Significance
The epidemic of multidrug-resistant gram-negative pathogens represents a major public health concern, highlighting the urgent need for novel antibiotics. Enzymes associated with multifaceted killing mechanisms, such as LpxH in the lipid A biosynthetic pathway in gram-negative bacteria, are particularly attractive, as their inhibition not only disrupts vital bacterial pathways, but also leads to accumulation of toxic lipid metabolites and pathway-independent cell death. Here we report the molecular structure of LpxH in complex with a sulfonyl piperazine inhibitor, structure-aided optimization of LpxH-targeting antibiotics, and the profound synergy of LpxH inhibitors with bacterial outer membrane permeability enhancers. These results set the stage for the rapid development of LpxH-targeting antibiotics.
Keywords: LpxH, lipid A, antibiotic, gram-negative bacteria
Abstract
The UDP-2,3-diacylglucosamine pyrophosphate hydrolase LpxH is an essential lipid A biosynthetic enzyme that is conserved in the majority of gram-negative bacteria. It has emerged as an attractive novel antibiotic target due to the recent discovery of an LpxH-targeting sulfonyl piperazine compound (referred to as AZ1) by AstraZeneca. However, the molecular details of AZ1 inhibition have remained unresolved, stymieing further development of this class of antibiotics. Here we report the crystal structure of Klebsiella pneumoniae LpxH in complex with AZ1. We show that AZ1 fits snugly into the L-shaped acyl chain-binding chamber of LpxH with its indoline ring situating adjacent to the active site, its sulfonyl group adopting a sharp kink, and its N-CF3–phenyl substituted piperazine group reaching out to the far side of the LpxH acyl chain-binding chamber. Intriguingly, despite the observation of a single AZ1 conformation in the crystal structure, our solution NMR investigation has revealed the presence of a second ligand conformation invisible in the crystalline state. Together, these distinct ligand conformations delineate a cryptic inhibitor envelope that expands the observed footprint of AZ1 in the LpxH-bound crystal structure and enables the design of AZ1 analogs with enhanced potency in enzymatic assays. These designed compounds display striking improvement in antibiotic activity over AZ1 against wild-type K. pneumoniae, and coadministration with outer membrane permeability enhancers profoundly sensitizes Escherichia coli to designed LpxH inhibitors. Remarkably, none of the sulfonyl piperazine compounds occupies the active site of LpxH, foretelling a straightforward path for rapid optimization of this class of antibiotics.
The emergence of multidrug- and pandrug-resistant nosocomial Gram-negative pathogens has become a major public health threat by significantly increasing patient morbidity and mortality as well as healthcare costs (1), prompting the World Health Organization (WHO) to declare a list of priority gram-negative bacteria for accelerated development of novel antimicrobial therapeutics (2).
Gram-negative bacteria are characterized by the presence of a unique outer membrane in their cell envelope. The outer membrane, consisting of phospholipid in the inner leaflet and lipid A in the outer leaflet, serves as a permeability barrier to shield gram-negative bacteria from the damage of external detergents and antibiotics. Constitutive biosynthesis of lipid A in the Raetz pathway is required for the viability of nearly all gram-negative bacteria (Fig. 1A) (3). As this pathway has never been exploited by commercial antibiotics, lipid A enzymes have emerged as attractive novel antibiotic targets (4). Indeed, potent antibiotics targeting LpxC, the second enzyme in the pathway, have been discovered (5, 6); these compounds display impressive antimicrobial activity against susceptible and multidrug-resistant gram-negative bacteria in vitro (7–19) and in animal models (11, 12, 20, 21), highlighting the therapeutic potential of disrupting lipid A biosynthesis as an effective treatment to combat drug-resistant gram-negative infections.
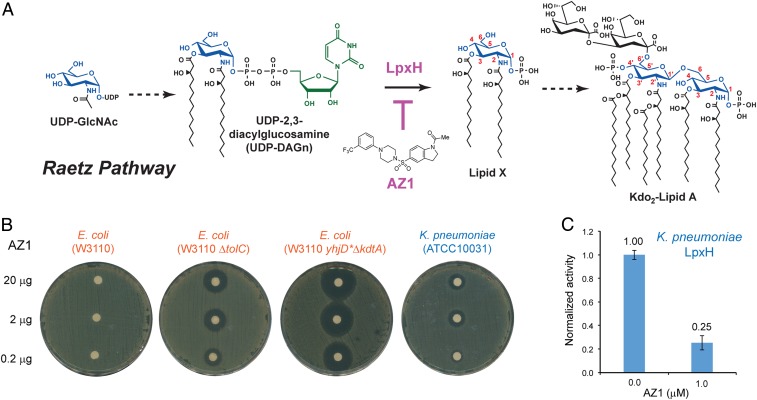
Fig. 1.
The LpxH-targeting sulfonyl piperazine antibiotic AZ1. (A) Inhibition of the UDP-diacylglucosamine pyrophosphohydrolase LpxH in the Raetz pathway of lipid A biosynthesis by AZ1. (B) Bacterial disk diffusion assays of AZ1. AZ1 is inactive against wild-type E. coli, but displays noticeable antibiotic activity against E. coli mutants with efflux pump deletion (ΔtolC) or with a compromised outer membrane (yhjD*ΔkdtA), and against wild-type K. pneumoniae. (C) Inhibition of K. pneumoniae LpxH by AZ1 at a concentration of 1.0 μM. Error bars represent SEM. n = 3.
Despite the success of LpxC inhibitors, targeting downstream lipid A enzymes, such as LpxH, might be uniquely advantageous, as inhibition of downstream lipid A enzymes not only disrupts the essential pathway of lipid A biosynthesis, but also leads to the accumulation of toxic lipid A intermediates in the bacterial inner membrane (22, 23), resulting in an independent mechanism of bacterial killing. LpxH, a calcineurin-like phosphatase (CLP), catalyzes the hydrolysis of UDP-2,3-diacyl-glucosamine (UDP-DAGn) to yield lipid X and UMP (22, 24, 25). Intriguingly, such a chemical transformation is performed by three functional orthologs in distinct gram-negative bacteria, with LpxH found in ∼70% of gram-negative bacteria and all the WHO priority gram-negative pathogens, LpxI in α-proteobacteria (26), and LpxG in Chlamydiae (27).
Recently, a small-molecule inhibitor of LpxH containing the sulfonyl piperazine scaffold (referred to as AZ1 below) was discovered through a functional high-throughput screening campaign by AstraZeneca (23). Although our structure-activity relationship analysis of the sulfonyl piperazine LpxH inhibitors has revealed a pharmacophore of AZ1 (28), the molecular details of the LpxH–AZ1 interaction have remained unknown, hindering the further development of LpxH-targeting antibiotics. Here we identify AZ1 as a potent inhibitor of Klebsiella pneumoniae LpxH, elucidate the molecular details of the K. pneumoniae LpxH-AZ1 interaction, and, based on structural and ligand dynamics information, demonstrate a strategy to enhance the potency of the sulfonyl piperazine LpxH inhibitors. Insights gained from this study set the stage for the accelerated development of LpxH inhibitors as novel antibiotics against multidrug-resistant gram-negative bacterial pathogens.
