Abstract
Introduction
Acne arises during puberty, in part, due to elevated hormones and growth factors which stimulate de novo lipogenesis (DNL) in primary sebocytes to significantly increase sebum production. Oral isotretinoin is an effective acne therapy, reducing sebum production through inducing apoptosis in sebocytes. However, isotretinoin is teratogenic and has additional unwanted side effects, including an initial acne flare-up, which limits its utility. The biguanide, metformin has been found to alleviate severe acne in women with polycystic ovary syndrome (PCOS) through normalization of their insulin and androgen hormone levels. Metformin’s broader effectiveness to improve acne in non-PCOS populations lacks significant clinical support. In an effort to determine whether biguanides directly affect sebogenesis, we investigated their ability to alter DNL in cell-based assays in vitro.
Methods
De novo lipogenesis was measured in human primary sebocytes using [14C]-acetate labeling. Lipid species analysis was performed by extracting newly synthesized lipids and subjecting them to thin layer chromatography. Gene expression changes in sebocytes were identified through qPCR analysis of isolated RNA. Metabolic parameters including oxygen consumption rate, lactate production and activation of adenosine monophosphate-dependent protein kinase (AMPK) were assessed in human primary sebocytes.
Results
Using human primary sebocytes, we found that biguanides, isotretinoin and azithromycin induced an acute dose and time-dependent increase in [14C]-acetate labeling of neutral lipids, while AICAR, an AMPK activator, inhibited this DNL response. Biguanides did not activate AMPK in sebocytes, however, they significantly reduced oxygen consumption rate and increased lactate production. Treatment with biguanides, but not isotretinoin, significantly upregulated ACSS2 gene expression in primary sebocytes and showed synergism with lipogenic activators to induce DNL genes.
Discussion
These changes are consistent with an acute increase in sebocyte lipogenesis and support the potential of biguanides to cause an initial flare-up in patients suffering from severe acne.
Keywords: sebocytes, acne, metformin, de novo lipogenesis, sebaceous gland
Introduction
Acne vulgaris is a common skin disorder that affects over 50 million people in the US and is primarily caused by the overproduction of sebum by sebaceous glands, hyperkeratinization of follicular epithelium, Propionibacterium acnes (P. acnes) proliferation and inflammation.1,2 In particular, excess sebum production, a complex mixture of triglyceride, wax esters, fatty acids and squalene, coupled with increased follicular keratinocyte proliferation can clog the hair follicle and provide a favorable environment for the growth of anaerobic P. acnes. The combination of bacterial products and immune cell response through cytokine secretion causes inflammation and exacerbates acne lesion formation. Clinically, sebum production has been shown to correlate with acne severity and generally higher levels of sebum are found in people suffering from acne.3–5
Oral isotretinoin (13-cis-retinoic acid) is a highly effective acne medication that addresses all four factors in the development of acne, however, its effect on sebum production may be secondary to its ability to induce apoptosis in sebocytes.6,7 Unfortunately, isotretinoin can be teratogenic and causes severe unwanted side effects that can limit its usage. Additionally, oral isotretinoin can induce an acne flare-up in the early stages of treatment, resulting in increased comedone formation and worsening of the disorder.8–11 While the underlying mechanism for acne flare-up is poorly understood, sebocytes rely on de novo lipogenesis for sebum production12 and isotretinoin has been shown to acutely activate lipogenesis in sebocytes exacerbating existing acne conditions.13
The onset of acne typically begins during puberty where the increase in hormones and growth factors stimulate de novo lipogenesis and increase sebum production.14 Similarly, women with polycystic ovary syndrome (PCOS) often suffer from severe acne, hirsutism, anovulation and insulin resistance due to hyperandrogenism and hyperinsulinemia. These patients are commonly prescribed antiandrogens and metformin to control the symptoms of the disease.15–18 Metformin has been shown to have beneficial effects in reducing insulin and testosterone levels and also improves ovulation and insulin resistance.19 Additionally, after six months of metformin treatment to normalize these hormones, metformin has shown a beneficial effect on acne. Clinical trials specifically investigating the role of metformin in reducing inflammatory acne in women with PCOS have shown improvement in acne scores as early as 6 to 12 weeks.20,21 Based on these results and concerns over the safety of isotretinoin, there has been considerable speculation that metformin could be a safe and effective anti-acne therapeutic in other populations.22–25
In non-PCOS populations the few clinical trials performed to assess metformin’s effects on acne have shown limited success.23–25 The most significant metformin effects were in general metabolic characteristics as with PCOS patients including insulin resistance, BMI and fasting blood glucose levels, suggesting metformin’s acne effects are secondary to its role in regulating metabolism. Mechanistically, metformin can activate AMPK to inhibit energy-dependent processes, including gluconeogenesis and fatty acid synthesis, favoring energy producing activities such as fatty acid oxidation.26–28 In sebocytes this would result in decreased de novo lipogenesis and a reduction in sebum production with the potential to reduce acne severity. In an effort to clarify the role of biguanides in sebogenesis, we utilized cultured human primary sebocytes to test their direct effects on these cells in vitro.
Using human primary sebocytes isolated from dissected sebaceous glands, we can mimic the sebogenic process in primary culture by inducing the cells with a combination of an LXR agonist and insulin.2,29 This results in a significant upregulation of de novo lipogenesis within the first 48 hrs as measured by acetate incorporation into newly synthesized neutral lipids. This assay format can identify antagonists as well as agonists of de novo lipogenesis and sebogenesis. Using this system, we found the surprising result that biguanides induce an upregulation of lipid synthetic pathways and de novo lipogenesis similar to isotretinoin, suggesting that biguanides may induce an acne flare-up in some patients when beginning treatment for severe acne.
Materials and Methods
Sebocyte Isolation
All procedures involving human tissues were approved by IRB (Pearl IRB, LLC, Indianapolis, IN) and were with the written informed consent of adult donors. Surgical waste material from elective cosmetic surgeries was used in these studies with no specific patient recruitment based on age, gender, BMI or diabetes status. Sebaceous glands were micro-dissected from thermolysin-separated epidermal layers of facial skin from elective plastic surgeries. The isolated glands were trypsinized and cells were liberated by gentle pipetting before collecting by centrifugation at 300xg for 5 mins. The cells were suspended in growth medium and seeded in collagen-1 coated flasks to propagate with medium refreshment every 2–3 days. Sebocytes were expanded in culture through three passages (<15 population doublings) and used in experiments at passage 3 or 4. Donor demographics of the sebocyte lots used in this study are described in Table 1.
Table 1.
