Abstract
PURPOSE
Rare cancers are challenging for researchers, as clinicians and scientists have difficulty recruiting sufficient patient cases to power studies appropriately. Likewise, patients often are frustrated by a lack of specific information or evidence base for their cancer and, although eager to participate in research, have limited opportunities. We established CART-WHEEL.org, an online patient-entered database, to directly engage patients in the research process, collect rare cancer data, and facilitate their entry into additional research.
PATIENTS AND METHODS
Patients access CART-WHEEL.org directly online. Clinical information is collected from users via a streamlined questionnaire developed collaboratively with consumer groups to ensure accessibility and relevance. Data collected include the following: patient demographics, comorbidities, and risk factors and tumor diagnostic, biomarker, and treatment history. Patients can download a medical summary for personal use; consent for research use of data; and indicate willingness to be contacted about other research or clinical trials. We describe data collected to date and its validation, and we provide examples of how CART-WHEEL.org can facilitate rare cancer research.
RESULTS
From January 2010 to March 2018, 558 patients provided consent and entered their rare cancer data. One hundred distinct rare tumor types and patients from 22 countries were included. Validation of data entered by 21 patients with sarcoma against a hospital database demonstrated accuracy sufficient to facilitate future research in key fields, such as tumor site (95%) and histopathologic diagnosis (90%). Examples of CART-WHEEL–based disease-specific projects, subsequent recruitment to other rare cancer projects, and rare cancer patient cases of interest are described.
CONCLUSIONS
Online platforms like CART-WHEEL.org can engage consumers directly, facilitating collection of patient-entered rare cancer data for hypothesis generation, and connect patients with researchers to enable specific rare cancer research and clinical trials.
INTRODUCTION
Defined by an incidence of < 6 per 100,000 person-years, rare cancers account for one fifth of cancer diagnoses and one third of cancer deaths, so they are a paradoxically common problem.1 However, treatment of most rare cancers is plagued by limited evidence, as research is complicated by small patient numbers and a lack of dedicated resources that have contributed to a slower rate of improvement in survival compared with more common cancers.2 The aims of better identifying patients with rare cancer and increasing their participation in clinical trials and basic research require novel platforms; international collaboration; and involvement from all stakeholders, including patients.3-5 Involvement of patients in cancer research is an important component of health services that has been gaining traction in recent years.6-9 Patients with rare diseases (including cancers) reportedly use the Internet disproportionately more than those with common diseases to address knowledge gaps and connect with other patients for support and information.10 Similarly, researchers of rare cancer can better capitalize on modern connectivity through consumer-driven databases available online to overcome the barrier of geography when investigating diseases with low incidence.2
The Centre for Analysis of Rare Tumors, or CART-WHEEL.org, is a Web-based platform that can help bridge the gap between patients and researchers. It allows patients to enter and share their clinical data and to consent to being contacted for additional research opportunities to facilitate rare tumor research.11 It is supported by the not-for-profit data-linkage platform BioGrid Australia and by cancer patient organizations, such as Rare Cancers Australia. CART-WHEEL.org was designed to assist patients, their next-of-kin, or their guardians to participate in rare tumor research. This report describes the CART-WHEEL.org database and consent process; outlines the validation of the patient-entered data; provides an example of how the database has been used to facilitate research into rare tumors; and describes clinical patient cases that highlight the plight currently faced by patients with rare cancer as well as progress made in the field.
CONTEXT
Key Objective
Online platforms have the potential to facilitate patient engagement in research, with particular benefits for traditionally underserved populations. We developed CART-WHEEL.org to assess whether an online database could be used to collect natural history data directly from patients with rare cancers and streamline their enrollment into specific research programs and clinical trials.
Knowledge Generated
Since enrolment, CART-WHEEL.org has collected patient data on more than 100 rare tumor types from patients living in 22 different countries and has directed more than 10% of participants with solid tumors into traditional biomarker and genomic research. In partnership with the International Waldenström's Macroglobulinemia Foundation, the platform hosts a growing database of more than 382 patients with Waldenström's macroglobulinemia.
Relevance
Unlike traditional hospital-based research, online platforms are not limited by geography and can synergize with existing online patient networks to accelerate recruitment of patients with rare diseases, including cancer, to facilitate research.
PATIENT AND METHODS
CART-WHEEL.org provides an online platform linking patients with a rare cancer to relevant researchers for recruitment to specific clinical trials or research studies. Potential participants locate CART-WHEEL.org directly or via associated consumer groups, learn about processes involved in rare tumor research, and enter their medical information into a streamlined online questionnaire. Collected clinical information is stored securely, and patients can download a summary of their entered information. Approved researchers involved in rare cancer projects can request de-identified aggregate data. Expert consumers and consumer groups have an ongoing role in developing and testing the questionnaire and consent process to optimize usability and minimize barriers to participation.
