Abstract
Background
Intercropping, an essential cultivation pattern in modern agricultural systems, increases crop yields and soil quality. Cassava and peanut intercropping systems exhibit advantages in solar utilization and cadmium absorption, etc. However, the inner mechanisms need to be elucidated. In this study, Illumina MiSeq platform was used to reveal the rhizospheric microbes and soil quality in cassava/peanut intercropping systems, and the results provided a reference for the application of this method in studying other intercropping systems.
Results
Both intercropping cassava/peanut (IP) and intercropping peanut/cassava (IC) systems significantly increased available N, available K, pH value, and urease activity, comparing with that in monocropping cassava (MC) and monocropping peanut (MP) system. However, there were few effects on the total N, total P, total K, available P, organic matter, protease activity, catalase activity, sucrase activity, and acid phosphatase activity. Both IP and MP soils contained more bacteria and fungi than those in the IC and MC soils, which were mainly made of Proteobacteria and Actinobacteria. Intercropping remarkably increased the number of Nitrospirae in IP and IC soils comparing those in MC and MP soils. Redundancy analysis (RDA) revealed that the abundances of DA101, Pilimelia, and Ramlibacter were positively correlated to the soil quality. These results suggest that intercropping enhances the available nitrogen content of soil through increasing the quantity of rhizospheric microbes, especially that of DA101 and Pilimelia.
Conclusions
The cassava/peanut intercropping system improves soil quality through increasing the available nitrogen content and abundance of DA101, Pilimelia, and Ramlibacter in the soil.
Keywords: Cassava, Intercropping, Microbial community, Peanut, Rhizospheric soil
Background
Intercropping, an old and effective planting method of growing one crop/animals alongside another, can increase yields, reduce pests [1] and weeds [2], etc. There are many intercropping models, such as chickpea/maize [3], maize/lablab [4], rubber/plantain [5], even marine finfish in shrimp ponds [6]. Intercropping showed many advantages, like higher yield [7, 8], higher light interception and utilization rate [9], phytoremediation of heavy metal contaminated soils [10], enhancing iron nutrition [11] and phosphorus availability [12], etc.
Cassava (Manihot esculenta Crantz) is an important food crop in the world [13], especially in Africa [14]. Since its importance, breeding high yield and low cyanide cultivars [14], cultivation [15, 16], genome evolution [17] as well as diseases control were widely studied [18]. Peanut (Arachis hypogaea Linn.) is a legume crop that has edible seeds and oils. In cassava, cultivation and nitrogen fixation [19], abiotic stress-responsive ESTs [20], disease resistance genes [21] were well studied. Interestingly, continuous cropping of peanut changes the soil bacterial community [22]. Previous studies showed that peanut/maize intercropping changed rhizosphere and nutrient concentrations in shoots [23], but showed no effects on Aspergillus flavus in soil [24].
Recently, the inner mechanisms of intercropping are found related to microorganisms [25]. The progress in molecular [26] and microbiome techniques [27] provided new tools for the elucidation of the mechanisms of intercropping. For instance, molecular mechanisms of microbial disease control in intercropping were related to signals triggered by neighboring plants [28]. Sugarcane-soybean intercropping increased microbial diversity [29]. Microbiome-dependent immunity was related to soil organic matter content [30]. It was reported in previous studies that intercropping can change the soil microecology, as indicated by increasing farmland biodiversity [31]. Intercropping can effectively improve the mobilization and uptake of nitrogen (N), phosphorus (P), potassium (K), and micronutrients via interspecific interactions in the rhizosphere soil [23]. In addition, legume/cereal intercropping systems could improve the utilization of phosphorus (P) by root exudation of organic acids from legume crops which also improve legume N uptake by enhanced nodulation of legume crops [32]. Soil microbe and soil enzyme activities play essential roles in nutrient cycling, organic matter decomposition, and suppression of soil-borne pathogens in the rhizosphere [33, 34]. Plants can release root exudates, thereby affecting the rhizospheric microbial community [35]. Changes can influence the potential activities of soil enzymes in microbial community composition. Due to the quantitative and qualitative differences between the root exudates of intercropping and monocropping systems, differences in the microbial community can be observed [36]. Many studies have investigated the changes in the biochemical and microbial characteristics of rhizospheric soils caused by intercropping [37]. For the alfalfa/rye intercropping system, intercropping affected the soil microbial composition and soil enzymatic activities [38]. Through phospholipid fatty acid (PLFA) analysis, it was found that the soil urease activities, invertase activities, and the soil gram-negative (G-) bacterial abundance were significantly increased in the peanut/Atractylodes lancea system [39]. Many studies have shown that the maize/peanut intercropping system can facilitate the acquisition of Fe and Zn by peanut and improve the yields of both crops [23].
Cassava/peanut intercropping is a typical intercropping cultivation mode in southern China. Since the original spacing of the cassava crop remains unchanged when intercropped with peanut, thereby it provides a distinct yield advantage. Although researches were conducted regarding the selection of the cassava/peanut intercropping model and the associated yield benefits, study on the molecular mechanisms underlying this cultivation system remains insufficient. Previous studies on cassava/peanut intercropping mainly focused on the uptake and utilization of nutrients, photosynthesis, agronomic traits, yield, efficiency, and nutrient conversion efficiency in the soil [40]. However, the influence of the microecological soil environment in the cassava/peanut intercropping system remains unknown. In this study, the inner mechanisms of cassava/peanut intercropping were elucidated through the analysis of rhizospheric soil quality and microbial community using the Illumina MiSeq platform. We found that cassava/peanut intercropping enhances the quantity of DA101 and Pilimelia, then facilitate nitrogen use efficiency in plants. The results of this study provide a reference to applying this pattern in studying other intercropping systems.
Results
Cassava/peanut intercropping enhanced soil physicochemical properties
As shown in Table 1, after three continuous years planting cassava and peanut between March and July of each year, both in the monocropping and intercropping system, the soil physicochemical properties were significantly changed. The available N, P, K increased nearly 20-folds, and the organic matter increased by almost 40% compared to the control soils (P < 0.01). IP (IP, i.e., planting in peanut former cassava field) and IC (IC, i.e., planting cassava in former peanut field) cultivation patterns significantly promoted the soil nutrient contents, especially in available N (P < 0.01) and pH value.
Table 1.
Basic soil physicochemical properties in the rhizospheric soils of MP, MC, IP and IC cultivation patterns
| Treatments | Total N (g kg − 1) | Total P (g kg − 1) | Total K (g kg − 1) | Available N (g kg − 1) | Available P (g kg − 1) | Available K (g kg − 1) | Organic matter (g kg − 1) | pH |
|---|---|---|---|---|---|---|---|---|
| MP | 1.49 ± 0.017B | 1.10 ± 0.017AB | 6.05 ± 0.059A | 0.124 ± 0.0012C | 0.036 ± 0.0028A | 0.277 ± 0.0017AB | 23.13 ± 0.8647A | 5.10 ± 0.1157B |
| MC | 1.75 ± 0.015A | 1.08 ± 0.045AB | 5.95 ± 0.130A | 0.126 ± 0.0003 BC | 0.034 ± 0.0029A | 0.208 ± 0.0015B | 25.00 ± 1.249A | 5.72 ± 0.1058A |
| IP | 1.76 ± 0.055A | 0.99 ± 0.020B | 6.29 ± 0.064A | 0.135 ± 0.0017A | 0.027 ± 0.0015A | 0.310 ± 0.0029A | 25.77 ± 1.384A | 6.12 ± 0.1099A |
| IC | 1.77 ± 0.003A | 1.18 ± 0.023A | 6.01 ± 0.113A | 0.131 ± 0.0013AB | 0.036 ± 0.0058A | 0.230 ± 0.0011AB | 25.13 ± 0.982A | 6.10 ± 0.0917A |
Note: MC monocropping cassava, MP monocropping peanut, IC planting cassava in former peanut field, IP planting peanut in former cassava field
To figure out which enzymes play a crucial role in the intercropping system, five major soil enzyme activities were measured (Table 2). The catalase, sucrase, protease, and acid phosphatase activities in the rhizospheric soil of IC and IP cultivation patterns showed no difference with those in the monocropping cassava (MC) and monocropping peanut (MP) plants. However, urease activity was highest in IC, which was 78.5% more than that in MC. These results suggested that intercropping cassava in the peanut field significantly enhances urease activity and available N contents.
