Significance
Cytokinesis concludes cell division by physically separating the daughter cells. Defects in cytokinesis result in tetraploid, binucleated cells that are genetically unstable and represent an important cause of tumorigenesis. The abnormal presence of lagging chromatin within the intercellular bridge (ICB) connecting the daughter cells is detected by an evolutionarily conserved abscission checkpoint, which delays abscission and stabilizes the ICB for hours, thereby preventing the formation of binucleated cells and DNA damage. Here, we show that the reductase MsrB2 enzymatically controls the polymerization of actin filaments in the ICB and prevents ICB instability specifically in the presence of lagging chromatin. This work thus reveals that actin reduction by MsrB2 is a key component of the abscission checkpoint.
Keywords: actin, cytoskeleton, cytokinesis, oxidoreduction, abscission checkpoint
Abstract
Abscission is the terminal step of cytokinesis leading to the physical separation of the daughter cells. In response to the abnormal presence of lagging chromatin between dividing cells, an evolutionarily conserved abscission/NoCut checkpoint delays abscission and prevents formation of binucleated cells by stabilizing the cytokinetic intercellular bridge (ICB). How this bridge is stably maintained for hours while the checkpoint is activated is poorly understood and has been proposed to rely on F-actin in the bridge region. Here, we show that actin polymerization is indeed essential for stabilizing the ICB when lagging chromatin is present, but not in normal dividing cells. Mechanistically, we found that a cytosolic pool of human methionine sulfoxide reductase B2 (MsrB2) is strongly recruited at the midbody in response to the presence of lagging chromatin and functions within the ICB to promote actin polymerization there. Consistently, in MsrB2-depleted cells, F-actin levels are decreased in ICBs, and dividing cells with lagging chromatin become binucleated as a consequence of unstable bridges. We further demonstrate that MsrB2 selectively reduces oxidized actin monomers and thereby counteracts MICAL1, an enzyme known to depolymerize actin filaments by direct oxidation. Finally, MsrB2 colocalizes and genetically interacts with the checkpoint components Aurora B and ANCHR, and the abscission delay upon checkpoint activation by nuclear pore defects also depends on MsrB2. Altogether, this work reveals that actin reduction by MsrB2 is a key component of the abscission checkpoint that favors F-actin polymerization and limits tetraploidy, a starting point for tumorigenesis.
The actin cytoskeleton plays a fundamental role in the initial steps of cytokinesis and is essential for cleavage furrow contraction (1–3). The cells are then connected by a thin cytoplasmic canal, the intercellular bridge (ICB), that is eventually cut in a complex process called abscission (4, 5). Abscission occurs on one side of the midbody, the central part of the ICB (6, 7), and relies on endosomal sorting complex required for transport (ESCRT)-III filaments that likely drive the final constriction (8–10). The ESCRT machinery is first recruited at the midbody and later concentrates at the abscission site itself, where the microtubules of the ICB have been cleared by the microtubule-severing enzyme Spastin (11–13). Clearing actin filaments (F-actin) from the ICB is equally important for successful abscission (14). Indeed, the ESCRT-III assembles normally at the midbody but not at the abscission site when actin is not properly depolymerized (14, 15). Two parallel pathways (Rab11/p50RhoGAP and Rab35/OCRL) contribute to prevent excessive actin polymerization in the ICB (5, 16, 17). In addition, we recently found that the Rab35 GTPase recruits the oxidase MICAL1 at the abscission site, in order to clear actin in the ICB (18, 19). MICAL1 belongs to the MICAL monooxygenase family (20–23), directly oxidizes Met44 and Met47 of F-actin into methionine-R-sulfoxides, and triggers rapid depolymerization of the filaments in vitro (18, 24–28). Thus, regulated oxidation promotes local F-actin clearance, ESCRT-III recruitment, and abscission.
There are nevertheless physiological conditions in which abscission is delayed, notably when the abscission checkpoint/NoCut checkpoint is activated (10, 29–36). This evolutionarily conserved checkpoint depends on several kinases, including Aurora B, and is triggered by different cytokinetic stresses, such as persisting, ultrathin DNA bridges (UFBs) and lagging chromatin positive for nuclear envelop markers (hereafter referred as “chromatin bridges”) within the ICB, as well as high membrane tension or nuclear pore defects (32, 37–47). Chromatin bridges spanning between the two daughter nuclei across the ICB can result from DNA replication stress, incomplete DNA decatenation, or telomere attrition and activate the checkpoint to delay abscission, presumably giving additional time for resolving these abnormalities (10, 33–35, 48). A defective checkpoint results in cytokinetic failure and binucleated cells (tetraploidy) (32, 38, 44) or chromosome breaks and DNA damage (40, 43, 45–47, 49), and these distinct outcomes apparently depend on which component of the checkpoint has been removed for reasons that are not fully understood (10, 33, 35, 36). In several instances, experimental inactivation of the checkpoint (e.g., Aurora B or abscission/NoCut checkpoint regulator [ANCHR] inactivation) leads to ICB instability and eventually binucleated cells in the presence of chromatin bridges, and to an acceleration of abscission in the absence of chromatin bridges (32, 38, 44, 50). Interestingly, activation of the checkpoint was originally associated with increased F-actin at or close to the ICB, and it was speculated that it could help to maintain ICB stability for hours until chromatin bridges have been resolved (32). More recently, actin patches located at the entry points of the ICBs have been described to depend on the activation of the Src kinase (46), but whether F-actin is essential for the checkpoint has not been directly tested. In addition, little is known about the mechanisms that directly control F-actin levels in the ICB region when the abscission checkpoint is activated.
We hypothesized that oxidation-mediated clearance of F-actin by MICAL1 might be counteracted by actin reduction by methionine sulfoxide reductases (MsrBs) during cell division. MsrBs belong to a family of enzymes found in all living organisms (51, 52), in particular dSelR in Drosophila and its orthologs MsrB1-3 in humans, that selectively reduce methionine-R-sulfoxide in vitro (25, 28, 53). In vivo, Drosophila dSelR counteracts dMical-mediated actin disassembly in bristle development, axon guidance, and muscle organization (53). In addition, MsrB1 antagonizes Mical1 and regulates actin-rich processes in stimulated mouse macrophages (25, 28). However, whether MsrBs play a role during cell division is unknown. Here, we report that human MsrB2 controls the timing of abscission, F-actin levels, and ICB stability and plays a critical role to prevent tetraploidy when the abscission checkpoint is activated by lagging chromatin.
Results
Drosophila dSelR and Human MsrB2 Are Negative Regulators of Cytokinetic Abscission.
