Abstract
Introduction
Proapoptotic peptide, (KLAKLAK)2, exhibits strong anti-tumor effect with the help of cell-penetrating peptides such as Pep2, targeting TLR2 with high expression in acute myeloid leukemia (AML). However, the applications are limited due to the peptide’s instability and high cost of synthesis. Recombinant PP7 bacteriophage-like particles (VLPs) can protect the peptides from degradation by proteases, based on their ability to display foreign peptides.
Methods
Here, we evaluated the feasibility of PP7 VLPs carrying Pep2 and (KLAKLAK)2 (2PP7-Pep2-KLAK VLPs) expressed in E. coli. We further investigated the characteristics including size, toxicity, thermal stability, penetrating ability, anti-tumor activity, and potential anti-tumor mechanism of 2PP7-Pep2-KLAK VLPs.
Results
2PP7-Pep2-KLAK VLPs was expressed in E. coli BL21(DE3) successfully with high yield and thermal stability. They penetrated the AML cells THP-1 rapidly after 30 min of incubation. Moreover, 2PP7-Pep2-KLAK VLPs were non-replicative, non-infectious, and non-toxic against normal cells, but inhibited the proliferation of THP-1 cells by inducing cell apoptosis after 24 h of exposure. This effect extends through 120 h of exposure, indicating their anti-proliferation effect was superior to that of synthetic peptides. In addition to the mitochondrial apoptotic pathway, the anti-tumor activity of 2PP7-Pep2-KLAK VLPs was also correlated with down-regulation of expression of enhancer of zeste homolog 2 (EZH2) and trimethylation of histone H3K27.
Conclusions
We identified the feasibility to prepare the stable, active Pep2-KLAK peptide by using PP7 bacteriophage as the vehicle. We revealed this peptide was an inhibitor of EZH2. 2PP7-Pep2-KLAK VLPs may have significant clinical implications in the treatment of MLL-AF9 AML as an epigenetic modulator.
Electronic supplementary material
The online version of this article (10.1007/s12195-019-00605-z) contains supplementary material, which is available to authorized users.
Keywords: Acute myeloid leukemia, Proapoptotic peptide, Toll-like receptor 2 (TLR2), PP7 bacteriophage, Virus-like particle (VLP), Enhancer of zeste homolog 2 (EZH2)
Introduction
Acute myeloid leukemia (AML) is caused by malignant proliferation of bone marrow hematopoietic stem cells. Its morbidity and mortality are estimated to account for approximately 35 and 49% of the total leukemia cases in America.21 With recent advancements in research, numerous notable peptides have shown efficacy in AML treatment,8,10,13 for example, a Wilms’ Tumor Gene 1 (WT1) 126–134 Peptide, which had completed the clinical phase 2 trial and demonstrated the potential clinical efficacy in AML patients.8 They overcome the side effects of chemotherapy such as immunosuppression, hepatic and renal toxicity, and the shortcomings of limited donor sources for hematopoietic stem cell transplantation, to become one of the leading treatments for AML.
Proapoptotic peptide was discovered in 1996 as an antimicrobial peptide.7 It is an L-type peptide, composed of L(KLAKLAK)2 and has less toxicity to normal cells. In view of the poor stability of L-type polypeptide, Ellerby et al. replaced it with D-type peptide in 1999, that is, D(KLAKLAK)2, which was used in the field of cancer treatment and showed strong anti-tumor effect in Kaposi sarcoma and breast cancer.5 In 2005, Marks et al. applied it to the treatment of AML, and discovered the peptides were less effective against fresh AML cells.11 This may be ascribed to low delivery efficiency of proapoptotic peptide mediated by CD33 monoclonal antibody in AML cells. Thus, a more effective vehicle for the delivery of proapoptotic peptide in AML cells was required.
Some studies have shown that in AML patients, the expression level of Toll-like receptor 2 (TLR2) was higher than chronic myeloid leukemia, acute lymphoid leukemia, and chronic lymphoid leukemia patients, in addition to healthy individuals.10,16,18,19 We deduced it may be plausible to deliver proapoptotic peptide through TLR2 to the surface of AML cells. A TLR2-targeting cell-penetrating peptide, Pep2,10 was chosen to perform the high-effective delivery of proapoptotic peptide.
For peptides, high cost of synthesis and rapid degradation by intracellular or extracellular proteases limit their clinical application.17 The ability of bacteriophage PP7 virus-like particles (VLPs) to display peptides on their surface solves these problems, and greatly improves the effectiveness of displayed peptides.14
PP7 bacteriophage is a RNA bacteriophage consisting of 90 capsid protein dimers. Previous studies showed that the second capsid protein AB ring or N-terminal position of PP7 capsid protein single-chain dimer had the ability to display peptide.25 Thus, 90 peptides can be displayed on the surface of one PP7 VLP rationally, which greatly improves the stability and validity of peptide. However, the peptides presented by PP7 VLPs are L-type peptides, whether their activity mirrors D-type peptide has not been determined. In addition, if the AML targeting peptide Pep2 and proapoptotic peptide are displayed on the surface of PP7 VLPs simultaneously, the function of Pep2 peptide to assist proapoptotic peptide entering TLR2+ cells has not been formally investigated.
In this study, the cDNA of Pep2 and proapoptotic peptides (Pep2-KLAK peptide) linked by one linker, was inserted into the coding gene of PP7 coat protein dimer, and expressed in E. coli strain BL21 (DE3). The expression level, assembly capability, and size of PP7 VLPs carrying the Pep2-KLAK peptide (2PP7-Pep2-KLAK VLPs) were analyzed, and the cell-penetrating ability, cytotoxicity, and anti-tumor effect of the inserted Pep2-KLAK peptide in AML cells THP-1 were investigated. We analyzed the results and studied the potential mechanism of its anti-tumor effect.
Materials and Methods
Bacterial Strains and Cell Lines
Escherichia coli strains TOP10 and BL21 (DE3) (Tiangen, Beijing, China) were cultured in LB medium and used for constructed vector amplification and protein expression. AML cell line THP-1 and KG-1 was purchased from DSMZ (Brauschweig, Germany), and cultured in RPMI1640 medium with 10% fetal bovine serum (Gibco, USA). All experiments were performed in logarithmic phase of cell growth.