Results
AZ1 Is a Potent Inhibitor of the LpxH Enzyme from K. pneumoniae.
As a first step toward the therapeutic development of sulfonyl piperazine LpxH inhibitors, we examined the inhibition of LpxH by AZ1 and its antibiotic activity against a set of gram-negative bacteria using the bacterial disk diffusion assay. We found AZ1 does not inhibit LpxH enzymes from Haemophilus influenzae and Pseudomonas aeruginosa even at a 100 μM concentration (SI Appendix, Fig. S1), consistent with the reported lack of AZ1 activity against H. influenzae and P. aeruginosa (23). Also in line with the previous report (23), we found that AZ1 showed no measurable activity against wild-type E. coli (W3110) in disk diffusion assays, but displayed clear killing zones for an E. coli mutant with a compromised efflux pump (W3110 ΔtolC) (Fig. 1B). To evaluate whether the blockage of compound entry into the bacteria or the efflux of the compound plays a more prominent role in restricting the antibiotic activity of AZ1, we constructed an E. coli strain containing a leaky outer membrane, W3110 yhjD*ΔkdtA. Similar to the KPM121 strain reported previously (29), the outer leaflet of the outer membrane of this strain contains only free lipid A molecules lacking all core sugars and O-antigen repeats. We found that AZ1 had a much larger killing zone against this leaky E. coli strain than against the efflux pump deletion strain W3110 ΔtolC, indicating that outer membrane impermeability plays a more prominent role than the efflux machinery in limiting the access of AZ1 to its target (Fig. 1B). Excitingly, we found that AZ1 has detectable activity against wild-type K. pneumoniae in bacterial disk diffusion assays (Fig. 1B), suggesting that AZ1 may be an effective inhibitor of K. pneumoniae LpxH as well as of E. coli LpxH.
Encouraged by this observation, we cloned and purified recombinant LpxH from K. pneumoniae and found that K. pneumoniae LpxH displayed robust enzymatic activity, with a fitted KM of 68.1 ± 6.7 μM and Vmax of 554.1 ± 51.0 μmol/mg/min (SI Appendix, Fig. S2). When assayed in the presence of 100 μM UDP-DAGn, we found that AZ1 inhibited 75% of the activity of K. pneumoniae LpxH at a concentration of 1 μM (Fig. 1C), similar to that reported for E. coli LpxH (83% activity inhibition at 1 μM AZ1) (28). Importantly, although purified E. coli LpxH gradually precipitates in solution, K. pneumoniae LpxH is stable and readily yields protein crystals amenable for structural analysis.
Structure of K. pneumoniae LpxH in Complex with AZ1.
After establishing that K. pneumoniae LpxH is potently inhibited by AZ1, we probed the molecular details of its interaction with AZ1. As a reference, we first crystallized K. pneumoniae LpxH in complex with its product lipid X and determined its structure at 1.92-Å resolution (Fig. 2A and SI Appendix, Table S1). The overall structure of the K. pneumoniae LpxH-lipid X complex is similar to those of the H. influenzae and P. aeruginosa LpxH-lipid X complexes (24, 30); it features a central CLP architecture and a unique insertion cap, with a di-manganese cluster coordinated by conserved metal binding residues from the CLP signature motifs in the active site and a prominent L-shaped acyl chain-binding chamber located adjacent to the active site and between the core CLP domain and the insertion “lid.” The 1-phospho-glucosamine head group of lipid X is recognized in the active site through hydrophilic interactions, whereas the 2-N-acyl chain is buried in the hydrophobic acyl chain-binding chamber with the 3-O-acyl chain extending out to the solvent-exposed surface (Fig. 2A).
Fig. 2.
Structural basis of K. pneumoniae LpxH inhibition by AZ1. (A) Crystal structure of K. pneumoniae LpxH in complex with its product, lipid X. (B) Crystal structure of K. pneumoniae LpxH in complex with AZ1. LpxH is shown in the cartoon model, lipid X and AZ1 are shown in the stick model, and the active site Mn2+ cluster is shown in the sphere model. The core CLP architecture and the insertion lid are shown in green and coral, respectively. The hydrophobic acyl-chain binding chamber and the buried 2-N-acyl chain and solvent-exposed 3-O-acyl of lipid X are labeled in A, and surface hydrophobic lid domain residues interacting with AZ1 are labeled in B. The purple meshes represent omit (2mFo-DFc) maps of lipid X and AZ1 contoured at 1σ. (C) Overlay of AZ1 with the 2-N-acyl chain of lipid X in the K. pneumoniae LpxH complex structures. (D) Interactions of AZ1 with K. pneumoniae LpxH residues.
By including AZ1 in early steps of the K. pneumoniae LpxH purification procedure, we succeeded in obtaining the K. pneumoniae LpxH-AZ1 complex crystals that diffracted to 2.26 Å (Fig. 2B and SI Appendix, Table S1). The density of the AZ1 molecule is very well defined by the omit density map (SI Appendix, Fig. S3A); it binds exclusively in the acyl chain-binding chamber of LpxH and is nearly completely shielded from the solvent. The conformation of AZ1 in the LpxH-bound state almost overlaps entirely with the L-shaped 2-N-acyl chain of lipid X (Fig. 2C). AZ1 has a sharp kink at the sulfonyl group, which coincides with the precipitous turn of the buried 2-N-acyl chain of lipid X; the N-acetyl indoline ring of AZ1 extends toward the active site; and its N-phenyl substituted piperazine group reaches out to the far side of the L-shaped acyl chain-binding chamber away from the active site (Fig. 2C). Numerous conserved van der Waals interactions are observed among the trifluoromethyl-substituted phenyl group, the piperazine group, and the indoline ring of AZ1 and the surrounding hydrophobic residues (F82, L83, Y125, F128, I137, F141, I152, A153, and M156) from K. pneumoniae LpxH (Fig. 2D). In addition, the guanidinium sidechain of R80 extends over the indoline ring of AZ1, forming a classic parallel cation-π stacking interaction (31). Finally, hydrogen bonds are observed between the AZ1 acetyl group and the sidechain of N79 of LpxH, and between the AZ1 sulfonamide group and the sidechain of R157 and the backbone amide group of W46 of LpxH. These structural observations provide an excellent molecular interpretation of our previously reported pharmacophore model of AHHRR, of which HHRR refers to hydrophobic (H) and aromatic (R) groups from the phenyl, piperazine, and indoline moieties of AZ1 and A refers to the hydrogen bond acceptor of the acetyl carbonyl group (28). Replacement of the sulfonamide group with an amide group diminishes AZ1 activity, highlighting the importance of this group for maintaining the proper molecular geometry and hydrogen bonds (28).
It is important to note that our structural analysis of the K. pneumoniae LpxH-AZ1 complex has revealed an AZ1-binding pose opposite to the docking model proposed by Bohl et al. (32) based on the structural analysis of a mutant form of E. coli LpxH, in which the trifluoromethyl phenyl group is located close to the active site.
