Abstract
Background
Liver metastases are the most common cause of death for patients with colorectal cancer and affect up to half of the patients. Liver resection is an established method that can potentially be curative. For patients with extrahepatic disease (EHD), the role of liver surgery is less established.
Methods
This is a retrospective study based on data from the national quality registry SweLiv. Data were obtained between 2009 and 2015. SweLiv is a validated registry and has been in use since 2009, with coverage above 95%. Patients with liver metastases and EHD were analyzed and cross-checked against the national death cause registry for survival analysis.
Results
During the study period, 2,174 patients underwent surgery for colorectal liver metastases (CRLM), and 277 patients with EHD were treated with resection or ablation. The estimated median survival time for the entire cohort from liver resection/ablation was 40 months (95% CI, 32–47). The survival time for patients treated with liver resection was 45 months compared to 26 months for patients treated with ablation (95% CI 38–53, 18–33, P=0.001). A subgroup analysis of resected patients revealed that the group with pulmonary metastases had a significantly longer estimated median survival (50 months; 95% CI, 39–60) than the group with lymph node metastases (32 months; 95% CI, 7–58) or peritoneal carcinomatosis (28 months; 95% CI, 14–41) (P=0.022 and 0.012, respectively). Other negative prognostic factors were major liver resection and nonradical liver resection.
Conclusions
For patients with liver metastases and limited EHD, liver resection results in prolonged survival compared to what can be expected from chemotherapy alone.
Keywords: Colorectal liver metastases, extrahepatic disease (EHD), liver resection
Introduction
Liver metastases are the primary causes of death for patients with colorectal cancer and affect up to half of the patients during the course of the disease (1). It is well established that the resection of metastases confined to the liver can result in prolonged survival and a potential cure. The resection of colorectal liver metastases (CRLM) may result in an over 50% 5-year survival rate (2,3). For non-operated patients with stage IV colorectal cancer given palliative chemotherapy, the 5-year survival rate is only approximately 5%, and the median survival time is 22 months (4-6).
The role of surgery in patients with liver metastases who also have extrahepatic metastases [extrahepatic disease (EHD)] is less established. The prognosis depends on the location of EHD as well as the total number of metastases. Approximately one-third of patients considered suitable for resection have EHD confined to a single site. Pulmonary metastases, lymph nodes in the hepatoduodenal ligament or peritoneal carcinomatosis are the most commonly reported sites. The median overall survival time after resection has been reported to be between 19 and 39 months (7,8). For patients with EHD in two sites, the median survival after resection is, however, considerably lower (i.e., 13 months) (7). For the group of patients with EHD confined to paraaortic lymph nodes, the median overall survival time after surgery is reported to be 32 months, and the 3-year survival rate is 35% (9). After the resection of EHD, up to 65% of patients experience recurrence, with a median recurrence-free survival time of 5 months, and the median overall survival time after surgery for EHD may reach 39 months (8,10).
The aim of this study was to evaluate the outcomes of patients with CRLM and EHD undergoing liver resection or ablation from a validated nationally maintained registry.
Methods
This study was a retrospective analysis of data from the Swedish liver registry (SweLiv), a national quality registry. SweLiv has been in full use since January 2009. The data were obtained between 2009 and 2015. During this period, 2,174 patients underwent surgery for CRLM, and 277 (13%) had EHD. The registry aims to include all patients with primary and secondary liver tumors and all liver surgical and ablative interventions. SweLiv has a coverage of above 95% of all liver resections and ablations performed in Sweden and has been validated.
In Sweden, liver surgery is centralized to six hepatobiliary centers. Regional liver tumor boards are held weekly at each center, with the objective of discussing all patients who may benefit from liver surgery. SweLiv is divided into three main parts. In the first part, preoperative data regarding comorbidity, extent of the tumor burden and the planned treatment are recorded. In the second part, data regarding actual liver surgery/ablation are registered. In the third part, data regarding the follow-up and eventual postoperative complications are registered.
