroxy-2-(di-n-propylamino)tetralin on resident intruder aggression in the rat. Eur J Pharmacol. 1992;212(2–3):295–298. doi: 10.1016/0014-2999(92)90348-8. [DOI] [PubMed] [Google Scholar]
- 95.Monn J, Prieto L, Taboada Martinez L, Montero Salgado C, Shaw BW. Mglu2 agonists. Patent WO2011084437, 2011.
- 96.Mos J, Olivier B, Poth M, van Aken H. The effects of intraventricular administration of eltoprazine, 1-(3-trifluoromethylphenyl)piperazine hydrochloride and 8-hydroxy-2-(di-n-propylamino)tetralin on resident intruder aggression in the rat. Eur J Pharmacol. 1992;212:295–298. doi: 10.1016/0014-2999(92)90348-8. [DOI] [PubMed] [Google Scholar]
- 97.Svenningsson P, Rosenblad C, Af Edholm Arvidsson K, Wictorin K, Keywood C, Shankar B, et al. Eltoprazine counteracts l-DOPA-induced dyskinesias in Parkinson’s disease: a dose-finding study. Brain J Neurol. 2015;138:963–73. [DOI] [PMC free article] [PubMed]
- 98.Schipper J, Tulp MT, Sijbesma H. Neurochemical profile of eltoprazine. Drug Metabol Drug Interact. 1990;8:85–114. doi: 10.1515/DMDI.1990.8.1-2.85. [DOI] [PubMed] [Google Scholar]
- 99.Cirrito JR, Disabato BM, Restivo JL, Verges DK, Goebel WD, Sathyan A, et al. Serotonin signaling is associated with lower amyloid-β levels and plaques in transgenic mice and humans. Proc Natl Acad Sci USA. 2011;108:14968–14973. doi: 10.1073/pnas.1107411108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sheline YI, West T, Yarasheski K, Swarm R, Jasielec MS, Fisher JR, et al. An antidepressant decreases CSF Aβ production in healthy individuals and in transgenic AD mice. Sci Transl Med. 2014;6:236–244. doi: 10.1126/scitranslmed.3008169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 102.Pollock BG, Mulsant BH, Rosen J, Sweet RA, Mazumdar S, Bharucha A, et al. Comparison of citalopram, perphenazine, and placebo for the acute treatment of psychosis and behavioral disturbances in hospitalized, demented patients. Am J Psychiatry. 2002;159:460–465. doi: 10.1176/appi.ajp.159.3.460. [DOI] [PubMed] [Google Scholar]
- 103.Porsteinsson AP, Drye LT, Pollock BG, Devanand DP, Frangakis C, Ismail Z, et al. Effect of citalopram on agitation in Alzheimer disease: the CitAD randomized clinical trial. JAMA. 2014;311:682–691. doi: 10.1001/jama.2014.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.US FDA. FDA drug safety communication: abnormal heart rhythms associated with high doses of Celexa (citalopram hydrobromide). 2018. http://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-abnormal-heart-rhythms-associated-high-doses-celexa-citalopram. Accessed 20 Aug 2019.
- 105.McCarrell JL, Bailey TA, Duncan NA, Covington LP, Clifford KM, Hall RG, et al. A review of citalopram dose restrictions in the treatment of neuropsychiatric disorders in older adults. Ment Health Clin. 2019;9:280–286. doi: 10.9740/mhc.2019.07.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hasnain M, Howland RH, Vieweg WVR. Escitalopram and QTc prolongation. J Psychiatry Neurosci. 2013;38:E11. doi: 10.1503/jpn.130055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sanchez C, Reines EH, Montgomery SA. A comparative review of escitalopram, paroxetine, and sertraline: are they all alike? Int Clin Psychopharmacol. 2014;29:185–196. doi: 10.1097/YIC.0000000000000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Qirjazi E, McArthur E, Nash DM, Dixon SN, Weir MA, Vasudev A, et al. Risk of ventricular arrhythmia with citalopram and escitalopram: a population-based study. PloS One. 2016;11. [DOI] [PMC free article] [PubMed]
- 109.ClinicalTrials.gov. Escitalopram treatment for BPSD in Alzheimer’s disease in comparison to risperidone. https://clinicaltrials.gov/ct2/show/NCT01119638. Accessed 20 Aug 2019.