To investigate whether AZ1 might adopt a different binding orientation when bound to E. coli LpxH, we synthesized a photo-crosslinking probe of AZ1 by inserting a diazirine group between the trifluoromethyl group and its attached phenyl ring (JH-LPH-13; SI Appendix, Fig. S4A). This design was based on our previous observation that the trifluoromethyl group can be replaced with bulky hydrophobic groups (28). The diazirine group is a proximity-labeling reagent, which on UV activation forms a reactive carbene species that crosslinks with nearby residues from the target protein (33). We found that JH-LPH-13 potently inhibited E. coli LpxH at a concentration of 1 μM (∼80% inhibition; SI Appendix, Fig. S4B), confirming that introduction of the diazirine group of JH-LPH-13 did not interfere with the compound binding to E. coli LpxH. Our LC-MS/MS analysis of the UV-crosslinking product of JH-LPH-13 with E. coli LpxH identified F141 (F141 in K. pneumoniae LpxH) as the predominant site of modification (F141mod), with two F141mod-containing peptides accounting for ∼83% of the total ion current of all modified peptide fragments (75.9% for peptide 1 and 7.1% for peptide 2). Following F141, residue L137 (I137 in K. pneumoniae LpxH) was also modified (L137mod), with the L137mod-containing peptide accounting for 7.4% of the total ion current of modified peptides (SI Appendix, Fig. S4C). These three peptide fragments, with modifications at either F141 or L137 in E. coli LpxH, accounted for >90% of all ion currents for the modified peptides, confirming that the trifluoromethyl phenyl group of AZ1 is indeed located away from the E. coli LpxH active site (SI Appendix, Fig. S4D), an observation consistent with our structurally observed binding mode of AZ1 in the K. pneumoniae LpxH-bound complex.
Lead Optimization from the Cryptic Ligand Envelope.
In our previous structural analysis of LpxC inhibitors, we found substantial ligand dynamics in the inhibitor-bound LpxC complex in solution, even though the corresponding crystal structure reveals a single ligand-bound conformation (34). These multiple ligand conformations in solution together delineate a cryptic ligand envelope that expands the ligand footprint observed in the crystalline state and has enabled the design of novel LpxC inhibitors with significantly improved binding affinity and antibiotic activity compared with the parent compound (34). We carried out a parallel solution-phase NMR analysis of AZ1 bound to K. pneumoniae LpxH. Intriguingly, we similarly found that despite the observation of a single ligand conformation in the crystal structure of the K. pneumoniae LpxH-AZ1 complex determined at 2.26-Å resolution, the solution 19F NMR spectrum readily revealed the presence of two 19F signals of the trifluoromethyl group of AZ1 when it was bound to K. pneumoniae LpxH (Fig. 3 A, Lower). Fitting of the two signals yielded a major conformation in 84.5% of the population and a minor conformation in 15.5%. This is in sharp contrast to the free compound, in which a single 19F signal was observed (Fig. 3 A, Upper). Taken together, these observations support the notion that AZ1 binds to K. pneumoniae LpxH in two distinct conformations in solution.
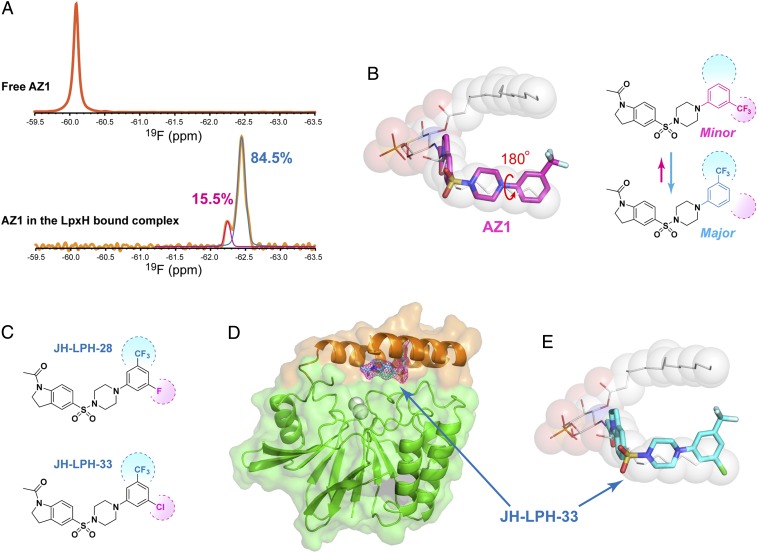
Fig. 3.
Lead optimization from the expanded, cryptic inhibitor envelope in solution. (A) Solution 19F NMR measurements reveal a dynamic equilibrium of two conformational states of AZ1 bound to K. pneumoniae LpxH. Shown are spectra of AZ1 in the apo state (Top) and in the K. pneumoniae LpxH-bound state (Bottom). (B) Schematic illustration of two distinct conformational states of AZ1 in the K. pneumoniae LpxH complex, which together delineate an expanded inhibitor envelope. (C) Sulfonyl piperazine compounds JH-LPH-28 and JH-LPH-33, designed to achieve full interactions with the expanded, cryptic inhibitor envelope. (D) Crystal structure of the K. pneumoniae LpxH/JH-LPH-33 complex validates the predicted binding mode of JH-LPH-33. K. pneumoniae LpxH is shown in the cartoon model, JH-LPH-33 in the stick model, and the active site Mn2+ cluster in the sphere model. The core CLP architecture and the insertion lid are shown in green and coral, respectively. The purple mesh represents the omit (2mFo-DFc) map of JH-LPH-33 contoured at 1σ. (E) Overlay of JH-LPH-33 with lipid X in the K. pneumoniae LpxH complexes, illustrating occupancy of the terminal methyl position of the 2-N-acyl chain of lipid X by the chloro substitution of JH-LPH-33.
AZ1 contains a rotatable bond between the trifluoromethyl-substituted phenyl ring and the piperazine group. As ring flipping of sidechains of phenylalanine and tyrosine residues has been extensively reported in studies of protein dynamics (35), we entertained the notion that the two different fluorine signals might represent two distinct rotameric ring conformations of the trifluoromethyl phenyl group, with the major rotamer observed in the crystal structure with the trifluoromethyl group pointing up toward the protein surface and the minor rotamer with the trifluoromethyl phenyl ring flipping down (Fig. 3B). In the latter case, the trifluoromethyl group would penetrate deeply into the acyl chain-binding chamber, occupying a cavity in which the terminal methyl group of the 2-N–linked acyl chain resides. Together, these two rotameric conformations of the AZ1-binding mode would delineate an expanded, cryptic inhibitor envelope that is invisible in the crystal structure but readily detectable in solution. If that were the case, then we reasoned that AZ1 should be able to tolerate an additional substitution at the meta-position of the phenyl ring.
Furthermore, a smaller hydrophobic group than the trifluoromethyl group might be preferred, as the acyl-chain terminal methyl pocket is occupied in only a minor population by the trifluoromethyl group (pink in Fig. 3B). To test this hypothesis and fully exploit the interactions with the cryptic ligand envelope, we synthesized two AZ1 derivatives, JH-LPH-28 and JH-LPH-33 (Fig. 3C), that contain either a fluoro substitution or a chloro substitution at the meta-position of the trifluoromethyl phenyl ring. Our crystal structure analysis of JH-LPH-33 in complex with K. pneumoniae LpxH at 2.25-Å resolution indeed confirmed our predicted binding mode, with the chloro group filling in the hydrophobic pocket occupied by the terminal methyl group of the 2-N–linked acyl chain shared by the substrate UDP-DAGn and product lipid X, and with the trifluoromethyl group pointing upward as seen for AZ1 in the crystal structure (Fig. 3 D and E and SI Appendix, Table S1 and Fig. S3B).
