Abstract
Purpose
National guidelines define adequate axillary lymph node dissections as those yielding ≥ 10 lymph nodes (LNs). We aimed to identify the optimal LN yield among node-positive patients.
Methods
Using the National Cancer Data Base (2010–2015), we categorized node-positive patients as follows: (1) neoadjuvant chemotherapy (NAC, cN1–3 or ypN1mi-3) or (2) upfront surgery (pN1–3). A restricted cubic splines model was used to estimate LN retrieval thresholds associated with change in overall survival (OS).
Results
129,685 patients were identified: 21.2% NAC, 78.8% upfront surgery. Low, moderate, and high retrieval thresholds were estimated to be 1–6, 7–21, and > 21 LNs (upfront surgery), and 1–7, 8–22, and > 22 LNs (NAC). In an adjusted model, high versus low LN yield was associated with greater receipt of adjuvant chemotherapy (upfront surgery OR 1.96, p < 0.001) and greater use of adjuvant radiation (upfront surgery OR 1.08, p = 0.02; NAC OR 1.23, p = 0.002). After adjustment, high versus low LN retrieval was associated with improved OS (upfront surgery HR 0.86, p < 0.001; NAC HR 0.77, p < 0.001). Worse OS was associated with retrieving fewer LNs, likely as a result of an under-staged axilla and missed opportunity for adjuvant therapy, while better OS was independently associated with retrieval of up to approximately 20 LNs, after which survival did not improve.
Conclusion
In node-positive breast cancer, the number of nodes retrieved is significantly associated with an increased positive nodal count and greater use of adjuvant therapy. Removal of approximately 20 LNs may improve survival by both more accurate nodal staging and increased adjuvant therapy use.
Keywords: Breast cancer, Node-positive, Axillary lymph node dissection, Staging, Guidelines, Overall survival
Introduction
Sentinel lymph node biopsy (SLNB) has largely replaced axillary lymph node dissection (ALND) for axillary staging in many circumstances. Settings in which pathologically node-positive disease does not require ALND include ACOSOG Z0011-eligible patients [1], isolated micrometastases in SLNs, and clinically node-negative patients who undergo mastectomy, have limited LN involvement, and receive postmastectomy radiation (PMRT) [2]. However, ALND continues to be guideline-concordant care for clinically positive nodes having upfront surgery, failed SLN mapping, patients not meeting Z0011 or AMAROS criteria, or those who remain pathologic node-positive after neoadjuvant chemotherapy. The goals of ALND are to establish nodal stage, optimize regional control, and to predict prognosis on which to base adjuvant treatment recommendations [2, 3].
The National Comprehensive Cancer Network (NCCN) defines an adequate ALND as retrieval of ≥ 10 lymph nodes (LNs) to accurately stage the axilla [2]. Two historic studies, published over 25 years ago, evaluated the LN thresholds required to ensure a node-negative axilla [4–6]. These studies found that an increased number of LNs examined correlated with greater positive nodal detection. The first study found significantly improved axillary recurrence-free and overall survival (OS) when ≥ 10 LNs were retrieved. Above 10 LNs, the frequency of positive node detection plateaued, and thereby the authors concluded 10 negative LNs retrieved was adequate to deem a patient “node-negative” [4]. The second study determined that a cutoff point for a T1 tumor is when 10 LNs are sampled from level 1 and found to be uninvolved [5, 6]. These two studies intended to define the adequate number of LNs retrieved to ensure a node-negative axilla.
Since the vast majority of ALNDs are now performed in node-positive patients, we sought to identify the number of LNs needed to adequately stage the node-positive axilla, guide treatment decisions, determine if a LN retrieval threshold exists, and when is enough, enough? Further, we sought to determine whether an association exists between the number of LNs retrieved and OS in the node-positive axilla.
Methods
We identified adult patients (≥ 18–75 years) between 2010 and 2015 with node-positive, invasive breast cancer from the National Cancer Data Base (NCDB), Fig. 1. Patients age > 75 years old, with metastatic disease, treated with neoadjuvant radiation or endocrine therapy, and those with missing data regarding type of surgery, survival, or staging were excluded. Patients receiving neoadjuvant endocrine therapy were excluded primarily because the treatment response has been shown to be significantly different from that achieved with NAC (which is more likely to result in a pathologic complete response). In addition, it is often offered to older, frailer women with potentially more comorbidities, and including these women may introduce unintended heterogeneity to our study populations. The study cohort was divided into two groups based on treatment sequence: upfront surgery or NAC. Node positivity for the upfront surgery cohort was limited to pathologic N1–3 disease; node positivity for the NAC cohort was defined as either clinical N1–3 (regardless of pN status) or ypN1–3 (ypN1mi-3). A small proportion of patients underwent treatment with a neoadjuvant systemic therapy other than chemotherapy (coded as “other systemic therapy” in the NCDB). These patients were included in the NAC group because this treatment was likely given as an alternative to neoadjuvant chemotherapy with similar intent (e.g., anti-HER2 therapy).
Fig. 1.