The project was approved by the Melbourne Health Human Research Ethics Committee (MH-2007.270). To our knowledge, this project is the first ethically approved, academically overseen, internationally available database for patient-entered clinical, molecular, and familial data covering all rare cancer types.
Database Design and Hosting
Data collected through CART-WHEEL.org are stored in the Rare Tumor Database managed by BioGrid Australia. Participant identifiers and clinical information are stored in separate databases. BioGrid Australia has completed extensive work in overcoming barriers to safeguard privacy and ethics while allowing data linkages to facilitate collaborative medical and bioinformatics research.12
Patient-entered clinical data are collected through a streamlined questionnaire, with data fields selected to collect information about the natural history of rare tumors and their management to provide a foundation for future rare cancer research. Other rare disease platforms that facilitate patient-entered data have demonstrated advantages, including decreased burden on clinical teams and ease of data collection and access.13 Collected data fields include the following: demographic information (age, sex, country of residence); tumor diagnostic information (date of diagnosis, primary site, tumor histologic type, genetic testing); treatment information (surgeries and biopsies, radiation therapies, drug therapy received, including drug access—such as participation in clinical trials, and adverse effects experienced); risk factors for the development of tumors (family history of cancer, germline mutation testing, history of an inherited familial cancer syndrome, smoking and alcohol consumption); and comorbidities. Comorbidities are recorded using a patient-friendly version of the Charlson comorbidity index.14 The questionnaire allows development of disease-specific projects, with custom fields that allow approved researchers and consumer groups to develop their own projects within the CART-WHEEL.org database.
Patient Consent
The consent process has been designed to minimize barriers for participant involvement.12 Patients originally were provided with participant information online and a consent form that could be printed, completed, and returned to CART-WHEEL.org by e-mail or post. Online push button consent was implemented in June 2017. The consent process is dynamic, allowing for different levels of involvement and patient-initiated modifications at any time. The base level of consent permits the storage of consumers’ self-entered medical information in the Rare Tumor Database and its deidentified use by approved researchers. Patients can grant permission to be contacted by BioGrid Australia about other relevant ethically approved research projects or clinical trials; allow a nominated clinician to enter, view, and edit their data, which is stored in parallel to their own entered data; and provide permission for BioGrid Australia to obtain copies of histopathology reports or other supporting medical information for data validation.
Data Quality Validation
Quality of patient-entered data was assessed in a cohort of patients attending a sarcoma clinic that had an existing clinical database overseen by a trained data manager, allowing data validation. Attendees to the clinic were recruited during 5 months by a clinical research fellow who provided information about CART-WHEEL.org and the validation study. Those interested were provided with a paper-based CART-WHEEL.org brochure, patient information and consent form, and recruitment letter describing this substudy. Twenty-one patients registered with CART-WHEEL.org, entered data, and provide consent. For all patients, selected data fields (demographics, tumor site and histology, date of diagnosis, and treatment) were compared for accuracy, and the hospital sarcoma database was used as the gold standard.
Data Access for Researchers
Researchers involved in an ethically approved rare cancer project can apply to access data held in CART-WHEEL.org using the BioGrid Australia standard process. This process is described in detail at BioGrid Australia online.15
RESULTS
Accrual and Participant Demographics
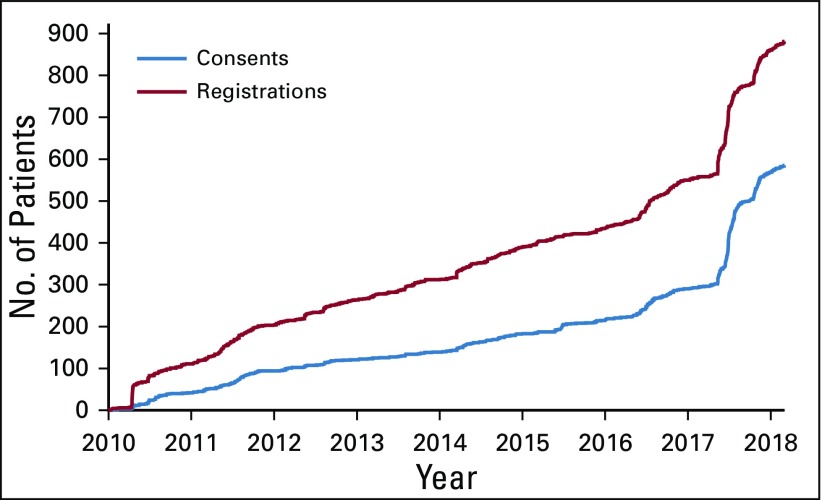
From January 2010 to March 2018, 881 patients registered, and 558 patients completed the consent process and entered data into the questionnaire (Fig 1). From 2010 until 2015, recruitment averaged 72 registrations per year; recruitment increased to 116 in 2016 and to 311 in 2017. This increase correlated with recruitment to the substudy, Waldenström's Macroglobulinemia Study Involving CART-WHEEL.org, or Whimsical (described in the Projects section). The average time from diagnosis to registration for patients who used CART-WHEEL.org was 2.9 years (range, 0-33.4 years).