Designed Sulfonyl Piperazine Analogs Show Enhanced LpxH Inhibition and Antibiotic Activity.
In an exciting finding, both JH-LPH-28 and JH-LPH-33 inhibited LpxH more potently than AZ1. The fluoro-substituted JH-LPH-28 displayed IC50 values of 0.11 μM against K. pneumoniae LpxH and 0.083 μM against E. coli LpxH; these values were 3.3-fold and 1.7-fold lower than the corresponding IC50 values of AZ1, respectively: 0.36 μM against K. pneumoniae LpxH and 0.14 μM against E. coli LpxH (Fig. 4 A and B). The chloro-substituted JH-LPH-33 was even more active, displaying IC50 values of 0.026 μM against K. pneumoniae LpxH and 0.046 μM against E. coli LpxH and improving the potency of AZ1 by 13.8-fold and 3.0-fold, respectively (Fig. 4 A and B).
Fig. 4.
Optimized sulfonyl piperazine compounds JH-LPH-28 and JH-LPH-33 display superior LpxH inhibition and antibiotic activity over AZ1. (A) IC50 values of AZ1, JH-LPH-28, and JH-LPH-33 against K. pneumoniae LpxH. (B) IC50 values of AZ1, JH-LPH-28 and JH-LPH-33 against E. coli LpxH. (C) MIC values of AZ1, JH-LPH-28, and JH-LPH-33 against wild-type K. pneumoniae. (D) MIC values of AZ1, JH-LPH-28, and JH-LPH-33 against wild-type E. coli in the presence of 10 μg/mL PMBN. Error bars represent SEM (n = 3).
To elucidate the mode of inhibition of JH-LPH-33, we measured its dose-dependent inhibition of K. pneumoniae LpxH at two different substrate concentrations: 50 and 250 μM of UDP-DAGn. Increasing the UDP-DAGn concentration from 50 to 250 μM shifted the mean IC50 value from 0.018 ± 0.001 μM to 0.043 ± 0.002 μM (SI Appendix, Fig. S5). Such a ∼2.4-fold increase in the IC50 value is consistent with competitive inhibition (Eq. 3), which predicts a 2.7-fold increase in the IC50 value, for a mean KM value of 68.1 ± 6.7 μM. IC50 curve fitting using the competitive binding model yielded a mean KI value of 0.0100 ± 0.0004 μM.
Accompanying the significant enhancement of in vitro inhibition of LpxH, JH-LPH-28 and JH-LPH-33 showed strikingly improved antibiotic activities. When tested against wild-type K. pneumoniae (ATCC 10031) in cell culture, AZ1 did not suppress bacterial growth at 64 μg/mL; in contrast, the fluoro analog JH-LPH-28 potently inhibited bacterial growth at 2.8 μg/mL, and the chloro analog JH-LPH-33 was even more effective, displaying a minimum inhibitory concentration (MIC) value of 1.6 μg/mL (Fig. 4C).
Somewhat surprisingly, despite the significant antibiotic activity of JH-LPH-28 and JH-LPH-33 against wild-type K. pneumoniae and their similar inhibition of E. coli LpxH at nanomolar concentrations in vitro, neither compound displayed measurable antibiotic activity against wild-type E. coli (e.g., the MIC of JH-LPH-33 alone was >64 μg/mL against E. coli; SI Appendix, Fig. S6 A and B), suggesting that E. coli might be more effective at either blocking the cellular entry of these compounds or at secreting the compounds out through efflux pumps. It has been previously reported that AZ1 is inactive for wild-type E. coli but displays notable antibiotic activity against efflux-deficient (ΔtolC) E. coli strains (23).
Interestingly, although our own disk diffusion assays confirmed the role of efflux pumps (Fig. 1B), they also revealed that increasing the membrane permeability by compromising the outer membrane of E. coli (i.e., yhjD*ΔkdtA) has a much more pronounced effect on the antibiotic activity of AZ1 than deleting the efflux pump (i.e., ΔtolC) (Fig. 1B). This observation prompted us to examine whether PMBN (36), a polymyxin B nonapeptide that enhances outer membrane permeability in gram-negative bacteria, could render wild-type E. coli susceptible to AZ1, JH-LPH-28, and JH-LPH-33. We found that PMBN itself did not exhibit significant antibiotic activity at concentrations up to 40 μg/mL (SI Appendix, Fig. S6A), but coadministration of PMBN at 10 μg/mL profoundly sensitized the wild-type E. coli strain (W3110) to sulfonyl piperazine LpxH inhibitors. AZ1, which did not display any detectable effect toward wild-type E. coli by itself, now showed robust antibiotic activity, with an MIC value of 2.3 μg/mL toward E. coli (Fig. 4D). Likewise, both JH-LPH-28 and JH-LPH-33 displayed outstanding antibiotic activity, with MIC values of 0.83 μg/mL and 0.66 μg/mL, respectively (Fig. 4D). Taken together, these observations provide strong evidence that coadministration of LpxH inhibitors with effective membrane permeability enhancers is an efficient solution for clinical applications of LpxH-targeting antibiotics against multidrug-resistant gram-negative human pathogens.
Discussion
The recent discovery of the sulfonyl piperazine LpxH inhibitor AZ1 through a high-throughput screening campaign (23) has established the feasibility of developing small-molecule antibiotics targeting the essential lipid A enzyme LpxH in the Raetz pathway to combat multidrug-resistant gram-negative bacterial infections. Despite advancements in the structural analysis of LpxH enzymes from H. influenzae (24), P. aeruginosa (30), and E. coli (32), along with the establishment of a pharmacophore model of sulfonyl piperazine inhibitors (28), the molecular details of the LpxH–AZ1 interaction have remained elusive until now. Our structural analysis of the K. pneumoniae LpxH-AZ1 complex together with the photo-crosslinking experiments have provided unambiguous evidence for the binding orientation of AZ1, clarifying the confusion engendered by a previous docking model (32).
Furthermore, although our crystal structure analysis of the K. pneumoniae LpxH-AZ1 complex reveals a single compound conformation, our solution NMR study readily unveils the existence of two distinct compound conformations that together delineate an expanded, cryptic inhibitor envelope invisible in the crystalline state. Using such information, we have successfully designed more potent AZ1 analogs—JH-LPH-28 and JH-LPH-33—that display not only enhanced LpxH inhibition over AZ1, but also strikingly improved antibiotic activity against wild-type K. pneumoniae. Although we cannot rule out the possibility that the observed minor state ligand signal could arise from an alternative protein conformation (e.g., an open state of the lid domain), our success in designing more potent AZ1 analogs and our structural validation of the predicted binding pose of JH-LPH-33 with a di-substituted phenyl ring strongly suggest that the minor ligand state signal more likely arises from ligand dynamics rather than from a conformational switch of the protein. Intriguingly, our structural analysis has shown that none of the functional groups of AZ1 or its derivatives described here reaches the active site of LpxH, suggesting that a fragment-based discovery approach (37) to extend AZ1 to the LpxH active site should readily enhance the antibiotic activity of sulfonyl piperazine inhibitors and bring these compounds closer to clinical applications.
