Abstract
Our objective was to determine if c-reactive protein (CRP) and ferritin values alone and in combination are associated with mortality among hospitalized children. All hospitalized patients at our institution with a CRP or ferritin assay in 2015 and 2016 were included. Area under the receiver operating curves (AUROC) were examined, optimal cut-points determined, and patients were stratified into low, intermediate, or high-risk groups based on elevation of zero, one, or both biomarkers. 14,928 CRP and 653 ferritin values were obtained, with both obtained for 172 patients. AUROC for maximum CRP value was 0.76 [0.68–0.85] with a cut-point of 7.1 mg/dL for in-hospital mortality and 0.90 [0.83–0.98] for maximum ferritin with a cut-point of 373 ng/mL. Elevation of both ferritin and CRP was associated with the highest inpatient mortality (21.7%) and greatest organ dysfunction, followed by either biomarker alone. Additional, prospective study of these biomarkers in combination in warranted.
Introduction
Pediatricians face the challenge of distinguishing life-threatening illness requiring aggressive supportive care and treatment from transient physiologic disturbances less-dependent on timely intervention. The weight of these decisions is substantial given the risks and costs associated with many time-dependent therapies, such as fluid resuscitation, broad-spectrum antibiotics, admission to intensive care, and invasive life support such as mechanical ventilation. Highly granular data housed in the electronic health record affords an opportunity to examine the utility of commonly ordered biomarkers for risk stratification in both general and specified populations. Establishing objective risk thresholds with observational data can facilitate earlier, more accurate identification of high versus low risk patients, guiding resource intensity and potentially forestalling deterioration. CRP and ferritin are ubiquitous, relatively low-cost tests that have been associated with infectious and non-infectious, inflammation-mediated disease. Using these biomarkers to stratify inpatient children according to low, medium and high-risk groups would add probabilistic weight to clinical gestalt.
C-reactive protein (CRP) and ferritin are acute-phase reactants measurable by assays that are relatively low-cost and widely available in both developed and resource-limited settings. Both biomarkers have demonstrated association with severity of a variety of inflammation-mediated illnesses, including infection and rheumatologic disease. CRP opsonizes pathogens and necrotic host-tissue, aiding phagocytosis. It is also involved in the complement cascade, binding to C1q.(1, 2) Ferritin safely sequesters iron for use in vital cellular processes and the manufacturing of heme, whereas unliganded iron potentiates the formation of tissue-damaging free radicals.(3) Ferritin has also been implicated in stimulating cytokine cascades generating NF-kB, independent of iron content.(4)
Moderately elevated ferritin has been independently associated with mortality in children with sepsis and very high ferritin levels have been associated with a stepwise increase in mortality risk among all hospitalized children.(5, 6) A recent prospective cohort study of children with sepsis admitted to the intensive care unit demonstrated the potential utility of a risk contingency table constructed by dichotomizing both CRP and ferritin into low and high mortality risk categories.(7) We hypothesized that ferritin and CRP obtained in hospitalized children could be used to construct a computable phenotype for systemic inflammation and that simultaneous elevation of both biomarkers would be associated with higher risk for death than an elevation in either biomarker alone. Additionally, we aimed to examine whether trajectories of these biomarkers are associated with survival and whether CRP is predictive of readmission.
Methods
Study Design
This is a single-center, retrospective cohort study conducted using data extracted from the electronic health record of a free-standing, 296-bed quaternary children’s hospital in the United States. The study center serves a region of approximately five million people and admits a full spectrum of pediatric diseases. Approximately 20,000 children are admitted annually to the institution, with approximately 3,000 admitted to the general pediatric intensive care unit (PICU) per year.
The primary aim was to examine whether mortality was higher when both ferritin and CRP are elevated during hospitalization compared to no elevation or elevation of only one of the biomarkers. We also examined whether a similar association existed for organ dysfunction. Additionally, the association between each biomarker and mortality was adjusted for the presence of infection as determined by a positive culture of any source during hospitalization. Finally, we examined whether an elevated CRP at the time of discharge was associated with 30-day or 90-day readmission.
Assays
From January 1, 2015 to June 21, 2016, CRP was measured in the clinical lab with SYNCHRON System’s Near Infrared Particle Immunoassay rate methodology, using a particle bound goat and mouse anti-CRP antibody and ferritin was measured using a two-site enzyme-labeled antibody assay (Beckman Coulter, Brea, California, USA). On June 22,2016, the clinical lab began analyzing both CRP and ferritin on the Dimension Vista 500 analytical system (Siemens Healthcare Diagnostics, Malvern, Pennsylvania, USA).
Data Collection
Data were extracted from an enterprise data warehouse using the business intelligence platform SAP BusinessObjects (SAP, Walldorf, Germany) and organized in Microsoft Excel (Microsoft Corp., Redmond, WA). An initial query identified all values of CRP and ferritin for inpatients admitted to our institution in 2015 and 2016. Demographic information was then extracted for this cohort, including age at admission and sex. Hospital length of stay and total PICU length of stay per hospitalization were identified. Results of all cultures obtained during hospitalization at our study center were examined, including blood, urine, cerebrospinal fluid (CSF), respiratory (endotracheal aspirate or bronchoalveolar lavage) and other sources (including pleural fluid, peritoneal fluid and wounds). Any identifiable growth was considered a positive result. Patients who died at any point in time were identified by querying death dates for the cohort and hospital, 90-day and 1-year mortality were categorized accordingly.