Construction of pETDuet-2PP7-Pep2 and pETDuet-2PP7-Pep2-KLAK Plasmids
The plasmid pETDuet-2PP7 containing the single-chain dimer gene of PP7 phage coat protein with one Kpn I site was constructed as described before.23 The plasmid pETDuet-2PP7-Pep2-KLAK was constructed based on the plasmid pETDuet-2PP7. Briefly, the genes containing Pep2 or Pep2-KLAK peptides were amplified by PCR using primers 2PP7-Pep2-for and PP7-r or 2PP7-Pep2-KLAK-for and PP7-r (Supplementary Table S1), respectively. The correct constructs were verified by DNA sequencing analysis.
Preparation of 2PP7-Pep2 VLPs and 2PP7-Pep2-KLAK VLPs
The plasmids mentioned above were isolated from 3-mL overnight cultures of single colony using a TIANpure Mini Plasmid Kit and transformed into the competent BL21 (DE3) cells. Transformed cells were cultured in 5 ml of LB medium supplemented with ampicillin (50 mg/L) for 8 h. The pre-cultures were inoculated to 500 mL of LB medium supplemented with ampicillin. When these cultures were grown to an OD600 of approximately 0.6 at 37 °C, IPTG was added with a final concentration of 1 mM and the cultures were incubated overnight at 37 °C. The cells were harvested by centrifugation at 12,000 rpm for 1 min at 4 °C, resuspended in a final volume of 20 mL TBS (10 mM Tris–HCl, 100 mM NaCl, 1 mM MgCl2, 0.1 mM EDTA, pH 7. 4), and disrupted by sonication (JP96-II, Shanghai, China) on ice for 200 times (on for 3 s, off for 6 s). The homogenate was centrifuged at 12,000 rpm, 4 °C for 30 min. The supernatant was collected, and 20 mL chloroform was added and mixed with the solution. The mixture was centrifuged at 12,000 rpm, 4 °C for 15 min, and the supernatant was collected. The nanoparticles in the supernatant were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on 12% gels. Then the supernatant was incubated with DNase I (20 U/mL) and RNase A (10 mg/mL) at 37 °C for 3 h, and purified by gel filtration chromatography (BioLogic DuoFlow QuadTec system, Bio-Rad, USA).
Transmission Electron Microscopy (TEM)
The recombinant PP7 virus-like particles (VLPs) were adsorbed on carbon-coated glow-discharged copper grids for 2 min, and were negatively stained with 2% uranyl acetate for 2 min. The VLPs were visualized using a Hitachi H7500 (Japan) transmission electron microscope (60 kV, ×100,000).
Determination of Particle Size and Size Distribution
The VLPs dissolved in TBS were used for the calculation of size and zeta potential by Malvern particle size analyzer (Zetasizer Nano ZS90, Malvern Inc., Worcestershire, UK). Before loading the sample, the solution containing dispersed nanoparticles was filtered through a 0.45 μm filter.
Thermal Stability Assay
The thermal stabilities of 2PP7-Pep2-KLAK VLPs were determined by measuring the fraction of protein remaining soluble after 2 min at 20, 37, 42, 49, 54, 59, 64, 70, 75, 80, 85, 90, 95, 100 °C.2 The amount of protein in the soluble fraction was determined by Bicinchoninic acid (BCA) assay.1 The values shown are the averages of three independent measurements.
Cytotoxicity of 2PP7-Pep2-KLAK VLPs to Human Peripheral Blood Mononuclear Cells (PBMCs)
Human PBMCs (5 × 103/well) separated from blood specimens from healthy donors were inoculated to a 96-well plate, and incubated with various concentrations (0, 2, 4, 8, 16, 32, 64, 128, 256 nM) of 2PP7-Pep2-KLAK VLPs for 24 h or 48 h in triplicate. 10 μL CCK (cell counting kit)-8 solution (Dojindo, Mashikimachi, Kumamoto, Japan) was carefully added to each well 4 h before detection. The absorbance of each well was measured at 450 nm with an ELISA plate reader (Multiskan Go, Thermo, USA).
Cell Penetrating Assay
The VLPs were labeled with FITC (Sigma, USA) according to the manufacture’s instruction. The purified FITC-labeled VLPs were added to 1 × 106 THP-1 and KG-1 cells per well with a final concentration of 50 nM, respectively. Unlabeled 2PP7-Pep2-KLAK VLPs, labeled 2PP7-Pep2 VLPs and 2PP7-KLAK VLPs were used as negative control. After incubation for 0, 0.5, 1, 2 h, respectively, the cells were washed three times with PBS to remove the VLPs-containing medium. Intracellular distribution of the VLPs was monitored under 200 (10 × 20) magnification with a fluorescence microscope with an excitation filter BG12 and an absorption filter OG4. Besides, the FITC fluorescence intensity in THP-1 or KG-1 cells after 2 h incubation was detected by flow cytometry (Beckman-Coulter CytoFLEX S, USA).
CCK-8 Assay
THP-1 cells (1 × 104/well) were inoculated in a 96-well plate, and incubated with various concentrations of 2PP7-Pep2 VLPs or 2PP7-Pep2-KLAK VLPs (0, 10, 20, 40, 80, 160, 200, 250 nM) for 48 h in triplicate, or with 80 nM 2PP7-Pep2-KLAK VLPs or 10 μM Pep2-KLAK peptide for 0, 24, 48, 72, 96, 120 h, respectively. 10 μL CCK-8 was carefully added to each well 4 h before detection. The absorbance of each well was measured at 450 nm with an ELISA plate reader.
Annexin-V-FITC/PI Apoptosis Assay
THP-1 cells (1 × 106/well) were inoculated in a 12-well plate, and incubated with 80 nM 2PP7-Pep2 VLPs or 2PP7-Pep2-KLAK VLPs for 48 h in triplicate. Following that, the cells was harvested and stained with annexin-V-FITC/PI apoptosis detection kit (BD Bioscience, USA). The fluorescence intensity of the cells was detected by flow cytometry and rate of apoptotic cells was determined.
Real-Time Quantitative PCR (RT-qPCR)
THP-1 cells were cultured in a six-well plate at 5 × 106 cells/well. 2PP7-Pep2-KLAK VLPs or 2PP7-Pep2 VLPs (80 nM) were then added to each well and incubated for 48 h. Total RNA was extracted from the harvested cells using TRIzol reagent (Invitrogen, USA). Reverse transcription was performed using PrimeScript™ RT Reagent Kit with gDNA Eraser (TaKaRa, Dalian, China). EZH2 expression levels were quantified by real-time PCR with the TB Green Premix Ex Taq™ II using an Applied Biosystems 7500 Fast instrument. All reactions were run in triplicate. GAPDH was used as an internal control. The relative expression levels of the mRNAs were calculated with the 2−ΔΔCt method20 and the differences in mRNA concentrations between the treated and control groups were expressed as fold changes.
