Abstract
Background
Although most studies proved that thoracic esophageal cancer surgery with supraclavicular lymph nodes (SCLNs) metastasis could benefit, less than 30% of the 5-year survival rate remained controversy on its surgical treatment. In this study, we aimed to analyze the prognosis of SCLNs on the different segments of thoracic esophageal cancer, which will supply a reference for the treatment of this disease.
Methods
Retrospectively collected the clinical data of 163 patients with thoracic esophageal squamous cancer (ESCC) and compared the effects of SCLNs on prognosis in different segments.
Results
Patients with SCLNs metastasis had a worse prognosis than the negative group (P<0.001). In the upper thoracic group, there was no significant difference in OS between SCLNs positive group and negative group (P=0.077); however, in the middle and lower thoracic group, SCLNs positive group had a worse prognosis than the negative group (P<0.001) and lymph nodes positive in other sites (except for SCLNs) (P=0.039). Multivariate analysis found that SCLNs metastasis was an independent risk factor affecting the prognosis of ESCC in the middle and lower thoracic segments (P=0.007).
Conclusions
For patients with upper thoracic ESCC, SCLNs appear to be regional nodes. For the middle and lower thoracic ESCC, SCLNs should be defined as distant metastasis, and neoadjuvant therapy first may be an available therapy.
Keywords: Supraclavicular lymph nodes (SCLNs), thoracic esophageal squamous cancer (thoracic ESCC), complications, prognosis
Introduction
Lymph node metastasis is an important factor affecting the prognosis of esophageal cancer (1,2). The eighth edition of UICC and AJCC esophageal cancer TNM staging indicates that regardless of the location of the primary tumor, celiac axis nodes and paraesophageal nodes in the neck are included in the regional lymph nodes. However, the supraclavicular lymph nodes (SCLNs) remain as distant metastases (M1). Many clinicians believe that distant metastatic lesions should not be treated surgically, but some studies have shown that patients with SCLNs metastases have a better prognosis than other organs metastases (3,4). Therefore, whether there is a need for SCLNs dissection for thoracic esophageal cancer is still controversial (5,6). In a study involving 1,309 cases of esophageal cancer, Tachimori et al. (7) founded that 190 (14.5%) had SCLNs metastases. The 5-year survival was 73.7% for patients with N0, 40.4% for node-positive patients without SCLNs disease, and 24.1% for patients with SCLNs metastasis. Multivariate analysis showed that SCLNs metastasis was not an independent risk factor for postoperative survival of thoracic esophageal cancer (P=0.062). They believed SCLNs appear to be regional nodes like other regional nodes.
However, they did not have a multivariate analysis of the prognosis of different segmental thoracic esophageal cancer. To this end, we conducted a retrospective clinical study to evaluate the survival benefit of dissection of metastases to the SCLNs in different segmental thoracic esophageal cancer.
Methods
Patients
One hundred sixty-three patients with thoracic esophageal squamous cancer (ESCC) who were diagnosed by pathological examination in the Affiliated Hospital of Jiangnan University and Affiliated Cancer Hospital of Nanjing Medical University from January 1, 2014, to December 31, 2017, were included. All patients underwent esophagectomy with three-field lymph node dissection. Inclusion criteria: (I) all patients were diagnosed as thoracic ESCC by gastroscope biopsy; (II) no clear distant organ metastasis; (III) preoperative cardiac and pulmonary function tests were normal, and there were no absolute contraindications for surgery; (IV) preoperative SCLNs were not significantly enlarged; (V) all patients signed informed consent before surgery. Exclusion criteria: (I) cervical lymph nodes or SCLNs were obviously enlarged and fixed, and could not be completely resected; (II) patients with distant metastases and unsuitable for surgical treatment; (III) unable to tolerate surgery with poor heart and lung function; (IV) patients who received neoadjuvant therapy before surgery.
Methodology
Observation indicators
All patients were divided into positive and negative groups according to the SCLNs; upper segment (20–25 cm from the incisor) and middle (25–30 cm from the incisor) and lower segment (30cm from the incisor to the dentate line) group according to tumor location. Observed and recorded: (I) operative time, blood loss, postoperative hospital stay; (II) postoperative complications: pulmonary infection, anastomotic leakage, arrhythmia, acute renal failure, chyle leakage, etc.; (III) postoperative pathological conditions: lymph node metastasis rate (number of lymph node-positive patients/total number of patients), degree of lymph node metastasis (positive lymph nodes/total number of lymph nodes), tumor size, T stage, differentiation, vascular invasion, neural invasion, cutting edge condition, etc.