- 110.Barak Y, Plopski I, Tadger S, Paleacu D. Escitalopram versus risperidone for the treatment of behavioral and psychotic symptoms associated with Alzheimer’s disease: a randomized double-blind pilot study. Int Psychogeriatr. 2011;23:1515–1519. doi: 10.1017/S1041610211000743. [DOI] [PubMed] [Google Scholar]
- 111.Berry AS, Shah VD, Baker SL, Vogel JW, O’Neil JP, Janabi M, et al. Aging affects dopaminergic neural mechanisms of cognitive flexibility. J Neurosci. 2016;36:12559–12569. doi: 10.1523/JNEUROSCI.0626-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Berry AS, Shah VD, Baker SL, et al. Aging affects dopaminergic neural mechanisms of cognitive flexibility. J Neurosci. 2016;36(50):12559–12569. doi: 10.1523/JNEUROSCI.0626-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.US FDA. Novel drug approvals for 2015. http://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2015. Accessed 27 Aug 2019.
- 114.Lundbeck H. Otsuka and Lundbeck announce improvement of agitation symptoms related to Alzheimer’s-type dementia following treatment with brexpiprazole relative to placebo. 2017. https://investor.lundbeck.com/news-releases/news-release-details/otsuka-and-lundbeck-announce-improvement-agitation-symptoms/
- 115.Das S, Barmwal P, Winston AB, Mondal S, Saha I. Brexpiprazole: so far so good. 2016;6(1):39–54. [DOI] [PMC free article] [PubMed]
- 116.ClinicalTrials.gov. A 12-week extension trial to evaluate the safety and tolerability of brexpiprazole in the treatment of subjects with agitation associated with dementia of the Alzheimer’s type. https://clinicaltrials.gov/ct2/show/NCT03594123. Accessed 24 Aug 2019.
- 117.Davis R, Dmitrienko A, Glass S, Kozauer S, Saillard J, Weingart M, et al. F46. Lumateperone (ITI-007): favorable safety profile in an open label safety switching study from standard-of-care antispychotic therapy in patients with schizophrenia. Schizophr Bull. 2018;44:S236–7.
- 118.Davis RE, Vanover KE, Zhou Y, Brašić JR, Guevara M, Bisuna B, et al. ITI-007 demonstrates brain occupancy at serotonin 5-HT2A and dopamine D2 receptors and serotonin transporters using positron emission tomography in healthy volunteers. Psychopharmacology. 2015;232:2863–2872. doi: 10.1007/s00213-015-3922-1. [DOI] [PubMed] [Google Scholar]
- 119.Intra-Cellular Therapies Inc. Intra-Cellular Therapies announces update on ITI-007-201 clinical trial for treatment of agitation in patients with probable Alzheimer’s disease. https://ir.intracellulartherapies.com/news-releases/news-release-details/intra-cellular-therapies-announces-update-iti-007-201-clinical. Accessed 24 Aug 2019.
- 120.Tzavara ET, Bymaster FP, Davis RJ, Wade MR, Perry KW, Wess J, et al. M4 muscarinic receptors regulate the dynamics of cholinergic and dopaminergic neurotransmission: relevance to the pathophysiology and treatment of related CNS pathologies. FASEB J. 2004;18:1410–1412. doi: 10.1096/fj.04-1575fje. [DOI] [PubMed] [Google Scholar]
- 121.Kamei H, Kameyama T, Nabeshima T. (+)-SKF-10,047 and dextromethorphan ameliorate conditioned fear stress via dopaminergic systems linked to phenytoin-regulated sigma 1 sites. Eur J Pharmacol. 1996;309:149–158. doi: 10.1016/0014-2999(96)00346-9. [DOI] [PubMed] [Google Scholar]
- 122.Nguyen L, Robson MJ, Healy JR, Scandinaro AL, Matsumoto RR. Involvement of sigma-1 receptors in the antidepressant-like effects of dextromethorphan. PLoS One. 2014;9:89985. doi: 10.1371/journal.pone.0089985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shin E-J, Bach J-H, Lee SY, Kim JM, Lee J, Hong J-S, et al. Neuropsychotoxic and neuroprotective potentials of dextromethorphan and its analogs. J Pharmacol Sci. 2011;116:137–148. doi: 10.1254/jphs.11R02CR. [DOI] [PubMed] [Google Scholar]
- 124.Taylor CP, Traynelis SF, Siffert J, Pope LE, Matsumoto RR. Pharmacology of dextromethorphan: Relevance to dextromethorphan/quinidine (Nuedexta®) clinical use. Pharmacol Ther. 2016;164:170–182. doi: 10.1016/j.pharmthera.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 125.Morris H, Wallach J. From PCP to MXE: a comprehensive review of the non-medical use of dissociative drugs. Drug Test Anal. 2014;6(7–8):614–632. doi: 10.1002/dta.1620. [DOI] [PubMed] [Google Scholar]
- 126.Garay RP, Grossberg GT. AVP-786 for the treatment of agitation in dementia of the Alzheimer’s type. Expert Opin Investig Drugs. 2017;26:121–132. doi: 10.1080/13543784.2017.1267726. [DOI] [PubMed] [Google Scholar]
- 127.Drugs@FDA. FDA-approved drugs. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&varApplNo=021879. Accessed 16 Dec 2019.