FIG 1.
Consents and Registrations to CART-WHEEL.org. From January 2010 to January 2018, 881 patients registered to CART-WHEEL.org. A total of 585 patients consented to the use of their data for research purposes. An increase in recruitment in 2017 relates to targeted recruitment via CART-WHEEL.org for the Waldenström’s Macroglobulinemia Whimsical study.
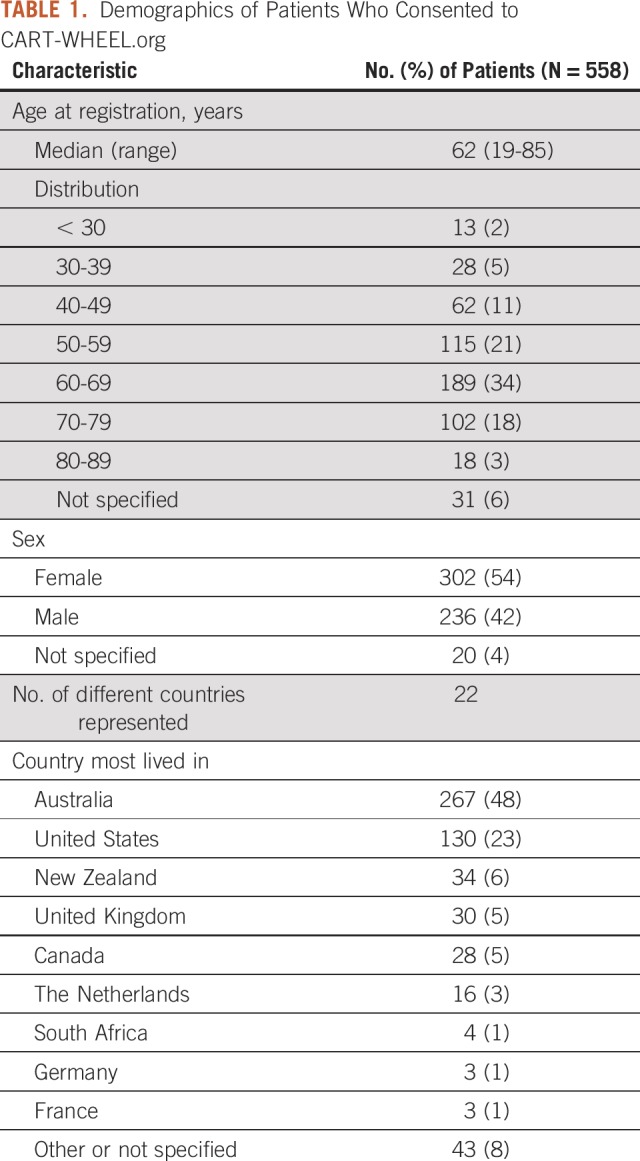
Table 1 lists the demographics of the patients who consented to CART-WHEEL.org use. The largest proportion of patients (34%) were age 60-69 years, and 18% of patients were younger than age 50. There were more women (54%) than men (42%); 4% of patients did not specify sex. At the time of this analysis, CART-WHEEL.org captured patient information from 22 countries; the majority were from Australia (48%) and the United States (23%).
TABLE 1.
