Abstract
Malignant melanoma of the skin is the leading cause of death from skin cancer and ranks fifth in cancer incidence among all cancers in the United States. While melanoma mortality has remained steady for the past several decades, melanoma incidence has been increasing, particularly among fair-skinned individuals. According to the American Cancer Society, nearly 10,000 people in the United States will die from melanoma this year. Individuals with dark skin complexion are protected damage generated by UV-light due to the high content of UV-blocking melanin pigment in their epidermis as well as better capacity for melanocytes to cope with UV damage. There is now ample evidence that suggests that the melanocortin 1 receptor (MC1R) is a major melanoma risk factor. Inherited loss-of-function mutations in MC1R are common in melanoma-prone persons, correlating with a less melanized skin complexion and poorer recovery from mutagenic photodamage. We and others are interested in the MC1R signaling pathway in melanocytes, its mechanisms of enhancing genomic stability and pharmacologic opportunities to reduce melanoma risk based on those insights. In this chapter, we review melanoma risk factors, the MC1R signaling pathway, and the relationship between MC1R signaling and DNA repair.
1. Melanoma: A growing problem
1.1. Epidemiology
Melanoma incidence has been increasing for the past 50 years (Fig. 1) and is now the fifth most common cause of cancer in the United States (Tellez et al., 2016). The US incidence has risen from 8.2 new cases per 100,000 in 1975 to 23 new cases per 100,000 in 2015, while the highest incidence globally is in Australia and New Zealand (Azoury & Lange, 2014; Berwick et al., 2016). Concurrent with its increasing incidence is the rising treatment costs of melanoma, which was estimated to total $457 million in 2011 (Guy, Machlin, Ekwueme, & Yabroff, 2015) and are expected to reach $1.6 billion by 2030 (Guy, Machlin, et al., 2015; Guy, Thomas, et al., 2015). In the United States, an estimated 91,270 individuals will be diagnosed and 9320 will die of melanoma in 2018 according to the American Cancer Society. Per the SEER database, Caucasians are disproportionately affected out of all racial groups. Though tremendous strides are being made in the therapy of melanoma (particularly in molecularly targeted therapy and immuno-therapies), there is still a great need to reduce the morbidity and mortality of melanoma.
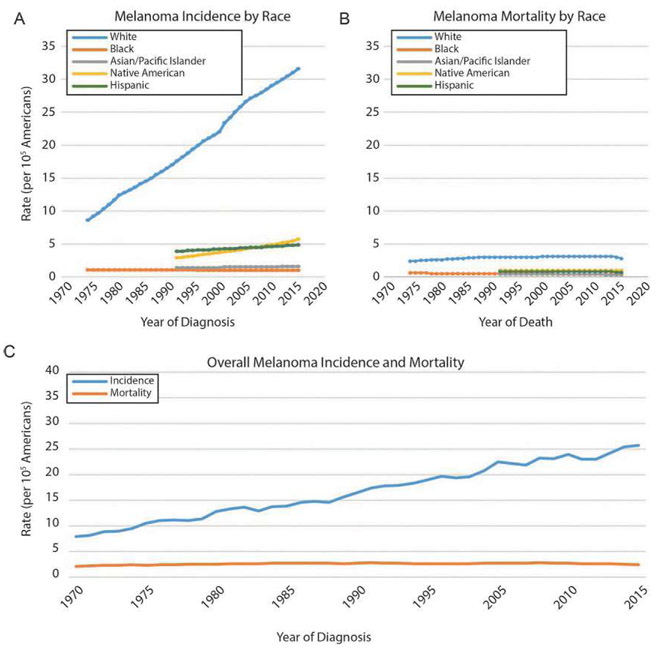
Fig. 1.
US incidence and mortality of cutaneous malignant melanoma by race, 1975–2015. (A) Melanoma incidence has been increasing steadily over the past four decades among non-Hispanic Caucasians, while all other ethnicities show very moderate, if any, growth in melanoma incidence. (B) Melanoma mortality has remained steady in each ethnic group in the time frame shown, with the mortality being highest among whites. (C) Overall, melanoma incidence has been increasing steadily since the early 1970s, almost exclusively a consequence of increased incidence among whites. Mortality has been effectively unchanged in the same period. Source: NCI SEER (Surveillance, Epidemiology, and End Results, http://seer.cancer.gov); rates are per 105 individuals.
Of the three most common types of skin cancer (basal cell carcinoma, squamous cell carcinoma and melanoma), melanoma accounts for 75% of all skin cancer related deaths with the death rate at 2.4 deaths per 100,000 (Schadendorf et al., 2015). If detected early enough and excised before the disease has had the chance to spread beyond the primary site, the 5-year survival rate is almost 92%. However, melanoma is quick to metastasize and long-term prognosis diminishes with increasing tumor stage (Azoury & Lange, 2014). Like other carcinogen-induced malignancies, incidence of melanoma increases with age (Fig. 2), presumably due to the time it takes for a susceptible cell (in this case a melanocyte) to accumulate the appropriate set of mutations and other genetic changes to fuel carcinogenesis. Beginning in the fifth decade of fife, melanoma incidence and mortality are higher in males than in females; the reasons for this are not well understood (Schadendorf et al., 2015); however, melanoma can affect people at any age. In fact, melanoma is now among the most common type of cancers in young adults (Tellez et al., 2016). It is also diagnosed in childhood and adolescence, accounting for 300–500 cases annually (Reguerre et al., 2016; Stefanaki, Chardalias, Soura, Katsarou, & Stratigos, 2017). In children, melanomas can be somewhat atypical. Instead of the usual black or dark brown growth noted on the skin (often arising in the context of a nevus), pediatric melanomas may be amelanotic and subtle (Mitkov et al., 2016; Stefanaki et al., 2017). The tumor biology of melanoma in children shares some features with that of adults but also has variable oncogene expression (e.g., c-KIT) (Lorimer et al., 2016). Because its incidence is increasing and because it can affect individuals of any age, it is imperative to identify and modify melanoma risk factors in order to curb the rising toll on morbidity, mortality and healthcare burden. A variety of environmental and inherited risk factors has been implicated in melanoma risk (Table 1).
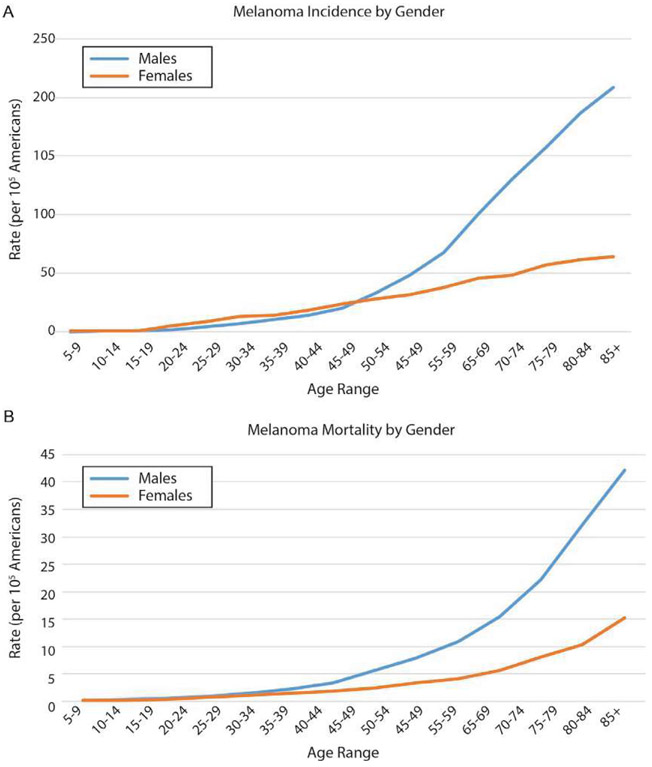
Fig. 2.
US incidence and mortality of cutaneous malignant melanoma by gender, 2011–2015, all races and all ages. (A) Melanoma incidence increases with age in both males and females, but the rate of increase in males is considerably higher than the rate seen in females. For females, melanoma incidence increases steadily from the late teens onward, while for males the incidence is actually less than it is for females until the fourth decade of life when the incidence balloons and quickly separates from the female melanoma incidence. (B) Similarly, melanoma mortality is equivalent between younger males and females, and while male and female melanoma mortality begin to increase around the mid-40s, the rate of increase is considerably greater in males than it is in females. Source: NCI SEER (Surveillance, Epidemiology, and End Results, http://seer.cancer.gov); rates are per 105 individuals.
Table 1.
Environmental and inherited melanoma risk factors.
| Environmental risk factors | Inherited risk factors | ||
|---|---|---|---|
| UV exposure | Intermittent intense sun exposure is associated with elevated risk of melanoma | Lighter hued phenotype | Fair skin, blue/green eyes, blond/red hair lead to increased susceptibility to UV-induced DNA damage |
| Indoor tanning has led to increased incidences of melanoma, particularly among women x < 30 year old | History of skin cancer | Personal and family history of melanoma and non-melanoma skin cancer leads to increased risk for developing melanoma | |
| Nevi | Large diameter congenital nevi, atypical nevi, and elevated number of nevi elevates the risk of melanoma development | ||
| Environmental exposure | More common in printing, industrial, and electronic industry, heavy metals such as chromium is affiliated with increased risk of developing melanoma | Immunodeficient state | T-cell immunodeficient diseases like HIV leads to elevated risk of developing melanoma |
| Pharmacological immunosuppression | Graft rejection prevention requires medications to suppress T cell functions, leading to a threefold risk of melanoma development in transplant patients on immunosuppressants | DNA repair mechanism defect | Xeroderma pigmentosum has a defect in nucleotide excision repair pathway, leading to inability to repair UV-induced DNA damages |
| Melanocortin 1 receptor defect (MC1R) | Heterozygosity or homozygosity in the allele for MC1R leads to inability to tan and repair UV-induced DNA damages | ||
A variety of intrinsic and extrinsic risk factors has been associated with melanoma.
1.2. UV exposure
It is generally accepted that the major environmental contributor for melanoma development is ultraviolet (UV) exposure. It is estimated that over 80% of melanoma cases are attributable to UV exposure in Australia, New Zealand, the Unites States, Canada, Nordic countries, and the United Kingdom (Berwick et al., 2016). The world health organization (WHO) classifies UV as a Group 1 human carcinogen, their highest cancer risk category, because of the very strong evidence finking UV with melanoma and other skin malignancies (Lazovich et al., 2016; Seidenberg, Mahalingam-Dhingra, Weinstock, Sinclair, & Geller, 2015). The natural source of UV is the sun, though artificial UV sources such as tanning salons are increasingly important.
Multiple epidemiological studies have shown that intermittent UV exposure, particularly in high sunburn-inducing doses, leads to increased risk of developing melanoma (Park et al., 2012). In contrast, chronic sun exposure, as might occur with an outdoor occupation, is more associated with keratinocyte malignancies such as squamous cell carcinoma or basal cell carcinoma (Kricker et al., 2007; Moan, Baturaite, Porojnicu, Dahlback, & Juzeniene, 2012; Nielsen, Masback, Olsson, & Ingvar, 2012). A major factor in skin cancer risk is sun-seeking behavior. Societal norms of favoring a tanned appearance began in the 1920s–1940s and have persisted to the modern era (Chang et al., 2014). The desire for a darker complexion (particularly in fair-skinned people) promotes sunbathing and indoor tanning, however, these activities are clearly linked with higher risk of melanoma and non-melanoma skin cancers (Molinaro et al., 2015; O’Leary, Diehl, & Levins, 2014; Weinstock & Fisher, 2010). Decreasing tanning bed use and rigorous use of topical sunscreens in outdoor activities can attenuate UV exposure and decrease incidence of melanoma and other skin (Ghiasvand, Weiderpass, Green, Lund, & Veierod, 2016; Green, Williams, Logan, & Strutton, 2011; Olsen et al., 2015).
1.3. Indoor tanning
Indoor tanning devices were developed in 1960s in response to the growing demand for having a “healthy tan” look. Early tanning devices emitted mainly in the UV-B range because that UV energy yielded a longer-lasting tan (Autier et al., 1991), however, with the growing recognition of the carcinogenic potential of UV-B, tanning devices began favoring blending more UV-A into their emissions (Geller et al., 2002; Ghiasvand et al., 2016; Swerdlow et al., 1988). Yet, despite incorporating more UV-A, tanning beds were still associated with elevated risk of developing melanoma (Autier et al., 1994). More recent studies suggest that people who ever used a tanning device have roughly a 50% higher chance of developing basal cell carcinoma and more than a 100% increased risk for squamous cell carcinoma (Karagas et al., 2002; Schulman & Fisher, 2009; Zhang et al., 2012). Age at first use of a tanning device matters, with highest risk being associated with use in adolescence. The same pattern holds true for melanoma (Bataille, Winnett, Sasieni, Newton Bishop, & Cuzick, 2004; Clough-Gorr, Titus-Ernstoff, Perry, Spencer, & Ernstoff, 2008) with tanning bed users <30 years old were more 6-times more likely to developing early-onset melanoma (Cust et al., 2011; Ghiasvand et al., 2016). A recent meta-analysis of melanoma and tanning bed use suggested that first exposure to indoor tanning before 35 years of age raises lifetime melanoma risk by 75% (Schulman & Fisher, 2009). Legislation prohibiting tanning beds has been enacted in various nations (e.g., Australia) and regulation of indoor tanning for minors either has been enacted or is being considered in many US states. Clearly, increasing awareness of the risks of skin cancer from tanning bed use would serve to diminish the incidence of melanoma.
1.4. Immunodeficiency
Solid organ transplant recipients who require pharmacologic suppression of T cell immunity to prevent graft rejection are among the highest risk patients for melanoma and other skin cancers. The relative risk of developing squamous cell carcinoma, basal cell carcinoma and melanoma are 65-, 10- and 3-fold, respectively (Arron et al., 2016; Berwick et al., 2016; Garrett et al., 2017). In addition, transplant patients with prior history of melanoma were noted to have a 27-fold increased risk of death due to melanoma-specific deaths, 30% increase in overall mortality, and 5-fold increased risk of incidence post-transplant (Arron et al., 2016). Fortunately, the cumulative incidence of melanoma-related events at the 5-year and 10-year post-transplant mark is low for those who had melanoma prior to transplant (1.2% and 3%, respectively) as well as in those who never had melanoma before transplant with the incidence (0.5% and 0.9%, respectively) (Arron et al., 2016). Nonetheless, these data highlight the importance of immunosurveillance in melanoma resistance and emphasize that defective immunity is a bona fide melanoma risk factor.
Other T cell deficiencies also heighten melanoma risk. Human Immunodeficiency (HIV), which infects millions of individuals worldwide through sexual activity, exposure to infected blood or perinatal transmission, depletes CD4+ T-cells when inadequately recognized and treated (Chang, Doiron, & Maurer, 2017). HIV patients have elevated incidences of skin cancers including squamous cell carcinoma, basal cell carcinoma and melanoma. In fact, melanoma is the third most common type of skin cancer in HIV patients after Kaposi’s sarcoma and squamous cell carcinoma (Chang et al., 2017). Prior cancer treatment is also a clear melanoma risk factor, presumably because of impaired immunosurveillance as a consequence of chemotherapy. In pediatric cancer survivors, for example, melanoma is the most common type of skin cancer with increasing incidence of 3% per year (Stefanaki et al., 2017).
1.5. Chemical exposure
Employment in specific industries has been linked with heightened melanoma risk. Specifically, working in the printing industry, industrial chemical plants, electronic factories, and electrical maintenance have all been linked to the disease (Berwick et al., 2016). It is hypothesized that exposure to melanoma-relevant carcinogens may explain much of this risk. Heavy metals such as hexavalent chromium (a known class I human carcinogen), for example, may be important because of their interactions with melanin pigments expressed in melanocytes (Berwick et al., 2016).
1.6. Fair skin complexion
Individuals with fighter skin color, green or blue eyes, freckles, and blonde or red hair are more prone to developing melanoma. These individuals also tend to be particularly UV-sensitive and burn rather than tan after UV exposure. Much of this phenotype is explained by a relative deficit of the dark brown/black pigment known as eumelanin, which deposits in the epidermis and shields the skin from UV by physically blocking penetration of incoming UV photons. Skin complexion can be described semi-quantitatively by the Fitzpatrick scale, which describes skin complexion on a scale of one (lightest) to six (darkest) (Fig. 3). In general, skin pigmentation is most correlated with the amount of eumelanin in the epidermis (described in more detail below). Individuals with low Fitzpatrick indices are fair-skinned and UV sensitive as reflected by tendency to burn rather than tan after UV exposure, and they have an increased skin cancer risk compared with individuals with higher Fitzpatrick indices. In fact, fair skin complexion is among the most important inherited melanoma risk factors, and the increasing incidence of melanoma has been most notable in this group. The Surveillance Epidemiology and End Results program in 2018 noted that Caucasians have the highest incidences of Melanoma (34.4 per 100,000 in men and 20.9 per 100,000 in women). Furthermore, Australia and New Zealand have the highest incidence of skin cancer given a large population of fair-skinned populace living in environs with high ambient UV radiation (Elliott, Whiteman, Olsen, & Gordon, 2017). Skin complexion is a polygenic phenotype, contributed to by a variety of loci including the melanocortin 1 receptor (MC1R), tyrosinase, dopachrome tautomerase, and tyrosinase-related protein 1 (Jablonski & Chaplin, 2017; Rees, 2003).
