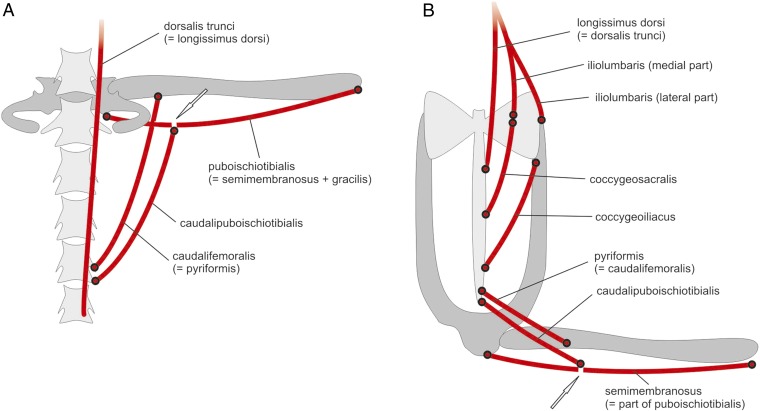
We recognize a frog largely because of its shape—short body, long legs, and absence of a tail. These features are modifications of the “vertebrate body plan,” a head, a body, four appendages, and a tail. However, evolving lineages of vertebrates have modified that theme in incredibly diverse ways. Research by Senevirathne et al. (1) shows that there are exciting ways to explore the origin of vertebrate novelties. The features that characterize vertebrates are innovations, new structures. The head is “new,” a product of the inception of neural crest cells streaming into the “head” region and forming new structures (e.g., jaws, gill bars, teeth, and the like) (2, 3); vertebrae—the fundamental postcranial segmental units for which the subphylum is named—are new, because bone is “new” and restricted to vertebrates, invented by the inception of mineralization in specific mesodermal sites (4). Modifying the body plan is the stuff of evolution and the great biodiversity of vertebrate animals. Concepts about developmental processes, adaptation, and many other features emerged as consequences of the study of body plan diversification. Vertebrate animals have been the foci of study for hundreds, if not thousands, of years. Aristotle (5, 6) referred to many vertebrates, including frogs, in his discussions of both animal structure and the essence of life, from which diversity arose. The evolution of the first terrestrial vertebrates (class Amphibia, subphylum Vertebrata) has therefore received considerable attention! The closest relatives to frogs (Anura, “no tail”) are salamanders (Caudata, “tailed”), which have followed more closely the ancestral vertebrate (tetrapod) plan of having a moderately long body, four relatively short limbs of nearly the same length, and a tail, and caecilians (Gymnophiona, “naked snake”), elongate, limbless, and usually tailless amphibians. The oldest known salamander, frog, and caecilian fossils are Jurassic (some 200 Ma) (7–9). Wake (10) illustrated the earliest frog and caecilian and a very early salamander. The frog already had a shortened vertebral column (11 precaudals) and long hind legs and an elongate pelvic girdle with fused caudal vertebrae—a urostyle. The caecilian had a highly elongated body with some 44 vertebrae, an enlarged sacral vertebra, 10 to 12 tail vertebrae, and tiny (but nearly complete except for digits) pectoral and pelvic limbs. The salamander had ∼12 vertebrae, a long tail, and substantial paired limbs. Head structure in all three fossils resembled that of extant relatives. Lineage diversification clearly began much more than 200 Ma. What is the basis for this diversification of amphibian body shapes? The ancestors of these animals had the “typical” tetrapod body plan. Prikryl et al. (11) provided schematics of the caudosacral and pelvic regions, and musculature, of generalized extant salamanders (Fig. 1A) and frogs (Fig.1B). The salamander pelvic girdle contacts the sacrum and supports the hind limbs. The frog pelvis is unique, with flared sacral extensions and elongate iliac rods that support the ischium posteriorly, all surrounding the urostyle. In a tour de force of investigation of evolution and development, Senevirathne et al. (1) show how the dynamic nature of processes of diversification as reflected by development in amphibians can now be investigated by focusing on a particular feature of a particular lineage, and combining “traditional” and genetic tools. They examined the development of a fundamental morphological feature of frogs, the urostyle—the structure of the pelvic girdle that is unique to frogs—and its association with the vertebral column and the limbs and with tail loss. They placed their investigation and its results in the context of the evolution of the structure and the evolution of frogs. Their work illustrates the power of a combined genetic, developmental, morphological, and evolutionary approach to a major question in biology: the process of diversification and the maintenance of biodiversity.
Fig. 1.
