Significance
T cell immunity is important in the control of chronic viral infections and cancer. Recent studies have identified a population of programmed cell death 1 (PD-1+) stemlike CD8 T cells that provide the proliferative burst after PD-1–directed immunotherapy. In this study, we have examined the migratory properties of these stemlike CD8 T cells using the mouse model of chronic lymphocytic choriomeningitis virus infection. We show that these quiescent stemlike CD8 T cells do not circulate and are resident in lymphoid tissues, providing a protective niche for their maintenance during chronic infection. These results raise important questions regarding the role of specialized niches within lymphoid tissues and for developing new immunotherapeutic approaches targeting these PD-1+ stemlike CD8 T cells.
Keywords: chronic viral infection, T cell exhaustion, PD-1, stemlike CD8 T cells, T cell migration
Abstract
The migratory patterns of virus-specific CD8 T cells during chronic viral infection are not well understood. To address this issue, we have done parabiosis experiments during chronic lymphocytic choriomeningitis virus (LCMV) infection of mice. We found that despite the high frequency of virus-specific CD8 T cells in both lymphoid and nonlymphoid tissues there was minimal migration of virus-specific CD8 T cells between the chronically infected conjoined parabiont mice. This was in contrast to parabionts between mice that had undergone an acute LCMV infection where virus-specific CD8 T cells established equilibrium demonstrating circulation of memory T cells generated after viral clearance. We have identified a population of PD-1+ TCF1+CXCR5+Tim-3- stemlike virus-specific CD8 T cells that reside in lymphoid tissues and act as resource cells for maintaining the T cell response during chronic infection. These are the cells that proliferate and give rise to the more terminally differentiated PD-1+ CXCR5-Tim-3+ CD8 T cells. Both the stemlike CD8 T cells and their terminally differentiated progeny showed minimal migration during chronic infection and the few LCMV-specific CD8 T cells that were present in circulation were the recently emerging progeny from the stemlike CD8 T cells. The PD-1+ TCF1+CXCR5+ stemlike CD8 T cells were truly resident in lymphoid tissues and did not circulate in the blood. We propose that this residency in specialized niches within lymphoid tissues is a key aspect of their biology and is essential for maintaining their quiescence and stemlike program under conditions of a chronic viral infection.
Persistent antigenic stimulation during chronic viral infection and cancer results in CD8 T cell dysfunction that is associated with expression of inhibitory receptors such as programmed cell death 1 (PD-1) (1–3). A better understanding of T cell exhaustion has come from recent studies that have characterized the various T cell states that exist during chronic viral infection and defined the lineage relationships between these different T cell subsets (1). A key finding has been the identification of a novel subset of PD-1+ TCF1+CXCR5+ virus-specific CD8 T cells that act as stem cells to sustain the CD8 T cell response during chronic lymphocytic choriomeningitis virus (LCMV) infection of mice (4–7). These LCMV-specific PD-1+ stemlike CD8 T cells maintain their population by a slow self-renewal and also differentiate into more effectorlike and terminally differentiated CD8 T cells. Thus, these virus-specific PD-1+ stemlike CD8 T cells function as resource cells during chronic infection to keep the virus-specific CD8 T cell response going and in the absence of these PD-1+TCF1+ CD8 T cells the LCMV-specific CD8 T cell response wanes in chronically infected mice (4, 6, 7). Importantly, the rapid proliferative burst of CD8 T cells that is seen after PD-1 blockade comes exclusively from this stemlike CD8 T cell subset that has the ability to proliferate and differentiate into more effectorlike T cells (4–6). The presence of these PD-1+TCF1+ stemlike CD8 T cells and their important role in effective antitumor immunity has also been reported recently in murine tumor models and in human cancer (8–12).
Interestingly, in addition to their functional, transcriptional, and epigenetic differences (4–7, 13), these two CD8 T cell subsets also exhibit striking differences in their tissue distribution. The PD-1+ TCF1+ stemlike CD8 T cell subset is mainly present in the lymphoid organs, particularly in the white pulp of the spleen and lymph nodes, while the terminally differentiated CD8 T cell subset that is derived from the stemlike cells is found in both lymphoid and nonlymphoid organs at sites of viral infection (4). An important question that has not been addressed is the in vivo migratory properties of virus-specific CD8 T cells during chronic infection. This is of particular interest in the context of the stemlike and terminally differentiated CD8 T cells and to determine if they are similar or different in terms of their migratory properties. In this study, we have examined this issue using the mouse model of chronic LCMV infection.
Results
Expression of Genes Involved in Migration by PD-1+ CD8 T Cell Subsets during Chronic LCMV Infection.
