Abstract
To investigate whether automated volumetric radiomic analysis of breast cancer vascularization (VAV) can improve survival prediction in primary breast cancer. 314 consecutive patients with primary invasive breast cancer received standard clinical MRI before the initiation of treatment according to international recommendations. Diagnostic work-up, treatment, and follow-up was done at one tertiary care, academic breast-center (outcome: disease specific survival/DSS vs. disease specific death/DSD). The Nottingham Prognostic Index (NPI) was used as the reference method with which to predict survival of breast cancer. Based on the MRI scans, VAV was accomplished by commercially available, FDA-cleared software. DSD served as endpoint. Integration of VAV into the NPI gave NPIVAV. Prediction of DSD by NPIVAV compared to standard NPI alone was investigated (Cox regression, likelihood-test, predictive accuracy: Harrell’s C, Kaplan Meier statistics and corresponding hazard ratios/HR, confidence intervals/CI). DSD occurred in 35 and DSS in 279 patients. Prognostication of the survival outcome by NPI (Harrell’s C = 75.3%) was enhanced by VAV (NPIVAV: Harrell’s C = 81.0%). Most of all, the NPIVAV identified patients with unfavourable outcome more reliably than NPI alone (hazard ratio/HR = 4.5; confidence interval/CI = 2.14-9.58; P = 0.0001). Automated volumetric radiomic analysis of breast cancer vascularization improved survival prediction in primary breast cancer. Most of all, it optimized the identification of patients at higher risk of an unfavorable outcome. Future studies should integrate MRI as a “gate keeper” in the management of breast cancer patients. Such a “gate keeper” could assist in selecting patients benefitting from more advanced diagnostic procedures (genetic profiling etc.) in order to decide whether are a more aggressive therapy (chemotherapy) is warranted.
Subject terms: Prognostic markers, Breast cancer
Introduction
Breast cancer is the most common malignant neoplasm of women in the western world. Despite substantial advances in diagnosis and treatment, morbidity and mortality of breast cancer remain high1. By adjusting the treatment to the individual cancer biology, precision medicine aims to both improve disease survival and to reduce the rate of unnecessary cytotoxic treatment2,3.
To achieve this goal, accurate biomarkers are essential tools for precision medicine. They help us to understand tumor biology and allow estimation of patient outcome4. The Nottingham Prognostic Index (NPI) is a well-established and widely used method for the prediction of survival of primary breast cancer. It is based on three classic biomarkers of breast cancer: tumor size, tumor grade, and the number of metastatic loco-regional lymph nodes5–8.
Breast MRI (MRI) is a functional imaging method that has been validated in clinical practice for over 30 years9. Objective semi-quantitative analysis of MRI by automated, FDA-cleared software has been intensely evaluated over the last decade and has been clinically established for several years10–12. As automated analysis of MRI provides objective measures of pathological tissue vascularization and quantifies tumor response to cytotoxic treatment, it can be considered a typical imaging biomarker4.
In the past several years, the feasibility of MRI as a prognostic biomarker has been demonstrated11–19. Hereby, breast MRI dynamically investigates the cancer in real-time within the whole tumor volume in vivo. This concept offers some advantages over traditional biomarkers which are typically evaluated on a small tumor sample ex vivo. To achieve a high prognostic accuracy, the complex imaging data of breast MRI has to be investigated by a dedicated method. This method of correlating the extensive MRI imaging data with outcome parameters is referred to as radiomic analysis20.
This study aimed to investigate whether automated volumetric radiomic analysis of breast cancer vascularization (VAV) can improve survival prediction in primary breast cancer.
Methods
Patients
This investigation was designed as a retrospective observational single-center study. Data were collected at an academic, tertiary care institution run by interdisciplinary breast cancer specialists from the departments of gynaecology, oncology, pathology, radiation oncology, and radiology. This interdisciplinary breast center is certified according to national quality management criteria. The local ethical review board approved this study and waived the necessity for informed consent.
The following inclusion criteria were applied
Patients were recruited over a consecutive time period
Indication for MRI: Pre-treatment staging of lesions rated as category IV and V according to the Breast Imaging Reporting and Data System (BI-RADS) upon mammography and/or ultrasound
Histologically verified diagnosis of invasive breast cancer
Treatment and follow-up at our center.
No neoadjuvant chemotherapy performed.
In case of recurrent or in situ breast cancer and known malignant neoplasms other than breast cancer, patients were excluded due to potential bias on MRI enhancement results or on outcome data. In addition to image-based analyses, data on demographic characteristics (sex, age, date of diagnosis), clinicopathological features, and individual patient outcome were collected.
Patient outcome
The end point was defined according to the “Standardized Definitions for Efficacy End Points in Adjuvant Breast Cancer Trials” document21. Disease specific death (DSD) was used as the primary end point. The latter is an established variant to “overall survival”21,22. For DSD only, “death from breast cancer” is considered as “event”. So different from OS, cases with either “death from non-breast cancer cause” or “death from unknown cause” were not considered, but were censored. The remaining patients were defined as showing “disease specific survival” (DSS).
The analysis of patient outcome was based on routine follow-up at our center after completion of treatment. Survival time was documented as the interval [months] between the initial diagnosis and the last follow-up. The minimum follow-up time after treatment was 27 months for inclusion into the DSS group. Classification of patient outcome was established by the medical record of the last follow-up. In order to achieve valid results upon survival analysis, a mean survival time of at least 80 months was specified23.
Clinicopathological features
Board-certified pathologists investigated the clinicopathological features, including standard measures of tumor size and disease extension (T1-T3, T4a–T4d). The number of resected lymph nodes was documented and further classified as N0–N324. Histological subtypes were specified as proposed by the World Health Organization Classification of Tumours24. Tumor aggressiveness was classified as Grade 1 to 3 according to25. Further details on clinicopathological features, including those not relevant for the NPI, are provided within the supplementary material.
Percentage of tumor cells expressing steroid receptors (estrogen/progesterone receptors: ER/PR) was evaluated (cut-off 1% of positive cells)24,26. The expression of the HER2 (Human epidermal growth factor receptor) was analyzed as specified in27. Ki-67 was not generally recommended at date of study conception, and so it was not included in the present analysis. Further details on the clinicopathological features are provided within the supplementary material.
Magnetic resonance imaging
For data acquisition, we used standard MRI protocols, with an overall examination time of 14 minutes. To achieve homogenous enhancement data, every patient was imaged with the same imaging protocol using two 1.5 Tesla whole-body magnetic resonance imaging scanners (Magnetom Symphony; Siemens Healthineers, Erlangen, Germany) and a dedicated, vendor-supplied, bilateral, four-channel breast coil.