For patients admitted to the PICU, organ dysfunction was examined by calculating Pediatric Logistic Organ Dysfunction 2 (PELOD2) scores based on the worst values for each parameter during hospitalization, per the intended design of the score.(8) All PELOD2 data were identified as structured data elements harbored by our data warehouse. PaO2 to FiO2 ratios were determined by using the values of PaO2 and FiO2 recorded closest in time to one another. We examined whether a final elevated CRP greater than an identified cut-point within 24 hours of discharge date was associated with readmission to our institution. Readmissions were counted if either ferritin or CRP was obtained during the subsequent hospitalization, considering the presence of these biomarkers an indication that a comparable clinical process was ensuing during the return hospitalization.
Analysis
Data exploration was performed by constructing a bagplot of maximum ferritin and CRP. A bagplot is analogous to a bivariate boxplot.(9) A center point is calculated based on determination of the Tukey depth, through which any biplane will split the data approximately in half, and a darker-shaded, inner “bag” is plotted which encloses approximately 50% of data points. An outer “fence” encompassing a distribution of points is then constructed by inflating the bag by specified factor. A factor of 2.58 was selected for this analysis such that the fence would encompass approximately 99% of points if the data were of a normal distribution. Both variables were converted to log scale for data exploration given high skew of the untransformed distributions.
Receiver operating characteristic (ROC) curves were constructed for both the presenting and maximum CRP and ferritin during hospitalization with mortality as the outcome and discrimination assessed by evaluating the area under the ROC (AUROC). Cut-points were identified using the nearest method (selecting the point on the ROC curve closest to [0,1] or maximum sensitivity and specificity). Confidence intervals were generated for the cut-points with 1000 bootstrap replicates. Contingency tables were constructed based on values of CRP and ferritin and dichotomized according to the respective cut-points. Logistic regression was used to examine for association between hospital and 90-day mortality and each dichotomized biomarker, adjusting for sex and culture positivity as covariates. The chi-squared test was used to compare proportional data, the Wilcoxon rank sum and Kruskal-Wallis tests were used to compare nonparametric, continuous data. Alpha was set to 0.05 and all P values were two-tailed. Analyses were performed in Stata 14.0 (StataCorp, College Station, TX, USA) and R (www.r-project.org and R Studio Inc., Boston, MA, USA).
Results
During the study period, there were 14,928 CRP and 653 ferritin assays performed for inpatients, with results available for both assays during the same hospitalization for 172 patients (Table 1). Patients with a CRP were younger and more likely to be male compared to patients with a ferritin value. Median LOS was longer in the ferritin group and mortality higher compared to patients with a CRP value. Among the group with values for both maximum CRP and maximum ferritin, bagplot visualization demonstrated that mortality was generally distributed across higher values of both ferritin and CRP (Supplemental Figure 1).
Table 1.
Cohort characteristics
| CRP | Ferritin | CRP and Ferritin | ||
|---|---|---|---|---|
| # Total Values | 14,927 | 653 | -- | |
| Unique Hospitalizations | 5,313 | 317 | 172 | |
| Unique Patients, N | 4,142 | 297 | 172 | |
| Characteristic, median (IQR) or n (%) | ||||
| Age (months) | 80.4(21.4, 156.2) | 125.4 (38.6,189.3) | 110.3 (42.2,184.1) | |
| Female | 1901 (45.9) | 156 (52.5) | 84 (48.8) | |
| CRP | Ferritin | |||
| Presenting Value* | 1.19(0.32,5.01) | 47.4(17.2,146.4) | 1.29 (0.32,6.72) | 85.9 (18.4,209.3) |
| Maximum Value* | 1.72 (0.32,6.45) | 49 (17.2,153.3) | 2.1 (0.32,13.5) | 85.9(18.4,243.1) |
| Positive Culture | 836 (20.2) | 68 (22.9) | 49 (28.5) | |
| Blood | 180 (4.3) | 29 (9.8) | 15 (8.7) | |
| Urine | 269 (6.5) | 29 (9.8) | 16 (9.3) | |
| Respiratory | 503 (12.1) | 34 (11.5) | 33 (19.2) | |
| Cerebrospinal Fluid | 20 (4.8) | 2 (0.7) | 2(1.2) | |
| Other (Pleural or Peritoneal) | 23 (5.6) | 9 (3.0) | 7(4.1) | |
| Hospital Length of Stay (days) | 3.0 (2.0, 7.0) | 5.6 (3.6,12.1) | 4.9 (2.5,13.4) | |
| PICU Admission | 1522 (36.7) | 81 (27.2) | 61 (35.5) | |
| PICU Length of Stay (days) | 2.4(1.2,5.9) | 7.3(1.5,19.2) | 5.9(1.5,15.1) | |
| Hospital Mortality | 46 (1.1) | 12 (4.0) | 8 (4.7) | |
| 90-day Mortality | 66 (1.6) | 15 (5.0) | 10 (5.8) | |
| 1-Year Mortality | 95 (2.3) | 16 (5.4) | 11 (6.4) | |
Ferritin (ng/mL), CRP (mg/dL)
Presenting CRP was not associated with any mortality outcome by ROC analysis (Table 2). The AUROC was greatest for maximum CRP and maximum ferritin and hospital mortality. Bootstrapped cut-points were statistically significant for 90-day mortality for both maximum CRP and ferritin. Figure 2 displays the ROC curves for 90-day mortality for maximum CRP and ferritin. For all patients with a CRP, the maximum value during hospitalization was dichotomized to greater than or less than and equal to 7.1 mg/dL. Ferritin was similarly dichotomized surrounding the cut-point of 373 ng/mL. Figure 1 displays the contingency table based on these cut-points. Elevation of both ferritin and CRP during hospitalization was associated with the highest mortality, followed by elevation of either biomarker alone. A similar relationship was observed when the contingency table was constructed using the same cut-points applied to the last CRP or ferritin value obtained for each patient (Supplemental Figure 2). No patient with both maximum ferritin and CRP measuring below the cut-point died during hospitalization. PELOD2 scores, calculated for the subset of patients in each quadrant with a PICU admission, were significantly greater in the combined high--risk and intermediate-risk quadrants (n=37) relative to the low-risk quadrant (n=24; P<0.001).