Western Blot Analysis
The purified VLPs was transferred from 12% SDS-PAGE gels onto a 0.45 µm polyvinylidene fluoride (PVDF) membrane. These membranes were incubated with anti-PP7 coat protein (prepared using wild-type PP7 phage coat protein as the immunogen by our lab, 1:2000 dilution) overnight at 4 °C and with HRP-labeled goat anti-mouse secondary antibody at 37 °C for 1 h,followed by ECL detection. The specific protein band was visualized using the Amersham Imager 600 (GE, USA).
Proteins were extracted from the THP-1 cells at 48 h incubation with both VLPs. Proteins (40 mg) were fractioned by SDS-PAGE and the target proteins were analyzed using the anti-EZH2 (ab186006), GAPDH (ab9485, Abcam), H3 (D1H2) or H3K27me3 (C36B11, Cell signaling) antibodies. GAPDH was used as an internal control. All experiments were performed in triplicate.
Statistical Analysis
Statistical analyses were performed using GraphPad Prism Software (Version 5.01). Data were considered statistically significant when p < 0.05.
Results
Cloning of 2PP7-Pep2-KLAK in Prokaryotic Expression Vector
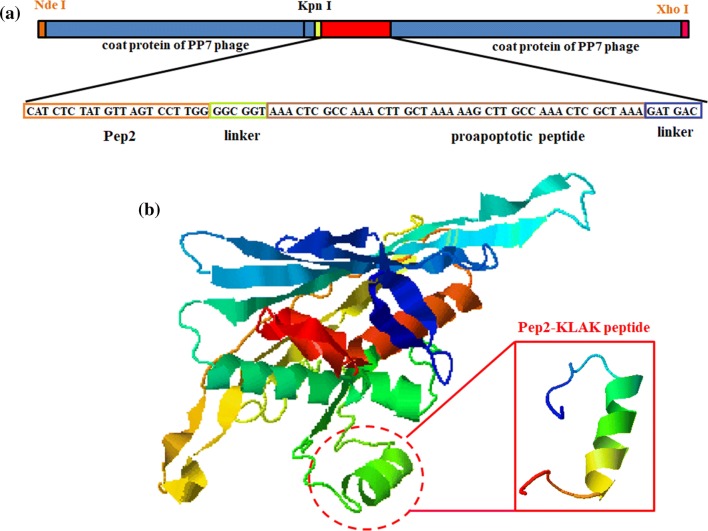
The cDNA of LMWP was inserted between the KpnI and XhoI of pETDuet-2PP7 (Fig. 1a), and the constructed vector was named as pETDuet-2PP7-Pep2-KLAK. The positive clones were screened by colony PCR, and verified by sequencing. The predicted secondary structure of Pep2-KLAK peptide in PP7 coat protein was mainly α-helix and coils, which was identical with the predicted result of the peptide alone (Fig. 1b).
Figure 1.
Construction of the plasmid pETDuet-2PP7-Pep2-KLAK. (a) The inserted site of Pep2-KLAK peptide in the pETDuet-2PP7 and its DNA sequence. (b) The second structure of 2PP7-Pep2-KLAK protein and Pep2-KLAK peptide predicted using I-TASSER. The Pep2-KLAK peptide in the PP7 coat protein dimer was marked by red circle.
Expression and Purification of the Recombinant VLPs
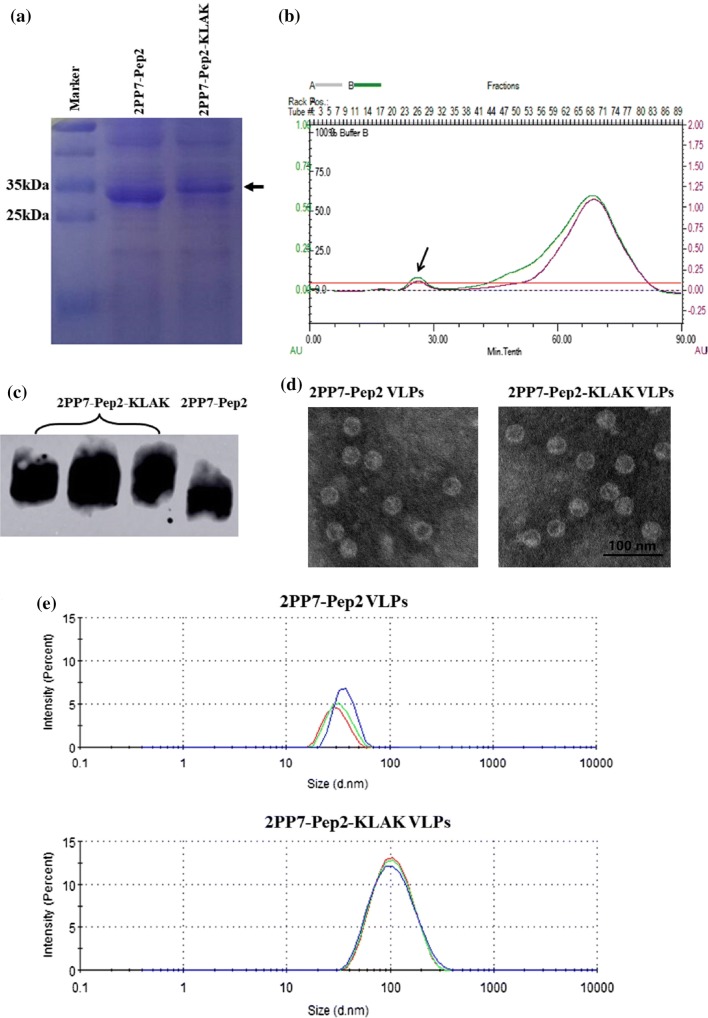
The recombinant 2PP7-Pep2-KLAK VLPs was expressed intracellularly from the plasmids pETDuet-2PP7-Pep2-KLAK, and represented a dominant protein species in the supernatant of bacteria lysis after centrifugation (Fig. 2a). SDS-PAGE results revealed that the molecular weight of single-chain dimer of PP7 phage coat protein carrying Pep2-KLAK peptide was approximately 30 kDa, which was almost identical with the predicted molecular weight (30.85846 kDa, Fig. 2a). The VLPs were then purified by gel filtration chromatography (Fig. 2b). The western blotting results showed the VLPs existed in the first elution peak (Figs. 2b and 2c), and the expression level of the purified 2PP7-Pep2-KLAK VLPs prepared from the supernatant of 1 L of overnight culture after sonication was approximately 3.23 mg/L. These results demonstrated that PP7 VLPs carrying Pep2-KLAK peptide was expressed in E. coli BL21 (DE3) with high yield.