Patient flow diagram (NCDB 2015 dataset)
Patient characteristics were summarized with N (%) for categorical variables and median (interquartile range, IQR) for continuous variables for all patients and by treatment sequence: (1) NAC (cN1–3 or ypN1mi-3), and (2) upfront surgery (pN1–3). Groups were compared using χ2 or Fisher’s exact tests for categorical variables, and t-tests or Wilcoxon Rank Sum tests for continuous variables, as appropriate. For each treatment group, the number of LNs retrieved, number of pathologically positive nodes, and treatment variables were summarized for each pN stage (1–3, 4–9, ≥ 10).
Two multivariable Cox proportional hazards models with restricted cubic splines (RCS) were created to characterize the functional association of the number of LNs retrieved with OS for the NAC and upfront surgery groups separately [7, 8]. The RCS method allowed for a flexible multivariable model of the nonlinear relationship of LN number and survival to be employed without assuming the existence of potential cut points. 3-, 4-, and 5-knot models were examined, and the 4-knot models (knots at 5th, 35th, 65th, and 95th percentiles) were selected for both cohorts based on the Akaike Information Criteria [9]. Each model identified the number of LNs removed corresponding to the critical point of the log hazard ratio function. Bootstrap simulation with a Monte Carlo Markov Chain procedure was used to estimate each threshold value as the LN retrieval associated with a marked change in OS over 1000 iterations. Final thresholds and confidence intervals were estimated from all iterations as mean (2.5–97.5th percentile). Patients were characterized as having “low,” “moderate,” and “high” LN retrieval based on these thresholds for both NAC and upfront surgery groups.
Cox proportional hazards regression analyses were utilized to estimate the association of categorized LNs retrieved (low, moderate, high) and OS, after adjustment for other covariates. A robust sandwich covariance estimator was included to account for the correlation of patients treated at the same hospital. Logistic regression was used to estimate the association of LN retrieval with utilization of adjuvant therapy, after adjustment for covariates. Sensitivity analyses excluding patients who were potentially eligible for the ACOSOG Z0011 trial was conducted. Due to similarity in results, only analyses for the full cohort are reported here. In addition, we evaluated those potentially eligible for the NSABP-B51 and Alliance A011202 trials to quantify those cohorts in this study. A p-value < 0.05 was considered significant, and no adjustments were made for multiple comparisons. Statistical analyses were conducted using SAS, version 9.4 (SAS Institute, Cary, NC). Due to use of de-identified data, our institutional review board granted the study exempt status.
Results
We identified 129,685 node-positive breast cancer patients: 27,485 (21.2%) received NAC and 102,200 (78.8%) underwent upfront surgery. Patient demographics, tumor characteristics, and treatments received are shown in Table 1 by treatment sequence. For the overall cohort, the median age was 56 years and median tumor size was 2.4 cm. Roughly one-third (34.4%) of all patients had clinically positive nodes at presentation (cN1), with expectedly higher rates in the NAC group (64.7%), compared to the upfront surgery group (26.3%). The overall median number of nodes retrieved was 11 (12 for NAC, 11 for upfront surgery), and the median number of pathologically positive LNs was 2 in both groups. This cohort had an overall high receipt of chemotherapy (80.4%) and radiation (92.1% among lumpectomy patients and 60.3% among mastectomy patients). When comparing patients by pN stage (Table 2), patients with a higher pN stage had a higher number of LNs retrieved in both groups, suggesting that with additional LNs retrieved, additional positive nodes are identified.
Table 1.
Patients with node-positive invasive, non-metastatic breast cancer in the National Cancer Data Base, 2010–2015
| All patients N = 129,685 (100%) |
Neoadjuvant chemotherapy N = 27,485 (21.2%) |
Upfront surgery N = 102,200 (78.8%) |
p-value | |
|---|---|---|---|---|
| Age (years) | ||||