Table 2.
Receiver operating curve characteristics for c-reactive protein, ferritin and hospital, 90-day and 1-year mortality
| Biomarker | Hospital Mortality | 90-day Mortality | 1-year Mortality | ||||||
|---|---|---|---|---|---|---|---|---|---|
| AUROC [95% Cl] | Cutpoint [95% Cl] | P* | AUROC [95% Cl] | Cutpoint [95% Cl] | P* | AUROC [95% Cl] | Cutpoint [95% Cl] | P* | |
| N = 4142 | |||||||||
| Presenting CRP | 0.45 [0.35–0.54] | -- | -- | 0.56 [0.49–0.64] | -- | -- | 0.55 [0.49–0.62] | -- | -- |
| Maximum CRP | 0.76 [0.68–0.85] | 7.1 [4.2–12.0] | <0.001 | 0.73 [0.66–0.81] | 7.1 [3.8–10.1] | <0.001 | 0.69 [0.63–0.75] | 6.9 [1.8–7.4] | <0.001 |
| N = 297 | |||||||||
| Presenting Ferritin | 0.80 [0.69–0.90] | 123.4 [0–342.4] | 0.27 | 0.79 [0.69–0.89] | 123.4 [0–247.3] | 0.05 | 0.79 [0.69–0.88] | 123.4 [27.7–219.1] | 0.01 |
| Maximum Ferritin | 0.90 [0.83–0.98] | 373 [0–1056.4] | 0.28 | 0.88 [0.79–0.97] | 373.7 [24.7–722.7] | 0.04 | 0.87 [0.78–0.96] | 135.0 [0–423.7] | 0.36 |
Refers to significance of bootstrapped cut-point
Figure 2.
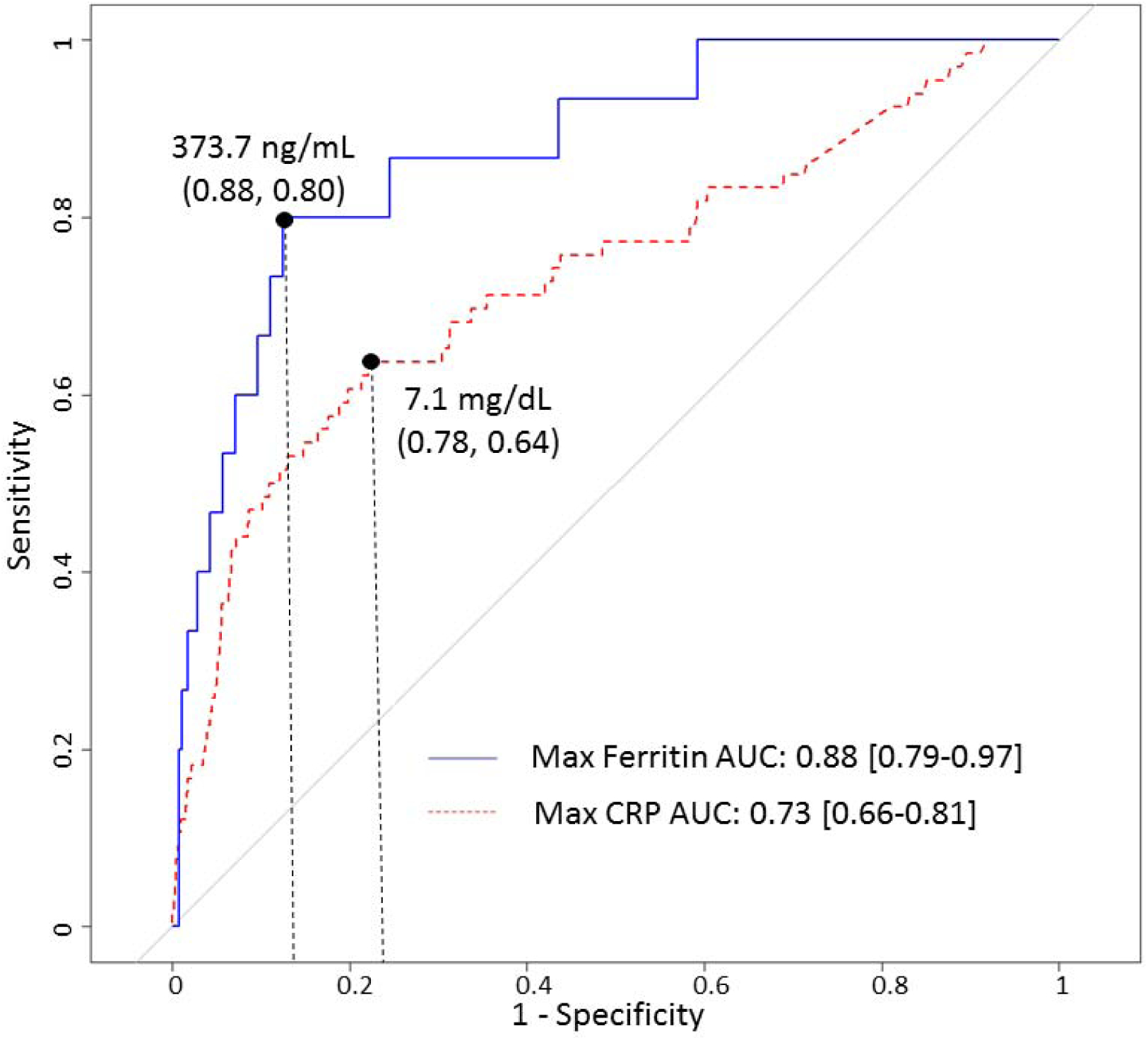
Receiver operating characteristic curves for 90-day mortality and maximum ferritin (red line) and CRP (blue line), with cut-points displayed for each biomarker and parentheses noting (sensitivity, specificity) at each cut-point.