Demographics of Patients Who Consented to CART-WHEEL.org
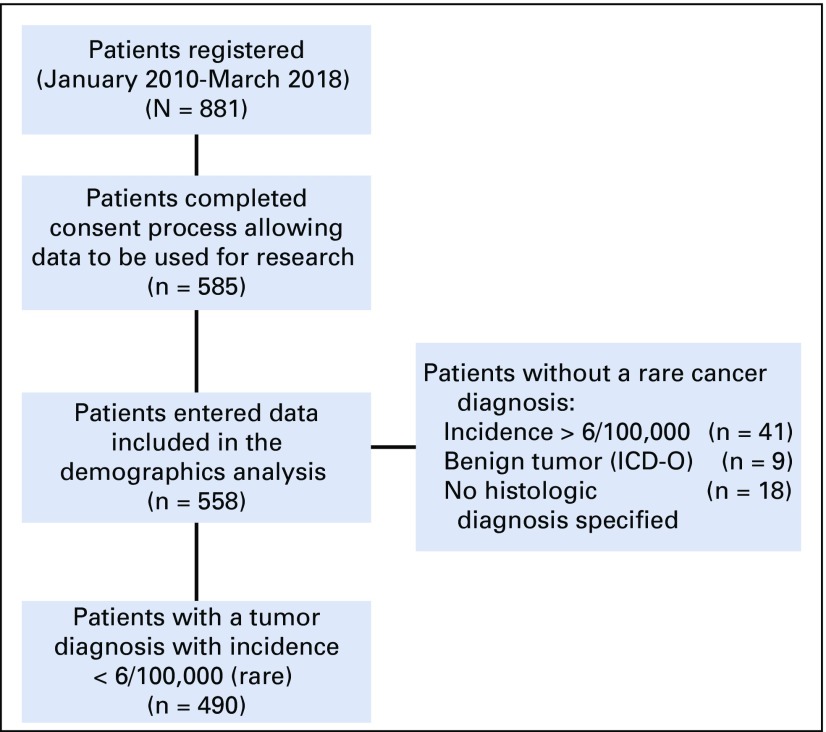
Of the 558 consenting patients, 490 (88%) entered a diagnosis of a rare malignant tumor (Fig 2). Forty-one patients (7%) entered a diagnosis of a tumor histology with an incidence of > 6 per 100,000 (potentially including rare subtypes of more common tumors), 9 patients entered a benign diagnosis, and 18 patients did not specify a histologic diagnosis. All 558 patients were included in the demographic analysis. Only the patients who entered a diagnosis of a rare malignant tumor were included in analysis of time points and treatment events.
FIG 2.
Patients registered and consented to CART-WHEEL.org. All patients who consented and entered data were included in demographic analysis. Only patients with a rare malignant tumor diagnosis were included in analysis of other events. ICD-O, International Classification of Diseases for Oncology.
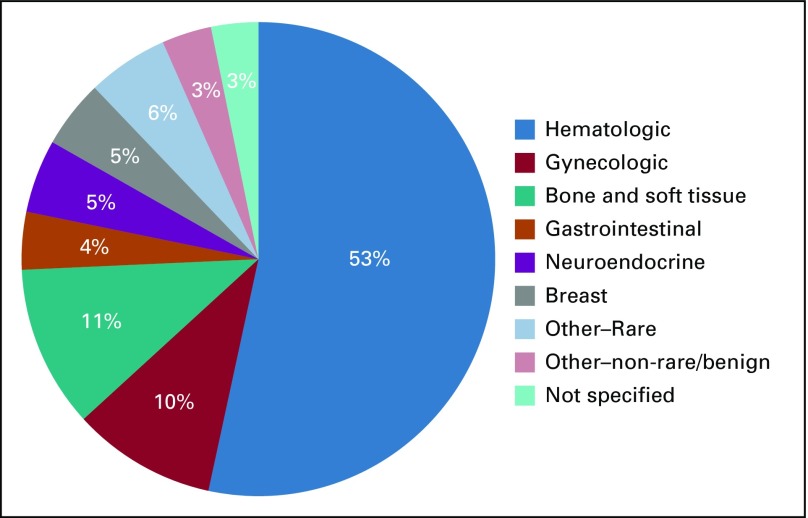
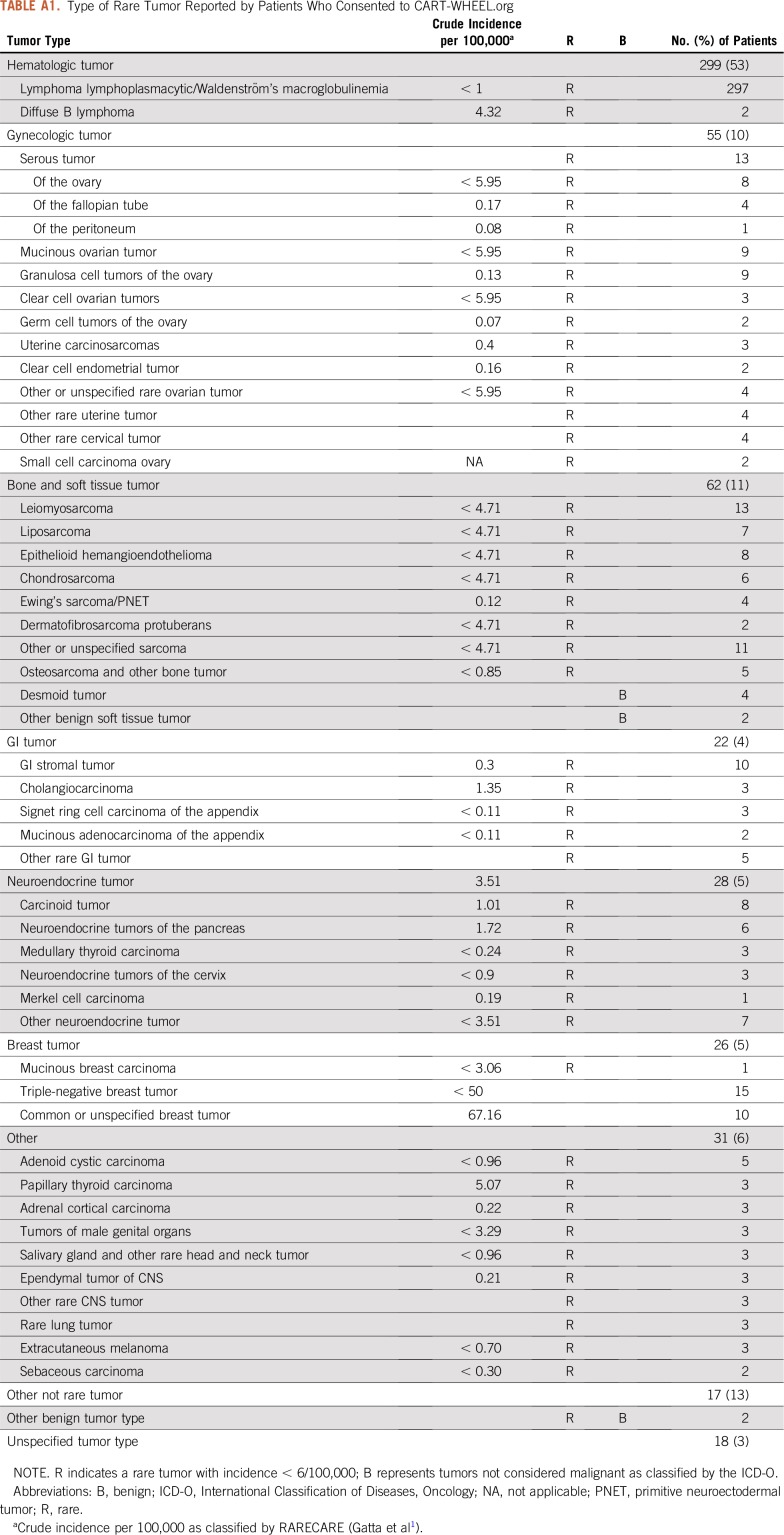
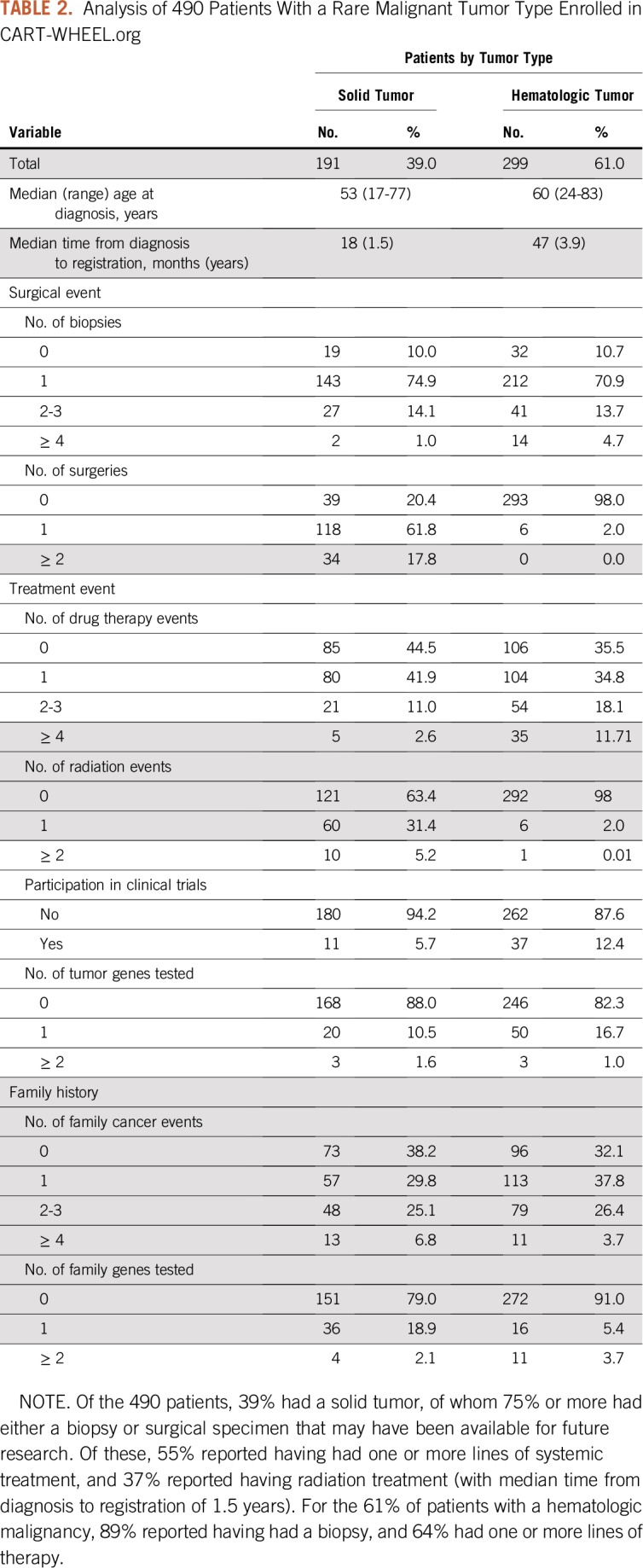
CART-WHEEL.org collected data representing 100 different tumor types, including 82 rare malignant tumor types, 4 rare benign tumor types, and 14 tumor types that were not rare (one of which was benign). Figure 3 shows the spectrum of tumor types represented by these 558 patients, and Appendix Table A1 (online only) details the spread of diagnoses. Two patients had 2 diagnoses, such that data were collected for 560 tumors. Half of patients (54%) had a hematologic