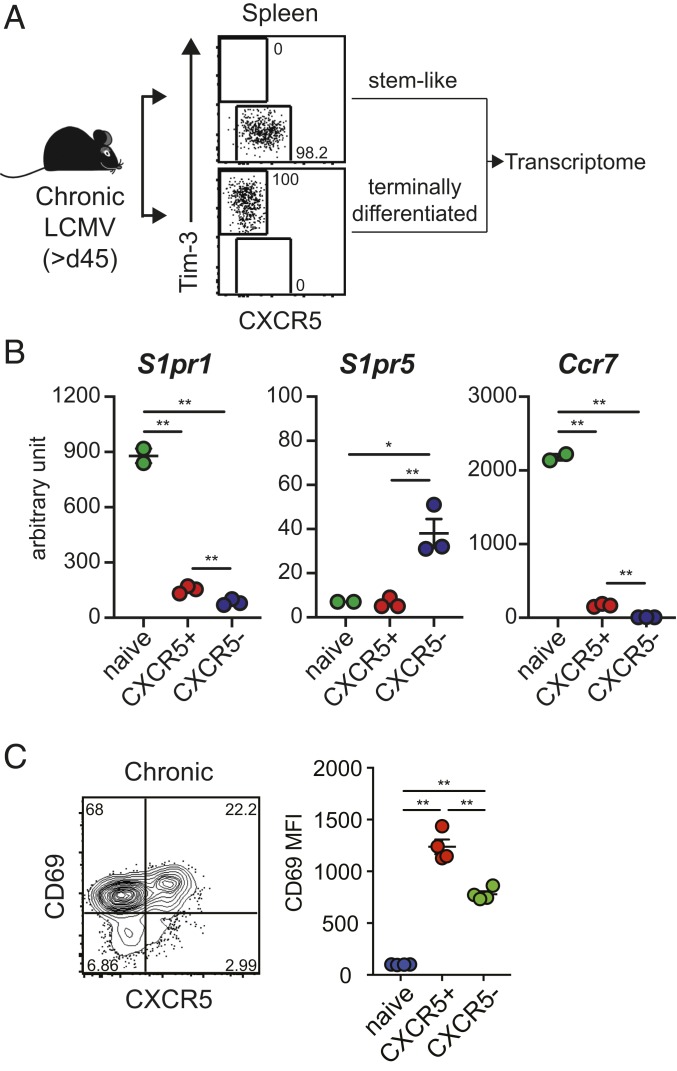
The LCMV chronic infection model of transient CD4 T cell depletion was used in this study (14). This model is particularly well suited to study CD8 T cell exhaustion since there is lifelong viremia and virus persists at high levels in both lymphoid and nonlymphoid tissues. To investigate the migratory properties of the PD-1+ CD8 T cell subsets during chronic LCMV infection, we first compared mRNA expression levels of genes associated with lymphocyte migration in CXCR5+Tim-3- stemlike and CXCR5-Tim-3+ terminally differentiated CD8 T cell subsets isolated from the spleens of chronically infected mice [>45 d postinfection (p.i.)] (GSE84105) (Fig. 1A). Naive CD44lo CD8 T cells were isolated from uninfected mice and used as controls for the gene expression analysis. Compared to naive CD8 T cells, both the stemlike and terminally differentiated PD-1+ CD8 T cell subsets exhibited substantial down-regulation of S1pr1 (Fig. 1B), a receptor that regulates the egress of lymphocytes from lymphoid organs to the circulation (15–17), suggesting that these two populations may be retained in tissues. Interestingly, the terminally differentiated CD8 T cells showed higher expression of S1pr5, which is another receptor modulating the egress of lymphocytes into the blood (18). This S1pr5 receptor is not expressed by naive CD8 T cells and the selective expression of this receptor by the CXCR5-Tim-3+ CD8 T cell subset suggests that these more differentiated cells could be using S1PR5 for migration into the blood. In terms of molecules needed for homing to lymph nodes and spleen, the homing receptor Ccr7 was down-regulated in both chronic CD8 T cell subsets compared to naive CD8 T cells, but the stemlike CD8 T cells expressed significantly (P = 0.0002) higher levels of Ccr7 message compared to their more terminally differentiated counterparts. This is consistent with the preferential location of the PD-1+ stemlike CD8 T cell subset in the T cell zones of the spleen. In addition, we have previously shown that CCR7 expression by the stemlike CD8 T cells is sufficient to allow these cells to respond in vitro to the chemokines CCL19 and CCL21 (4). In addition to the down-regulation of S1pr1 expression, both stemlike and terminally differentiated CD8 T cells showed high expression of CD69 protein (Fig. 1C), which antagonizes S1P-mediated egress when residual S1P1 expression exists (19–21). It is worth noting that the expression of CD69 on the stemlike CD8 T cell subset is significantly higher (P = 0.001) compared to that on the terminally differentiated CD8 T cell subset (Fig. 1 C, Right). Taken together, these data suggest that both stemlike and terminally differentiated CD8 T cell subsets display resident attributes. However, the very low expression of both S1pr1 and S1pr5 and the higher expression of CD69 and Ccr7 on stemlike CD8 T cells compared to terminally differentiated CD8 T cells (Fig. 1 B and C) strongly suggests that these PD-1+ TCF1+ virus-specific CD8 T cells may be more resident in the secondary lymphoid organs.
Fig. 1.
Expression of genes involved in migration by LCMV-specific CD8 T cell subsets during chronic viral infection. (A) PD-1+ CXCR5+Tim-3- stemlike and PD-1+ CXCR5−Tim-3+ terminally differentiated CD8 T cells were isolated from the spleens of LCMV chronically infected mice (>45 d p.i.). Naive CD44lo CD8 T cells were isolated from uninfected mice and their gene expression analyzed. (B) Expression of migration-associated genes in each CD8 T cell subset as determined by Affymetrix microarray analysis (GSE84105). Data are shown as arbitrary units. (C) Representative expression of CXCR5 and CD69 on GP33-specific CD8 T cells (Left) and mean fluorescence intensity of CD69 expression of CD8 T cell subsets (Right) in the spleen of chronically infected mice (>45 d p.i.). Representative data from more than three experiments are shown. Graphs show the mean and SEM Student’s t test, where *P < 0.05; where **P < 0.01.
Parabiosis Experiment to Analyze the In Vivo Migration of LCMV-Specific CD8 T Cells during Chronic Viral Infection.