This imaging protocol was in accordance with international recommendations28: For the present analysis, dynamic T1-weighted gradient-echo images only were investigated, with 60 seconds temporal resolution and an examination time of eight minutes (fast low angle shot/FLASH; flip angle 80°, repetition time 113 ms, echo time 5 ms, voxel size: 1.1 × 0.9, slice thickness 3 mm). This dynamic sequence was acquired before and after intravenous bolus injection (automatic injector: Spectris, Medrad, Pittsburgh, USA) of gadopentetate dimeglumine (Bayer HealthCare, Leverkusen, Germany) at a dosage of 0.1 mmol/kg body weight followed by 20 ml saline solution. A delay of 30 seconds after contrast medium application was set before post-contrast images were acquired under identical tuning conditions for a total of 7 measurements. Further technical details of the MRI protocol are provided within the supplementary material.
Volumetric analysis of breast cancer vascularization (VAV)
Raw data of MRI were analyzed by automated, FDA-approved, commercially available software (BreVis, Siemens Healthineers, Erlangen, Germany). The software was operated by a reader blinded to clinicopathological features and patient outcome. The reader received special training in handling the software and was supervised by two radiologists with at least 7 years of experience in breast MRI. This kind of software has been scientifically evaluated since 2005 in multiple institutions, and has been in clinical use for several years. A detailed description can be found in the literature10–12.
The only user-dependent step during analysis of MRI was defining the volume of interest. This was achieved by encircling a rectangular cuboid around the tumor (see Fig. 1)29. Based on our experience with the given protocol over many years, the minimum initial enhancement threshold was set to 30% (change of signal intensity first minute after contrast relative to baseline in [%]). As a consequence, perifocal tissue or necrotic tumor components were automatically excluded from further analysis. In case of multiple tumors per breast, the largest one was defined as index lesion.
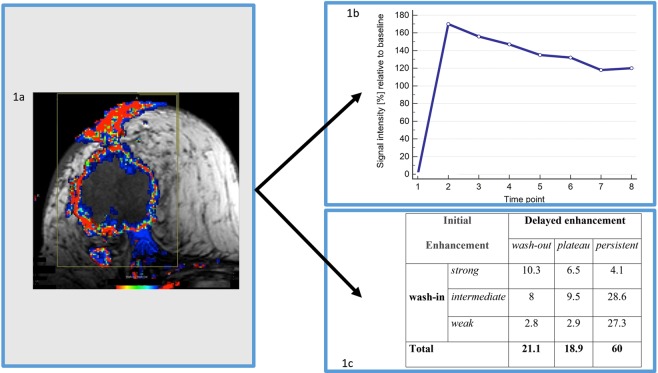
Figure 1.
Clinical case illustrating the assessment of VAV. MRI of a 51 year old female patient (invasive ductal cancer, G2, diameter 5.8 cm, T4c, HER2 negative, hormonal receptor negative, 5 positive lymph nodes, NPI = 6.2). (A) Heat map of the color-coded CAD overlay of the T1 pre-contrast scan reflecting heterogeneity of vascularization. The colors are coded from red to blue and reflect the wash-out ratio (see main text for definition). A large heterogeneously enhancing mass with infiltration of skin and thoracic wall and central necrosis is visualized, consistent with T4c. Note the discrepancy between the vital tumor assessed by MRI (color coded parts. TTV) and the larger morphologic extensions including the central necrosis. (B) Vascularization of the most suspect tumor compartment. A strong wash-in (170%) followed by a significant wash-out (50%) is demonstrated. The time to peak enhancement (TTP) was one minute. (C) Heterogeneity of vascularization summarized in a 3×3 matrix. There was a wide range of vascularisation patterns present within the same tumor. A relevant proportion of the TTV (10.3%) exhibited a pattern typical of hypervascularization and vascular shunting (category I: strong wash-in and wash-out). Nevertheless, the majority of the tumor demonstrated patterns indicative of a less pronounced neovascularization (weak or intermediate wash-in and persistent: 55.9% of the TTV). Both the NPI and the VAV were suggestive of a poor outcome. This patient died from breast cancer 18 months after initial diagnosis.
Based on established dynamic enhancement patters29, the vascularization pattern of every voxel within the tumor was characterized. In total, the software automatically extracted 14 quantitative and semi-quantitative radiomic features for the VAV. These parameters assessed three aspects of tumor vascularization:
-
I.
Total enhancing tumor volume (TTV) characterized the amount of vital cancer tissue and was measured as [cm3]. It was defined as the sum of tumor voxels surpassing the initial enhancement threshold (see above). Accordingly, the TTV addressed only the vital cancer tissue and did not consider areas of necrosis. Therefore, the TTV has also been called “functional tumor volume” previously (Table 1, parameter #1)18.
-
II.Heterogeneity of vascularization (within the TTV). Breast cancer is a heterogeneous disease and various vascularization patterns can be present in the same cancer in parallel. Accordingly, a wide range of enhancement patterns can be observed within the TTV16. This aspect was investigated by the heterogeneity of vascularization. Hereby, every voxel of the tumor was investigated as follows:
- Initially, the enhancement patterns within each voxel were identified following international breast MRI standards29,30. The initial enhancement phase investigates the first minute after contrast application. It was categorized as weak (30% to 50%), intermediate (50% to 100%), or strong “wash-in” (>100%), with percentages expressing the change of signal intensity during the first minute after contrast media application, relative to baseline29,30.
- The delayed enhancement phase investigates the change of contrast enhancement between the first and the last minute after contrast media application. It was categorized as persistent (>10%), plateau (+/−10%), or wash-out (−10%). Percentages express the change of signal intensity during the last minute after contrast media application, relative to the first postcontrast scan29,30.
- Combining the initial phase (n = 3) and delayed phase categories (n = 3) yielded nine enhancement patterns to classify the vascularization of each voxel within the TTV (for instance as “weak wash-in” and “persistent”, etc.).
- Finally, the tumor volume was subdivided according to these nine enhancement patterns. Hereby, the proportion relative to the TTV [%] was identified for every enhancement pattern (Table 1, parameters #2–10).
-
III.
Vascularization of the most suspect tumor compartment. In this analysis only one particular compartment of the entire tumor volume was investigated. For this reason, the TTV was divided into clusters (size: 3×3 voxels). Within every cluster, the ratio of the corresponding initial versus delayed enhancement was computed (wash-out ratio). According to the literature, the cluster achieving the highest wash-out ratio was defined as the “most suspicious tumor compartment”11.