Table 2.
The activities of five major enzymes in the rhizospheric soils of MP, MC, IP and IC cultivation patterns
| Treatments | Urease activity (IU L− 1) | Protease activity (U L− 1) | Catalase activity (IU L− 1) | Sucrase activity (U L− 1) | Acid phosphatase activity (U L− 1) |
|---|---|---|---|---|---|
| MP | 2.997 ± 0.0696B | 14.416 ± 2.667A | 17.742 ± 1.3336A | 1.132 ± 0.1276A | 1.540 ± 0.1157A |
| MC | 2.278 ± 0.0606C | 13.807 ± 2.018A | 18.047 ± 1.0089A | 1.203 ± 0.2498A | 1.424 ± 0.0386A |
| IP | 3.082 ± 0.0411B | 12.921 ± 1.155A | 18.489 ± 0.5776A | 1.404 ± 0.1178A | 1.920 ± 0.4649A |
| IC | 4.067 ± 0.0312A | 10.845 ± 1.159A | 19.528 ± 0.5793A | 1.381 ± 0.1715A | 1.596 ± 0.0501A |
Note: MC monocropping cassava, MP monocropping peanut, IC planting cassava in former peanut field, IP planting peanut in former cassava field
Cassava/peanut intercropping increased the quantity of culturable microbial in the rhizospheric soil
Since the rhizospheric soil physicochemical properties changed after IC and IP cultivation, we tested the microbial quantity to characterize the relationship between microbes and soil physicochemical properties (Table 3). IP cultivation pattern induced a significant increase in the bacterial abundance, fungal abundance, and total microbial amount comparing with that in MC. In contrast, microbial Shannon-Wiener diversity index in the rhizospheric soil of IP was less than that of MC. These results indicated that intercropping peanut in cassava fields increased microbial quantity but decreased microbial diversity. IC significantly reduced the bacterial abundance, fungal abundance, and total microbial amount comparing with that of MP. However, the microbial Shannon-Wiener diversity index in the rhizospheric soil of IC was increased. These results indicated that intercropping cassava in peanut fields decreased microbial amount but increased microbial diversity.
Table 3.
Microbial quantity in the rhizospheric soils of MP, MC, IP and IC cultivation patterns
| Treatments | Bacteria (105 g − 1) | Fungi (102 g − 1) | Actinomyces (105 g − 1) | Total microbial population (105 g − 1) | Shannon-Wiener diversity index |
|---|---|---|---|---|---|
| MP | 13.333 ± 0.6667A | 36.417 ± 1.6915A | 2.667 ± 0.4167A | 16.036 ± 0.9451B | 0.4615 ± 0.0284B |
| MC | 5.333 ± 0.7265B | 15.917 ± 1.3411B | 3.500 ± 0.4330A | 8.849 ± 1.0433C | 0.6818 ± 0.0126A |
| IP | 17.000 ± 1.4649A | 37.333 ± 0.7949A | 4.083 ± 0.2205A | 21.121 ± 1.3025A | 0.5042 ± 0.0299B |
| IC | 5.917 ± 0.3005B | 16.583 ± 0.6821B | 2.750 ± 0.1443A | 8.683 ± 0.1669C | 0.6359 ± 0.0170A |
Note: MC monocropping cassava, MP monocropping peanut, IC planting cassava in former peanut field, IP planting peanut in former cassava field
Cassava/peanut intercropping changed the microbial community
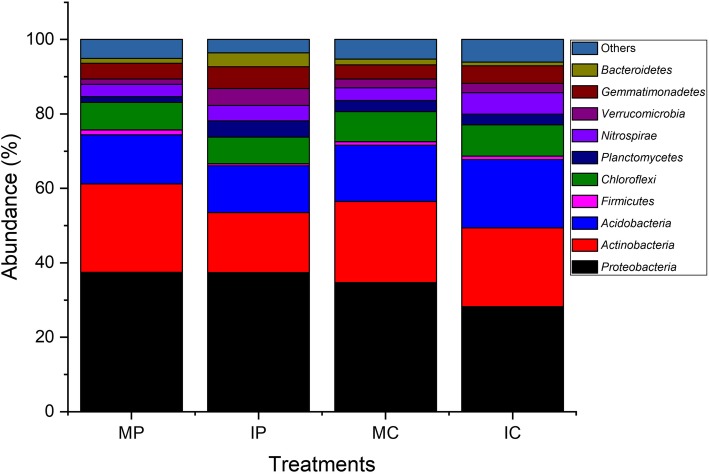
To identify the change of microbial community in the intercropping systems, a total of 43,519 valid sequences were yielded by 16S rRNA gene sequencing using the Illumina MiSeq platform, which represented the vast diversity of the bacterial community (Figs. 1, 2). The taxonomic distribution at the phylum level is shown in Fig. 1. Proteobacteria was the most abundant phylum, accounting for 28.24 to 37.45% of the total valid reads in all the samples, with an average relative abundance of 34.45%. Actinobacteria was the second most abundant phylum, with an average relative abundance of 20.70%. The other dominant phyla were Acidobacteria (12.55–18.32%, with an average value of 14.79%), Chloroflexi (7.19–8.40%, with an average value of 7.76%), Gemmatimonadetes (3.80–5.83%, with an average value of 4.63%), Nitrospirae (3.27–5.81%, with an average value of 4.16%), Planctomycetes (1.60–4.36%, with an average value of 2.96%), Verrucomicrobia (1.46–4.51%, with an average value of 2.71%), and Bacteroidetes (1.03–3.75%, with an average value of 1.91%). Importantly, the percentages of Nitrospirae, Verrucomicrobia and Gemmatimonadetes in the rhizospheric soils of the IP and IC intercropping systems were more than those in the monocropping systems. Bacteroidetes and Planctomycetes were also more abundant in the rhizospheric soil of the intercropping system IP than those in the monocropping system MP. These phyla were also less abundant in MC than that in IC. Other phyla, such as Proteobacteria, Actinobacteria, Acidobacteria and Chloroflexi did not exhibit a significant difference between the monoculture and intercropping systems. On the genera levels (Fig. 2), four kinds of soils showed different dominant genera, for instance, Aquicella, Chthonomonas, Kribbella, DA101 and Nitrospira were more abundant in the rhizospheric soil of the cassava plants in the IP and IC. Actinoallomurus and Streptomyces were still more abundant in the MC. Comparing with the rhizospheric soil of cassava, the rhizospheric soil of peanut exhibited higher diversity of dominant genera. There were 15 dominant genera in the rhizospheric soil of the intercropped peanut plants, including Optitutus, A4, Chthoniobacter, Flavisolibater, Dokdonella, and Pilimelia. However, these genera were not highly abundant in the peanut plants of monoculture system, which had different dominant genera, such as Chryseobacterium, Alicyclobacillus, Escherichia, Ralstonia, and Hypomicrobium. Thus, these results clearly demonstrated that the cassava/peanut intercropping changed the microbial community, which became distinct from that of the monocropping system. Furthermore, the microbial community in the rhizospheric soils of peanut and cassava plants is different due to different planting patterns, i.e. monocropping and intercropping systems.