The discovery of AZ1 required the use of efflux pump-deficient (ΔtolC) E. coli strains (23), indicating that AZ1 is actively transported outside of the bacteria. Here we have identified the bacterial outer membrane as another major deterrent for LpxH-targeting antibiotics. We found that coapplication of the outer membrane permeability enhancer PMBN potentiated the antibiotic activity of JH-LPH-33 in E. coli (Fig. 4D and SI Appendix, Fig. S6A), as did pentamidine (SI Appendix, Fig. S6B), a recently discovered bacterial outer membrane permeabilizer (38). These observations highlight the therapeutic potential of LpxH-targeting antibiotics in combination with outer membrane permeabilizers for treating multidrug-resistant gram-negative bacterial infections.
Materials and Methods
Chemical Synthesis.
Synthesis of the photo-crosslinking probe 9 (JH-LPH-13).
To establish the binding mode of AZ1, we synthesized a trifluoromethylaryl diazirine probe 9 (JH-LPH-13) (SI Appendix, Scheme S1A; details provided in SI Appendix, Materials and Methods) (39). In brief, trifluoroacetylation of the known aryl bromide 1 (40) provided the trifluoromethyl ketone 2. Compound 2 was converted to the corresponding oxime 3, which was activated by TsCl to afford the tosylate 4. Compound 4 was treated with liquid ammonia to give the diaziridine 5. Oxidation of 5 with freshly prepared Ag2O provided the diazirine 6. Boc-deprotection of 6 and subsequent coupling of the resulting piperazine 7 with 1-acetyl-5-indolinesulfonyl chloride (8) successfully afforded 9 (JH-LPH-13).
Synthesis of AZ1 and analogs JH-LPH-28 and JH-LPH-33.
AZ1 was synthesized as described previously (28). The AZ1 analogs JH-LPH-28 and JH-LPH-33 were prepared by coupling known substituted phenyl piperazines with 1-acetyl-5-indolinesulfonyl chloride (SI Appendix, Scheme S1B; details provided in SI Appendix, Materials and Methods) (28).
Construction of E. coli Mutant Strains.
To construct the E. coli mutant strain with a compromised outer membrane, we took advantage of the R134C point mutation in the yhjD gene that suppresses the lethal phenotype of a kdo-deficient strain (29). Since yhjD is not an essential gene, it was first replaced with a Cam-SacB cassette derived from plasmid pYA4373 (41) in the wild-type E. coli strain (W3110) using the one-step chromosomal gene inactivation protocol with the helper plasmid pKD46 (42). The Cam-SacB cassette was then replaced with a yhjD-R134C mutated DNA fragment with the same strategy, using sucrose as the counterselection to yield strain W3110 yhjD*. The chromosomal deletion of the essential gene kdtA was first constructed in strain DY330 carrying a plasmid overexpressing MsbA as reported previously (43), except that the kdtA gene was replaced with a removable kanamycin cassette used in constructing the E. coli Keio collection, a single-gene knockout mutant library (44). After the transfer of ΔkdtA::kan into W3110 yhjD* by P1 transduction, the kanamycin cassette was removed using the helper plasmid pCP20 (42), and the mutated strain was designated W3110 yhjD*ΔkdtA. The mutation in yhjD and deletion of kdtA were verified by PCR and sequencing.
To construct W3110 ΔtolC strain, ΔtolC::kan mutation in tolC deletion mutant strain (JW5503-1) from the Keio collection was transferred to the wild-type W3110 strain via P1 transduction. The kanamycin cassette was removed similarly as described above, and the deletion of tolC was verified by PCR and sequencing.
Bacterial Disk Diffusion Assays.
Bacterial strains of wild-type K. pneumoniae (ATCC 10031), wild-type E. coli (W3110), E. coli with the efflux pump deletion (W3110 ΔtolC), and E. coli with a leaky outer membrane (W3110 yhjD*ΔkdtA) were used. Each strain was grown overnight in Lysogeny broth (LB) medium, and 1 μL of the overnight culture was diluted to 100 μL. The diluted culture was spread on the LB plates with sterile cotton-tipped wooden applicators. Small filter paper disks were placed on the plate and different amounts of AZ1 (20, 2, and 0.2 μg) were spotted onto the disks. After incubation overnight at 37 °C, the plates were imaged for the zone of inhibition.
MIC Assay.
The MIC assay protocol was adapted from the broth microdilution methods described by the National Committee for Clinical Laboratory Standards (45) using 96-well plates. Overnight bacterial cultures were prediluted to an OD600 of 0.6 and further diluted 100-fold into cation-adjusted Mueller–Hinton medium. The cells were incubated at 37 °C for 22 h in the presence of different concentrations of inhibitors and 7% DMSO. MIC values were reported as the lowest compound concentration that inhibited bacterial growth. The standard checkerboard assays were conducted to examine the synergy of PMBN and pentamidine with JH-LPH-33 following 1.25- to 2-fold serial dilutions of individual compounds against E. coli W3110.
Construction and Purification of GB1-LpxH-His10 for Enzymatic Assays.
Our preliminary investigations showed that E. coli LpxH exhibited poor stability by itself; therefore, we constructed GB1-fused LpxH constructs with enhanced stability (46) for enzymatic assays. In brief, genes encoding E. coli, K. pneumoniae, H. influenzae, and P. aeruginosa LpxH enzymes were cloned into a modified pET30 vector (EMD Millipore) containing GB1 between the NdeI and BamHI sites and a His10 tag after the BamHI site. The DNA fragments encoding different LpxH orthologs were inserted immediately after GB1 with the infusion cloning method, so the final expression constructs contained GB1-LpxH-His10. The constructs were verified by DNA sequencing and were used to transform BL21 STAR (DE3) competent E. coli cells (Thermo Fisher Scientific) for expression of LpxH.
Expression and purification of recombinant LpxH were conducted as described previously (28). In brief, cells were grown in the LB medium at 37 °C until an OD600 of 0.5, induced with 1 mM isopropyl-β-D-thiogalactopyranoside (IPTG) for 3 h, and then harvested by centrifugation. All the purification steps were carried out at 4 °C. Bacterial cell pellets from 8 L of induced culture were resuspended and lysed in 120 mL of the lysis buffer containing 20 mM Hepes (pH 8.0) and 200 mM NaCl using a French press. Cell debris were removed by centrifugation at 10,000 × g for 40 min. To the supernatant, n-dodecyl- β-D-maltopyranoside (DDM) was added to reach a final concentration of 1.5% (wt/vol; 29 mM). After 2 h of incubation, the insoluble fraction was removed by ultra-centrifugation at 100,000 × g for 1 h.