Follow-up and statistical methods
Follow-up was conducted by a combination of telephone return visits and outpatient review. Retrospective statistical analysis was performed by reviewing the original medical records. Statistical analysis was performed using SPSS 17.0: an independent sample t-test analyzed the continuous variables; the categorical variables were analyzed by the chi-square test or Fisher exact test; survival was analyzed using the Kaplan-Meier method, and values were compared using the log-rank test. Variables with a p value of less than 0.1 were included in the Cox proportional hazards model. P<0.05 indicates statistical significance.
Results
General data result of thoracic ESCC
Among the 163 patients with thoracic ESCC, 113 were male, and 50 were female, aged 49–80 years, mean age was 64.7±7.0 years, with a median age of 65.0 years. The long diameter of the tumor was 1.2–10 cm, and the upper thoracic, middle thoracic and lower thoracic segments were 62, 80, and 21, respectively. Gross pathological types: 88 cases of ulcer type, 14 cases of sputum umbrella type, 26 cases of erosive type, 30 cases of medulla type, and 5 cases of constriction type. Postoperative TNM staging: T1 40 cases, T2 34 cases, T3 63 cases, T4 26 cases; N0 92 cases, N1 33 cases, N2 28 cases, N3 10 cases; stage I 37 cases, stage II 41 cases, stage III 43 cases, stage IV 42 cases. All patients had no cancer involvement in the upper and lower margins, 34 cases of vascular invasion and 31 cases of neural invasion (Table 1). There were no significant differences in gender, age, operation time, blood loss, and postoperative hospital stay between the SCLNs positive and the negative group (all P>0.05). In addition, there were no significant differences in gender, age, operative time, blood loss, and postoperative hospital stay between the upper thoracic and middle, lowed thoracic ESCC groups (all P>0.05).
Table 1. Baseline data of 163 patients with thoracic ESCC.
| Factor | Case | Percentage (%) |
|---|---|---|
| Sex | ||
| Male | 113 | 69.3 |
| Female | 50 | 30.7 |
| Age | ||
| ≤60 y | 47 | 28.8 |
| >60 y | 116 | 71.2 |
| Location | ||
| Upper | 62 | 38.0 |
| Middle | 80 | 49.1 |
| Lower | 21 | 12.9 |
| TNM stage | ||
| I | 37 | 22.7 |
| II | 41 | 25.2 |
| III | 43 | 26.4 |
| IV | 42 | 25.7 |
ESCC, esophageal squamous cancer.
Postoperative complication rate and mortality of thoracic ESCC
Complications occurred in 55 patients after operation, the incidence was 33.7% (55/163), and there was no significant difference between tumor location and complication rate (P=0.512). Pulmonary infection was the most common complication (20.2%, 33/163), followed by anastomotic leakage (12.3%, 20/163); 12 cases of arrhythmia; 5 patients developed respiratory failure, 3 of which underwent tracheotomy; 2 patients developed acute renal failure; 1 case of thoracic infection; 1 case of chyle leakage (Table 2). No patient died within 30 days after surgery, so the perioperative mortality rate was 0%.
Table 2. Complications after three-field lymph node dissection in patients with thoracic ESCC.
| Complication | Case | Percentage (%) |
|---|---|---|
| Pulmonary infection | 33 | 20.2 |
| Anastomotic leakage | 20 | 12.3 |
| Arrhythmia | 12 | 7.4 |
| Respiratory failure | 5 | 3.1 |
| Thoracic infection | 4 | 2.5 |
| Acute renal failure | 2 | 1.2 |
| Chyle leakage | 1 | 0.6 |
ESCC, esophageal squamous cancer.
Lymph nodes metastases of thoracic ESCC
A total of 3,182 lymph nodes were dissected from the study, and the averages of 19.5 lymph nodes were cleaned per case. Postoperative pathology showed lymph node metastasis in 71 patients, and the lymph node metastasis rate was 43.6% (71/163); the number of positive lymph nodes was 240, and the degree of lymph node metastasis was 7.5% (240/3,182). There were 35 cases of SCLNs metastasis; the positive rate of SCLNs was 21.5% (35/163). There was no significant difference in the supraclavicular, upper and lower mediastinal lymph node metastasis between upper thoracic and middle and lower thoracic ESCC (P=0.291, 0.611, 0.092 respectively); the middle and lower thoracic ESCC has higher metastasis rate in middle mediastinal lymph node (P=0.001) and abdominal lymph node (P<0.001) (Table 3).