Figure 1.
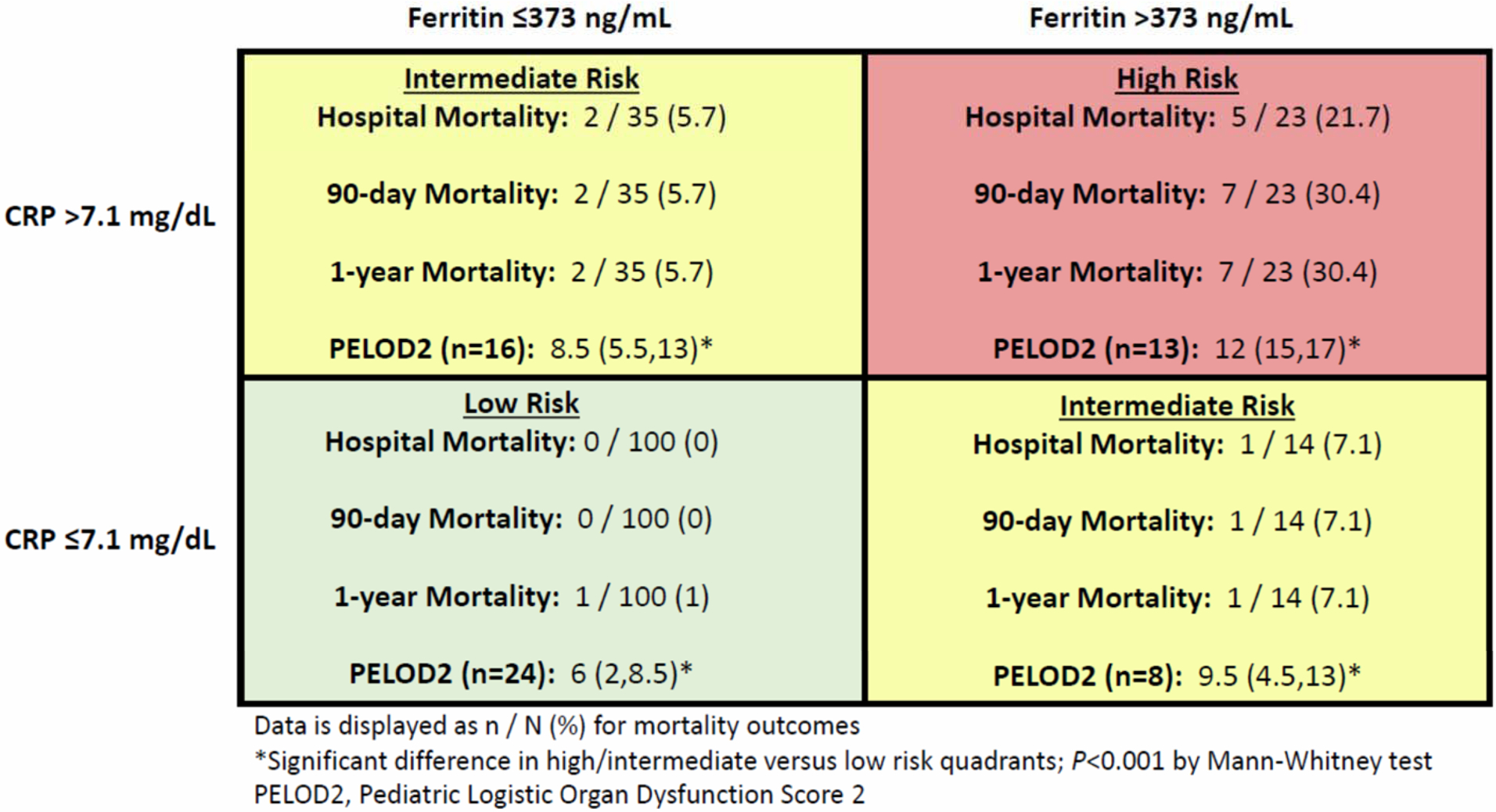
Risk contingency table for mortality and organ dysfunction based on cut-points for C-reactive protein and ferritin and patients’ maximum value for each biomarker.