Figure 2.
Identification of PP7 VLPs carrying Pep2-KLAK peptide. (a) Expression of 2PP7-Pep2-KLAK VLPs and 2PP7-Pep2 VLPs in the supernatant of BL21 (DE3) after sonication. The target proteins are marked by black arrows. (b) Purification chromatogram of 2PP7-Pep2-KLAK VLPs by gel filtration chromatography. The peaks of the target proteins are marked by black arrows. (c) Western blot of the pure VLPs. (d) Assembly of the PP7 coat protein dimer carrying Pep2-KLAK peptide into whole VLPs. The VLPs was observed using TEM at 60 kV and ×100,000 screen magnification. (e) Size analysis of VLPs by particle size analyzer.
Identification of the Recombinant 2PP7-Pep2-KLAK VLPs by WB, TEM and Particle Size Analyzer
In order to identify the purified protein 2PP7-Pep2-KLAK VLPs, the murine antibody of anti-coat protein of PP7 phage was used in the WB. The results showed only one specific band appeared in every lane, which demonstrated that the PP7 coat protein dimer carrying Pep2-KLAK peptide could be detected by this antibody (Fig. 2c). It was also verified by TEM whether the PP7 coat protein dimer carrying Pep2-KLAK peptide could assemble into the whole VLPs. TEM results indicated that this coat protein dimer could form the whole VLPs with the diameter of about 100 nm (Fig. 2d), which was larger than that of wild-type PP7 VLPs (about 30 nm)23 and 2PP7-Pep2 (about 35 nm, Fig. 2d). Moreover, this result was confirmed by particle size analyzer (Fig. 2e).
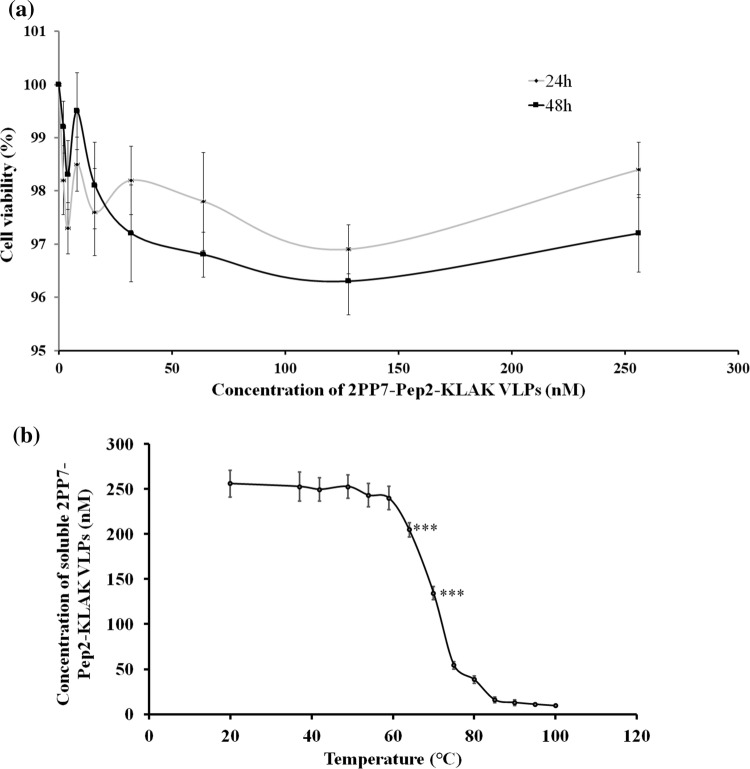
Cytotoxicity of 2PP7-Pep2-KLAK VLPs for Human PBMCs and Their Thermal Stability
The cytotoxicity of 2PP7-Pep2-KLAK VLPs in human PBMCs was measured by CCK-8 assay. The results showed non dose-dependent cytotoxicity in the VLPs (Fig. 3a). Even after exposure of 256 nm for 48 h, cell viability was higher than 95%, demonstrating low cytotoxicity of recombinant 2PP7-Pep2-KLAK VLPs for human PBMCs.
Figure 3.
Cytotoxicity and thermal stability of 2PP7-Pep2-KLAK VLPs. (a) Cytotoxicity of 2PP7-Pep2-KLAK VLPs. Human PBMCs (5 × 103/well) were incubated with various concentrations (0, 2, 4, 8, 16, 32, 64, 128, 256 nM) of 2PP7-Pep2-KLAK VLPs for 24 h or 48 h in triplicate. The cytotoxicity was expressed as cell viability. (b) Thermal stability of 2PP7-Pep2-KLAK VLPs at 20, 37, 42, 49, 54, 59, 64, 70, 75, 80, 85, 90, 95, 100 °C. ***p < 0.001.