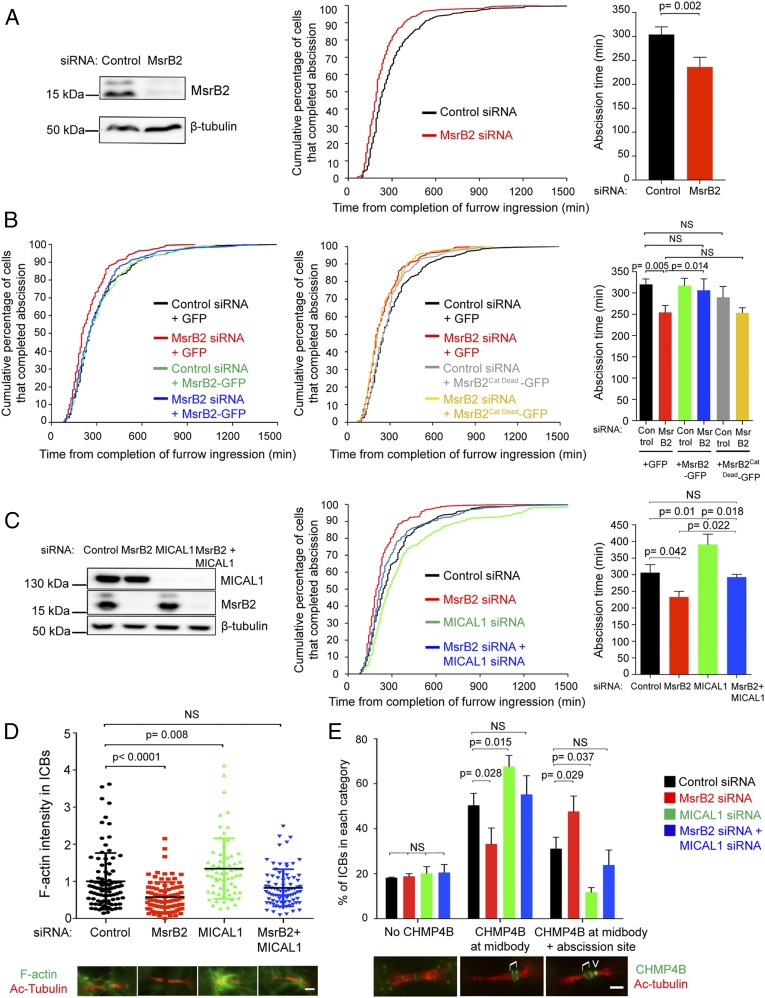
While depletion of dMical delays cytokinetic abscission (18), we now found that depletion of dSelR conversely accelerated abscission in Drosophila S2 cells (SI Appendix, Fig. S1A). Similarly, depletion of MsrB2 accelerated abscission in human HeLa cells (Fig. 1A). Time-lapse phase-contrast microscopy revealed that cell rounding, furrow ingression, and ICB formation occurred normally in MsrB2-depleted cells, but the timing of abscission was advanced by ∼70 min (Fig. 1A and SI Appendix, Fig. S1B). The abscission acceleration was fully rescued by expression of a small interfering RNA (siRNA)-resistant version of MsrB2, but not of a catalytically dead mutant (Fig. 1B and SI Appendix, Fig. S1C), indicating that the reductase activity of MsrB2 is critical for controlling the timing of abscission. In contrast, MsrB3 depletion did not perturb the timing of abscission (SI Appendix, Fig. S2A). Furthermore, neither the overexpression of MsrB1 nor the overexpression of MsrB3B was able to rescue the accelerated abscission observed in MsrB2-depleted cells (SI Appendix, Fig. S2B). Altogether, the methionine sulfoxide reductases dSelR and MsrB2 play a specific and evolutionarily conserved role as negative regulators of cytokinetic abscission. In the rest of the manuscript, we focus on human cells to understand how MsrB2 controls abscission and its physiological significance.
Fig. 1.
MsrB2 is a negative regulator of cytokinetic abscission and counteracts MICAL1-mediated actin oxidation and ESCRT-III recruitment. (A, Left) Lysates from HeLa cells treated with control or MsrB2 siRNAs were blotted for MsrB2 and β-tubulin (loading control). (Middle and Right) Distribution of the abscission time (P < 0.001, nonparametric and distribution-free Kolmogorov–Smirnov [KS] test) and mean abscission time ± SD in control- and MsrB2-depleted cells (N = 3 independent experiments). n = 244 to 247 cells per condition. (B) Distribution of abscission time (Left and Middle) and mean abscission time ± SD (Right) for control- and MsrB2-depleted cells transfected with indicated plasmids (N = 3 independent experiments). n = 217 to 227 cells per condition. No statistical difference between black and either green, blue, or gray curves. No statistical difference between red and yellow curve. P = 0.001 between black and either red or yellow curves (KS test). (C, Left) Lysates from cells treated with control, MsrB2, MICAL1, or MsrB2+MICAL1 siRNAs were blotted for MICAL1, MsrB2, and β-tubulin (loading control). (Middle and Right) Distribution of the abscission time and mean abscission time ± SD for the same cell populations described in the Left (N = 3 independent experiments). n = 233 to 245 cells per condition. No statistical significance between black and blue curves, P < 0.001 between black and red curve, P = 0.066 between black and green curve (KS test). (D) F-actin intensity in the ICBs from the same cell populations used in C (N = 3 independent experiments). n = 64 to 89 ICBs per condition. Mean ± SD. (Bottom) Representative images of F-actin in the ICBs for the corresponding conditions. (Scale bar: 2 μm.) (E) Quantification of ICBs with either No CHMP4B (Bottom Left image), with CHMP4B only at the midbody (Bottom Middle image), or with CHMP4B both at midbody and abscission site (Bottom Right image) for each cell population described in C (N = 3 independent experiments). n = 151 to 153 ICBs per condition. Mean ± SD. Brackets and arrowhead mark the midbody and the abscission site, respectively. (Scale bar: 2 μm.) NS, not significant. P values (Student t tests) are indicated.
MsrB2 Counteracts MICAL1-Mediated Actin Oxidation and ESCRT-III Recruitment during Abscission.
To test whether MsrB2 could counteract MICAL1 function during cytokinesis, we compared the timing of abscission in cells depleted for MsrB2, MICAL1, or both (Fig. 1C). MICAL1 depletion delayed abscission by ∼85 min, as expected (18), whereas codepletion of MICAL1 and MsrB2 restored normal timing of abscission (Fig. 1C). MICAL1 depletion was previously found to trigger an increase of F-actin in ICBs (Fig. 1D and ref. 18). In contrast, F-actin levels were diminished in MsrB2-depleted ICBs, compared to controls (Fig. 1D). Interestingly, F-actin levels were restored to normal levels in cells depleted for both MsrB2 and MICAL1 (Fig. 1D). Finally, we previously reported that delayed abscission after MICAL1 depletion was due to defective recruitment of ESCRT-III at the abscission site (18). This is evidenced by a decreased proportion of late ICBs with CHMP4B both at the midbody and abscission site, concomitant with an increased proportion of late ICBs with CHMP4B only at the midbody (Fig. 1E). We observed the opposite phenotype in cells depleted for MsrB2: a decreased proportion of late ICBs with CHMP4B only at the midbody and conversely an increased proportion of late ICBs with CHMP4B both at the midbody and abscission site (Fig. 1E). Remarkably, ESCRT-III localization was as in control cells after codepletion of MICAL1 and MsrB2 (Fig. 1E). We conclude that MsrB2 counteracts MICAL1 activity in controlling F-actin levels during late cytokinesis, ESCRT-III recruitment at the abscission site, and the timing of abscission. Our results also suggest that MsrB2 depletion accelerates abscission by decreasing F-actin levels, which then favors the localization of ESCRT-III at the abscission site.
MsrB2 Selectively Reduces Actin Monomers Whereas MICAL1 Only Oxidizes Actin Filaments In Vitro.
Purified dSelR and MsrB1/B2 are known to counteract MICALs on F-actin polymerization in vitro, but this has been documented in bulk, actin-pyrene assays in which the behavior of individual filaments cannot be assayed (25, 28, 53). As such, it is unclear whether MsrB2 acts on MICAL1-oxidized actin filaments and/or on MICAL1-oxidized actin monomers. To address this issue, we took advantage of microfluidics to visualize individual, fluorescently labeled actin filaments, while exposing them successively to different protein solutions. We carried out in vitro experiments with the recombinant, active MICAL1 catalytic domain (18) and the active MsrB2 amino acids (aa) 24 to 182, previously used by others (54). We first subjected F-actin to MICAL1 in order to oxidize the filaments (Fig. 2 A and B). We then exposed the filaments to buffer and observed the rapid depolymerization of their free barbed ends, approximately five times faster than nonoxidized filaments, as expected for oxidized filaments (18, 27) (Fig. 2B). We finally exposed the filaments to MsrB2, along with actin monomers at the critical concentration in order to prevent the filaments from depolymerizing. When exposing the filaments to buffer again, after exposure to MsrB2, their depolymerization resumed at the same rate as before their exposure to MsrB2 (Fig. 2B). These results indicate that, once an actin filament is oxidized, MsrB2 cannot revert its subunits to their initial, slowly depolymerizing state and show that MsrB2 is unable to act on filaments.
Fig. 2.