In SweLiv, EHD is organized into the following groups: lymph node metastases, pulmonary metastases, peritoneal carcinomatosis and metastases in other sites. Neoadjuvant chemotherapy was defined in this study as chemotherapy administered before liver surgery.
Minor liver resection was defined as ≥2 liver segments, and major liver resection was defined as ≤3 liver segments (according to the Couinaud classification).
Patients with EHD were cross-checked against the national death cause registry for survival analysis.
This study was approved by the Ethical Review Board, Gothenburg, (Dnr 189-15). No informed consent was given from the participants specific for this study, but all patients approve being recorded in Sweliv prior liver surgery/ablation.
Statistical analysis
The results are expressed as the mean ± standard deviation or median (range), as appropriate. Statistical analysis was performed using ANOVA, and categorical data were analyzed using the chi2 test. The follow-up time is expressed as the median (range) and was defined as the time from diagnosis of the primary tumor, time from the diagnosis of liver metastases or time from liver surgery to the date of data extraction from the registries or the date of death. Survival was calculated from liver resection or ablation. Survival was estimated using Kaplan-Meier analysis and is expressed as the median (95%). Pairwise survival comparisons were made using the log-rank test. Survival analysis was performed using Cox regression analysis. On multivariable Cox regression, variables with a P value < on univariable analysis was included. Analyses were performed using IBM SPSS (version 25; IBM Corp., Armonk, NY, USA). A P value less than 0.05 was considered significant.
Results
Of the 2,174 patients recorded in SweLiv who were treated with liver resection or ablation, 277 (13%) also had EHD. The basic demographic data and data regarding EHD can be found in Table 1. The location of the primary tumor was stated for 119 patients. Sixty-six patients (55%) had the primary tumor located in the colon, and 53 (45%) had the primary tumor located in the rectum.
Table 1. Characteristics of the included patients.
| Characteristics | Value |
|---|---|
| Age, years (mean ± SD) | 63±11 |
| Sex distribution (male/female) | 154 (56%)/123 (44%) |
| Location of the primary tumor (colon/rectum) | 66 (55%)/53 (45%) |
| Number of patients with EHD who received chemotherapy after the diagnosis of liver metastases | 206 (74%) |
| Number of sites EHD 1/2/3 | 246/29/2 |
| Pulmonary metastases ± any other site | 179 (65%) |
| Lymph node* ± any other site | 69 (25%) |
| Peritoneal carcinomatosis ± any site | 29 (10%) |
No significant difference was found regarding baseline characteristics for patients with lung metastases, lymph node metastases and patients with carcinomatosis. *, 87% had localized lymph node metastases, 11% had distant lymph node metastases, 2% had localized and distant lymph node metastases. EHD, extrahepatic disease.
The median American Society of Anesthesiologists (ASA) score was 2 (range, 1–4), and the preoperative functional level estimated with the Eastern Cooperative Oncology Group (ECOG) was 0 (range, 0–2) (11). The median number of liver metastases on preoperative CT or MRI was 2 (range, 1–20), and the median size of the largest liver metastasis was 25 mm (range, 2.5–150 mm). The number of EHD sites was a median of 1 (range, 1–3): 246 patients (89%) had 1 site of EHD, 29 (10%) had 2 sites of EHD, and 2 patients (1%) had three sites of EHD. Thirty (11%) patients had, in addition to EHD, preoperatively suspected local extrahepatic tumor spread with the involvement of adjacent organs, such as the gallbladder or duodenum. This was confirmed in 11 (4%) patients in the postoperative pathological examination.
Two hundred six (74%) patients received chemotherapy within 3 months before liver surgery or ablation. One hundred fifty-six patients (76%) responded to chemotherapy with regression or remission, 31 (15%) had stable disease, 4 (2%) showed progression, and 4 (2%) showed an effect that was not evaluable. For 11 patients (5%), the response to chemotherapy was not stated.
The time from diagnosis of the primary tumor to the diagnosis of liver metastases was 4 months (range, 0–85 months). The follow-up time from diagnosis of the primary tumor was 42 months (range, 9–142 months), and the time from the diagnosis of liver metastases was 33 months (range, 4–96 months). The follow-up time from liver surgery was 26 months (range, 0.3–86 months); at the end of the follow-up period, 138 (50%) patients were alive, and 139 (50%) patients had died.
