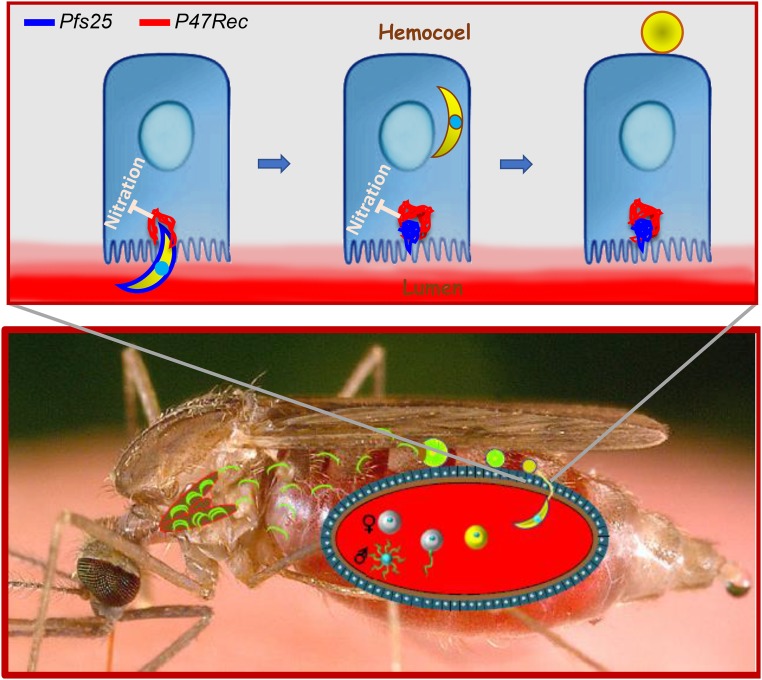
Malaria is one of the three most lethal infectious diseases. Unlike AIDS and tuberculosis, malaria is unique in that the parasite must complete a complex differentiation program in its mosquito vector for transmission to occur (1) (Fig. 1). Despite significant progress made in the last couple of decades, our mechanistic understanding of how the parasite develops in the mosquito is incomplete. A very strong bottleneck occurs in the mosquito gut (2). Out of the many hundred gametocytes that are typically ingested by the mosquito with an infected blood meal, only few parasites (single digits) succeed to form oocysts, even in high-transmission areas. As such, the parasite’s midgut stages constitute prime targets for interference with gut traversal and for curtailing transmission.
Fig. 1.
Plasmodium cycle in the mosquito and possible role of the mosquito Pfs47 receptor (P47Rec). (Lower) A mosquito acquires the Plasmodium parasite when it takes a blood meal from an infected individual. Soon after arrival in the mosquito gut, the Plasmodium sexual forms complete gametogenesis, followed by fertilization. The resulting zygotes differentiate into motile ookinetes that migrate within the blood bolus and about 24 h later cross the midgut to form oocysts on the surface of the midgut epithelium that faces the hemocoel. About 10 d later, thousands of sporozoites that develop within each oocyst are liberated into the hemocoel and invade the salivary gland. Transmission occurs when a mosquito carrying salivary gland sporozoites bites an individual. (Upper) The mosquito P47Rec cytoskeletal protein lines the microvilli of the midgut epithelium. Upon entry, the ookinete induces accumulation of P47Rec around itself and this process inhibits nitration of the parasite by an unknown mechanism. P47Rec colocalizes with Pfs25 which was shed off at the entry site, however not with the parasite during cell traversal. Inhibition of nitration protects the ookinete from attack by the mosquito immune system when it emerges on the side facing the hemocoel, leading to its differentiation into a viable oocyst (right cell). A nitrated ookinete would be destroyed upon exit from the cell (not shown).
In PNAS, Molina-Cruz et al. (3) report on the discovery of a key component of the mechanism utilized by the malaria parasite to evade the mosquito immune system. In 2013 the authors’ laboratory reported on an important finding (4) that stemmed from the analysis of a prior genetic cross between African (GB4) and Brazilian (7G8) Plasmodium falciparum clones. When fed to the African hyperimmune L3-5 Anopheles gambiae strain, the African parasites developed normally while the Brazilian parasites were completely melanized. Five P. falciparum recombinant clones from the cross developed normally in L3-5 mosquitoes, while four others were heavily melanized. Genetic mapping indicated that a 172-kb region coding for 41 genes on chromosome 13 contained the gene responsible for the fate of parasite melanization. Further sequencing, infection, and knockout (KO) experiments led to the identification of the Pfs47 gene that encodes an ookinete surface protein. Pfs47 KO parasites developed normally in L3-5 mosquitoes when their TEP1 (a key mosquito complement-like immune protein) expression was knocked down and Pfs47 KO induced robust nitration of ookinetes while traversing midgut epithelial cells. These and other observations indicated that the Pfs47 protein somehow protects the ookinete from the mosquito immune system.
That the fate of different P. falciparum strains (Brazilian and African) when they infect the same mosquito (L3-5) is so different implies that they are recognized differently, possibly by a mosquito immune component. This hypothesis was tested by the authors’ laboratory by examining how parasites from different continents (Africa, Asia, and the Americas) interact with mosquitoes from these regions (5). Significantly, parasites infected mosquitoes from the same region much better than mosquitoes from a different continent and, importantly, these differences were abolished if the host mosquito’s immune complement system was silenced. Sequence analysis of 364 worldwide isolates revealed that Pfs47 has a strong geographic differentiation and very high index of fixation (5, 6). Does Pfs47 polymorphism directly determine parasite fate in different mosquito vectors? This was tested by constructing different recombinant P. falciparum parasites, each carrying a Pfs47 haplotype from a different part of the world but otherwise having the same genetic background (5). The authors found that replacement of the Pfs47 haplotype was enough to change compatibility with different vectors. Moreover, when a parasite infects a compatible vector, disruption of the immune complement system does not ameliorate infection. Based on these findings, the authors proposed the “lock-and-key theory” (5), the key being the Pfs47 protein and the lock a mosquito protein with which Pfs47 interacts and that is different in mosquitoes from different parts of the world. Thus, in order to expand geographically (e.g., by human migration) the parasite would have to adapt to the local mosquito “lock protein” in order to be competitive and survive.