MsrB2 selectively reduces actin monomers whereas MICAL1 only oxidizes actin filaments in vitro. (A) MICAL1 and MsrB2 constructs used in this figure. aa, amino acid. (B) MsrB2 amino acids 24 to 182 cannot reduce F-actin. (Upper) Time-lapse of the depolymerization of a single filament oxidized by MICAL1, sequentially exposed to buffer (a phase during which the filament depolymerized) and MsrB2 amino acids 24 to 182 (supplemented with actin at its critical concentration, 0.1 µM, such that the filament length remained constant during this phase). (Lower) The barbed end (BE) depolymerization rate is constant despite exposing the filament to MsrB2 amino acids 24 to 182. The depolymerization rates are normalized by the average depolymerization rate of oxidized filaments that have not been exposed to MsrB2 amino acids 24 to 182. n = 30 filaments, points: mean ± SD. (C) MsrB2 amino acids 24 to 182, but not a catalytically dead mutant (CD) or its catalytic core domain (CC), can reduce MICAL1-oxidized G-actin to allow repolymerization. (Upper) Sketch of the procedure and typical fields of view (cropped): 2 µM F-actin are sequentially incubated with MICAL1 (or buffer, for 90 min) and MrB2 constructs (or buffer, for various times). Fractions of this solution are diluted 20-fold into F-buffer supplemented with 0.3% methylcellulose and injected into an open chamber for visualization. (Lower) Quantification of the density of actin filaments. For each experiment and time point, we measured the total F-actin length in 4 to 12 individual fields of view, 10,000 µm2 each. Points: one to four independent experiments, mean ± SD. (D) MICAL1 does not oxidize G-actin. (Upper) Time-lapse images showing barbed end polymerization from a solution of profilin-actin containing NADPH, with or without MICAL1, incubated for up to 60 min, prior to elongation (the 0.6-µM actin solution was supplemented with 1 µM profilin to prevent spontaneous nucleation and to maintain a stable pool of G-actin). (Lower) Polymerization rate is independent of incubation time and presence of MICAL1. n = 20 filaments (two experiments), points: mean ± SD. (E) Regulation of actin turnover by the MICAL1/MsrB2 redox balance: MICAL1 oxidizes actin filaments, driving their depolymerization and the formation of oxidized monomers while MsrB2 reduces the oxidized monomers, allowing them to repolymerize into filaments. ADP, adenosine 5′-diphosphate; WT, wild-type.
We further investigated how MsrB2 functions by incubating filaments with MICAL1 followed by MsrB2 in bulk, and periodically flowing samples in open chambers for visualization. As expected, addition of MICAL1 catalytic domain led to full depolymerization of actin filaments into monomers after 90 min (Fig. 2C). Remarkably, addition of MsrB2 amino acids 24 to 182 to the solution of oxidized monomers allowed for the repolymerization of the actin filaments (Fig. 2C). This was not observed if a catalytically dead version of MsrB2 (C169G, “CD” MsrB2) (55, 56) was added (Fig. 2 A and C, graph). Interestingly, the core catalytic domain (which is defined as the region highly similar among MsrB1, MsrB2, and MsrB3, amino acids 75 to 182, “CC” MsrB2) (Fig. 2A) was unable to efficiently restore actin polymerization (Fig. 2C, graph), revealing that the N-terminal amino acids 24 to 74 of MsrB2 are required for proper actin reduction. We conclude that MsrB2 acts on MICAL1-oxidized monomers and produces reduced actin molecules competent for polymerization into filaments. Conversely, we observed that actin monomers supplemented with profilin (in order to maintain a stable pool of profilin-actin monomers that prevent the spontaneous nucleation of F-actin, as in cells) incubated with the catalytic domain of MICAL1 for up to 1 h were still able to polymerize as fast as untreated monomers (Fig. 2D). Thus, MICAL1 does not efficiently oxidize actin monomers, as proposed for Drosophila dMical using bulk assays (24). Altogether, our results indicate that MICAL1 acts on actin filaments to induce their oxidation and depolymerization whereas MsrB2 acts on actin monomers to reduce them and promote their polymerization (Fig. 2E). Therefore, MICAL1 and MsrB2 do not compete for the same substrate but favor different sides of the actin polymerization/depolymerization cycle, and together modulate actin turnover.
A Nonmitochondrial, Cytosolic Pool of MsrB2 Controls the Timing of Abscission.
Human MsrB2 has been previously localized within mitochondria (in the matrix), and the first 23 amino acids are indeed predicted to act as a mitochondrial targeting sequence (MTS) (ref. 54 and Fig. 3A). We thus wondered how MsrB2 could control cytokinetic abscission since actin monomers reside in the cytosol. Since no antibody specific for MsrB2 is available for immunofluorescence, we used a C-terminal green fluorescent protein (GFP)-fusion MsrB2 (Fig. 3A) to localize MsrB2, as previously described in ref. 54, and we confirmed that the bulk of MsrB2-GFP is localized to mitochondria (Fig. 3B). In addition, we found an unnoticed, diffuse MsrB2 pool within the cytoplasm, detectable in all cells, whatever the level of overexpression (Fig. 3 B, Insets and C for quantification). This was not an artifact resulting from the saturation of the mitochondrial import machinery since the mitochondrial matrix marker Mito-dsRed (MTS of cytochrome-c fused to dsRed) coexpressed with MsrB2-GFP was fully localized into mitochondria (Fig. 3B). The MTS of MsrB2 alone (amino acids 1 to 23 or MsrB21-23) (Fig. 3A), fused to GFP, was localized only in mitochondria in ∼60% of the cells, and both in the cytosol and mitochondria in the remaining 40% of the cells (Fig. 3C). Interestingly, MsrB2 MTS followed by residues preceding the core catalytic domain (amino acids 1 to 74 or MsrB21-74) (Fig. 3A) displayed this dual localization in essentially all cells (Fig. 3C). Conversely, a truncated version of MsrB2 lacking the MTS (amino acids 24 to 182 or MsrB224-182) (Fig. 3A) remained cytosolic (Fig. 3C). These results were confirmed by quantifying the mitochondria:cytosol ratio for the different constructions: MsrB21-23-GFP (MTS alone) localized to mitochondria as much as Tom20’s MTS-GFP whereas MsrB224-182-GFP (lacking the MTS) was cytosolic like GFP alone, and both MsrB21-74-GFP and full-length MsrB2-GFP distributed between the cytosol and the mitochondria (SI Appendix, Fig. S3A). We conclude that the N-terminal, noncatalytic domain (amino acids 1 to 74) is responsible for the localization of MsrB2 in the cytosol, in addition to the mitochondria.
Fig. 3.
MsrB2 localizes to both mitochondria and cytosol, and the cytosolic pool of MsrB2 controls the timing of abscission. (A) MsrB2 constructs used in this study. aa, amino acid; Cyto, cytosolic; GFP, green fluorescent protein; MTS, mitochondrial targeting sequence. (B, Top) Cells expressing MsrB2-GFP (green) were stained with Tom22 (red). (Bottom) Cells coexpressing MsrB2-GFP (green) and Mito-dsRed (red). (Scale bars: 10 μm.) (C, Upper) Cells expressing MsrB2 MTS-GFP (Top, green) or MsrB2 amino acids 1–74-GFP (Middle, green) or MsrB2 amino acids 24–182-GFP (Bottom, green) were stained with Tom22 (red). (Scale bars: 10 μm.) (Lower) Percentage of MsrB2-GFP, MsrB2 MTS-GFP, MsrB2 amino acids 1–74-GFP, or MsrB2 amino acids 24–182-GFP transfected cells displaying both mitochondria and cytosol localization (N = 3 independent experiments). n = 1,500 cells per condition. (D and E) Distribution of the abscission time and mean abscission time ± SD in control- and MsrB2-depleted cells transfected with indicated plasmids (N = 3 independent experiments). n = 171 to 224 cells per condition. In D, no statistical significance between black and blue curves, P < 0.001 between black and red curves, P = 0.014 between black and green curves (KS test). In E, no statistical significance between black and green curves, or red and blue curves, P < 0.001 between black and either red or blue curves (KS test). NS, not significant. P values (Student t tests) are indicated.
To decide which pool of MsrB2 controls abscission, we measured the timing of abscission in MsrB2-depleted cells that expressed only the cytosolic version of MsrB2 (MsrB224-182 or Cyto MsrB2) (Fig. 3A) and found that it fully restored normal abscission (Fig. 3D and SI Appendix, Fig. S3B). In contrast, a cytosolic version of MsrB2 lacking a functional enzymatic activity (Cyto MsrB2Cat Dead) was unable to rescue the accelerated abscission phenotype resulting from endogenous MsrB2 depletion (Fig. 3E and SI Appendix, Fig. S3B). Altogether, our results indicate that the nonmitochondrial pool of MsrB2 is responsible for the control of cytokinetic abscission. This is consistent with the notion that the cytosolic pool of MsrB2 reduces actin in the cytosol and thus negatively regulates abscission.