For both CRP and ferritin, maximum and last values were significantly different between and among survivors and non-survivors (Figure 3). A CRP >7.1 mg/dL was significantly associated with hospital mortality alone (OR 4.6 [2.4–8.9]; P<0.001) and after adjusting for sex and any positive culture (OR 6.6 [3.3–12.9]; P<0.001). A CRP >7.1 mg/dL was also associated with 90-day mortality alone (OR 3.7 [2.2–6.3]; P<0.001) and after adjustment (OR 5.6 [3.2–9.6]; P<0.001). Ferritin >373 ng/mL was associated with both hospital mortality (OR 14.0 [2.7–72.6]; P=0.002) and 90-day mortality (OR 15.5 [3.6–66.2]; P<0.001). Adjustment could not be performed for ferritin and hospital mortality due to collinearity with the outcome and positive culture, though ferritin >373 ng/mL was associated with 90-day mortality after adjusting for sex and positive culture (OR 32.2 [3.9–267.6]; P=0.001).
Figure 3.
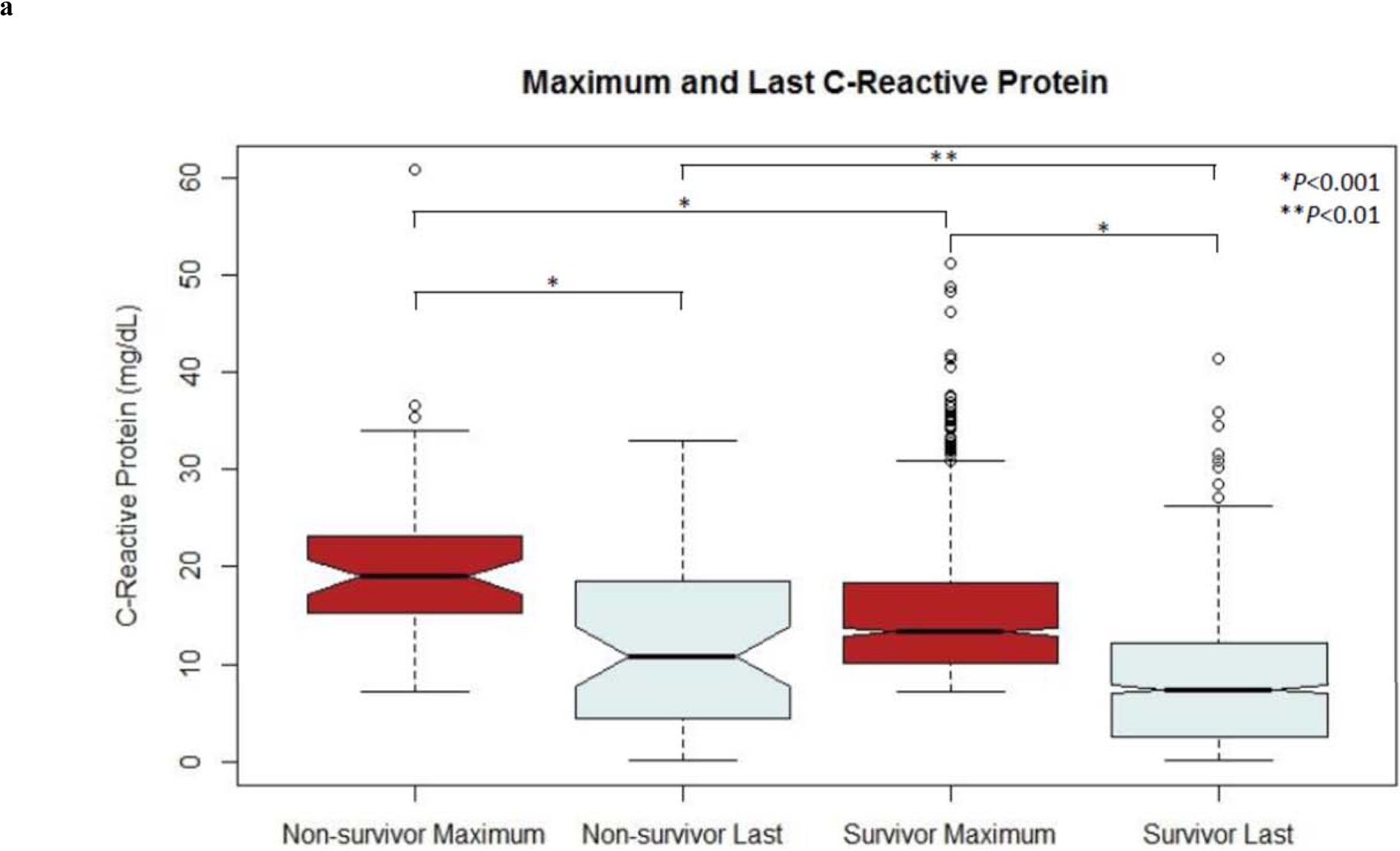
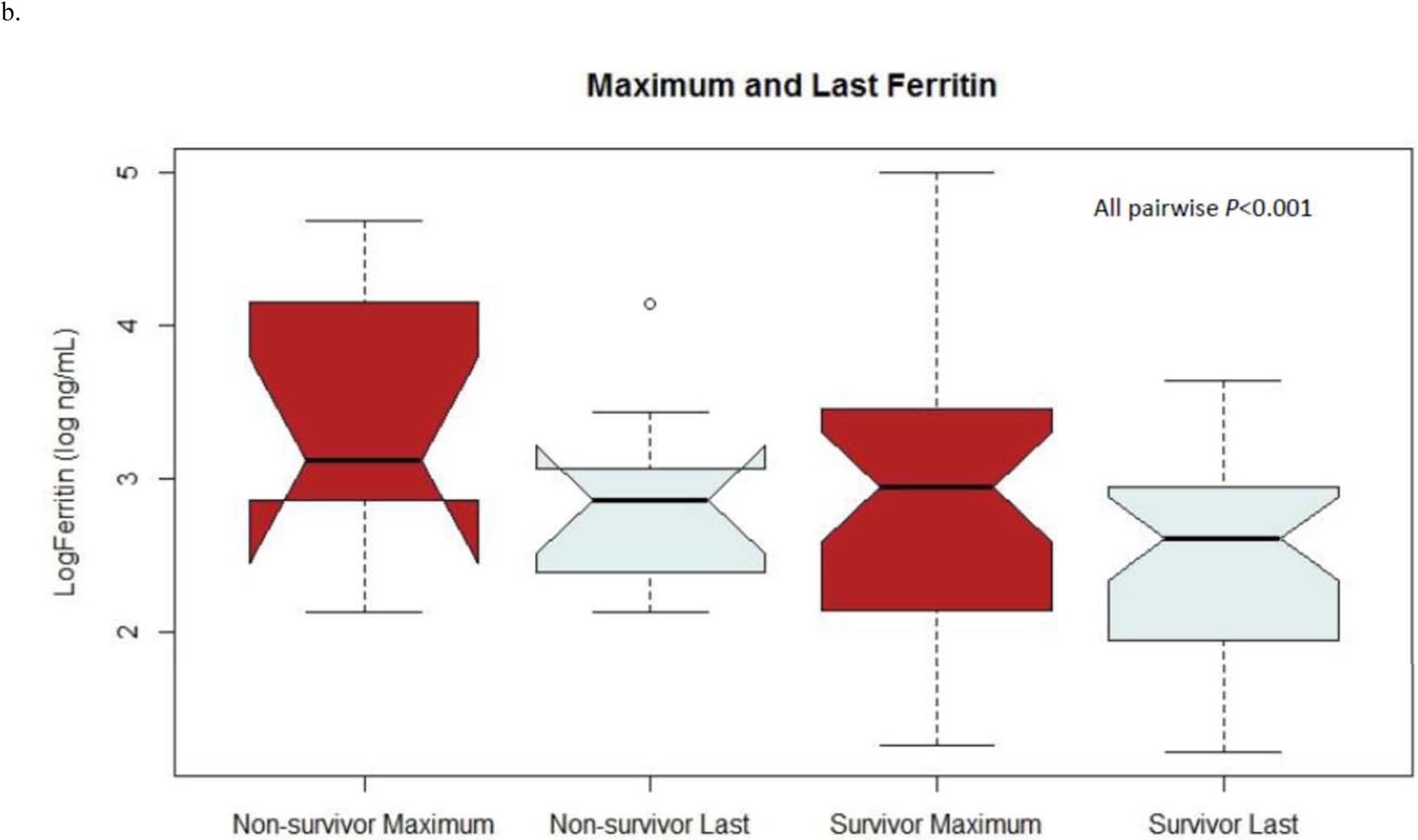
Notch plots of maximum and last values of (a) CRP and (b) ferritin for both survivors and non-survivors
For patients with a positive culture, there were no significant differences in the AUROCs for Ferritin and CRP in predicting 90-day mortality (AUROC 0.76 versus 0.71, respectively; P=0.71). An elevated CRP >1.29 mg/dL within 24 hours of discharge was not associated with odds of 30-day readmission (OR 1.36 [0.92–2.01]; P=0.12] or 90-day readmission (OR 1.28 [0.92–1.77]; P=0.15). Similarly, an elevated CRP >7.1 mg/dL within 24 hours of discharge was not associated with 30-day readmission (OR 0.71 [0.34–1.49]; P=0.37) or 90-day readmission (OR 0.93 [0.51–1.71]; P=0.82).
Discussion
In this retrospective study of electronic health record data, we demonstrate the combined utility of CRP and ferritin as a computable phenotype of systemic inflammation. The combination of these biomarkers effectively stratified patients at moderate and high risk for inflammation-associated organ dysfunction and mortality among diagnostically diverse patients admitted to a tertiary children’s hospital. Construction of a simple contingency table illustrates the additive association of elevation of both of these biomarkers with disease burden. These findings are compatible with previous studies associating mortality and elevated ferritin and CRP levels (2, 5, 7). To the best of our knowledge, the present study identified the lowest cut-point yet reported for ferritin, 373 ng/mL, associated with increased risk of organ dysfunction and death in children. We also demonstrate the utility of structured electronic record data for calculating the PELOD2, a valid, objective marker of organ dysfunction.