Additionally, the thermal stability of the 2PP7-Pep2-KLAK VLPs was determined. The results showed that the concentrations of 2PP7-Pep2-KLAK VLPs decreased significantly at above 64 °C (p < 0.05, Fig. 3b), indicating that they resisted heating up to about 64 °C. The stability of these VLPs is similar to the other VLPs reported in our previous articles.23
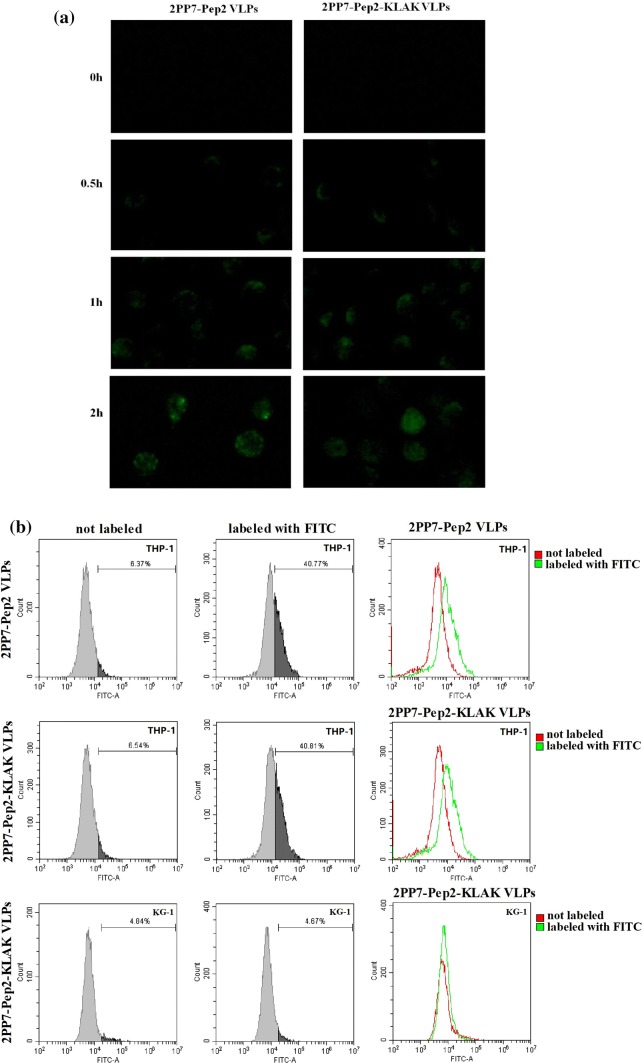
Cell-Penetrating Ability of 2PP7-Pep2-KLAK VLPs
To explore whether recombinant PP7 VLPs carrying Pep2-KLAK peptide can penetrate cells, the 2PP7-Pep2-KLAK VLPs labeled with FITC were incubated with human acute myeloid leukemia cell line THP-1. After 30 min, THP-1 cells incubated with 2PP7-Pep2 VLPs or 2PP7-Pep2-KLAK VLPs exhibited a weak level of fluorescence (Fig. 4a). As the incubation time increased, the fluorescence intensity in THP-1 cells strengthened. At 2 h, the fluorescence from the groups of 2PP7-Pep2 VLPs or 2PP7-Pep2-KLAK VLPs labeled with FITC was found almost everywhere in the cells (Fig. 4), whereas no fluorescence occurred in the groups of 2PP7 VLPs or 2PP7-KLAK VLPs labeled with FITC, or unlabeled 2PP7-Pep2-KLAK VLPs (data not shown), which may be attributed to the Pep2 peptide rapidly binding to the TLR2 on the surface of THP-1 cells. This result further proved that the Pep2-KLAK peptide could be expressed on the surface of 2PP7-Pep2-KLAK VLPs and retained strong cell-penetrating ability.
Figure 4.
Cell-penetrating ability of 2PP7-Pep2 VLPs and 2PP7-Pep2-KLAK VLPs in THP-1 cells. (a) Strong cell-penetrating ability of both VLPs was observed in THP-1 cells by the fluorescence microscope. Pictures were taken under fluorescence (×400). (b) The cell-penetrating ability of both VLPs in THP-1 cells was evaluated by flow cytometry.
Additionally, we tested the expression of TLR2 in leukemia cell lines K562, MV4;11, KASUMI-1, HL-60, MOLM-13, KG1 and THP-1. As shown in the Fig. S1, the expression of TLR2 in THP1 cells was the highest, and that in KG1 cells was negative. Following that, we compared the fluorescence intensity between THP-1 (TLR2high) and KG-1 (TLR2negative) cells after the 2PP7-Pep2-KLAK VLPs labeled with FITC was added to these cells for 2 h. The results showed no fluorescence occurred in KG-1 cells (Fig. 4b). Furthermore, the strong cell-penetrating ability of the PP7 VLPs may enhance in vivo delivery efficiency of proapoptotic peptide and lay the foundation for targeted delivery of other drugs in suspension cells with the high expression of TLR2.
Anti-Proliferation Effect of the 2PP7-Pep2-KLAK VLPs on Human AML Cells
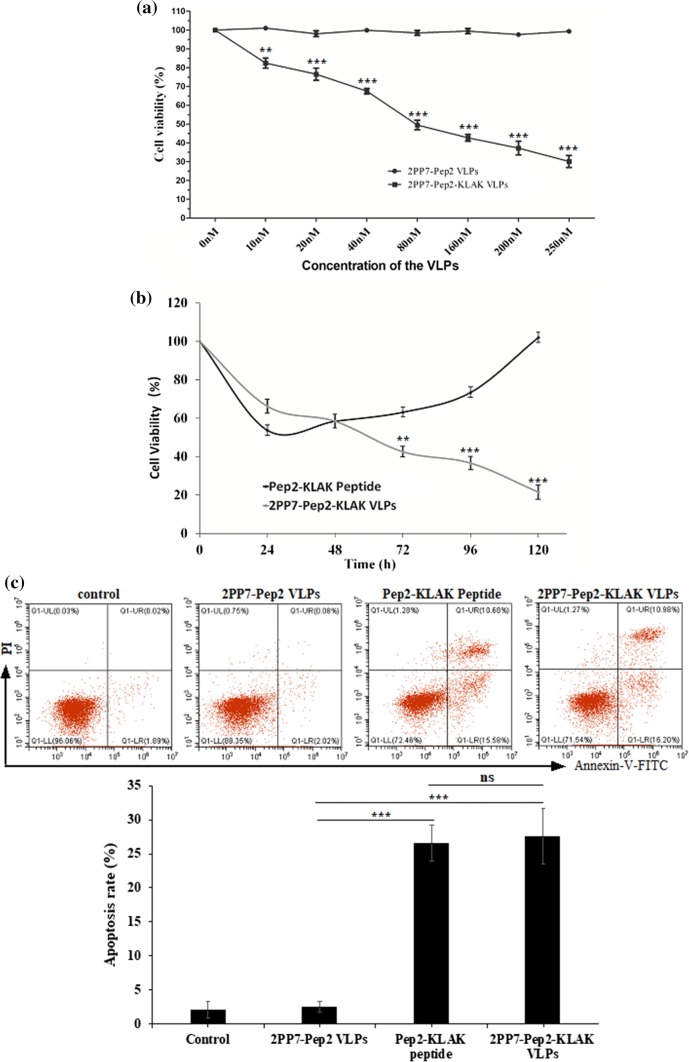
To determine the effect of 2PP7-Pep2-KLAK VLPs on human AML cells, THP-1 cells were exposed to various concentrations of the 2PP7-Pep2-KLAK VLPs for 48 h. As shown in Fig. 5a, the 2PP7-Pep2-KLAK VLPs, with the concentration ranging from 10 to 250 nM, decreased cell viability in a dose-dependent manner. Moreover, the cell viability treated with the 2PP7-Pep2-KLAK VLPs was even lower than 50% at the concentration of 80 nM. The IC50 value of the 2PP7-Pep2-KLAK VLPs is 83.2 nM. In contrast, no obvious cell viability decreased in THP-1 cells as the concentration of the 2PP7-Pep2 VLPs increased.