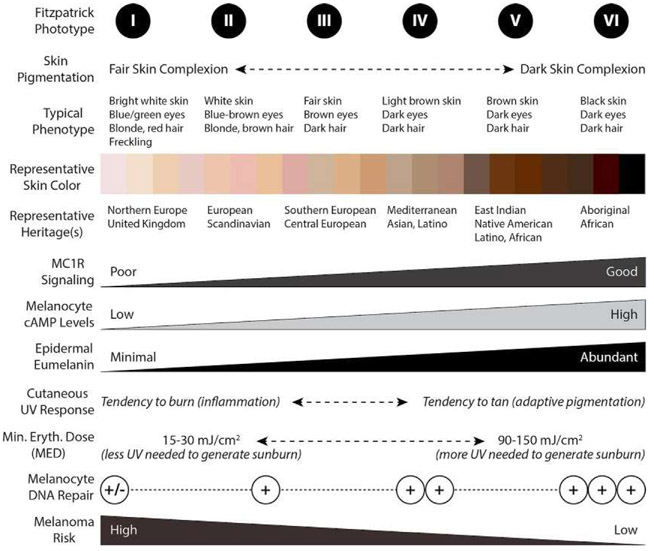
Fig. 3.
Relationship between skin complexions, Fitzpatrick scale score, epidermal melanin composition, quality of MC1R signaling, UV tanning/burn response, DNA repair and melanoma risk. Skin complexion can be described by Fitzpatrick phototype, with individuals of least pigmentation having phototype I and persons of darkest complexion having phototype VI. Though many genes determine pigmentation, skin complexion and UV responses are heavily regulated by the MC1R signaling and epidermal eumelanin composition. Robust MC1R signaling leads to induction of cAMP in melanocytes which promotes eumelanin production responsible for a vigorous tanning response (adaptive pigmentation) and better protection from subsequent UV insults. MC1R signaling also enhances melanocyte genomic stability by improving the efficiency of DNA repair. Thus, melanoma risk is heavily influenced by skin pigmentation and MC1R signaling.
1.7. Family history of skin cancer
There are melanoma-prone kindreds with inherited defects in tumor suppressors that place individuals at risk for melanoma. The prime example, Familial Atypical Multiple Moles Melanoma (FAMMM) syndrome, has two of the highest risk genes leading to melanogenesis: CDKN2A and CD4K germline mutations (Muller et al., 2016; Soura, Eliades, Shannon, Stratigos, & Tsao, 2016). CDKN2A is a tumor suppressor gene that encodes the p16 protein that has crucial roles in cell cycle regulation and senescence via inducing apoptosis. CDKN2A mutations account for roughly a quarter of known inherited melanoma predisposition (Goldstein et al., 2017; Ribero, Glass, & Bataille, 2016). CDK4, which encodes cyclin-dependent kinase 4 that regulates the G1/S cell cycle checkpoint, is also a melanoma risk factor when mutant (Abdel-Rahman et al., 2011; Tsao et al., 2000). These germline mutations result in the development of atypical nevi and a high lifetime melanoma risk (Azoury & Lange, 2014; Soura et al., 2016). In addition to CDK2NA and CDK4 mutations, other high-risk melanoma susceptibility genes include BAP1, POT1, ACD, TERF2IP, TERT, BRAF, and NRAS and account for nearly 40% of the melanoma-prone families’ genetic mutations (Goldstein et al., 2017). Melanoma is not only limited to these melanoma prone families, but can also occur in rarer family cancer syndromes of retinoblastoma, Li Fraumeni syndrome, neurofibromatosis type I, and central nervous system tumors (Ribero et al., 2016).
1.8. Nevi (moles)
Roughly 30% or more of melanomas arise from preexisting nevi (moles). Nevi share some of the molecular features of melanoma—namely MAPK activation through gain-of-function BRAF mutations (Pollock et al., 2003)—but are incompletely transformed collections of benign melanocytes. Additional genetic and/or epigenetic events are required for a benign nevus to transform into a malignant melanoma. Congenital melanocytic nevi (CMN) are benign clusters of melanocytes found at birth that are permanent and grow in proportion with the child (Kinsler et al., 2017). Melanoma risk varies based on size of the CMN: Small CMN have a 1–2% melanoma risk whereas large or giant nevi have a higher risk (at 10–15%) (Kinsler et al., 2017). Other congenital nevi, Spitz nevi (benign melanocytic neoplasm characterized by epithelioid or spindle melanocytes) and Mongolian spots (lumbosacral bluish hyperpigmented area that disappear over time) are not considered premalignant states (Dika, Ravaioli, Fanti, Neri, & Patrizi, 2017). Additionally, clinically atypical nevi (based on the ABCDE criteria: asymmetry, border irregularity, color changes, enlarging diameter, and evolution) are more likely to develop into melanoma. There is also a strong correlation with the elevated number of nevi (benign >30 and atypical >5) and an elevated risk of developing melanoma (Avril et al., 2001; Bett, 2005). However, a recent study of two US Veterans Affairs centers in 2016 revealed that a sizable number of patients (66% out of 566 patients) in their study had few benign and atypical nevi yet developed melanoma (Geller et al., 2016). Regardless if one lacks nevi or has multiple nevi, consistent and regular examinations of the skin b will help detect at-risk nevi to enable timely management to prevent or identify melanomas at an early, treatable stage.
2. Melanocortin 1 receptor (MC1R)
2.1. MC1R structure and polymorphisms
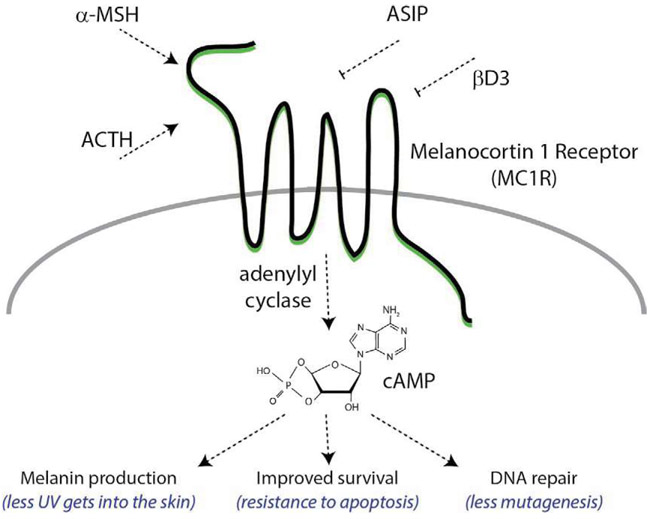
The melanocortin 1 receptor (MC1R) is a 7-transmembrane G protein-coupled receptor expressed on the plasma membrane of melanocytes that regulates proliferation, differentiation, DNA-repair and UV sensitivity. MC1R interacts with a variety of protein ligands including the high-affinity melanocortin agonists α-melanocyte stimulating hormone (αMSH) (Swope et al., 2012) and adrenocorticotropic hormone (ACTH) (Abdel-Malek et al., 2000) as well as the antagonists agouti signaling protein (ASIP) (Abdel-Malek et al., 2000; Suzuki et al., 1997) and β-defensin 3 (Candille et al., 2007). A classic G protein-coupled receptor (Fig. 4), MC1R signals by engaging adenylyl cyclase with subsequent production of the second messenger cAMP. In melanocytes, cAMP elevation promotes melanin production and positions the cell to tolerate environmental stressors such as UV by increasing cellular capacity to identify and repair DNA damage and to resist apoptosis (Kadekaro et al., 2005). The human MC1R locus is highly polymorphic, with genetic variability likely selected for based on epidermal melanin expression and capacity for sunlight-induced vitamin D production (Mohania et al., 2017).
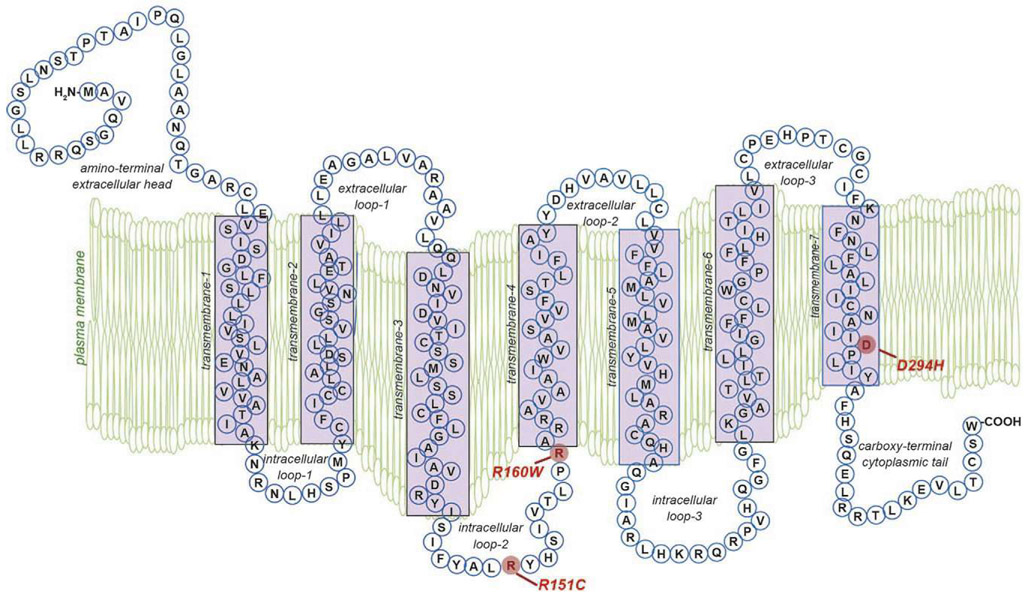
Fig. 4.
Physical structure of the MC1R protein. The MC1R is a 7-transmembrane Gs protein-coupled receptor (GPCR). Extracellular and transmembrane domains engage MC1R ligands while intracellular and transmembrane domains regulate adenylyl cyclase interactions and signaling. Residues identified in red include the common polymorphisms of the “RHC” phenotype that significantly reduce MC1R function and lead to reduced cAMP signaling and preferential production of the red/blonde pheomelanin pigment.
MC1R genetic polymorphisms are a major source of the variation in human hair color and skin pigmentation (Smith et al., 1998). Roughly 200 MC1R allelic variants differentiate the response to ultraviolet (UV) radiation and skin cancer susceptibility between pigmentary phenotypes (Garcia-Borron, Sanchez-Laorden, & Jimenez-Cervantes, 2005). Specific MC1R polymorphisms have been associated with the presence of fair and/or freckled skin, impairment or loss of the adaptive tanning response to UV damage, and high likelihood of sunburn. This complexion is described as the red hair color (RHC) phenotype (Box, Wyeth, O’Gorman, Martin, & Sturm, 1997; Valverde, Healy, Jackson, Rees, & Thody, 1995). The MC1R variants associated with the RHC phenotype have also been correlated with a high risk of melanoma and non-melanoma skin cancers (Scherer & Kumar, 2010). RHC associated alleles have been characterized for population penetrance as high (R) or low (r) using odds ratios based on RHC as the outcome phenotype (Duffy et al., 2004; Sturm et al., 2003). Of these, the R151C, R160W, and D294H polymorphisms are described as highly penetrant alleles with incredibly high associations with red hair and fair skin (odds ratios for expression of RHC phenotype for individuals with these polymorphisms relative to the consensus sequence are between 50 and 120 (Duffy et al., 2004)) and occurring at a relatively high frequency in the RHC phenotype population (Garcia-Borron et al., 2005). Other highly penetrant alleles occurring more rarely are the D84E and R142H alleles (Garcia-Borron et al., 2005). The low penetrant (r) MC1R allelic variants are the V60L, V92M, and R163Q polymorphisms and are observed in approximately 2–6% of the RHC human population (Garcia-Borron et al., 2005; Healy et al., 2001). It has been reported that the R160W, D294H, V60L, R151C, and R142H alleles are present in around 30% of individuals of northern European descent and account for over 60% of the incidence of red hair in those individuals (Healy et al., 2001). MC1R polymorphisms that correlate with fair complexion, UV sensitivity and skin cancer risk generally lead to loss-of-function of the MC1R protein and a blunted second messenger response, which will be elaborated on below. With reduced MC1R signaling comes an attenuated ability of melanocytes to cope with UV. MC1R-defective melanocytes are less protected from UV-mediated oxidative stress (Kadekaro et al., 2012), UV-induced DNA damage (Jarrett, Horrell, et al., 2014; Kadekaro et al., 2005; Yin, Sturm, & Smith, 2014) and have blunted UV-adaptive pigment synthesis (Valverde et al., 1995). All of these impaired MC1R-directed functions lead to increased cellular damage and increased potential for malignant transformation.
2.2. Melanocortin-mediated MC1R cAMP signaling
The MC1R is activated by a family of biosynthetically-related peptide hormones that include the melanocortin (MC) isoforms α-, β-, and γ-melanocortin (also called melanocyte stimulating hormone; MSH) and adrenocorticotrophic hormone (ATCH) (Garcia-Borron, Abdel-Malek, & Jimenez-Cervantes, 2014). Of these, human MC1R has the greatest affinity for α-MSH and ATCH (Suzuki, Cone, Im, Nordlund, & Abdel-Malek, 1996). When MC1R binds one of these agonists, the Gs protein and adenylate cyclase are sequentially activated leading to an increased level of intracellular cyclic AMP (cAMP). This initiates a series of downstream signaling events, largely mediated by the cAMP-dependent protein kinase A (PKA)-signaling pathway (Suzuki et al., 1999) (Fig. 5). When levels of intracellular cAMP increase, the result is activated PKA which phosphorylates members of the cAMP-responsive element binding protein (CREB) family of transcription factors (Iordanov et al., 1997). Phosphorylated CREB activates the expression program of microphthalmia-associated transcription factor (MITF), a myc-like transcription factor which regulates gene expression of a variety of enzymes that drive the melanogenic program that will be described more in depth below. Activated PKA also activates the mitogen activated protein kinase (MAPK) cascade subsequently activating p38 (Smalley & Eisen, 2002). cAMP signaling is regulated intracellularly by cAMP-degrading phosphodiesterases (PDEs) which degrade cAMP in to AMP. MC1R signaling is terminated by β-arrestins (ARRBs), as well as other MCR subfamily-specific regulatory partners discussed further below (Garcia-Borron et al., 2014).
Fig. 5.
Complexity of native MC1R signaling. MC1R signaling is regulated by cognate agonists and antagonists. Activators of MC1R include the high-affinity melanocortin ligands alpha melanocyte stimulating hormone (α-MSH) and adrenocorticotropic hormone (ACTH). α-MSH and ACTH are generated in response to UV-induced DNA damage in keratinocytes. MC1R agonists stimulate MC1R to activate adenylyl cyclase and generate c-AMP. Downstream responses to c-AMP signaling include increased synthesis of melanin pigment, increased cellular survival through resistance to apoptosis, and increased efficiency of DNA repair to reduce mutagenic potential of UV-induced photodamage. MC1R signaling is antagonized by agouti signaling protein (ASIP) and β-defensin 3 (βD3).
3. Melanins
3.1. Eumelanin vs. pheomelanin
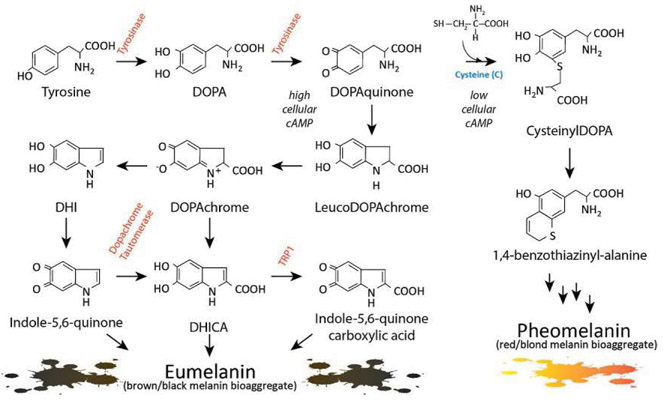
There are two major pigment types present in the skin: eumelanin and pheomelanin. Each is made exclusively in melanocytes and each derives from the amino acid tyrosine, which is oxidized and cyclized through the actions of tyrosinase and other pigment enzymes (Fig. 6). Tyrosinase is the rate-limiting enzyme for melanogenesis and plays a role in converting the amino acid Tyrosine to DOPA and then to DOPA quinone. Eumelanin is a dark brown/black bioaggregate of melanin pigments derived from 5,6-dihydroxyindole-2-carboxy]ic acid (DHICA) and 5,6-dihydroxyindole (DHI) which themselves derive from DOPA and DOPA quinone (Ito & Wakamatsu, 2003). Eumelanin is an inert pigment capable of efficiently absorbing UV photons as they enter the epidermis (Hoogduijn et al., 2004). Skin darkness is directly related to the amount of eumelanin present in the interfollicular epidermis. Thus the darker the skin complexion, the more eumelanin is contained in the skin and the less UV is able to penetrate the epidermis. Heavy deposition of eumelanin in the epidermis explains in large part why dark-skinned individuals are relatively protected from acute and chronic UV pathologies. Fair-skinned individuals, on the other hand, are deficient in epidermal eumelanin and express pheomelanin preferentially (Rees & Healy, 1997). Pheomelanin is also derived from tyrosine, but there is incorporation of a cysteine during its biosynthesis. Retention of the sulfur atom donated by cysteine is thought to be responsible for pheomelanin’s reddish/orange color and its pro-oxidative chemical nature. Pheomelanin is much less able to block UV energy and in fact may synergize with UV photons to promote free radical formation and carcinogenesis in the skin (Mitra et al., 2012). Though skin complexion is multigenic, it is well established that MC1R and cAMP signaling lead to greater eumelanin production with elevated eumelanin to pheomelanin ratio and subsequent increased photoprotection (Del Bino et al., 2015; Scott, Suzuki, & Abdel-Malek, 2002).