Diagram of the posterior vertebral column and musculature of salamanders (A) and frogs (B). Note the configuration of the sacral–pelvic girdle complex and its association with the single sacral vertebra in salamanders and that of the reorganized frog pelvic girdle. The association of the muscles with the sacrum–pelvic girdle and the hind limb facilitates walking and jumping locomotor patterns. Reprinted with permission from ref. 11.
To understand the evolution of the urostyle, Senevirathne et al. (1) focus on the hypochor, a rod-like column of cells that develops below the notochord in amniote vertebrates. Early development of the hypochord and its signaling properties and responses have been described in early development of zebrafish (12).The salamander hypochord is apparently transient, because Löfberg and Collazo (13) report its development and its position and potential influence on the dorsal aorta but report disappearance after 8 d, owing to extensive apoptosis. Wake and Wake (14) found no evidence for a hypochord in the caecilian Dermophis mexicanus in their examination of early vertebrogenesis and the notochord (perhaps correlated with the absence of girdles, limbs, and tail). Senevirathne et al. (1) specifically assessed the origin of the ossifying hypochord in order to assess its origin, its association with the notochord (physically and in signaling), with the terminal vertebrae (the coccygeal component of the urostyle), with the dorsal aorta, and with the process of metamorphosis. They parsed the questions about the hypochord in terms of problem and of technique, employing both “traditional” and new genetic tools. Clearing and staining (alizarin red S for bone and Alcian blue for cartilage) a series of whole tadpoles was used to develop a staged series of embryos and tadpoles for examination of the development of the cartilage and bone of the vertebral column, the urostyle, and limbs. Histological preparations revealed the cell structure of the developing structures. Maintaining stage-54 tadpoles in a solution containing thyroxin and a control series in a solution without thyroxin, then raising them for 2 mo, finally clearing and staining them, was used to assess the effect of thyroxin on urostyle development. A series of tadpoles at stage 54 were stained with phosphomolybdic acid and the specimens were scanned by computed tomography to follow development of the urostyle. Scans were analyzed and segmented. Six tadpoles of each of four stages were immunohistochemically stained to examine cell death, neurons, and muscle remodeling. Antibodies used included Caspase-3 to observe apoptosis, acetylated tubulin for neurons, and Laminen for muscle fibers. Whole-mount in situ hybridization and whole-mount immunohistochemistry were done with a diversity of enzymes and antibodies. The abundant illustrations in the publication reflect the copious body of specimens which were examined and data taken and analyzed.
The data from this extensive exploration indicate the changes in the postsacral vertebrae that will form the coccyx occur during prometamorphosis, and the hypochord begins its ossification then as well. By metamorphic climax the postsacral vertebrae have fused together and with the ossifying hypochord, and the notochord degenerates. Much modification of the osteocytes and chondrocytes of the structures occurs during the process. The thyroxin experiment showed that treated tadpoles had incomplete coccygeal development and no development of the hypochord. The larval hypochordal cells undergo chondrogenesis and osteogenesis in the presence of thyroxin and contribute to the ossifying hypochord. The whole-mounts and sectioned immunohistochemistry revealed that the fibers present in the tadpole that give rise to the muscles associated with the adult urostyle (which facilitate jumping) undergo either extensive turnover or reshaping during metamorphosis. Similarly, the experiments with acetylated tubulin, and so on, illustrated that the spinal cord and peripheral nervous system were remodeled during the metamorphic process. When the tadpole tail starts degenerating, spinal nerves also degenerate such that spinal nerve X exits through the coccygeal foramen, and more posterior nerves degenerate as the coccyx and hypochord fuse. The examination of the relationship of the ossifying hypochord to the dorsal aorta showed that as the hypochord enlarges it occludes the dorsal aorta at it posteriormost point, probably initiating the bifurcation that gives rise to the femoral arteries and the loss of blood to the tadpole tail, causing its resorption. The experiments on cell death and cell proliferation produced expected results: As the hypochord increased its maximum length tadpole tail reduction began, and phagocytic cells and markers for apoptosis arose. At last, a detailed picture emerges of the developmental events that give rise to the loss of the tadpole tail, the development of the coccyx–hypochord association that facilitates the formation of the urostyle, the modification of the tadpole postaxial vascular pattern, and the development of the adult hind-limb musculature and its association with the urostyle (and other pelvic elements).