One hundred seventy-two (81%) patients had tumor-free surgical margins (R0) in the liver, whereas 23 (11%) had an R1 resection; for 17 patients (8%), the radicality was difficult to assess in the pathology report. For 32 patients (13%), the radicality was not stated. The estimated operative blood loss was 400 mL (range, 0–6,000 mL). Eleven (4%) patients had a postoperative complication ≥3b (according to the Clavien-Dindo classification) within 30 days of surgery (12). For a description of the surgical procedures, see Table 2.
Table 2. Description of the surgical procedures.
| Surgical procedure | Value |
|---|---|
| Number of patients with liver resection | 244 (88%) |
| Number of patients with ablation (radiofrequency or microwave; percutaneously or laparoscopically) | 33 (12%) |
| Number of patients with minor liver resection (less than three liver segments) | 139 (57%) |
| Number of patients with major liver resection (three or more liver segments) | 99 (41%) |
| Number of patients with two-stage hepatectomy | 10 (4%) |
| Number of patients with HIPEC | 2 (1%) |
| Number of patients with synchronous resection of either the primary tumor or EHD | 29 (12%) |
| Number of patients with reresection/reablation | 31 (11%) |
Some patients were categorized into more than one group; for example, those treated with HIPEC were also grouped according to the size of the liver resected. HIPEC, hyperthermic intraperitoneal chemotherapy; EHD, extrahepatic disease.
The estimated median survival time for the entire cohort from liver resection/ablation was 40 months (95% CI, 32–47). The survival time for patients treated with liver resection was 45 months compared to 26 months for patients treated with ablation (95% CI, 38–53, 18–33, P=0.001).
Outcomes in patients treated with liver resection
Further analysis of patients treated with liver resection revealed that the site of EHD affected survival. The longest estimated survival time from liver surgery was observed in the group with pulmonary metastases (50 months; 95% CI, 39–60) and was significantly longer than that observed in the group with lymph node metastases, whose estimated survival time was 32 months (95% CI, 7–58), as well as in the group with peritoneal carcinomatosis, whose estimated survival time was 28 months (95% CI, 14–41) (P=0.022 and 0.012, respectively; Figure 1).
Figure 1.
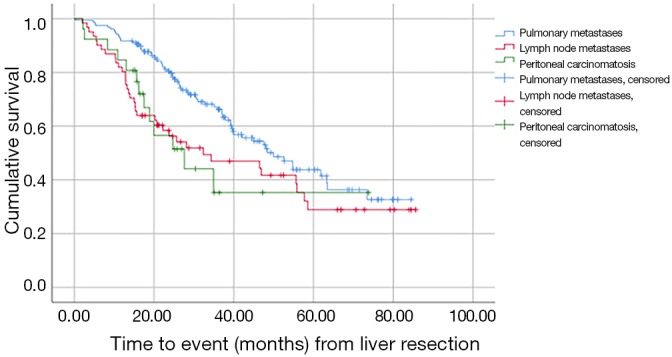
Comparison of survival among patients with pulmonary metastases, lymph node metastases and peritoneal carcinomatosis.
Additionally, the number of sites of EHD affected survival, although the difference was not statistically significant. Patients with one site of EHD had an estimated survival of 48 months (95% CI, 37–58) compared to 35 months (95% CI, 12–58) for patients with two or three sites of EHD (P=0.230; Figure 2).
Figure 2.
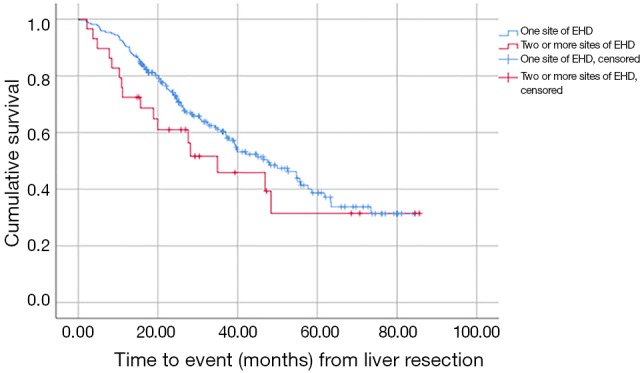
Comparison of survival among patients with 1 site of EHD or 2 or more sites of EHD. EHD, extrahepatic disease.