Fig. 6.
Melanin synthesis. There are two main types of melanin: the dark brown/black UV-protective eumelanin and the red/blonde sulfated pheomelanin pigment. Each is derived from progressive cyclization and oxidation of the amino acid tyrosine. Tyrosinase, the rate-limiting enzyme for melanogenesis, catalyzes the first two stages of melanin biosynthesis. When MC1R is functional and cAMP levels are high, melanocytes produce eumelanin preferentially (bottom left section). In contrast, when MC1R is dysfunctional and cAMP levels are low, cysteine is incorporated and pheomelanin is made instead (right section). Inherited deficiencies of melanogenic enzymes such as tyrosinase, dopachrome tautomerase, and tyrosinase-related protein-1 are associated with hypopigmentary disorders of albinism.
3.2. MC1R antagonists
In addition to its interactions with native agonists (α-MSH, ACTH), MC1R signaling is also influenced by at least two receptor antagonists: agouti signaling protein (ASIP) and β-defensin 3 (βD3). ASIP is a paracrine signaling molecule produced in the hair follicle of mammals (Lu et al., 1994); its expression changes the melanin composition and pigmentation of hair presumably for camouflage purposes. Before it was determined to be a direct ligand of MC1R, ASIP was shown to regulate hair color toward a black coat color phenotype (Millar, Miller, Stevens, & Barsh, 1995). It was later determined that its pigmentary effects were dependent upon the presence of a functional MC1R (Ollmann, Lamoreux, Wilson, & Barsh, 1998), irrespective of MC1R polymorphisms (Abdel-Malek et al., 2001). In fact, ASIP is an inverse antagonist (Lu et al., 1994; Wilson et al., 1995), inhibiting MC1R signaling directly (Graham, Wakamatsu, Hunt, Ito, & Thody, 1997) and also functioning as a competitive MC1R antagonist that prevents α-MSH binding to MC1R (Blanchard et al., 1995; Suzuki et al., 1997). Though its role in mammalian coat color pigmentation is well documented, it is unclear whether ASIP is expressed in human skin or pertinent to human MC1R signaling regulation.
The defensins are a group of antimicrobial peptides that link the innate and adaptive immune responses (Boman, 1995). There are two major classes of defensins—α and β (Schneider, Unholzer, Schaller, Schafer-Korting, & Korting, 2005)—however, only β-defensins have been identified in the skin (Bensch, Raida, Magert, Schulz-Knappe, & Forssmann, 1995; Robles et al., 1998; Schroder & Harder, 1999; Weinberg, Krisanaprakornkit, & Dale, 1998). Of the three β-defensins, only βD3 has been shown to bind MC1R (Harder, Bartels, Christophers, & Schroder, 2001). Before the function of β-defensins was elucidated, Clarence Cook Little showed that the dominant inheritance of black coat color in dogs is inherited independently of mutations in MC1R. He initially suggested that this phenotype was due to an unusual allele of agouti (Little, 1957). However, it was later shown that overexpression of the canine homolog of βD3, β-defensin 103, promoted the black coat color phenotype (Candille et al., 2007). Like ASIP, β-defensin homologs act as competitive inhibitors that prevent α-MSH binding to MC1R and attenuate MC1R signaling as a result. βD3’5 activity requires the presence of a functional MC1R protein and cannot be rescued by other melanocyte surface receptors (Nix et al., 2013). However, unlike ASIP, βD3 appears to be a competitive antagonist rather than an inverse antagonist, unable to down-regulate MC1R signaling beyond preventing MC1R’s interactions with melanocortins (Candille et al., 2007; Swope et al., 2012).
4. Role of ultraviolet light in DNA damage
4.1. UV mutagenesis
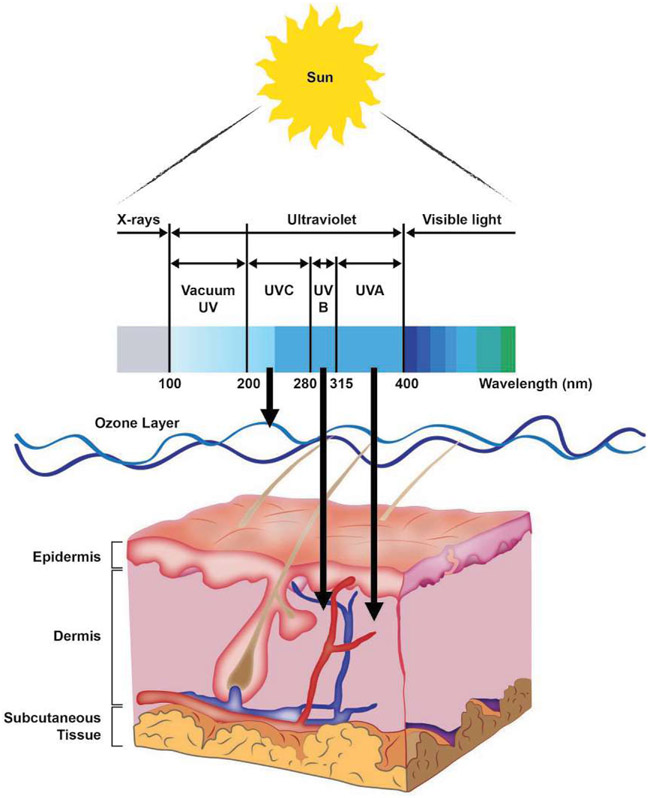
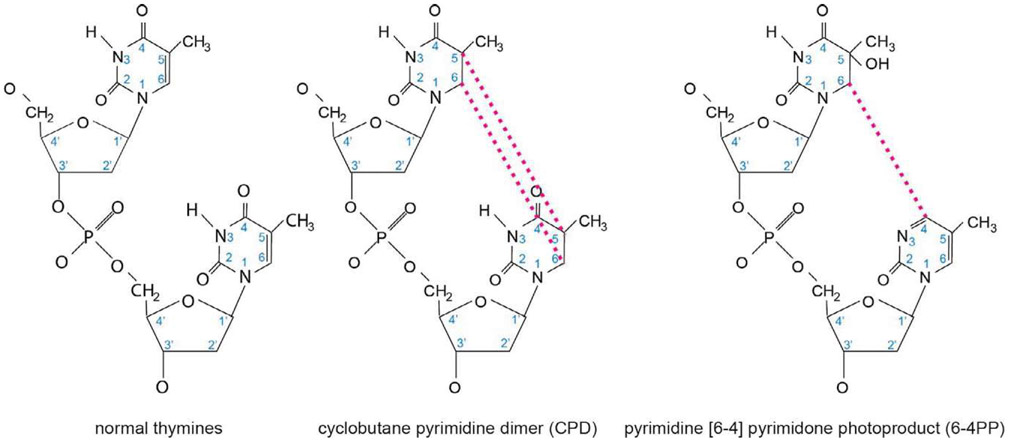
UV is an energetic form of light found just short of the visible light in the electromagnetic spectrum. Based on photon wavelength, UV can be divided into three components: UV-A (320–400nm), UV-B (290–320nm), and UV-C (<290 nm) (Diffey, 2002) (Fig. 7). The sun is the natural source of UV on Earth, though artificial sources such as tanning beds and UV lamps are increasingly relevant. There are two principle covalent DNA adducts produced by UV-light: the 6–4 photoproduct (6–4 PP) and the cyclobutane pyrimidine dimer (CPD) (Fig. 8). The shortest wavelengths of solar UV radiation (<290nm), which carry the most energy per photon, are efficiently absorbed by the ozone layer. As a result, ambient solar UV lacks UV-C components and tends to be a blend of UV-A and UV-B in most geographies (Diffey, 2002). Longer wavelengths of UV (320–400 nm) penetrate deeper into the skin and are associated with the production of oxidative free radicals (Pfeifer, You, & Besaratinia, 2005), but because their photons carry less energy, they are less able to cause UV photodamage to DNA directly as the shorter UV wavelengths. Therefore, with respect to covalent photodamage, UV-B, the middle wavelengths of ultraviolet light, is the source of most solar DNA damage. However, accumulating evidence suggests that UV-A contributes to the generation of a small quantity of UV covalent DNA adducts (Woollons et al., 1999), as might be relevant in sunbeds that use predominantly UV-A (but a small amount of UV-B) light. Filtering the small quantity of UV-B light out of a sunbed eliminates almost all of the CPD generating potential of the light, but not all, indicating both UV-A and UV-B light contribute to CPD generation, and likely UV-mediated mutagenesis.
Fig. 7.
UV radiation and the skin. The ultraviolet (UV) region of the electromagnetic spectrum of light can be divided into three components, UV-A, UV-B and UV-C, based on photon wavelength. The most energetic of these is UV-C, which has the shortest wavelength. However, the ozone layer of the atmosphere absorbs essentially all UV-C, and as a result ambient solar UV exposure is predominantly UV-A (90–95%) and UV-B (5–10%). Longer wavelength UV-A penetrates deeply into the skin, reaching well into the dermis. In contrast, UV-B affects primarily the epidermis. UVA is efficient at generating reactive oxygen species that damage DNA through oxidative base modifications. UV-B is directly absorbed by pyrimidine bases in DNA to produce photoproducts. Mutations and cancer can result from UV-generated changes to DNA if left unrepaired.
Fig. 8.
Chemical structures of UV photoproducts. When UV-light strikes DNA at dipyrimidine nucleotides, such as the thymine dimer shown on the left, there are two common DNA lesions formed. First, the 5′ and 6′ positions of the pyrimidine ring structures can form a cyclobutane ring between the adjacent thymines, generating a DNA lesion referred to as a cyclobutane pyrimidine dimer (CPD) as shown in the middle panel. This lesion is the less helically distorting, and therefore more poorly repaired, of the two adducts shown, and is the most commonly generated adduct when DNA is exposed to UV-light. The second most commonly generated DNA adduct is the pyrimidine [6–4] pyrimidone photoproduct (6–4PP), a lesion formed when the 6′ carbon of one thymine covalently binds the 4′ carbon of an adjacent thymine, as shown in the panel on the right. The 6–4PP is considerably more helically distorting than the CPD lesion, and is therefore more readily recognized by NER, more rapidly repaired, and consequently less mutagenic than a CPD lesion.
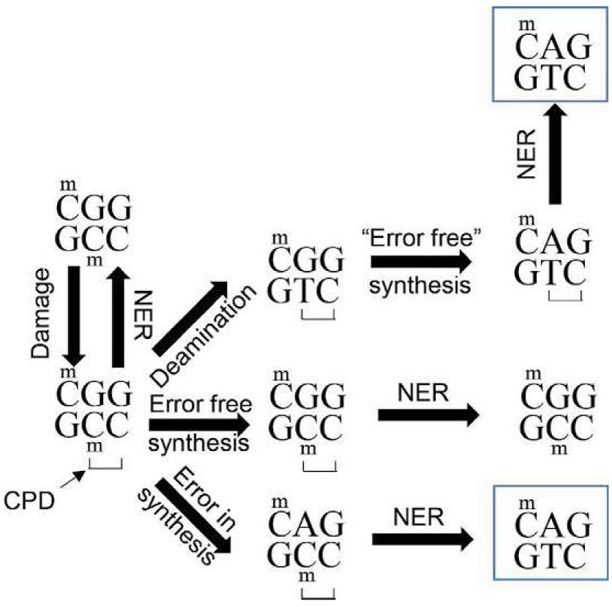
When DNA is irradiated with shorter wavelength UV light (either UV-B or UV-C), multiple lesions are generated. The most common of these are cyclobutane pyrimidine dimers (CPDs) and 6–4 photoproducts (6–4PPs), with CPDs outnumbering 6–4PPs (Mitchell & Nairn, 1989). CPDs and 6–4PPs are mutagenic because they lead to mispairing of bases during replication and they can evoke translesional DNA synthesis, which is error-prone (Fig. 9). The most common mutation introduced by UV is the C → T transition mutation (Pfeifer et al., 2005), and the CPD lesion is responsible for the bulk of these mutagenic events (You et al., 2001). The location of adducts formed by UV light is not randomly distributed throughout the genome. CPDs generated by solar light form preferentially at dipyrimidines that contain a 5-methylcytosine (Tommasi, Denissenko, & Pfeifer, 1997), likely due to the longer wavelength absorption of 5-methylcytosine relative to cytosine (Pfeifer et al., 2005; You, Li, & Pfeifer, 1999). Deamination of these adducted 5-methylcyotsines into thymine is one of the major contributors to the C → T transition mutation (Lee & Pfeifer, 2003), and this deamination occurs frequently at methylated cytosine residues within a CPD (Tu, Dammann, & Pfeifer, 1998). After deamination of 5-methylcytosine to thymine in a CPD, bypass polymerases can insert an A across from the newly formed T (a “correct” synthesis) and fix the mutation into one of the daughter cells (Lee & Pfeifer, 2003). The preference of CPD formation at dipyrimidines containing a 5-methylcytosine (TmC and CmC) is enriched significantly (15-fold) when the dipyrimidine is followed by a guanine (Tommasi et al., 1997), indicating that the PyCG (P = pyrimidine, a C or T) trinucleotide is a target for CPD formation, and may in fact be a mutational hotspot. Indeed, evaluating the mutational spectra of p53 in non-melanoma skin cancer (NMSC), many of the C → T mutations in the gene are present in the context of PymCG sequences (Pfeifer et al., 2005).
Fig. 9.
Potential fates of UV-damaged DNA. UV-photodamage, in this case a CPD lesion, predominantly occur at TCG and CCG sequences when the middle cytosine nucleotide is methylated as shown. Whether the CPD will generate a mutation depends on whether it will be resolved before the next round of cellular replication. The CPD may be repaired efficiently by NER, resulting in a return to the initial sequence in an error free manner. Additionally, damage tolerant polymerases such as DNA polymerase η may synthesize across the CPD adduct in an error free manner, allowing more time for NER machinery to remove the adduct. However, damage tolerant polymerases have lower fidelity than traditional replicative polymerases, and when a damage tolerant polymerase adds a nucleotide incorrectly across from a methylated cytosine that is part of a CPD adduct, the most commonly incorporated nucleotide is A. After misincorporation of this A, NER would use it as the template to replace the adducted C, and instead would incorporate T, generating a C-T transition mutation. Finally, methylated cytosines that are part of a CPD lesion are prone to deamination. This deamination produces a T, and if the deaminated T in the CPD is bypassed “correctly” by DNA polymerase η, a C to T transition will occur. NER is still required to remove the CPD adduct, but the mutation is already fixed.
There is an additional mechanism related to the generation of mutations from UV-derived DNA adducts. When an adduct is encountered by a normal, high-fidelity DNA polymerase (DNA Pol α, δ, or ε) the adduct stalls the polymerase, stopping replication and often inducing apoptosis (Lange, Takata, & Wood, 2011; Schmitt, Matsumoto, & Loeb, 2009). In order to prevent this death, cells employ “damage-tolerant” or “lesion-bypass” polymerases that are able to synthesize across from adducted DNA (a process known as translesion synthesis, TLS). There are several TLS polymerases used in the bypass of photo-adducts: Polη has a high success rate of inserting A’s across a T-T CPD (Johnson, Washington, Prakash, & Prakash, 2000). Interestingly, Polη is the only known polymerase whose deficiency creates a predisposition for cancer. XP-V (XP variant) is one of the complementation groups of Xeroderma Pigmentosum, and is a result of the loss of POLH, the gene encoding Polη. Even though damage tolerant polymerases do reduce the potential harm of these lesions (as the CPD adduct remains after synthesis), they lack proofreading endonuclease ability, and misincorporation of T in place of the C involved in a TC photoproduct can occur, which also generates the C → T transition mutation mentioned above (DiGiovanna & Kraemer, 2012; Lange et al., 2011).