Fig. 1.
Taxonomic classification of bacterial reads in the rhizospheric soils of different cultivation patterns. MC, monocropping cassava; MP, monocropping peanut; IC, intercropping peanut/cassava; IP, intercropping cassava/peanut
Fig. 2.
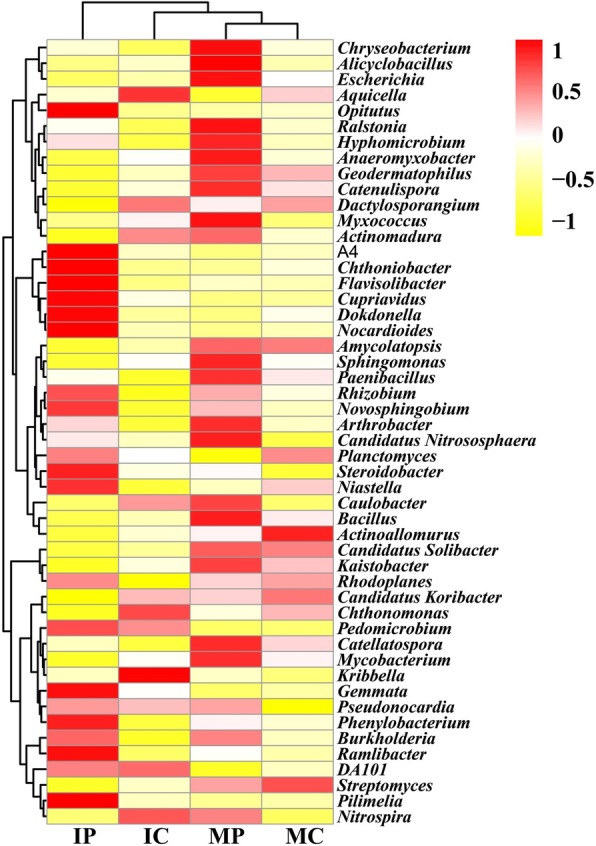
Heatmap analysis of the dominant genera in the rhizospheric soils of different cultivation patterns. MC, monocropping cassava; MP, monocropping peanut; IC, intercropping peanut/cassava; IP, intercropping cassava/peanut
Principal component analysis (PCA) and redundancy analysis (RDA) of microbial communities and physicochemical properties in different cultivation patterns
Principal component analysis showed that samples from monocropping and intercropping systems were separated from each other. The MP and IP were distributed in quadrant 2 and quadrant 1, 4, respectively (Fig. 3). It suggested that the same crop in different planting patterns influences the microbial communities accordingly. This finding may be associated with the environmental parameters in the different planting patterns. Microbial community exhibited a high correlation with intrinsic ecological parameters. Relationships between the important ecological parameters and the microbial community were discerned by RDA (Fig. 4). The length of the arrow corresponding to an ecological parameter indicated the strength of the ecological parameter concerning the overall microbial community. The results of RDA suggested that there were significant differences in the bacterial communities in the four planting patterns. As shown in Fig. 4, the available N, catalase, organic matter, sucrase activity, acid phosphatase activity, urease activity, total N, pH, total K, and available K were positively correlated with the RDA axis 1, and they were strongly and significantly associated with the overall microbial communities in IC and IP. In contrast, the total P, available P, and protease activity were negatively correlated with the RDA axis 1. These results revealed that available N, pH, catalase activity, and sucrase activity had the most significant impact on the microbial communities. Additionally, the abundances of some microbial genera, such as DA101, Pilimelia, and Ramlibacter, were positively correlated with available N, which were also the dominant genera in the soil of intercropped peanut plants (Fig. 1).
Fig. 3.
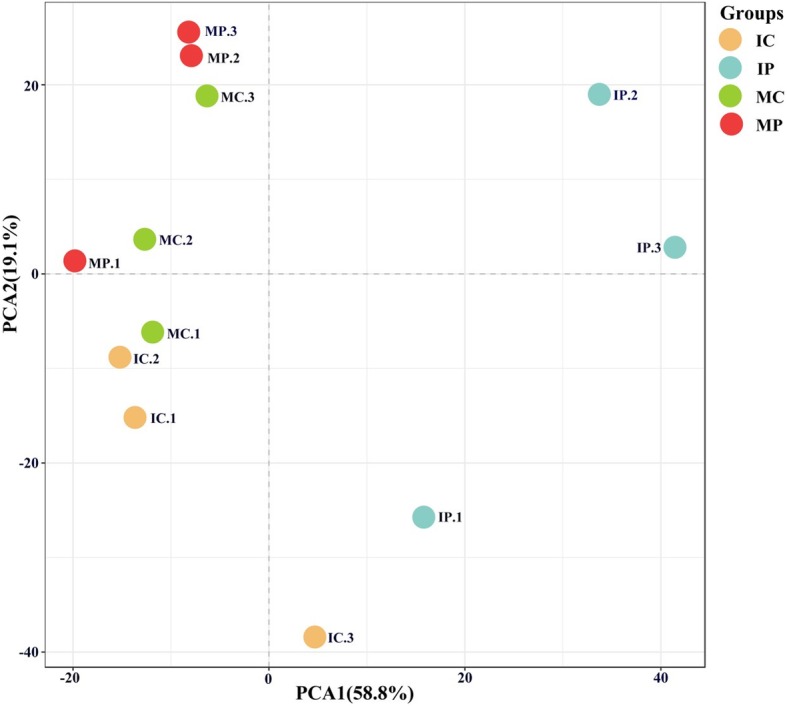
Principal component analyses of bacterial community in the rhizospheric soils of monocropping and intercropping systems based on Euclidean distance. MC, monocropping cassava; MP, monocropping peanut; IC, intercropping peanut/cassava; IP, intercropping cassava/peanut
Fig. 4.