Table 3. Lymph node metastasis after three-field lymph node dissection in patients with thoracic ESCC.
| Lymph node metastasis | Upper | Middle/lower | P |
|---|---|---|---|
| Total lymph node (P/N) | 28/34 | 43/58 | 0.746 |
| Supraclavicular lymph nodes (P/N) | 16/46 | 19/82 | 0.291 |
| Upper mediastinal lymph node (P/N) | 15/47 | 21/80 | 0.611 |
| Middle mediastinal lymph node (P/N) | 3/59 | 25/76 | 0.001 |
| Lower mediastinal lymph node (P/N) | 1/61 | 9/92 | 0.092 |
| Abdominal lymph node (P/N) | 0/62 | 23/78 | <0.001 |
ESCC, esophageal squamous cancer.
Survival of 163 patients with thoracic ESCC
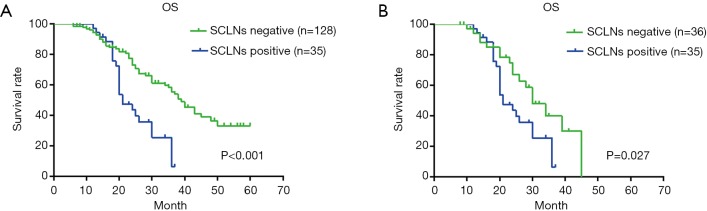
One hundred sixty-three patients with thoracic ESCC were followed up, 12 were lost to follow-up, the follow-up rate was 92.6%, the median survival time was 35.0 months, and the 1-, 3-, and 5-year survival rates were 93.0%, 45.0%, and 26.0%, respectively (Figure 1). Univariate analysis showed that there was a significant difference in OS of thoracic ESCC in terms of vascular invasion, degree of differentiation, tumor size, T stage, TNM stage, total lymph nodes metastasis and SCLNs metastasis (Table 4). The OS of patients with positive SCLNs was 21.0±1.5 months, and the negative patients was 39.0±2.8 months (P<0.001); moreover, the OS of patients with SCLNs metastasis had a statistically significant shorten compared with patients with other lymph node metastasis (except for SCLNs) (21.0±1.5 vs. 30.0±3.3, P=0.027) (Figure 2). Multivariate analysis found that the T stage (P=0.038), TMN stage (P=0.008), and SCLNs metastasis (P=0.019) were independent risk factors for postoperative survival of thoracic ESCC (Table 5).
Figure 1.
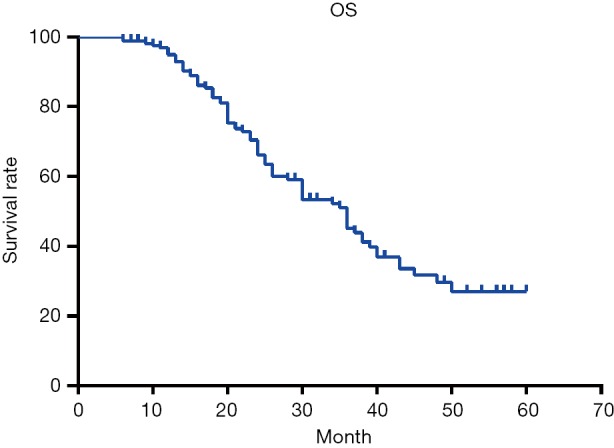
Survival curve of all thoracic ESCC patients with three-field lymph node dissection. ESCC, esophageal squamous cancer.
Table 4. Univariate analysis of factors for survival in all thoracic ESCC.
| Factor | HR | 95% CI | P value |
|---|---|---|---|
| Sex (M/F) | 0.910 | 0.563–1.470 | 0.699 |
| Age (≤60/>60) | 1.131 | 0.685–1.867 | 0.630 |
| Location (U/M-L) | 1.109 | 0.694–1.774 | 0.664 |
| Vascular invasion (P/N) | 1.975 | 1.184–3.293 | 0.009 |
| Nerve invasion (P/N) | 1.604 | 0.965–2.665 | 0.068 |
| Differentiation (H-HM/M-ML) | 0.609 | 0.384–0.964 | 0.034 |
| Tumor size (<5/≥5 cm) | 2.016 | 1.266–3.208 | 0.003 |
| T stage (T1–2/T3–4) | 2.686 | 1.664–4.335 | <0.001 |
| TNM stage (H-HM/M-ML) | 3.759 | 2.220–6.365 | <0.001 |
| Total lymph nodes (P/N) | 2.337 | 1.445–3.754 | <0.001 |
| Supraclavicular lymph nodes (P/N) | 2.671 | 1.621–4.402 | <0.001 |
ESCC, esophageal squamous cancer; M, male; F, female; U, upper; M, middle; L, lower; H, high differentiation; M, medium differentiation; L, low differentiation; P, positive; N, negative.