4.2. UV and melanoma link
The relationship between UV-light and melanoma is not as straightforward as it is for non-melanoma skin cancer. While there are multiple factors that contribute to melanoma risk including family history of melanoma, fair skin complexion, dysplastic nevi, immunosuppression and UV-light exposure, epidemiological studies indicate that a major risk factor for the development of melanoma is childhood exposure to UV-light especially in the context of severe sunburns (Lo & Fisher, 2014; Whiteman, Whiteman, & Green, 2001). It is not known whether this causality is due to a very high dose of UV that promotes mutagenesis, contribution of inflammatory signals or a combination of both. At the genetic level, although the p53 gene is mutated much less frequently in melanomas than in non-melanoma skin cancer(Giglia-Mari & Sarasin, 2003), the majority of the p53 mutations in melanoma are still UV-signature C → T or CC → TT mutations (Zerp, van Elsas, Peltenburg, & Schrier, 1999). Additionally, whole-exomic sequencing (WES) determined that as many as half of all driver mutations in melanomas are C → T and G → T mutations (Hodis et al., 2012), both of which can be produced by UV-light (Alexandrov et al., 2013). However, not all mutations commonly found in melanoma are a result of UV-exposure. The most common mutation in melanoma, BRAFV600E, is less common in areas of the body highly exposed to UV than it is in areas intermittently exposed, and is generally believed not to be a UV-signature mutation (Curtin et al., 2005; Lo & Fisher, 2014). This suggests that UV-exposure is responsible for many, but not all, of the mutagenic events that drive melanoma. Nonetheless, DNA mutational burden in melanomas is among the highest of all cancers, and the vast majority of mutations are C → T transition mutations, the hallmark of UV-damage (Lawrence et al., 2013) and the principle driving force behind the increased mutagenic burden of melanomas (Pleasance et al., 2010). Additionally, the mutational burden in melanomas increases with increasing stage of the disease, while the percentage of mutations that are C → T or CC → TT remains constant throughout the disease progression (Shain et al., 2015), suggesting that UV-light plays a critical role in disease progression, and it is equally involved in damage induction in early and late stages.
5. Repair of DNA damage
5.1. Melanoma and DNA repair defects
The nucleotide excision repair (NER) pathway is the major genomic maintenance system whereby melanocytes and other cells repair UV-mediated DNA damage. NER is vital to the recognition and removal of helically distorting DNA adducts such as UV photoproducts. Defects in DNA repair mechanisms, particularly NER, are a clear melanoma risk factor (Masaki et al., 2014). Genetic loss of NER capacity occurs in three documented diseases: Xeroderma Pigmentosum (XP), Cockayne Syndrome (CS) and trichothiodystrophy (TTD). Of these, XP is well established as a bona fide inherited cancer risk factor, particularly for UV-induced malignancies such as squamous cell carcinoma (SCC) and melanoma. Indeed, the risk of developing skin cancer in XP patients is at least 1000-fold higher than in non-XP individuals and the latency for such tumors is reduced by decades. Whereas UV-dependent malignancies peak in the fifth and sixth decades of life in the general population, they occur before the age of 20 in many XP patients. XP is a homozygous recessive disorder, although for any given patient, the mutation is typically a compound heterozygous mutation. Interestingly, melanomas formed in XP individuals are almost exclusively in sun-exposed regions of the body, have a high incidence of p53 mutation, and have a very strong UV-signature mutation content, suggesting that these cancers were caused by UV-light exposure. NER deficiency would prevent repair of these adducts and promote carcinogenesis (Giglia-Mari & Sarasin, 2003; Spatz, Giglia-Mari, Benhamou, & Sarasin, 2001). While clear evidence exists relating a homozygous loss of NER to cancer risk, there is a growing field of research investigating the impact of polymorphisms and resultant reduced gene doses of NER factors on sporadic melanoma and skin cancer risk. Over two dozen proteins are involved in NER, and several of these proteins have been analyzed for the potential for polymorphisms in their genes to increase risk of developing melanoma, with some recent studies summarized by a meta-analysis of multiple of population studies (Mocellin, Verdi, & Nitti, 2009) described briefly in Table 2.
Table 2.
NER gene polymorphisms and their contribution to melanoma risk.
Polymorphisms are identified by their reference SNP ID (rs number). All mutations indicated were either synonymous or missense SNPs, there were no nonsense mutations.
5.2. Nucleotide excision repair (NER)
The nucleotide excision repair (NER) pathway is a DNA repair mechanism whose principle function is to remove helically distorting DNA lesions prior to synthesis of new DNA to reduce the mutagenic risk these lesions possess. Substrate lesions for the NER pathway include those generated by metabolites of chemical carcinogens such as polycyclic aromatic hydrocarbons (PAHs) in tobacco smoke, cyclobutane pyrimidine dimers (CPDs) and 6–4 photoproducts (6–4PPs) induced by exposure to UV light, and platinum adducts from chemotherapeutic agents such as cisplatin (Gillet & Scharer, 2006). There are over two dozen different protein factors in mammals that participate in NER, including the XPA-G and XPV factors that are singly defective in the eight corresponding complementation groups of the human disease Xeroderma Pigmentosum (XP). Transcriptional regulation of NER by the tumor suppressor factor p53 impacts the XPC and DDB2 gene products in response to DNA damage (Adimoolam & Ford, 2002; Amundson, Patterson, Do, & Fornace, 2002; Fitch, Cross, & Ford, 2003; Smith & Seo, 2002). There are two components to NER: the global genome nucleotide excision repair (GG-NER) pathway is responsible for removing DNA damage from throughout the genome. The other sub-pathway is known as transcription-coupled nucleotide excision repair (TC-NER) and is responsible for removal of damage that occurs on the transcribed strand of actively transcribed DNA (Vermeulen & Fousteri, 2013).
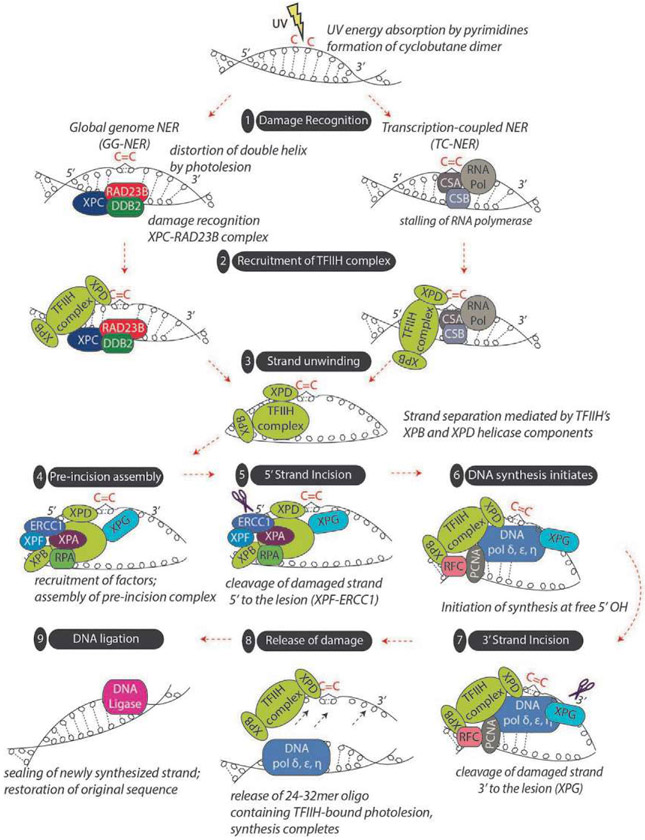
The first step in the NER pathway is the recognition of a helically distorting DNA adduct by one of the NER DNA damage recognition factors (Fig. 10). The manner of recognition is different between two subpathways. In GG-NER the principle DNA recognition protein is XPC (Sugasawa et al., 1998), while the UV-DDB protein complex contributes to the recognition of UV-derived photo-adducts (Sugasawa, 2016). The UV-generated DNA lesions (primarily CPDs) are poor substrates for XPC, but DDB2 recognizes them with a much higher affinity (Fujiwara et al., 1999; Sugasawa et al., 2005). DDB2 binds directly to photo-adducts via a pocket in the protein that interacts with the adducted bases (Scrima et al., 2008) and promotes recruitment of XPC to the damaged DNA (Fitch, Nakajima, Yasui, & Ford, 2003; Wang, Zhu, Wani, Chen, & Wani, 2004). For lesions in which XPC is the sole damage recognition factor, the mechanism of damage recognition by XPC is quite different. In order to be able to respond to the multitude of DNA adducts that are substrates for NER, XPC, stabilized by its obligate binding partner HR23B, recognizes the helical distortion in DNA produced by a DNA adduct, rather than the lesion itself (Sugasawa, Shimizu, Iwai, & Hanaoka, 2002), and binds to the undamaged strand opposite the lesion. Recent evidence suggests that the rate-limiting step in NER is the efficiency with which DNA damage can be recognized, implicating the primary recognition protein, XPC, as the rate-limiting enzyme in GG-NER (Luijsterburg et al., 2010; Nishi et al., 2009; Yeo, Khoo, Fagbemi, & Scharer, 2012). The other sub-pathway of NER, TC-NER, is responsible for removing DNA adducts exclusively on the transcribed strand of actively transcribed DNA. The first step in TC-NER occurs when an actively transcribing RNA polymerase II complex is stalled by a DNA adduct. This stabilizes CSB, a component of the transcription complex, and this stabilization of CSB promotes recruitment of the subsequent NER machinery (Spivak & Ganesan, 2014; Svejstrup, 2002; Vermeulen & Fousteri, 2013).
Fig. 10.
Overview of nucleotide excision repair (NER). Helically distorting DNA damage, such as that produced by UV light is repaired by the NER pathway, which can be subdivided into global genome NER (GG-NER) and transcription-coupled NER (TC-NER). GG-NER occurs anywhere in the genome and is initiated by recognition of helical-distorting DNA damage such as a cyclobutane pyrimidine dimer (CPD). In GG-NER, XPC, as part of a multiprotein complex including RAD23B, serves as the primary recognition factor. XPC is further assisted by the UV damage-binding protein 2 (DDB2) to specifically recognize CPD adducts. Without DDB2, CPDs are poorly recognized by XPC. In contrast, TC-NER is initiated by the stalling of RNA polymerase at a CPD present on the template strand of an actively transcribed gene and involves the cofactors CSA and CSB. TC-NER and GG-NER only differ in their first step, damage recognition; the pathways converge downstream into a common NER mechanism beginning with pre-incision complex formation. In both GG-NER and TC-NER, TFIIH (a multi-complex protein containing the XPB and XPD helicases as well as eight other subunits) is recruited to the site of damage. Using its XPB and XPD helicase components, TFIIH unwinds the DNA around the photolesion, initiating strand separation to enable the recruitment of other NER factors to form a “pre-incision complex.” XPA, replication protein A (RPA), XPG, XPF, and ERCC1 are each recruited to the complex, and at some point, initiation factors exit the region. Next, there is incision of the photolesion-containing strand some distance away from the DNA lesion. Strand incision is accomplished by XPF-ERCC1 working in complex to cleave the strand 5′ to the damage and by XPG 3′ to the damage. Evidence suggests that 5′ strand cleavage may actually occur before 3′ strand cleavage, and in fact, DNA polymerase may begin filling in the gap from the 5′ side before the 3′ incision step. After 5′ and 3′ strand incision, a 24-32mer oligonucleotide harboring the photolesion is generated that is removed (associated with TFIIH) from chromatin. The resultant gap is then filled in by DNA polymerases together with PCNA, RFC, and RPA using the undamaged sister strand to ensure fidelity of repair. Finally, DNA ligation is achieved by DNA ligases I, III or XRCC1 to complete the process.
After the DNA damage recognition steps, the pathways converge to complete the repair process. To accomplish this, the respective DNA damage recognition proteins (XPC in GG-NER and CSB in TC-NER) each recruit the next component of the NER pathway, the TFIIH transcriptional complex containing the helicases XPB and XPD, to the site of the damage (Fig. 10). XPB is responsible for opening the DNA upstream of the DNA lesion, permitting XPD to travel 5′ to 3′ across the damaged strand until it encounters the DNA adduct (Riedl, Hanaoka, & Egly, 2003; Sugasawa, Akagi, Nishi, Iwai, & Hanaoka, 2009). The unwinding permits subsequent NER factors access to the DNA damage site, including the endonuclease XPG (which is recruited through interactions with TFIIH), the single strand binding protein RPA, and XPA. XPA is an indispensable component of the NER pathway, but the role it plays is still somewhat vague. XPA can interact with a multitude of NER factors including XPC, THIIH, RPA, XPF/ERCC1, and it may serve to verify that all necessary factors are in place ahead of the DNA incision step. The other directional endonuclease complex, XPF/ERCC1, is recruited to the damage site through interactions with XPA (Orelli et al., 2010). After recruitment of the XPF/ERCC1 complex, the pre-incision complex is complete, and XPF/ERCC1 cuts 5′ of the damaged nucleotide. This incision generates a 3′ hydroxyl group that serves as a template for the repair polymerase (of which there are several) to bind and initiate DNA synthesis. After synthesis has begun, XPG performs the second single-stranded cut 3′ of the damaged site (Staresincic et al., 2009) , releasing the damaged photoproduct along with some number of undamaged neighboring bases proximal to the damage. The original integrity of the DNA is restored when the repair polymerase synthesizes an approximately 30 nucleotide segment of DNA using the undamaged sister strand as a template for high-fidelity repair and a DNA ligase seals the strand (Shivji, Podust, Hubscher, & Wood, 1995).
A recent investigation into the post-incision steps in NER reveals that the process is more complicated than previously thought. While replicative (error free) DNA polymerases δ and ε were initially thought to be the principle polymerases in the gap filling step, the error prone polymerase κ also plays a role in repair synthesis. All three polymerases are recruited by different accessory factors, suggesting there is a different context to the recruitment of each polymerase in the repair synthesis step of NER (Ogi et al., 2010). Additionally, the final step in repair—ligation of the damaged strand after repair synthesis—can be accomplished by one of two ligases, DNA ligase I or IIIα. The choice of which ligase to use depends on the replicative status of the cell, with quiescent cells utilizing DNA ligase IIIα and proliferating cells using DNA ligase I (Moser et al., 2007).
6. Intersection of melanocytes and NER
6.1. MC1R-cAMP signaling and NER
The MC1R is a major determinant of melanocytic responses to UV damage. In addition to its role in promoting melanin synthesis, the melanocortin signaling axis promotes melanocyte genomic stability by enhancing the repair of DNA damage. Seminal work from the Abdel-Malek lab identified that the MC1R controls melanocyte DNA repair of UV DNA damage (Hauser et al., 2006; Kadekaro et al., 2005). Although it had been known for over a decade that MC1R signaling accelerates NER kinetics, the mechanisms by which the cAMP-mediated repair phenomenon occurs have only recently begun to be elucidated and appear to be complex and multifactorial. The enhancement of UV-induced DNA damage has been shown to be reliant upon both the nuclear receptor subfamily 4 group A member 2 (NR4A2) (Smith et al., 2008) and ataxia telangiectasia mutated and Rad3 related (ATR) signaling pathways (Jarrett, Horrell, etal., 2014). MC1R signaling leads to the induction of the NR4A2 which translocates to sites of damage in a p38-dependent manner and colocalizes with XPC and XPE at sites of DNA damage (Wong, Ainger, Leonard, & Sturm, 2012). Activation of MC1R has also been shown to facilitate repair via an increase in DNA damage response proteins. Treatment with α-MSH leads to an increase in XPC and γH2AX levels promoting formation of DNA repair complexes in primary human melanocytes (Swope et al., 2014).
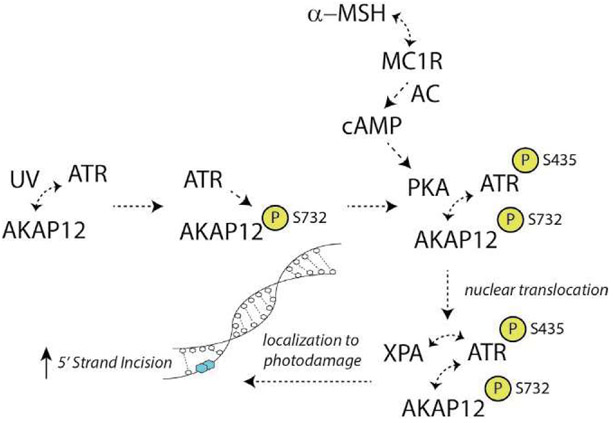
We reported that a critical molecular event linking MC1R signaling to DNA repair is a phosphorylation event of the global cell damage response protein ataxia telangiectasia and Rad3-related (ATR) (Jarrett, Horrell, et al., 2014) (Fig. 11). When cAMP levels are induced either by MSH-MC1R interactions or pharmacologically with adenylyl cyclase activation, cAMP-dependent protein kinase (PKA) becomes activated and phosphorylates ATR on its Serine 435 (S435) residue (Jarrett, Horrell, et al., 2014). We found that the xeroderma pigmentosum A (XPA) protein and ATR-pS435 localize to UV photoproducts in a greatly accelerated and enhanced manner if melanocytes have been stimulated through MC1R signaling. Interestingly, instead of ATR’s classical role in cell damage responses, which is activation of Chk1, PKA-mediated phosphorylation of ATR on S435 promotes binding of ATR with the key NER factor XPA and co-localize with UV photodamage in the nucleus. Our findings suggest that PKA-mediated phosphorylation of ATR at S435 is an important event that regulates early recruitment and assembly of XPA and possibly other DNA repair proteins to sites of UV damage in order to optimize NER. More recently, we identified A kinase anchoring protein 12 (AKAP12) as the critical molecular scaffold that facilitates PKA-mediated ATR phosphorylation on S435 (Jarrett, Wolf Horrell, & D’Orazio, 2016). Like other AKAPs, AKAP12 binds regulatory subunits of PKA through its conserved amphipathic protein interaction domain. Our evidence suggests that AKAP12 acts as a scaffold for ATR to enhance the PKA-ATR interaction. This interaction is important for NER enhancement and attenuating UV-induced mutagenesis (Jarrett et al., 2016).