- 128.Cummings JL, Lyketsos CG, Peskind ER, Porsteinsson AP, Mintzer JE, Scharre DW, et al. Effect of dextromethorphan-quinidine on agitation in patients with Alzheimer disease dementia: a randomized clinical trial. JAMA. 2015;314:1242–1254. doi: 10.1001/jama.2015.10214. [DOI] [PubMed] [Google Scholar]
- 129.Avanir Pharmaceuticals Inc. Avanir Pharmaceuticals announces accelerated development path for AVP-786 following successful pre-IND meeting with FDA. https://www.avanir.com/press/avanir-pharmaceuticals-announces-accelerated-development-path-avp-786-following-successful. Accessed 16 Dec 2019.
- 130.Dwoskin LP, Rauhut AS, King-Pospisil KA, Bardo MT. Review of the pharmacology and clinical profile of bupropion, an antidepressant and tobacco use cessation agent. CNS Drug Rev. 2006;12:178–207. doi: 10.1111/j.1527-3458.2006.00178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Axsome Therapeutics. About AXS-05. https://axsome.com/axs-pipeline/about-axs-05/. Accessed 16 Dec 2019.
- 132.Javitt DC. Glutamate and schizophrenia: phencyclidine, N-methyl-d-aspartate receptors, and dopamine-glutamate interactions. Int Rev Neurobiol. 2007;78:69–108. doi: 10.1016/S0074-7742(06)78003-5. [DOI] [PubMed] [Google Scholar]
- 133.Mehta MA, Schmechtig A, Kotoula V, McColm J, Jackson K, Brittain C, et al. Group II metabotropic glutamate receptor agonist prodrugs LY2979165 and LY2140023 attenuate the functional imaging response to ketamine in healthy subjects. Psychopharmacology. 2018;235:1875–1886. doi: 10.1007/s00213-018-4877-9. [DOI] [PubMed] [Google Scholar]
- 134.Kinon BJ, Millen BA, Zhang L, McKinzie DL. Exploratory analysis for a targeted patient population responsive to the metabotropic glutamate 2/3 receptor agonist pomaglumetad methionil in schizophrenia. Biol Psychiatry. 2015;78:754–762. doi: 10.1016/j.biopsych.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 135.Mechri A, Saoud M, Khiari G, d’Amato T, Dalery J, Gaha L. Glutaminergic hypothesis of schizophrenia: clinical research studies with ketamine. L’Encephale. 2001;27:53–59. [PubMed] [Google Scholar]
- 136.Felder CC, Schober DA, Tu Y, Quets A, Xiao H, Watt M, et al. Translational pharmacology of the metabotropic glutamate 2 receptor-preferring agonist LY2812223 in the animal and human brain. J Pharmacol Exp Ther. 2017;361:190–197. doi: 10.1124/jpet.116.237859. [DOI] [PubMed] [Google Scholar]
- 137.McColm J, Brittain C, Suriyapperuma S, Swanson S, Tauscher-Wisniewski S, Foster J, et al. Evaluation of single and multiple doses of a novel mGlu2 agonist, a potential antipsychotic therapy, in healthy subjects. Br J Clin Pharmacol. 2017;83:1654–1667. doi: 10.1111/bcp.13252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Célanire S, Duvey G, Poli S, Rocher J-P. mGluR2 activators and mGluR5 blockers advancing in the clinic for major CNS disorders. Annu Rep Med Chem. 2012;47:71–88. [Google Scholar]
- 139.TVM Life Science Ventures VII announces investment in Mediti Pharma Inc. for the development of a novel treatment for Alzheimer’s disease psychosis. 2016. https://www.tvm-lifescience.com/tvm-life-science-ventures-vii-announces-investment-in-mediti-pharma-inc-for-the-development-of-a-novel-treatment-for-alzheimers-disease-psychosis/. Accessed 16 Dec 2019.