Figure 2.
Survival curve by involved lymph node location in all patients. (A) Survival curve of all patients with positive SCLNs and negative SCLNs, median survival time was 21.0±1.5 and 39.0±2.8 months, respectively; (B) survival curve of patients with SCLNs metastasis and other lymph node metastasis (except for SCLNs), median survival time was 21.0±1.5 and 30.0±3.3 months, respectively. SCLN, supraclavicular lymph node.
Table 5. Multivariate analysis of factors for survival in all thoracic ESCC.
| Factor | HR | 95% CI | P value |
|---|---|---|---|
| Differentiation (H-HM/M-ML) | 0.866 | 0.516–1.452 | 0.585 |
| Tumor size (<5/≥5 cm) | 0.967 | 0.563–1.661 | 0.902 |
| T stage (T1–2/T3–4) | 1.845 | 1.035–3.289 | 0.038 |
| Vascular invasion (P/N) | 1.336 | 0.769–2.321 | 0.304 |
| Nerve invasion (P/N) | 1.041 | 0.576–1.880 | 0.895 |
| TNM stage (I–II/III–IV) | 3.187 | 1.353–7.510 | 0.008 |
| Total lymph nodes (P/N) | 0.533 | 0.246–1.153 | 0.110 |
| Supraclavicular lymph nodes (P/N) | 2.162 | 1.133–4.126 | 0.019 |
ESCC, esophageal squamous cancer; H, high differentiation; M, medium differentiation; L, low differentiation; P, positive; N, negative.
Survival of upper thoracic and middle, lower thoracic ESCC patients
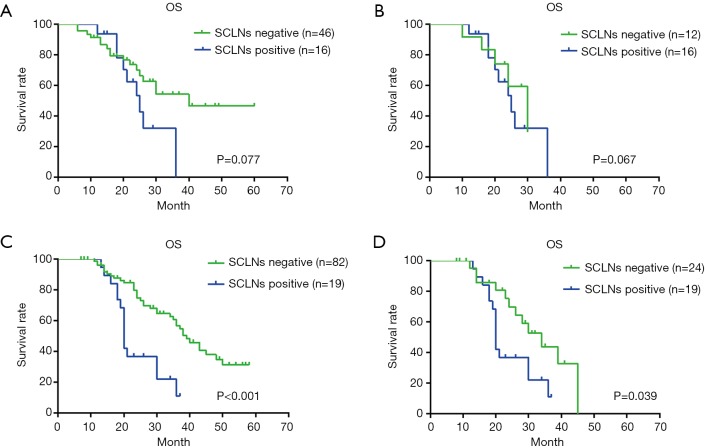
In the upper thoracic group, univariate analysis showed that there was no significant difference in OS between SCLNs positive group and negative group, SCLNs positive group and lymph nodes positive in other sites (except for SCLNs) group (P=0.077, 0.067 respectively) (Figure 3), but there was significant difference in terms of T stage, TNM stage and vascular invasion (P=0.025, 0.014, 0.022 respectively) (Table 6). In the middle and lower thoracic group, there was a significant difference in OS between the tumor size (P=0.013), T stage (P=0.001), TNM stage (P<0.001), degree of differentiation (P=0.033), total lymph nodes metastasis (P=0.002)and SCLNs metastasis (P<0.001) (Table 6); in patients with lymph node metastasis, the prognosis of patients with positive SCLNs was significantly worse than lymph nodes positive in other sites (except for SCLNs) (P=0.039) (Figure 3); multivariate analysis found that T stage, total lymph nodes metastasis, TNM stage, and SCLNs metastasis were independent risk factors affecting postoperative survival of ESCC in the middle and lower thoracic segments (Table 7).
Figure 3.
Survival curve by involved lymph node location in upper, middle, and lower thoracic ESCC patients. (A) Survival curve of patients with positive SCLNs and negative SCLNs in the upper thoracic ESCC patients, median survival time was 25.0±3.0 and 40.0±3.6 months, respectively; (B) survival curve of patients with SCLNs metastasis and other lymph node metastasis (except for SCLNs) in the upper thoracic ESCC patients, median survival time was 25.0±3.0 and 30.0±4.6 months, respectively; (C) survival curve of patients with positive SCLNs and negative SCLNs in middle and lower thoracic ESCC patients, median survival time was 20.0±0.4 and 39.0±3.0 months, respectively; (D) survival curve of patients with SCLNs metastasis and other lymph node metastasis (except for SCLNs) in middle and lower thoracic ESCC patients, median survival time was 20.0±0.4 and 34.0±4.9 months, respectively. ESCC, esophageal squamous cancer; SCLN, supraclavicular lymph node.