MsrB2 Depletion Leads to Binucleated Cells in the Presence of Chromatin Bridges.
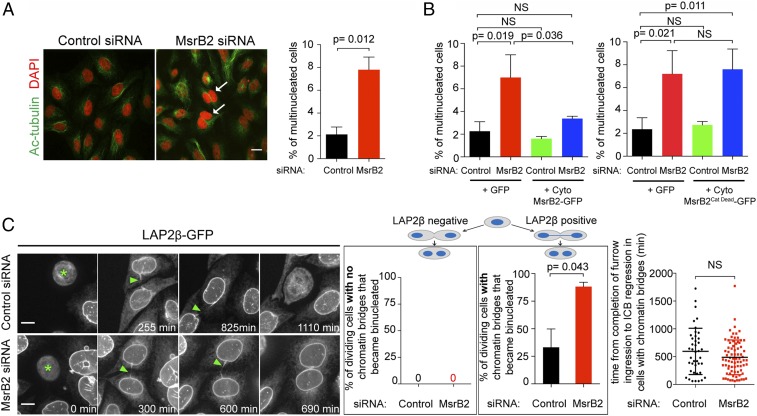
We noticed that a relatively small but reproducible proportion of cells were binucleated after MsrB2 depletion (Fig. 4A). This phenotype was fully rescued by the expression of a catalytically active (but not a catalytically dead) cytosolic version of MsrB2 (Fig. 4B). However, this phenotype was not significantly rescued by either the expression of catalytically active MsrB1 (P = 0.47, n = 1,004 cells) or MsrB3B (P = 0.98, n = 1,003 cells), reinforcing the idea that, among MsrBs, MsrB2 has a specific role in cytokinesis. The modest increase in binucleated cells together with an accelerated abscission observed after MsrB2 depletion prompted us to investigate whether MsrB2 might participate to the abscission checkpoint. Indeed, these two features are observed after inactivation of a subset of checkpoint components (e.g., Aurora B, ANCHR, and ALIX) where binucleated cells arise only in the minor proportion of dividing cells harboring abnormal chromatin bridges (32, 38, 44). We thus turned to time-lapse spinning disk confocal microscopy in a cell line that stably expresses a reliable and sensitive marker of chromatin bridges, the nuclear envelope protein LAP2β-GFP (32). When the checkpoint is unperturbed (control RNAi), cells with LAP2β-negative ICBs never became binucleated, and only 30% of the cells with LAP2β-positive ICBs became binucleated (Fig. 4C, black bar and Movie S1). These results were in line with previous reports from other laboratories (32, 44). In contrast, almost all of the cells harboring chromatin bridges became binucleated in MsrB2-depleted cells, and this never happened in cells without chromatin bridges (Fig. 4C, red bar and Movie S2). In both control- and MsrB2-depeleted cells, binucleated cells resulted from the regression of the ICB containing a chromatin bridge, ∼10 h after complete furrow ingression (Fig. 4 C, Right). Of note, if cells were arrested in cytokinesis by means that did not trigger the abscission checkpoint (i.e., by depleting CEP55), MsrB2 depletion did not destabilize these ICBs (SI Appendix, Fig. S3C). The percentage of binucleated cells upon CEP55 and MsrB2 codepletion was indeed purely additive (SI Appendix, Fig. S3C). We conclude that MsrB2 is essential for the normal stabilization of the ICB and to prevent tetraploidy, but only in cells presenting a chromatin bridge. Altogether, our results suggest that MsrB2 is a component of the abscission checkpoint.
Fig. 4.
MsrB2 depletion leads to binucleated cells exclusively in the presence of chromatin bridges. (A, Left) Control- and MsrB2-depleted cells were stained with DAPI (blue) and acetylated-tubulin (Ac-tubulin) (green). White arrows indicate multinucleated cells. (Scale bar: 20 μm.) (Right) Percentage of multinucleated cells after control or MsrB2 depletion (N = 3 independent experiments). n = 1,500 cells per condition. Mean ± SD. (B) Percentage of multinucleated cells after control or MsrB2 depletion and transfection with indicated plasmids (N = 3 independent experiments). n = 1,500 cells per condition. Mean ± SD. (C, Left) Snapshot of time-lapse spinning-disk confocal microscopy movies of LAP2β-GFP cells treated with either control or MsrB2 siRNAs. Green asterisks and green arrowheads mark metaphase cells and chromatin bridges, respectively. (Scale bars: 5 μm.) (Middle) Percentage of dividing control- and MsrB2-depleted cells without (left box) or with (right box) chromatin bridges that eventually became binucleated (N = 3 independent experiments). n = 860 to 946 cell divisions per condition. (Right) Time from completion of furrow ingression to ICB regression in control- and MsrB2-depleted cells displaying chromatin bridges (N = 3 independent experiments). n = 41 to 78 cells per condition. Mean ± SD. NS, not significant. P values (Student t tests) are indicated.
MsrB2 Is Recruited to the Midbody in the Presence of Chromatin Bridges and Controls F-Actin Levels.
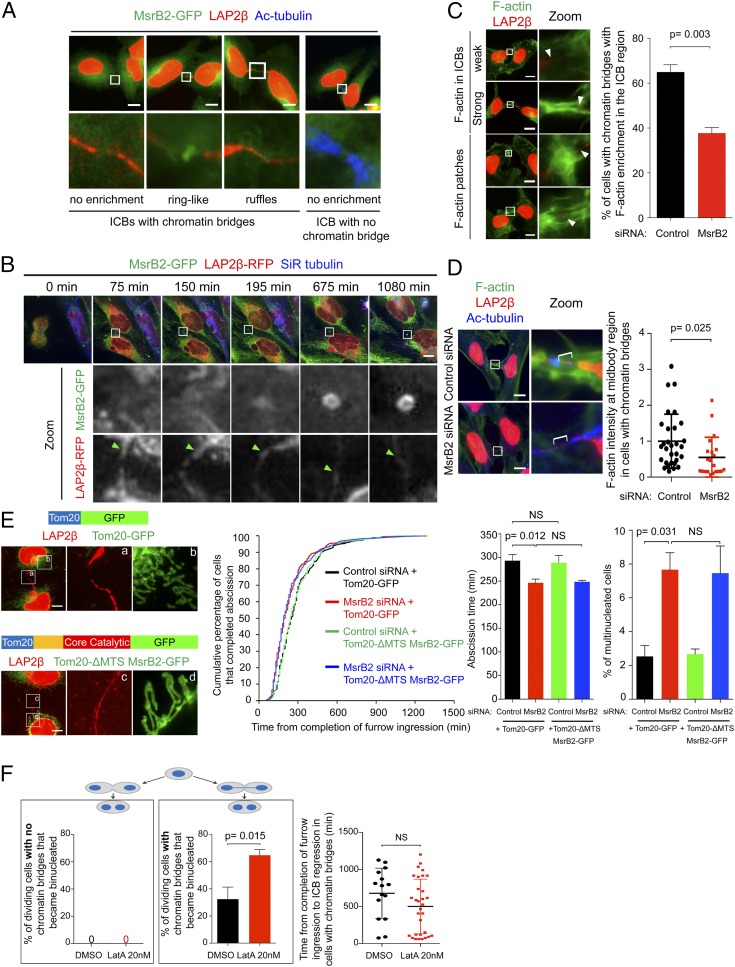
We next investigated MsrB2 localization in dividing cells with or without chromatin bridges. In fixed samples, MsrB2 was present as a ring-like structure at the midbody surrounding LAP2β-positive chromatin bridges (57.3% of ICBs, n = 96) (Fig. 5 A, Left). We also sometimes detected MsrB2 in plasma membrane ruffles in the ICB region (23.9% of ICBs, n = 96) (Fig. 5 A, Left). In dividing cells with no chromatin bridges, MsrB2 was not seen enriched at the midbody or at ruffles (none of 47 ICBs analyzed) (Fig. 5 A, Right). Time-lapse spinning disk confocal microscopy further confirmed that MsrB2-GFP was diffuse and at low levels in the midbody region of dividing cells with no chromatin bridges (22 of 26 cases) (SI Appendix, Fig. S4A and Movie S3) and displayed a more pronounced localization at the midbody in the remaining cases (4 of 26). Importantly, a strong signal of MsrB2-GFP at the midbody appeared several hours after furrow ingression in the majority of cells presenting a chromatin bridge (9 of 10 cases) (Fig. 5B and Movie S4). Thus, MsrB2 is strongly recruited at the midbody in the presence of chromatin bridges.