Table 1.
Summary of parameters used for MRI analysis.
| Parameter | Unit | Definition | |||
|---|---|---|---|---|---|
| 1 | Total tumor volume (TTV) | cm3 | Sum of tumor voxels with wash-in > 30%§ | ||
| Heterogeneity of vascularization | wash-in | delayed enhancement | |||
| category: | |||||
| 2 | A | % (of TTV) | weak (30% to 50%) | persistent | |
| 3 | B | plateau | |||
| 4 | C | wash-out | |||
| 5 | D | intermediate (50% to 100%) | persistent | ||
| 6 | E | plateau | |||
| 7 | F | wash-out | |||
| 8 | G | strong (>100%) | persistent | ||
| 9 | H | plateau | |||
| 10 | I | wash-out | |||
| Most suspect tumor compartment | |||||
| parameter: | |||||
| 11 | time to peak enhancement (TTP) | minutes | |||
| 12 | peak enhancement | %§ | maximum contrast uptake by the tumor during the entire dynamic scan | ||
| 13 | wash-in | contrast uptake by the tumor one minute after contrast application | |||
| 14 | wash-out ratio | n.a. | ratio of wash-in and wash-out | ||
Note: The software investigates the signal intensity of every tumor voxel (size 1.1×0.9×3 mm3) during the dynamic MRI scan (one pre- and seven post-contrast scans at one-minute temporal resolution). Thus, the software calculated 11 quantitative (parameters 1 to 11: [cm3] or [minutes]) and three semi-quantitative parameters (parameters 11 to 14). This enabled identification of the total tumor volume (TTV), volumetric analysis of tumor vascularization with a focus on tissue heterogeneity (VA: Parameters 2 to 10), and a detailed investigation of the most suspect tumor compartment (parameters 11 to 14)
Notes: Wash-in: Initial contrast uptake by the tumor during the first minute after contrast application. Values refer to the baseline signal before contrast administration. Delayed enhancement: Change of lesion signal behaviour between the seventh and first minute after contrast application. Three categories are defined: persistent (>10% further signal increase), plateau (stable signal ±10%) and wash-out (>10% signal decrease). §Percentages normalized to baseline signal intensity before application of contrast media.
The “most suspicious tumor compartment” was characterized by the following four radiomic features (Table 1, parameters #11–14): “wash-out ratio” (see above), “wash-in” (signal intensity first minute after contrast relative to baseline [%]), “peak enhancement” (maximum contrast uptake by the tumor during the whole dynamic scan), and time to “peak enhancement” (TTP [minutes]).
A clinical case illustrating the workflow of the volumetric analysis of breast cancer vascularization is given in Fig. 1. Table 1 summarizes all 14 parameters provided by the VAV.
Statistical analysis
Disease specific death (DSD) was defined as the primary study endpoint. Association of clinicopathological features, patient age, NPI, and NPIVAV with survival outcome was explored by cox regression and likelihood ratio tests.
All tests were two-sided. In this exploratory study, an alpha error below 10% was defined as appropriate to reject the null hypothesis. Considering the total number of variables (n = 14), we applied a conventional Bonferroni correction for alpha-error accumulation. Accordingly, the significance level was set to P = 0.007.
NPI
According to7, the NPI was calculated as follows:
Hereby, S is the size of the index lesion in centimeters, N is the nodal status (0 nodes = 1; 1–4 nodes = 2;>4 nodes = 3), and G is the tumor grade (Grade I = 1; Grade II = 2; Grade III = 3).
The association of NPI with survival was investigated by cox regression, and predictive values were saved. The corresponding performance of NPI to predict DSD was validated by the Harrell’s C index. The latter is an extension of the area under the ROC curve for censored survival data31.
VAV
Association of the 14 VAV parameters with the endpoint was analyzed by univariate cox regression.
In addition, multivariate analysis was performed. Only VAV revealing significant association with DSD upon univariate analysis were eligible for this step. Multivariate cox regression with backward feature selection was applied (P for enter/removal: 0.001/0.05). Feature selection removed redundant or irrelevant VAV. Furthermore, feature selection enabled simplification and – by reducing overfitting – generalization of the model. Corresponding predictive values of the multivariate model were saved and investigated by the Harrell’s C index as described for NPI.
NPIVAV
NPIVAV was designed as a compound measure of NPI and VAV. VAV revealing significant association with DSD upon univariate analysis and the NPI were eligible as covariates. In order to construct NPIVAV, multivariate cox regression with backward feature selection was applied as described above. Predictive values were saved. Performance of NPIVAV to predict DSD was investigated as described for NPI (Harrell’s C index).
Comparison of outcome prediction
Finally, predictions of DSD by NPI and VAV were compared. For this purpose, the corresponding predictive values by the cox regressions were compared32.
Kaplan Meier survival analysis was used to graphically illustrate differences between NPIVAV and NPI. Hereby, the cut-off criterion for the prediction of DSD was optimized to the value that maximized Youden’s J statistic on the time-dependent data. For the latter, we used the predictive values of the corresponding cox regression analysis of NPI and NPIVAV33,34.
The differences between the corresponding Kaplan Meier survival curves were explored by hazard ratios (HR), corresponding confidence intervals (CI), and the logrank test23.
Statement of human rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required.
Results
A detailed listing of background clinical data and clinicopathological factors is given in the supplementary material. This also contains a detailed uni- and multivariate analysis of study data including subgroup analysis.
Characteristics of patients and subgroups
There were 314 patients with primary breast cancer who were included (DSS = 279; DSD = 35). Mean survival time was 84.6 months (CI: 81.9–87.2, standard error: 1.3). Range of follow-up interval was 27–93 months for DSS. On average, DSD occurred 27.9 months after initial diagnosis (range: 3–70 months).
Mean patient age was 57.6 years (SD: 11.7). Mean age within the DSS (mean: 57.4 years; SD: 11.7) and the DSD group was similar (mean: 59.6 years; SD: 11.5). Patient age could not be identified as a predictive parameter upon univariate analysis (P = 0.23). Inclusion of patient age into the NPI did not significantly increase prediction of DSD (P = 0.13). The same results were observed when including patient age into the NPIVAV (P = 0.23).
Clinicopathological features
Clinicopathological features showed expected distributions between the DSS and DSD group: There was a significant association with T- and N-Stage versus survival outcome (P < 0.001). Notably, the DSD exhibited larger tumors (e.g., T3: HR = 9.4; P < 0.0001) with a higher proportion of nodal-positive cases (e.g., N2: HR = 3.8; P = 0.0002). There was no significant association with Grading (P = 0.96) and histological subtypes (P > 0.77) on survival outcome. Key results of the clinicopathological features, stratified by the endpoints, are summarized in Table 2.