- 140.Burnet P, Eastwood S, Bristow G, Godlewska B, Sikka P, Walker M, et al. D-amino acid oxidase (DAO) activity and expression are increased in schizophrenia. Mol Psychiatry. 2008;13:658–660. doi: 10.1038/mp.2008.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhou Q, Sheng M. NMDA receptors in nervous system diseases. Neuropharmacology. 2013;74:69–75. doi: 10.1016/j.neuropharm.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 142.Sershen H, Hashim A, Dunlop DS, Seckow RF, Cooper TB, et al. Modulating NMDA receptor function with D-amino acid oxidase inhibitors: understanding functional activity in PCP-treated mouse model. Neurochem Res. 2016;41(1–2):398–408. doi: 10.1007/s11064-016-1838-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lin C-H, Chen P-K, Chang Y-C, Chuo L-J, Chen Y-S, Tsai GE, et al. Benzoate, a D-amino acid oxidase inhibitor, for the treatment of early-phase Alzheimer disease: a randomized, double-blind, placebo-controlled trial. Biol Psychiatry. 2014;75:678–685. doi: 10.1016/j.biopsych.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 144.Chen Q, Xiao EY, Goldman AL, Bharadwaj R, Healy K, et al. Poster session III Wednesday, December 9, 2015. Neuropsychopharmacology. 2015;40(1):443–611. [Google Scholar]
- 145.Pipeline—SyneuRx. http://www.syneurx.com/en/pipeline/. Accessed 16 Dec 2019.
- 146.Volpicelli LA, Levey AI. Muscarinic acetylcholine receptor subtypes in cerebral cortex and hippocampus. Prog Brain Res. 2004;145:59–66. doi: 10.1016/S0079-6123(03)45003-6. [DOI] [PubMed] [Google Scholar]
- 147.Leanza G, Gulino R, Zorec R. Noradrenergic hypothesis linking neurodegeneration-based cognitive decline and astroglia. Front Mol Neurosci. 2018;11:254. doi: 10.3389/fnmol.2018.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Szot P, White SS, Greenup JL, Leverenz JB, Peskind ER, Raskind MA. Changes in adrenoreceptors in the prefrontal cortex of subjects with dementia: evidence of compensatory changes. Neuroscience. 2007;146:471–480. doi: 10.1016/j.neuroscience.2007.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sharp SI, Ballard CG, Chen CPL-H, Francis PT. Aggressive behavior and neuroleptic medication are associated with increased number of alpha1-adrenoceptors in patients with Alzheimer disease. Am J Geriatr Psychiatry. 2007;15:435–7. [DOI] [PubMed]
- 150.Szot P, White SS, Greenup JL, Leverenz JB, Peskind ER, Raskind MA. Compensatory changes in the noradrenergic nervous system in the locus ceruleus and hippocampus of postmortem subjects with Alzheimer’s disease and dementia with Lewy bodies. J Neurosci. 2006;26:467–478. doi: 10.1523/JNEUROSCI.4265-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wang LY, Shofer JB, Rohde K, Hart KL, Hoff DJ, McFall YH, et al. Prazosin for the treatment of behavioral symptoms in patients with Alzheimer disease with agitation and aggression. Am J Geriatr Psychiatry. 2009;17:744–751. doi: 10.1097/JGP.0b013e3181ab8c61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Greve MJ, DesJarlais D, Ahmed I. Successful treatment of agitation and aggression with prazosin in an elderly patient with dementia and comorbid heart disease. J Clin Gerontol Geriatr. 2016;7:109–111. doi: 10.1016/j.jcgg.2015.06.001. [DOI] [Google Scholar]
- 153.van Zwieten PA. Antihypertensive drugs interacting with alpha- and beta-adrenoceptors: a review of basic pharmacology. Drugs. 1988;35(Suppl. 6):6–19. doi: 10.2165/00003495-198800356-00003. [DOI] [PubMed] [Google Scholar]
- 154.The Binding Database. Compounds in cluster. http://www.bindingdb.org/bind/searchby_monomerids.jsp?monomerids=29568,50063906,50403647,50403649,50408679,50411350,50411351,50411352,50411353,50411354&title=10+similar+compounds+to+monomer+50122826. Accessed 24 Aug 2019.