Pediatricians caring for hospitalized children must balance appropriate escalation of treatment with the need to minimize exposure to iatrogenic harm and avoid unnecessary expenditures in increasingly safety-driven, cost-conscious healthcare systems. Many acute pediatric illnesses, such as viral bronchiolitis, improve with time and supportive care though can be occasionally difficult to distinguish from cases of life-threatening systemic inflammation, severe sepsis and septic shock. Identifying objective thresholds of biomarkers indicative of inflammation-related mortality risk has the potential to serve clinicians by guiding them when to apply additional resources in the care of acutely ill children.
Approximately 1 in 5 patients with a CRP elevated >7.1 mg/dL and ferritin >373 mg/mL died during hospitalization in the present study; this number climbed to nearly 1 in 3 when the follow-up time was extended to 90-days. The present study included all patients with both CRP and ferritin values available. A previous study examining these biomarkers among children with severe sepsis identified cut-points of 4.08 mg/dL for CRP and 1,980 ng/mL for ferritin. In a comparable contingency table, the high-risk group (elevations of both biomarkers above the identified cut-points) was associated with mortality in 6 of 13 patients (46.15%).(7)
In the present study, both ferritin and CRP were independently associated with 90-day mortality after adjusting for any positive culture, suggesting that that these biomarkers were indicative of non-infectious inflammation in a significant proportion of severe cases. Hyperferritinemia has been independently associated with mortality in children in several previous studies. Among children with sepsis in a resource-limited setting, ferritin >500 ng/mL was associated with 58% mortality after adjusting for severity of presentation.(5) In a separate cohort of children with sepsis, hyperferritinemia was associated with disease severity, including longer hospital length of stay, longer intensive care unit length of stay, fewer mechanical ventilation-free hours, higher inotrope score and higher Pediatric Index of Mortality 2 score.(10) Bennett et al. demonstrated a that children with ferritin greater than 3000 ng/mL were significantly more likely to receive critical care and suffer mortality during hospitalization compared to children with ferritin between 1000–3000 ng/mL.(6)
Though the mechanistic underpinnings of ferritin’s role in severe disease have not been entirely elucidated, a “Hyperferritinemic Syndrome” has been proposed, implicating ferritin as part of the pathogenic mechanisms underlying systemic inflammatory response triggered by a number of pathways.(4, 11, 12) Apart from sepsis, elevated serum ferritin is a hallmark of inflammatory states such as hemophagocytic lymphohistiocytosis and macrophage activation syndrome, which may be triggered, but are not necessarily mediated, by infection.(4, 13) CRP has been implicated in a number of inflammation-related diseases, including atherosclerosis/coronary artery disease, sepsis and serious bacterial infections, and tissue injury secondary to trauma or surgery.(14, 15) The many physiologic roles of CRP include mediating coagulation and platelet aggregation, neutrophil activation, apoptotic signaling, stimulation of pro- and anti-inflammatory cytokines, complement activation and antigen presentation.(14) The precise mechanisms resulting in the association between CRP and ferritin with mortality in the present cohort are unclear and it is possible that elevations of these biomarkers reflect related but distinct inflammatory pathways and insults.
In the present cohort, maximum CRP and ferritin values were higher in non-survivors as compared to survivors, as were the last measured values of both CRP and ferritin. Notably, a decline in both CRP and ferritin was appreciated between the maximum and last measured values among both survivors and non-survivors. Certain conditions, such as burn injuries, are associated with CRP elevations of unclear significance.(16) It has been proposed that the trajectory of the biomarker, rather than the absolute value, may be more meaningful in such circumstances. Jeschke et al. demonstrated similar elevations of CRP among children with burns during the first week following injury, though non-survivors demonstrated greater elevation in the first two weeks following injury and less decline up to 180-days post-injury compared to survivors.(17) Among children with severe sepsis, at risk patients (those with an initially elevated CRP) were observed to be more likely to die if CRP increased over time, whereas decreasing CRP over time is associated with survival.(7) Of note, in the present study children with a persistently elevated CRP at the time of discharge were not more likely to be readmitted compared to children with a normal CRP prior to leaving the hospital. This likely reflects the importance of the clinical circumstances surrounding elevated CRP, such as whether an appropriate antibiotic has been selected, tissue damage halted, and the relative trajectory prior to discharge.
Strengths of the present study include the detailed information collected from the electronic health record and the inclusion of all children, irrespective of diagnosis. To the best of our knowledge, this is the largest study examining concomitant CRP and ferritin and risk of mortality among children. That our findings among all hospitalized children are compatible with previous work examining children with sepsis lends plausibility to the results of the present study. The ferritin cut-point of 373 ng/mL is only slightly greater than our lab’s reported normal range (30 to 300 ng/mL), though is in line with the threshold previously reported by Garcia et al. of 500 ng/mL in children with sepsis and septic shock.(5) The CRP cut-point of 7.1 mg/dL is comparable to threshold reported for patients with worse outcome by Carcillo et al. (4.08 mg/dL) and comparable to the median value reported Rey et al. for children with severe sepsis (7.6 mg/dL).(2, 7) The use of structured electronic data to calculate PELOD2 score demonstrated has potential for other applications, including unit acuity surveillance and as a non-mortality outcome metric that is automatically refreshed with the electronic record.