malignancy, predominantly Waldenström's macroglobulinemia (WM; 53%), reflecting strong consumer engagement for disease-specific substudies via the Whimsical project. The next most common cancer types were bone and soft tissue tumors (62 of 560 patients, or 11%) and gynecologic cancers (55 of 560 patients, or 10%). Table 2 shows the key time points and treatment events recorded by the 490 patients who had rare tumor diagnoses. For patients with solid tumors, only 11% reported receiving more than one line of chemotherapy, and only 5% had been included in a clinical trial. These data may reflect the short time since diagnosis of the cohort or may represent real-world data, because many patients may not be treated at a major cancer center.
FIG 3.
The types of rare tumors reported by patients consented to CART-WHEEL.org. More than half of the patients consented to CART-WHEEL.org reported having a hematologic malignancy, reflecting consumer support of the Whimsical project specific to Waldenström’s macroglobulinemia. Sarcoma and gynecologic rare cancers were the next most common types of cancers. An additional 35 rare tumor types were reported.
TABLE 2.
Analysis of 490 Patients With a Rare Malignant Tumor Type Enrolled in CART-WHEEL.org
Data Validation Against a Hospital Sarcoma Database
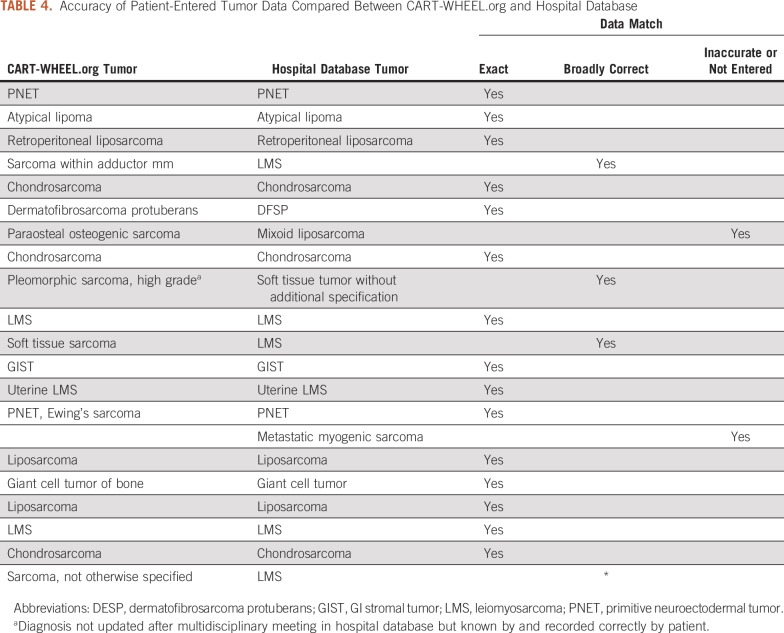