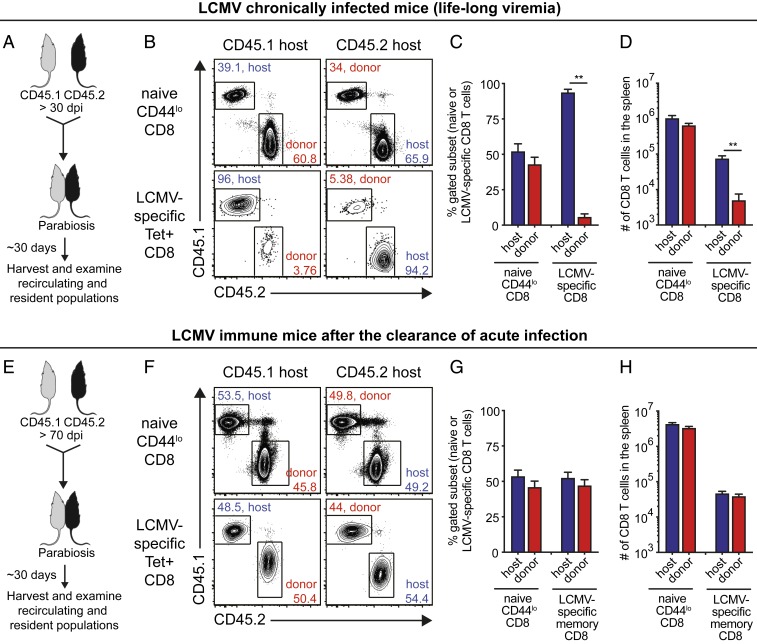
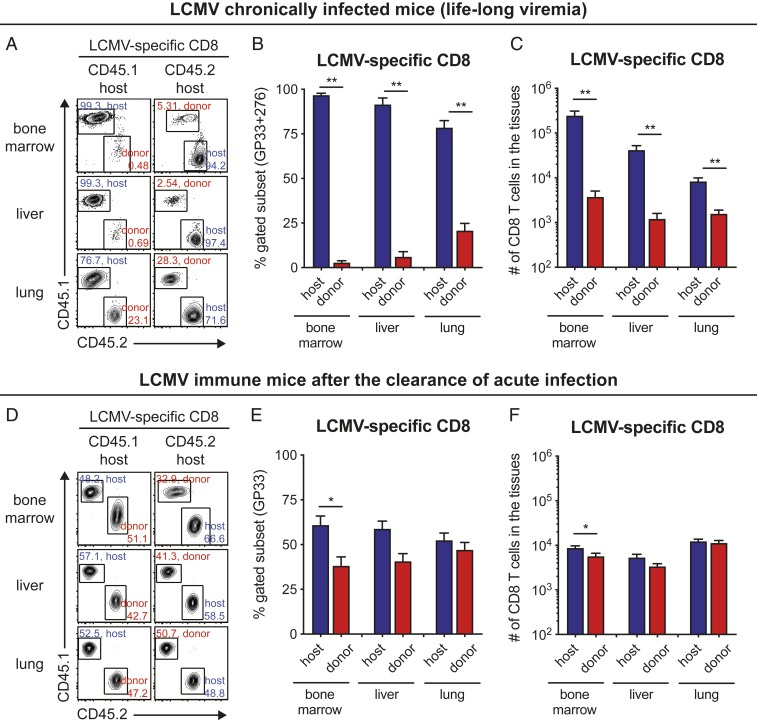
To more directly investigate the in vivo migratory properties of the stemlike and terminally differentiated CD8 T cells, we conjoined two congenically distinct mice by parabiosis surgery at >30-d postchronic LCMV infection (Fig. 2 A–D). As a control, we also performed the same surgery using mice that had cleared an acute infection (>70 d) after challenge with the LCMV Armstrong strain (Fig. 2 E–H). This allowed us to compare the migration of LCMV-specific CD8 T cells during an established chronic infection versus the migration pattern of LCMV-specific CD8 T cells after clearance of an acute infection. At around 30 d after the surgery, we determined host and donor populations of naive (CD44lo) and LCMV-specific CD8 T cells in the spleens of the chronically infected parabionts. The donor population represents the immigrant cells from the other attached parabiont. Naive CD8 T cells between chronically infected parabionts equilibrated in the spleen (Fig. 2 B–D), confirming successful vascular anastomosis. In contrast, LCMV-specific CD8 T cells did not reach equilibrium in the spleen between chronically infected parabionts, indicating the impaired circulating ability of virus-specific CD8 T cells during chronic viral infection. On average, only 5.8% of donor CD8 T cells were observed in the spleen of the host parabionts (Fig. 2 C, Right). Moreover, the minimal migration of virus-specific CD8 T cells during chronic viral infection was also observed in other tissues such as bone marrow, liver, and lung (Fig. 3 A–C). In contrast to the results seen in chronically infected mice, LCMV-specific CD8 T cells established equilibrium in the spleen (Fig. 2 F–H) and other tissues (Fig. 3 D–F) between the two acutely infected conjoined parabionts, indicating dynamic circulation of memory CD8 T cells generated after clearance of an acute infection. However, there is also some degree of T cell residency in mice that have cleared the acute infection (SI Appendix, Fig. S1). This migratory pattern in LCMV immune mice has been demonstrated before (22–26) and serves as an important control in our study highlighting the striking difference between acute and chronic infection.
Fig. 2.