Table 2.
Summary table of tumor characteristics.
| Parameter | Outcome | Total | ||
|---|---|---|---|---|
| DSS | DSD | |||
| T-Stage | T1a | 26 | 1 | 27 |
| 9.30% | 2.90% | 8.60% | ||
| T1b | 43 | 3 | 46 | |
| 15.40% | 8.60% | 14.60% | ||
| T1c | 117 | 6 | 123 | |
| 41.90% | 17.10% | 39.20% | ||
| T2 | 80 | 17 | 97 | |
| 28.70% | 48.60% | 30.90% | ||
| T3 | 7 | 4 | 11 | |
| 2.50% | 11.40% | 3.50% | ||
| T4 | 6 | 4 | 10 | |
| 2.20% | 11.40% | 3.20% | ||
| Histological subtype | Invasive ductal (not otherwise specified) | 212 | 27 | 239 |
| 76.00% | 77.10% | 76.10% | ||
| Invasive lobular | 23 | 4 | 27 | |
| 8.20% | 11.40% | 8.60% | ||
| Mixed (Invasive lobular and ductal) | 38 | 4 | 42 | |
| 13.60% | 11.40% | 13.40% | ||
| Invasive medullary | 3 | 0 | 3 | |
| 1.10% | 0.00% | 1.00% | ||
| Invasive mucinous | 3 | 0 | 3 | |
| 1.10% | 0.00% | 1.00% | ||
| Histological Grading | G1 | 12 | 0 | 12 |
| 4.30% | 0.00% | 3.80% | ||
| G2 | 108 | 13 | 121 | |
| 38.70% | 37.10% | 38.50% | ||
| G3 | 159 | 22 | 181 | |
| 57.00% | 62.90% | 57.60% | ||
| Total | 279 | 35 | 314 | |
| 100.00% | 100.00% | 100.00% | ||
Note: tumor characteristics stratified by the clinical outcome given as DSD and DSS (disease specific survival and death).
aA more detailed overview of clinicopathological characteristics is given in the supplementary material.
NPI
Mean NPI was 4.4 (95% CI = 4.3–4.5). In patients with DSD, the mean NPI (5.2; CI = 4.8–5.6) was significantly higher compared to the DSS group (4.3; CI = 4.1–4.4; P < 0.001). Harrell’s C was 75.3% for NPI. Descriptive statistics of the NPI are summarized in Table 3.
Table 3.
Descriptive statistics of parameters retained in the NPIVAV model and patient age.
| Survival | Total tumor volume (TTV [cm3]) | Heterogeneity of vascularization* | Time-to-peak enhancement (TTP [min]) | NPI | Age [years] | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| DSS | DSD | DSS | DSD | DSS | DSD | DSS | DSD | DSS | DSD | |
| Minimum | 0.1 | 0.5 | 2.4 | 5.4 | 1 | 1 | 2.2 | 3.2 | 27 | 36 |
| Maximum | 313.1 | 249.7 | 80 | 45.3 | 4 | 6 | 7.2 | 7.5 | 87 | 82 |
| Mean | 7.1 | 29.9 | 29.3 | 23 | 1:18 | 1:42 | 4.3 | 5.2 | 57.4 | 59.6 |
| Median | 2.9 | 7.6 | 27 | 23.2 | 1 | 1 | 4.3 | 4.7 | 58 | 61 |
| SD | 21.1 | 60.4 | 14.1 | 8.6 | 0:42 | 1:12 | 1 | 1.2 | 11.7 | 11.5 |
Note: Given are all four parameters retained in the NPIVAV model and patient age (see Table 4). Corresponding results of descriptive statistics are listed. Except patient age (P = 0.23), all parameters were predictive of the endpoint (for P-values, see Table 4). *Weak wash-in (initial phase) and persistent (delayed phase) [%] (details see Table 1 and main text).
VAV
VAV revealed significant association with the endpoint: Time to peak enhancement (TTP: P = 0.003), total tumor volume [cm³] (P < 0.0001) and one parameter focusing on the heterogeneity of vascularization (category A: P = 0.006) were significant predictors of DSD. Predictive performance of VAV reached Harrell’s C = 74.0% and was equal to that of NPI (P = 0.92). Key VAV stratified by the endpoints are summarized in Table 3.
In addition, peak enhancement was slightly higher in the DSD group (DSD/DSS = 123.5/110%; P = 0.07), as was wash-in (DSD/DSS: 119.6/107.1%; P = 0.08). Both parameters, however, missed the significance level.
NPI versus NPIVAV
Feature selection retained the NPI and all three parameters of VAV in the final NPIVAV model (Table 4). Corresponding model fit was superior compared to NPI alone (Chi-squared: 51.5 vs. 27.9). Harrell’s C was 81.0% for NPIVAV and surpassed the value for NPI by 5.6%.
Table 4.
NPIVAV model.
| Covariate | HR | CI | P | Coefficient | SE |
|---|---|---|---|---|---|
| Total tumor volume (TTV) | 1.01 | 1.00 to 1.01 | 0.04 | 0.01 | 0.00 |
| Heterogeneity of vascularization* | 0.95 | 0.92 to 0.98 | 0.002 | −0.05 | 0.02 |
| Time-to-peak enhancement (TTP) | 1.84 | 1.40 to 2.42 | <0.0001 | 0.61 | 0.14 |
| Nottingham Prognostic Index (NPI) | 2.01 | 1.48 to 2.73 | <0.0001 | 0.70 | 0.16 |
Note: Given is the NPIVAV model as provided by the Cox regression. After applying feature selection, four covariates were retained. Besides NPI this included three parameters for MRI analysis (for details, see Table 1).
CI: 95% confidence interval of the hazard ratio. SE: standard error of the coefficient. *Weak wash-in (initial phase) and persistent (delayed phase) [%] (details see Table 1 and main text).
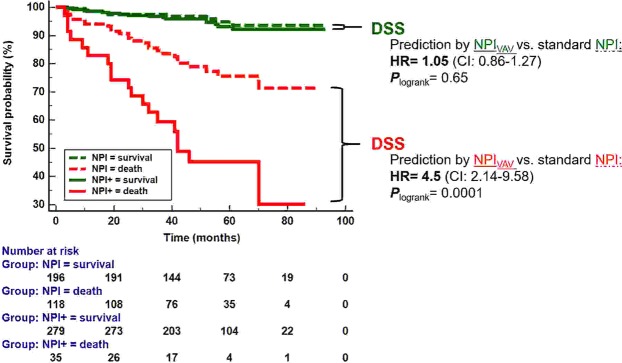
The improved survival prediction by VAV was also demonstrated by the Kaplan Meier analysis (Fig. 2):
Figure 2.