Figure 5.
Anti-proliferation effect of 2PP7-Pep2-KLAK VLPs in THP-1 cells. (a) Cytotoxicity of 2PP7-Pep2-KLAK VLPs in THP-1 cells in different doses (0, 10, 20, 40, 80, 160, 200, 250 nM). (b) Cytotoxicity of 2PP7-Pep2-KLAK VLPs in THP-1 cells in different time (0, 24, 48, 72, 96, 120 h). The cytotoxicity was expressed as cell viability. (c) Cell apoptosis effect of the 2PP7-Pep2-KLAK VLPs on THP-1 cells. THP-1 cells were exposed to 80 nM of the Pep2-KLAK peptide, 2PP7-Pep2 VLPs or 2PP7-Pep2-KLAK VLPs for 48 h, stained with FITC-conjugated annexin-V and PI, and quantified by flow cytometric analysis. **p < 0.01; ***p < 0.001.
In addition, the cell viability treated with 80 nM of the Pep2-KLAK peptide decreased after 24 h of exposure significantly, but increased from 48 h (Fig. 5b). This may be attributed to the rapid degradation of this peptide. However, the cytotoxicity of the 2PP7-Pep2-KLAK VLPs (80 nM) was time-dependent, and lasted at least 120 h (Fig. 5b). The results demonstrated the Pep2-KLAK peptide displayed on the surface of the VLPs was more stable and effective than the synthetic peptide.
To determine whether the reduction in cell viability was due to apoptosis, an annexin-V binding assay was performed. Compared with the 2PP7-Pep2 VLPs treatment, 80 nM of the 2PP7-Pep2-KLAK VLPs treatment increased the apoptosis of THP-1 cells significantly, which was similar to the Pep2-KLAK peptide treatment (Fig. 5c). These results indicate that the 2PP7-Pep2-KLAK VLPs-induced reduction in cell viability is largely due to apoptosis.
Effect of 2PP7-Pep2-KLAK VLPs on EZH2 Expression in MLL-AF9 AML Cells
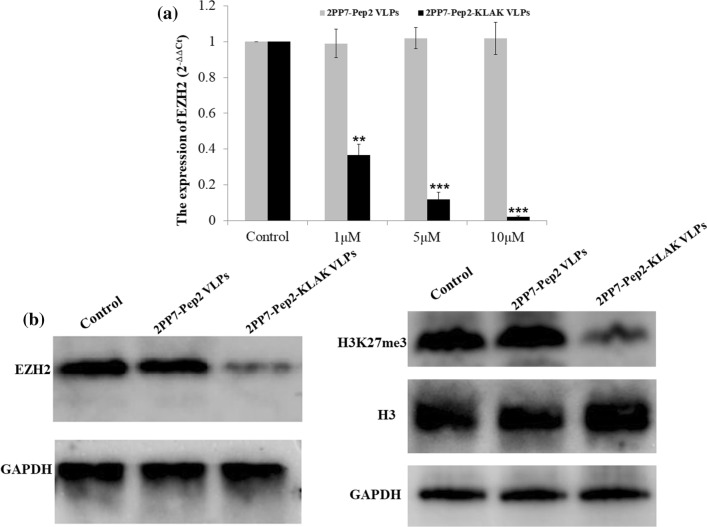
Two previous studies have suggested that the maintenance of MLL-AF9 AML is reliant on EZH2,12,24 a catalytic component of the polycomb repressive complex 2, suggesting EZH2 carries oncogenic function and is therefore a plausible therapeutic target in established AML. One of the known main functions of EHZ2 was to trimethylate histone H3 at lysine 27 (H3K27me3) to repress the transcription of target genes. In this study, MLL-AF9 AML cell line THP-1 was chosen as the model, and the effect of the 2PP7-Pep2-KLAK VLPs on EZH2 in THP-1 cells was evaluated. RT-qPCR and WB results demonstrated that 2PP7-Pep2-KLAK VLPs down-regulated the mRNA and protein levels of EZH2 simultaneously, compared to the control and 2PP7-Pep2 VLPs groups (Fig. 6).
Figure 6.
Effect of 2PP7-Pep2-KLAK VLPs on EZH2 in THP-1 cells. THP-1 cells were exposed to 80 nM of 2PP7-Pep2 VLPs or 2PP7-Pep2-KLAK VLPs. After 48 h, the cells were collected by centrifugation. The mRNA and protein levels of EZH2 were detected by RT-qPCR (a) and WB (b), respectively. Also, the expression of H3K27me3 was quantified by WB (b).
To further verify this result, H3K27me3 level was detected by WB. The results showed 2PP7-Pep2-KLAK VLPs decreased the methylation of H3 at lysine 27 (Fig. 6b), which may be due to the decreased EZH2.
Discussion
Proapoptotic peptide (KLAKLAK)2 has been proven to be effective in the treatment of several cancers including AML.11 However, it cannot penetrate the cell membrane itself, and must rely on other substances to help it enter the cell.11 Among these substances, cell-penetrating peptides, such as TAT peptide, are most commonly used. Though TAT peptide exhibits high effective cell-penetrating ability, it has comparatively poor selectivity and targeting to cells,3 making proapoptotic peptide delivery toxic to normal cells. Therefore, it is paramount for AML treatment to find an AML-targeted cell-penetrating peptide capable of delivering proapoptotic peptide that is highly effective and selective.