One hundred fifty-one patients attending a tertiary hospital sarcoma clinic were approached by a research fellow about the data validation project; 127 patients (84%) provided verbal agreement to participate, and 22 patients (17%) completed both the questionnaire and the written consent process. One patient subsequently diagnosed with a benign soft tissue tumor was excluded from this analysis. Five shared questions between the CART-WHEEL.org questionnaire and the hospital sarcoma database were compared for accuracy (Tables 3 and 4). These concerned the diagnosis and management of sarcoma and were considered most relevant for researchers wanting to generate a future rare cancer research project. Date of diagnosis was accurately reported by 18 patients (86%). Incorrect dates could be explained by delay between initial investigations and confirmation of the diagnosis, or by patients who underwent multiple biopsies and were unsure which was diagnostic. A correct diagnosis broadly fitting with sarcoma was entered by 19 patients (90%), with 15 (71%) entering the same histopathologic diagnosis as contained in the hospital database. Primary site of disease was accurately recorded by 20 patients (95%). Both the hospital sarcoma database and the CART-WHEEL.org questionnaire collected personal and family cancer history to identify potential genetic risks. Fifteen patients (71%) entered family history data consistent with the hospital sarcoma database, and some provided additional family history not previously recorded. Overall, 96% (representing 49 of 51 patients) of the patient-entered data about treatment was accurate, reflecting the ability of patients to understand and accurately input information about complex treatment pathways.