Supernatant from the centrifugation was diluted to a final volume of 240 mL with the lysis buffer and added to a column containing 20 mL of HisPur Ni-NTA resin (Thermo Fisher Scientific) preequilibrated with 100 mL of the purification buffer containing 20 mM Hepes (pH 8.0), 200 mM NaCl, and 0.0174% (wt/vol) 0.34 mM DDM. The column was washed with 250 mL of the purification buffer containing 50 mM imidazole, and LpxH was eluted with 150 mL of the purification buffer containing 300 mM imidazole. The eluted protein sample was concentrated and purified to homogeneity with size-exclusion chromatography (Superdex 200; GE Healthcare Life Sciences) in the purification buffer, except that the DDM concentration was reduced to 0.013% (wt/vol; 0.25 mM). The concentrated stock enzyme solution (1 to 2 mg/mL) was mixed with glycerol (50% vol/vol final concentration), aliquoted, and flash-frozen with liquid nitrogen for storage at −80 °C.
LpxE-Coupled LpxH Activity Assay.
The LpxE-coupled LpxH activity assay was conducted as described previously (28). In brief, two reaction mixtures were prepared containing 20 mM Tris⋅HCl pH 8.0, 0.5 mg/mL BSA, 0.02% Triton X-100, 1 mM MnCl2, 1 mM DTT, and 10% DMSO, with either 100 μM substrate (UDP-DAGn) or LpxH (5 ng/mL of E. coli LpxH, 10 ng/mL of K. pneumoniae LpxH, 10 ng/mL of H. influenzae LpxH, or 50 ng/mL P. aeruginosa LpxH) and the desired concentration of the inhibitor. The reaction mixtures were preincubated at 37 °C for 10 min, then an equal volume of the LpxH mixture was added to the substrate mixture to start the reaction at 37 °C. At the desired reaction time points, an aliquot of 20 μL of reaction mixture was removed and added to a well in 96-well half-area plate containing 5 mM EDTA (final concentration) to quench the LpxH reaction. Purified Aquifex aeolicus LpxE was then added to a final concentration of 5 μg/mL. The plate was incubated at 37 °C for 30 min, followed by the addition of formic acid to a final concentration of 3.75 M to quench the reaction. The malachite green reagent (Sigma Aldrich; catalog no. MAK307) was added with a fivefold dilution, and absorbance at 620 nm was measured after a 30-min incubation at room temperature. The IC50 value was extracted from fitting of the dose–response curve of vi/v0 = 1/(1 + [I]/IC50) [Eq. 1]. The UDP-DAGn concentration was varied from 7 to 150 μM to determine the mean KM value (68.1 ± 6.7 μM) of K. pneumoniae LpxH using the steady-state Michaelis-Menten equation: v = vmax*[S]/(KM + [S]) [Eq. 2].
To determine the mode of compound inhibition, additional IC50 assays for K. pneumoniae LpxH were conducted with 50 μM and 250 μM UDP-DAGn. The observed increase in mean IC50 value from 0.018 ± 0.001 μM to 0.043 ± 0.002 μM is consistent with competitive inhibition: IC50 = KI*(1 + [S]/KM) [Eq. 3].
Construction and Purification of K. pneumoniae LpxH-TEV-His10 for Structural Analysis.
For crystallographic studies, K. pneumoniae LpxH was cloned into a modified pET21b (Novagen/Millipore Sigma) vector, yielding the LpxH fusion protein with a C-terminal TEV protease site (ENLYFQGS) and His10 tag, similar to the previously reported H. influenzae LpxH construct (24, 25). The K. pneumoniae LpxH construct used for crystallization of lipid X-bound and AZ1-bound complexes contained a valine residue immediately after the start codon due to a cloning artifact, which was eliminated through mutagenesis in the construct used for crystallization of the JH-LPH-33–bound complex.
Vector-transformed BL21 STAR (DE3) E. coli cells (Thermo Fisher Scientific) were grown in LB medium at 37 °C to an OD600 of 0.5, induced with 1 mM IPTG for an additional 5 h at 30 °C, and then harvested by centrifugation. Protein purification was carried out at 4 °C. Cell pellets from 8 L of induced culture were resuspended and lysed in 120 mL of the lysis buffer containing 50 mM phosphate-citrate, 20 mM MES (pH 6.0), 600 mM NaCl, 10% sucrose, 5 mM 2-mercaptoethanol, 10 mM imidazole, and 0.1% Triton X-100 using a French press. Cell debris was removed by centrifugation at 10,000 × g for 40 min, and the supernatant was loaded onto a column containing 20 mL of HisPur Ni-NTA resin (Thermo Fisher Scientific) was preequilibrated with 100 mL of the lysis buffer. After extensive column washing with the purification buffer containing 20 mM phosphate-citrate, 20 mM MES (pH 6.0), 300 mM NaCl, 5% glycerol, 5 mM 2-mercaptoethanol, and 40 mM imidazole, LpxH was eluted from the column with a stepwise increase in imidazole concentration from 40 to 400 mM in the purification buffer. The sample was concentrated for further purification with size-exclusion chromatography (Superdex 200; GE Healthcare Life Sciences) in the FPLC buffer containing 20 mM MES (pH 6.0), 800 mM NaCl, 1 mM DTT, and 5% glycerol. The peak fractions containing K. pneumoniae LpxH were buffer-exchanged into a buffer containing 20 mM MES (pH 6.0), 200 mM NaCl, 1 mM DTT, and 5% glycerol and then concentrated to 8 mg/mL for crystallization.
Crystallization of the K. pneumoniae LpxH-Lipid X Complex.
Protein crystals were grown using the sitting-drop vapor diffusion method at 20 °C. Each drop was prepared by mixing 1 μL of the protein solution with 1 μL of the reservoir solution containing 200 mM calcium chloride dihydrate, 100 mM Hepes (pH 7.0), and 33% PEG 400. Diffraction quality protein crystals were typically harvested after 2 wk and soaked with the reservoir solution also containing 20% glycerol and 100 μM MnCl2 for cryoprotection.
Cocrystallization of K. pneumoniae LpxH with Sulfonyl Piperazine Antibiotics AZ1 and JH-LPH-33.
To obtain K. pneumoniae LpxH cocrystals with sulfonyl piperazine antibiotics, individual compounds were incubated with K. pneumoniae LpxH after the Ni-NTA affinity column purification step for 30 min. The complex sample was then purified to homogeneity by size-exclusion chromatography (Superdex 200; GE Healthcare) as described above and concentrated to 4 to 8 mg/mL for cocrystallization. An excess amount of compound in DMSO (2 molar equivalents to K. pneumoniae LpxH) was added before setting up crystal trays, yielding a protein-compound solution containing 20 mM MES (pH 6.0), 200 mM NaCl, 1 mM DTT, 5% glycerol, and 1.5% DMSO. Diffraction-quality crystals were obtained using the sitting-drop vapor diffusion method at 20 °C by mixing 1 μL of the protein solution with 1 μL of the reservoir solution containing 50 mM sodium chloride, 20 mM magnesium chloride hexahydrate, 100 mM sodium citrate (pH 6.0), and 22% PEG 400. The LpxH-compound cocrystals were typically harvested after 2 wk and were soaked with the reservoir solution containing 20% glycerol and 100 μM MnCl2 for cryoprotection.