| Median (IQR) | 56 (48–64) | 52 (44–60) | 57 (49–65) | < 0.001 |
| Histology | ||||
| Ductal | 114,318 (88.2%) | 24,719 (89.9%) | 89,599 (87.7%) | < 0.001 |
| Lobular | 14,107 (10.9%) | 2094 (7.6%) | 12,013 (11.8%) | |
| Other | 1260 (1%) | 672 (2.4%) | 588 (0.6%) | |
| Grade | ||||
| 1 | 17,100 (13.2%) | 1706 (6.2%) | 15,394 (15.1%) | < 0.001 |
| 2 | 58,271 (44.9%) | 10,614 (38.6%) | 47,657 (46.6%) | |
| 3 | 54,314 (41.9%) | 15,165 (55.2%) | 39,149 (38.3%) | |
| ER status | ||||
| ER+ | 105,413 (81.3%) | 18,727 (68.1%) | 86,686 (84.8%) | < 0.001 |
| PR status | ||||
| PR+ | 93,712 (72.3%) | 15,932 (58%) | 77,780 (76.1%) | < 0.001 |
| HER2 status | ||||
| HER2+ | 22,301 (17.2%) | 6646 (24.2%) | 15,655 (15.3%) | < 0.001 |
| Clinical T stage | ||||
| T1 | 52,368 (40.4%) | 4044 (14.7%) | 48,324 (47.3%) | < 0.001 |
| T2 | 49,495 (38.2%) | 12,034 (43.8%) | 37,461 (36.7%) | |
| T3 | 11,687 (9%) | 6444 (23.4%) | 5243 (5.1%) | |
| T4 | 5172 (4%) | 4124 (15%) | 1048 (1%) | |
| Clinical N stage | ||||
| N0 | 65,036 (50.1%) | 4537 (16.5%) | 60,499 (59.2%) | < 0.001 |
| N1 | 44,613 (34.4%) | 17,772 (64.7%) | 26,841 (26.3%) | |
| N2 | 6657 (5.1%) | 2785 (10.1%) | 3872 (3.8%) | |
| N3 | 3127 (2.4%) | 1545 (5.6%) | 1582 (1.5%) | |
| Tumor Size (mm) | ||||
| Median (IQR) | 24 (15–35) | 35 (22–55) | 22 (15–32) | < 0.001 |
| Number of lymph nodes retrieved | ||||
| Median (IQR) | 11 (5–17) | 12 (6–17) | 11 (4–17) | < 0.001 |
| Number of positive lymph nodes | ||||
| Median (IQR) | 2 (1–4) | 2 (1–6) | 2 (1–3) | < 0.001 |
| Surgery type | ||||
| Lumpectomy | 49,850 (38.4%) | 7142 (26%) | 42,708 (41.8%) | < 0.001 |
| Mastectomy | 79,835 (61.6%) | 20,343 (74%) | 59,492 (58.2%) | |
| Pathologic T stage | ||||
| T1 | 59,278 (45.7%) | 13,457 (49%) | 45,821 (44.8%) | < 0.001 |
| T2 | 55,301 (42.6%) | 8927 (32.5%) | 46,374 (45.4%) | |
| T3 | 12,048 (9.3%) | 3498 (12.7%) | 8550 (8.4%) | |
| T4 | 3058 (2.4%) | 1603 (5.8%) | 1455 (1.4%) | |
| Pathologic N stage | ||||
| N0 | 5048 (3.9%) | 5048 (18.4%) | 0 (0%) | < 0.001 |
| N1 | 91,101 (70.2%) | 13,751 (50%) | 77,350 (75.7%) | |
| N2 | 23,098 (17.8%) | 6045 (22%) | 17,053 (16.7%) | |
| N3 | 10,438 (8%) | 2641 (9.6%) | 7797 (7.6%) | |
| Received any chemotherapy | ||||
| Yes | 104,256 (80.4%) | 27,401 (99.7%)a | 76,855 (75.2%) | < 0.001 |
| Received any radiation therapy | ||||
| Yes | 94,028 (72.5%) | 22,513 (81.9%) | 71,515 (70%) | < 0.001 |
| Chemotherapy type | ||||
| Adjuvant chemotherapy only | 77,332 (59.6%) | 477 (1.7%)a | 76,855 (75.2%) | < 0.001 |
| Neoadjuvant chemotherapy | 26,924 (20.8%) | 26,924 (98%) | 0 (0%) | |
| No chemotherapy | 25,429 (19.6%) | 84 (0.3%)a | 25,345 (24.8%) | |
| Radiation sites | ||||
| None | 35,388 (27.3%) | 4918 (17.9%) | 30,470 (29.8%) | < 0.001 |
| Breast | 30,843 (23.8%) | 4665 (17%) | 26,178 (25.6%) | |
| Breast/lymph nodes | 26,479 (20.4%) | 5817 (21.2%) | 20,662 (20.2%) | |
| Chest wall | 7082 (5.5%) | 2286 (8.3%) | 4796 (4.7%) | |
| Chest wall/lymph nodes | 27,714 (21.4%) | 9247 (33.6%) | 18,467 (18.1%) | |
| Z0011 eligibilityb | ||||
| Eligible | 15,807 (12.2%) | 0 (0%) | 15,807 (15.5%) | |
| Ineligible | 113,878 (87.8%) | 27,485 (100%) | 86,393 (84.5%) | |
| NSABP B-51 Eligibilityc | < 0.001 | |||
| Eligible | 4715 (3.6%) | 4715 (17.2%) | 0 (0%) | |
| Ineligible | 124,970 (96.4%) | 22,770 (82.8%) | 102,200 (100%) | |
| Alliance (A011202) eligibilityd | < 0.001 | |||
| Eligible | 3470 (2.7%) | 3470 (12.6%) | 0 (0%) | |
| Ineligible | 126,215 (97.3%) | 24,015 (87.4%) | 102,200 (100%) | |
IQR interquartile range, NAC neoadjuvant chemotherapy, ER estrogen receptor, PR progesterone receptor, HER2 human-epidermal-growth-factor-receptor-2
N = 561 patients underwent treatment with a neoadjuvant systemic therapy other than chemotherapy (e.g., anti-HER2 therapy) and are included in the NAC group. 84/561 received no chemotherapy and 477/561 underwent adjuvant chemotherapy
Z0011 Eligibility (must have all: cT1–2, cN0, upfront surgery, lumpectomy, breast or whole breast/lymph nodes radiation, 1 or 2 positive lymph nodes, < 10 lymph nodes retrieved)
\NSABP B-51 Eligibility (must have all: cN+, NAC, pN0, any number of LN removed)
\Alliance (A011202) Eligibility (must have all: cN+, NAC, cN0 (unknown in NCDB), pN+, 1–8 LN removed)
Table 2.