Li et al. found that Pep2 is an effective cell penetrating peptide, which can specifically bind to TLR2.10 It was found that TLR2 was highly expressed on the surface of AML cells, which provided an experimental basis for the targeting delivery of proapoptotic peptides to AML cells by Pep2. However, we found that the inhibitory effect of Pep2-KLAK peptide on the proliferation of AML cells disappeared after 48 h incubation with THP-1 cells, which may be closely related with the instability of this peptide. Therefore, PP7 VLPs carrying Pep2-KLAK peptide was prepared based on the ability of PP7 phages to display peptides on their surface, and they were expressed in E. coli with high yield and high thermal stability.
In addition, when 2PP7-Pep2-KLAK VLPs were added to THP-1 cells, they quickly bound to and entered leukemia cells. At 2 h, there has been a large number of VLPs in THP-1 cells, but this phenomenon was not found in AML cell line-KG-1, which had minimal expression of TLR2. At the same time, the results of cytotoxicity test showed that 2PP7-Pep2-KLAK VLPs exhibits more durable inhibition of the proliferation of leukemia cells, for up to 120 h, with no obvious toxicity to PBMCs and KG-1 which almost did not express TLR2. Since Pep2 is a target peptide of TLR2,10 the high expression of TLR2 on the surface of AML cells may be the prerequisite for Pep2-KLAK peptide to enter cells and exert its toxicity.
TLR2, as an innate immune receptor, is highly expressed on activated memory T cells and AML cells.4,9,16,19 The mRNA expression of TLR2 was significantly higher in patients with no response than in AML patients with complete remission and incomplete marrow recovery, and was higher in patients with acute myelomonocytic and monoblastic leukemia, than in patients with other types of AML.19 The expression was significantly lower after chemotherapy as compared to before treatment.16 Eriksson et al found that activating the TLR1/TLR2 complex by the agonist Pam3CSK4 in MLL-AF9-driven human AML resulted in induction of apoptosis by p38 MAPK-dependent activation of caspase-3 and myeloid differentiation in a NF-κB-dependent manner.6 However, our studies found that Pep2 alone did not induce the apoptosis of THP-1 cells, and this may be due to the limited activation of TLR2 and no activation of TLR1, indicating that anti-leukemic effect targeting TLR alone may be not so effective.
Nevertheless, to some degree, the anti-tumor effect of proapoptotic peptide was obviously stronger than Pep2, even though the entry of this peptide into the cells was dependent on the help of Pep2 or other vehicles.18 Previous studies proved that proapoptotic peptide killed cancer cells through disrupting the function of mitochondria.15,22 Here, we investigated whether 2PP7-Pep2-KLAK VLPs had an influence on the expression of EZH2, the maintenance factor of fusion gene MLL-AF9 in THP-1 cells. Surprisingly, they decreased the expression of EZH2 significantly at the transcriptional level, as well as at the translational level. Due to the decreased EZH2, H3K27me3 level was also down-regulated significantly. Following that, which genes they modulated the expression of has been studied.
In short, in this study, we successfully prepared PP7 bacteriophage-like nanoparticles carrying TLR2-targeted peptide Pep2 and proapoptotic peptide with dual activities of targeting and cytotoxicity to acute myeloid leukemia cells. Moreover, 2PP7-Pep2-KLAK VLPs could serve as a potential targeting and effective drug for the therapy of MLL-AF9 AML.
Conclusions
In this study, our work identified the feasibility to prepare the stable, active Pep2-KLAK peptide by using PP7 bacteriophage as the vehicle, and revealed that this peptide was more stable than the synthetic peptide. Moreover, this peptide induced the apoptosis of THP-1 cells carrying MLL-AF9 fusion gene through down-regulating the expression of EZH2. Therefore, 2PP7-Pep2-KLAK VLPs may have significant clinical implications in the treatment of MLL-AF9 AML as many epigenetic modulators are currently in clinical trials.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This work was supported by the grants from the National Natural Science Foundation of China (81570157, 81770915, 81700167), the Project of Shandong Provincial Natural Science Foundation of China (ZR2015HL036, ZR2019BH009) and the Project of Shandong Province Higher Educational Science and Technology Program of China (J14LK14). Zhenbo Hu was sponsored by the Alexander Von Humboldt Foundation, Germany. No human or animal studies were carried out by the authors for this article.
Conflict of Interest
Yanli Sun, Jiaqiu Li, Yanhua Sun, Ronglan Zhao, Lujuan Wang, Wei Song, Zhanzhao Wang, Jialing Wang, Liuya Wei, Yao Zhao, Yang Song and Zhenbo Hu declare that they have no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yanli Sun and Jiaqiu Li have equally contributed to this study.
Contributor Information
Yanli Sun, Email: zbxzhg320@163.com.
Zhenbo Hu, Email: zhenbohux@163.com.