With the “key” identified (Pfs47), the next question was, What is the “lock” in which the key fits? This is the subject of the article by Molina-Cruz et al. in PNAS (3). The premise that the Pfs47 protein interacts with a cellular component of the mosquito midgut epithelium was tested by fractionating midgut proteins on a polyacrylamide gel, transferring them to a membrane, and incubating this membrane with a recombinant Pfs47 (rPfs47). Using an antibody to rPfs47 it was determined that Pfs47 interacts with a ∼31-kDa mosquito protein that was further identified by mass spectroscopy and termed AgP47Rec for An. gambiae Pfs47 receptor. Further tests determined that the parasite rPfs47 and mosquito rAgP47Rec proteins interact in vitro with high affinity. Importantly, down-regulation of AgP47Rec gene expression significantly reduced the success of P. falciparum parasite infection, confirming the functional role of the mosquito receptor in protecting ookinetes during the traversal of the mosquito epithelial cells. Cell fractionation and immunofluorescence assays showed that the AgP47Rec protein is preferentially localized in the submicrovillar cytoskeleton (the site of parasite invasion) and that the receptor protein accumulates at the site of ookinete invasion. The previous evidence that parasite infectivity is dependent on the match between parasite and mosquito geographic origins was tested by producing recombinant AgP47Rec proteins from African, Asian, and American mosquitoes and measuring their interactions with recombinant Pfs47 of parasites from Africa and America. Indeed, the proteins from the same continent interacted best, while African rPfs47 interacted less well with Asian rAgP47Rec and poorly with American rAgP47Rec. However, the American parasite rPfs47 interacted in vitro equally well with all three receptors. Phylogenetic analysis and in silico three-dimensional modeling of the P47Rec haplotypes confirmed the key–lock model and furthermore drew discussions on how ancestral gorilla Plasmodium parasites were horizontally transferred to humans and geographically expanded via bridge vector species.
In summary, Molina-Cruz et al. (3) identify the “lock” (AgP47Rec) that opens one of the mosquito’s immune doors, facilitating parasite protection from attack. This paper also clarifies one important aspect of the puzzle: the molecular basis for parasite adaptation to different vectors of the world. However, this is not the only door that protects the parasite from mosquito immunity. For instance, the refractory L3-5 An. gambiae and the susceptible G3 An. gambiae have an identical AgP47Rec sequence, yet the fate of 7G8 (Brazilian) parasites is diametrically opposite when infecting these mosquitoes. Moreover, while the African parasite rPfs47 bound similarly to the African and Asian mosquito rAgP47Rec (3), African parasite infection of an African mosquito is much higher than that of an Asian mosquito (5). A key determinant of ookinete survival in the mosquito is nitration of its surface that occurs while the parasite is traversing a mosquito epithelial cell and marks it for destruction by the mosquito immune system. It is known that one role of parasite Pfs47 is to suppress nitration by the mosquito immune system (4). How does Pfs47 function to do this? How does the interaction of Pfs47 with a midgut peripheral cytoskeletal protein (AgP47Rec) suppress cell immunity? We look forward to learning the answers to these questions.
Acknowledgments
Our research is supported by National Institute of Allergy and Infectious Diseases of the National Institutes of Health grants R01AI031478 and R01AI127405 and by a grant from the Bill & Melinda Gates Foundation (OPP1179809). Support from the Johns Hopkins Malaria Research Institute and the Bloomberg Family Foundation is gratefully acknowledged.
Footnotes
The authors declare no competing interest.
See companion article on page 2597 in issue 5 of volume 117.
References
- 1.Ghosh A., Edwards M. J., Jacobs-Lorena M., The journey of the malaria parasite in the mosquito: Hopes for the new century. Parasitol. Today (Regul. Ed.) 16, 196–201 (2000). [DOI] [PubMed] [Google Scholar]
- 2.Smith R. C., Vega-Rodríguez J., Jacobs-Lorena M., The Plasmodium bottleneck: Malaria parasite losses in the mosquito vector. Mem. Inst. Oswaldo Cruz 109, 644–661 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Molina-Cruz A., et al. , Plasmodium falciparum evades immunity of anopheline mosquitoes by interacting with a Pfs47 midgut receptor. Proc. Natl. Acad. Sci. U.S.A 117, 2597–2605 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Molina-Cruz A., et al. , The human malaria parasite Pfs47 gene mediates evasion of the mosquito immune system. Science 340, 984–987 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Molina-Cruz A., et al. , Plasmodium evasion of mosquito immunity and global malaria transmission: The lock-and-key theory. Proc. Natl. Acad. Sci. U.S.A. 112, 15178–15183 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anthony T. G., Polley S. D., Vogler A. P., Conway D. J., Evidence of non-neutral polymorphism in Plasmodium falciparum gamete surface protein genes Pfs47 and Pfs48/45. Mol. Biochem. Parasitol. 156, 117–123 (2007). [DOI] [PubMed] [Google Scholar]