Node retrieval, number of positive nodes, and treatment received by pN stage and treatment group
| Number of positive nodes | ||||||
|---|---|---|---|---|---|---|
| Upfront surgery | Neoadjuvant chemotherapy | |||||
| 1–3 | 4–9 | ≥ 10 | 0–3 | 4–9 | ≥ 10 | |
| N (%) | 77,110 (75.5%) | 17,388 (17.0%) | 7702 (7.5%) | 17,928 (65.2%) | 6536 (23.8%) | 3021 (11.0%) |
| Median # nodes retrieved w/IQR | 8 (3–15) | 14 (10–19) | 20 (16–25) | 10 (4–15) | 13 (9–17) | 18 (14–23) |
| Median # positive nodes w/IQR | 1 (1–2) | 5 (4–7) | 14 (11–18) | 1 (1–2) | 5 (4–7) | 13 (11–17) |
| N/(%) received adjuvant chemotherapy | 54,257 (70.4%) | 15,673 (90.1%) | 6925 (89.9%) | – | – | – |
| N/(%) of HR + received adjuvant endocrine therapy | 60,155 (90.0%) | 12,786 (88.4%) | 5497 (87.3%) | 10,878 (88.9%) | 4434 (90.7%) | 1839 (89.3%) |
| N/(%) lumpectomy (without XRT) | 2812 (7.8%) | 462 (9.4%) | 208 (12.8%) | 348 (6.3%) | 74 (6.2%) | 28 (6.3%) |
| N/(%) lumpectomy (with XRT) | 33,380 (92.2%) | 4429 (90.6%) | 1417 (87.2%) | 5145 (93.7%) | 1128 (93.8%) | 419 (93.7%) |
| N/(%) mastectomy (without XRT) | 23,862 (58.3%) | 2237 (17.9%) | 1104 (18.2%) | 3556 (28.6%) | 573 (10.7%) | 393 (15.3%) |
| N/(%) mastectomy (with XRT) | 17,056 (41.7%) | 10,260 (82.1%) | 4973 (81.8%) | 8879 (71.4%) | 4761 (89.3%) | 2181 (84.7%) |
Unadjusted data table, IQR interquartile range, HR+ hormone receptor positive, XRT radiation therapy
Cox proportional hazards models with RCS estimated the number of LNs corresponding to the critical points of the log hazard ratio function to be at 6.83 (95% CI 6.37–6.92) and 21.00 (95% CI 20.09–21.68) in the upfront surgery group, and 7.77 (95% CI 7.21–7.93) and 22.33 (95% CI 20.48–23.59) in the NAC group (Fig. 2). Based on these critical thresholds, low, moderate, and high LN retrieval groups were defined as 1–7, 8–22, and > 22 LNs for the NAC group, and 1–6, 7–21, and > 21 LNs for those who underwent upfront surgery. Worse OS was seen with retrieval of fewer than 8 LNs (NAC), or 7 LNs (upfront surgery), while OS increased with retrieval of 8–22 LNs (NAC) or 7–21 LNs (upfront surgery), after which point survival did not improve with removal of additional nodes. For the overall cohort, high compared to low LN retrieval was independently associated with improved OS (HR 0.84, 95% CI 0.79–0.90, p < 0.001). This improvement was similarly seen in the moderate versus low LN retrieval groups (HR 0.91, 95% CI 0.89–0.96, p < 0.001) overall. Subset analysis showed similar significant improvement in OS in both upfront surgery and NAC groups (Table 3).
Fig. 2.