Sebocyte Donor Demographics
| Lot Number | Gender | Age | BMI | Type 2 Diabetes | Medications |
|---|---|---|---|---|---|
| SEB060314D | Female | 55 | 24.8 | N | Synthroid, Tribenzor, Bystolic |
| SEB121913C | Female | 66 | 25.1 | N | Metoprolol, Nexium |
| SEB042613 | Female | 60 | 27.3 | N | None |
| SEBM022614B | Female | 59 | 27.6 | N | None |
| SEBM111913E | Female | 63 | 30.3 | N | None |
| SEBM091813B | Male | 58 | 35.7 | N | None |
| SEB031412A | Female | 66 | 32.0 | Y | Lipitor, Januvia, Micardis, Glucophage, Gabapentin |
| SEB013019A | Female | 49 | 33.5 | Y | Metformin, Fenoxene, Montelukast, Rosuvastatin, Synthroid |
Abbreviation: BMI, Body mass index, kg/m2.
Scinitiplate [14C]-Acetate Assay
Sebocytes were seeded at 10,000 cells per well in a collagen I-coated 96-well Scintiplate (PerkinElmer, Boston, MA) and allowed to reach confluence. The growth medium was removed and Sebocyte Treatment Medium (STM, DMEM/F12, biotin, pantothenate) was added containing compounds plus an LXR agonist, 1 µM T0901317. The cells were incubated overnight and the treatments reapplied in STM containing 1 µM human insulin. 1 µCi of [14C]-acetate (PerkinElmer, Boston, MA) was added per well and the uptake assay incubated for 4 hrs. To normalize the data and assess cell viability, Cell Titer Blue (CTB) reagent (Promega, Madison, WI) was added to each well after 2 hrs of uptake and incubated with the cells for the remaining 2 hrs. CTB fluorescence was determined using 560nm excitation and 590nm emission wavelengths and a 570nm cutoff. To determine [14C]-acetate uptake and incorporation into lipids, the medium was removed and cells washed twice with PBS. The final PBS wash was removed and the monolayer allowed to air dry before measuring incorporation by scintillation proximity counting.30
[14C]-Acetate Lipid Analysis
Sebocytes were seeded in a clear-bottom, black wall 96-well plate and treated as described above. After treatment, cells were harvested with trypsin/EDTA and transferred to glass vials for lipid extraction according to the BUME method.31 Sequential addition of equal volumes of butanol/methanol (3:1 v/v), heptane/ethyl acetate (3:1 v/v) and 1% acetic acid were added to each vial, carefully mixed and the two phases allowed to separate. An aliquot of the upper, organic phase was mixed with scintillation fluid for scintillation counting. The remaining organic phase was dried under nitrogen and mixed with lipid standards for separation and quantitation by TLC. Lipid samples spotted on silica TLC plates were separated using a petroleum ether/diethyl ether/acetic acid (80/20/1 v/v) mobile phase. Lipid standards were visualized by iodine vapor and areas corresponding to the standards scraped into 3 mL of scintillation fluid to determine acetate labeling of each lipid species.
RNA Isolation and qPCR Analysis
Sebocytes were seeded at 120,000 cells per well in 12-well plates and allowed to adhere overnight. Treatments were in STM containing 1 µM T0901317 ± 1mM metformin, 25 µM phenformin, 5 µM 13-cis-retinoic acid, or 10 µg/mL azithromycin. Sebocytes were treated for 48 hrs and total RNA isolated using an RNeasy kit (Qiagen, Carlsbad, CA). Reverse transcription reactions were prepared using 1 µg of total RNA and qPCR analysis was performed in duplicate using 10 ng/well cDNA to determine the transcript levels of SREBF1 (Hs01088691_m1), FASN (Hs01005622_m1), ACSS2 (Hs00218766_m1), SCD (Hs01682761_m1), HMGCR (Hs00168352_m1), SQLE (Hs01123768_m1), ACLY (Hs00982738_m1), ACACA (Hs1046047_m1), ELOVL6 (Hs00907564_m1), KRT7 (Hs00559840_m1), MUC1 (Hs00159357_m1) and TBP (Hs00427620_m1) Taqman probes (Life Technologies, Waltham, MA).
Oxygen Consumption Rate Analysis
Three different donor lots of sebocytes were seeded at 60,000 cells per well in a black, clear-bottom collagen-coated 96-well plate and allowed to adhere overnight. Medium was replaced with phenol red-free STM containing treatments, including 1mM metformin, 25 µM phenformin, 5 µM 13-cis-retinoic acid, 10 µg/mL azithromycin, or 250 µM AICAR and incubated for 2 hrs at 37°C in triplicate for each donor lot. Pre-treatments were removed and reapplied in phenol red-free STM. MitoXpress Xtra Reagent (Agilent Technologies, Inc., Santa Clara, CA) was added to each well and sealed with mineral oil. Oxygen consumption rate (OCR) was determined using dual-read TRF lifetime detection on a FLUORstar plate reader (BMG LABTECH, Cary, NC) taking readings every 2 mins for 3 hrs with excitation 340 ±50 nm, emission 655 ±50 nm, delay times of 30 µs and 70 µs, and integration time of 30 µs.
Lactate Production Analysis
Three different donor lots of human primary sebocytes were seeded at a density of 40,000 cells per well in a 12-well plate using growth medium and allowed to adhere overnight. Growth medium was removed and cells were treated in STM containing 1 µM T0901317 ± 25 µM phenformin or 5 µM 13-cis-retinoic acid in triplicate for 24 hrs at 37°C. Conditioned medium was collected and lactate concentrations determined using the L-Lactate Assay Kit protocol (Cayman Chemical, Ann Arbor, MI).
Phospho-AMPK, Phospho-S6RP and Phospho-mTOR Analysis
Human primary sebocytes were seeded at 15,000 cells per well in a 96-well collagen-coated plate and allowed to adhere overnight. Growth medium was replaced with STM containing 1% FBS and the cells incubated for 24 hrs at 37°C. Treatment of 1mM metformin, 25 µM phenformin, 10 µM 13-cis-retinoic acid, 20 µg/mL azithromycin, 250 µM AICAR, or 100 nM rapamycin were applied in STM containing 1% FBS for 16 hrs; controls included no glucose or serum containing DMEM (Glucose Starve) and STM containing 10% FBS (Fed). Treatments were removed and protein lysates prepared for HTRF ELISAs using Phospho-AMPK(Thr172) and Phospho-S6RP (Ser235/236) cellular kits (CisBio US, Bedford, MA) or colorimetric ELISA using Phospho-mTOR (Ser2448) cellular kit (Cell Signaling Technology, Danvers, MA). Phospho-AMPK and phospho-S6RP signals were determined by HTRF using an Infinite f500 plate reader (Tecan, Morrisville, NC). Phospho-mTOR signals were determined by absorbance at 450nm using a SpectraMax iD3 plate reader (Molecular Devices, San Jose, CA).