The response to preoperative chemotherapy significantly affected the estimated median survival. Patients who responded to chemotherapy had significantly longer survival (48 months; 95% CI, 37–58) compared to those who progressed (13 months; 95% CI, 1–25; P=0.004). The difference in survival between patients with a response and stable disease (38 months; 95% CI, 31–44; P=0.210) did not reach statistical significance.
Patients treated with neoadjuvant chemotherapy had a significantly longer survival time (50 months; 95% CI, 37–63) than patients treated with chemotherapy for downsizing purposes (23 months; 95% CI, 14–33; P<0.05).
Uni- and multivariable survival analysis
Univariable analysis indicates that lung metastases compared to lymph node metastases or peritoneal carcinomatosis had an improved survival [hazard ratio (HR) 1.45; 95% CI, 1.12–1.88; P=0.005]. Furthermore, that patients treated with neoadjuvant chemotherapy had a more favorable outcome than patients treated with chemotherapy in downsizing purpose (HR 0.36; 95% CI, 0.20–0.65; P=0.001). Patients with response to preoperative chemotherapy had a more favorable outcome, as well as those treated with a minor liver resection, compared to a major liver resection.
One multivariable analysis only response to chemotherapy remained significant (HR 2.69; 95% CI, 1.49–4.68; P=0.001), although the purpose of chemotherapy and type of resection were borderline significant (Table 3).
Table 3. Cox regression analysis.
| Covariate | Univariable analysis | Multivariable analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Coefficient | HR | 95% CI | P value | Coefficient | HR | 95% CI | P value | ||
| Site of extrahepatic disease (EHD) | 0.37 | 1.45 | 1.12–1.88 | 0.005 | |||||
| Number of sites of EHD (1 or 2–3 sites) | −0.32 | 0.73 | 0.43–1.23 | 0.232 | |||||
| Purpose of preoperative chemotherapy (neoadjuvant/downsizing) | −1.02 | 0.36 | 0.20–0.65 | 0.001 | −0.77 | 0.46 | 0.2–1.08 | 0.074 | |
| Response to preoperative chemotherapy (response/stable/progress/not evaluable) | 0.495 | 1.64 | 1.02–2.64 | 0.042 | 0.99 | 2.69 | 1.49–4.68 | 0.001 | |
| Type of resection (minor/major) | 0.47 | 1.59 | 1.09–2.34 | 0.017 | −0.54 | 0.59 | 0.32–1.07 | 0.081 | |
| Radicality of the liver resection (R0/R1 or difficult to determine) | 0.55 | 1.74 | 1.1–2.74 | 0.018 | |||||
Discussion
This retrospective analysis of data from the prospectively maintained national Swedish quality registry SweLiv shows that the resection of liver metastases despite the presence of EHD may result in prolonged survival.
Previous studies have shown that in patients with colorectal liver metastasis with extrahepatic metastasis, the resection of liver metastases or extrahepatic metastases can result in a 5-year survival of nearly 30% (13), which is in accordance with the findings in the present study.
The site of EHD seems to be important for prognosis, and the longest survival period was observed in the group with pulmonary metastases. In this study, not all metastases were confirmed with a pathological diagnosis, and the diagnosis of EHD may have been established by CT or MRI, possibly including some patients with nonspecific pulmonary nodules. This result is, however, consistent with those of previous studies (14). In this study, the number of patients who underwent a resection of pulmonary metastases was not defined; however, in previous studies in which some patients underwent the resection of only liver metastases and not pulmonary metastases, the prognosis was still improved compared with that for patients treated with only palliative chemotherapy (15). This may indicate that liver metastases are the determining factor for survival and, therefore, resection can prolong survival despite leaving EHD in situ.