- 155.Jones SB, Smith JM, Jones AW, Bylund DB. Alpha-1 adrenergic receptor binding in aortas from rat and dog: comparison of [3H]prazosin and beta-iodo-[125I]-4-hydroxyphenyl-ethyl-aminomethyl-tetralone. J Pharmacol Exp Ther. 1987;241:875–881. [PubMed] [Google Scholar]
- 156.Liu CS, Chau SA, Ruthirakuhan M, Lanctôt KL, Herrmann N. Cannabinoids for the treatment of agitation and aggression in Alzheimer’s disease. CNS Drugs. 2015;29:615–623. doi: 10.1007/s40263-015-0270-y. [DOI] [PubMed] [Google Scholar]
- 157.Rossi S, Motta C, Musella A, Centonze D. The interplay between inflammatory cytokines and the endocannabinoid system in the regulation of synaptic transmission. Neuropharmacology. 2015;96:105–112. doi: 10.1016/j.neuropharm.2014.09.022. [DOI] [PubMed] [Google Scholar]
- 158.Bedse G, Romano A, Lavecchia AM, Cassano T, Gaetani S. The role of endocannabinoid signaling in the molecular mechanisms of neurodegeneration in Alzheimer’s disease. J Alzheimers Dis. 2015;43:1115–1136. doi: 10.3233/JAD-141635. [DOI] [PubMed] [Google Scholar]
- 159.Aso E, Juvés S, Maldonado R, Ferrer I. CB2 cannabinoid receptor agonist ameliorates Alzheimer-like phenotype in AβPP/PS1 mice. J Alzheimers Dis. 2013;35:847–858. doi: 10.3233/JAD-130137. [DOI] [PubMed] [Google Scholar]
- 160.Cassano T, Calcagnini S, Pace L, De Marco F, Romano A, Gaetani S. Cannabinoid receptor 2 signaling in neurodegenerative disorders: from pathogenesis to a promising therapeutic target. Front Neurosci. 2017;11:30. doi: 10.3389/fnins.2017.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Vilela FC, Giusti-Paiva A. Cannabinoid receptor agonist disrupts behavioral and neuroendocrine responses during lactation. Behav Brain Res. 2014;263:190–197. doi: 10.1016/j.bbr.2014.01.037. [DOI] [PubMed] [Google Scholar]
- 162.Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153:199–215. doi: 10.1038/sj.bjp.0707442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Van den Elsen GAH, Ahmed AIA, Verkes R-J, Kramers C, Feuth T, et al. Tetrahydrocannabinol for neuropsychiatric symptoms in dementia. Neurology. 2015;84(23):2338–2346. doi: 10.1212/WNL.0000000000001675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.van den Elsen GAH, Ahmed AIA, Verkes R-J, Feuth T, van der Marck MA, Olde Rikkert MGM. Tetrahydrocannabinol in behavioral disturbances in dementia: a crossover randomized controlled trial. Am J Geriatr Psychiatry. 2015;23:1214–1224. doi: 10.1016/j.jagp.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 165.Woodward MR, Harper DG, Stolyar A, Forester BP, Ellison JM. Dronabinol for the treatment of agitation and aggressive behavior in acutely hospitalized severely demented patients with noncognitive behavioral symptoms. Am J Geriatr Psychiatry. 2014;22:415–419. doi: 10.1016/j.jagp.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 166.Volicer L, Stelly M, Morris J, McLaughlin J, Volicer BJ. Effects of dronabinol on anorexia and disturbed behavior in patients with Alzheimer’s disease. Int J Geriatr Psychiatry. 1997;12:913–919. doi: 10.1002/(SICI)1099-1166(199709)12:9<913::AID-GPS663>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 167.Walther S, Mahlberg R, Eichmann U, Kunz D. Delta-9-tetrahydrocannabinol for nighttime agitation in severe dementia. Psychopharmacology. 2006;185(4):524–528. doi: 10.1007/s00213-006-0343-1. [DOI] [PubMed] [Google Scholar]
- 168.Mahlberg R, Walther S. Actigraphy in agitated patients with dementia: monitoring treatment outcomes. Z Gerontol Geriatr. 2007;40:178–184. doi: 10.1007/s00391-007-0420-z. [DOI] [PubMed] [Google Scholar]
- 169.Walther S, Schüpbach B, Seifritz E, Homan P, Strik W. Randomized, controlled crossover trial of dronabinol, 2.5 mg, for agitation in 2 patients with dementia. J Clin Psychopharmacol. 2011;31:256–8. [DOI] [PubMed]
- 170.Nikas SP, Alapafuja SO, Papanastasiou I, Paronis CA, Shukla VG. Novel 1′,1′-chain substituted hexahydrocannabinols: 9β-hydroxy-3-(1-hexyl-cyclobut-1-yl)-hexahydrocannabinol (AM2389) a highly potent cannabinoid receptor 1 (CB1) agonist. J Med Chem. 2010;52(19):6996–7010. doi: 10.1021/jm100641g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Amanullah S, MacDougall K, Sweeney N, Coffin J, Cole J. Synthetic cannabinoids in dementia with agitation: case studies and literature review. Clin Neuropsychiatry. 2013;10:142–147. [Google Scholar]