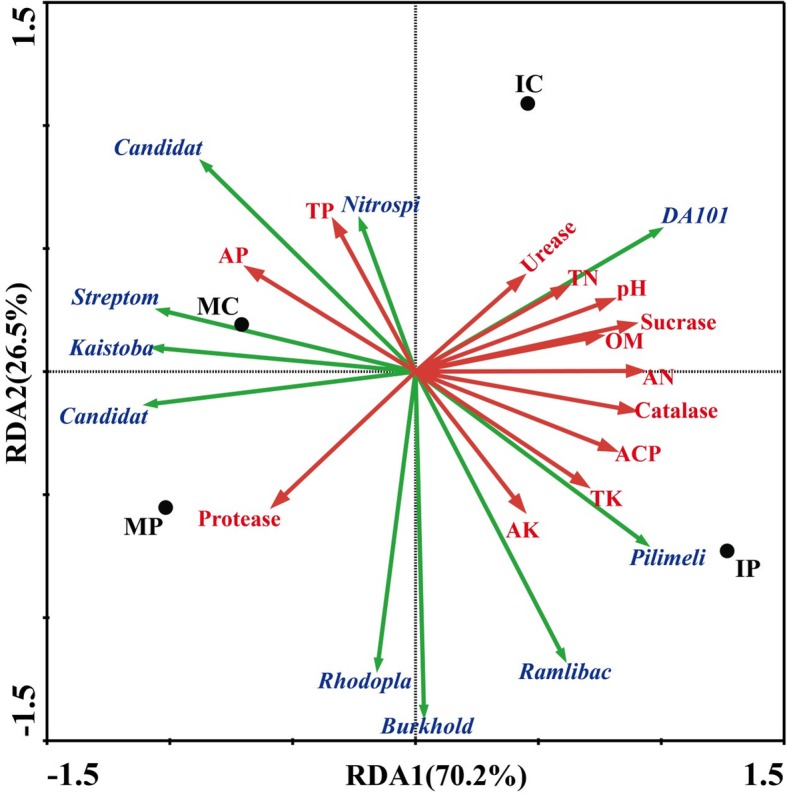
Redundancy analysis (RDA) of sequencing data of 16S rRNA gene and physicochemical properties in the rhizospheric soils of different cultivation patterns. MC, monocropping cassava; MP, monocropping peanut; IC, Intercropping peanut/cassava; IP, intercropping cassava/peanut
Discussion
Cassava/peanut intercropping system changed physicochemical properties of rhizospheric soils
Intercropping systems show great importance in agronomy, plant physiology, and ecology [41]. In general, intercropping systems change the bacterial diversity of soils [42], decrease disease rates [43, 44], and increase yields and cadmium accumulation [45]. In this study, rhizospheric soils both in IP and IC systems showed higher available N and pH than those in MP and MC systems, respectively. These were consistent with previous studies. For instance, intercropping of green garlic (Allium sativum L.) with cucumber (Cucumis sativus L.) increased organic matter and available N, P and K contents in soils [46]. Eggplant/garlic relay intercropping system increased available N contents from 61.95 mg kg− 1 to 76.30 mg kg− 1 [47]. In sugarcane-soybean intercropping system, available N increased by 10% [29]. The increase of available N was related to the urease activities, which was the only significant change in IC and IP comparing with that in MC and MP (Table 2). The role of urease is to catalyze urea hydrolysis into ammonia and carbon dioxide [48, 49], which is common in higher plants, bacteria, fungi, and algae. Thus, we speculate that the changes of soil physicochemical properties may be related to urease produced by microbial community since both the dominant genera and quantity of the microbial community were changed in IC and IP comparing with that in MC and MP (Table 3).
Improvement of physicochemical properties of rhizospheric soils was related to the microbial community in cassava/peanut intercropping system
Through MiSeq sequencing analysis of the 16S rRNA gene, we found that the percentage of Nitrospirae, Verrucomicrobia, and Gemmatimonadetes in the rhizospheric soils of IP and IC intercropping systems were higher than those in the monocropping systems (Fig. 1). This finding is quite different from the result of a previous study using a different detection technique [50]. In the previous study, denaturing gradient gel electrophoresis (DGGE) was used to analyze microbial community structure and diversity, and only ten kinds of fungi and bacteria were identified [50]. In this study, the new MiSeq sequencing technique was used, and a total of 50 species were identified (Fig. 3). Proteobacteria and Actinobacteria were the most abundances bacteria in different intercropping systems [49], while other species were varying. For instance, Firmicutes was the dominant genus in mulberry/alfalfa intercropping soils [49], but it accounted for only 0.54–1.33% in this study (Fig. 1). These results suggested that each intercropping system had different mechanisms, especially in the composition of bacterial community. Based on genus analysis, PCA and RDA analysis, it was clear that the differences in the IC and IP systems comparing with those in MC and MP systems (Figs. 2, 3 and 4) were related to the abundance of DA101, Pilimeli, and Ramlibac. Then these bacteria increased enzyme activities and improved the physicochemical properties of rhizospheric soil (Fig. 5). Unclassified DA101 belongs to the family Chthoniobacteraceae, phylum Verrucomicrobia, which was found in the soils of pepper tree [51], and grassland [52]. The abundance of DA101 was increased in the soil of intercropping system, but it was negatively correlated with the total N content and pH at significant level [53]. In this study, we found that DA101 abundance was significantly and positively correlated with the available N content in cassava/peanut intercropping system, which was consistent with the change in the abundance of the phylum Verrucomicrobia in the intercropping system (Fig. 2-4). These results indicated that the available N content, and not the total N content was related to DA101 abundance. Recently, researchers assembled a draft genome of Candidatus Udaeobacter copiosus, which is a representative of the DA101 clade, and speculated that this organism is a soil oligotrophic bacterium which reduces its requirement for soil organic carbon by acquiring amino acids and vitamins from the environment [54]. The functions of DA101 need to be investigated more deeply. Ramlibacter tataouinensis TTB310T (strain TTB310) is a beta-proteobacterium isolated from sand particles [55]. Different species from this genus were isolated in tropical forest soil [56] and the rhizospheric soil of Mugunghwa [57]. Furthermore, studies revealed that the genus of Ramlibacter has phosphatase activity [58] and ginsenoside-converting activity [59]. The relationship between these bacteria and enzymes activities should be studied further to reveal their functions.
Fig. 5.
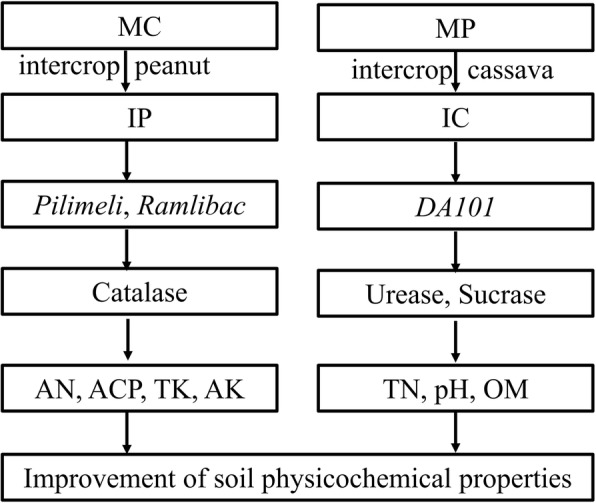
Models of IP and IC enhanced soil physicochemical properties. MC, monocropping cassava; MP, monocropping peanut; IC, peanut/cassava intercropping; IP, cassava/peanut intercropping
Conclusions
The inner mechanism of cassava/peanut intercropping system was elucidated through analyzing the physicochemical properties of rhizospheric soils and microbial community. We found that the cassava/peanut intercropping system increased the quantities of DA101, Pilimelia, and Pilimelia, and thereby increased the available N content in the soil and improved soil quality.
Methods
Cassava and peanut plants
Cassava variety Huanan 205 and shade-tolerant peanut variety Guihua 836 were provided by the Cash Crops Research Institute of the Guangxi Academy of Agricultural Sciences. All cassava and peanut experiments were performed in the Lijian Scientific Base, which is certified for the field cultivation experiment by local government.
Experimental site and soil
The experiments were performed in the Lijian Scientific Base (23°14′25′′N, 108°03′42′′E) of the Guangxi Academy of Agricultural Sciences, Nanning City, Guangxi province, China. The field site was previously used for monocropping cassava and peanut. The tested soil was acid red loam, with total nitrogen content (total N), total phosphorus content (total P), total potassium content (total K), available nitrogen content (available N), available phosphorus content (available P), available potassium content (available K), the organic matter content and pH value of 1.34 g kg− 1, 0.53 g kg − 1, 12.6 g kg − 1,0.0705 g kg − 1, 0.0139 g kg − 1, 0.098 g kg − 1, 16.2 g kg − 1, and 5.8, respectively.