The number of sites of EHD did not significantly affect survival. However, most patients had only one site of EHD. This differs from previous studies in which the number of sites of EHD, as well as the total number of metastases, had a prognostic effect (8,16). Despite the lack of statistical significance, there was a tendency towards less favorable outcomes for patients with more than one site of EHD. The limited number of patients with multiple sites of EHD probably explains the discrepancy between our results and earlier results.
It is difficult to draw a firm conclusion regarding the effect of chemotherapy for patients who have EHD and are treated with liver surgery. In the survival analysis, the comparison was made based on the date of liver resection; hence, patients with synchronous and metachronous CRLM were compared in the same group.
One must also consider both the purpose of preoperative chemotherapy and the extent of liver metastases. In this study, patients treated with chemotherapy for downsizing purposes experienced shorter survival than those treated with chemotherapy for neoadjuvant purposes. Therefore, patients with both liver metastases, who require downsizing chemotherapy for resection, and EHD may not benefit from liver surgery to the same extent as patients with less advanced liver metastases.
For the diverse cohort of patients with stage 4 colorectal cancer, the role of perioperative chemotherapy is not well established. Some studies indicate no significant difference in survival for patients treated with neoadjuvant chemotherapy, although the data indicate that adjuvant chemotherapy may result in prolonged disease-free survival as well as overall survival (17,18).
However, since the potential benefit of preoperative chemotherapy for this diverse group of patients is complex, we firmly believe that all patients with CRLM and EHD should be discussed on a tumor board, with liver surgeon, as well as a medical oncologist present. For patients treated with preoperative chemotherapy and that respond to the treatment, resection should be considered.
Both the non-pulmonary site of EHD and R1/uncertain radicality were more strongly associated with shorter survival than major hepatectomy as opposed to minor hepatectomy. When major/minor hepatectomy was excluded as a negative prognostic factor and only the EHD site (lymph node or peritoneal carcinomatosis) and radicality were included, the median survival for patients with those two factors was only 19 months. This may indicate that in patients with advanced tumor burden in the liver (i.e., in need of major liver resection), where R0 resection is unlikely, and at the same time have lymph node or peritoneal metastasis, liver surgery is questionable.
This study has some limitations that must be acknowledged. It was not possible to determine how many patients had surgery for extrahepatic metastases, and it is possible that this subgroup of patients experienced further improved survival. Furthermore, it could not be determined whether a subgroup of patients with pulmonary metastases had only benign nodules.
Despite these limitations, the results from the current study show that improved survival is possible in patients with EHD.
Conclusions
Patients with CRLM and EHD may benefit from liver resection; therefore, all patients with CRLM and EHD should be evaluated for possible gains from liver surgery.
Acknowledgments
None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Ethical Review Board, Gothenburg, (Dnr 189-15). No informed consent was given from the participants specific for this study, but all patients approve being recorded in Sweliv prior liver surgery/ablation.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Abdalla EK, Adam R, Bilchik AJ, et al. Improving resectability of hepatic colorectal metastases: expert consensus statement. Ann Surg Oncol 2006;13:1271-80. 10.1245/s10434-006-9045-5 [DOI] [PubMed] [Google Scholar]