Statistical Analysis
Statistical analysis was performed by GraphPad Prism software using Student’s 2-tailed t-test for two group analysis, and ANOVA Tukey’s multiple comparison test for analysis of multiple groups. Data are presented as mean ± standard deviation (SD). P‐values < 0.05 were considered statistically significant.
Results
Biguanides Induce Acute de novo Lipogenesis in Human Primary Sebocytes
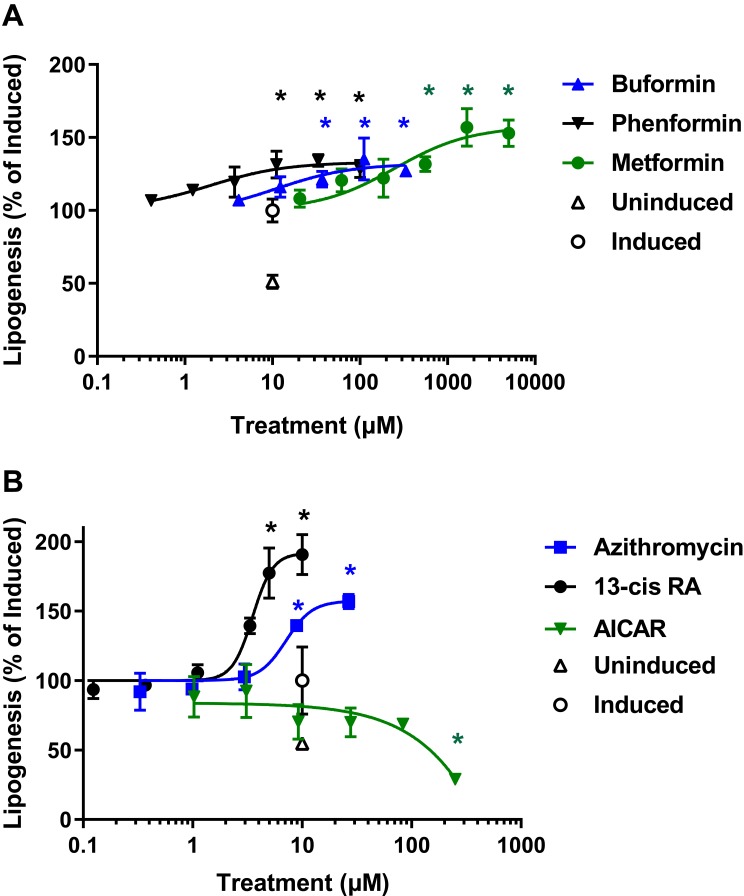
To determine the effects of biguanide treatment on sebogenesis, human primary sebocytes previously isolated from facial skin-derived sebaceous glands were seeded in 96-well plates using growth medium. Upon confluence, the cells were induced to stimulate sebogenesis by overnight incubation with 1 µM T0901317, an LXRα agonist, in the presence of increasing concentrations of biguanides based on their relative potencies. Following the pretreatment, the compounds were reapplied in medium containing 1 µM insulin and [14C]-acetate, and the incorporation of acetate into cellular lipids monitored by scintillation counting. The implementation of an assay using collagen-coated Scintiplates rapidly and effectively measured these effects without the need for lipid extraction (Figure 1A).30 In contrast to the expected decrease in lipogenesis by biguanides, we found a significant dose-dependent increase in de novo lipogenesis above that induced by T0901317 + insulin. These effects were similar to those seen with 13-cis-retinoic acid (isotretinoin) and azithromycin, which has been shown to stimulate lipogenesis in sebocyte-like Meibomian cells (Figure 1B). However, as expected, the AMPK activator, AICAR, inhibited de novo lipogenesis induced by insulin and the LXR agonist.
Figure 1.
Biguanides, isotretinoin and azithromycin stimulate acute de novo lipogenesis in human primary sebocytes. Sebocytes seeded in Scintiplates were incubated overnight with T0901317 in the presence of increasing concentrations of biguanides, isotretinoin, azithromycin or AICAR. Test articles were reapplied in the presence of insulin with T0901317 and [14C]-acetate for 4 hrs. Sebocytes were washed with PBS and the cell monolayers allowed to dry before [14C]-acetate incorporation was determined by scintillation counting. (A) Dose response of phenformin, buformin and metformin; (B) Dose response of isotretinoin (13-cis RA), azithromycin and AICAR. Controls are uninduced and induced with insulin plus T0901317. Values are plotted as mean ± SD compared to induced control (100%) of three independent determinations; *denotes p < 0.05 compared to induced control.
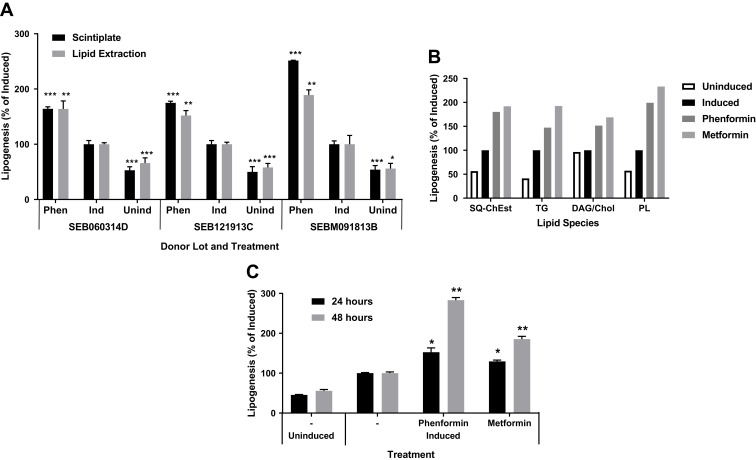
The induction in lipogenesis by biguanides was verified by using an adapted high throughput BUME lipid extraction procedure to specifically isolate acetate-labeled lipids.32 Sebocytes from multiple donors showed similar lipogenic effects using both analysis methods, suggesting that biguanides induce an unexpected acute lipogenic response in human primary sebocytes (Figure 2A). TLC analysis of the extracted lipids showed a corresponding general increase in all neutral lipid species, including triglyceride, squalene and cholesterol esters, and phospholipids (Figure 2B). Additionally, this response was both time and dose dependent, increasing the response out to 48 hrs of treatment with biguanides (Figure 2C).
Figure 2.