Fig. 5.
MsrB2 is recruited to the midbody in the presence of chromatin bridges and controls F-actin levels. (A) Cells expressing MsrB2-GFP (green) were stained with LAP2β (red) and acetylated-tubulin (blue). Indicated zoomed regions are also presented (Lower). The acetylated-tubulin channel has been displayed only for the ICB with no chromatin bridge (Right). (Scale bars: 10 μm.) (B) Snapshots of a time-lapse spinning-disk confocal microscopy movie of cells expressing MsrB2-GFP (green) and LAP2β-RFP (red), labeled with SiR-tubulin (blue). Green arrowheads indicate chromatin bridges. (Scale bar: 10 μm.) (C, Left) Representative images of cells stained with phalloidin (green) and LAP2β (red). White arrowheads indicate chromatin bridges. (Scale bars: 10 μm.) (Right) Percentage of control- and MsrB2-depleted cells with LAP2β-positive chromatin bridges with F-actin enrichment in the ICB region (N = 3 independent experiments). n = 114 to 115 chromatin bridges containing ICBs. Mean ± SD. (D, Left) Representative images of control- and MsrB2-depleted cells stained with phalloidin (green), LAP2β (red), and acetylated-tubulin (blue). (Scale bars: 10 μm.) (Right) Quantification of F-actin intensity in the midbody region (brackets) in control- or MsrB2-depleted cells with LAP2β-positive chromatin bridges (N = 3 independent experiments). n = 21 to 30 chromatin bridges-positive ICBs per condition. Mean ± SD. (E, Left) Representative images of cells expressing Tom201-35-GFP (green, Upper) or Tom201-35-ΔMTS MsrB2-GFP (green, Bottom) stained with LAP2β (red). (Scale bars: 10 μm.) a–d correspond to the indicated zoomed regions in E, Left. (Middle) Distribution of abscission time and mean abscission time ± SD for control- and MsrB2-depleted cells transfected with indicated plasmids (N = 3 independent experiments). n = 226 to 240 cells per condition. No statistical difference between black and green curves or between red and blue curves. P = 0.001 between black and red curves (KS test). (Right) Percentage of multinucleated cells after control or MsrB2 depletion and transfection with indicated plasmids (N = 3 independent experiments). n = 1,500 cells per condition. Mean ± SD. (F, Left) Percentage of dividing cells without (left box) or with (right box) chromatin bridges that became binucleated after treatment with either dimethyl sulfoxide (DMSO) or 20 nM LatA (N = 3 independent experiments). n = 485 to 555 cell divisions per condition. (Right) Time from completion of furrow ingression to ICB regression in DMSO- or 20 nM LatA-treated cells displaying chromatin bridges (N = 3 independent experiments). n = 15 to 31 cells per condition. Mean ± SD. NS, not significant. P values (Student t tests) are indicated.
We next analyzed the effect of MsrB2 depletion on F-actin among dividing cells presenting a chromatin bridge, using fluorescently labeled phalloidin. MsrB2 depletion decreased the number of cells with F-actin enrichment in the ICB region (strong F-actin in the ICB and F-actin patches) (Fig. 5C). Quantification further revealed a twofold decrease of F-actin levels within the intercellular bridge, in the midbody area (Fig. 5D). Of note, MsrB2 depletion did not induce a global decrease of F-actin in the cell bodies (SI Appendix, Fig. S4B). Thus, MsrB2 promotes high levels of polymerized F-actin in the presence of chromatin bridges within ICBs and prevents ICB regression when the checkpoint is activated.
Since MsrB2 is strongly recruited at the midbody upon checkpoint activation by lagging chromatin, this ICB-localized pool of MsrB2 might directly reduce actin within the ICBs. Alternatively, the cytosolic pool of MsrB2 outside the ICB might reduce actin in the cell bodies of dividing cells, which could indirectly regulate bridge stability. To decide between these two hypotheses, we investigated whether preventing MsrB2 recruitment to the midbody would rescue the cytokinetic defects observed upon MsrB2 depletion. To this end, we designed an siRNA-resistant version of MsrB2-GFP deleted of its MTS (to prevent its entry into mitochondria, thus identical to Cyto-MsrB2 in Fig. 4B) fused at its N terminus to the transmembrane domain of the outer mitochondrial membrane protein Tom20 (to immobilize MsrB2 to the cytosolic surface of the mitochondria). The resulting Tom20-ΔMTS MsrB2-GFP (SI Appendix, Fig. S4C) was fully retained at the surface of mitochondria and was not recruited to the midbody in dividing cells with lagging chromatin (Fig. 5 E, Left). Remarkably, neither the increase of binucleated cells or the accelerated abscission observed upon MsrB2 depletion was rescued by Tom20-ΔMTS MsrB2-GFP (Fig. 5 E, Middle and Right). Altogether, these results indicate that the localization of MsrB2 at the midbody is key for controlling cytokinetic abscission and bridge stability upon checkpoint activation.
To directly test the importance of actin polymerization in the abscission checkpoint, we recorded cells treated with low doses (20 nM) of LatrunculinA (LatA), a concentration that was chosen to not perturb ICB stability after furrow ingression in normal cells. Indeed, cells with no chromatin bridges did not become binucleated after this LatA treatment (Fig. 5F and Movie S5). In contrast, a strong increase of binucleation, due to ICB regression, was observed in LatA-treated cells that presented a chromatin bridge (Fig. 5F and Movie S6). As after MsrB2 depletion, this happened very late, 9 to 10 h after furrow ingression. Altogether, we conclude that appropriate levels of F-actin are crucial for maintaining ICB stability and thus preventing tetraploidization when the abscission checkpoint is activated by lagging chromatin.
MsrB2 Colocalizes and Genetically Interacts with the Checkpoint Components Aurora B and ANCHR.
To further establish that MsrB2 is a component of the abscission checkpoint, we analyzed the relationships between MsrB2 and two core constituents of the checkpoint: Aurora B and ANCHR (32, 38). We first observed that MsrB2 largely colocalized with active, phosphorylated Aurora B at the midbody when the checkpoint was activated by lagging chromatin (Fig. 6A). In addition, the localization of active Aurora B at the midbody did not depend on MsrB2 (SI Appendix, Fig. S4D). In previous experiments (Fig. 3D), we noticed that the overexpression of cytosolic MsrB2 by itself was sufficient to delay abscission, presumably because of the higher levels of MsrB2 compared to normal cells. Remarkably, the delay induced by MsrB2 was found to be fully dependent on Aurora B activity (Fig. 6B). Indeed, Aurora B inhibition alone accelerated abscission, as expected (32, 50), and the delay in abscission observed after MsrB2 overexpression was abolished by Aurora B inhibition (Fig. 6B). Aurora B inhibition was actually epistatic to MsrB2 overexpression (Fig. 6B). The fact that Aurora B and MsrB2 genetically interact suggests that they act in the same pathway for regulating abscission.
Fig. 6.