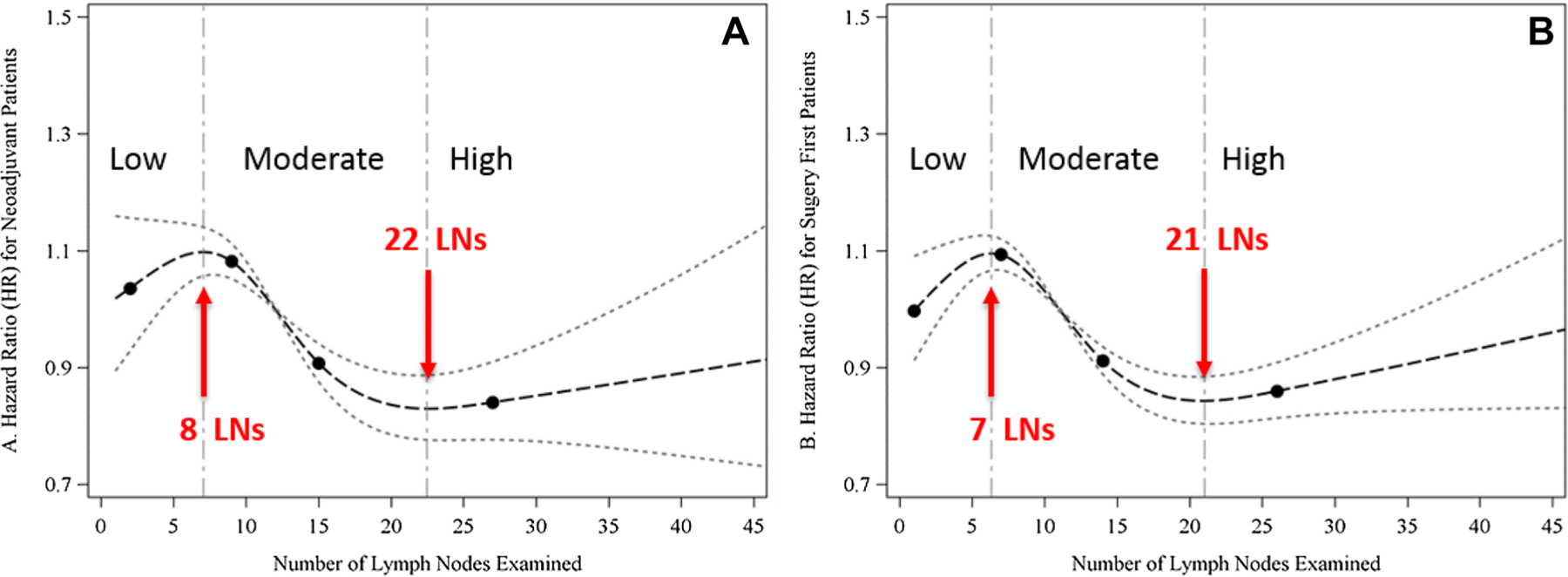
Association between total number of lymph nodes retrieved and survival, in patients undergoing neoadjuvant chemotherapy (a), or upfront surgery (b), from adjusted Cox proportional hazards model with restricted cubic splines
Table 3.
Cox proportional hazard and generalized logistic regression models predicting overall survival and receipt of adjuvant chemotherapy, radiation therapy, and endocrine therapy by treatment cohort
| Upfront surgery | Neoadjuvant chemotherapy | |||
|---|---|---|---|---|
| Overall survival | Overall survival | |||
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| LN Retrieval Group | ||||
| Low | Reference | Reference | ||
| Moderate | 0.93 (0.87–0.99) | 0.01 | 0.87 (0.80–0.94) | < 0.001 |
| High | 0.86 (0.79–0.93) | < 0.001 | 0.77 (0.69–0.88) | < 0.001 |
| Adjuvant chemotherapy | Adjuvant chemotherapy | ||||
|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | ||
| LN Retrieval Group | |||||
| Low | Reference | N/Aa | N/Aa | – | |
| Moderate | 1.76 (1.69–1.83) | < 0.001 | N/Aa | N/Aa | – |
| High | 1.96 (1.82–2.10) | < 0.001 | N/Aa | N/Aa | – |
| Adjuvant radiation | Adjuvant radiation | |||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| LN Retrieval Group | ||||
| Low | Reference | Reference | ||
| Moderate | 1.02 (0.98–1.07) | 0.33 | 1.33 (1.23–1.44) | < 0.001 |
| High | 1.08 (1.01–1.16) | 0.02 | 1.23 (1.08–1.40) | 0.002 |
| Adjuvant endocrine | Adjuvant endocrine | |||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| LN Retrieval Group | ||||
| Low | Reference | Reference | ||
| Moderate | 1.06 (1.01–1.12) | 0.03 | 1.25 (1.12–1.40) | < 0.001 |
| High | 1.05 (0.96–1.13) | 0.29 | 1.17 (0.98–1.40) | 0.08 |
HR > 1 indicates higher risk of death (compromised survival) and HR < 1 indicates lower risk of death (improved survival) compared to the reference level. OR > 1 indicates higher odds of receipt of adjuvant treatment and OR < 1 indicates lower odds of receipt of adjuvant treatment compared to the reference level
All models adjusted for age, race/ethnicity, grade, estrogen and progesterone receptor status, HER2 status, pT stage, pN stage, type of surgery, facility type, facility location, and insurance status. All models also account for the correlation of patients treated at the same hospital
Receipt of additional adjuvant chemotherapy is unknown for the neoadjuvant chemotherapy group
After adjustment for the number of positive LNs, high versus low LN retrieval was strongly associated with a greater likelihood of receipt of adjuvant chemotherapy (OR 1.96, 95% CI 1.82–2.10, p < 0.001) for those in the upfront surgery group. Similarly, high versus low LN retrieval was associated with a slightly greater receipt of adjuvant radiation in both the upfront surgery group (OR 1.08, 95% CI 1.01–1.16, p = 0.02) and the NAC group (OR 1.23, 95% CI 1.08–1.40, p = 0.002). Patients undergoing upfront mastectomy had markedly increased rates of radiation with increasing nodal burden (41.7% for 1–3 positive nodes vs. 81.8% RT for ≥ 10 positive nodes). Notably, a high versus low LN retrieval was not associated with adjuvant endocrine therapy in either group.