Biguanides induce a time dependent increase in de novo neutral lipid synthesis. Sebocytes from three donors in Scintiplates and culture plates were incubated overnight (or 48 hrs) with T0901317 plus phenformin or metformin. Biguanides were reapplied with insulin plus T0901317 and [14C]-acetate for 4 hrs. Scintiplates were analyzed as described previously. Culture plate sebocytes were harvested with trypsin/EDTA and lipid extracts prepared for liquid scintillation counting and TLC analysis of lipid species. (A) [14C]-acetate incorporation induced by phenformin (Phen) using Scintiplate and lipid extraction assays. (B) TLC lipid species analysis: SQ-ChEst, squalene and cholesterol esters; TG, triacylglycerols; DAG/Chol, diacylglycerols and cholesterol; PL, phospholipids. (C) Time course of Scintiplate assay. Values are mean ± SD of three independent cultures. *p<0.05, **p<0.01, ***p<0.001.
Biguanides Display AMPK-Independent Activities in Human Sebocytes
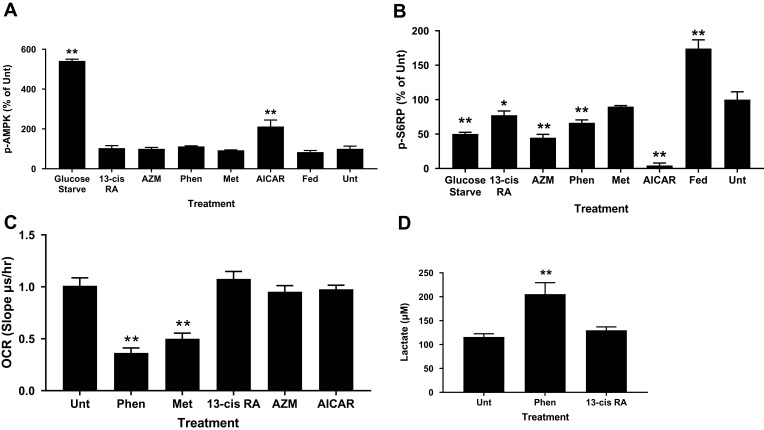
Biguanides exert their effects through complex mechanisms of action, many of which are AMPK-dependent and regulate cellular metabolism, including de novo lipogenesis. We tested whether biguanides had an effect on AMPK activation in human primary sebocytes by quantifying AMPK phosphorylation. Primary sebocytes were treated with metformin, phenformin, AICAR, azithromycin or 13-cis-retinoic acid for 24 hrs and the level of AMPK phosphorylation was compared with untreated, serum-fed or glucose-starved cells. Figure 3A shows that only glucose-starvation and AICAR treatment significantly increased AMPK phosphorylation under these conditions. S6-kinase (p70S6K) is known to be inhibited by activated AMPK, however, AMPK-independent inhibition through different mechanisms, including mTORC1, have also been identified. p70S6K activity was assessed in these same extracts, and as expected, serum feeding increased phosphorylation of S6 ribosomal protein (S6RP) while glucose starvation inhibited it (Figure 3B). Direct activation of AMPK by AICAR also greatly inhibited S6-kinase activity. However, metformin showed no effect on S6-kinase activity and phenformin, 13-cis-retinoic acid and azithromycin slightly inhibited S6-kinase in the apparent absence of AMPK activation. These results are consistent with the slight changes in phosphorylated mTOR at S2448 caused by biguanides and the significant reductions by AICAR and glucose starvation (Figure S1). The S2448 site is sensitive to AMPK-dependent phosphorylation at the nearby T2446 residue which precludes S2448 phosphorylation.
Figure 3.
Biguanides show AMPK-independent activity on S6-kinase, OCR and lactate production. Sebocytes were treated with phenformin (Phen), metformin (Met), isotretinoin (13-cis RA), azithromycin (AZM), or AICAR for 16 hrs before preparing protein lysates for (A) phospho-AMPK and (B) phospho-S6RP ELISAs. Controls are untreated (Unt-1% FBS), 10% FBS medium (Fed), and glucose starved. Values are mean ± SD of three independent determinations normalized to Unt. (C) Sebocytes were incubated with treatments for 2 hrs prior to OCR analysis. Values are mean ± SD of three different donors in triplicate. (D) Sebocytes were treated for 24 hrs with insulin plus T0901317 before lactate concentration determined in the conditioned medium. Values are mean ± SD of three different donors in triplicate. *p<0.05, **p<0.01 compared to untreated control.
To ensure that biguanides were able to directly affect the cells, we tested whether phenformin and metformin had any effect on basal mitochondrial respiration. Biguanides are known to inhibit mitochondrial complex 1 and the electron transport chain resulting in increased levels of NADH and reduced ATP production. We found that both metformin and phenformin readily inhibited the oxygen consumption rate (OCR) of primary sebocytes and, as a result, increased cellular lactate production, while 13-cis-retinoic acid, azithromycin and AICAR had no effect (Figure 3C and D).
Biguanide-Induced de novo Lipogenesis Results from Synergism with Lipogenic Activators at the Transcript Level
To determine if biguanides induced changes in lipogenic gene transcript levels, primary sebocytes were treated with biguanides in the presence or absence of 1 µM T0901317 for 48 hrs and qPCR analysis of selected mRNA performed. SREBF1 mRNA transcript levels were increased nearly 3-fold with T0901317 treatment, however, neither biguanide significantly increased SREBF1 expression beyond the level induced by the LXR agonist (Table 2). In contrast, the addition of phenformin to T0901317 significantly increased the expression levels of ACSS2, ACLY and all lipogenesis genes. The addition of metformin, however, only increased the expression levels of ACSS2 and SCD, while slightly decreasing the expression of HMGCR and SQLE. Phenformin and metformin increased the expression of ACSS2 in the absence of LXR induction even to a level higher than that of T0901317 treatment alone. Additionally, the treatment of cells with azithromycin and T0901317 significantly increased FASN, SCD, SQLE and ACLY transcript levels. All of these changes correlate with the results of the de novo lipogenesis assays. 13-cis-retinoic acid, however, significantly reduced the expression of all of the genes except SCD and HMGCR after 48 hrs of treatment. Gene expression markers of sebocyte differentiation were assessed using these same samples. Phenformin, metformin and retinoic acid shifted the gene expression phenotype towards the undifferentiated state as shown by increasing KRT7 expression and decreasing MUC1 expression levels (Table 2). Phenformin was able to promote these changes in the absence of T0901317 treatment. Azithromycin treatment unexpectedly upregulated the expression levels of both KRT7 and MUC1.
Table 2.