Table 6. Univariate analysis of factors for survival in upper, middle, and lower thoracic ESCC.
| Factor | Upper | Middle/lower | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| Sex (M/F) | 0.622 | 0.290–1.334 | 0.223 | 1.205 | 0.632–2.297 | 0.572 | |
| Age (≤60/>60) | 0.600 | 0.248–1.449 | 0.256 | 1.448 | 0.781–2.685 | 0.240 | |
| Vascular invasion (P/N) | 2.800 | 1.658–6.772 | 0.022 | 1.697 | 0.894–3.219 | 0.106 | |
| Nerve invasion (P/N) | 2.078 | 0.609–7.089 | 0.243 | 1.611 | 0.901–2.881 | 0.107 | |
| Differentiation (H-HM/M-ML) | 0.725 | 0.314–1.675 | 0.452 | 0.540 | 0.307–0.950 | 0.033 | |
| Tumor size (<5/≥5 cm) | 1.966 | 0.909–4.253 | 0.086 | 2.108 | 1.170–3.799 | 0.013 | |
| T stage (T1–2/T3–4) | 2.646 | 1.130–6.195 | 0.025 | 2.728 | 1.518–4.900 | 0.001 | |
| TNM stage (I–II/III–IV) | 3.060 | 1.255–7.465 | 0.014 | 4.046 | 2.121–7.718 | <0.001 | |
| Total lymph nodes (P/N) | 2.035 | 0.915–4.527 | 0.081 | 2.510 | 1.398–4.507 | 0.002 | |
| Supraclavicular lymph nodes (P/N) | 2.055 | 0.926–4.562 | 0.077 | 3.150 | 1.659–5.982 | <0.001 | |
ESCC, esophageal squamous cancer; M, male; F, female; H, high differentiation; M, medium differentiation; L, low differentiation; P, positive; N, negative.
Table 7. Multivariate analysis of factors for survival in upper, middle, and lower thoracic ESCC.
| Factor | Upper | Middle/lower | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| Vascular invasion (P/N) | 2.827 | 1.083–7.376 | 0.034 | – | – | – | |
| Differentiation (H-HM/M-ML) | – | – | – | 0.900 | 0.489–1.657 | 0.736 | |
| Tumor size (<5/≥5 cm) | 0.834 | 0.300–2.321 | 0.729 | 0.947 | 0.496–1.911 | 0.939 | |
| T stage (T1–2/T3–4) | 2.111 | 0.739–6.031 | 0.163 | 2.220 | 1.113–4.425 | 0.024 | |
| TNM stage (I–II/III–IV) | 1.613 | 0.441–5.895 | 0.470 | 4.968 | 1.627–15.170 | 0.005 | |
| Total lymph nodes (P/N) | 1.119 | 0.328–3.823 | 0.857 | 0.344 | 0.129–0.921 | 0.034 | |
| Supraclavicular lymph nodes (P/N) | 1.380 | 0.422–4.507 | 0.594 | 3.152 | 1.376–7.222 | 0.007 | |
ESCC, esophageal squamous cancer; M, male; F, female; H, high differentiation; M, medium differentiation; L, low differentiation; P, positive; N, negative.
Discussion
In the early 1980s, Japanese scholars took place three-field lymph node dissection as the standard procedure for lymph node dissection of esophageal cancer. They believed that three-field lymph node dissection could take care of the cervical lymph nodes and SCLNs dissection, reducing the risk of local recurrence after surgery, and improving the distance survival rate (8-10). However, many retrospective studies have found that the incidence of complications after three-field lymph node dissection is high, which limits the development of three-field lymph node dissection for esophageal cancer (11,12). In our study, the incidence of complications after thoracic ESCC with three-field lymph node dissection was 33.7% (55/163), pulmonary infection was the most common complication (20.2%, 33/163), and other complications included anastomotic hemorrhoids, respiratory failure, acute renal failure, arrhythmia, etc., but the incidence was fairly low. Compared with the related literature in recent years, the incidence of surgical complications was within the acceptable range, and the perioperative mortality rate was not significantly increased (13,14). Moreover, with the advancement of anesthesia technology, the improvement of surgical techniques, and the application of the concept of enhanced recovery after surgery (ERAS), the safety of thoracic ESCC with three-field lymph node dissection has been improved, and many centers have been carried out.