Discussion
In contemporary breast cancer care, SLNB provides accurate axillary staging with fewer side effects for the many patients who ultimately have pathologically negative nodes. However, ALND is still recommended for patients with clinically positive nodes (cN1–3 disease) undergoing upfront surgery, inflammatory breast cancer, and select patients with a positive SLNB [10]. In addition, guideline-concordant care includes ALND for patients with clinically positive nodes undergoing NAC, although emerging data support SLNB alone after NAC in specific clinical scenarios [11–13]. Historically, a node-negative axilla was confidently deemed node-negative when ≥ 10 negative LNs were retrieved [4, 5]. However, the number of LNs needed to accurately stage the axilla has yet to be defined specifically in the node-positive axilla. Our findings demonstrate that, in this setting, adequate axillary dissection includes retrieval of approximately 20 LNs, and has the potential to guide adjuvant treatment decisions, and improve survival of individuals with high-risk breast cancer.
Extent of LN metastases is one of the strongest prognostic indicators for breast cancer, conferring significantly worse survival for those with regional disease, and a continued decline in survival with additional positive nodes [14, 15]. As such, LN status remains an essential variable in the AJCC prognostic staging guidelines and remains an important determinant of adjuvant treatment decision-making [16, 17]. In our study, inferior survival was independently associated with retrieval of fewer than 7 or 8 LNs (upfront surgery vs. NAC) and improved to a threshold of 21 or 22 LNs (upfront surgery vs. NAC), after which point, survival did not improve with the retrieval of additional nodes. While we do not interpret these data to suggest a therapeutic survival benefit to additional axillary clearance, it is likely an indicator of improved accuracy of axillary staging and its influence on adjuvant treatment decision-making.
Chen et al. similarly found improved OS with increased LN retrieval, even in a population of pathologically node-negative women [18]. Among stage II/III breast cancer patients receiving NAC followed by modified radical mastectomy (MRM) with pathologically negative nodes, having higher numbers of LNs retrieved was associated with improved survival (4–9 nodes: reference; 10–19 nodes: HR 0.19; 20 + nodes: HR 0.41; p = 0.002) [18]. Similar to our findings, the authors conclude this is likely a reflection of staging accuracy (reduced false-negative staging). Further-more, a greater extent of axillary dissection may be a proxy for overall more aggressive care.
In addition to improved survival, higher LN retrieval in our study was independently associated with increased receipt of adjuvant radiation and chemotherapy. NCCN guidelines strongly recommend regional nodal irradiation for patients with ≥ 4 positive LN [10] based on large randomized trials [19, 20]. Additionally, PMRT has been associated with improved outcomes for women with any nodal involvement [21, 22]. Our study found significantly increased rates of radiation receipt in upfront mastectomy patients with higher nodal burden (1–3 positive nodes, 41.7% vs. 4–9 positive nodes, 82.1%), and similar findings were observed in the NAC group.
Adjuvant chemotherapy receipt was independently associated with higher nodal retrieval in our study. While most node-positive patients are candidates for chemotherapy [10], the NCCN recommends decision-making be based on individual recurrence risk and predicted response to therapy. Multiple models provide risk assessment and predict potential benefits of systemic therapy, many of which include lymph node status [23, 24]. Our data demonstrated a significant increase in adjuvant chemotherapy with both moderate and high LN retrieval, as compared to low retrieval. Similarly, a considerably higher rate of chemotherapy receipt was seen in 4–9 positive nodes (90.1%), when compared to 1–3 positive nodes (70.4%), again demonstrating the importance of accurate axillary staging in the node-positive patient. These data suggest that retrieval of only a few additional positive nodes (upstaging from pN1 to pN2) greatly alters adjuvant treatment receipt.
Defining adequacy of an ALND requires defining the optimal number of LNs retrieved whereby removal of additional nodes no longer alters treatment decisions or prognosis. In the node-negative axilla, that has been determined to be 10 LNs [4, 5]. In our node-positive patients, we found that evaluating a higher number of nodes was required to reach a threshold after which removal of additional nodes did not impact treatment decisions or OS. Similar to our findings, others have also shown that removing more LNs (> 15, but not > 25 LNs) appears to be associated with improved survival [25]. More recently, a study by Wang et al. also sought to determine the optimal threshold for LN retrieval in the node-positive patient [26]. In this review of > 9000 breast cancer patients (SEER database) who underwent MRM and were found to have at least 3 positive LNs, examination of at least 12 LNs was determined to be the optimal threshold based on its association with cancer-specific survival. They similarly found a significant relationship between the number of examined and number of positive LNs identified, as well as increased examined LNs as independently associated with improved cancer-specific survival (p = 0.001) [26].