Parabiosis experiment to analyze the in vivo migration of LCMV-specific CD8 T cells during chronic infection: spleen data. (A) Parabiosis surgery was performed to conjoin two congenically distinct LCMV chronically infected mice at >30 d p.i. and these were euthanized around 30 d after the parabiosis surgery. (B–D) Representative fluorescence-activated cell sorting (FACS) plots (B) and a summary graph (C and D) showing the proportion (C) and number (D) of host and donor cells among naive CD44lo and LCMV GP33-GP276-specific CD8 T cells in the spleens of chronically infected parabionts. (E) Parabiosis surgery between two congenically distinct mice that had cleared an acute LCMV infection (70 d after infection with LCMV Armstrong strain). (F–H) Representative FACS plots (F) and a summary graph (G and H) showing the proportion (G) and number (H) of host and donor cells among naive CD44lo and GP33-specific CD8 T cells in the spleen of acutely infected parabiont. Data are combined results of three independent experiments (total n = 7) and mean and SEM are shown. Student’s t test, where **P < 0.01.
Fig. 3.
Parabiosis experiment to analyze the in vivo migration of virus-specific CD8 T cells during chronic LCMV infection: bone marrow, liver, and lung data. (A–C) Representative FACS plots (A) and a summary graph (B and C) showing the proportion (B) and number (C) of host and donor cells among LCMV GP33-GP276-specific CD8 T cells in the indicated tissues of chronically infected parabionts. (D–F) Representative FACS plots (D) and a summary graph (E and F) showing the proportion (E) and number (F) of host and donor cells among GP33-specific CD8 T cells in the indicated tissues of acutely infected parabiont. Data are combined results of three independent experiments (total n = 7) and mean and SEM are shown. Student’s t test, where *P < 0.05; where **P < 0.01.
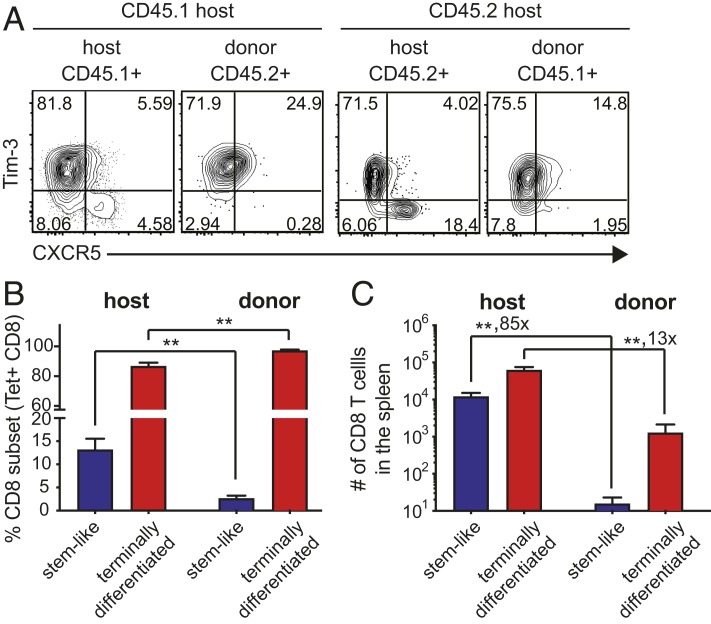
We next examined the phenotype of host and donor virus-specific CD8 T cell subsets in the conjoined parabionts during chronic infection. As expected, the host LCMV-specific CD8 T cell population in the spleens of the chronically infected parabionts consisted of both the CXCR5+Tim-3- and CXCR5-Tim3+ subsets but the donor LCMV-specific CD8 T cells in these mice consisted almost exclusively of the more differentiated CXCR5-Tim-3+ CD8 T cells (Fig. 4). Although both subsets exhibited minimal circulation in the setting of chronic viral infection, these results show that it is the PD-1+ TCF1+CXCR5+ stemlike CD8 T cells that are truly resident in the lymphoid tissues and are not circulating in these chronically infected mice.
Fig. 4.
Migration of PD-1+ LCMV-specific CD8 T cell subsets during chronic viral infection. (A–C) Representative FACS plots (A) and summary graphs (B and C) showing the proportion (B) and number (C) of CXCR5+Tim-3- stemlike and CXCR5−Tim-3+ terminally differentiated CD8 T cells in the spleens of chronically infected parabionts. Data are combined results of three independent experiments (total n = 7) and mean and SEM are shown. Student’s t test, where **P < 0.01.
Analysis of Virus-Specific CD8 T Cell Subsets in the Blood during CD4 T Cell Helped and Unhelped Models of Chronic LCMV Infection.