Limitations to the present study include the retrospective, single-center observational design. Selection bias may be present in that the combination of CRP and ferritin biomarkers were ordered for patients with more severe illness, though it is notable that patients with values below the identified cut-points had significantly less organ dysfunction compared to patients with elevated levels of both biomarkers. Culture data were limited to the results harbored by the study instution’s electronic records and did not include the results of cultures sent by outside facilities. The true prevalence of bacterial disease may be underrepresented given the tendency to initiate treatment prior to transfer to the study institution, impacting the likelihood of culture growth. The change in CRP and ferritin assays mid-way through the study period may have influenced cut-points, though laboratory-defined normal ranges did not change substantially and we expect the impact of this change to be minimal.
In conclusion, we describe an electronic-record-based computable phenotype for systemic inflammation using CRP and ferritin associated with organ dysfunction and mortality among all hospitalized children. A prospective, observational study is necessary to further clarify the utility of these biomarkers for risk stratification of hospitalized children. Calculating PELOD2 using structured electronic record data may provide an efficient approach to unit surveillance and outcomes assessment.
Supplementary Material
Funding Source:
This work was supported by NIH grants NICHD T32 HD40686 (CMH) and NIGMS 5R01GM108618-04 (JAC).
Footnotes
Financial Disclosure: The authors have no financial relationships relevant to this article to disclose.
Conflicts of Interest: The authors have no conflicts of interest relevant to this article to disclose.
References
- 1.McWilliam S, Riordan A: How to use: C-reactive protein. Arch Dis Child Educ Pract Ed 2010; 95:55–58 [DOI] [PubMed] [Google Scholar]
- 2.Rey C, Los Arcos M, Concha A, et al. : Procalcitonin and C-reactive protein as markers of systemic inflammatory response syndrome severity in critically ill children. Intensive Care Med 2007; 33:477–484 [DOI] [PubMed] [Google Scholar]
- 3.B.Kell D, Pretorius E: Serum ferritin is an important inflammatory disease marker, as it is mainly a leakage product from damaged cells. Metallomics 2014; 6:748–773 [DOI] [PubMed] [Google Scholar]
- 4.Rosário C, Zandman-Goddard G, Meyron-Holtz EG, et al. : The hyperferritinemic syndrome: macrophage activation syndrome, Still’s disease, septic shock and catastrophic antiphospholipid syndrome. BMC Med 2013; 11:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garcia PCR, Longhi F, Branco RG, et al. : Ferritin levels in children with severe sepsis and septic shock. Acta Paediatr Oslo Nor 1992 2007; 96:1829–1831 [DOI] [PubMed] [Google Scholar]
- 6.Bennett TD, Hayward KN, Farris RWD, et al. : Very high serum ferritin levels are associated with increased mortality and critical care in pediatric patients. Pediatr Crit Care Med J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Soc 2011; 12:e233–236 [DOI] [PubMed] [Google Scholar]
- 7.Carcillo JA, Sward K, Halstead ES, et al. : A Systemic Inflammation Mortality Risk Assessment Contingency Table for Severe Sepsis. Pediatr Crit Care Med J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Soc 2017; 18:143–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leteurtre S, Duhamel A, Salleron J, et al. : PELOD-2: an update of the PEdiatric logistic organ dysfunction score. Crit Care Med 2013; 41:1761–1773 [DOI] [PubMed] [Google Scholar]
- 9.Rousseeuw PJ, Ruts I, Tukey JW: The Bagplot: A Bivariate Boxplot. Am Stat 1999; 53:382–387 [Google Scholar]
- 10.Tonial CT, Garcia PCR, Schweitzer LC, et al. : Cardiac dysfunction and ferritin as early markers of severity in pediatric sepsis. J Pediatr (Rio J) 2017; 93:301–307 [DOI] [PubMed] [Google Scholar]
- 11.Schulert GS, Canna SW: Convergent pathways of the hyperferritinemic syndromes. Int Immunol 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carcillo JA, Simon DW, Podd BS: How We Manage Hyperferritinemic Sepsis-Related Multiple Organ Dysfunction Syndrome/Macrophage Activation Syndrome/Secondary Hemophagocytic Lymphohistiocytosis Histiocytosis. Pediatr Crit Care Med J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Soc 2015; 16:598–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allen CE, Yu X, Kozinetz CA, et al. : Highly elevated ferritin levels and the diagnosis of hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer 2008; 50:1227–1235 [DOI] [PubMed] [Google Scholar]
- 14.Lelubre C, Anselin S, Zouaoui Boudjeltia K, et al. : Interpretation of C-Reactive Protein Concentrations in Critically Ill Patients [Internet]. BioMed Res Int 2013; 2013Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3826426/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sapin F, Biston P, Piagnerelli M: Predictive value of C-reactive protein in critically ill patients after abdominal surgery. Clinics 2017; 72:23–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosanova MT, Tramonti N, Taicz M, et al. : Assessment of C-reactive protein and procalcitonin levels to predict infection and mortality in burn children. Arch Argent Pediatr 2015; 113:36–41 [DOI] [PubMed] [Google Scholar]
- 17.Jeschke MG, Gauglitz GG, Finnerty CC, et al. : Survivors versus nonsurvivors postburn: differences in inflammatory and hypermetabolic trajectories. Ann Surg 2014; 259:814–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