Gene Expression Changes in Primary Sebocytes After 48 hrs
| Gene | Uninduced | Induced | ||||||
|---|---|---|---|---|---|---|---|---|
| Unt | Phen | Met | Unt | Phen | Met | 13-cis-RA | AZM | |
| SREBF1 | 0.36 ± 0.01** | 0.35 ± 0 0.01** | 0.35 ± 0.01** | 1.00 ± 0.08 | 1.13 ± 0.11 | 0.86 ± 0.01 | 0.60 ± 0.10** | 1.23 ± 0.12 |
| ACSS2 | 1.27 ± 0.09* | 2.06 ± 0.03** | 1.93 ± 0.08** | 1.00 ± 0.03 | 3.52 ± 0.52* | 1.27 ± 0.05** | 0.49 ± 0.06** | 1.38 ± 0.21 |
| FASN | 0.70 ± 0.03* | 0.79 ± 0.04* | 0.76 ± 0.10* | 1.00 ± 0.08 | 2.38 ± 0.35* | 1.10 ± 0.12 | 0.44 ± 0.07** | 1.71 ± 0.25* |
| SCD | 0.40 ± 0.002** | 0.56 ± 0.01** | 0.47 ± 0.02** | 1.00 ± 0.04 | 2.13 ± 0.10** | 1.16 ± 0.03** | 1.04 ± 0.08 | 1.67 ± 0.10** |
| HMGCR | 1.34 ± 0.05** | 1.30 ± 0.01** | 1.27 ± 0.09* | 1.00 ± 0.01 | 1.86 ± 0.15** | 0.85 ± 0.03** | 0.87 ± 0.09 | 1.02 ± 0.09 |
| SQLE | 1.40 ± 0.13* | 1.24 ± 0.06** | 1.21 ± 0.02** | 1.00 ± 0.05 | 1.32 ± 0.05** | 0.75 ± 0.03** | 0.61 ± 0.06** | 0.87 ± 0.04* |
| ACLY | 1.10 ± 0.20 | 1.22 ± 0.02** | 1.17 ± 0.05* | 1.00 ± 0.05 | 2.50 ± 0.35* | 0.96 ± 0.03 | 0.71 ± 0.09* | 1.24 ± 0.08* |
| ACACA | 0.82 ± 0.11 | 0.95 ± 0.08 | 0.84 ± 0.07* | 1.00 ± 0.08 | 1.29 ± 0.09* | 0.99 ± 0.04 | 0.79 ± 0.09* | 1.37 ± 0.19 |
| ELOVL6 | 1.12 ± 0.25 | 1.19 ± 0.01 | 1.18 ± 0.05* | 1.00 ± 0.09 | 1.47 ± 0.08** | 1.01 ± 0.06 | 0.83 ± 0.04* | 1.33 ± 0.19 |
| KRT7 | 0.75 ± 0.09* | 1.37 ± 0.09** | 1.15 ± 0.15 | 1.00 ± 0.04 | 1.51 ± 0.09** | 1.23 ± 0.09* | 8.56 ± 1.07** | 2.66 ± 0.82* |
| MUC1 | 1.08 ± 0.26 | 0.71 ± 0.09* | 0.88 ± 0.07 | 1.00 ± 0.08 | 0.58 ± 0.13* | 0.67 ± 0.11* | 0.50 ± 0.08** | 3.64 ± 0.37** |
Notes: Uninduced, treated 48 hrs in Sebocyte Treatment Medium; Induced, treated 48 hrs with 1 µM T0901317 in Sebocyte Treatment Medium. Values are relative gene expression levels compared to induced control and shown as mean ± SD; *denotes p < 0.05 and **p<0.01 compared to induced control.
Abbreviations: Unt, untreated; Phen, 10 µM phenformin; Met, 1 mM metformin; 13-cis RA, 5 µM 13-cis-retinoic acid; AZM, 25 µM azithromycin.
Discussion
Human primary sebocytes can be induced to stimulate sebogenesis in culture and thereby provide a cell system to dissect molecular mechanisms and identify sebogenesis inhibitors as potential acne therapeutics.2,29,33,34 Several methods of activation have been described, including the addition of serum, lipids and small molecules. Our system relies on using an LXR agonist, T0901317, combined with insulin to stimulate sebogenesis.2 We have adapted a scintillation proximity assay using 96-well Scintiplates to monitor [14C]-acetate incorporation into newly synthesized cellular lipids.30 This assay directly correlates with a similar 96-well format using a butanol-based lipid extraction method to specifically isolate neutral lipids for quantification and lipid species analysis via TLC.2,32 In these systems, phenformin, buformin and metformin cause an acute increased flux of de novo lipogenesis in human primary sebocytes that is dose and time dependent, and show an increase in neutral lipid species by TLC. These surprising results stand in stark contrast to previous reports showing biguanide inhibition of LXR/SREBP lipogenic signaling through activation of AMPK.35–37 Consistent with those previous reports, we found that AICAR effectively activated AMPK and inhibited lipogenesis in the primary sebocyte assay. Biguanides, however, did not activate AMPK in primary sebocytes, but did show AMPK-independent inhibition of mitochondrial function by reducing oxygen consumption, increased lactate production and modest inhibition of S6-kinase possibly through mTORC1 inhibition.38,39
Activation of LXR by T0901317 is known to increase SREBP1-regulated genes necessary for increased lipogenesis, including FASN, SCD and ACACA.31,40 In human primary sebocytes, T0901317 induced a 3-fold increase in SREBF1 expression, leading to increased FASN and SCD expression. Surprisingly, co-treatment with T0901317 and phenformin further upregulated FASN, SCD, HMGCR, SQLE and ACACA expression levels, which is consistent with the increased de novo lipogenesis seen in the cell-based assays. Unlike other acute sebogenic agonists, isotretinoin and azithromycin, biguanides induced a large increase in ACSS2, cytosolic acetyl-CoA synthetase, and a modest increase in ACLY, ATP-citrate lyase, gene expression on their own, which is enhanced by the addition of T0901317. Both enzymes regulate the level of acetyl-CoA through metabolism of acetate or citrate providing the necessary building blocks for de novo lipogenesis. In the absence of a lipogenesis activator this likely has little effect on sebogenesis. In fact we do not see an increase in sebogenesis when cells are treated only with biguanides. However, in the presence of lipogenic activators such as T0901317, there is a synergistic effect upregulating several genes in the de novo lipogenesis pathway.