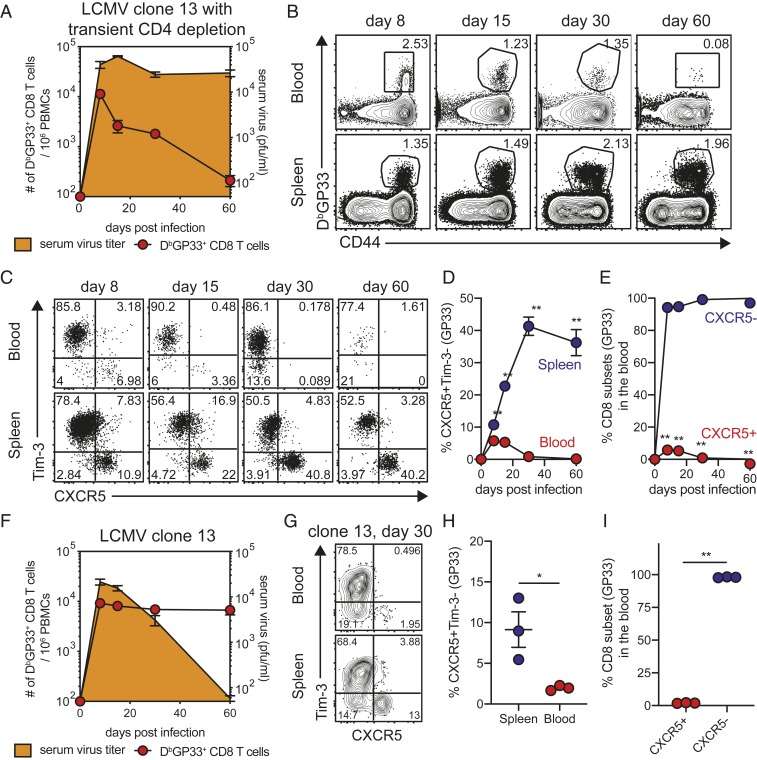
The data we have shown so far in Figs. 1–4 have come from a model of LCMV clone 13 infection where mice are treated with anti-CD4 T cell antibody (GK1.5) at the time of infection. This results in a transient depletion of CD4 T cells during the early stages of infection followed by nearly full recovery of total CD4 T cells within 4 wk p.i. However, while total CD4 T cell numbers come back to near-normal levels these mice remain highly deficient in the number of LCMV-specific CD4 T cells (14). In this CD4 T cell-unhelped model of LCMV clone 13 infection, there is lifelong viremia with high levels of virus in almost every tissue in the mouse. These chronically infected mice contain LCMV-specific CD8 T cells in all infected tissues but the number of virus-specific CD8 T cells in the blood becomes low over time (Fig. 5 A and B). Also, it is worth noting that most of the cells in the blood are the more differentiated CXCR5-Tim-3+ CD8 T cells with minimal to no detectable CXCR5+Tim-3- stemlike CD8 T cells in the blood after day 30 p.i. (Fig. 5 C–E). In contrast, the spleens of these mice have a substantial proportion of the stemlike LCMV-specific CD8 T cells consistent with the resident nature of these cells in lymphoid organs (Fig. 5 C and D). Taken together, these data are in complete agreement with the results of the parabiosis experiments shown in Figs. 2–4.
Fig. 5.
Analysis of LCMV-specific CD8 T cell subsets during CD4 T cell helped- and unhelped models of LCMV clone 13 infection. (A) Mice were infected with LCMV Cl13 strain with the treatment of anti-CD4 depleting antibodies (clone GK1.5) at the time of infection. Kinetics of serum virus titers and GP33-specific CD8 T cells in the blood are shown. (B) Representative FACS plots of GP33-specific CD8 T cells in the blood and spleen of CD4-depleted chronically infected mice. (C and D) Representative FACS plots (C) and a summary graph (D) showing the kinetics of CXCR5+Tim-3- stemlike CD8 T cells in the blood and spleen of CD4-depleted chronically infected mice. (E) Kinetics of the two CD8 T cell subsets in the blood of mice chronically infected in the absence of CD4 help. (F) Mice were infected with LCMV Cl13 strain without the treatment of anti-CD4 depleting antibodies. Kinetics of serum virus titers and GP33-specific CD8 T cells in the blood are shown. (G and H) Representative FACS plots (G) and a summary graph (H) showing the proportion of CXCR5+Tim-3- stemlike CD8 T cells 30 d p.i. in the blood and spleen of chronically infected mice with the CD4 help. (I) Proportion of two CD8 T cell subsets 30 d p.i. in the blood of mice chronically infected in the presence of CD4 help. Representative data from two experiments (n = 3–4/experiment) are shown. Graphs show the mean and SEM Student’s t test, where *P < 0.05; where **P < 0.01.
We next examined the LCMV clone 13 chronic infection model where there is no depletion of CD4 T cells. In this CD4 T cell-helped model, there is eventual control of viremia between day 30 and 60 p.i. (Fig. 5F) and reduction of LCMV from most systemic tissues but virus continues to persist long-term in tissues like the kidney (27). In this chronic infection model, there is a higher frequency of LCMV-specific CD8 T cells in the blood (Fig. 5F) but importantly also in this instance the vast majority of the virus-specific CD8 T cells in the blood are the more differentiated CXCR5- CD8 T cells day 30 p.i. (Fig. 5 G–I). These results show that the CXCR5+ stemlike CD8 T cell subset is resident in lymphoid tissues in both the CD4 helped and CD4 unhelped models of chronic LCMV infection.
Characterization of CD8 T Cells Circulating in the Blood during Chronic LCMV Infection.
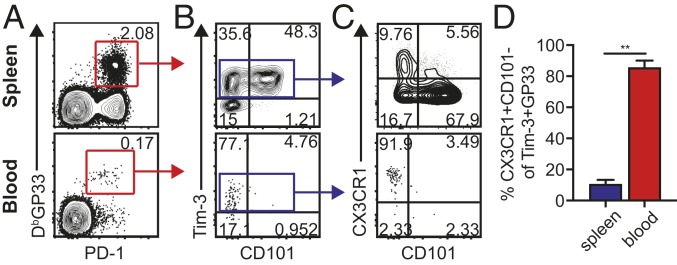
We have recently shown that the more differentiated LCMV-specific CXCR5-Tim-3+ CD8 T cells can be subdivided into two distinct populations based on the expression of CD101 (28). The CD101+Tim-3+ CD8 T cells are more exhausted and terminally differentiated while the CD101-Tim-3+ CD8 T cells are the proliferating and newly emerging cells derived from the quiescent CXCR5+TCF1+ stemlike cells. Thus, the CD8 T cell-differentiation pathway and lineage relationship during chronic LCMV infection are as follows: TCF1+ stemlike CD8 T cells → CD101-Tim-3+ transitory cells → CD101+Tim-3+ terminally differentiated cells (28). These transitory CD101-Tim-3+ cells have a more effectorlike signature (28, 29) and also express CX3CR1, which has a role in migration of cells to inflamed endothelium (30, 31). So, we next investigated which CD8 T cells were circulating in the blood of chronically infected mice. We found that while the frequency of tetramer-positive CD8 T cells in the blood is very low compared to the spleen (Fig. 6A), the vast majority of these circulating LCMV-specific CD8 T cells in chronically infected mice were CD101-Tim-3+ and expressed CX3CR1 (Fig. 6). As shown in this figure, these newly emerging CD8 T cells comprised only about 10% of the LCMV-specific CD8 T cells in the spleen but were >80% of the virus-specific CD8 T cells in the blood. Thus, it is the newly emerging progeny derived from the stemlike CD8 T cells that come out into the blood on their way to various sites of viral infection. These data are from the transient CD4 T cell-depletion model of LCMV chronic infection. We also examined this in the LCMV clone 13 infection model with no manipulation of CD4 T cells. Similarly, we found that most of the PD-1+Tim-3+ virus-specific CD8 T cells in the blood were CD101-CX3CR1+ (SI Appendix, Fig. S2).