Prediction of good (DSS) and poor (DSD) patient outcome: Comparison of NPIVAV vs. standard NPI. NPIVAV enabled a better identification of patients at risk for DSD compared to standard NPI (HR = 4.5, CI: 2.14–9.58). This was also evident by a faster decline of the corresponding survival curve (Plogrank = 0.0001). Optimized identification of high risk patients by the NPIVAV did not come on the price of a worse identification of patients with a more favorable outcome. Indeed, the likelihood of DSS was alike if NPIVAV was used to predict DSS, compared to standard NPI alone (HR = 1.05, CI: 0.86–1.27, Plogrank = 0.65).
Prediction of DSD
The survival curves of patients in whom NPIVAV predicted DSD significantly differed from those in whom NPI alone predicted DSD (logrank test: P = 0.0001). DSD was more likely to occur, if this event was predicted by NPIVAV, compared to NPI alone (HR = 4.5; CI = 2.14–9.58).
Probability of DSS
The survival curves of patients classified as DSS either by NPIVAV or by NPI were not significantly different (logrank test: P = 0.65). The risk of DSS was similar, if DSS was predicted by NPIVAV, compared to the patients in whom NPI alone predicted DSD (HR = 1.05; CI: 0.86–1.27).
Discussion
Breast MRI contributed to the prediction of survival in patients with primary invasive breast cancer. Automated volumetric radiomic analysis of breast cancer vascularization improved survival prediction compared to the Nottingham Prognostic Index alone (NPI). The NPI is a well-established and widely used method of predicting survival of primary breast cancer. Most of all, the use of MRI optimized the stratification of patients at risk of an unfavourable survival outcome
MRI showed a prognostic accuracy equal to that of the NPI
To our knowledge, we are the first group to report a head-to-head comparison of MRI and NPI in the risk stratification of patients. One advantage of prognostic MRI is that it is performed as a one-stop-shop procedure within one session. Our approach was based on a standard MRI protocol, as recommended by international guidelines, and required only 14 minutes of magnet time28. Based on this fast examination, both the staging — including the assessment of tumor extension plus multifocal and bilateral disease — and the risk estimation can be accomplished. Whereas classical risk factors such as nodal metastasis and histological grading are nominal or ordinal by nature, MRI provides quantitative and semi-quantitative parameters. This information is semi-automatically assessed by the software, which is an advantage over standard pathological assessment. For instance, the evaluation of grading is known to be limited by significant inter-pathologist variability35. Prognostic MRI information moreover is available in real-time. This is a potential advantage for clinical workflow and patient management. Of note, however, some NPI cannot be assessed on core biopsy samples, but require surgical resection for definitive classification. This is frequently the case in nodal staging, resulting in the delayed availability of these biomarkers for prognostic purposes. MRI both volumetrically and dynamically investigates the whole tumor volume in vivo. This is an advantage over most prognostic biomarkers that evaluate small tumor samples ex vivo. As MRI is not a snapshot diagnosis, it is well suited to investigate tumor heterogeneity. In fact, volumetric MRI features were among the best prognostic parameters in our analysis.
Our findings support the evaluation of MRI as a prognostic biomarker in precision medicine
The concept of precision medicine aims to adapt cancer treatments to an individual’s tumor characteristics. Thus, the goal of precision medicine is to both maximize the effectiveness of treatment and to minimize the side effects of cytotoxic drugs2. As every known biomarker thus far has limitations, there is intensive ongoing research into the development of new tissue biomarkers. One of the most promising fields is gene expression profiling of breast cancer2,3,36,37. Gene expression profiling is already used in systemic breast cancer treatment planning3, and empiric evidence shows that the decision towards more aggressive treatments such as cytotoxic drugs can be based on distinct gene profiles37. MRI could actually tread the same path: In our analysis, the strength of MRI was a more accurate identification of patients with a less favorable outcome. Thus, our data could be used to stratify patient risk. Certainly, it would be unwise to apply a more aggressive treatment based only on the MRI information at the time being. Nevertheless, MRI might be used as a “gate keeper”. If MRI indicates an unfavorable outcome, further biomarkers such as genetic profiling might be warranted, in order to decide whether a more aggressive therapy is indicated.
Possible biological correlates for our MRI findings will be discussed
Tumor size is one of the best-established prognostic parameters of breast cancer38. Typically, the measurement of the breast cancer size is performed as linear measurements in two spatial dimensions. Both the NPI and the T-staging are based on this method5,8,25. The breast carcinoma may grow in a diffuse pattern, as is often observed in invasive lobular subtypes39. Consequently, the tumor size of breast cancer cannot always be accurately measured by two-dimensional linear measurement. However, TTV is a volumetric method and thus can evaluate the tumor size in any spatial extent.
However, TTV is not only a metric parameter of tumor size. According to the research by Hylton et al. it can also be interpreted as a functional biomarker of vascularization18. Neoangiogenesis is regarded as a pivotal step in the development of cancer40. At the same time, neoangiogenesis significantly determines the potential of a breast cancer to metastasize and hereby limits patient outcome41,42. One method to quantify tumor vascularization is the determination of its microvascular density (MVD). It is therefore conclusive that microvascular density can also be considered as a prognostic biomarker of breast cancer42. Interestingly, contrast enhancement assessed by MRI was closely linked to MVD in previous radiopathological correlations studies43. Thus, the prognostic significance of TTV may also be explained in this functional context. However, tumor size alone is not a sufficient parameter to optimally predict the prognosis of a patient. This explains why additional parameters were identified as independent predictors of survival status in the NPIVAV model44.
Different from the classical pathological analysis as well as from modern methods (such as genetic profiling), MRI can examine the entire tumor volume16,19,25,36,45. For this reason, the method can also be used to assess the intratumoral heterogeneity. The latter is a major aspect in breast cancer biology46. We identified “heterogeneity of vascularization” within the tumor as a significant parameter of patient outcome. Hereby, a higher volume of compartments exhibiting slow wash-in and persistent enhancement corresponded to a more favorable prognosis. Of note, this MRI pattern has been described by Leong et al. as typical for breast cancers45. Considering the relationship of MRI enhancement and the MVD (see above), this finding seems conclusive: Buadu reported that breast cancer with slower initial and delayed contrast enhancement in MRI exhibited lower MVD in radiopathological correlation43. Since a lower MVD is regarded as a predictor of a relatively better outcome, this finding seems conclusive42.