As shown in Table 3, our study found that 163 patients with thoracic ESCC had a lymph node metastasis rate of 43.6% (71/163), lymph node metastasis of 7.5% (240/3,182), and 35 cases had SCLNs metastasis. The positive rate of SCLNs was 21.5% (35/163). Our results were similar to those reported in the previous studies (15). The esophageal submucosa has rich reticular lymphatic vessels along the esophagus, and the number of longitudinal lymphatic vessels is 6 times that of transverse lymphatic vessels. Therefore, once the carcinoma invades the submucosal layer, it can occur along the lymphatic vessels in the esophageal wall, long-distance transfer in the superior or inferior direction, or extensive lymph node metastasis as a “jumping model” (16), this is also one of the theoretical basis for promoting three-field lymph node dissection in radical surgery for thoracic ESCC (17-19).
The eighth edition of UICC/AJCC staging manuals define SCLNs metastases as distant metastases (M1); however, several retrospective studies have suggested that SCLNs should be reclassified as regional lymph nodes in thoracic esophageal cancer for better stratification of postoperative survival (7,20). After digging deeper into these issues, we found they did not have a multivariate analysis of the prognosis of different segmental thoracic esophageal cancer. Our study proved that in thoracic ESCC, the prognosis of the SCLNs positive group was significantly worse than that of the negative group (P<0.001) and other lymph node metastasis (except for SCLNs) (P=0.027). Subgroup analysis showed in patients with upper thoracic ESCC, there was no significant difference between OS and SCLNs metastasis (P=0.077), and the OS of SCLNs positive group was similar to other lymph nodes positive (except for SCLNs) group (P=0.067). In patients with middle and lower thoracic ESCC, the prognosis of patients with positive SCLNs was significantly worse than that of negative patients (P<0.001) and lymph node-positive patients in other sites (except for SCLNs) (P=0.039), moreover, SCLNs metastasis were an independent risk factor of postoperative survival in the middle and lower thoracic ESCC. Our results were consistent with the latest study (including 6,178 patients with thoracic esophageal cancer) (21). Thus, we believed that a revision of the eighth edition of UICC/AJCC staging manuals to include SCLNs in thoracic ESCC was warranted.
For advanced esophageal cancer, studies have shown that neoadjuvant therapy combined with surgery may improve the prognosis (22-24). Motoyama et al. (25) found that neoadjuvant therapy can improve pathological remission (PR) in patients with esophageal cancer who received three-field lymph node dissection. In a study of 98 cases of esophageal cancer (68 received neoadjuvant therapy), Lin et al. (26) found after neoadjuvant chemoradiotherapy, the pathologic complete response rate (pCR) was 47.1% (32/68), the 2-year survival rate was 69.1% in patients undergoing neoadjuvant chemoradiotherapy combined surgery compared to 40.0% in patients undergoing single-line neoadjuvant chemoradiotherapy. In Western countries, neoadjuvant chemoradiotherapy followed by surgery has become the standard therapy for patients with resectable esophageal cancer. Some scholars have pointed out that neoadjuvant therapy may increase the difficulty of surgery and increase the incidence of complications due to local tissue adhesion. However, Motoyama et al. (25) found that the incidence of surgical complications did not increase after neoadjuvant therapy. In addition, Ma et al. (27) found that compared with the minimally invasive surgery group, the operation time, blood loss, perioperative mortality, ICU occupancy rate, and duration of ICU stay of neoadjuvant therapy combined with minimally invasive surgery group may slightly prolonged or elevated, but there is no significant difference between the two groups. Therefore, we believed patients of middle and lower thoracic ESCC with SCLNs metastases might benefit from neoadjuvant chemoradiotherapy followed by surgery.
In summary, we believe that the right thoracic approach to the three-field lymph node dissection for the treatment of thoracic ESCC is safe and feasible. In the upper thoracic ESCC patients, SCLNs appear to be regional nodes, and if there is no distant organ metastasis, the three-field lymph node dissection can be used. In patients with middle and lower thoracic ESCC, SCLNs should be defined as distant metastasis (M1) and combined with recent advances in neoadjuvant therapy in advanced esophageal cancer, and we considered neoadjuvant therapy followed by surgery might be an available treatment method.