Solutions to several persistent issues emerge from the research such as fundamental questions about the appearance of novelty in frog evolution, and general questions relating to pattern and process of the evolutionary origins of new structures and of consequent lineage diversification. This is groundwork for extensive comparative biology (in anamniotes, but also examining the fused terminal vertebrae of birds and primates), as well as uses of combined techniques and approaches to analyze development and evolution. The way that the investigators present their work in the context of the “big picture” of the evolution of novelty by focusing on a particular innovation, one that presumably gave rise to the frog body form and function, is an exceptional example of clear delineation of the development and relationships of the several components of the urostyle. Their data provide resolution to the ongoing debate in the literature of whether the hypochord is mesodermal or endodermal in origin; it is endodermal, and it signals the mesodermal coccygeal vertebrae to together form the urostyle.
At the same time, the research by Senevirathne et al. (1) opens a number of questions at several levels of consideration. As the authors note, more information is needed on the signaling systems and the timing of metamorphosis in the development of the urostyle. Also, given that amniotes lack a hypochord, what are their mechanisms for positioning the dorsal aorta and its bifurcation? What mechanisms provide either fixation or flexibility of numbers of vertebrae? How is tail length controlled in tetrapods, given such phenomena as tail loss and postmetamorphic addition of vertebrae (in some salamanders)? There are many such wide-ranging questions about vertebrate body plan diversification. Similarly, questions arise about the genetic architecture of development of specific structures, and how and why they vary. Clearly, the work by Senevirathne et al. (1) provides an intellectual and technical road map for developing and exploring the answers to major questions in evolutionary biology and development.
Acknowledgments
I thank Tomas Prikryl and Zbynek Rocek for permission to use their figure of schematics of salamander and frog caudosacral and pelvic structures and musculature and for the scan of the original. I appreciate the many years of funding of my research in amphibian evolution, morphology, and development by the National Science Foundation.
Footnotes
The author declares no competing interest.
See companion article on page 3034 in issue 6 of volume 117.
References
- 1.Senevirathne G., Baumgart S., Shubin N., Hanken J., Shubin N. H., Ontogeny of the anuran urostyle and the developmental context of evolutionary novelty. Proc. Natl. Acad. Sci. U.S.A. 117, 3034–3044 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gans C., Northcutt R. G., Neural crest and the origin of vertebrates: A new head. Science 220, 268–273 (1983). [DOI] [PubMed] [Google Scholar]
- 3.Northcutt R. G., Gans C., The genesis of neural crest and epidermal placodes: A reinterpretation of vertebrate origins. Q. Rev. Biol. 58, 1–28 (1983). [DOI] [PubMed] [Google Scholar]
- 4.Wagner D. O., Aspenberg P., Where did bone come from? Acta Orthop. 82, 393–398 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aristotle, On the Parts of Animals. Translated by William Ogle. Books I–IV. (Internet Classics Archive, http://classics.mit.edu/Aristotle/parts_animals.html).
- 6.Aristotle, On the Generation of Animals. Translated by A. L. Peck (Loeb Classical Library 366, Harvard University Press, 1942). [Google Scholar]
- 7.Evans S. A., Milner A. R., Mussett F., The earliest known salamanders (Amphibia: Caudata): A record from the Middle Jurassic of England. Geobios 21, 539–552 (1988). [Google Scholar]
- 8.Shubin N. H., Jenkins F. A., An early Jurassic jumping frog. Nature 37, 49–52 (1995). [Google Scholar]
- 9.Jenkins F. A., Walsh D. M., An early Jurassic caecilian with limbs. Nature 365, 246–249 (1993). [Google Scholar]
- 10.Wake M. H., Amphibian locomotion in evolutionary time. Zoology 100, 141–151 (1998). [Google Scholar]
- 11.Prikryl T., Aerts P., Havelková P., Herrel A., Rocek Z., Pelvic and thigh musculature in frogs (Anura) and origin of anuran jumping locomotion. J. Anat. 214, 100–139 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Latimer A. J., Dong X., Markov Y., Appel B., Delta-Notch signaling induces hypochord development in zebrafish. Development 129, 2555–2563 (2002). [DOI] [PubMed] [Google Scholar]
- 13.Löfberg J., Collazo A., Hypochord, an enigmatic embryonic structure: Study of the axolotl embryo. J. Morphol. 232, 57–66 (1997). [DOI] [PubMed] [Google Scholar]
- 14.Wake M. H., Wake D. B., Early developmental morphology of vertebrae in caecilians (Amphibia: Gymnophiona): Resegmentation and phylogenesis. Zool. Anal. Compl. Syst. 103, 68–88 (2000). [Google Scholar]