The analysis of tumor heterogeneity also enabled the identification of the most suspicious compartment. It was identified based on the parameters wash-in and wash-out. In the context of the aforementioned MVD, both parameters could be regarded as parameters of tissue vascularisation42,43. Within this compartment, a slightly faster wash-in, a slightly higher maximum enhancement, and, above all, a significantly longer TTP were observed in the DSD group. Wash-in can be interpreted as a parameter of tissue perfusion47. It reflects both the blood flow and the intravascular blood volume (for instance figure 5 in47), and correlates with the MVD42,43,47. After the perfusion phase, the contrast medium is transmitted through the blood vessels into the interstitium. This event is referred to as leakage and here the maximum enhancement is typically observed47. However, it needs to be emphasized that an exact separation of the different pharmacokinetic compartments is not feasible with standard breast MRI protocols47. Leakage is not only determined by the quantity (vessel surface), but also by the quality (permeability) of the vessels47. The quantity (vessel surface) correlates with the MVD41,47. The permeability is triggered by numerous factors, among others by VEGF48. VEGF is a cytokine that plays a central role in the process of angiogenesis48. These considerations might explain why the maximum enhancement in the DSD group was slightly higher and slightly delayed compared to the DSS cases. Interestingly, the parameter TTP is rarely used in breast MRI diagnostics. In different fields of radiology, such as in neuro imaging, the TTP is a standard parameter47. This is particularly relevant since early breast MRI research has already shown that TTV is superior to more commonly used qualitative kinetic criteria49. In this context, an in-depth re-evaluation of this parameter may be of promise.
Some limitations of our work will be discussed
The literature reports a large number of breast cancer biomarkers. Hereby, the NPI was introduced in 1982 and remains a widely used method to predict survival in primary breast cancer5,6,8. A completely different approach is taken by multigene assays. These rather new biomarkers focus on genetic analysis and are increasingly used in clinical routine50. Interestingly, it has already been shown in the literature that there is a close correlation between MRI parameters with multigene assays such as MammaPrint, Oncotype DX, and PAM5051. So future studies might investigate to what extent MRI analysis could substitute genetic tests, or whether MRI might actually improve the prognostic information provided by multigene assays.
We investigated radiomic parameters of tumor vascularization. We excluded morphologic features from our analysis20. Previous studies have shown that also morphologic MRI data provide prognostic information17,52. The presence of avascular, seemingly necrotic compartments have for instance been associated with surrogates of poor outcome52. So future studies should investigate textural radiomic features as well in order to determine whether predictive accuracy of VAV can be further increased.
Breast cancer can metastasize after many years and lead to DSD53. It cannot be excluded that some of our patients died after the end of data collection. This is a potential bias of the study.
We conceptualized this work as a cross-sectional study. In this respect, our data represent a typical patient collective at our practice. Therefore - as well as in the context of the patient number - no formal subgroup analysis according to histopathological parameters (clinicopathological factors, etc.) as well as the treatment regime was performed. Therefore, we cannot exclude that these two parameters had an impact on our results. In the same way, we decided to exclude patients with NAC from the analysis. These patients were predominantly included in clinical trials and were therefore treated with a therapy not yet established. Nevertheless, this implies a potential limitation of our study: Results do not apply on NAC patients.
In distinction to the classical visual MRI analysis, the influence of the reader on the VAV is significantly lower54. The software works largely automated and the only investigator-dependent step is the definition of the volume of interest. Nevertheless, some observer-dependent bias within our data cannot be excluded. Furthermore, the reproducibility of our results is also influenced by the software itself. Although the software is established on the market, our results might not be completely reproducible on other systems. Finally, the MRI protocol itself can also have influence on our results55. We aimed to control for this bias and adopted our imaging protocol to international standards and used well established criteria to assess the tumor vascularization16,18,28,29,45,56. Future studies should investigate the repeatability of our results with different software, MRI protocols and identify the potential impact of the reader on our results.
Conclusion
In conclusion, automated volumetric radiomic analysis of breast cancer vascularization improved the prediction of survival of patients with primary invasive breast cancer. Most of all it improved the identification of patients at higher risk of an unfavorable outcome.
These results are based on a standard, clinical MRI followed by real-time analysis by a commercially available, FDA-cleared computer algorithm. Accordingly, the method can be incorporated into the clinical routine.
One potential role of MRI would be a “gate keeper”, in order to identify patients requiring a more advanced diagnosis (genetic profiling etc.) and/or more aggressive therapies (chemotherapy). This approach should be evaluated in future trials.
Supplementary information
Acknowledgements
The authors dedicate this work to the memory of Werner Alois Kaiser. His death marked the loss of an unprejudiced, creative, and critical scientist, a remarkable clinical radiologist, a generous, untiring mentor and a dear and dependable friend.Among many other research projects, he inspired us to study prognostic breast MRI long before this became a hot research topic and the buzz word “radiomics” was invented. The database created during this project is also the basis of the present study. Accordingly, some subjects of our study have been used for previous projects in a different clinical and/or scientific context (e.g. 12,16).
Author contributions
Conceptualization: M.D., P.A.T.B. (lead) with contributions by all co-authors. Data curation and software: M.D., P.A.T.B., R.Z., I.B.R. Formal analysis M.D., P.A.T.B., S.E., R.Z. Investigation: M.D., P.A.T.B., R.Z., I.B.R. Methodology: M.D. & P.A.T.B. (lead) with contributions by all co-authors. Project administration: M.D., P.A.T.B., R.S.W., M.U. Resources: M.D., P.A.T.B., I.B.R., R.S.W., M.U. Software: M.D., P.A.T.B., R.Z. Supervision: M.D., P.A.T.B., R.Z. Validation: M.D., P.A.T.B. Visualization: M.D., P.A.T.B., Writing (original draft; review and editing): All authors.
Data availability
All relevant data are within the paper and its supplementary files.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-60393-9.
References
- 1.SEER Stat Fact Sheets: Female Breast Cancer. http://seer.cancer.gov/statfacts/html/breast.html (2019).