Study limitations
As with other retrospective studies, this study has certain limitations. On the one hand, there are more or fewer biases in the retrospective study itself; on the other hand, the sample size is small, and with no adenocarcinoma of esophagus, in addition, the small number of patients with SCLNs metastases may have limited the statistical power of the analyses; therefore, the sample size needs to be further increased and included adenocarcinoma of esophagus. Moreover, the prospective researches on neoadjuvant therapy for the middle and lower thoracic ESCC with SCLNs metastases are quite lacking, and we hope that each center should pay attention to the clinical research of such patients to improve their prognosis.
Acknowledgments
We appreciate the aid of Large-scale Data Analysis Center of Cancer Precision Medicine-LinkDoc for clinical and pathological data collected.
Funding: This research was supported by Wuxi Key Medical Talents (ZDRC011).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by Medical Ethics Committee of Affiliated Hospital of Jiangnan University (No. LS2013044) and written informed consent was obtained from all patients.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Zhang H, Tang P, Miao X, et al. Does tumor size improve the accuracy of prognostic prediction in patients with esophageal squamous cell carcinoma after surgical resection? Oncotarget 2016;7:66623-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang Z, Zhou D, Cai Q, et al. Clinical characteristics and prognostic factors of esophageal carcinoma associated with multiple primary carcinomas: a report of 268 cases. Transl Cancer Res 2018;7:996-1005. 10.21037/tcr.2018.07.11 [DOI] [Google Scholar]
- 3.Deng HY, Zheng X, Alai G, et al. Tumor location is an independent prognostic factor of esophageal adenocarcinoma based on the eighth edition of TNM staging system in Chinese patients. Ann Transl Med 2019;7:365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen MQ, Xu BH, Zhang YY. Analysis of prognostic factors for esophageal squamous cell carcinoma with distant organ metastasis at initial diagnosis. J Chin Med Assoc 2014;77:562-6. 10.1016/j.jcma.2014.05.014 [DOI] [PubMed] [Google Scholar]
- 5.Wang H, Bi L, Zhang L, et al. Prognostic value of tumor length in predicting survival for patients with esophageal cancer. Transl Cancer Res 2018;7:506-14. 10.21037/tcr.2018.05.07 [DOI] [Google Scholar]
- 6.Ji X, Cai J, Chen Y, et al. Lymphatic spreading and lymphadenectomy for esophageal carcinoma. World J Gastrointest Surg 2016;8:90-4. 10.4240/wjgs.v8.i1.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tachimori Y, Ozawa S, Numasaki H, et al. Supraclavicular node metastasis from thoracic esophageal carcinoma: A surgical series from a Japanese multi-institutional nationwide registry of esophageal cancer. J Thorac Cardiovasc Surg 2014;148:1224-9. 10.1016/j.jtcvs.2014.02.008 [DOI] [PubMed] [Google Scholar]
- 8.Tachimori Y, Ozawa S, Numasaki H, et al. Efficacy of lymph node dissection by node zones according to tumor location for esophageal squamous cell carcinoma. Esophagus 2016;13:1-7. 10.1007/s10388-015-0515-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taniyama Y, Nakamura T, Mitamura A, et al. A strategy for supraclavicular lymph node dissection using recurrent laryngeal nerve lymph node status in thoracic esophageal squamous cell carcinoma. Ann Thorac Surg 2013;95:1930-7. 10.1016/j.athoracsur.2013.03.069 [DOI] [PubMed] [Google Scholar]
- 10.Yamasaki M, Miyata H, Miyazaki Y, et al. Evaluation of the nodal status in the 7th edition of the UICC-TNM classification for esophageal squamous cell carcinoma: proposed modifications for improved survival stratification: impact of lymph node metastases on overall survival after esophagectomy. Ann Surg Oncol 2014;21:2850-6. [DOI] [PubMed] [Google Scholar]
- 11.Bailey SH, Bull DA, Harpole DH, et al. Outcomes after esophagectomy: a ten-year prospective cohort. Ann Thorac Surg 2003;75:217-22; discussion 222. 10.1016/S0003-4975(02)04368-0 [DOI] [PubMed] [Google Scholar]