- 2.John SK, Robinson SM, Rehman S, et al. Prognostic factors and survival after resection of colorectal liver metastasis in the era of preoperative chemotherapy: an 11-year single-centre study. Dig Surg 2013;30:293-301. 10.1159/000354310 [DOI] [PubMed] [Google Scholar]
- 3.Shimada H, Tanaka K, Endou I, et al. Treatment for colorectal liver metastases: a review. Langenbecks Arch Surg 2009;394:973-83. 10.1007/s00423-009-0530-8 [DOI] [PubMed] [Google Scholar]
- 4.Beppu T, Miyamoto Y, Sakamoto Y, et al. Chemotherapy and targeted therapy for patients with initially unresectable colorectal liver metastases, focusing on conversion hepatectomy and long-term survival. Ann Surg Oncol 2014;21 Suppl 3:S405-13. 10.1245/s10434-014-3577-x [DOI] [PubMed] [Google Scholar]
- 5.Nuzzo G, Guilante F, Ardito F, et al. Liver resection for primarily unresectable colorectal metastases downsized by chemotherapy. J Gastrointest Surg 2007;11:318-24. 10.1007/s11605-006-0070-2 [DOI] [PubMed] [Google Scholar]
- 6.Stillwell AP, Buettner PG, Ho YH. Meta-analysis of survival of patients with stage IV colorectal cancer managed with surgical resection versus chemotherapy alone. World J Surg 2010;34:797-807. 10.1007/s00268-009-0366-y [DOI] [PubMed] [Google Scholar]
- 7.Aoki T, Umekita N, Tanaka S, et al. Prognostic value of concomitant resection of extrahepatic disease in patients with liver metastases of colorectal origin. Surgery 2008;143:706-14. 10.1016/j.surg.2008.02.004 [DOI] [PubMed] [Google Scholar]
- 8.Pulitanò C, Bodingbauer M, Aldrighetti L, et al. Liver resection for colorectal metastases in presence of extrahepatic disease: results from an international multi-institutional analysis. Ann Surg Oncol 2011;18:1380-8. 10.1245/s10434-010-1459-4 [DOI] [PubMed] [Google Scholar]
- 9.Pulitanò C, Bodingbauer M, Aldrighetti L, et al. Colorectal liver metastasis in the setting of lymph node metastasis: defining the benefit of surgical resection. Ann Surg Oncol 2012;19:435-42. 10.1245/s10434-011-1902-1 [DOI] [PubMed] [Google Scholar]
- 10.Wei AC, Coburn NG, Devitt KS, et al. Survival Following Resection of Intra- and Extra-Hepatic Metastases from Colorectal Cancer: A Phase II Trial. Ann Surg Oncol 2016;23:2644-51. 10.1245/s10434-016-5189-0 [DOI] [PubMed] [Google Scholar]
- 11.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5:649-55. 10.1097/00000421-198212000-00014 [DOI] [PubMed] [Google Scholar]
- 12.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. 10.1097/01.sla.0000133083.54934.ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elias D, Sideris L, Pocard M, et al. Results of R0 resection for colorectal liver metastases associated with extrahepatic disease. Ann Surg Oncol 2004;11:274-80. 10.1245/ASO.2004.03.085 [DOI] [PubMed] [Google Scholar]
- 14.Robertson V, Neal CP, Jones M, et al. Indeterminate Pulmonary Nodules in Resected Liver Metastases from Colorectal Cancer: A Comparison of Patient Outcomes. World J Surg 2017;41:1834-9. 10.1007/s00268-017-3930-x [DOI] [PubMed] [Google Scholar]
- 15.Mise Y, Kopetz S, Mehran RJ, et al. Is complete liver resection without resection of synchronous lung metastases justified? Ann Surg Oncol 2015;22:1585-92. 10.1245/s10434-014-4207-3 [DOI] [PubMed] [Google Scholar]
- 16.Elias D, Libarale G, Vernerey D, et al. Hepatic and extrahepatic colorectal metastases: when resectable, their localization does not matter, but their total number has a prognostic effect. Ann Surg Oncol 2005;12:900-9. 10.1245/ASO.2005.01.010 [DOI] [PubMed] [Google Scholar]
- 17.Brandi G, Derenzini E, Falcone A, et al. Adjuvant systemic chemotherapy after putative curative resection of colorectal liver and lung metastases. Clin Colorectal Cancer 2013;12:188-94. 10.1016/j.clcc.2013.04.002 [DOI] [PubMed] [Google Scholar]
- 18.Leung U, Gonen M, Allen PJ, et al. Colorectal Cancer Liver Metastases and Concurrent Extrahepatic Disease Treated With Resection. Ann Surg 2017;265:158-65. 10.1097/SLA.0000000000001624 [DOI] [PMC free article] [PubMed] [Google Scholar]