Several studies have also shown that metformin and phenformin reduce TCA cycle intermediates and glucose-derived citrate while increasing glucose-derived lactate.41,42 These effects are mediated through biguanide inhibition of mitochondrial complex 1 and reduction in oxidative phosphorylation, forcing cells to utilize other sources for fatty acid synthesis. Deficiency in respiration, hypoxia or cancer transformation can force cells into glycolytic metabolism, increasing ACSS2, ACLY and FASN to compensate for the loss of mitochondrial respiration and capture pyruvate-derived acetyl-CoA as citrate for use in addition to exogenous acetate for cytosolic fatty acid synthesis.42,43 Biguanide inhibition of mitochondrial complex 1 in human primary sebocytes reduces cellular respiration and leads to a similar acute response to increase de novo lipogenesis from acetate and citrate. Over a longer time period it would be expected that the reduced level of glucose-derived intermediates and oxidative respiration would lead to a decrease in the overall mass of neutral lipid synthesis as seen in other cell types.42
In support of the utility of biguanides to reduce sebocyte maturation and differentiation, we noted a shift towards less mature sebocytes after a 48 hr treatment with biguanides. Metformin and phenformin significantly induced KRT7 and decreased MUC1 transcript levels, markers of undifferentiated and mature sebocytes, respectively, indicating a trend towards a less differentiated sebocyte population.44–46 Isotretinoin demonstrated the most significant changes in these differentiation markers, heavily favoring the undifferentiated state. This is consistent with previous studies showing isotretinoin inhibiting sebogenesis by causing cell death and altering the sebocyte population makeup towards the undifferentiated state.47,48 Azithromycin is known to stimulate lipogenesis in Meibomian cells, which are sebocyte-like cells responsible for producing lipids to maintain tear-film stability.49,50 Azithromycin showed a similar effect in human primary sebocytes, significantly increasing acetate incorporation into lipids and lipogenic gene transcripts. However, the antibiotic caused a significant increase in both KRT7 and MUC1 transcript levels, suggesting a possible increase in the early differentiation state and terminal sebocyte differentiation resulting in increased lipogenesis.
The role of metformin in improving whole body metabolism is well established from its long history as a first-line type 2 diabetes therapy.51–53 Through AMPK-dependent and –independent mechanisms, metformin alters cellular energy utilization to favor energy production over energy utilization. However, most of the studies involving metformin’s effects on fatty acid metabolism have been carried out in hepatocytes, adipocytes or skeletal muscle cells and have focused on long term effects and not on the acute effects of biguanide treatment. Because metformin can improve insulin resistance and prevent progression to impaired glucose tolerance, it is often prescribed off-label for PCOS patients with hyperinsulinemia.54,55 In the absence of direct clinical data, anecdotal reports by PCOS patients with severe acne suggest that at the initiation of metformin treatment to improve insulin resistance, some have suffered from acne flare-up.56–60 However, after longer treatment and normalization of metabolism, their acne improved. This response is similar to the initiation of isotretinoin treatment and can result in patients discontinuing treatment or reducing compliance.8,9,11,61–63 To determine if metformin causes a similar flare-up, it will be necessary to follow PCOS patients beginning metformin treatment and assess sebum production and acne severity in the early weeks of treatment.
Conclusion
Based on our in vitro results using human primary sebocytes, we have identified an acute response to biguanide treatment that might result in an acne flare-up in patients starting metformin therapy, especially PCOS patients. These patients with severe acne have high levels of lipogenic inducers, such as androgens and insulin, which correlate to the induced state in our in vitro system. Adding biguanides to such an induced system would be expected to significantly increase acute sebogenesis by altering gene expression to upregulate de novo lipogenesis, resulting in more sebum production and the worsening of acne.
Disclosure
This study was funded by ZenBio, Inc. and authors JN and BMB are employees of ZenBio. The authors report no other conflicts of interest in this work.
References
- 1.Degitz K, Placzek M, Borelli C, et al. Pathophysiology of acne. J Dtsch Dermatol Ges. 2007;5:316–323. doi: 10.1111/j.1610-0387.2007.06274.x [DOI] [PubMed] [Google Scholar]
- 2.Hunt DW, Winters GC, Brownsey RW, et al. Inhibition of sebum production with the acetyl coenzyme A carboxylase inhibitor olumacostat glasaretil. J Invest Dermatol. 2017;137:1415–1423. doi: 10.1016/j.jid.2016.12.031 [DOI] [PubMed] [Google Scholar]
- 3.Harris HH, Downing DT, Stewart ME, et al. Sustainable rates of sebum secretion in acne patients and matched normal control subjects. J Am Acad Dermatol. 1983;8:200–203. doi: 10.1016/S0190-9622(83)70023-X [DOI] [PubMed] [Google Scholar]
- 4.Mourelatos K, Eady EA, Cunliffe WJ, et al. Temporal changes in sebum excretion and propionibacterial colonization in preadolescent children with and without acne. Br J Dermatol. 2007;156:22–31. doi: 10.1111/j.1365-2133.2006.07517.x [DOI] [PubMed] [Google Scholar]
- 5.Pappas A, Johnsen S, Liu JC, et al. Sebum analysis of individuals with and without acne. Dermatoendocrinol. 2009;1:157–161. doi: 10.4161/derm.1.3.8473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lumsden KR, Nelson AM, Dispenza MC, et al. Isotretinoin increases skin-surface levels of neutrophil gelatinase-associated lipocalin in patients treated for severe acne. Br J Dermatol. 2011;165:302–310. doi: 10.1111/j.1365-2133.2011.10362.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nelson AM, Zhao W, Gilliland KL, et al. Neutrophil gelatinase-associated lipocalin mediates 13-cis retinoic acid-induced apoptosis of human sebaceous gland cells. J Clin Invest. 2008;118:1468–1478. doi: 10.1172/JCI33869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vallerand IA, Lewinson RT, Farris MS, et al. Efficacy and adverse events of oral isotretinoin for acne: a systematic review. Br J Dermatol. 2018;178:76–85. doi: 10.1111/bjd.15668 [DOI] [PubMed] [Google Scholar]