Structural Analysis of K. pneumoniae LpxH Complexes with Lipid X, AZ1, and JH-LPH-33.
Datasets of the K. pneumoniae LpxH complexes with lipid X, AZ1, and JH-LPH-33 were collected at the Northeastern Collaborative Access Team (NECAT) 24-ID-C and 24-ID-E beamlines at the Advanced Photon Source at Argonne National Laboratory. The X-ray diffraction data were processed using XDS (47). The phase data for the crystal structures of the K. pneumoniae LpxH complexes were obtained by molecular replacement with the PHASER module in the PHENIX suite (48) using PDB ID code 5K8K as the search model. Restraints of the inhibitors were generated using eLBOW (49) and edited manually. Iterative model building and refinement were carried out using Coot (50) and PHENIX (48). The 2mFo-DFc omit maps were generated using PHENIX (48).
Photo-Crosslinking Experiment for Identification of Binding Mode.
Purified E. coli LpxH at a concentration of 50 μM in 20 mM Hepes (pH 8.0), 200 mM NaCl, 0.2 mM DTM, and 2% DMSO was incubated with 100 μM of JH-LPH-13, the AZ1 photo-crosslinking probe, for 30 min at 4 °C. After incubation, the reaction mixture was illuminated at 365 nm at 4 °C for 10 min. The crosslinked sample was then trypsinized, and the resulting peptide fragments were analyzed by LC-MS/MS to identify residues modified by the photo-crosslinking probe JH-LPH-13.
Solution NMR Measurements.
Purified K. pneumoniae LpxH at 20 μM concentration in a buffer containing 800 mM NaCl, 5% glycerol, and 20 mM MES (pH 6.0) was incubated with a fivefold molar ratio of AZ1 with a final DMSO concentration of 10% for 2 h at 4 °C. The sample was then concentrated and buffer-exchanged 200-fold with the aforementioned buffer containing 5% DMSO. The sample was then passed through a PD-10 desalting column (GE) in the same buffer to further remove any unbound AZ1. The resulting protein complex sample was concentrated to 50 μM and loaded into a 4-mm NMR tube, which was inserted into a 5 mm NMR tube with D2O added between the two NMR tubes as the lock. For the AZ1 compound control, 100 μM AZ1 in DMSO was loaded into a 5-mm NMR tube. 19F spectra were collected at 25 °C on a 500-MHz Bruker spectrometer equipped with a 19F cryoprobe. NMR data were processed using NMRPipe (51), and figures were generated using iNMR (www.inmr.net).
Data Availability.
The X-ray structure coordinates of the K. pneumoniae LpxH complexes with lipid X, AZ1, and JH-LPH-33 have been deposited in the Protein Data Bank, www.wwpdb.org (PDB ID codes 6PH9, 6PIB, and 6PJ3, respectively).
Supplementary Material
Acknowledgments
This work was supported in part by grants from the National Institute of General Medical Sciences (GM115355), the National Institute of Allergy and Infectious Diseases (AI139216), and the Bridge Fund from Duke University School of Medicine. X-ray diffraction data were collected at the Northeastern Collaborative Access Team beamlines 24-ID-C and 24-ID-E, which are funded by the National Institute of General Medical Sciences (Grant P30 GM124165). The Pilatus 6M detector on the 24-ID-C beam line is funded by the NIH Office of Research Infrastructure Programs (Hight-End Instrumentation Grant S10 RR029205). This research used resources of the Advanced Photon Source, a US Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by the Argonne National Laboratory under Contract DE-AC02-06CH11357.
Footnotes
Competing interest statement: P.Z. and J.H. are inventors of a provisional patent covering the designed LpxH inhibitors.
This article is a PNAS Direct Submission.
Data deposition: The X-ray structure coordinates of the K. pneumoniae LpxH complexes with lipid X, AZ1, and JH-LPH-33 have been deposited in the Protein Data Bank, www.wwpdb.org (PDB ID codes 6PH9, 6PIB, and 6PJ3).
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1912876117/-/DCSupplemental.
References
- 1.Boucher H. W., et al. , Bad bugs, no drugs: No ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 48, 1–12 (2009). [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization , Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics (2017). https://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-ET_NM_WHO.pdf. Accessed 1 June 2019.
- 3.Raetz C. R. H., Whitfield C., Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 71, 635–700 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou P., Zhao J., Structure, inhibition, and regulation of essential lipid A enzymes. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1862, 1424–1438 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barb A. W., Zhou P., Mechanism and inhibition of LpxC: An essential zinc-dependent deacetylase of bacterial lipid A synthesis. Curr. Pharm. Biotechnol. 9, 9–15 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang J., Zhang L., Li X., Xu W., UDP-3-O-(R-3-hydroxymyristoyl)-N-acetylglucosamine deacetylase (LpxC) inhibitors: A new class of antibacterial agents. Curr. Med. Chem. 19, 2038–2050 (2012). [DOI] [PubMed] [Google Scholar]
- 7.Lee C. J., et al. , Species-specific and inhibitor-dependent conformations of LpxC: Implications for antibiotic design. Chem. Biol. 18, 38–47 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee C. J., et al. , Structural basis of the promiscuous inhibitor susceptibility of Escherichia coli LpxC. ACS Chem. Biol. 9, 237–246 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liang X., et al. , Syntheses, structures and antibiotic activities of LpxC inhibitors based on the diacetylene scaffold. Bioorg. Med. Chem. 19, 852–860 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liang X., Lee C. J., Zhao J., Toone E. J., Zhou P., Synthesis, structure, and antibiotic activity of aryl-substituted LpxC inhibitors. J. Med. Chem. 56, 6954–6966 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Onishi H. R., et al. , Antibacterial agents that inhibit lipid A biosynthesis. Science 274, 980–982 (1996). [DOI] [PubMed] [Google Scholar]
- 12.Brown M. F., et al. , Potent inhibitors of LpxC for the treatment of Gram-negative infections. J. Med. Chem. 55, 914–923 (2012). [DOI] [PubMed] [Google Scholar]
- 13.Montgomery J. I., et al. , Pyridone methylsulfone hydroxamate LpxC inhibitors for the treatment of serious gram-negative infections. J. Med. Chem. 55, 1662–1670 (2012). [DOI] [PubMed] [Google Scholar]
- 14.Oddo A., Holl R., Design and stereoselective synthesis of a C-aryl furanoside as a conformationally constrained CHIR-090 analogue. Carbohydr. Res. 359, 59–64 (2012). [DOI] [PubMed] [Google Scholar]
- 15.Warmus J. S., et al. , Structure based design of an in vivo active hydroxamic acid inhibitor of P. aeruginosa LpxC. Bioorg. Med. Chem. Lett. 22, 2536–2543 (2012). [DOI] [PubMed] [Google Scholar]
- 16.Szermerski M., et al. , Synthesis, biological evaluation and molecular docking studies of benzyloxyacetohydroxamic acids as LpxC inhibitors. Bioorg. Med. Chem. 22, 1016–1028 (2014). [DOI] [PubMed] [Google Scholar]
- 17.Löppenberg M., et al. , Synthesis and biological evaluation of flexible and conformationally constrained LpxC inhibitors. Org. Biomol. Chem. 11, 6056–6070 (2013). [DOI] [PubMed] [Google Scholar]