Fig. 6.
Circulation of PD-1+Tim-3+CX3CR1+CD101- CD8 T cells during chronic LCMV infection. (A and B) Representative FACS plots of GP33-specific CD8 T cells 60 d p.i. in the spleen and blood of chronically infected mice (A) and their CD101 and Tim-3 expression (B). (C and D) Representative FACS plots of CD101 and CX3CR1 expression on Tim-3+ GP33-specific CD8 T cells and a summary graph (D) showing the proportion of CX3CR1+CD101- CD8 T cells among Tim-3+ GP33-specific CD8 T cells. Representative data from two experiments (total n = 8). These data are from the LCMV CD4 T cell-unhelped chronic infection model with lifelong viremia. Graphs show the mean and SEM. Student’s t test, where **P < 0.01.
Discussion
This study shows that under conditions of a long-term chronic viral infection with high levels of viremia and systemic infection involving multiple tissues, there are very few virus-specific CD8 T cells in the blood. This is despite the high frequency of virus-specific CD8 T cells in both lymphoid and nonlymphoid organs of these chronically infected mice. Thus, there is clearly a residency program in the vast majority of virus-specific CD8 T cells during chronic LCMV infection. The few CD8 T cells that appear in the blood are the more effectorlike CD8 T cells that have been recently generated following the proliferation and differentiation of the stemlike CD8 T cells residing in lymphoid organs (4–7, 28). It is interesting that PD-1 blockade substantially increases the number of virus-specific CD8 T cells in the blood by acting on the PD-1+ stemlike CD8 T cells and increasing their proliferation and differentiation (32–35). As we have shown before this increased proliferative burst requires not only removing the PD-1 brake but also providing appropriate costimulatory signals through CD28-B7 interactions (36). Similar observations have been made in human cancer where the frequency of tumor-specific CD8 T cells in the blood appears to be low at steady state but increases substantially in the blood in patients that respond to PD-1 therapy (37–40).
Taken together, the results of the parabiosis experiments and the analysis of circulating LCMV-specific CD8 T cells in the blood during chronic infection show that most of the terminally differentiated exhausted CD8 T cells are resident at sites of viral infection in multiple tissues and the stemlike CD8 T cells are resident in lymphoid organs. An important question is if these two otherwise strikingly distinct CD8 T cell populations are following the same or different programs of tissue residency. Another question of interest is whether antigen is playing a role in this residency program. Both of these CD8 T cell populations express PD-1 and since TCR signaling is the major regulator of PD-1 expression, it is highly likely that these tissue-resident CD8 T cells are in contact with antigen. Several studies have defined tissue-resident memory CD8 T cells (Trm) after clearance of an acute infection or vaccination (41, 42). It will be of interest to examine the similarities and differences in the residency programs of these acute CD8 Trm cells and the chronic CD8 T cell subsets.
Probably the most important point to emerge from our studies is that the PD-1+ TCF1+CXCR5+ stemlike CD8 T cells are not only found in lymphoid tissues during chronic viral infection but that these cells are actually resident at these lymphoid sites and do not circulate in the blood during an established chronic infection. The only time they are in circulation is during the early phase of the chronic infection (Fig. 5 C–E) when these cells are being generated from naive CD8 T cells (4). Once the chronic phase is established the PD-1+ stemlike CD8 T cells acquire the resident program. This is a key aspect of the biology of these T cells; it is critical for them to remain at these protected sites in a specialized niche. This environment allows them to maintain their quiescence and retain their stemlike features of pluripotency and proliferative potential. Future studies are needed to better define how such lymphoid niches are generated and maintained under conditions of chronic infections and cancer.
Materials and Methods
Mice and Viral Infections.
All animal experiments were performed in accordance with Emory University Institutional Animal Care and Use Committee. C57BL/6J and CD45.1 congenic female mice were purchased from Jackson Laboratory. Mice were infected with LCMV Armstrong strain [2 × 105 PFU, intraperitoneally (i.p.)] for acute infection or LCMV clone 13 strain (2 × 106 PFU, intravenously) with or without the transient CD4+ T cell depletion by treatment with 300 µg anti-CD4 antibody (GK1.5 clone) i.p. twice. This transient CD4 T cell depletion during the early stages of infection led to lifelong viremia (14). However, full components of CD4 T cell response come back to normal within 4–6 wk p.i. although LCMV-specific CD4 T cells were highly limited.
Parabiosis Surgery.