- 172.Kogan NM, Mechoulam R. Cannabinoids in health and disease. Dialog Clin Neurosci. 2007;9:413–430. doi: 10.31887/DCNS.2007.9.4/nkogan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Blytt KM, Bjorvatn B, Husebo B, Flo E. Clinically significant discrepancies between sleep problems assessed by standard clinical tools and actigraphy. BMC Geriatr. 2017;17:253. doi: 10.1186/s12877-017-0653-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Cho HJ, Lavretsky H, Olmstead R, Levin MJ, Oxman MN, Irwin MR. Sleep disturbance and depression recurrence in community-dwelling older adults: a prospective study. Am J Psychiatry. 2008;165:1543–1550. doi: 10.1176/appi.ajp.2008.07121882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Cohen-Mansfield J, Garfinkel D, Lipson S. Melatonin for treatment of sundowning in elderly persons with dementia: a preliminary study. Arch Gerontol Geriatr. 2000;31:65–76. doi: 10.1016/S0167-4943(00)00068-6. [DOI] [PubMed] [Google Scholar]
- 176.Cakir S, Kulaksizoglu IB. The efficacy of mirtazapine in agitated patients with Alzheimer’s disease: a 12-week open-label pilot study. Neuropsychiatr Dis Treat. 2008;4:963–966. doi: 10.2147/NDT.S3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Dolder CR, Nelson MH, Iler CA. The effects of mirtazapine on sleep in patients with major depressive disorder. Ann Clin Psychiatry. 2012;24:215–224. [PubMed] [Google Scholar]
- 178.Schmid DA, Wichniak A, Uhr M, Ising M, Brunner H, Held K, et al. Changes of sleep architecture, spectral composition of sleep EEG, the nocturnal secretion of cortisol, ACTH, GH, prolactin, melatonin, ghrelin, and leptin, and the DEX-CRH test in depressed patients during treatment with mirtazapine. Neuropsychopharmacology. 2006;31:832–844. doi: 10.1038/sj.npp.1300923. [DOI] [PubMed] [Google Scholar]
- 179.Anttila SA, Leinonen EV. A review of the pharmacological and clinical profile of mirtazapine. CNS Drug Rev. 2001;7:249–264. doi: 10.1111/j.1527-3458.2001.tb00198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Van der Mey M, Windhorst AD, Klok RP, Herscheid JDM, Kennis LE, Bischoff F, et al. Synthesis and biodistribution of [11C]R107474, a new radiolabeled alpha2-adrenoceptor antagonist. Bioorg Med Chem. 2006;14:4526–4534. doi: 10.1016/j.bmc.2006.02.029. [DOI] [PubMed] [Google Scholar]
- 181.Buskova J, Busek P, Nevsimalova S. Gabapentin in the treatment of dementia-associated nocturnal agitation. Med Sci Monit. 2011;17:CS149–51. [DOI] [PMC free article] [PubMed]
- 182.Reddy DS. An enigmatic role of tonic inhibition in gabapentin therapy. EBioMedicine. 2019;42:14–15. doi: 10.1016/j.ebiom.2019.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Dolphin AC. Voltage-gated calcium channels and their auxiliary subunits: physiology and pathophysiology and pharmacology. J Physiol. 2016;594:5369–5390. doi: 10.1113/JP272262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Taylor CP. Emerging perspectives on the mechanism of action of gabapentin. Neurology. 1994;44(6):10–16. [PubMed] [Google Scholar]
- 185.Chouinard G, Beauclair L, Belanger MC. Gabapentin: long-term antianxiety and hypnotic effects in psychiatric patients with comorbid anxiety-related disorders. Can J Psychiatry. 1998;43(3):305. doi: 10.1177/070674379804300315. [DOI] [PubMed] [Google Scholar]
- 186.Sammaritano M, Sherwin A. Effect of anticonvulsants on sleep. Neurology. 2000;54(5):16–24. [PubMed] [Google Scholar]
- 187.Roane DM, Deinberg TE, Meckler L, Miner CR, Scicutella A. Treatment of dementia-associated agitation with gabapentin. J Neuropsychiatry Clin Neurosci. 2000;12(1):40–43. doi: 10.1176/jnp.12.1.40. [DOI] [PubMed] [Google Scholar]
- 188.Kim Y, Wilkins KM, Tampl RR. Use of gabapentin in the treatment of behavioural and psychological symptoms of dementia. Drugs Aging. 2008;25(3):187–196. doi: 10.2165/00002512-200825030-00002. [DOI] [PubMed] [Google Scholar]
- 189.Rochon PA, Vozoris N, Gill SS. The harms of benzodiazepines for patients with dementia. CMAJ. 2017;189:517–518. doi: 10.1503/cmaj.170193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zvejniece L, Vavers E, Svalbe B, Veinberg G, Rizhanova K, Liepins V, et al. R-phenibut binds to the α2-δ subunit of voltage-dependent calcium channels and exerts gabapentin-like anti-nociceptive effects. Pharmacol Biochem Behav. 2015;137:23–29. doi: 10.1016/j.pbb.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 191.ClinicalTrials.gov. Citalopram for agitation in Alzheimer’s disease. https://clinicaltrials.gov/ct2/show/NCT00898807. Accessed 16 Dec 2019.