- 2.Kurian AW, Friese CR. Precision Medicine in Breast Cancer Care: An Early Glimpse of Impact. JAMA Oncology. 2015;1:1109. doi: 10.1001/jamaoncol.2015.2719. [DOI] [PubMed] [Google Scholar]
- 3.Friese CR, et al. Chemotherapy decisions and patient experience with the recurrence score assay for early-stage breast cancer: Breast Cancer Recurrence Scores. Cancer. 2017;123:43–51. doi: 10.1002/cncr.30324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biomarkers Definitions Working Group Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clinical Pharmacology & Therapeutics. 2001;69:89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- 5.Fong Y, et al. The Nottingham Prognostic Index: five- and ten-year data for all-cause Survival within a Screened Population. The Annals of The Royal College of Surgeons of England. 2015;97:137–139. doi: 10.1308/003588414X14055925060514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haybittle JL, et al. A prognostic index in primary breast cancer. Br J Cancer. 1982;45:361–366. doi: 10.1038/bjc.1982.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Todd JH, et al. Confirmation of a prognostic index in primary breast cancer. Br J Cancer. 1987;56:489–492. doi: 10.1038/bjc.1987.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blamey RW, et al. Survival of invasive breast cancer according to the Nottingham Prognostic Index in cases diagnosed in 1990–1999. European Journal of Cancer. 2007;43:1548–1555. doi: 10.1016/j.ejca.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 9.Kaiser W. MRI of the female breast. First clinical results. Arch. Int. Physiol. Biochim. 1985;93:67–76. doi: 10.3109/13813458509080627. [DOI] [PubMed] [Google Scholar]
- 10.Pediconi F, et al. Color-coded automated signal intensity curves for detection and characterization of breast lesions: preliminary evaluation of a new software package for integrated magnetic resonance-based breast imaging. Invest Radiol. 2005;40:448–457. doi: 10.1097/01.rli.0000167427.33581.f3. [DOI] [PubMed] [Google Scholar]
- 11.Baltzer, P. A. et al. Computer Assisted Analysis of MR-Mammography Reveals Association Between Contrast Enhancement and Occurrence of Distant Metastasis. Technology in cancer research & treatment (2012). [DOI] [PubMed]
- 12.Kim Jin Joo, Kim Jin You, Kang Hyun Jung, Shin Jong Ki, Kang Taewoo, Lee Seok Won, Bae Young Tae. Computer-aided Diagnosis–generated Kinetic Features of Breast Cancer at Preoperative MR Imaging: Association with Disease-free Survival of Patients with Primary Operable Invasive Breast Cancer. Radiology. 2017;284(1):45–54. doi: 10.1148/radiol.2017162079. [DOI] [PubMed] [Google Scholar]
- 13.Johansen R, et al. Predicting survival and early clinical response to primary chemotherapy for patients with locally advanced breast cancer using DCE-MRI. Journal of Magnetic Resonance Imaging. 2009;29:1300–1307. doi: 10.1002/jmri.21778. [DOI] [PubMed] [Google Scholar]
- 14.Li SP, et al. Use of dynamic contrast-enhanced MR imaging to predict survival in patients with primary breast cancer undergoing neoadjuvant chemotherapy. Radiology. 2011;260:68–78. doi: 10.1148/radiol.11102493. [DOI] [PubMed] [Google Scholar]
- 15.Pickles MD, Lowry M, Gibbs P. Pretreatment Prognostic Value of Dynamic Contrast-Enhanced Magnetic Resonance Imaging Vascular, Texture, Shape, and Size Parameters Compared With Traditional Survival Indicators Obtained From Locally Advanced Breast Cancer Patients. Investigative Radiology. 2016;51:177–185. doi: 10.1097/RLI.0000000000000222. [DOI] [PubMed] [Google Scholar]
- 16.Dietzel M, et al. Association between survival in patients with primary invasive breast cancer and computer aided MRI. J Magn Reson Imaging. 2013;37:146–155. doi: 10.1002/jmri.23812. [DOI] [PubMed] [Google Scholar]
- 17.Dietzel M, et al. Potential of MR mammography to predict tumor grading of invasive breast cancer. Rofo. 2011;183:826–833. doi: 10.1055/s-0031-1273244. [DOI] [PubMed] [Google Scholar]
- 18.Hylton NM, et al. Neoadjuvant Chemotherapy for Breast Cancer: Functional Tumor Volume by MR Imaging Predicts Recurrence-free Survival—Results from the ACRIN 6657/CALGB 150007 I-SPY 1 TRIAL. Radiology. 2016;279:44–55. doi: 10.1148/radiol.2015150013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hylton NM, et al. Locally Advanced Breast Cancer: MR Imaging for Prediction of Response to Neoadjuvant Chemotherapy—Results from ACRIN 6657/I-SPY TRIAL. Radiology. 2012;263:663–672. doi: 10.1148/radiol.12110748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gillies RJ, Kinahan PE, Hricak H. Radiomics: Images Are More than Pictures, They Are Data. Radiology. 2016;278:563–577. doi: 10.1148/radiol.2015151169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hudis CA, et al. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. J. Clin. Oncol. 2007;25:2127–2132. doi: 10.1200/JCO.2006.10.3523. [DOI] [PubMed] [Google Scholar]
- 22.Kweldam CF, Wildhagen MF, Bangma CH, van. Leenders GJLH. Disease-specific death and metastasis do not occur in patients with Gleason score ≤6 at radical prostatectomy. BJU International. 2015;116:230–235. doi: 10.1111/bju.12879. [DOI] [PubMed] [Google Scholar]
- 23.Survival analysis. MedCalc https://www.medcalc.org/manual/kaplan-meier.php (2019).
- 24.Fattaneh, A. & Tavassoli, P. Tumours of the breast. in World Health Organization Classification of Tumours. Pathology and Genetics.Tumours of the Breast and Female Genital Organs 9–112 (IARC Press, 2003).
- 25.Edge, S., Byrd, D., Carducci, M. & Wittekind, C. TNM Classification of Malignant Tumours. (Springer, 2009).
- 26.Hammond, M. E. H. et al. American Society of Clinical Oncology/College of American Pathologists Guideline Recommendations for Immunohistochemical Testing of Estrogen and Progesterone Receptors in Breast Cancer. Arch. Pathol. Lab. Med.134(6), 907–922 (2010). [DOI] [PMC free article] [PubMed]
- 27.Wolff AC, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch. Pathol. Lab. Med. 2007;131:18–43. doi: 10.5858/2007-131-18-ASOCCO. [DOI] [PubMed] [Google Scholar]
- 28.Mann RM, Kuhl CK, Kinkel K, Boetes C. Breast MRI: guidelines from the European Society of Breast Imaging. Eur Radiol. 2008;18:1307–1318. doi: 10.1007/s00330-008-0863-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morris, E. A. et al. ACR BI-RADS® Magnetic Resonance Imaging. in ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System (American College of Radiology, 2013).