TABLE 3.
Data Categories Compared Between CART-WHEEL.org and Hospital Database
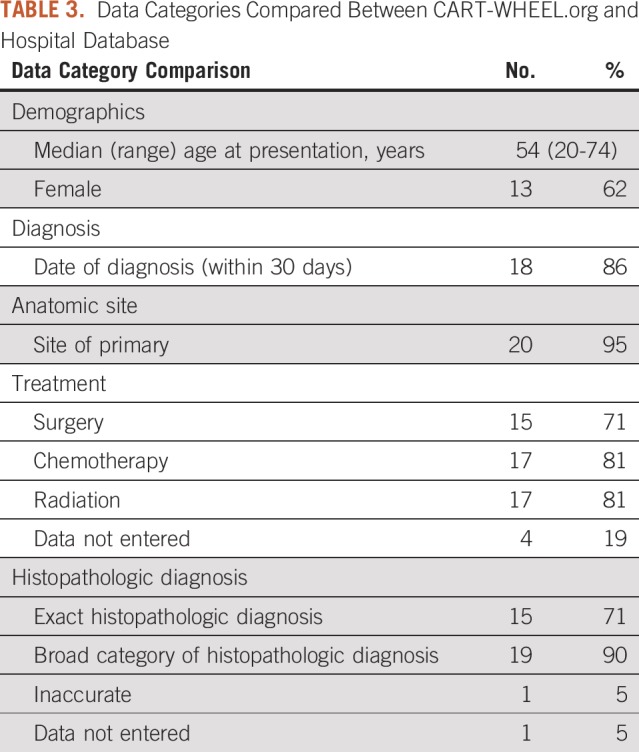
TABLE 4.
Accuracy of Patient-Entered Tumor Data Compared Between CART-WHEEL.org and Hospital Database
CART-WHEEL.org Projects
A key aspect of CART-WHEEL.org is the ability for clinicians and patient-researchers to collaborate in developing ethically approved disease-specific projects with customized questions. This feature allows consumer groups to play a more active role in research and accelerate data collection for specific rare cancers. An example project is Whimsical, which is led by clinician and expert consumer investigators in partnership with the International Waldenström’s Macroglobulinemia Foundation and its Australian affiliate, WMozzies. WM is a very rare cancer, with an incidence of < 0.3 per 100,000 making it difficult to study using conventional methodology. However, its readily trackable biomarkers, hemoglobin and immunoglobin M, are easily recognized and recorded by patients. For Whimsical, patient investigators were integral in designing and testing WM-specific questions that track and display these biomarkers and in the decision to include the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire C30 into the general CART-WHEEL.org questionnaire. Active promotion by consumer groups resulted in strong recruitment of patients with WM to CART-WHEEL.org. An early data analysis was presented by Tohidi-Esfahani et al16 at the 2017 meeting of the American Society of Hematology. At that time, a total of 206 patients with WM (137 of whom were recruited within a 10-week period) had provided data about symptoms, biomarkers, and treatment. The ongoing project reached 382 participants in April 2019 and has an eventual accrual target of 1,000 patients. When completed, the project aims to provide a comprehensive international description of real-world treatment patterns for this rare disease.
Referral to Subsequent Rare Cancer Research Projects
Thirty-one participants identified through CART-WHEEL.org subsequently have been contacted and enrolled in additional rare cancer research via the Walter and Eliza Hall Medical Research Institute’s Stafford Fox Rare Cancer Program. This second program includes a remote consent option that enables CART-WHEEL.org participants, with appropriate consent, to be invited for inclusion in a traditional cancer biomarker program involving detailed analysis of their biospecimens. This program includes next-generation sequencing and, if they are undergoing surgery or biopsy, the development of long-lived cell lines. The purpose of this preclinical research is to improve translational opportunities for all patients with rare cancers.17
A quality assurance review of the clinical data of the first 100 patient cases enrolled in the CART-WHEEL.org database revealed that these data could help generate new hypotheses and research directions. For instance, of the 24 GI and genitourinary neuroendocrine tumors so far accrued to CART-WHEEL.org, five patient cases have family histories of neuroendocrine tumors in which germline testing of known predisposition genes had not yet revealed a mutation. In addition, three patient cases have relatives with spindle cell carcinomas in whom additional investigation could be warranted. One of the hallmarks of an unrecognized germline mutation is a family history that includes rare tumors occurring in more than one individual or multiple primary tumors occurring within an individual, so CART-WHEEL.org can provide a fertile platform for discovery of novel cancer predisposition genes.