- 192.ClinicalTrials.gov. Safety and tolerability study of two fixed-doses of brexpiprazole in the treatment of subjects with agitation associated with dementia of the Alzheimer’s type. .https://clinicaltrials.gov/ct2/show/NCT01862640. Accessed 16 Dec 2019.
- 193.ClinicalTrials.gov. Safety and tolerability study of flexible dosing of brexpiprazole in the treatment of subjects with agitation associated with dementia of the Alzheimer’s type. https://clinicaltrials.gov/ct2/show/NCT01922258?term=NCT01922258&draw=2&rank=1. Accessed 16 Dec 2019.
- 194.ClinicalTrials.gov. Delta-THC in dementia. https://clinicaltrials.gov/ct2/show/NCT01608217. Accessed 16 Dec 2019.
- 195.ClinicalTrials.gov. Delta-THC in behavioral disturbances in dementia. https://clinicaltrials.gov/ct2/show/NCT01302340. Accessed 16 Dec 2019.
- 196.ClinicalTrials.gov. Efficacy, safety and tolerability study of AVP-923 (dextromethorphan/quinidine) for treatment of symptoms of agitation in Alzheimer’s patients. https://clinicaltrials.gov/ct2/show/NCT01584440. Accessed 16 Dec 2019.
- 197.Morphy R, Rankovic Z. Designed multiple ligands: an emerging drug discovery paradigm. J Med Chem. 2005;48:6523–6543. doi: 10.1021/jm058225d. [DOI] [PubMed] [Google Scholar]
- 198.Morphy R, Kay C, Rankovic Z. From magic bullets to designed multiple ligands. Drug Discov Today. 2004;9(15):641–651. doi: 10.1016/S1359-6446(04)03163-0. [DOI] [PubMed] [Google Scholar]
- 199.Kołaczkowski M, Marcinkowska M, Bucki A, Pawłowski M, Mitka K, Jaśkowska J, et al. Novel arylsulfonamide derivatives with 5-HT6/5-HT7 receptor antagonism targeting behavioral and psychological symptoms of dementia. J Med Chem. 2014;57:4543–4557. doi: 10.1021/jm401895u. [DOI] [PubMed] [Google Scholar]
- 200.Bucki A, Marcinkowska M, Śniecikowska J, Więckowski K, Pawłowski M, Głuch-Lutwin M, et al. Novel 3-(1,2,3,6-tetrahydropyridin-4-yl)-1H-indole-based multifunctional ligands with antipsychotic-like, mood-modulating, and procognitive activity. J Med Chem. 2017;60:7483–7501. doi: 10.1021/acs.jmedchem.7b00839. [DOI] [PubMed] [Google Scholar]
- 201.Kołaczkowski M, Marcinkowska M, Bucki A, Śniecikowska J, Pawłowski M, Kazek G, et al. Novel 5-HT6 receptor antagonists/D2 receptor partial agonists targeting behavioral and psychological symptoms of dementia. Eur J Med Chem. 2015;92:221–235. doi: 10.1016/j.ejmech.2014.12.045. [DOI] [PubMed] [Google Scholar]
- 202.Kołaczkowski M, Marcinkowska M, Bucki A, Pawłowski M, Krukowski A, Rusiecki R, et al. Sulphonamide derivatives of alicyclic amines for the treatment of central nervous system diseases. Patent WO2013001505, 2013.
- 203.Kołaczkowski M, Marcinkowska M, Bucki A, Pawłowski M, Kazek G, Bednarski M, et al. Indoleamine derivatives for the treatment of central nervous system diseases. Patent WO2013001499, 2013.
- 204.Foster DJ, Choi DL, Conn PJ, Rook JM. Activation of M1 and M4 muscarinic receptors as potential treatments for Alzheimer’s disease and schizophrenia. Neuropsychiatr Dis Treat. 2014;10:183–191. doi: 10.2147/NDT.S55104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Bubser M, Bridges TM, Dencker D, Gould RW, Grannan M, Noetzel MJ, et al. Selective activation of M4 muscarinic acetylcholine receptors reverses MK-801-induced behavioral impairments and enhances associative learning in rodents. ACS Chem Neurosci. 2014;5:920–942. doi: 10.1021/cn500128b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Lebois EP, Schroeder JP, Esparza TJ, Bridges TM, Lindsley CW, et al. Disease-modifying effects of M1 muscarinic acetylcholine receptor activation in an Alzheimer’s disease mouse model. ACS Chem Neurosci. 2017;8(6):1177–1187. doi: 10.1021/acschemneuro.6b00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Partnered Programs—Sosei Heptares. https://soseiheptares.com/our-pipeline/rd-pipeline/partnered-programs.html. Accessed 16 Dec 2019.