Fig. 11.
Model of cAMP-enhanced NER. With UV exposure, ATR (ataxia telangiectasia and rad3-related) is activated and physically interacts with AKAP12 (A kinase anchoring protein 12). ATR phosphorylates AKAP12 on the S732 moiety, an event required for nuclear translocation of the complex. With MC1R (melanocortin 1 receptor) interactions with high-affinity agonists (e.g., alpha melanocyte stimulating hormone; α-MSH), AC (adenylyl cyclase) is activated and the second messenger cAMP is formed. cAMP-dependent protein kinase (protein kinase A; PKA) is activated, interacts with AKAP12 and ATR and phosphorylates ATR on the S435 residue. This post-translational modification accelerates and enhances association of the complex with the core NER factor XPA (xeroderma pigmentosum A protein) and together the ATR/AKAP12/XPA translocates to photodamage in the chromatin. Activation of this pathway results in more robust 5′ strand incision to optimize NER.
Given the central role of XPA and ATR in NER, the discovery of AKAP12 as a scaffold protein that facilitates XPA’s transport to damage introduces a novel regulatory mechanism for NER. We hypothesize that in melanocytes AKAP12 may be an integral platform for the docking and integration of damage sensing and repair proteins, particularly in the context of MC1R/cAMP signaling. In so doing, AKAP12 provides an appropriate “microenvironment” to enable interactions between ATR and XPA and optimize assembly of DNA repair complexes. It is uncertain how AKAP12 enhances NER; however, we provide evidence that AKAP12 promotes the interaction between XPA and ERCC1-XPF (Jarrett et al., 2016). As ERCC1-XPF is the endonuclease that is catalyzes the 5′ incision step of NER, we reasoned AKAP12 might be functionally necessary for cAMP-enhancement of ERCC1-XPF incision. An ERCC1-XPF-mediated incision assay was studied using a fluorescently labeled stem-loop substrate incubated with chromatin fractions isolated from HEK293 cells expressing either AKAP12-WT or AKAP12 deletion variants. Using a stem-loop DNA substrate as a surrogate for this incision step, we showed that cAMP-enhanced 5′ strand incision and this was dependent upon AKAP12 expression. We reason that it might be possible that AKAP12 may more efficiently assemble the pre-incision NER complex. In addition, the recent identification of AKAP12 interacting with ATR at collapsed replication forks (Jarrett, Horrell, et al., 2014) suggests other signals may promote AKAP12 functioning with ATR. This raises the possibility of functional relevance for AKAP12 in genomic maintenance pathways beyond NER.
6.2. Influence of MC1R ligands on NER
MC1R’s cAMP-stimulating ability is regulated by both positive and negative ligands. MC1R-signaling is enhanced by either α-MSH or the related ACTH (Suzuki et al., 1996) and is inhibited by either ASIP (Suzuki et al., 1997) or βD3 (Candille et al., 2007). Our work has shown that in primary human melanocytes, either α-MSH or ACTH increased repair of UV-induced DNA damage and diminished UV-induced mutagenesis (Jarrett, Wolf Horrell, Boulanger, & D’Orazio, 2015). Interestingly, cells possessing MC1R loss-of-function mutants demonstrated no NER benefit from MC1R-ligand activation, although forskolin, a direct activator of adenylyl cyclase independent of upstream MC1R signaling, rescued NER as it bypasses defective MC1R (Jarrett et al., 2015). In contrast, incubating MC1R-intact cells with either ASIP or βD3 slowed DNA repair and raised levels of UV-induced mutagenesis (Jarrett et al., 2015). In fact, ASIP reduced DNA repair levels below baseline, which support previous observations that ASIP may be an inverse agonist of MC1R (Chai et al., 2003). When we treated MC1R-intact melanocytes with βD3, no impact on basal DNA repair was observed, however, any α-MSH-induced increases DNA repair was blocked (Jarrett et al., 2015), supporting the concept that βD3 acts as a neutral MC1R antagonist (Candille et al., 2007). Together, these findings demonstrate that melanocytic NER is dynamic process and NER is influenced by basal MC1R signaling ability and by the expression of MC1R agonists and antagonists in the skin after UV injury.
6.3. Relevance of MC1R signaling pathway beyond UV
As NER is the DNA repair pathway that is active against helical-distorting lesions, we tested whether MC1R-cAMP signaling may affect the repair of other types of damage that are NER-relevant. Since platinum-containing chemotherapeutics form helix-distorting lesions via both intra- and interstrand crosslinks, we evaluated the effect of cAMP signaling on melanocyte DNA repair responses to platinum compounds (Jarrett et al., 2016). By using an intrastrand lesion-specific cisplatin-DNA antibody that recognizes the 1,2-d(GpG) cross-links, we were able to measure the repair of cisplatin-damaged DNA. We found that induction of cAMP signaling, either induced through MSH-MC1R signaling or by forskolin, increased the repair of cisplatin-induced DNA adducts in melanocytes and in HEK293 cells. As MC1R’s cAMP-stimulating ability is regulated by both positive and negative ligands, we further tested MC1R’s ligands to influence the repair of cisplatin damage. MSH-enhanced clearance of cisplatin DNA damage was inhibited by MC1R antagonists HBD3 and ASIP, supporting the hypothesis that repair of cisplatin-damaged DNA is regulated through MC1R-ligand interactions. Of interest, cAMP-mediated enhancement of cisplatin damage was greater than for that of UV-induced DNA damage. This infers differential NER-cAMP repair profiles for different DNA lesions, perhaps explained by variable conformational alterations in DNA or extent of lesions generated by damaging agents.
6.4. Additional mechanisms of regulating melanocyte NER
In addition to the increased rate of NER produced through melanocortin-MC1R signaling, there are additional mechanisms by which melanocytes can modulate NER activity. First, it has been observed that palmitoylation of MC1R can promote cAMP production in melanocytes that harbor a mutated MC1R such as R151C, which do not respond properly to MSH signaling (Chen et al., 2017). This is possible because the palmitoylation event occurs at a cytoplasmic moiety on MC1R that is present even when MC1R polymorphisms render the protein incapable of responding to MSH. This post-translational modification of MC1R, produced most efficiently by the protein-acyl transferase ZDHHC13, increases cAMP levels and, in turn, increases the efficiency of removal of UV-induced photolesions (Chen et al., 2017). MC1R mutants that are incapable of being palmitoylated (like the C315S mutant) have a reduced efficiency of removing photolesions, implicating this modification as a critical component in MC1R signaling.
Beyond the MC1R-cAMP pathway, melanocytes possess other means to enhance repair rates and reduce mutation risk. The endothelin-B receptor (ETBR) is plasma membrane G protein-coupled receptor whose primary agonist endothelin-1 (ET-1) produces a range of responses in melanocytes, including increasing the rate of repair of DNA photo-adducts (Kadekaro et al., 2005; von Koschembahr, Swope, Starner, & Abdel-Malek, 2015). Mechanistically, ET-1 activates the JNK and p38 signaling pathways in a calcium-dependent fashion and subsequently activates ATF-2, a JNK and p38 target protein (von Koschembahr et al., 2015). ATF-2 is a transcription factor that is involved in DNA damage response signaling (Bhoumik, Lopez-Bergami, & Ronai, 2007) and whose activation by JNK promotes enhanced DNA repair (Hayakawa, Depatie, Ohmichi, & Mercola, 2003). However, ATF-2 activation is not sufficient to transduce ET-1 signaling into its full potential for stimulation of CPD repair. Inhibition of p38 by the chemical inhibitor SB 203580 had no significant impact on ATF-2 activation upon ET-1 treatment, but it did impact CPD repair, suggesting an additional, currently undetermined, mechanism behind ET-1 mediated CPD repair enhancement (von Koschembahr et al., 2015). ET-1 enhancement of DNA repair does not require MC1R proficiency, and cAMP levels are unaffected by ET-1 treatment, and therefore this mechanism of enhanced repair can be considered a completely separate pathway than that of the αMSH/MC1R signaling axis (von Koschembahr et al., 2015). Our group verified that ET-1 treatment of melanocytes did not induce PKA-mediated phosphorylation of ATR on S435, suggesting a distinct mechanism of NER enhancement than is promoted by MC1R signaling (Jarrett et al., 2015).
NER function in melanocytes is also mediated by MED23, a member of the Mediator family of coactivator proteins that connect transcription factors to RNA polymerase II. Inhibition of MED23 impairs MITF expression, which results in a loss of pigmentation but almost counterintuitively an increase in NER proficiency and a reduction in the initial burden of photo-damage following irradiation (Xia et al., 2017). Finally, the vitamin nicotinamide has also been shown to enhance the repair of UV-induced DNA damage, as well as oxidative DNA damage, in primary melanocytes (Thompson, Surjana, Halliday, & Damian, 2014). What is clear is that melanocytic NER capacity is not a static phenomenon, but rather can be regulated by a variety of signaling pathways.
6.5. Translational implications: Enhancement of melanocyte genomic stability for melanoma prevention
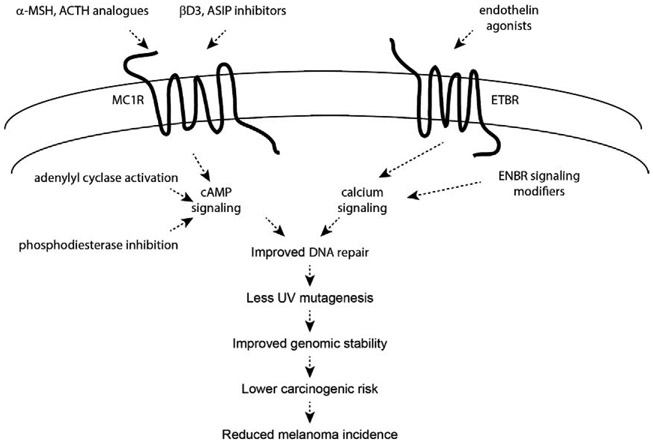
Melanoma has among the highest somatic mutational burdens of all human cancers (Lawrence et al., 2014) and UV-induced mutations account for the great majority of genetic changes that occur with melanoma progression (Hodis et al., 2012; Shain et al., 2015). Therefore, the more efficiently a melanocyte can resist and/or repair UV damage, the better positioned it will be to avoid UV mutagenesis and carcinogenic progression. We and others have documented that NER, the genomic maintenance pathway that rids DNA of UV photolesions and other helical-distorting damage, can be regulated in melanocytes by a variety of signaling pathways including the MC1R and endothelin-ETBR signaling axes (Abdel-Malek et al., 2009; Bohm et al., 2005; Hauser et al., 2006; Jarrett, Carter, Shelton, & D’Orazio, 2017; Jarrett et al., 2015; Kadekaro et al., 2005; von Koschembahr et al., 2015). On the surface, it seems illogical that NER would not already be maximally efficient in epidermal melanocytes that, because of their anatomic placement, are at heightened risk for exposure to UV and other environmental carcinogens that interact with the skin. Certainly, melanocyte NER is active at a baseline level without exogenous activation of the MSH-MC1R-cAMP or the endothelin-ETBR signaling pathways, but it can be markedly enhanced when these damage-induced pathways are recruited (Fig. 12). It is possible that NER might not be maintained at maximum efficiency in melanocytes because of the potential for active damage scanning to conflict with replication or transcription processes. Moreover, maintaining NER at optimal levels (i.e., even when there has been no UV damage) would represent an undue energetic and metabolic burden in terms of ATP consumption or nucleotide pool levels. We posit that the increased melanoma risk associated with loss-of-function MC1R polymorphisms may, in addition to attenuated pigmentary responses, be due to sub-optimal DNA repair. There is no “cAMP repair boost” in MC1R defective persons, allowing UV damage to linger and cause mutagenesis in melanocyte genomes. Therefore, over years, UV-exposed melanocytes in the skin of MC1R-defective individuals would accumulate more mutations and be at heightened risk of malignant degeneration. Recent data documenting a 42% higher somatic mutational burden in melanomas isolated from MC1R-defective (vs. MC1R-intact) persons support this concept (Robles-Espinoza et al., 2016).
Fig. 12.
Melanoma-preventive strategies based on enhancing melanization and DNA repair in melanocytes. Individuals with inherited MC1R signaling defects are at increased risk of melanoma because of ineffective epidermal melanization and sub-optimal nucleotide excision repair (NER), leading to more UV penetration into the skin and less capacity to reverse mutagenic photodamage. Discovering that melanocyte NER can be improved by MC1R and ETBR signaling pathways offers opportunities to regulate DNA repair and genomic stability pharmacologically. MC1R and ETBR signaling could be targeted by agonists or inhibitors of antagonists, but may not be effective in persons carrying defective MC1R receptors. Alternatively, signaling pathways could be pharmacologically manipulated (e.g., adenylyl cyclase activation or phosphodiesterase inhibition in the case of cAMP signaling). If effective approaches can be developed to improve melanocyte UV resistance, these would theoretically reduce melanoma risk by reducing the mutagenic burden of UV.
Knowing that DNA repair can be optimized by up-regulating cAMP levels introduces the possibility of pharmacologically stimulating MC1R responses to protect melanocytes from UV-induced mutagenesis by modulating intracellular cAMP levels. This could theoretically be done in a variety of ways that differ based on whether MC1R itself will be targeted. Since MC1R is restricted to melanocytes among epidermal cells, cutaneously applied agents designed to activate MC1R signaling (such as topical MSH analogues (Abdel-Malek et al., 2009)) would be expected to yield cAMP increases only in melanocytes. Though this approach would limit off-target cAMP-mediated toxicities, it would be expected to be of limited benefit for MC1R-defective persons who are at high risk for melanoma. For such individuals, a more effective approach might be to apply small molecules topically that manipulate cAMP levels in an MC1R-independent manner by activating adenylyl cyclases or by inhibiting phosphodiesterases. We have used forskolin in our studies as an MC1R-independent adenylyl cyclase activator in preclinical work to demonstrate rescue of UV-protective melanocyte responses including eumelanin induction (Amaro-Ortiz, Vanover, Scott, & D’Orazio, 2013; D’Orazio et al., 2006; Spry et al., 2009) and enhancement of DNA repair (Jarrett et al., 2017, 2016; Jarrett, Horrell, et al., 2014; Jarrett, Wolf Horrell, Boulanger, & D’Orazio, 2015). Though manipulation of epidermal cAMP is highly effective, this approach lacks melanocyte specificity, and the cAMP increase could have off-target effects as cAMP levels in the cell produced by different pathways often have selective functions, whereas increasing cAMP in a pathway-independent manner as is done with forskolin will activate all downstream responders to cellular cAMP. Careful toxicity and feasibility studies would be required to understand the risks and benefits of this approach. In any case, it is evident that it is possible to reduce UV damage and mutagenesis in melanocytes (and therefore melanoma risk) by rationally targeting the cAMP UV-protective pathway. Furthermore, the discovery of non-MC1R based signaling pathways that enhance melanocyte DNA repair and UV resistance (Chen et al., 2017; Thompson et al., 2014; von Koschembahr et al., 2015; Xia et al., 2017) offers potentially new opportunities for melanoma prevention, especially for individuals with inherited defects in MC1R signaling.
Acknowledgments
This work was supported by the following NIH grants: R01 CA131075, P30 CA177558, 2T32 CA160003-06A1, and 5T32 CA165990-06. We thank the Melanoma Research Alliance, the Regina Drury Pediatric Research Endowed Chair Fund, the Wendy Will Case Cancer Research Fund, the Markey Cancer Foundation, the Children’s Miracle Network, and the Jennifer and David Dickens Melanoma Research Foundation. We thank the Markey Cancer Center’s Research Communications Office for assistance with figure preparation.
Footnotes
Conflicts of interest
The authors would like to state that there are no conflicts of interest that arose during the preparation of this manuscript.