These data, and our current study, support the concept that a lower LN retrieval may result in under-staging, leading to lower receipt of adjuvant therapy and thereby potentially worse OS. We do not interpret our data to imply a high LN retrieval provides a direct survival benefit but rather that the associated improvement in survival is due to a more accurately staged axilla and an increased receipt of adjuvant therapies, leading to improved outcomes. In addition, the number of nodes retrieved may represent a proxy for institutional commitment to breast cancer care, specifically, as a marker of quality of the surgeon, pathologic evaluation and identification of nodes within a specimen, or multi-disciplinary decision-making.
Alternatively, it is also possible that removing more LNs results in stage migration (Will Rogers phenomenon). For example, a patient may be classified at pN2 if only 9 positive LNs were removed, while removing only 1 additional positive node would upstage them to pN3, even with only 1 LN difference. If included in the pN2 cohort, this patient with 9 positive LNs would likely have a worse survival than those with only 4 positive LNs (also pN2); in contrast, if the patient were found to have 10 positive LNs (classified as pN3), they would likely have a better survival than a patient with 20 positive LNs; thus resulting in stage migration (improved survival in both populations with the less and more severe disease stages based on the defined diagnostic criteria) [27]. However, we used LN retrieval as a continuous variable to determine our statistical cutoffs for each group. Although selecting different cutoffs for each group may yield different results (or stage migration), we feel the statistical analysis applied provides reliable and reproducible support for our findings.
Our study represents one of the largest analyses of node-positive patients to evaluate the association of LN retrieval with treatment decisions and outcomes. However, there are several limitations to using the NCDB, including the absence of recurrence or breast cancer-specific survival data, both of which may be more relevant endpoints than OS. In addition, the data on non-surgical therapies in NCDB (e.g., chemotherapy, endocrine therapy, etc.) and completion of planned therapies may be inadequate [28]. Unfortunately, the NCDB does not specify the intent of the axillary procedure (SLNB alone vs. ALND). Lastly, classification of patients as cN+ does not mandate biopsy proven disease, thus potentially including patients in this cohort that were erroneous classified as node-positive (e.g., cN+, ypN0).
As such, we repeated our analysis excluding potentially Z0011-eligible patients, excluding those with < 10 LN retrieved as a proxy for SLNB, and found similar results overall. These data likely do not apply to the Z0011 cohort as the majority of this cohort received adjuvant chemotherapy and radiation, and additional nodal retrieval would not change adjuvant treatment decision-making. Additionally, we evaluated the potentially eligible patients for the currently enrolling NSABP B-51 and Alliance (A011202) trials. As these require NAC, only 3.6% and 2.7% of patients overall were potentially B-51 and Alliance eligible, respectively, indicating these were small portions of our overall dataset and thus precluding any meaningful subset analysis in this important group of patients.
Over time we will continue to offer more NAC, performing more SLNB and targeted axillary dissections, and are studying the omission of radiation in select patients. However, in the initially or persistently node-positive axilla following NAC, we need to ensure adequacy of axillary staging, by performing a complete level I and II anatomic axillary node dissection, as it is consistently shown to drive adjuvant treatment decisions and impact OS.
Conclusion
In a node-positive breast cancer cohort, a higher yield of excised LNs resulted in an increased positive nodal count. This higher LN retrieval is associated with increased receipt of adjuvant chemotherapy and radiation. OS is also strongly associated with the overall number of LNs retrieved up to a threshold of approximately 20 nodes, with a survival advantage in high versus low retrieval cohorts, which is seen for both the upfront surgery and NAC populations.
Funding
Dr. O. Fayanju is supported by the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH) under Award Number 1KL2TR002554 (PI: Svetkey). Dr. R. Greenup is supported by the NIH BIRCWH K12HD043446 (PI: Andrews). This work is also supported by the Duke Cancer Institute through NIH Grant P30CA014236 (PI: Kastan).
Footnotes
Conflict of interest All authors declare that they have no conflict of interest to disclose.
Ethical approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent Due to use of national de-identified data (National Cancer Data Base, NCDB), our institutional review board granted the study exempt status and no individual informed consent was needed.