Experimental design and management
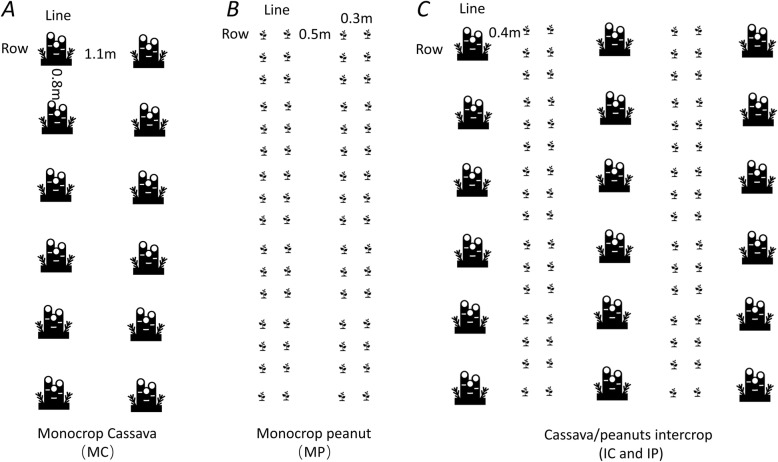
In March 2016–2018, cassava and peanut were planted simultaneously in the field. Monocropping cassava (MC), monocropping peanut (MP) crops were compared with peanut/cassava intercropping (IC, i.e., planting cassava in former peanut field) and cassava/peanut intercropping (IP, i.e., planting peanut in former cassava field) (Fig. 6). For MC, cassava planted with a row spacing of 1.1 m × 0.8 m and with equivalent line spacing. For MP, the peanut planted in a narrow-wide row spacing pattern. The line spacing for peanut in the wide line was 0.5 m, and the row spacing in the narrow row was 0.3 m × 0.16 m. For IC and IP, two lines of peanut were planted alongside one line of cassava. The line spacing between cassava and peanut was 0.4 m. The row spacing for cassava and peanut intercropping was 1.1 m × 0.8 m and 0.3 m × 0.16 m, respectively. The experiment was arranged in plots (6 m × 8 m) in a randomized design with three replicates in each treatment.
Fig. 6.
Diagram of experimental design
Peanut was supplied with 450 kg ha− 1 compound granulated NPK fertilizers (N-P2O5-K2O = 15–15-15) and 750 kg. ha− 1 fused calcium-magnesium phosphate fertilizer (available P2O5 18%). Cassava was supplied with 750 kg Ha− 1 compound granulated NPK fertilizers. The crops were irrigated two times during crop growth period based on crop water requirement and soil water content. Pesticide and herbicide were applied about two months after sowing.
Soil sampling
In July 2016–2018, the time of harvesting mature peanut, ten plants of cassava and peanut per treatments were uprooted. The rhizospheric soil from both loose soil and cohesive soil from the plant roots were collected, mixed, and separated into three sealed virus-free bags for the following assays. One sample was maintained in the refrigerator at 4 °C and used for determining the culturable soil microbe. One sample was maintained in the refrigerator at − 80 °C and used for extracting soil DNA and high-throughput sequencing. The last sample was dried naturally, grounded, and sieved for determining the soil physicochemical properties.
Soil physicochemical property analysis
The physicochemical properties were measured according to the previous reports [49]. The available N contents were measured by the alkaline hydrolysis diffusion method. The available P was measured by sodium bicarbonate extraction/Mo-Sb colorimetry method. The available K was measured by ammonium acetate extraction/flame photometry method. Organic matter content was measured by the potassium bichromate titrimetric method. Soil enzyme activities were measured according to the previous reports. Catalase activity [60], sucrase activity, proteinase activity, urease activity [48], and acid phosphatase activity [3] were measured by permanganate titration, sodium thiosulfate titration, ninhydrin colorimetry, indophenol blue colorimetry, and the disodium phosphate benzene colorimetric method, respectively.
Determination of soil microbial quantity
Soil microbial quantity was measured by the conventional microculture method. Bacteria, fungi, and actinomycetes were cultured in beef extract-peptone medium, Martin medium, and Gao 1 medium, respectively. The Shannon-Wiener index was used to calculate the biodiversity index (H): H = −Σ(ni/N) × ln(ni/N). In this formula, ni is the microbial quantity of species i, and N is the total microbial quantity [61].
Soil DNA extraction, PCR amplification, high-throughput sequencing and analysis
Total genomic DNA was extracted from the samples using the FastDNA SPIN Kit (MP Biomedicals, Santa Ana, USA) according to the manufacturer’s instructions. The DNA concentration and purity were monitored on 1% agarose gels, and the DNA was diluted to 1 ng/μL using sterile water. The DNA was stored at − 80 °C until subsequent PCR amplification. The total genomic DNA was subjected to PCR amplification using the primer pair 515f/806r, which amplified the V4 region of the 16S rDNA gene [62], following a previously described protocol [63]. PCR was carried out in 30-μL reactions with 15 μL of Phusion® high-fidelity PCR master mix, 0.2 μM of forward and reverse primers, and approximately 10 ng of template DNA. Thermal cycling consisted of an initial denaturation at 98 °C for 1 min; followed by 30 cycles of denaturation at 98 °C for 10 s, annealing at 50 °C for 30 s, and elongation at 72 °C for 30 s; and a final step at 72 °C for 5 min. The PCR products were mixed with equal volumes of 1× loading buffer (containing SYBR Green), and electrophoresis conducted on a 2% agarose gel for detection. Samples with a bright band at 400–450 bp were chosen for further experiments. The PCR products were mixed at equal concentrations. Then, the mixture of PCR products were purified with the GeneJET Gel Extraction Kit (Thermo Scientific). Sequencing libraries were generated using the NEB Next® Ultra™ DNA Library Prep Kit for Illumina (NEB, USA) following the manufacturer’s recommendations, and the index codes were added. The library quality was assessed on a Qubit 2.0 fluorometer (Thermo Scientific) and an Agilent 2100 bioanalyzer system. Finally, the library was sequenced on an Illumina MiSeq platform by the Novogene Corporation (Beijing, China), and 250/300-bp paired-end reads were generated.
Illumina MiSeq sequencing analysis
Paired-reads from the original DNA fragments were merged based on a previously described method [64]. Sequencing reads were assigned to each sample according to the individual unique barcodes, then analyzed with the QIIME (Quantitative Insights Into Microbial Ecology) software package and the UPARSE pipeline [65]. The reads were first filtered by QIIME quality filters. Default settings for Illumina processing in QIIME were used. Then, the UPARSE pipeline was used to select operational taxonomic units (OTUs) with 97% similarity. For each OTU, a representative sequence was selected and used to assign taxonomic composition by the RDP classifier. Then, the estimated species richness was determined by rarefaction analysis [66]. Redundancy analysis (RDA) was performed to analyze the correlation between environmental factors and microbial community.
Data analysis
Principal component analysis (PCA) was performed with SIMCA software v.13.0 (Umetrics, Sweden) [67]. The means and standard errors of three repeats in MC, MP, IC, and IP planting patterns were analyzed by One-way variance analysis with SPSS24.0, and Duncan’s test of the homogeneity of variance was performed with the confidence level of 0.01 [68].
Acknowledgements
We thank for Wilking Biotechnologe Co., Ltd., (Nanning, Guangxi, China) for data collection and analysis, and thanks to Dr. Ning Yu for helps in the graph drawing.