- 9.Borghi A, Mantovani L, Minghetti S, et al. Acute acne flare following isotretinoin administration: potential protective role of low starting dose. Dermatology. 2009;218:178–180. doi: 10.1159/000182270 [DOI] [PubMed] [Google Scholar]
- 10.Demircay Z, Kus S, Sur H. Predictive factors for acne flare during isotretinoin treatment. Eur J Dermatol. 2008;18:452–456. doi: 10.1684/ejd.2008.0441 [DOI] [PubMed] [Google Scholar]
- 11.Katz RA, Jorgensen H, Nigra TP. Flare of cystic acne from oral isotretinoin. J Am Acad Dermatol. 1983;8:132–133. doi: 10.1016/S0190-9622(83)80302-8 [DOI] [PubMed] [Google Scholar]
- 12.Esler WP, Tesz GJ, Hellerstein MK, et al. Human sebum requires de novo lipogenesis, which is increased in acne vulgaris and suppressed by acetyl-CoA carboxylase inhibition. Sci Transl Med. 2019:11. doi: 10.1126/scitranslmed.aau8465 [DOI] [PubMed] [Google Scholar]
- 13.Burney W, Bosanac SS, Nguyen C, et al. Short-term exposure of human sebocytes to 13-cis-retinoic acid induces acnegenic changes. Br J Dermatol. 2018;179:1201–1202. doi: 10.1111/bjd.16837 [DOI] [PubMed] [Google Scholar]
- 14.Melnik BC, Schmitz G. Role of insulin, insulin-like growth factor-1, hyperglycaemic food and milk consumption in the pathogenesis of acne vulgaris. Exp Dermatol. 2009;18:833–841. doi: 10.1111/j.1600-0625.2009.00924.x [DOI] [PubMed] [Google Scholar]
- 15.Wang QY, Song Y, Huang W, et al. Comparison of drospirenone- with cyproterone acetate-containing oral contraceptives, combined with metformin and lifestyle modifications in women with polycystic ovary syndrome and metabolic disorders: a prospective randomized control trial. Chin Med J (Engl). 2016;129:883–890. doi: 10.4103/0366-6999.179783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ibanez L, de Zegher F. Low-dose flutamide-metformin therapy for hyperinsulinemic hyperandrogenism in non-obese adolescents and women. Hum Reprod Update. 2006;12:243–252. doi: 10.1093/humupd/dmi054 [DOI] [PubMed] [Google Scholar]
- 17.Harborne L, Fleming R, Lyall H, et al. Descriptive review of the evidence for the use of metformin in polycystic ovary syndrome. Lancet. 2003;361:1894–1901. doi: 10.1016/S0140-6736(03)13493-9 [DOI] [PubMed] [Google Scholar]
- 18.Kolodziejczyk B, Duleba AJ, Spaczynski RZ, et al. Metformin therapy decreases hyperandrogenism and hyperinsulinemia in women with polycystic ovary syndrome. Fertil Steril. 2000;73:1149–1154. doi: 10.1016/S0015-0282(00)00501-X [DOI] [PubMed] [Google Scholar]
- 19.Tan S, Hahn S, Benson S, et al. Metformin improves polycystic ovary syndrome symptoms irrespective of pre-treatment insulin resistance. Eur J Endocrinol. 2007;157:669–676. doi: 10.1530/EJE-07-0294 [DOI] [PubMed] [Google Scholar]
- 20.Israni DA, Mehta TY, Goyal RK. Effect of metformin therapy in female visiting dermatologist for acne vulgaris having endocrine and sonographic characteristics of polycystic ovary syndrom (PCOS). Asian J Pharm Clin Res. 2013;6:76–82. [Google Scholar]
- 21.Sharma S, Mathur DK, Paliwal V, et al. Efficacy of metformin in the treatment of acne in women with polycystic ovarian syndrome: a newer approach to acne therapy. J Clin Aesthet Dermatol. 2019;12:34–38. [PMC free article] [PubMed] [Google Scholar]
- 22.Cong TX, Hao D, Wen X, et al. From pathogenesis of acne vulgaris to anti-acne agents. Arch Dermatol Res. 2019;311:337–349. doi: 10.1007/s00403-019-01908-x [DOI] [PubMed] [Google Scholar]
- 23.Fabbrocini G, Izzo R, Faggiano A, et al. Low glycaemic diet and metformin therapy: a new approach in male subjects with acne resistant to common treatments. Clin Exp Dermatol. 2016;41:38–42. doi: 10.1111/ced.12673 [DOI] [PubMed] [Google Scholar]
- 24.Lee JK, Smith AD. Metformin as an adjunct therapy for the treatment of moderate to severe acne vulgaris. Dermatol Online J. 2017;23:11. [PubMed] [Google Scholar]
- 25.Robinson S, Kwan Z, Tang MM. Metformin as an adjunct therapy for the treatment of moderate to severe acne vulgaris: a randomized open-labeled study. Dermatol Ther. 2019;e12953. doi: 10.1111/dth.12953 [DOI] [PubMed] [Google Scholar]
- 26.Canto C, Gerhart-Hines Z, Feige JN, et al. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou G, Myers R, Li Y, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jenkins Y, Sun TQ, Markovtsov V, et al. AMPK activation through mitochondrial regulation results in increased substrate oxidation and improved metabolic parameters in models of diabetes. PLoS One. 2013;8:e81870. doi: 10.1371/journal.pone.0081870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fontao F, Barnes L, Kaya G, et al. From the cover: high susceptibility of lrig1 sebaceous stem cells to tcdd in mice. Toxicol Sci. 2017;160:230–243. doi: 10.1093/toxsci/kfx179 [DOI] [PubMed] [Google Scholar]
- 30.Ung T, Mason JL, Robinson RG, et al. A cellular assay for inhibitors of the fatty acid biosynthetic pathway using scintillating microplates. Assay Drug Dev Technol. 2015;13:285–292. doi: 10.1089/adt.2015.644 [DOI] [PubMed] [Google Scholar]
- 31.Grefhorst A, Elzinga BM, Voshol PJ, et al. Stimulation of lipogenesis by pharmacological activation of the liver X receptor leads to production of large, triglyceride-rich very low density lipoprotein particles. J Biol Chem. 2002;277:34182–34190. doi: 10.1074/jbc.M204887200 [DOI] [PubMed] [Google Scholar]
- 32.Lofgren L, Stahlman M, Forsberg GB, et al. The BUME method: a novel automated chloroform-free 96-well total lipid extraction method for blood plasma. J Lipid Res. 2012;53:1690–1700. doi: 10.1194/jlr.D023036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schneider MR, Zouboulis CC. Primary sebocytes and sebaceous gland cell lines for studying sebaceous lipogenesis and sebaceous gland diseases. Exp Dermatol. 2018;27:484–488. doi: 10.1111/exd.13513 [DOI] [PubMed] [Google Scholar]
- 34.Zhang MF, Cai XL, Jing KP, et al. Differentiation model establishment and differentiation-related protein screening in primary cultured human sebocytes. Biomed Res Int. 2018;2018:7174561. doi: 10.1155/2018/7174561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee J, Hong SW, Park SE, et al. AMP-activated protein kinase suppresses the expression of LXR/SREBP-1 signaling-induced ANGPTL8 in HepG2 cells. Mol Cell Endocrinol. 2015;414:148–155. doi: 10.1016/j.mce.2015.07.031 [DOI] [PubMed] [Google Scholar]