Parabiosis surgery was performed as described previously (43) with minor modifications. Briefly, mice were anesthetized with isofluorane/oxygen vaporizer 1.5–2% via nose cones after initial anesthesia in the induction box at 3%. After applying the drying preventive ointment on mouse eyes, skin on lateral flanks was shaved and disinfected with alcohol prep pads and Prevantix pads. Mice received analgesic, slow-release formula of Buprenex subcutaneously. All principles of sterile surgery were observed. Mirrored incisions were made from the olecranon to the knee joint of both mice. Mice were connected through the exposed knee joints of each animal and at the olecranon with the suture 18-in PS-3 cutting Vicryl undyed suture. Mice are connected along dorsal and ventral aspects using continuous suturing. Closely monitored parabionts were then allowed to rest for 25–36 d to allow vascular connection. The parabiosis surgery using chronically infected mice was performed earlier than that using acutely infected mice because from our previous experiments, survival of the chronically infected mice was significantly better when the surgery was performed at day 30 p.i. than at day 70 p.i. Furthermore, it should be noted that residence vs. recirculating migration patterns are largely established by day 30 after acute LCMV infection, and one would not anticipate that many recirculating cells would become resident after day 30 but before day 70 (22).
Flow Cytometry.
Flow cytometric analysis was performed on FACS Canto II and LSR II (BD Biosciences). Lymphocytes were isolated from tissues including spleen, blood, liver, bone marrow, and lung as described previously (27). Directed ex vivo staining was performed as described previously (44) with fluorochome-conjugated antibodies against CD8, CD45.1, CD45.2, CD4, CD19, CXCR5, Tim-3, CD69, CD44, PD-1, and CD62L. Antibodies were purchased from BD Biosciences, Affymetrix eBioscience, and BioLegend. For detecting LCMV-specific CD8 T cell responses, tetramers were prepared as described previously (45). Cell viability was determined with the LIVE/DEAD Fixable Aqua dead-cell stain kit (Invitrogen). FACS data were analyzed with FlowJo software (TreeStar).
Statistical Analysis.
All experiments were analyzed using Prism 7 (GraphPad Software). Statistical differences were assessed using a two-tailed unpaired or paired Student’s t test. P values of <0.05 and <0.01 indicated the significant difference between relevant groups.
Data Availability.
Microarray data of naive CD44lo CD8 T cells isolated from uninfected mice and PD-1+ CXCR5+Tim-3- stemlike and PD-1+ CXCR5-Tim-3+ terminally differentiated CD8 T cells isolated from the spleens of LCMV chronically infected mice (>45 d p.i.) are available in the Gene Expression Omnibus (GEO) database (https://www.ncbi.nlm.nih.gov/geo/) under the accession number GSE84105 (4).
Supplementary Material
Acknowledgments
This work was supported by Grants R01 AI030048 and P01 AI056299 from the National Institute of Allergy and Infectious Diseases of the NIH (to R.A.).
Footnotes
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1917298117/-/DCSupplemental.
References
- 1.Hashimoto M., et al. , CD8 T cell exhaustion in chronic infection and cancer: Opportunities for interventions. Annu. Rev. Med. 69, 301–318 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Sharma P., Allison J. P., The future of immune checkpoint therapy. Science 348, 56–61 (2015). [DOI] [PubMed] [Google Scholar]
- 3.McLane L. M., Abdel-Hakeem M. S., Wherry E. J., CD8 T cell exhaustion during chronic viral infection and cancer. Annu. Rev. Immunol. 37, 457–495 (2019). [DOI] [PubMed] [Google Scholar]
- 4.Im S. J., et al. , Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature 537, 417–421 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He R., et al. , Follicular CXCR5- expressing CD8(+) T cells curtail chronic viral infection. Nature 537, 412–428 (2016). [DOI] [PubMed] [Google Scholar]
- 6.Utzschneider D. T., et al. , T cell factor 1-expressing memory-like CD8(+) T cells sustain the immune response to chronic viral infections. Immunity 45, 415–427 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Wu T., et al. , The TCF1-Bcl6 axis counteracts type I interferon to repress exhaustion and maintain T cell stemness. Sci. Immunol. 1, eaai8593 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sade-Feldman M., et al. , Defining T cell states associated with response to checkpoint immunotherapy in melanoma. Cell 175, 998–1013.e20 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siddiqui I., et al. , Intratumoral Tcf1(+)PD-1(+)CD8(+) T cells with stem-like properties promote tumor control in response to vaccination and checkpoint blockade immunotherapy. Immunity 50, 195–211.e10 (2019). [DOI] [PubMed] [Google Scholar]
- 10.Miller B. C., et al. , Subsets of exhausted CD8+ T cells differentially mediate tumor control and respond to checkpoint blockade. Nat. Immunol. 20, 326–336 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brummelman J., et al. , High-dimensional single cell analysis identifies stem-like cytotoxic CD8+ T cells infiltrating human tumors. J. Exp. Med. 215, 2520–2535 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jansen C. S., et al. , An intra-tumoral niche maintains and differentiates stem-like CD8 T cells. Nature 576, 465–470 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jadhav R. R., et al. , Epigenetic signature of PD-1+ TCF1+ CD8 T cells that act as resource cells during chronic viral infection and respond to PD-1 blockade. Proc. Natl. Acad. Sci. U.S.A. 116, 14113–14118 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matloubian M., Concepcion R. J., Ahmed R., CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J. Virol. 68, 8056–8063 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skon C. N., et al. , Transcriptional downregulation of S1pr1 is required for the establishment of resident memory CD8+ T cells. Nat. Immunol. 14, 1285–1293 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cyster J. G., Schwab S. R., Sphingosine-1-phosphate and lymphocyte egress from lymphoid organs. Annu. Rev. Immunol. 30, 69–94 (2012). [DOI] [PubMed] [Google Scholar]