References
- Abdel-Malek ZA, Ruwe A, Kavanagh-Starner R, Kadekaro AL, Swope V, Haskell-Luevano C, et al. (2009). Alpha-MSH tripeptide analogs activate the melanocortin 1 receptor and reduce UV-induced DNA damage in human melanocytes. Pigment Cell & Melanoma Research, 22(5), 635–644. 10.1111/j.1755-148X.2009.00598.x. [DOI] [PubMed] [Google Scholar]
- Abdel-Malek ZA, Scott MC, Furumura M, Lamoreux ML, Ollmann M, Barsh GS, et al. (2001). The melanocortin 1 receptor is the principal mediator of the effects of agouti signaling protein on mammalian melanocytes. Journal of Cell Science, 114, 1019–1024 Pt. 5. [DOI] [PubMed] [Google Scholar]
- Abdel-Malek Z, Scott MC, Suzuki I, Tada A, Im S, Lamoreux L, et al. (2000). The melanocortin-1 receptor is a key regulator of human cutaneous pigmentation. Pigment Cell Research, 13(Suppl. 8), 156–162. [DOI] [PubMed] [Google Scholar]
- Abdel-Rahman MH, Pilarski R, Massengill JB, Christopher BN, Noss R, & Davidorf FH (2011). Melanoma candidate genes CDKN2A/p16/INK4A, p14ARF, and CDK4 sequencing in patients with uveal melanoma with relative high-risk for hereditary cancer predisposition. Melanoma Research, 21(3), 175–179. 10.1097/CMR.0b013e328343eca2. [DOI] [PubMed] [Google Scholar]
- Adimoolam S, & Ford JM (2002). p53 and DNA damage-inducible expression of the xeroderma pigmentosum group C gene. Proceedings of the National Academy of Sciences of the United States of America, 99(20), 12985–12990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, et al. (2013). Signatures of mutational processes in human cancer. Nature, 500(7463), 415–421. 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaro-Ortiz A, Vanover JC, Scott TL, & D’Orazio JA (2013). Pharmacologic induction of epidermal melanin and protection against sunburn in a humanized mouse model. Journal of Visualized Experiments, (79). 10.3791/50670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amundson SA, Patterson A, Do KT, & Fornace AJ Jr. (2002). A nucleotide excision repair master-switch: p53 regulated coordinate induction of global genomic repair genes. Cancer Biology & Therapy, 1(2), 145–149. [DOI] [PubMed] [Google Scholar]
- Arron ST, Raymond AK, Yanik EL, Castenson D, McCulloch CE, Clarke CA, et al. (2016). Melanoma outcomes in transplant recipients with pretransplant melanoma. Dermatologic Surgery, 42(2), 157–166. 10.1097/DSS.0000000000000602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autier P, Dore JF, Lejeune F, Koelmel KF, Geffeler O, Hille P, et al. (1994). Cutaneous malignant melanoma and exposure to sunlamps or sunbeds: An EORTC multicenter case-control study in Belgium, France and Germany. EORTC melanoma cooperative group. International Journal of Cancer, 58(6), 809–813. [DOI] [PubMed] [Google Scholar]
- Autier P, Joarlette M, Lejeune F, Lienard D, Andre J, & Achten G (1991). Cutaneous malignant melanoma and exposure to sunlamps and sunbeds: A descriptive study in Belgium. Melanoma Research, 1(1), 69–74. [DOI] [PubMed] [Google Scholar]
- Avril MF, Chompret A, Verne-Fourment L, Terrier-Lacombe MJ, Spatz A, Fizazi K, et al. (2001). Association between germ cell tumours, large numbers of naevi, atypical naevi and melanoma. Melanoma Research, 11(2), 117–122. [DOI] [PubMed] [Google Scholar]
- Azoury SC, & Lange JR (2014). Epidemiology, risk factors, prevention, and early detection of melanoma. The Surgical Clinics of North America, 94(5), 945–962. vii. 10.1016/j.suc.2014.07.013. [DOI] [PubMed] [Google Scholar]
- Baccarelli A, Calista D, Minghetti P, Marinelli B, Albetti B, Tseng T, et al. (2004). XPD gene polymorphism and host characteristics in the association with cutaneous malignant melanoma risk. British Journal of Cancer, 90(2), 497–502. 10.1038/sj.bjc.6601385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bataille V, Winnett A, Sasieni P, Newton Bishop JA, & Cuzick J (2004). Exposure to the sun and sunbeds and the risk of cutaneous melanoma in the UK: A case-control study. European Journal of Cancer, 40(3), 429–435. https://doi.org/S095980490300861X [pii]. [DOI] [PubMed] [Google Scholar]
- Bensch KW, Raida M, Magert HJ, Schulz-Knappe P, & Forssmann WG (1995). hBD-1: A novel beta-defensin from human plasma. FEBS Letters, 368(2), 331–335. [DOI] [PubMed] [Google Scholar]
- Bertram CG, Gaut RM, Barrett JH, Randerson-Moor J, Whitaker L, Turner F, et al. (2004). An assessment of a variant of the DNA repair gene XRCC3 as a possible nevus or melanoma susceptibility genotype. The Journal of Investigative Dermatology, 122(2), 429–432. 10.1046/j.0022-202X.2003.12541.x. [DOI] [PubMed] [Google Scholar]
- Berwick M, Buller DB, Cust A, Gallagher R, Lee TK, Meyskens F, et al. (2016). Melanoma epidemiology and prevention. Cancer Treatment and Research, 167, 17–49. 10.1007/978-3-319-22539-5_2. [DOI] [PubMed] [Google Scholar]
- Bett BJ (2005). Large or multiple congenital melanocytic nevi: Occurrence of cutaneous melanoma in 1008 persons. Journal of the American Academy of Dermatology, 52(5), 793–797. 10.1016/j.jaad.2005.02.024. [DOI] [PubMed] [Google Scholar]
- Bhoumik A, Lopez-Bergami P, & Ronai Z (2007). ATF2 on the double-activating transcription factor and DNA damage response protein. Pigment Cell Research, 20(6), 498–506. 10.1111/j.1600-0749.2007.00414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard SG, Harris CO, Ittoop OR, Nichols JS, Parks DJ, Truesdale AT, et al. (1995). Agouti antagonism of melanocortin binding and action in the B16F10 murine melanoma cell line. Biochemistry, 34(33), 10406–10411. [DOI] [PubMed] [Google Scholar]
- Blankenburg S, Konig IR, Moessner R, Laspe P, Thoms KM, Krueger U, et al. (2004). No association between three xeroderma pigmentosum group C and one group G gene polymorphisms and risk of cutaneous melanoma. European Journal of Human Genetics, 13(2), 253–255. [DOI] [PubMed] [Google Scholar]
- Bohm M, Wolff I, Scholzen TE, Robinson SJ, Healy E, Luger TA, et al. (2005). Alpha-melanocyte-stimulating hormone protects from ultraviolet radiation-induced apoptosis and DNA damage. The Journal of Biological Chemistry, 280(7), 5795–5802. 10.1074/jbc.M406334200. [DOI] [PubMed] [Google Scholar]
- Boman HG (1995). Peptide antibiotics and their role in innate immunity. Annual Review of Immunology, 13, 61–92. 10.1146/annurev.iy.13.040195.000425. [DOI] [PubMed] [Google Scholar]
- Box NF, Wyeth JR, O’Gorman LE, Martin NG, & Sturm RA (1997). Characterization of melanocyte stimulating hormone receptor variant alleles in twins with red hair. Human Molecular Genetics, 6(11), 1891–1897. https://doi.org/dda242 [pii]. [DOI] [PubMed] [Google Scholar]
- Candille SI, Kaelin CB, Cattanach BM, Yu B, Thompson DA, Nix MA, et al. (2007). A -defensin mutation causes black coat color in domestic dogs. Science, 318(5855), 1418–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai BX, Neubig RR, Millhauser GL, Thompson DA, Jackson PJ, Barsh GS, et al. (2003). Inverse agonist activity of agouti and agouti-related protein. Peptides, 24(4), 603–609. [DOI] [PubMed] [Google Scholar]
- Chang AY, Doiron P, & Maurer T (2017). Cutaneous malignancies in HIV. Current Opinion in HIV and AIDS, 12(1), 57–62. 10.1097/COH.0000000000000338. [DOI] [PubMed] [Google Scholar]
- Chang C, Murzaku EC, Penn L, Abbasi NR, Davis PD, Berwick M, et al. (2014). More skin, more sun, more tan, more melanoma. American Journal of Public Health, 104(11), e92–e99. 10.2105/AJPH.2014.302185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Zhu B, Yin C, Liu W, Han C, Chen B, et al. (2017). Palmitoylation-dependent activation of MC1R prevents melanomagenesis. Nature, 549(7672), 399–403. 10.1038/nature23887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough-Gorr KM, Titus-Ernstoff L, Perry AE, Spencer SK, & Ernstoff MS (2008). Exposure to sunlamps, tanning beds, and melanoma risk. Cancer Causes & Control, 19(7), 659–669. 10.1007/s10552-008-9129-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin JA, Fridlyand J, Kageshita T, Patel HN, Busam KJ, Kutzner H, et al. (2005). Distinct sets of genetic alterations in melanoma. The New England Journal of Medicine, 353(20), 2135–2147. https://doi.org/353/20/2135 [pii]. [DOI] [PubMed] [Google Scholar]
- Cust AE, Armstrong BK, Goumas C, Jenkins MA, Schmid H, Hopper JL, et al. (2011). Sunbed use during adolescence and early adulthood is associated with increased risk of early-onset melanoma. International Journal of Cancer, 128(10), 2425–2435. 10.1002/ijc.25576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debniak T, Scott RJ, Huzarski T, Byrski T, Masojc B, van de Wetering T, et al. (2006). XPD common variants and their association with melanoma and breast cancer risk. Breast Cancer Research and Treatment, 98(2), 209–215. 10.1007/S10549-005-9151-2. [DOI] [PubMed] [Google Scholar]
- Del Bino S, Ito S, Sok J, Nakanishi Y, Bastien P, Wakamatsu K, et al. (2015). Chemical analysis of constitutive pigmentation of human epidermis reveals rather constant eumelanin to pheomelanin ratio. Pigment Cell & Melanoma Research, 28(6), 707–717. 10.1111/pcmr.12410. [DOI] [PubMed] [Google Scholar]
- Diffey BL (2002). Sources and measurement of ultraviolet radiation. Methods, 28(1), 4–13. [DOI] [PubMed] [Google Scholar]
- DiGiovanna JJ, & Kraemer KH (2012). Shining a light on xeroderma pigmentosum. The Journal of Investigative Dermatology, 132(3 Pt. 2), 785–796. https://doi.org/jid2011426 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dika E, Ravaioli GM, Fanti PA, Neri I, & Patrizi A (2017). Spitz nevi and other Spitzoid neoplasms in children: Overview of incidence data and diagnostic criteria. Pediatric Dermatology, 34(1), 25–32. 10.1111/pde.13025. [DOI] [PubMed] [Google Scholar]
- D’Orazio JA, Nobuhisa T, Cui R, Arya M, Spry M, Wakamatsu K, et al. (2006). Topical drug rescue strategy and skin protection based on the role of Mc1r in UV-induced tanning. Nature, 443(7109), 340–344. https://doi.org/nature05098 [pii]. [DOI] [PubMed] [Google Scholar]
- Duan Z, Shen H, Lee JE, Gershenwald JE, Ross MI, Mansfield PF, et al. (2002). DNA repair gene XRCC3 241Met variant is not associated with risk of cutaneous malignant melanoma. Cancer Epidemiology, Biomarkers & Prevention, 11( 10 Pt. 1), 1142–1143. [PubMed] [Google Scholar]
- Duffy DL, Box NF, Chen W, Palmer JS, Montgomery GW, James MR, et al. (2004). Interactive effects of MC1R and OCA2 on melanoma risk phenotypes. Human Molecular Genetics, 13(4), 447–461. [DOI] [PubMed] [Google Scholar]
- Elliott TM, Whiteman DC, Olsen CM, & Gordon LG (2017). Estimated healthcare costs of melanoma in Australia over 3 years post-diagnosis. Applied Health Economics and Health Policy, 15(6), 805–816. 10.1007/s40258-017-0341-y. [DOI] [PubMed] [Google Scholar]
- Fitch ME, Cross IV, & Ford JM (2003). p53 responsive nucleotide excision repair gene products p48 and XPC, but not p53, localize to sites of UV-irradiation-induced DNA damage, in vivo. Carcinogenesis, 24(5), 843–850. [DOI] [PubMed] [Google Scholar]
- Fitch ME, Nakajima S, Yasui A, & Ford JM (2003). In vivo recruitment of XPC to UV-induced cyclobutane pyrimidine dimers by the DDB2 gene product. The Journal of Biological Chemistry, 278(47), 46906–46910. 10.1074/jbc.M307254200. [DOI] [PubMed] [Google Scholar]
- Fujiwara Y, Masutani C, Mizukoshi T, Kondo J, Hanaoka F, & Iwai S (1999). Characterization of DNA recognition by the human UV-damaged DNA-binding protein. The Journal of Biological Chemistry, 274(28), 20027–20033. [DOI] [PubMed] [Google Scholar]
- Garcia-Borron JC, Abdel-Malek Z, & Jimenez-Cervantes C (2014). MC1R, the cAMP pathway, and the response to solar UV: Extending the horizon beyond pigmentation. Pigment Cell & Melanoma Research, 27(5), 699–720. 10.1111/pcmr.12257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Borron JC, Sanchez-Laorden BL, & Jimenez-Cervantes C (2005). Melanocortin-1 receptor structure and functional regulation. Pigment Cell Research, 18(6), 393–410. https://doi.org/PCR278 [pii]. [DOI] [PubMed] [Google Scholar]
- Garrett GL, Blanc PD, Boscardin J, Lloyd AA, Ahmed RL, Anthony T, et al. (2017). Incidence of and risk factors for skin Cancer in organ transplant recipients in the United States. JAMA Dermatology, 153(3), 296–303. 10.1001/jamadermatol.2016.4920. [DOI] [PubMed] [Google Scholar]
- Geller AC, Colditz G, Oliveria S, Emmons K, Jorgensen C, Aweh GN, et al. (2002). Use of sunscreen, sunburning rates, and tanning bed use among more than 10,000 US children and adolescents. Pediatrics, 109(6), 1009–1014. [DOI] [PubMed] [Google Scholar]
- Geller AC, Mayer JE, Sober AJ, Miller DR, Argenziano G, Johnson TM, et al. (2016). Total nevi, atypical nevi, and melanoma thickness: An analysis of 566 patients at 2 US centers. JAMA Dermatology, 152(4), 413–418. 10.1001/jamadermatol.2016.0027. [DOI] [PubMed] [Google Scholar]
- Ghiasvand R, Weiderpass E, Green AC, Lund E, & Veierod MB (2016). Sunscreen use and subsequent melanoma risk: A population-based cohort study. Journal of Clinical Oncology, 34(33), 3976–3983. 10.1200/JCO.2016.67.5934. [DOI] [PubMed] [Google Scholar]
- Giglia-Mari G, & Sarasin A (2003). TP53 mutations in human skin cancers. Human Mutation, 21(3), 217–228. 10.1002/humu.10179. [DOI] [PubMed] [Google Scholar]
- Gillet LC, & Scharer OD (2006). Molecular mechanisms of mammalian global genome nucleotide excision repair. Chemical Reviews, 106(2), 253–276. [DOI] [PubMed] [Google Scholar]
- Goldstein AM, Xiao Y, Sampson J, Zhu B, Rotunno M, Bennett H, et al. (2017). Rare germline variants in known melanoma susceptibility genes in familial melanoma. Human Molecular Genetics, 26(24), 4886–4895. 10.1093/hmg/ddx368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham A, Wakamatsu K, Hunt G, Ito S, & Thody AJ (1997). Agouti protein inhibits the production of eumelanin and phaeomelanin in the presence and absence of alpha-melanocyte stimulating hormone. Pigment Cell Research, 10(5), 298–303. [DOI] [PubMed] [Google Scholar]
- Green AC, Williams GM, Logan V, & Strutton GM (2011). Reduced melanoma after regular sunscreen use: Randomized trial follow-up. Journal of Clinical Oncology, 29(3), 257–263. https://doi.org/JCO.2010.28.7078. [pii]. [DOI] [PubMed] [Google Scholar]
- Guy GP Jr., Machlin SR, Ekwueme DU, & Yabroff KR (2015). Prevalence and costs of skin cancer treatment in the U.S., 2002-2006 and 2007-2011. American Journal of Preventive Medicine, 48(2), 183–187. 10.1016/j.amepre.2014.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy GP Jr., Thomas CC, Thompson T, Watson M, Massetti GM, Richardson LC, et al. (2015). Vital signs: Melanoma incidence and mortality trends and projections—United States, 1982-2030. MMWR. Morbidity and Mortality Weekly Report, 64(21), 591–596. [PMC free article] [PubMed] [Google Scholar]
- Han J, Colditz GA, Liu JS, & Hunter DJ (2005). Genetic variation in XPD, sun exposure, and risk of skin cancer. Cancer Epidemiology, Biomarkers & Prevention, 14(6), 1539–1544. [DOI] [PubMed] [Google Scholar]
- Han J, Colditz GA, Samson LD, & Hunter DJ (2004). Polymorphisms in DNA double-strand break repair genes and skin cancer risk. Cancer Research, 64(9), 3009–3013. [DOI] [PubMed] [Google Scholar]
- Harder J, Bartels J, Christophers E, & Schroder JM (2001). Isolation and characterization of human beta-defensin-3, a novel human inducible peptide antibiotic. The Journal of Biological Chemistry, 276(8), 5707–5713. 10.1074/jbc.M008557200. [DOI] [PubMed] [Google Scholar]