Abbreviations
- IC
Intercropping peanut/cassava
- IP
Intercropping cassava/peanut
- MC
Monocropping cassava
- MP
Monocropping peanut
- OTUs
Operational taxonomic units
- PCA
Principal component analysis
- RDA
Redundancy analysis
Authors’ contributions
TX conceived and designed the experiments and wrote the manuscript. TR and HL revised the manuscript and provided critical advice. TX, ZR, JJ, HL, HZ, WH, XF, and HZ performed the experiments. SG and LJ analyzed the data. All authors have read and approved the final manuscript.
Funding
These works were supported by the National Natural Science Foundation Project (31401318 & 31660371), Technology System for Modern Agriculture (CARS-13-Southern China), Guangxi Natural Science Foundation Project (2017GXNSFAA198144) and Scientific Project from the Guangxi Academy of Agricultural Sciences (2015JZ12 &2017JZ12), which had no role in the design of the study, the collection, analysis, and interpretation of data, or in writing the manuscript.
Availability of data and materials
Raw data of 16S rRNA gene obtained from all samples are accessible via NCBI SRA database under accession number PRJNA606845.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not Applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xiumei Tang, Email: tangxiumei196@163.com.
Ronghua Tang, Email: tronghua@163.com.
Longfei He, Email: lfhe@gxu.edu.cn.
References
- 1.Singh A, Weisser WW, Hanna R, Houmgny R, Zytynska SE. Reduce pests, enhance production: benefits of intercropping at high densities for okra farmers in Cameroon. Pest Manag Sci. 2017;73:2017–2027. doi: 10.1002/ps.4636. [DOI] [PubMed] [Google Scholar]
- 2.Liebman M, Dyck E. Crop rotation and intercropping strategies for weed management. Ecol Appl. 1993;3:92–122. doi: 10.2307/1941795. [DOI] [PubMed] [Google Scholar]
- 3.Li SM, Li L, Zhang FS, Tang C. Acid phosphatase role in chickpea/maize intercropping. Ann Bot. 2004;94:297–303. doi: 10.1093/aob/mch140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maluleke MH, Addo-Bediako A, Ayisi KK. Influence of maize/lablab intercropping on lepidopterous stem borer infestation in maize. J Econom Entomol. 2005;98:384–388. doi: 10.1093/jee/98.2.384. [DOI] [PubMed] [Google Scholar]
- 5.Tetteh EN, Abunyewa AA, Tuffour HO, Berchie JN, Acheampong PP, Twum-Ampofo K, Dawoe E, Logah V, Agbenyega O, Ennin SA, Nunoo I, Melenya C, Danquah EO, Barnes VR, Partey ST. Rubber and plantain intercropping: effects of different planting densities on soil characteristics. PLoS One. 2019;14:e0209260. doi: 10.1371/journal.pone.0209260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Damodaran D, Mojjada SK, Vase VK, Sukhdhane K, Abdul Azeez P, Kumar R. Intercropping of marine finfish in shrimp ponds: A maiden feasibility study. PLoS One. 2019;14:e0216648. doi: 10.1371/journal.pone.0216648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen P, Song C, Liu XM, Zhou L, Yang H, Zhang X, Zhou Y, Du Q, Pang T, Fu ZD, Wang XC, Liu WG, Yang F, Shu K, Du JB, Liu J, Yang WY, Yong TW. Yield advantage and nitrogen fate in an additive maize-soybean relay intercropping system. Sci Total Environ. 2019;657:987–999. doi: 10.1016/j.scitotenv.2018.11.376. [DOI] [PubMed] [Google Scholar]
- 8.Dong N, Tang MM, Zhang WP, Bao XG, Wang Y, Christie P, Li L. Temporal differentiation of crop growth as one of the drivers of intercropping yield advantage. Sci Rep. 2018;8:3110. doi: 10.1038/s41598-018-21414-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhi XY, Han YC, Xing FF, Lei YP, Wang GP, Feng L, Yang BF, Wang ZB, Li XL, Xiong SW, Fan ZY, Li YB. How do cotton light interception and carbohydrate partitioning respond to cropping systems including monoculture, intercropping with wheat, and direct-seeding after wheat? PLoS One. 2019;14:e0217243. doi: 10.1371/journal.pone.0217243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Conti L, Ceretta CA, Melo GWB, Tiecher TL, Silva LOS, Garlet LP, Mimmo T, Cesco S, Brunetto G. Intercropping of young grapevines with native grasses for phytoremediation of cu-contaminated soils. Chemosphere. 2019;216:147–156. doi: 10.1016/j.chemosphere.2018.10.134. [DOI] [PubMed] [Google Scholar]
- 11.Dai J, Qiu W, Wang N, Nakanishi H, Zuo Y. Comparative transcriptomic analysis of the roots of intercropped peanut and maize reveals novel insights into peanut iron nutrition. Plant Physiol Biochem. 2018;127:516–524. doi: 10.1016/j.plaphy.2018.04.024. [DOI] [PubMed] [Google Scholar]
- 12.Darch T, Giles CD, Blackwell MSA, George TS, Brown LK, Menezes-Blackburn D, Shand CA, Stutter MI, Lumsdon DG, Mezeli MM, Wendler R, Zhang H, Wearing C, Cooper P, Haygarth PM. Inter- and intra-species intercropping of barley cultivars and legume species, as affected by soil phosphorus availability. Plant Soil. 2018;427:125–138. doi: 10.1007/s11104-017-3365-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okezie BO, Kosikowski FV. Cassava as a food. Crit Rev Food Sci Nutr. 1982;17:259–275. doi: 10.1080/10408398209527349. [DOI] [PubMed] [Google Scholar]
- 14.Onabolu A, Bokanga M, Tylleskar T, Rosling H. High cassava production and low dietary cyanide exposure in mid-West Nigeria. Public Health Nutr. 2001;4:3–9. doi: 10.1079/PHN200049. [DOI] [PubMed] [Google Scholar]
- 15.Schulthess F, Chabi-Olaye A, Gounou S. Multi-trophic level interactions in a cassava-maize mixed cropping system in the humid tropics of West Africa. Bull Entomol Res. 2004;94:261–272. doi: 10.1079/BER2004296. [DOI] [PubMed] [Google Scholar]
- 16.Aina OO, Dixon AG, Akinrinde EA. Effect of soil moisture stress on growth and yield of cassava in Nigeria. Pak J Biol Sci. 2007;10:3085–3090. doi: 10.3923/pjbs.2007.3085.3090. [DOI] [PubMed] [Google Scholar]
- 17.Sardos J, McKey D, Duval MF, Malapa R, Noyer JL, Lebot V. Evolution of cassava (Manihot esculenta Crantz) after recent introduction into a South Pacific Island system: the contribution of sex to the diversification of a clonally propagated crop. Genome. 2008;51:912–921. doi: 10.1139/G08-080. [DOI] [PubMed] [Google Scholar]
- 18.Patil BL, Fauquet CM. Cassava mosaic geminiviruses: actual knowledge and perspectives. Mol Plant Pathol. 2009;10:685–701. doi: 10.1111/j.1364-3703.2009.00559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El-Akhal MR. Rincon a, Coba de la Pena T, Lucas MM, El Mourabit N, Barrijal S, Pueyo JJ. Effects of salt stress and rhizobial inoculation on growth and nitrogen fixation of three peanut cultivars. Plant Biol (Stuttg) 2013;15:415–421. doi: 10.1111/j.1438-8677.2012.00634.x. [DOI] [PubMed] [Google Scholar]