- 30.Dietzel M, Baltzer PAT. How to use the Kaiser score as a clinical decision rule for diagnosis in multiparametric breast MRI: a pictorial essay. Insights into Imaging. 2018;9:325. doi: 10.1007/s13244-018-0611-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Antolini L, Boracchi P, Biganzoli E. A time-dependent discrimination index for survival data. Stat Med. 2005;24:3927–3944. doi: 10.1002/sim.2427. [DOI] [PubMed] [Google Scholar]
- 32.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. doi: 10.2307/2531595. [DOI] [PubMed] [Google Scholar]
- 33.Oikonomou EK, et al. Non-invasive detection of coronary inflammation using computed tomography and prediction of residual cardiovascular risk (the CRISP CT study): a post-hoc analysis of prospective outcome data. The Lancet. 2018;392:929–939. doi: 10.1016/S0140-6736(18)31114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–35. doi: 10.1002/1097-0142(1950)3:1<32::AID-CNCR2820030106>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 35.Boiesen P, et al. Histologic grading in breast cancer–reproducibility between seven pathologic departments. South Sweden Breast Cancer Group. Acta Oncol. 2000;39:41–45. doi: 10.1080/028418600430950. [DOI] [PubMed] [Google Scholar]
- 36.Potosky AL, et al. Population-based study of the effect of gene expression profiling on adjuvant chemotherapy use in breast cancer patients under the age of 65 years: Breast Cancer Genetics and Chemotherapy. Cancer. 2015;121:4062–4070. doi: 10.1002/cncr.29621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barcenas CH, et al. Outcomes in patients with early-stage breast cancer who underwent a 21-gene expression assay: Outcomes With 21-Gene Expression. Cancer. 2017;123:2422–2431. doi: 10.1002/cncr.30618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verschraegen C, et al. Modeling the Effect of Tumor Size in Early Breast Cancer. Ann Surg. 2005;241:309–318. doi: 10.1097/01.sla.0000150245.45558.a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reed AEM, Kutasovic JR, Lakhani SR, Simpson PT. Invasive lobular carcinoma of the breast: morphology, biomarkers and’omics. Breast Cancer Research. 2015;17:12. doi: 10.1186/s13058-015-0519-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Folkman J. What Is the Evidence That Tumors Are Angiogenesis Dependent? J Natl Cancer Inst. 1990;82:4–7. doi: 10.1093/jnci/82.1.4. [DOI] [PubMed] [Google Scholar]
- 41.Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin. Oncol. 2002;29:15–18. doi: 10.1053/sonc.2002.37263. [DOI] [PubMed] [Google Scholar]
- 42.Uzzan B, Nicolas P, Cucherat M, Perret G-Y. Microvessel density as a prognostic factor in women with breast cancer: a systematic review of the literature and meta-analysis. Cancer Res. 2004;64:2941–2955. doi: 10.1158/0008-5472.CAN-03-1957. [DOI] [PubMed] [Google Scholar]
- 43.Buadu LD, et al. Breast lesions: correlation of contrast medium enhancement patterns on MR images with histopathologic findings and tumor angiogenesis. Radiology. 1996;200:639–649. doi: 10.1148/radiology.200.3.8756909. [DOI] [PubMed] [Google Scholar]
- 44.Sopik V, Narod SA. The relationship between tumour size, nodal status and distant metastases: on the origins of breast cancer. Breast Cancer Res Treat. 2018;170:647–656. doi: 10.1007/s10549-018-4796-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leong LCH, Gombos EC, Jagadeesan J, Fook-Chong SMC. MRI Kinetics With Volumetric Analysis in Correlation With Hormonal Receptor Subtypes and Histologic Grade of Invasive Breast Cancers. AJR Am J Roentgenol. 2015;204:W348–W356. doi: 10.2214/AJR.13.11486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Turashvili, G. & Brogi, E. Tumor Heterogeneity in Breast Cancer. Front Med (Lausanne) 4, (2017). [DOI] [PMC free article] [PubMed]
- 47.Cuenod CA, Balvay D. Perfusion and vascular permeability: basic concepts and measurement in DCE-CT and DCE-MRI. Diagn Interv Imaging. 2013;94:1187–1204. doi: 10.1016/j.diii.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 48.Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J. Clin. Oncol. 2002;20:4368–4380. doi: 10.1200/JCO.2002.10.088. [DOI] [PubMed] [Google Scholar]
- 49.Szabó BK, Aspelin P, Wiberg MK, Boné B. Dynamic MR imaging of the breast. Analysis of kinetic and morphologic diagnostic criteria. Acta Radiol. 2003;44:379–386. doi: 10.1080/j.1600-0455.2003.00084.x. [DOI] [PubMed] [Google Scholar]
- 50.Vieira, A. F. & Schmitt, F. An Update on Breast Cancer Multigene Prognostic Tests—Emergent Clinical Biomarkers. Front Med (Lausanne) 5, (2018). [DOI] [PMC free article] [PubMed]
- 51.Li H, et al. MR Imaging Radiomics Signatures for Predicting the Risk of Breast Cancer Recurrence as Given by Research Versions of MammaPrint, Oncotype DX, and PAM50 Gene Assays. Radiology. 2016;281:382–391. doi: 10.1148/radiol.2016152110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dietzel M, et al. The Necrosis Sign in Magnetic Resonance-Mammography: Diagnostic Accuracy in 1,084 Histologically Verified Breast Lesions. The Breast Journal. 2010;16:603–608. doi: 10.1111/j.1524-4741.2010.00982.x. [DOI] [PubMed] [Google Scholar]
- 53.Pan H, et al. 20-Year Risks of Breast-Cancer Recurrence after Stopping Endocrine Therapy at 5 Years. N Engl J Med. 2017;377:1836–1846. doi: 10.1056/NEJMoa1701830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grimm LJ, et al. Interobserver Variability Between Breast Imagers Using the Fifth Edition of the BI-RADS MRI Lexicon. American Journal of Roentgenology. 2015;204:1120–1124. doi: 10.2214/AJR.14.13047. [DOI] [PubMed] [Google Scholar]
- 55.Saha A, Yu X, Sahoo D, Mazurowski MA. Effects of MRI scanner parameters on breast cancer radiomics. Expert Syst Appl. 2017;87:384–391. doi: 10.1016/j.eswa.2017.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cheng Z, et al. Discrimination between benign and malignant breast lesions using volumetric quantitative dynamic contrast-enhanced MR imaging. Eur Radiol. 2018;28:982–991. doi: 10.1007/s00330-017-5050-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are within the paper and its supplementary files.