- 208.Rösler M. The efficacy of cholinesterase inhibitors in treating the behavioural symptoms of dementia. Int J Clin Pract Suppl. 2002;127:20–36. [PubMed] [Google Scholar]
- 209.Weinreb O, Amit T, Bar-Am O, Youdim MB. A novel anti-Alzheimer’s disease drug, ladostigil neuroprotective, multimodal brain-selective monoamine oxidase and cholinesterase inhibitor. Int Rev Neurobiol. 2011;100:191–215. doi: 10.1016/B978-0-12-386467-3.00010-8. [DOI] [PubMed] [Google Scholar]
- 210.Weinreb O, Amit T, Bar-Am O, Youdim MBH. Ladostigil: a novel multimodal neuroprotective drug with cholinesterase and brain-selective monoamine oxidase inhibitory activities for Alzheimer’s disease treatment. Curr Drug Targets. 2012;13:483–494. doi: 10.2174/138945012799499794. [DOI] [PubMed] [Google Scholar]
- 211.Weinstock M, Poltyrev T, Bejar C, Youdim MB. Effect of TV3326, a novel monoamine-oxidase cholinesterase inhibitor, in rat models of anxiety and depression. Psychopharmacology. 2002;160(3):318–324. doi: 10.1007/s00213-001-0978-x. [DOI] [PubMed] [Google Scholar]
- 212.Calhoun A, Ko J, Grossberg GT. Emerging chemical therapies targeting 5-hydroxytryptamine in the treatment of Alzheimer’s disease. Expert Opin Emerg Drugs. 2017;22:101–105. doi: 10.1080/14728214.2017.1293651. [DOI] [PubMed] [Google Scholar]
- 213.R&D Department. Adamed. https://adamed.com.pl/en/rd-department. Accessed 16 Dec 2019.
- 214.Wu C-S, Wang S-C, Gau SS-F, Tsai H-J, Cheng Y-C. Association of stroke with the receptor-binding profiles of antipsychotics-a case-crossover study. Biol Psychiatry. 2013;73:414–21. [DOI] [PubMed]
- 215.Douglas IJ, Smeeth L. Exposure to antipsychotics and risk of stroke: self controlled case series study. BMJ. 2008;337:1227. doi: 10.1136/bmj.a1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Paśko P, Rodacki T, Domagała-Rodacka R, Palimonka K, Marcinkowska M, Owczarek D. Second generation H1—antihistamines interaction with food and alcohol: a systematic review. Biomed Pharmacother. 2017;93:27–39. doi: 10.1016/j.biopha.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 217.Aftab A, Shah AA. Behavioral emergencies: special considerations in the geriatric psychiatric patient. Psychiatr Clin North Am. 2017;40:449–462. doi: 10.1016/j.psc.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 218.Pfister B, Jonsson J, Gustafsson M. Drug-related problems and medication reviews among old people with dementia. BMC Pharmacol Toxicol. 2017;18:52. doi: 10.1186/s40360-017-0157-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Nachimuthu S, Assar MD, Schussler JM. Drug-induced QT interval prolongation: mechanisms and clinical management. Ther Adv Drug Saf. 2012;3:241–253. doi: 10.1177/2042098612454283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Fenichel RR, Malik M, Antzelevitch C, Sanguinetti M, Roden DM, Priori SG, et al. Drug-induced torsades de pointes and implications for drug development. J Cardiovasc Electrophysiol. 2004;15:475–495. doi: 10.1046/j.1540-8167.2004.03534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Üçok A, Gaebel W. Side effects of atypical antipsychotics: a brief overview. World Psychiatry. 2008;7:58–62. doi: 10.1002/j.2051-5545.2008.tb00154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Garay RP, Citrome L, Grossberg GT, Cavero I, Llorca P-M. Investigational drugs for treating agitation in persons with dementia. Expert Opin Investig Drugs. 2016;25:973–983. doi: 10.1080/13543784.2016.1193155. [DOI] [PubMed] [Google Scholar]
- 223.Magierski R, Sobow T. Serotonergic drugs for the treatment of neuropsychiatric symptoms in dementia. Expert Rev Neurother. 2016;16:375–387. doi: 10.1586/14737175.2016.1155453. [DOI] [PubMed] [Google Scholar]