- 18.Hale M. R., et al. , Exploring the UDP pocket of LpxC through amino acid analogs. Bioorg. Med. Chem. Lett. 23, 2362–2367 (2013). [DOI] [PubMed] [Google Scholar]
- 19.McAllister L. A., et al. , Heterocyclic methylsulfone hydroxamic acid LpxC inhibitors as Gram-negative antibacterial agents. Bioorg. Med. Chem. Lett. 22, 6832–6838 (2012). [DOI] [PubMed] [Google Scholar]
- 20.Lin L., et al. , Inhibition of LpxC protects mice from resistant Acinetobacter baumannii by modulating inflammation and enhancing phagocytosis. mBio 3, e00312-12 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lemaitre N., et al. , Curative treatment of severe Gram-negative bacterial infections by a new class of antibiotics targeting LpxC. mBio 8, e00674-17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Babinski K. J., Kanjilal S. J., Raetz C. R., Accumulation of the lipid A precursor UDP-2,3-diacylglucosamine in an Escherichia coli mutant lacking the lpxH gene. J. Biol. Chem. 277, 25947–25956 (2002). [DOI] [PubMed] [Google Scholar]
- 23.Nayar A. S., et al. , Novel antibacterial targets and compounds revealed by a high-throughput cell wall reporter assay. J. Bacteriol. 197, 1726–1734 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cho J., Lee C. J., Zhao J., Young H. E., Zhou P., Structure of the essential Haemophilus influenzae UDP-diacylglucosamine pyrophosphohydrolase LpxH in lipid A biosynthesis. Nat. Microbiol. 1, 16154 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Young H. E., Donohue M. P., Smirnova T. I., Smirnov A. I., Zhou P., The UDP-diacylglucosamine pyrophosphohydrolase LpxH in lipid A biosynthesis utilizes Mn2+ cluster for catalysis. J. Biol. Chem. 288, 26987–27001 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Metzger L. E. 4th, Raetz C. R., An alternative route for UDP-diacylglucosamine hydrolysis in bacterial lipid A biosynthesis. Biochemistry 49, 6715–6726 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Young H. E., et al. , Discovery of the elusive UDP-Diacylglucosamine hydrolase in the lipid A biosynthetic pathway in Chlamydia trachomatis. MBio 7, e00090 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee M., et al. , Structure-activity relationship of sulfonyl piperazine LpxH inhibitors analyzed by an LpxE-coupled malachite green assay. ACS Infect. Dis. 5, 641–651 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mamat U., et al. , Single amino acid substitutions in either YhjD or MsbA confer viability to 3-deoxy-d-manno-oct-2-ulosonic acid-depleted Escherichia coli. Mol. Microbiol. 67, 633–648 (2008). [DOI] [PubMed] [Google Scholar]
- 30.Okada C., et al. , Crystal structures of the UDP-diacylglucosamine pyrophosphohydrase LpxH from Pseudomonas aeruginosa. Sci. Rep. 6, 32822 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gallivan J. P., Dougherty D. A., Cation-pi interactions in structural biology. Proc. Natl. Acad. Sci. U.S.A. 96, 9459–9464 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bohl T. E., et al. , The substrate-binding cap of the UDP-diacylglucosamine pyrophosphatase LpxH is highly flexible, enabling facile substrate binding and product release. J. Biol. Chem. 293, 7969–7981 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Das J., Aliphatic diazirines as photoaffinity probes for proteins: Recent developments. Chem. Rev. 111, 4405–4417 (2011). [DOI] [PubMed] [Google Scholar]
- 34.Lee C. J., et al. , Drug design from the cryptic inhibitor envelope. Nat. Commun. 7, 10638 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wagner G., Wüthrich K., Dynamic model of globular protein conformations based on NMR studies in solution. Nature 275, 247–248 (1978). [DOI] [PubMed] [Google Scholar]
- 36.Tsubery H., Ofek I., Cohen S., Fridkin M., Structure-function studies of polymyxin B nonapeptide: Implications to sensitization of gram-negative bacteria. J. Med. Chem. 43, 3085–3092 (2000). [DOI] [PubMed] [Google Scholar]
- 37.Shuker S. B., Hajduk P. J., Meadows R. P., Fesik S. W., Discovering high-affinity ligands for proteins: SAR by NMR. Science 274, 1531–1534 (1996). [DOI] [PubMed] [Google Scholar]
- 38.Stokes J. M., et al. , Pentamidine sensitizes Gram-negative pathogens to antibiotics and overcomes acquired colistin resistance. Nat. Microbiol. 2, 17028 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kwon D. Y., et al. , Synthesis and biological evaluation of manassantin analogues for hypoxia-inducible factor 1α inhibition. J. Med. Chem. 58, 7659–7671 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nara H., et al. , Design, synthesis, and biological activity of novel, potent, and highly selective fused pyrimidine-2-carboxamide-4-one-based matrix metalloproteinase (MMP)-13 zinc-binding inhibitors. Bioorg. Med. Chem. 24, 6149–6165 (2016). [DOI] [PubMed] [Google Scholar]
- 41.Sun W., Roland K. L., Branger C. G., Kuang X., Curtiss R. 3rd, The role of relA and spoT in Yersinia pestis KIM5 pathogenicity. PLoS One 4, e6720 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Datsenko K. A., Wanner B. L., One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U.S.A. 97, 6640–6645 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reynolds C. M., Raetz C. R., Replacement of lipopolysaccharide with free lipid A molecules in Escherichia coli mutants lacking all core sugars. Biochemistry 48, 9627–9640 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baba T., et al. , Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: The Keio collection. Mol. Syst. Biol. 2, 2006.0008 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Standards NCfCL , Methods for Dilution Antimicrobial Susceptibility Test for Bacteria that Grow Aerobically (Approved Standard, NCCLS document M7-A1, Wayne, PA, 1997). [Google Scholar]
- 46.Zhou P., Lugovskoy A. A., Wagner G., A solubility-enhancement tag (SET) for NMR studies of poorly behaving proteins. J. Biomol. NMR 20, 11–14 (2001). [DOI] [PubMed] [Google Scholar]
- 47.Kabsch W., XDS. Acta Crystallogr. D Biol. Crystallogr. 66, 125–132 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Adams P. D., et al. , PHENIX: A comprehensive python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moriarty N. W., Grosse-Kunstleve R. W., Adams P. D., Electronic ligand builder and optimization workbench (eLBOW): A tool for ligand coordinate and restraint generation. Acta Crystallogr. D Biol. Crystallogr. 65, 1074–1080 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Emsley P., Cowtan K., Coot: Model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004). [DOI] [PubMed] [Google Scholar]
- 51.Delaglio F., et al. , NMRPipe: A multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR 6, 277–293 (1995). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The X-ray structure coordinates of the K. pneumoniae LpxH complexes with lipid X, AZ1, and JH-LPH-33 have been deposited in the Protein Data Bank, www.wwpdb.org (PDB ID codes 6PH9, 6PIB, and 6PJ3, respectively).