MsrB2 colocalizes and genetically interacts with Aurora B and ANCHR. (A) Cells expressing MsrB2-GFP (green) were stained with p-Aurora B (red) and LAP2β (gray, Inset). Merged zoom of the midbody and individual red/green channels is also displayed, as indicated. (Scale bar: 10 μm.) (B) Distribution of abscission time (Left) and mean abscission time ± SD (Right) for GFP or Cyto MsrB2-GFP–expressing cells treated with either DMSO or Aurora B inhibitor ZM447439 (N = 3 independent experiments). n = 93 to 99 cells per condition. P = 0.035 between black and green curves. P = 0.038 between black and red curves. No statistical difference between green and blue curves (KS tests). (C) Cells expressing GFP-ANCHR and MsrB2-mCherry were stained with LAP2β (gray, Inset). Merged zoom of the midbody and individual red/green channels is also displayed, as indicated. (Scale bar: 10 μm.) (D) Distribution of abscission time (Left) and mean abscission time ± SD (Middle) for control, MsrB2, ANCHR, or MsrB2+ANCHR-depleted cells (N = 3 independent experiments). n = 241 to 247 cells per condition. P < 0.001 between black and either red, green, or blue curves. No statistical difference between red, green, and blue curves (KS tests). (Right) Percentage of multinucleated cells after control, MsrB2, ANCHR, or MsrB2+ANCHR depletion (N = 3 independent experiments). n = 1,500 cells per condition. Mean ± SD. (E, Left) Representative images of control and MsrB2-depleted cells stained for endogenous ANCHR (green) and LAP2β (red). (Insets) Correspond to the indicated zoomed regions (midbodies). (Scale bars: 10 μm.) (Right) Triplicate quantification for mean ANCHR fluorescent intensity (arbitrary units) in the midbodies of control- and MsrB2-depleted cells in the presence of LAP2β-positive chromatin bridges. n = 63 to 68 midbodies per condition. (F) Model: The reduction of G-actin by MsrB2 regulates its polymerization cycle by countering the effect of oxidation by MICAL1, thereby favoring the polymerization of actin filaments in the ICB. This F-actin pool delays the recruitment of ESCRT-III at the abscission site and stabilizes the ICB when a chromatin bridge is present. ICB, intercellular bridge. A green arrow means “favors” and a red arrow means “inhibits” localization and/or activity, whether the effect is direct or indirect. NS, not significant. P values (Student t tests) are indicated.
Similarly, MsrB2 colocalized with ANCHR at the midbody when the checkpoint was activated by lagging chromatin (Fig. 6C). As previously reported (38), ANCHR depletion by itself accelerated abscission (Fig. 6D and SI Appendix, Fig. S4E). Interestingly, depleting MsrB2 did not exacerbate this phenotype (Fig. 6D). Furthermore, the percentages of binucleated cells after MsrB2 depletion, ANCHR depletion, or codepletion were identical (Fig. 6D). In addition, MsrB2 promoted ANCHR localization at the midbody in cells with lagging chromatin (Fig. 6E). Altogether, these results are consistent with MsrB2 and ANCHR functioning in the same pathway during cytokinesis and in the checkpoint.
Finally, we tested whether MsrB2 might be important for the abscission checkpoint when it is activated by other means, other than lagging chromatin, such as nuclear pore defects. Partial depletion of the nuclear pore protein Nup153 delays abscission in an Aurora B-dependent manner (37), and we confirmed that it indeed delayed abscission using time-lapse microscopy (SI Appendix, Fig. S4F). Interestingly, this delay was significantly attenuated by codepleting MsrB2 (SI Appendix, Fig. S4F). These results indicate that the abscission delay observed when the checkpoint is activated independently of lagging chromatin also depends, at least in part, on MsrB2. Altogether, we propose that MsrB2 is a component of the abscission checkpoint.
Discussion
We report here the implication of methionine sulfoxide reductases and more generally of protein reduction in cell division. In the majority of dividing cells (cells without chromatin bridges), MsrB2 acted as a negative regulator of abscission in a reductase-dependent manner (Fig. 1 A–C). F-actin intensities at the ICBs and ESCRT-III localization data (Fig. 1 D and E) are consistent with the idea that depletion of MsrB2 accelerates abscission by reducing F-actin levels in ICBs, which favors ESCRT-III localization at the abscission site. Since MsrB2 counteracted MICAL1 function during cytokinetic abscission and had opposite effects on F-actin levels in ICBs, ESCRT-III recruitment, and timing of abscission, we propose that a balance of actin oxidation by MICAL1 and actin reduction by MsrB2 regulates F-actin polymerization during the terminal steps of cytokinesis. This is evolutionarily conserved because dMical depletion (18) and dSelR depletion (this study) had also opposite effects on abscission timing in Drosophila cells.
Importantly, MsrB2 depletion led to the formation of binucleated cells in dividing cells with chromatin bridges, but not in normal dividing cells (Fig. 4). This is a phenotype also observed upon inactivation of a subset of core components of the abscission checkpoint, such as Aurora B, ALIX, and ANCHR (32, 38, 44). The colocalization, as well as the genetic interactions between MsrB2, Aurora B, and ANCHR (Fig. 6 A–E), argues that these proteins function in the same pathway. Interestingly, MsrB2 was also involved in delaying abscission in the presence of nuclear pore defects (SI Appendix, Fig. S4F), which is a stress that activates the cytokinetic checkpoint independently of chromatin bridges (37). Altogether, our results indicate that MsrB2 represents a component of the abscission checkpoint. We notice that MsrB2 and ANCHR displayed strong similarities: Their depletion accelerated abscission in normal cells (ref. 38 and Fig. 1), they strongly localized at the midbody in the presence of chromatin bridges (ref. 38 and Fig. 6C), their depletion led to binucleated cells in the presence of chromatin bridges (ref. 38 and Fig. 4), their overexpression delayed abscission in an Aurora B-dependent manner (ref. 38 and Fig. 6B), and their presence contributed to delay abscission upon activation of the checkpoint by nuclear pore defects (ref. 38 and SI Appendix, Fig. S4F). Future studies will be required to understand how MsrB2 is recruited at the midbody and how MsrB2/ANCHR together with Aurora B cooperate to prevent tetraploidy in dividing cells with chromatin bridges. Inactivation of other components of the checkpoint can lead to chromosome breaks (40, 43, 45–47, 49), indicating that the checkpoint relies on several branches and is more complex than initially proposed. Nevertheless, we never observed accelerated chromatin bridge resolution or breakage after MsrB2 depletion. Instead, ICBs regressed after ∼10 h in the presence of chromatin bridges when MsrB2 is depleted.
The role of F-actin in the stabilization of the ICB when chromatin bridges are present has long been debated (4). The low-dose LatA experiment reported in Fig. 5F constitutes direct evidence that actin filaments are indeed important for stabilizing the ICB, selectively when the abscission checkpoint is activated.
In the original report showing that Aurora B is involved in the abscission checkpoint in human cells, it was noticed that actin patches at the entry points of ICBs into the cell bodies were present in dividing cells with chromatin bridges, and this was confirmed by others (32, 46). An important question is to understand whether and how actin patches are implicated in the checkpoint, especially for the integrity of the ICB when chromatin bridges are present. Inhibition of the tyrosine kinase Src has been recently reported to lead to the disappearance of the patches, in correlation with LAP2β-positive bridge breakage and DNA damage (46), arguing that Src could play a role in the abscission checkpoint. However, it should be pointed out that actin patches are not always seen when chromatin bridges are present in ICBs and that Src inhibition has strong effects on the overall actin cytoskeleton (57). In addition, the possible role of other pools of F-actin, in particular within ICBs, has not been investigated. Here, we find that MsrB2 depletion reduced the proportion of F-actin–rich ICBs positive for chromatin bridges and decreased F-actin levels at the midbody (Fig. 5 C and D). Remarkably, this correlated with an almost systematic destabilization of the ICB, leading to binucleation in cells displaying chromatin bridges (Fig. 4C). We thus propose that the strong recruitment of MsrB2 in chromatin-containing ICBs (Fig. 5 A and B) could favor the reduction of actin monomers and thereby promote actin polymerization in the midbody area in order to stabilize the ICBs (Fig. 6F).
We did not observe a global effect on the actin cytoskeleton upon MsrB2 depletion, and cell spreading occurred normally (SI Appendix, Fig. S1B). Importantly, a version of MsrB2 that could not localize to the ICB (Tom20-ΔMTS MsrB2-GFP) was unable to rescue the cytokinetic defects (neither accelerated abscission or binucleated cells) observed after depletion of endogenous MsrB2 (Fig. 5E). We thus favor a local and direct role of MsrB2 on actin polymerization and turnover through actin oxidoreduction within the ICB, which appears critical for the stability of ICBs when chromatin bridges are present. Of note, the presence of F-actin promoted by MsrB2 would also contribute to delay abscission by retarding the recruitment of ESCRT-III at the abscission site since F-actin clearance is necessary for ESCRT-III localization at the abscission site (5, 15–17) (Fig. 6F). This actin-dependent mechanism would act in parallel to other, well-described mechanisms directly acting on the ESCRT machinery, such as CHMP4C, ANCHR, and ULK3/IST1, upon checkpoint activation (38, 40, 42, 43).