- 20.Kumari A, Kumar A, Wany A, Prajapati GK, Pandey DM. Identification and annotation of abiotic stress responsive candidate genes in peanut ESTs. Bioinformation. 2012;8:1211–1219. doi: 10.6026/97320630081211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar D, Kirti PB. Pathogen-induced SGT1 of Arachis diogoi induces cell death and enhanced disease resistance in tobacco and peanut. Plant Biotechnol J. 2015;13:73–84. doi: 10.1111/pbi.12237. [DOI] [PubMed] [Google Scholar]
- 22.Chen M, Li X, Yang Q, Chi X, Pan L, Chen N, Yang Z, Wang T, Wang M, Yu S. Dynamic succession of soil bacterial community during continuous cropping of peanut (Arachis hypogaea L.) PLoS One. 2014;9:e101355. doi: 10.1371/journal.pone.0101355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inal A, Gunes A, Zhang F, Cakmak I. Peanut/maize intercropping induced changes in rhizosphere and nutrient concentrations in shoots. Plant Physiol Biochem. 2007;45:350–356. doi: 10.1016/j.plaphy.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 24.Horn BW, Greene RL, Dorner JW. Effect of corn and peanut cultivation on soil populations of Aspergillus flavus and A. parasiticus in southwestern Georgia. Appl Environ Microbiol. 1995;61:2472–2475. doi: 10.1128/AEM.61.7.2472-2475.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen M, Li X, Yang Q, Chi X, Pan L, Chen N, Yang Z, Wang T, Wang M, Yu S. Soil eukaryotic microorganism succession as affected by continuous cropping of peanut--pathogenic and beneficial fungi were selected. PLoS One. 2012;7:e40659. doi: 10.1371/journal.pone.0040659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nelkner J, Henke C, Lin TW, Patzold W, Hassa J, Jaenicke S, Grosch R, Puhler A, Sczyrba A, Schluter A. Effect of long-term farming practices on agricultural soil microbiome members represented by metagenomically assembled genomes (MAGs) and their predicted plant-beneficial genes. Genes (Basel). 2019:10, 424. [DOI] [PMC free article] [PubMed]
- 27.Wang G, Schultz P, Tipton A, Zhang J, Zhang F, Bever JD. Soil microbiome mediates positive plant diversity-productivity relationships in late successional grassland species. Ecol Lett. 2019;22:1221–1232. doi: 10.1111/ele.13273. [DOI] [PubMed] [Google Scholar]
- 28.Zhu S, Morel JB. Molecular mechanisms underlying microbial disease control in intercropping. Mol Plant-Microbe Interactions. 2019;32:20–24. doi: 10.1094/MPMI-03-18-0058-CR. [DOI] [PubMed] [Google Scholar]
- 29.Lian T, Mu Y, Jin J, Ma Q, Cheng Y, Cai Z, Nian H. Impact of intercropping on the coupling between soil microbial community structure, activity, and nutrient-use efficiencies. Peer J. 2019;7:e6412. doi: 10.7717/peerj.6412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haron MH, Tyler HL, Chandra S, Moraes RM, Jackson CR, Pugh ND, Pasco DS. Plant microbiome-dependent immune enhancing action of Echinacea purpurea is enhanced by soil organic matter content. Sci Rep. 2019;9:136. doi: 10.1038/s41598-018-36907-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang F, Shen J, Li L, Liu X. An overview of rhizosphere processes related with plant nutrition in major cropping systems in China. Plant Soil. 2004;260:89–99. doi: 10.1023/B:PLSO.0000030192.15621.20. [DOI] [Google Scholar]
- 32.Fang Q, Yu Q, Wang E, Chen Y, Zhang G, Wang J, Li L. Soil nitrate accumulation, leaching and crop nitrogen use as influenced by fertilization and irrigation in an intensive wheat–maize double cropping system in the North China plain. Plant Soil. 2006;284:335–350. doi: 10.1007/s11104-006-0055-7. [DOI] [Google Scholar]
- 33.Burns RG, DeForest JL, Marxsen J, Sinsabaugh RL, Stromberger ME, Wallenstein MD, Weintraub MN, Zoppini A. Soil enzymes in a changing environment: current knowledge and future directions. Soil Biol Biochem. 2013;58:216–234. doi: 10.1016/j.soilbio.2012.11.009. [DOI] [Google Scholar]
- 34.Pignataro A, Moscatelli MC, Mocali S, Grego S, Benedetti A. Assessment of soil microbial functional diversity in a coppiced forest system. App Soil Ecol. 2012;62:115–123. doi: 10.1016/j.apsoil.2012.07.007. [DOI] [Google Scholar]
- 35.Kourtev P, Ehrenfeld J, Häggblom M. Experimental analysis of the effect of exotic and native plant species on the structure and function of soil microbial communities. Soil Biol Biochem. 2003;35:895–905. doi: 10.1016/S0038-0717(03)00120-2. [DOI] [Google Scholar]
- 36.Baudoin E, Benizri E, Guckert A. Impact of artificial root exudates on the bacterial community structure in bulk soil and maize rhizosphere. J Soil Biol Biochem. 2003;35:1183–1192. doi: 10.1016/S0038-0717(03)00179-2. [DOI] [Google Scholar]
- 37.Bainard LD, Koch AM, Gordon AM, Klironomos JN. Growth response of crops to soil microbial communities from conventional monocropping and tree-based intercropping systems. Plant Soil. 2012;363:345–356. doi: 10.1007/s11104-012-1321-5. [DOI] [Google Scholar]
- 38.Sun YM, Zhang NN, Wang ET, Yuan HL, Yang JS, Chen WX. Influence of intercropping and intercropping plus rhizobial inoculation on microbial activity and community composition in rhizosphere of alfalfa (Medicago sativa L.) and Siberian wild rye (Elymus sibiricus L.) FEMS Microbiol Ecol. 2009;70:62–70. doi: 10.1111/j.1574-6941.2009.00752.x. [DOI] [PubMed] [Google Scholar]
- 39.Dai CC, Chen Y, Wang XX, Li PD. Effects of intercropping of peanut with the medicinal plant Atractylodes lancea on soil microecology and peanut yield in subtropical China. Agrofor Syst. 2012;87:417–426. doi: 10.1007/s10457-012-9563-z. [DOI] [Google Scholar]
- 40.Polthanee A, Wanapat S, Mangprom P. Row arrangement of peanut in cassava-peanut intercropping: II. Kaen Kaset: Nutrient removal and nutrient balance in soil; 1998. [Google Scholar]
- 41.Brooker RW, Bennett AE, Cong WF, Daniell TJ, George TS, Hallett PD, Hawes C, Iannetta PP, Jones HG, Karley AJ, Li L, McKenzie BM, Pakeman RJ, Paterson E, Schob C, Shen J, Squire G, Watson CA, Zhang C, Zhang F, Zhang J, White PJ. Improving intercropping: a synthesis of research in agronomy, plant physiology and ecology. New Phytol. 2015;206:107–117. doi: 10.1111/nph.13132. [DOI] [PubMed] [Google Scholar]
- 42.Cao X, Liu S, Wang J, Wang H, Chen L, Tian X, Zhang L, Chang J, Wang L, Mu Z, Qiao Z. Soil bacterial diversity changes in different broomcorn millet intercropping systems. J Basic Microbiol. 2017;57:989–997. doi: 10.1002/jobm.201700133. [DOI] [PubMed] [Google Scholar]
- 43.Boudreau MA. Diseases in intercropping systems. Annu Rev Phytopathol. 2013;51:499–519. doi: 10.1146/annurev-phyto-082712-102246. [DOI] [PubMed] [Google Scholar]