- 17.Matloubian M., et al. , Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 427, 355–360 (2004). [DOI] [PubMed] [Google Scholar]
- 18.Jenne C. N., et al. , T-bet-dependent S1P5 expression in NK cells promotes egress from lymph nodes and bone marrow. J. Exp. Med. 206, 2469–2481 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mackay L. K., et al. , Cutting edge: CD69 interference with sphingosine-1-phosphate receptor function regulates peripheral T cell retention. J. Immunol. 194, 2059–2063 (2015). [DOI] [PubMed] [Google Scholar]
- 20.Shiow L. R., et al. , CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature 440, 540–544 (2006). [DOI] [PubMed] [Google Scholar]
- 21.Lamana A., et al. , CD69 modulates sphingosine-1-phosphate-induced migration of skin dendritic cells. J. Invest. Dermatol. 131, 1503–1512 (2011). [DOI] [PubMed] [Google Scholar]
- 22.Masopust D., et al. , Dynamic T cell migration program provides resident memory within intestinal epithelium. J. Exp. Med. 207, 553–564 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Casey K. A., et al. , Antigen-independent differentiation and maintenance of effector-like resident memory T cells in tissues. J. Immunol. 188, 4866–4875 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Masopust D., Schenkel J. M., The integration of T cell migration, differentiation and function. Nat. Rev. Immunol. 13, 309–320 (2013). [DOI] [PubMed] [Google Scholar]
- 25.Mueller S. N., Gebhardt T., Carbone F. R., Heath W. R., Memory T cell subsets, migration patterns, and tissue residence. Annu. Rev. Immunol. 31, 137–161 (2013). [DOI] [PubMed] [Google Scholar]
- 26.Schenkel J. M., Fraser K. A., Masopust D., Cutting edge: Resident memory CD8 T cells occupy frontline niches in secondary lymphoid organs. J. Immunol. 192, 2961–2964 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wherry E. J., Blattman J. N., Murali-Krishna K., van der Most R., Ahmed R., Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J. Virol. 77, 4911–4927 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hudson W. H., et al. , Proliferating transitory T cells with an effector-like transcriptional signature emerge from PD-1+ stem-like CD8+ T cells during chronic infection. Immunity 51, 1043–1058.e4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zander R., et al. , CD4+ T cell help is required for the formation of a cytolytic CD8+ T cell subst that protects against chronic infection and cancer. Immunity 51, 1028–1042.e4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mionnet C., et al. , CX3CR1 is required for airway inflammation by promoting T helper cell survival and maintenance in inflamed lung. Nat. Med. 16, 1305–1312 (2010). [DOI] [PubMed] [Google Scholar]
- 31.Gerlach C., et al. , The chemokine receptor CX3CR1 defines three antigen-experienced CD8 T cell subsets with distinct roles in immune surveillance and homeostasis. Immunity 45, 1270–1284 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barber D. L., et al. , Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439, 682–687 (2006). [DOI] [PubMed] [Google Scholar]
- 33.Jin H. T., et al. , Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc. Natl. Acad. Sci. U.S.A. 107, 14733–14738 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.West E. E., et al. , PD-L1 blockade synergizes with IL-2 therapy in reinvigorating exhausted T cells. J. Clin. Invest. 123, 2604–2615 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Penaloza-MacMaster P., et al. , Interplay between regulatory T cells and PD-1 in modulating T cell exhaustion and viral control during chronic LCMV infection. J. Exp. Med. 211, 1905–1918 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kamphorst A. O., et al. , Rescue of exhausted CD8 T cells by PD-1-targeted therapies is CD28-dependent. Science 355, 1423–1427 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kamphorst A. O., et al. , Proliferation of PD-1+ CD8 T cells in peripheral blood after PD-1-targeted therapy in lung cancer patients. Proc. Natl. Acad. Sci. U.S.A. 114, 4993–4998 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang A. C., et al. , T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature 545, 60–65 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wieland A., et al. , T cell receptor sequencing of activated CD8 T cells in the blood identifies tumor-infiltrating clones that expand after PD-1 therapy and radiation in a melanoma patient. Cancer Immunol. Immunother. 67, 1767–1776 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang A. C., et al. , A single dose of neoadjuvant PD-1 blockade predicts clinical outcomes in resectable melanoma. Nat. Med. 25, 454–461 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Masopust D., Soerens A. G., Tissue-resident T cells and other resident leukocytes. Annu. Rev. Immunol. 37, 521–546 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu X., Wu P., Shen Y., Jiang X., Xu F., CD8+ resident memory T cells and viral infection. Front. Immunol. 9, 2093 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schenkel J. M., Fraser K. A., Vezys V., Masopust D., Sensing and alarm function of resident memory CD8+ T cells. Nat. Immunol. 14, 509–513 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simoni Y., et al. , Bystander CD8+ T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature 557, 575–579 (2018). [DOI] [PubMed] [Google Scholar]
- 45.Murali-Krishna K., et al. , Counting antigen-specific CD8 T cells: A reevaluation of bystander activation during viral infection. Immunity 8, 177–187 (1998). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Microarray data of naive CD44lo CD8 T cells isolated from uninfected mice and PD-1+ CXCR5+Tim-3- stemlike and PD-1+ CXCR5-Tim-3+ terminally differentiated CD8 T cells isolated from the spleens of LCMV chronically infected mice (>45 d p.i.) are available in the Gene Expression Omnibus (GEO) database (https://www.ncbi.nlm.nih.gov/geo/) under the accession number GSE84105 (4).