References
- 1.Bainor A, Chang L, McQuade TJ, Webb B, Gestwicki JE. Bicinchoninic acid (BCA) assay in low volume. Anal. Biochem. 2011;410(2):310–312. doi: 10.1016/j.ab.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 2.Caldeira JC, Peabody DS. Thermal stability of RNA phage virus-like particles displaying foreign peptides. J. Nanobiotechnol. 2011;9:22. doi: 10.1186/1477-3155-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen X, Chen J, Fu R, Rao P, Weller R, Bradshaw J, Liu S. Can the cellular internalization of cargo proteins be enhanced by fusing a Tat peptide in the center of proteins? A fluorescence study. J. Pharm. Sci. 2018;107(3):879–886. doi: 10.1016/j.xphs.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Cottalorda A, Mercier BC, Mbitikon-Kobo FM, Arpin C, Teoh DY, McMichael A, Marvel J. TLR2 engagement on memory CD8(+) T cells improves their cytokine-mediated proliferation and IFN-gamma secretion in the absence of Ag. Eur. J. Immunol. 2009;39(10):2673–2681. doi: 10.1002/eji.200939627. [DOI] [PubMed] [Google Scholar]
- 5.Ellerby HM, Arap W, Ellerby LM, Kain R, Andrusiak R, Rio GD, Krajewski S, Lombardo CR, Rao R, Ruoslahti E, Bredesen DE, Pasqualini R. Anti-cancer activity of targeted pro-apoptotic peptides. Nat. Med. 1999;5(9):1032–1038. doi: 10.1038/12469. [DOI] [PubMed] [Google Scholar]
- 6.Eriksson M, Peña-Martínez P, Ramakrishnan R, Chapellier M, Högberg C, Glowacki G. Agonistic targeting of TLR1/TLR2 induces p38 MAPK-dependent apoptosis and NFκB-dependent differentiation of AML cells. Blood Adv. 2017;1(23):2046–2057. doi: 10.1182/bloodadvances.2017006148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Javadpour MM, Juban MM, Lo WC, Bishop SM, Alberty JB, Cowell SM, Becker CL, McLaughlin ML. De novo antimicrobial peptides with low mammalian cell toxicity. J. Med. Chem. 1996;39(16):3107–3113. doi: 10.1021/jm9509410. [DOI] [PubMed] [Google Scholar]
- 8.Keilholz U, Letsch A, Busse A, Asemissen AM, Bauer S, Blau IW, Hofmann WK, Uharek L, Thiel E, Scheibenbogen C. A clinical and immunologic phase 2 trial of Wilms tumor gene product 1 (WT1) peptide vaccination in patients with AML and MDS. Blood. 2009;113(26):6541–6548. doi: 10.1182/blood-2009-02-202598. [DOI] [PubMed] [Google Scholar]
- 9.Komai-Koma M, Jones L, Ogg GS, Xu D, Liew FY. TLR2 is expressed on activated T cells as a costimulatory receptor. Proc. Natl. Acad. Sci. U.S.A. 2004;101(9):3029–3034. doi: 10.1073/pnas.0400171101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li K, Lv XX, Hua F, Lin H, Sun W, Cao WB, Fu XM, Xie J, Yu JJ, Li Z, Liu H, Han MZ, Hu ZW. Targeting acute myeloid leukemia with a proapoptotic peptide conjugated to a Toll-like receptor 2-mediated cell-penetrating peptide. Int. J. Cancer. 2014;134(3):692–702. doi: 10.1002/ijc.28382. [DOI] [PubMed] [Google Scholar]
- 11.Marks AJ, Cooper MS, Anderson RJ, Orchard KH, Hale G, North JM, Ganeshaguru K, Steele AJ, Mehta AB, Lowdell MW, Wickremasinghe RG. Selective apoptotic killing of malignant hemopoietic cells by antibody-targeted delivery of an amphipathic peptide. Cancer Res. 2005;65(6):2373–2377. doi: 10.1158/0008-5472.CAN-04-2594. [DOI] [PubMed] [Google Scholar]
- 12.Neff T, Sinha AU, Kluk MJ, Zhu N, Khattab MH, Stein L, Xie H, Orkin SH, Armstrong SA. Polycomb repressive complex 2 is required for MLL-AF9 leukemia. Proc. Natl Acad. Sci. U.S.A. 2012;109:5028–5033. doi: 10.1073/pnas.1202258109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oka Y, Tsuboi A, Nakata J, Nishida S, Hosen N, Kumanogoh A, Oji Y, Sugiyama H. Wilms’ tumor gene 1 (WT1) peptide vaccine therapy for hematological malignancies: from CTL epitope identification to recent progress in clinical studies including a cure-oriented strategy. Oncol. Res. Treat. 2017;40(11):682–690. doi: 10.1159/000481353. [DOI] [PubMed] [Google Scholar]
- 14.Peabody DS, Manifold-Wheeler B, Medford A, Jordan SK, do Carmo Caldeira J, Chackerian B. Immunogenic display of diverse peptides on virus-like particles of RNA phage MS2. J. Mol. Biol. 2008;380(1):252–263. doi: 10.1016/j.jmb.2008.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qiao ZY, Zhao WJ, Cong Y, Zhang D, Hu Z, Duan ZY, Wang H. Self-assembled ROS-sensitive polymer-peptide therapeutics incorporating built-in reporters for evaluation of treatment efficacy. Biomacromolecule. 2016;17(5):1643–1652. doi: 10.1021/acs.biomac.6b00041. [DOI] [PubMed] [Google Scholar]
- 16.Ramzi M, Khalafi-Nezhad A, Iravani Saadi M, Jowkar Z. Association between TLR2 and TLR4 expression and response to induction therapy in acute myeloid leukemia patients. Int. J. Hematol. 2018;12(4):303–312. [PMC free article] [PubMed] [Google Scholar]
- 17.Reissmann S. Cell penetration: scope and limitations by the application of cell-penetrating peptides. J. Pept. Sci. 2014;20(10):760–784. doi: 10.1002/psc.2672. [DOI] [PubMed] [Google Scholar]
- 18.Rybka J, Butrym A, Wróbel T, Jaźwiec B, Stefanko E, Dobrzyńska O, Poreba R, Kuliczkowski K. The expression of Toll-like receptors and development of severe sepsis in patients with acute myeloid leukemias after induction chemotherapy. Med. Oncol. 2014;31(12):319. doi: 10.1007/s12032-014-0319-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rybka J, Butrym A, Wróbel T, Jaźwiec B, Stefanko E, Dobrzyńska O, Poreba R, Kuliczkowski K. The expression of Toll-like receptors in patients with acute myeloid leukemia treated with induction chemotherapy. Leuk. Res. 2015;39(3):318–322. doi: 10.1016/j.leukres.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008;3(6):1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 21.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J. Clin. 2019;69(1):7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 22.Sun W, Li L, Li LJ, Yang QQ, Zhang ZR, Huang Y. Two birds, one stone: dual targeting of the cancer cell surface and subcellular mitochondria by the galectin-3-binding peptide G3-C12. Acta Pharmacol. Sin. 2017;38(6):806–822. doi: 10.1038/aps.2016.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun YL, Sun YH, Zhao RL, Gao KS. Intracellular delivery of messenger RNA by recombinant PP7 virus-like particles carrying low molecular weight protamine. BMC Biotechnol. 2016;16(1):46. doi: 10.1186/s12896-016-0274-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanaka S, Miyagi S, Sashida G, Chiba T, Yuan J, Mochizuki-Kashio M, Suzuki Y, Sugano S. Ezh2 augments leukemogenicity by reinforcing differentiation blockage in acute myeloid leukemia. Blood. 2012;120(5):1107–1117. doi: 10.1182/blood-2011-11-394932. [DOI] [PubMed] [Google Scholar]
- 25.Tumban E, Peabody J, Tyler M, Peabody DS, Chackerian B. VLPs displaying a single L2 epitope induce broadly cross-neutralizing antibodies against human papillomavirus. PLoS ONE. 2012;7(11):e49751. doi: 10.1371/journal.pone.0049751. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.