Oral presentation at the 2019 Society of Surgical Oncology Annual Cancer Symposium, March 28th, 2019 in San Diego, CA.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Giuliano AE, Hunt KK, Ballman KV et al. (2011) Axillary dissection vs. no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA 305:569–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Comprehensive Cancer Network (2018) NCCN, Invasive Cancer, Surgical Axillary Staging. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed 4 Jan 2019
- 3.Luini A, Gatti G, Ballardini B et al. (2005) Development of axillary surgery in breast cancer. Ann Oncol 16:259–262 [DOI] [PubMed] [Google Scholar]
- 4.Axelsson CK, Mouridsen HT, Zedeler K (1992) Axillary dissection of level I and II lymph nodes is important in breast cancer classification. The Danish Breast Cancer Cooperative Group (DBCG). Eur J Cancer 28A:1415–1418 [DOI] [PubMed] [Google Scholar]
- 5.Kiricuta CI, Tausch J (1992) A mathematical model of axillary lymph node involvement based on 1446 complete axillary dissections in patients with breast carcinoma. Cancer 69:2496–2501 [DOI] [PubMed] [Google Scholar]
- 6.Veronesi U, Luini A, Galimberti V, Marchini S, Sacchini V, Rilke F (1990) Extent of metastatic axillary involvement in 1446 cases of breast cancer. Eur J Surg Oncol 16:127–133 [PubMed] [Google Scholar]
- 7.Desquilbet L, Mariotti F (2010) Dose-response analyses using restricted cubic spline functions in public health research. Stat Med 29:1037–1057 [DOI] [PubMed] [Google Scholar]
- 8.Harrell FE (2015) Regression modeling strategies: with applications to linear models, logistic regression, and survival analysis. Springer International Publishing, New York [Google Scholar]
- 9.Akaike H (1998) Information theory and an extension of the maximum likelihood principle Selected Papers of Hirotogu Akaike. Springer, New York, NY, pp 199–213 [Google Scholar]
- 10.Gradishar WJ, Anderson BO, Aft R, et al. (2018) National Comprehensive Cancer Network NCCN, Invasive Cancer, Version 1.2018. In Kumar R, Shead DA (eds) [Google Scholar]
- 11.Caudle AS, Yang WT, Krishnamurthy S et al. (2016) Improved axillary evaluation following neoadjuvant therapy for patients with node-positive breast cancer using selective evaluation of clipped nodes: implementation of target axillary dissection. J Clin Oncol 34:1072–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuehn T, Bauerfeind I, Fehm T et al. (2013) Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol. 14:609–618 [DOI] [PubMed] [Google Scholar]
- 13.Boughey JC, Suman VJ, Mittendorf EA et al. (2013) Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (alliance) clinical trial. JAMA 310:1455–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nemoto T, Vana J, Bedwani RN et al. (1980) Management and survival of female breast cancer: results of a National Survey by the American College of Surgeons. Cancer 45:2917–2924 [DOI] [PubMed] [Google Scholar]
- 15.Carter CL, Allen C, Henson DE (1989) Relation of tumor size, lymph node status, and survival in 24,740 breast cancer cases. Cancer 63:181–187 [DOI] [PubMed] [Google Scholar]
- 16.Plichta JK, Campbell BM, Mittendorf EA et al. (2018) Anatomy and breast cancer staging: is it still relevant? Surg Oncol Clin N Am 27:51–67 [DOI] [PubMed] [Google Scholar]
- 17.AJCC Cancer Staging Manual (ed 8th) (2016) Springer International Publishing, New York [Google Scholar]
- 18.Chen S, Liu Y, Huang L et al. (2014) Lymph node counts and ratio in axillary dissections following neoadjuvant chemotherapy for breast cancer: a better alternative to traditional pN staging. Ann Surg Oncol 21:42–50 [DOI] [PubMed] [Google Scholar]
- 19.Whelan TJ, Olivotto IA, Parulekar WR et al. (2015) Regional nodal irradiation in early-stage breast cancer. N Engl J Med 373:307–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poortmans PM, Collette S, Kirkove C et al. (2015) Internal mammary and medial supraclavicular irradiation in breast cancer. N Engl J Med 373:317–327 [DOI] [PubMed] [Google Scholar]
- 21.Overgaard M, Nielsen HM, Overgaard J (2007) Is the benefit of postmastectomy irradiation limited to patients with four or more positive nodes, as recommended in international consensus reports? A subgroup analysis of the DBCG 82 b&c randomized trials. Radiother Oncol 82:247–253 [DOI] [PubMed] [Google Scholar]
- 22.EBCTCG (Early Breast Cancer Trialists’ Collaborative Group), McGale P, Taylor C et al. (2014) Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet 383:2127–2135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olivotto IA, Bajdik CD, Ravdin PM et al. (2005) Population-based validation of the prognostic model ADJUVANT! for early breast cancer. J Clin Oncol 23:2716–2725 [DOI] [PubMed] [Google Scholar]
- 24.Wishart GC, Azzato EM, Greenberg DC et al. (2010) PREDICT: a New UK prognostic model that predicts survival following surgery for invasive breast cancer. Breast Cancer Res 12:R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuru B (2006) Prognostic significance of total number of nodes removed, negative nodes removed, and ratio of positive nodes to removed nodes in node positive breast carcinoma. Eur J Surg Oncol 32(10):1082–1088 [DOI] [PubMed] [Google Scholar]
- 26.Wang X, Ji C, Chi H et al. (2018) How many ELNs are optimal for breast cancer patients with more than three PLNs who underwent MRM? A large population-based study. Onco Targets Ther 11:1005–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sormani MP (2009) The Will Rogers phenomenon: the effect of different diagnostic criteria. J Neurol Sci 287(Suppl 1):S46. [DOI] [PubMed] [Google Scholar]
- 28.Mallin K, Palis BE, Watroba N et al. (2013) Completeness of American Cancer Registry Treatment Data: implications for Quality of Care Research. J Am Coll Surg 216:428–437 [DOI] [PubMed] [Google Scholar]