- 36.Li Y, Xu S, Mihaylova MM, et al. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011;13:376–388. doi: 10.1016/j.cmet.2011.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yap F, Craddock L, Yang J. Mechanism of AMPK suppression of LXR-dependent Srebp-1c transcription. Int J Biol Sci. 2011;7:645–650. doi: 10.7150/ijbs.7.645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dowling RJ, Zakikhani M, Fantus IG, et al. Metformin inhibits mammalian target of rapamycin-dependent translation initiation in breast cancer cells. Cancer Res. 2007;67:10804–10812. doi: 10.1158/0008-5472.CAN-07-2310 [DOI] [PubMed] [Google Scholar]
- 39.Kalender A, Selvaraj A, Kim SY, et al. Metformin, independent of AMPK, inhibits mTORC1 in a rag GTPase-dependent manner. Cell Metab. 2010;11:390–401. doi: 10.1016/j.cmet.2010.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Darimont C, Avanti O, Zbinden I, et al. Liver X receptor preferentially activates de novo lipogenesis in human preadipocytes. Biochimie. 2006;88:309–318. doi: 10.1016/j.biochi.2005.08.010 [DOI] [PubMed] [Google Scholar]
- 41.Janzer A, German NJ, Gonzalez-Herrera KN, et al. Metformin and phenformin deplete tricarboxylic acid cycle and glycolytic intermediates during cell transformation and NTPs in cancer stem cells. Proc Natl Acad Sci U S A. 2014;111:10574–10579. doi: 10.1073/pnas.1409844111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Griss T, Vincent EE, Egnatchik R, et al. Metformin antagonizes cancer cell proliferation by suppressing mitochondrial-dependent biosynthesis. PLoS Biol. 2015;13:e1002309. doi: 10.1371/journal.pbio.1002309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yoshii Y, Furukawa T, Saga T, et al. Acetate/acetyl-CoA metabolism associated with cancer fatty acid synthesis: overview and application. Cancer Lett. 2015;356:211–216. doi: 10.1016/j.canlet.2014.02.019 [DOI] [PubMed] [Google Scholar]
- 44.Barrault C, Dichamp I, Garnier J, et al. Immortalized sebocytes can spontaneously differentiate into a sebaceous-like phenotype when cultured as a 3D epithelium. Exp Dermatol. 2012;21:314–316. doi: 10.1111/j.1600-0625.2012.01463.x [DOI] [PubMed] [Google Scholar]
- 45.Zouboulis CC, Xia LQ, Detmar M, et al. Culture of human sebocytes and markers of sebocytic differentiation in vitro. Skin Pharmacol. 1991;4:74–83. doi: 10.1159/000210927 [DOI] [PubMed] [Google Scholar]
- 46.Barrault C, Garnier J, Pedretti N, et al. Androgens induce sebaceous differentiation in sebocyte cells expressing a stable functional androgen receptor. J Steroid Biochem Mol Biol. 2015;152:34–44. doi: 10.1016/j.jsbmb.2015.04.005 [DOI] [PubMed] [Google Scholar]
- 47.Landthaler M, Kummermehr J, Wagner A, et al. Inhibitory effects of 13-cis-retinoic acid on human sebaceous glands. Arch Dermatol Res. 1980;269:297–309. doi: 10.1007/BF00406424 [DOI] [PubMed] [Google Scholar]
- 48.Melnik BC. p53: key conductor of all anti-acne therapies. J Transl Med. 2017;15:195. doi: 10.1186/s12967-017-1297-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu Y, Kam WR, Ding J, et al. Effect of azithromycin on lipid accumulation in immortalized human meibomian gland epithelial cells. JAMA Ophthalmol. 2014;132:226–228. doi: 10.1001/jamaophthalmol.2013.6030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu Y, Kam WR, Ding J, et al. Can tetracycline antibiotics duplicate the ability of azithromycin to stimulate human meibomian gland epithelial cell differentiation? Cornea. 2015;34:342–346. doi: 10.1097/ICO.0000000000000351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jia Y, Lao Y, Zhu H, et al. Is metformin still the most efficacious first-line oral hypoglycaemic drug in treating type 2 diabetes? A network meta-analysis of randomized controlled trials. Obes Rev. 2019;20:1–12. doi: 10.1111/obr.12753 [DOI] [PubMed] [Google Scholar]
- 52.Valencia WM, Botros D, Vera-Nunez M, et al. Diabetes treatment in the elderly: incorporating geriatrics, technology, and functional medicine. Curr Diab Rep. 2018;18:95. doi: 10.1007/s11892-018-1052-y [DOI] [PubMed] [Google Scholar]
- 53.Valencia WM, Florez H. Pharmacological treatment of diabetes in older people. Diabetes Obes Metab. 2014;16:1192–1203. doi: 10.1111/dom.12362 [DOI] [PubMed] [Google Scholar]
- 54.Chang HH, Hsueh YS, Cheng YW, et al. Association between polymorphisms of OCT1 and metabolic response to metformin in women with polycystic ovary syndrome. Int J Mol Sci. 2019;20:1720. doi: 10.3390/ijms20071720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Palomba S, Falbo A, Zullo F, et al. Evidence-based and potential benefits of metformin in the polycystic ovary syndrome: a comprehensive review. Endocr Rev. 2009;30:1–50. doi: 10.1210/er.2008-0030 [DOI] [PubMed] [Google Scholar]
- 56.Alwaysneedinghelp. Metformin causing breakouts In: Metformin Causing Breakouts in General Acne Discussion. acne.org; 2019; Available from: https://www.acne.org/forums/topics/382750-metformincausing-breakouts/#comment-3611000. Accessed 1 July 2019. [Google Scholar]
- 57.Graciemeow. After first month of metformin In: My Journey-Logs on Various Meds/Acne/Treatments. acne.org; 2013; Available from: https://www.acne.org/blogs/entry/27508-afterfirst-month-of-metformin/. Accessed 1 July 2019. [Google Scholar]
- 58.Graciemeow. When do I get a break In: My Journey-Logs on Various Meds/Acne/Treatments. acne.org; 2013; Available from: https://www.acne.org/blogs/entry/27452-when-do-i-get-a-break/. Accessed 1 July 2019. [Google Scholar]
- 59.LillyRose7. Metformin for acne In: Prescription Acne Medications. acne.org; 2013; Available from: https://www.acne.org/forums/topic/313080-metformin-for-acne/#comment-3343746. Accessed 1 July 2019. [Google Scholar]
- 60.Sunshinenmysoul3. Did this happen to anyone else taking metformin? In: Living with PCOS. BabyCenter, LLC; 2009; Available from: https://community.babycenter.com/post/a17686885/did_this_happen_to_anyone_else_taking _metformin. Accessed 1 July 2019. [Google Scholar]
- 61.Chivot M. [Acne flare-up and deterioration with oral isotretinoin]. Ann Dermatol Venereol. 2001;128:224–228. [PubMed] [Google Scholar]
- 62.Lehucher Ceyrac D, Chaspoux C, Sulimovic L, et al. [Aggravation of acne by isotretinoin. 6 cases, predictive factors]. Ann Dermatol Venereol. 1998;125:496–499. [PubMed] [Google Scholar]
- 63.Poli F, Revuz J. [Acne flare on isotretinoin: a pointer to diagnosis of hidradenitis suppurativa]. Ann Dermatol Venereol. 2019;146:4–8. doi: 10.1016/j.annder.2018.07.020 [DOI] [PubMed] [Google Scholar]