At the mechanistic level, our in vitro experiments revealed that the oxidase MICAL1 and the reductase MsrB2 control the amount of F-actin by each targeting separate actin pools. MICAL1 was found to oxidize actin filaments but not actin monomers; conversely, MsrB2 could reduce oxidized monomers but not filaments (Fig. 2). Thus, MICAL1 promotes actin filament depolymerization whereas MsrB2 recycles oxidized monomers into reduced actin molecules competent for polymerization (Fig. 2E). Interestingly, the N-terminal part of MsrB2 (amino acids 24 to 74), which precedes the catalytic domain, appeared to play a critical role for actin reduction (Fig. 2C, compare MsrB2 amino acids 24 to 182 vs. CC MsrB2). It could potentially activate the enzymatic activity of the core catalytic domain (amino acids 75 to 182) or alternatively promote actin binding to the enzyme. Crystallographic data show that the unusually extended N terminus of MsrB2 is structurally distinct from the enzymatic domain, and our results are consistent with the hypothesis that it could play a role in substrate specificity or affinity (58).
The N terminus domain of MsrB2 plays also a critical role for its subcellular localization. MsrB2 was previously reported to localize to mitochondria (54). We confirmed this localization and describe here an additional, cytosolic pool that depended on the domain preceding the core enzymatic domain (Fig. 3). We found that amino acids 1 to 23 acted as a mitochondrial targeting signal and that amino acids 24 to 74 were key for the retention of MsrB2 into the cytosol. It is conceivable that the latter region partially masks the MTS or interacts with yet-to-be-discovered cytosolic proteins that retain a fraction of MsrB2 outside mitochondria. This is not unique to MsrB2 since a number of mitochondrial proteins have been described to localize outside mitochondria as well (59). Importantly, our results argue that the cytosolic pool of MsrB2, but not the mitochondrial one, is critical for the control of abscission (Fig. 3 D and E). Of note, MsrB1 and MsrB3 were reported to be cytosolic and/or associate to the ER, and we did not find evidence that they were implicated in cytokinesis (SI Appendix, Fig. S2), suggesting that MsrBs exert nonoverlapping functions in human cells.
In conclusion, the methionine sulfoxide reductase MsrB2 represents a key component of the abscission checkpoint and prevents the formation of genetically unstable, tetraploid cells (Fig. 6F). This work also reveals a direct role of the actin cytoskeleton in the abscission checkpoint and highlights the importance of targeted actin reduction in cell physiology.
Materials and Methods
Detailed experimental procedures and data analysis used in this study are described in SI Appendix, Supplementary Methods and Information.
Cell Cultures.
The Drosophila Anillin-mCherry S2 cell line was generated and characterized in ref. 60 (kind gift from Gilles Hickson, St. Justin, Montréal, Canada) and grown in Schneider medium (Invitrogen) at 26 °C. HeLa cells cl2 from the ATCC were grown in Dulbecco’s modified Eagle’s medium (DMEM) GlutaMax (31966; Gibco, Invitrogen Life Technologies) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin (Gibco) in 5% CO2 condition at 37 °C. HeLa LAP2β-GFP + H2B-RFP and Actin-GFP + LAP2β-RFP stable cell lines have been characterized in ref. 32 (kind gifts from Daniel Gerlich, Institute of Molecular Biotechnology, Vienna, Austria) and were cultured in DMEM with 10% FBS, 0.5 μg/mL puromycin, and 0.5 mg/mL G418. For LatrunculinA experiments, Actin-GFP + LAP2β-RFP HeLa cells were treated with 20 nM LatrunculinA (Sigma-Aldrich).
Data Availability Statement.
All data associated with this paper are included in the manuscript and SI Appendix.
Supplementary Material
Acknowledgments
We thank the A.E. laboratory members, T. Wai, and I. Petropoulos for helpful discussions and reagents. We thank the imaging facilities Unit of Technology and Service Photonic BioImaging (Utechs PBI), Institut Pasteur and P.-H. Commere (Cytometry Utechs, Institut Pasteur). Work in the A.E. laboratory has been supported by Institut Pasteur, CNRS, and the Agence Nationale de la Recherche (ANR) (AbsCystem and RedoxActin). J.B. was supported by the Pasteur-Paris University (PPU) international PhD program and received a fellowship from Fondation ARC pour la Recherche sur le Cancer (DOC20180507410). H.W. has been supported by a postdoctoral fellowship from the Fondation ARC pour la Recherche sur le Cancer. T.A. has been supported by a postdoctoral fellowship from the Fondation pour la Recherche Médicale (FRM SPF201809006907) and the ANR (Cytosign).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
Data deposition: High quality versions of the figures are available on Zenodo (DOI: 10.5281/zenodo.3635406).
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1911629117/-/DCSupplemental.
References
- 1.Green R. A., Paluch E., Oegema K., Cytokinesis in animal cells. Annu. Rev. Cell Dev. Biol. 28, 29–58 (2012). [DOI] [PubMed] [Google Scholar]
- 2.D’Avino P. P., Giansanti M. G., Petronczki M., Cytokinesis in animal cells. Cold Spring Harb. Perspect. Biol. 7, a015834 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pollard T. D., Nine unanswered questions about cytokinesis. J. Cell Biol. 216, 3007–3016 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mierzwa B., Gerlich D. W., Cytokinetic abscission: Molecular mechanisms and temporal control. Dev. Cell 31, 525–538 (2014). [DOI] [PubMed] [Google Scholar]
- 5.Frémont S., Echard A., Membrane traffic in the late steps of cytokinesis. Curr. Biol. 28, R458–R470 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Dionne L. K., Wang X. J., Prekeris R., Midbody: From cellular junk to regulator of cell polarity and cell fate. Curr. Opin. Cell Biol. 35, 51–58 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crowell E. F., Gaffuri A. L., Gayraud-Morel B., Tajbakhsh S., Echard A., Engulfment of the midbody remnant after cytokinesis in mammalian cells. J. Cell Sci. 127, 3840–3851 (2014). [DOI] [PubMed] [Google Scholar]
- 8.Carlton J. G., Martin-Serrano J., Parallels between cytokinesis and retroviral budding: A role for the ESCRT machinery. Science 316, 1908–1912 (2007). [DOI] [PubMed] [Google Scholar]
- 9.Morita E., et al. , Human ESCRT and ALIX proteins interact with proteins of the midbody and function in cytokinesis. EMBO J. 26, 4215–4227 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stoten C. L., Carlton J. G., ESCRT-dependent control of membrane remodelling during cell division. Semin. Cell Dev. Biol. 74, 50–65 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Connell J. W., Lindon C., Luzio J. P., Reid E., Spastin couples microtubule severing to membrane traffic in completion of cytokinesis and secretion. Traffic 10, 42–56 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schiel J. A., et al. , Endocytic membrane fusion and buckling-induced microtubule severing mediate cell abscission. J. Cell Sci. 124, 1411–1424 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lafaurie-Janvore J., et al. , ESCRT-III assembly and cytokinetic abscission are induced by tension release in the intercellular bridge. Science 339, 1625–1629 (2013). [DOI] [PubMed] [Google Scholar]
- 14.Addi C., Bai J., Echard A., Actin, microtubule, septin and ESCRT filament remodeling during late steps of cytokinesis. Curr. Opin. Cell Biol. 50, 27–34 (2018). [DOI] [PubMed] [Google Scholar]
- 15.Terry S. J., Donà F., Osenberg P., Carlton J. G., Eggert U. S., Capping protein regulates actin dynamics during cytokinetic midbody maturation. Proc. Natl. Acad. Sci. U.S.A. 115, 2138–2143 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dambournet D., et al. , Rab35 GTPase and OCRL phosphatase remodel lipids and F-actin for successful cytokinesis. Nat. Cell Biol. 13, 981–988 (2011). [DOI] [PubMed] [Google Scholar]
- 17.Schiel J. A., et al. , FIP3-endosome-dependent formation of the secondary ingression mediates ESCRT-III recruitment during cytokinesis. Nat. Cell Biol. 14, 1068–1078 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frémont S., et al. , Oxidation of F-actin controls the terminal steps of cytokinesis. Nat. Commun. 8, 14528 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klinkert K., Echard A., Rab35 GTPase: A central regulator of phosphoinositides and F-actin in endocytic recycling and beyond. Traffic. 17, 1063–1077 (2016) [DOI] [PubMed] [Google Scholar]