- 44.Damicone JP, Edelson JV, Sherwood JL, Myers LD, Motes JE. Effects of border crops and intercrops on control of cucurbit virus diseases. Plant Dis. 2007;91:509–516. doi: 10.1094/PDIS-91-5-0509. [DOI] [PubMed] [Google Scholar]
- 45.Deng Q, Deng Q, Wang Y, Li L, Long X, Ren S, Fan Y, Lin L, Xia H, Liang D, Wang J, Zhang H, Lv X, Wang Y. Effects of intercropping with Bidens species plants on the growth and cadmium accumulation of Ziziphus acidojujuba seedlings. Environ Monit Assess. 2019;191:342. doi: 10.1007/s10661-019-7375-6. [DOI] [PubMed] [Google Scholar]
- 46.Xiao X, Cheng Z, Meng H, Liu L, Li H, Dong Y. Intercropping of green garlic (Allium sativum L.) induces nutrient concentration changes in the soil and plants in continuously cropped cucumber (Cucumis sativus L.) in a plastic tunnel. PLoS One. 2013;8:e62173. doi: 10.1371/journal.pone.0062173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang M, Wu C, Cheng Z, Meng H, Zhang M, Zhang H. Soil chemical property changes in eggplant/garlic relay intercropping systems under continuous cropping. PLoS One. 2014;9:e111040. doi: 10.1371/journal.pone.0111040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cordero I, Snell H, Bardgett RD. High throughput method for measuring urease activity in soil. Soil Biol Biochem. 2019;134:72–77. doi: 10.1016/j.soilbio.2019.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang MM, Wang N, Hu YB, Sun GY. Changes in soil physicochemical properties and soil bacterial community in mulberry (Morus alba L.)/alfalfa (Medicago sativa L.) intercropping system. Microbiologyopen. 2018;7:e00555. doi: 10.1002/mbo3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu HQ, Huang J, Liu ZF, Wei YX, Su BM, Li T. Effects of cassava-peanut intercropping on microbial amount, community structure and diversity in rhizosphere soils. [in Chinese] J Southern Agricul. 2016;47:185–190. [Google Scholar]
- 51.Dawkins K, Esiobu N. The invasive brazilian pepper tree (Schinus terebinthifolius) is colonized by a root microbiome enriched with Alphaproteobacteria and unclassified Spartobacteria. Front Microbiol. 2018;9:876. doi: 10.3389/fmicb.2018.00876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Felske A, Akkermans AD. Prominent occurrence of ribosomes from an uncultured bacterium of the Verrucomicrobiales cluster in grassland soils. Lett App Microbiol. 1998;26:219–223. doi: 10.1046/j.1472-765X.1998.00322.x. [DOI] [PubMed] [Google Scholar]
- 53.Shen C, Ge Y, Yang T, Chu H. Verrucomicrobial elevational distribution was strongly influenced by soil pH and carbon/nitrogen ratio. J Soils Sediments. 2017;17:2449–2456. doi: 10.1007/s11368-017-1680-x. [DOI] [Google Scholar]
- 54.Brewer TE, Handley KM, Carini P, Gilbert JA, Fierer N. Genome reduction in an abundant and ubiquitous soil bacterium ‘Candidatus Udaeobacter copiosus’. Nat Microbiol. 2016;2:1–7. doi: 10.1038/nmicrobiol.2016.198. [DOI] [PubMed] [Google Scholar]
- 55.De Luca G, Barakat M, Ortet P, Fochesato S, Jourlin-Castelli C, Ansaldi M, Py B, Fichant G, Coutinho PM, Voulhoux R, Bastien O, Marechal E, Henrissat B, Quentin Y, Noirot P, Filloux A, Mejean V, DuBow MS, Barras F, Barbe V, Weissenbach J, Mihalcescu I, Vermeglio A, Achouak W, Heulin T. The cyst-dividing bacterium Ramlibacter tataouinensis TTB310 genome reveals a well-stocked toolbox for adaptation to a desert environment. PLoS One. 2011;6:e23784. doi: 10.1371/journal.pone.0023784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang XJ, Feng GD, Yao Q, Wang YH, Yang SZ, Zhu HH. Ramlibacter humi sp. nov., isolated from tropical forest soil. Int J Syst Evol Microbiol. 2019;69:3460–3464. doi: 10.1099/ijsem.0.003641. [DOI] [PubMed] [Google Scholar]
- 57.Yan ZF, Trinh H, Moya G, Lin P, Li CT, Kook M, Yi TH. Ramlibacter rhizophilus sp. nov., isolated from rhizosphere soil of national flower Mugunghwa from South Korea. Int J Syst Evol Microbiol. 2017;67:3773–3777. doi: 10.1099/ijsem.0.002191. [DOI] [PubMed] [Google Scholar]
- 58.Skouri-Panet F, Benzerara K, Cosmidis J, Ferard C, Caumes G, De Luca G, Heulin T, Duprat E. In vitro and in silico evidence of phosphatase diversity in the biomineralizing bacterium Ramlibacter tataouinensis. Front Microbiol. 2017;8:2592. doi: 10.3389/fmicb.2017.02592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang L, An DS, Kim SG, Jin FX, Kim SC, Lee ST, Im WT. Ramlibacter ginsenosidimutans sp. nov., with ginsenoside-converting activity. J Microbiol Biotechnol. 2012;22:311–315. doi: 10.4014/jmb.1106.06041. [DOI] [PubMed] [Google Scholar]
- 60.Mueller S, Riedel H-D, Stremmel W. Determination of catalase activity at physiological hydrogen peroxide concentrations. Anal Biochem. 1997;245:55–60. doi: 10.1006/abio.1996.9939. [DOI] [PubMed] [Google Scholar]
- 61.Weaver MA, Krutz LJ, Zablotowicz RM, Reddy KN. Effects of glyphosate on soil microbial communities and its mineralization in a Mississippi soil. Pest Manag Sci. 2007;63:388–393. doi: 10.1002/ps.1351. [DOI] [PubMed] [Google Scholar]
- 62.Bergmann GT, Bates ST, Eilers KG, Lauber CL, Caporaso JG, Walters WA, Knight R, Fierer N. The under-recognized dominance of Verrucomicrobia in soil bacterial communities. Soil Biol Biochem. 2011;43:1450–1455. doi: 10.1016/j.soilbio.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, Owens SM, Betley J, Fraser L, Bauer M, Gormley N, Gilbert JA, Smith G, Knight R. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012;6:1621–1624. doi: 10.1038/ismej.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Magoc T, Salzberg SL. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics. 2011;27:2957–2963. doi: 10.1093/bioinformatics/btr507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, Sahl JW, Stres B, Thallinger GG, Van Horn DJ, Weber CF. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol. 2009;75:7537–7541. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang L, Fuchino H, Kawahara N, Narukawa Y, Hada N, Kiuchi F. Application of a new method, orthogonal projection to latent structure (OPLS) combined with principal component analysis (PCA), to screening of prostaglandin E2 production inhibitory flavonoids in Scutellaria root. J Nat Med. 2016;70:731–739. doi: 10.1007/s11418-016-1004-2. [DOI] [PubMed] [Google Scholar]
- 68.Guo M, Rupe MA, Wei J, Winkler C, Goncalves-Butruille M, Weers BP, Cerwick SF, Dieter JA, Duncan KE, Howard RJ, Hou Z, Loffler CM, Cooper M, Simmons CR. Maize ARGOS1 (ZAR1) transgenic alleles increase hybrid maize yield. J Exp Bot. 2013;65:249–260. doi: 10.1093/jxb/ert370. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw data of 16S rRNA gene obtained from all samples are accessible via NCBI SRA database under accession number PRJNA606845.