- Hauser JE, Kadekaro AL, Kavanagh RJ, Wakamatsu K, Terzieva S, Schwemberger S, et al. (2006). Melanin content and MC1R function independently affect UVR-induced DNA damage in cultured human melanocytes. Pigment Cell Research, 19(4), 303–314. https://doi.org/PCR315 [pii]. [DOI] [PubMed] [Google Scholar]
- Hayakawa J, Depatie C, Ohmichi M, & Mercola D (2003). The activation of c-Jun NH2-terminal kinase (JNK) by DNA-damaging agents serves to promote drug resistance via activating transcription factor 2 (ATF2)-dependent enhanced DNA repair. The Journal of Biological Chemistry, 278(23), 20582–20592. 10.1074/jbc.M210992200. [DOI] [PubMed] [Google Scholar]
- Healy E, Jordan SA, Budd PS, Suffolk R, Rees JL, & Jackson IJ (2001). Functional variation of MC1R alleles from red-haired individuals. Human Molecular Genetics, 10(21), 2397–2402. [DOI] [PubMed] [Google Scholar]
- Hodis E, Watson IR, Kryukov GV, Arold ST, Imielinski M, Theurillat JP, et al. (2012). A landscape of driver mutations in melanoma. Cell, 150(2), 251–263. https://doi.org/S0092-8674(12)00778-7 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogduijn MJ, Cemeli E, Ross K, Anderson D, Thody AJ, & Wood JM (2004). Melanin protects melanocytes and keratinocytes against H2O2-induced DNA strand breaks through its ability to bind Ca2+. Experimental Cell Research, 294(1), 60–67. [DOI] [PubMed] [Google Scholar]
- Iordanov M, Bender K, Ade T, Schmid W, Sachsenmaier C, Engel K, et al. (1997). CREB is activated by UVC through a p38/HOG-1-dependent protein kinase. The EMBO Journal, 16(5), 1009–1022. 10.1093/emboj/16.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S, & Wakamatsu K (2003). Quantitative analysis of eumelanin and pheomelanin in humans, mice, and other animals: A comparative review. Pigment Cell Research, 16(5), 523–531. [DOI] [PubMed] [Google Scholar]
- Jablonski NG, & Chaplin G (2017). The colours of humanity: The evolution of pigmentation in the human lineage. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 372(1724), 20160349 10.1098/rstb.2016.0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett SG, Carter KM, Shelton BJ, & D’Orazio JA (2017). The melanocortin signaling cAMP axis accelerates repair and reduces mutagenesis of platinum-induced DNA damage. Scientific Reports, 7(1), 11708 10.1038/s41598-017-12056-5. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Jarrett SG, Horrell EM, Christian PA, Vanover JC, Boulanger MC, Zou Y, et al. (2014). PKA-mediated phosphorylation of ATR promotes recruitment of XPA to UV-induced DNA damage. Molecular Cell, 54(6), 999–1011. 10.1016/j.molcel.2014.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Jarrett SG, Wolf Horrell EM, Boulanger MC, & D’Orazio JA (2015). Defining the contribution of MC1R physiological ligands to ATR phosphorylation at Ser435, A predictor of DNA repair in melanocytes. The Journal of Investigative Dermatology. 10.1038/jid.2015.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett SG, Wolf Horrell EM, & D’Orazio JA (2016). AKAP12 mediates PKA-induced phosphorylation of ATR to enhance nucleotide excision repair. Nucleic Acids Research, 44(22), 10711–10726. 10.1093/nar/gkw871. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Johnson RE, Washington MT, Prakash S, & Prakash L (2000). Fidelity of human DNA polymerase eta. The Journal of Biological Chemistry, 275(11), 7447–7450. [DOI] [PubMed] [Google Scholar]
- Kadekaro AL, Chen J, Yang J, Chen S, Jameson J, Swope VB, et al. (2012). Alpha-melanocyte-stimulating hormone suppresses oxidative stress through a p53-mediated signaling pathway in human melanocytes. Molecular Cancer Research, 10(6), 778–786. https://doi.org/1541-7786.MCR-11-0436 [pii]. [DOI] [PubMed] [Google Scholar]
- Kadekaro AL, Kavanagh R, Kanto H, Terzieva S, Hauser J, Kobayashi N, et al. (2005). Alpha-melanocortin and endothelin-1 activate antiapoptotic pathways and reduce DNA damage in human melanocytes. Cancer Research, 65(10), 4292–4299. [DOI] [PubMed] [Google Scholar]
- Karagas MR, Stannard VA, Mott LA, Slattery MJ, Spencer SK, & Weinstock MA (2002). Use of tanning devices and risk of basal cell and squamous cell skin cancers. Journal of the National Cancer Institute, 94(3), 224–226. [DOI] [PubMed] [Google Scholar]
- Kertat K, Rosdahl I, Sun XF, Synnerstad I, & Zhang H (2008). The Gln/Gln genotype of XPD codon 751 as a genetic marker for melanoma risk and Lys/Gln as an important predictor for melanoma progression: A case control study in the Swedish population. Oncology Reports, 20(1), 179–183. [PubMed] [Google Scholar]
- Kinsler VA, O’Hare P, Bulstrode N, Calonje JE, Chong WK, Hargrave D, et al. (2017). Melanoma in congenital melanocytic naevi. The British Journal of Dermatology, 176(5), 1131–1143. 10.1111/bjd.15301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kricker A, Armstrong BK, Goumas C, Litchfield M, Begg CB, Hummer AJ, et al. (2007). Ambient UV, personal sun exposure and risk of multiple primary melanomas. Cancer Causes & Control, 18(3), 295–304. 10.1007/s10552-006-0091-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange SS, Takata K, & Wood RD (2011). DNA polymerases and cancer. Nature Reviews Cancer, 11(2), 96–110. 10.1038/nrc2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence MS, Stojanov P, Mermel CH, Robinson JT, Garraway LA, Golub TR, et al. (2014). Discovery and saturation analysis of cancer genes across 21 tumour types. Nature, 505(7484), 495–501. 10.1038/nature12912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A, et al. (2013). Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature, 499(7457), 214–218. 10.1038/nature12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazovich D, Isaksson Vogel R, Weinstock MA, Nelson HH, Ahmed RL, & Berwick M (2016). Association between indoor tanning and melanoma in younger men and women. JAMA Dermatology, 152(3), 268–275. 10.1001/jamadermatol.2015.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DH, & Pfeifer GP (2003). Deamination of 5-methylcytosines within cyclobutane pyrimidine dimers is an important component of UVB mutagenesis. The Journal of Biological Chemistry, 278(12), 10314–10321. 10.1074/jbc.M212696200. [DOI] [PubMed] [Google Scholar]
- Li C, Hu Z, Liu Z, Wang LE, Strom SS, Gershenwald JE, et al. (2006). Polymorphisms in the DNA repair genes XPC, XPD, and XPG and risk of cutaneous melanoma: A case-control analysis. Cancer Epidemiology, Biomarkers & Prevention, 15(12), 2526–2532. [DOI] [PubMed] [Google Scholar]
- Li C, Liu Z, Wang LE, Strom SS, Lee JE, Gershenwald JE, et al. (2006). Genetic variants of the ADPRT, XRCC1 and APE1 genes and risk of cutaneous melanoma. Carcinogenesis, 27(9), 1894–1901. [DOI] [PubMed] [Google Scholar]
- Little CC (1957). The inheritance of coat color in dogs. Ithaca, N. Y: Comstock Publishing Associates, Cornell University Press; London: Constable & Co. Ltd. [Google Scholar]
- Lo JA, & Fisher DE (2014). The melanoma revolution: From UV carcinogenesis to a new era in therapeutics. Science, 346(6212), 945–949. 10.1126/science.1253735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorimer PD, White RL, Walsh K, Han Y, Kirks RC, Symanowski J, et al. (2016). Pediatric and adolescent melanoma: A National Cancer Data Base update. Annals of Surgical Oncology, 23(12), 4058–4066. 10.1245/s10434-016-5349-2. [DOI] [PubMed] [Google Scholar]
- Lu D, Willard D, Patel IR, Kadwell S, Overton L, Kost T, et al. (1994). Agouti protein is an antagonist of the melanocyte-stimulating-hormone receptor. Nature, 371(6500), 799–802. 10.1038/371799a0. [DOI] [PubMed] [Google Scholar]
- Luijsterburg MS, von Bornstaedt G, Gourdin AM, Politi AZ, Mone MJ, Warmerdam DO, et al. (2010). Stochastic and reversible assembly of a multiprotein DNA repair complex ensures accurate target site recognition and efficient repair. The Journal of Cell Biology, 189(3), 445–463. 10.1083/jcb.200909175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masaki T, Wang Y, DiGiovanna JJ, Khan SG, Raffeld M, Beltaifa S, et al. (2014). High frequency of PTEN mutations in nevi and melanomas from xeroderma pigmentosum patients. Pigment Cell & Melanoma Research, 27(3), 454–464. 10.1111/pcmr.12226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar SE, Miller MW, Stevens ME, & Barsh GS (1995). Expression and transgenic studies of the mouse agouti gene provide insight into the mechanisms by which mammalian coat color patterns are generated. Development, 121(10), 3223–3232. [DOI] [PubMed] [Google Scholar]
- Mitchell DL, & Nairn RS (1989). The biology of the (6-4) photoproduct. Photochemistry and Photobiology, 49(6), 805–819. [DOI] [PubMed] [Google Scholar]
- Mitkov M, Chrest M, Diehl NN, Heckman MG, Tollefson M, & Jambusaria-Pahlajani A (2016). Pediatric melanomas often mimic benign skin lesions: A retrospective study. Journal of the American Academy of Dermatology, 75(4). 10.1016/j.jaad.2016.05.015. 706–711.e4. [DOI] [PubMed] [Google Scholar]
- Mitra D, Luo X, Morgan A, Wang J, Hoang MP, Lo J, et al. (2012). An ultraviolet-radiation-independent pathway to melanoma carcinogenesis in the red hair/fair skin background. Nature, 491(7424), 449–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moan J, Baturaite Z, Porojnicu AC, Dahlback A, & Juzeniene A (2012). UVA, UVB and incidence of cutaneous malignant melanoma in Norway and Sweden. Photochemical & Photobiological Sciences, 11(1), 191–198. 10.1039/c1pp05215b. [DOI] [PubMed] [Google Scholar]
- Mocellin S, Verdi D, & Nitti D (2009). DNA repair gene polymorphisms and risk of cutaneous melanoma: A systematic review and meta-analysis. Carcinogenesis, 30(10), 1735–1743. https://doi.org/bgp207 [pii]. [DOI] [PubMed] [Google Scholar]
- Mohania D, Chandel S, Kumar P, Verma V, Digvijay K, Tripathi D, et al. (2017). Ultraviolet radiations: Skin defense-damage mechanism. Advances in Experimental Medicine and Biology, 996, 71–87. 10.1007/978-3-319-56017-5_7. [DOI] [PubMed] [Google Scholar]
- Molinaro AM, Ferrucci LM, Cartmel B, Loftfield E, Leffell DJ, Bale AE, et al. (2015). Indoor tanning and the MC1R genotype: Risk prediction for basal cell carcinoma risk in young people. American Journal of Epidemiology, 181(11), 908–916. 10.1093/aje/kwu356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser J, Kool H, Giakzidis I, Caldecott K, Mullenders LH, & Fousteri MI (2007). Seating of chromosomal DNA nicks during nucleotide excision repair requires XRCC1 and DNA ligase III alpha in a cell-cycle-specific manner. Molecular Cell, 27(2), 311–323. 10.1016/j.molcel.2007.06.014. [DOI] [PubMed] [Google Scholar]
- Muller C, Wendt J, Rauscher S, Burgstaller-Muehlbacher S, Sunder-Plassmann R, Scheurecker C, et al. (2016). Characterization of patients at high risk of melanoma in Austria. The British Journal of Dermatology, 174(6), 1308–1317. 10.1111/bjd.14407. [DOI] [PubMed] [Google Scholar]
- Nielsen K, Masback A, Olsson H, & Ingvar C (2012). A prospective, population-based study of 40,000 women regarding host factors, UV exposure and sunbed use in relation to risk and anatomic site of cutaneous melanoma. International Journal of Cancer, 131(3), 706–715. 10.1002/ijc.26408. [DOI] [PubMed] [Google Scholar]
- Nishi R, Alekseev S, Dinant C, Hoogstraten D, Houtsmuller AB, Hoeijmakers JH, et al. (2009). UV-DDB-dependent regulation of nucleotide excision repair kinetics in living cells. DNA Repair (Amst), 8(6), 767–776. 10.1016/j.dnarep.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Nix MA, Kaetin CB, Ta T, Weis A, Morton GJ, Barsh GS, et al. (2013). Molecular and functional analysis of human beta-defensin 3 action at melanocortin receptors. Chemistry & Biology, 20(6), 784–795. 10.1016/j.chembiol.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogi T, Limsirichaikul S, Overmeer RM, Volker M, Takenaka K, Cloney R, et al. (2010). Three DNA polymerases, recruited by different mechanisms, carry out NER repair synthesis in human cells. Molecular Cell, 37(5), 714–727. 10.1016/j.molcel.2010.02.009. [DOI] [PubMed] [Google Scholar]
- O’Leary RE, Diehl J, & Levins PC (2014). Update on tanning: More risks, fewer benefits. Journal of the American Academy of Dermatology, 70(3), 562–568. 10.1016/j.jaad.2013.11.004. [DOI] [PubMed] [Google Scholar]
- Ollmann MM, Lamoreux ML, Wilson BD, & Barsh GS (1998). Interaction of Agouti protein with the melanocortin 1 receptor in vitro and in vivo. Genes & Development, 12(3), 316–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen CM, Wilson LF, Green AC, Bain CJ, Fritschi L, Neale RE, et al. (2015). Cancers in Australia attributable to exposure to solar ultraviolet radiation and prevented by regular sunscreen use. Australian and New Zealand Journal of Public Health, 39(5), 471–476. 10.1111/1753-6405.12470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orelli B, McClendon TB, Tsodikov OV, Ellenberger T, Niedernhofer LJ, & Scharer OD (2010). The XPA-binding domain of ERCC1 is required for nucleotide excision repair but not other DNA repair pathways. The Journal of Biological Chemistry, 285(6), 3705–3712. 10.1074/jbc.M109.067538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SL, LeMarchand L, Wilkens LR, Kolonel LN, Henderson BE, Zhang ZF, et al. (2012). Risk factors for malignant melanoma in white and non-white/non-African American populations: The multiethnic cohort. Cancer Prevention Research (Philadelphia, Pa), 5(3), 423–434. https://doi.org/1940-6207.CAPR-11-0460[pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer GP, You YH, & Besaratinia A (2005). Mutations induced by ultraviolet light. Mutation Research, 571(1–2), 19–31. https://doi.org/S0027-5107(04)00480-4 [pii]. [DOI] [PubMed] [Google Scholar]
- Pleasance ED, Cheetham RK, Stephens PJ, McBride DJ, Humphray SJ, Greenman CD, et al. (2010). A comprehensive catalogue of somatic mutations from a human cancer genome. Nature, 463(7278), 191–196. https://doi.org/nature08658 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock PM, Harper UL, Hansen KS, Yudt LM, Stark M, Robbins CM, et al. (2003). High frequency of BRAF mutations in nevi. Nature Genetics, 33(1), 19–20. 10.1038/ng1054. [DOI] [PubMed] [Google Scholar]
- Povey JE, Darakhshan F, Robertson K, Bisset Y, Mekky M, Rees J, et al. (2007). DNA repair gene polymorphisms and genetic predisposition to cutaneous melanoma. Carcinogenesis, 28(5), 1087–1093. [DOI] [PubMed] [Google Scholar]
- Rees JL (2003). Genetics of hair and skin color. Annual Review of Genetics, 37, 67–90. [DOI] [PubMed] [Google Scholar]
- Rees JL, & Healy E (1997). Melanocortin receptors, red hair, and skin cancer. The Journal of Investigative Dermatology. Symposium Proceedings, 2(1), 94–98. [DOI] [PubMed] [Google Scholar]
- Reguerre Y, Vittaz M, Orbach D, Robert C, Bodemer C, Mateus C, et al. (2016). Cutaneous malignant melanoma in children and adolescents treated in pediatric oncology units. Pediatric Blood & Cancer, 63(11), 1922–1927. 10.1002/pbc.26113. [DOI] [PubMed] [Google Scholar]
- Ribero S, Glass D, & Bataille V (2016). Genetic epidemiology of melanoma. European Journal of Dermatology, 26(4), 335–339. 10.1684/ejd.2016.2787. [DOI] [PubMed] [Google Scholar]