- 20.Alto L. T., Terman J. R., MICALs. Curr. Biol. 28, R538–R541 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frémont S., Romet-Lemonne G., Houdusse A., Echard A., Emerging roles of MICAL family proteins–From actin oxidation to membrane trafficking during cytokinesis. J. Cell Sci. 130, 1509–1517 (2017). [DOI] [PubMed] [Google Scholar]
- 22.Manta B., Gladyshev V. N., Regulated methionine oxidation by monooxygenases. Free Radic. Biol. Med. 109, 141–155 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giridharan S. S., Caplan S., MICAL-family proteins: Complex regulators of the actin cytoskeleton. Antioxid. Redox Signal. 20, 2059–2073 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hung R. J., Pak C. W., Terman J. R., Direct redox regulation of F-actin assembly and disassembly by Mical. Science 334, 1710–1713 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee B. C., et al. , MsrB1 and MICALs regulate actin assembly and macrophage function via reversible stereoselective methionine oxidation. Mol. Cell 51, 397–404 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lundquist M. R., et al. , Redox modification of nuclear actin by MICAL-2 regulates SRF signaling. Cell 156, 563–576 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grintsevich E. E., et al. , Catastrophic disassembly of actin filaments via Mical-mediated oxidation. Nat. Commun. 8, 2183 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu H., Yesilyurt H. G., Yoon J., Terman J. R., The MICALs are a family of F-actin dismantling oxidoreductases conserved from Drosophila to humans. Sci. Rep. 8, 937 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mendoza M., et al. , A mechanism for chromosome segregation sensing by the NoCut checkpoint. Nat. Cell Biol. 11, 477–483 (2009). [DOI] [PubMed] [Google Scholar]
- 30.Norden C., et al. , The NoCut pathway links completion of cytokinesis to spindle midzone function to prevent chromosome breakage. Cell 125, 85–98 (2006). [DOI] [PubMed] [Google Scholar]
- 31.Amaral N., et al. , The Aurora-B-dependent NoCut checkpoint prevents damage of anaphase bridges after DNA replication stress. Nat. Cell Biol. 18, 516–526 (2016). [DOI] [PubMed] [Google Scholar]
- 32.Steigemann P., et al. , Aurora B-mediated abscission checkpoint protects against tetraploidization. Cell 136, 473–484 (2009). [DOI] [PubMed] [Google Scholar]
- 33.Nähse V., Christ L., Stenmark H., Campsteijn C., The abscission checkpoint: Making it to the final cut. Trends Cell Biol. 27, 1–11 (2017). [DOI] [PubMed] [Google Scholar]
- 34.Agromayor M., Martin-Serrano J., Knowing when to cut and run: Mechanisms that control cytokinetic abscission. Trends Cell Biol. 23, 433–441 (2013). [DOI] [PubMed] [Google Scholar]
- 35.Lens S. M. A., Medema R. H., Cytokinesis defects and cancer. Nat. Rev. Cancer 19, 32–45 (2019). [DOI] [PubMed] [Google Scholar]
- 36.Petsalaki E., Zachos G., Building bridges between chromosomes: Novel insights into the abscission checkpoint. Cell. Mol. Life Sci. 76, 4291–4307 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mackay D. R., Makise M., Ullman K. S., Defects in nuclear pore assembly lead to activation of an Aurora B-mediated abscission checkpoint. J. Cell Biol. 191, 923–931 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thoresen S. B., et al. , ANCHR mediates Aurora-B-dependent abscission checkpoint control through retention of VPS4. Nat. Cell Biol. 16, 550–560 (2014). [DOI] [PubMed] [Google Scholar]
- 39.Mackay D. R., Ullman K. S., ATR and a Chk1-Aurora B pathway coordinate postmitotic genome surveillance with cytokinetic abscission. Mol. Biol. Cell 26, 2217–2226 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carlton J. G., Caballe A., Agromayor M., Kloc M., Martin-Serrano J., ESCRT-III governs the Aurora B-mediated abscission checkpoint through CHMP4C. Science 336, 220–225 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Capalbo L., et al. , Coordinated regulation of the ESCRT-III component CHMP4C by the chromosomal passenger complex and centralspindlin during cytokinesis. Open Biol. 6, 160248 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Capalbo L., et al. , The chromosomal passenger complex controls the function of endosomal sorting complex required for transport-III Snf7 proteins during cytokinesis. Open Biol. 2, 120070 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Caballe A., et al. , ULK3 regulates cytokinetic abscission by phosphorylating ESCRT-III proteins. eLife 4, e06547 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Christ L., et al. , ALIX and ESCRT-I/II function as parallel ESCRT-III recruiters in cytokinetic abscission. J. Cell Biol. 212, 499–513 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petsalaki E., Zachos G., Clks 1, 2 and 4 prevent chromatin breakage by regulating the Aurora B-dependent abscission checkpoint. Nat. Commun. 7, 11451 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dandoulaki M., Petsalaki E., Sumpton D., Zanivan S., Zachos G., Src activation by Chk1 promotes actin patch formation and prevents chromatin bridge breakage in cytokinesis. J. Cell Biol. 217, 3071–3089 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bhowmick R., et al. , The RIF1-PP1 axis controls abscission timing in human cells. Curr. Biol. 29, 1232–1242.e5 (2019). [DOI] [PubMed] [Google Scholar]
- 48.Hong Y., et al. , LEM-3 is a midbody-tethered DNA nuclease that resolves chromatin bridges during late mitosis. Nat. Commun. 9, 728 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sadler J. B. A., et al. , A cancer-associated polymorphism in ESCRT-III disrupts the abscission checkpoint and promotes genome instability. Proc. Natl. Acad. Sci. U.S.A. 115, E8900–E8908 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mathieu J., et al. , Aurora B and cyclin B have opposite effects on the timing of cytokinesis abscission in Drosophila germ cells and in vertebrate somatic cells. Dev. Cell 26, 250–265 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tarrago L., Gladyshev V. N., Recharging oxidative protein repair: Catalysis by methionine sulfoxide reductases towards their amino acid, protein, and model substrates. Biochemistry (Mosc.) 77, 1097–1107 (2012). [DOI] [PubMed] [Google Scholar]
- 52.Achilli C., Ciana A., Minetti G., The discovery of methionine sulfoxide reductase enzymes: An historical account and future perspectives. Biofactors 41, 135–152 (2015). [DOI] [PubMed] [Google Scholar]
- 53.Hung R. J., Spaeth C. S., Yesilyurt H. G., Terman J. R., SelR reverses Mical-mediated oxidation of actin to regulate F-actin dynamics. Nat. Cell Biol. 15, 1445–1454 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim H. Y., Gladyshev V. N., Methionine sulfoxide reduction in mammals: Characterization of methionine-R-sulfoxide reductases. Mol. Biol. Cell 15, 1055–1064 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jung S., Hansel A., Kasperczyk H., Hoshi T., Heinemann S. H., Activity, tissue distribution and site-directed mutagenesis of a human peptide methionine sulfoxide reductase of type B: hCBS1. FEBS Lett. 527, 91–94 (2002). [DOI] [PubMed] [Google Scholar]
- 56.Kim H. Y., Gladyshev V. N., Different catalytic mechanisms in mammalian selenocysteine- and cysteine-containing methionine-R-sulfoxide reductases. PLoS Biol. 3, e375 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bursell L., et al. , Src kinase inhibition promotes the chondrocyte phenotype. Arthritis Res. Ther. 9, R105 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aachmann F. L., et al. , Structural and biochemical analysis of mammalian methionine sulfoxide reductase B2. Proteins 79, 3123–3131 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dinur-Mills M., Tal M., Pines O., Dual targeted mitochondrial proteins are characterized by lower MTS parameters and total net charge. PLoS One 3, e2161 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.El Amine N., Kechad A., Jananji S., Hickson G. R., Opposing actions of septins and Sticky on Anillin promote the transition from contractile to midbody ring. J. Cell Biol. 203, 487–504 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data associated with this paper are included in the manuscript and SI Appendix.