- 12.Kosugi S, Kanda T, Yajima K, et al. Risk factors that influence early death due to cancer recurrence after extended radical esophagectomy with three-field lymph node dissection. Ann Surg Oncol 2011;18:2961-7. 10.1245/s10434-011-1712-5 [DOI] [PubMed] [Google Scholar]
- 13.Ye T, Sun Y, Zhang Y, et al. Three-field or two-field resection for thoracic esophageal cancer: a meta-analysis. Ann Thorac Surg 2013;96:1933-41. 10.1016/j.athoracsur.2013.06.050 [DOI] [PubMed] [Google Scholar]
- 14.Shang QX, Chen LQ, Hu WP, et al. Three-field lymph node dissection in treating the esophageal cancer. J Thorac Dis 2016;8:E1136-49. 10.21037/jtd.2016.10.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanaka T, Matono S, Nagano T, et al. Esophagectomy with extended lymphadenectomy for submucosal esophageal cancer: long-term outcomes and prognostic factors. Ann Surg Oncol 2012;19:750-6. 10.1245/s10434-011-2023-6 [DOI] [PubMed] [Google Scholar]
- 16.Berger AC, Bloomenthal A, Weksler B, et al. Oncologic efficacy is not compromised, and may be improved with minimally invasive esophagectomy. J Am Coll Surg 2011;212:560-6; discussion 566-8. 10.1016/j.jamcollsurg.2010.12.042 [DOI] [PubMed] [Google Scholar]
- 17.Kumakura Y, Yokobori T, Yoshida T, et al. Elucidation of the Anatomical Mechanism of Nodal Skip Metastasis in Superficial Thoracic Esophageal Squamous Cell Carcinoma. Ann Surg Oncol 2018;25:1221-8. 10.1245/s10434-018-6390-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang F, Zheng YZ, Wang Z, et al. Nodal Skip Metastasis in Esophageal Squamous Cell Carcinoma Patients Undergoing Three-Field Lymphadenectomy. Ann Thorac Surg 2017;104:1187-93. 10.1016/j.athoracsur.2017.03.081 [DOI] [PubMed] [Google Scholar]
- 19.Liu J, Liu Q, Wang Y, et al. Nodal skip metastasis is associated with a relatively poor prognosis in thoracic esophageal squamous cell carcinoma. Eur J Surg Oncol 2016;42:1202-5. 10.1016/j.ejso.2016.05.025 [DOI] [PubMed] [Google Scholar]
- 20.Zheng Y, Wang Z, Wang F, et al. Proposed modifications of supraclavicular lymph node metastasis in the esophageal squamous cell carcinoma staging system for improved survival stratification. Oncotarget 2017;8:41563-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wen J, Chen D, Zhao T, et al. Should the clinical significance of supraclavicular and celiac lymph node metastasis in thoracic esophageal cancer be reevaluated? Thorac Cancer 2019;10:1725-35. 10.1111/1759-7714.13144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang H, Liu H, Chen Y, et al. Neoadjuvant Chemoradiotherapy Followed by Surgery Versus Surgery Alone for Locally Advanced Squamous Cell Carcinoma of the Esophagus (NEOCRTEC5010): A Phase III Multicenter, Randomized, Open-Label Clinical Trial. J. Clin. Oncol 2018;36:2796-803. 10.1200/JCO.2018.79.1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shah T, Kushnir V, Mutha P, et al. Neoadjuvant cryotherapy improves dysphagia and may impact remission rates in advanced esophageal cancer. Endosc Int Open 2019;7:E1522-7. 10.1055/a-0957-2798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Wilk BJ, Noordman BJ, Neijenhuis LKA, et al. Active Surveillance Versus Immediate Surgery in Clinically Complete Responders After Neoadjuvant Chemoradiotherapy for Esophageal Cancer: A Multicenter Propensity Matched Study. Ann. Surg 2019. [Epub ahead of print]. 10.1097/SLA.0000000000003636 [DOI] [PubMed] [Google Scholar]
- 25.Motoyama S, Sato Y, Sasaki T, et al. Efficacy and Safety of Neoadjuvant Chemoradiotherapy Following Esophagectomy with Japanese-style Extended 3-Field Lymphadenectomy for Thoracic Esophageal Cancer. Anticancer Res 2017;37:5837-43. [DOI] [PubMed] [Google Scholar]
- 26.Lin JW, Hsu CP, Yeh HL, et al. The impact of pathological complete response after neoadjuvant chemoradiotherapy in locally advanced squamous cell carcinoma of esophagus. J Chin Med Assoc 2018;81:18-24. 10.1016/j.jcma.2017.08.007 [DOI] [PubMed] [Google Scholar]
- 27.Ma S, Yan T, Liu D, et al. Neoadjuvant chemotherapy followed by minimally invasive esophagectomy is safe and feasible for treatment of esophageal squamous cell carcinoma. Thorac Cancer 2018;9:310-5. 10.1111/1759-7714.12590 [DOI] [PMC free article] [PubMed] [Google Scholar]