- Riedl T, Hanaoka F, & Egly JM (2003). The comings and goings of nucleotide excision repair factors on damaged DNA. The EMBO Journal, 22(19), 5293–5303. 10.1093/emboj/cdg489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles AI, Rodriguez-Puebla ML, Glick AB, Trempus C, Hansen L, Sicinski P, et al. (1998). Reduced skin tumor development in cyclin D1-deficient mice highlights the oncogenic ras pathway in vivo. Genes & Development, 12(16), 2469–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles-Espinoza CD, Roberts ND, Chen S, Leacy FP, Alexandrov LB, Pornputtapong N, et al. (2016). Germline MC1R status influences somatic mutation burden in melanoma. Nature Communications, 7, 12064 10.1038/ncomms12064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schadendorf D, Fisher DE, Garbe C, Gershenwald JE, Grob JJ, Halpern A, et al. (2015). Melanoma. Nature Reviews Disease Primers, 1, 15003 10.1038/nrdp.2015.3. [DOI] [PubMed] [Google Scholar]
- Scherer D, & Kumar R (2010). Genetics of pigmentation in skin cancer—A review. Mutation Research, 705(2), 141–153. https://doi.org/S1383-5742(10)00081-5 [pii]. [DOI] [PubMed] [Google Scholar]
- Schmitt MW, Matsumoto Y, & Loeb LA (2009). High fidelity and lesion bypass capability of human DNA polymerase delta. Biochimie, 91(9), 1163–1172. 10.1016/j.biochi.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JJ, Unholzer A, Schaller M, Schafer-Korting M, & Korting HC (2005). Human defensins. Journal of Molecular Medicine (Berlin, Germany), 83(8), 587–595. 10.1007/s00109-005-0657-1. [DOI] [PubMed] [Google Scholar]
- Schroder JM, & Harder J (1999). Human beta-defensin-2. The International Journal of Biochemistry & Cell Biology, 31(6), 645–651. https://doi.org/S1357272599000138. [pii]. [DOI] [PubMed] [Google Scholar]
- Schulman JM, & Fisher DE (2009). Indoor ultraviolet tanning and skin cancer: Health risks and opportunities. Current Opinion in Oncology, 21(2), 144–149. 10.1097/CCO.0b013e3283252fc5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott MC, Suzuki I, & Abdel-Malek ZA (2002). Regulation of the human melanocortin 1 receptor expression in epidermal melanocytes by paracrine and endocrine factors and by ultraviolet radiation. Pigment Cell Research, 15(6), 433–439. https://doi.org/2o051 [pii]. [DOI] [PubMed] [Google Scholar]
- Scrima A, Konickova R, Czyzewski BK, Kawasaki Y, Jeffrey PD, Groisman R, et al. (2008). Structural basis of UV DNA-damage recognition by the DDB1-DDB2 complex. Cell, 135(7), 1213–1223. 10.1016/j.cell.2008.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidenberg AB, Mahalingam-Dhingra A, Weinstock MA, Sinclair C, & Geller AC (2015). Youth indoor tanning and skin cancer prevention: lessons from tobacco control. American Journal of Preventive Medicine, 48(2), 188–194. 10.1016/j.amepre.2014.08.034. [DOI] [PubMed] [Google Scholar]
- Shain AH, Yeh I, Kovalyshyn I, Sriharan A, Talevich E, Gagnon A, et al. (2015). The genetic evolution of melanoma from precursor lesions. The New England Journal of Medicine, 373(20), 1926–1936. 10.1056/NEJMoa1502583. [DOI] [PubMed] [Google Scholar]
- Shivji MK, Podust VN, Hubscher U, & Wood RD (1995). Nucleotide excision repair DNA synthesis by DNA polymerase epsilon in the presence of PCNA, RFC, and RPA. Biochemistry, 34(15), 5011–5017. [DOI] [PubMed] [Google Scholar]
- Smalley KS, & Eisen TG (2002). Differentiation of human melanoma cells through p38 MAP kinase is associated with decreased retinoblastoma protein phosphorylation and cell cycle arrest. Melanoma Research, 12(3), 187–192. [DOI] [PubMed] [Google Scholar]
- Smith R, Healy E, Siddiqui S, Flanagan N, Steijlen PM, Rosdahl I, et al. (1998). Melanocortin 1 receptor variants in an Irish population. The Journal of Investigative Dermatology, 111(1), 119–122. 10.1046/j.1523-1747.1998.00252.x. [DOI] [PubMed] [Google Scholar]
- Smith AG, Luk N, Newton RA, Roberts DW, Sturm RA, & Muscat GE (2008). Melanocortin-1 receptor signaling markedly induces the expression of the NR4A nuclear receptor subgroup in melanocytic cells. The Journal of Biological Chemistry, 283(18), 12564–12570. [DOI] [PubMed] [Google Scholar]
- Smith ML, & Seo YR (2002). p53 regulation of DNA excision repair pathways. Mutagenesis, 17(2), 149–156. [DOI] [PubMed] [Google Scholar]
- Soura E, Eliades PJ, Shannon K, Stratigos AJ, & Tsao H (2016). Hereditary melanoma: Update on syndromes and management: Genetics of familial atypical multiple mole melanoma syndrome. Journal of the American Academy of Dermatology, 74(3), 395–407. quiz 408-310. 10.1016/j.jaad.2015.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spatz A, Giglia-Mari G, Benhamou S, & Sarasin A (2001). Association between DNA repair-deficiency and high level of p53 mutations in melanoma of Xeroderma pigmentosum. Cancer Research, 61(6), 2480–2486. [PubMed] [Google Scholar]
- Spivak G, & Ganesan AK (2014). The complex choreography of transcription-coupled repair. DNA Repair (Amst), 19, 64–70. 10.1016/j.dnarep.2014.03.025. [DOI] [PubMed] [Google Scholar]
- Spry ML, Vanover JC, Scott T, Abona-Ama O, Wakamatsu K, Ito S, et al. (2009). Prolonged treatment of fair-skinned mice with topical forskolin causes persistent tanning and UV protection. Pigment Cell & Melanoma Research, 22(2), 219–229. https://doi.org/PCR536 [pii]. [DOI] [PubMed] [Google Scholar]
- Staresincic L, Fagbemi AF, Enzlin JH, Gourdin AM, Wijgers N, Dunand-Sauthier I, et al. (2009). Coordination of dual incision and repair synthesis in human nucleotide excision repair. The EMBO Journal, 28(8), 1111–1120. 10.1038/emboj.2009.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanaki C, Chardalias L, Soura E, Katsarou A, & Stratigos A (2017). Paediatric melanoma. Journal of the European Academy of Dermatology and Venereology, 31(10), 1604–1615. 10.1111/jdv.14299. [DOI] [PubMed] [Google Scholar]
- Sturm RA, Duffy DL, Box NF, Newton RA, Shepherd AG, Chen W, et al. (2003). Genetic association and cellular function of MC1R variant alleles in human pigmentation. Annals of the New York Academy of Sciences, 994, 348–358. [DOI] [PubMed] [Google Scholar]
- Sugasawa K (2016). Molecular mechanisms of DNA damage recognition for mammalian nucleotide excision repair. DNA Repair (Amst), 44, 110–117. 10.1016/j.dnarep.2016.05.015. [DOI] [PubMed] [Google Scholar]
- Sugasawa K, Akagi J, Nishi R, Iwai S, & Hanaoka F (2009). Two-step recognition of DNA damage for mammalian nucleotide excision repair: Directional binding of the XPC complex and DNA strand scanning. Molecular Cell, 36(4), 642–653. 10.1016/j.molcel.2009.09.035. [DOI] [PubMed] [Google Scholar]
- Sugasawa K, Ng JM, Masutani C, Iwai S, van der Spek PJ, Eker AP, et al. (1998). Xeroderma pigmentosum group C protein complex is the initiator of global genome nucleotide excision repair. Molecular Cell, 2(2), 223–232. [DOI] [PubMed] [Google Scholar]
- Sugasawa K, Okuda Y, Saijo M, Nishi R, Matsuda N, Chu G, et al. (2005). UV-induced ubiquitylation of XPC protein mediated by UV-DDB-ubiquitin ligase complex. Cell, 121(3), 387–400. 10.1016/j.cell.2005.02.035. [DOI] [PubMed] [Google Scholar]
- Sugasawa K, Shimizu Y, Iwai S, & Hanaoka F (2002). A molecular mechanism for DNA damage recognition by the xeroderma pigmentosum group C protein complex. DNA Repair (Amst), 1(1), 95–107. [DOI] [PubMed] [Google Scholar]
- Suzuki I, Cone RD, Im S, Nordlund J, & Abdel-Malek ZA (1996). Binding of melanotropic hormones to the melanocortin receptor MC1R on human melanocytes stimulates proliferation and melanogenesis. Endocrinology, 137(5), 1627–1633. 10.1210/endo.137.5.8612494. [DOI] [PubMed] [Google Scholar]
- Suzuki I, Im S, Tada A, Scott C, Akcali C, Davis MB, et al. (1999). Participation of the melanocortin-1 receptor in the UV control of pigmentation. The Journal of Investigative Dermatology. Symposium Proceedings, 4(1), 29–34. [DOI] [PubMed] [Google Scholar]
- Suzuki I, Tada A, Ollmann MM, Barsh GS, Im S, Lamoreux ML, et al. (1997). Agouti signaling protein inhibits melanogenesis and the response of human melanocytes to alpha-melanotropin. The Journal of Investigative Dermatology, 108(6), 838–842. https://doi.org/S0022202X97862847 [pii]. [DOI] [PubMed] [Google Scholar]
- Svejstrup JQ (2002). Mechanisms of transcription-coupled DNA repair. Nature Reviews. Molecular Cell Biology, 3(1), 21–29. 10.1038/nrm703. [DOI] [PubMed] [Google Scholar]
- Swerdlow AJ, English JS, MacKie RM, O’Doherty CJ, Hunter JA, Clark J, et al. (1988). Fluorescent lights, ultraviolet lamps, and risk of cutaneous melanoma. BMJ, 297(6649), 647–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swope V, Alexander C, Starner R, Schwemberger S, Babcock G, & Abdel-Malek ZA,- (2014). Significance of the melanocortin 1 receptor in the DNA damage response of human melanocytes to ultraviolet radiation. Pigment Cell & Melanoma Research, 27(4), 601–610. 10.1111/pcmr.12252. [DOI] [PubMed] [Google Scholar]
- Swope VB, Jameson JA, McFarland KL, Supp DM, Miller WE, McGraw DW, et al. (2012). Defining MC1R regulation in human melanocytes by its agonist alpha-melanocortin and antagonists agouti signaling protein and beta-defensin 3. The Journal of Investigative Dermatology, 132(9), 2255–2262doi:jid2012135 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellez A, Rueda S, Conic RZ, Powers K, Galdyn I, Mesinkovska NA, et al. (2016). Risk factors and outcomes of cutaneous melanoma in women less than 50 years of age. Journal of the American Academy of Dermatology, 74(4), 731–738. 10.1016/j.jaad.2015.11.014. [DOI] [PubMed] [Google Scholar]
- Thompson BC, Surjana D, Halliday GM, & Damian DL (2014). Nicotinamide enhances repair of ultraviolet radiation-induced DNA damage in primary melanocytes. Experimental Dermatology, 23(7), 509–511. 10.1111/exd.12430. [DOI] [PubMed] [Google Scholar]
- Tomescu D, Kavanagh G, Ha T, Campbell H, & Melton DW (2001). Nucleotide excision repair gene XPD polymorphisms and genetic predisposition to melanoma. Carcinogenesis, 22(3), 403–408. [DOI] [PubMed] [Google Scholar]
- Tommasi S, Denissenko MF, & Pfeifer GP (1997). Sunlight induces pyrimidine dimers preferentially at 5-methylcytosine bases. Cancer Research, 57(21), 4727–4730. [PubMed] [Google Scholar]
- Tsao H, Zhang X, Kwitkiwski K, Finkelstein DM, Sober AJ, & Haluska FG (2000). Low prevalence of germline CDKN2A and CDK4 mutations in patients with early-onset melanoma. Archives of Dermatology, 136(9), 1118–1122. https://doi.org/dst0010 [pii]. [DOI] [PubMed] [Google Scholar]
- Tu Y, Dammann R, & Pfeifer GP (1998). Sequence and time-dependent deamination of cytosine bases in UVB-induced cyclobutane pyrimidine dimers in vivo. Journal of Molecular Biology, 284(2), 297–311. 10.1006/jmbi.1998.2176. [DOI] [PubMed] [Google Scholar]
- Valverde P, Healy E, Jackson I, Rees JL, & Thody AJ (1995). Variants of the melanocyte-stimulating hormone receptor gene are associated with red hair and fair skin in humans. Nature Genetics, 11(3), 328–330. 10.1038/ng1195-328. [DOI] [PubMed] [Google Scholar]
- Vermeulen W, & Fousteri M (2013). Mammalian transcription-coupled excision repair. Cold Spring Harbor Perspectives in Biology, 5(8), a012625 10.1101/cshperspect.a012625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Koschembahr AM, Swope VB, Starner RJ, & Abdel-Malek ZA (2015). Endothelin-1 protects human Melanocytes from UV-induced DNA damage by activating JNK and p38 signaling pathways. Experimental Dermatology, 24(4), 269–274. 10.1111/exd.12638. [DOI] [PubMed] [Google Scholar]
- Wang QE, Zhu Q, Wani G, Chen J, & Wani AA (2004). UV radiation-induced XPC translocation within chromatin is mediated by damaged-DNA binding protein, DDB2. Carcinogenesis, 25(6), 1033–1043. 10.1093/carcin/bgh085. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Krisanaprakornkit S, & Dale BA (1998). Epithelial antimicrobial peptides: Review and significance for oral applications. Critical Reviews in Oral Biology and Medicine, 9(4), 399–414. [DOI] [PubMed] [Google Scholar]
- Weinstock MA, & Fisher DE (2010). Indoor ultraviolet tanning: What the data do and do not show regarding risk of melanoma and keratinocyte malignancies. Journal of the National Comprehensive Cancer Network, 8(8), 867–872. quiz 873. https://doi.org/8/8/867 [pii]. [DOI] [PubMed] [Google Scholar]
- Whiteman DC, Whiteman CA, & Green AC (2001). Childhood sun exposure as a risk factor for melanoma: A systematic review of epidemiologic studies. Cancer Causes & Control, 12(1), 69–82. [DOI] [PubMed] [Google Scholar]
- Wilson BD, Ollmann MM, Kang L, Stoffel M, Bell GI, & Barsh GS (1995). Structure and function of ASP, the human homolog of the mouse agouti gene. Human Molecular Genetics, 4(2), 223–230. [DOI] [PubMed] [Google Scholar]
- Winsey SL, Haldar NA, Marsh HP, Bunce M, Marshall SE, Harris AL, et al. (2000). A variant within the DNA repair gene XRCC3 is associated with the development of melanoma skin cancer. Cancer Research, 60(20), 5612–5616. [PubMed] [Google Scholar]
- Wong SS, Ainger SA, Leonard JH, & Sturm RA (2012). MC1R variant allele effects on UVR-induced phosphorylation of p38, p53, and DDB2 repair protein responses in melanocytic cells in culture. The Journal of Investigative Dermatology, 132(5), 1452–1461. https://doi.org/jid2011473 [pii]. [DOI] [PubMed] [Google Scholar]
- Woollons A, Kipp C, Young AR, Petit-Frere C, Arlett CF, Green MH, et al. (1999). The 0.8% ultraviolet B content of an ultraviolet a sunlamp induces 75% of cyclobutane pyrimidine dimers in human keratinocytes in vitro. The British Journal of Dermatology, 140(6), 1023–1030. [DOI] [PubMed] [Google Scholar]
- Xia M, Chen K, Yao X, Xu Y, Yao J, Yan J, et al. (2017). Mediator MED23 links pigmentation and DNA repair through the transcription factor MITF. Cell Reports, 20(8), 1794–1804. 10.1016/j.celrep.2017.07.056. [DOI] [PubMed] [Google Scholar]
- Yeo JE, Khoo A, Fagbemi AF, & Scharer OD (2012). The efficiencies of damage recognition and excision correlate with duplex destabilization induced by acetylaminofluorene adducts in human nucleotide excision repair. Chemical Research in Toxicology, 25(11), 2462–2468. 10.1021/tx3003033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin K, Sturm RA, & Smith AG (2014). MC1R and NR4A receptors in cellular stress and DNA repair: Implications for UVR protection. Experimental Dermatology, 23(7), 449–452. 10.1111/exd.12420. [DOI] [PubMed] [Google Scholar]
- You YH, Lee DH, Yoon JH, Nakajima S, Yasui A, & Pfeifer GP (2001). Cyclobutane pyrimidine dimers are responsible for the vast majority of mutations induced by UVB irradiation in mammalian cells. The Journal of Biological Chemistry, 276(48), 44688–44694. 10.1074/jbc.M107696200. [DOI] [PubMed] [Google Scholar]
- You YH, Li C, & Pfeifer GP (1999). Involvement of 5-methylcytosine in sunlight-induced mutagenesis. Journal of Molecular Biology, 293(3), 493–503. 10.1006/jmbi.1999.3174. [DOI] [PubMed] [Google Scholar]
- Zerp SF, van Elsas A, Peltenburg LT, & Schrier PI (1999). p53 mutations in human cutaneous melanoma correlate with sun exposure but are not always involved in melanomagenesis. British Journal of Cancer, 79(5–6), 921–926. 10.1038/sj.bjc.6690147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Qureshi AA, Geller AC, Frazier L, Hunter DJ, & Han J (2012). Use of tanning beds and incidence of skin cancer. Journal of Clinical Oncology, 30(14), 1588–1593. 10.1200/JCO.2011.39.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]