DISCUSSION
In 2010, we launched CART-WHEEL.org with the aim of facilitating access for patients with rare cancer and consumer groups to rare cancer research to generate meaningful real-world data for these under-researched conditions. Since launch, more than 558 patients with more than 100 rare tumor types have entered their data and provided consent for its use in research. This includes an international database of patients with WM that, with 382 patients registered, represents nearly 130 million person-years in accrual. More than 10% of participants with solid tumors subsequently have been recruited into traditional biomarker and genomic research.
Decentralizing access to research for patients with rare cancers complements existing strategies of centralizing expertise and virtual networks typified by the development of European Reference Networks for Rare Adult Solid Cancers. A key recommendation of the Cancer Moonshot initiative has been the development of networks to facilitate direct patient engagement aiming to improve research involvement of currently underserved populations, such as rural or minority populations and those with rare cancers.18 The NCI Rare Tumor Patient Engagement Network currently oversees two major projects: The NCI Comprehensive Oncology Network Evaluating Rare Central Nervous System Tumors (ie, NCI-CONNECT) and MyPART: My Pediatric and Adult Rare Tumor Network. Like CART-WHEEL.org, these programs partner with patient advocacy groups and use online surveys and remote consent to enroll participants, collect natural history data, and preregister patients for rare cancer research and clinical trials. Unlike traditional hospital databases, online platforms are not limited by geography and can synergize with existing online patient networks to expand recruitment. Although early users of CART-WHEEL.org predominantly were based in Australia, partnerships with international consumer groups have resulted in an increasingly global demographic. Online recruitment and participant self-entry of data also minimize costs associated with a traditional data manager–run databases, making a project like this viable.
The limitations of this approach must be considered when making use of the collected data. Patient self-enrollment will lead to biases in recruitment. For online platforms, the recruited demographic will more closely reflect those people seeking help on the Internet and (in the case of CART-WHEEL.org) those who are proficient in English. Patients with very good or very poor outcomes may be less likely to enroll—the former because they are cured and are not seeking additional health information, and the latter because they are too unwell or have died. Enrollment therefore may be skewed toward certain cancer types or prognostic subgroups or to those who have responded exceptionally well (or poorly) to therapy. Although these factors may be difficult to control across cancer types, within specific cancer histologies, potential biases may be more predictable and can enrich for interesting subgroups that allow focused analysis.
A second concern, relating to patient self-entry of data, involves ensuring data accuracy, completeness, and follow-up. In our validation cohort, we demonstrated that participants who self-select to enroll and consent to CART-WHEEL.org were able to complete key data fields with a high degree of accuracy. The low completion rate of 17% of initially interested participants was disappointing. Identified factors contributing to this low rate included that only a single approach for the majority of participants (94%) and potential difficulties in completing the questionnaire, which may reflect usability or lack of patient knowledge but also may positively select for participants more able to provide higher-quality data. Of note, this low participation rate still exceeds international benchmarks for clinical trial participation in the adult cancer population.19,20 However, in recognition that complete and ongoing validation would be difficult and beyond the resources of this project, a protocol amendment has allowed participants to provide consent for CART-WHEEL.org to contact a nominated clinician and obtain access to medical records, including histology reports. This allows for a targeted approach to data validation so that patient cases potentially relevant to a specific research question or secondary research program can be audited selectively.
There is precedence for clinical information collected by lay people to generate meaningful findings for rare cancers. The International Hemangioendothelioma, Epithelioid Hemangioendothelioma, and Related Vascular Disorders (ie, HEARD) Support Group collected clinical data from more than 200 of its members that was analyzed by researchers and published in 2011.21 This report continues to be one of the largest clinical data sets for these rare vascular tumors, providing key information relating to disease demographics, clinical patterns, and outcomes, and continues to be highly cited today.22
In summary, CART-WHEEL.org is an online database that facilitates research access for patients with rare cancers. It provides a cost-effective platform where patients can share clinical information to help generate natural history data and to match patients with research projects. CART-WHEEL.org can overcome the barriers of geography and time of diagnosis when recruiting patients to research. The dynamic consent process allows additional verification of data, such as histopathology, if required. Project-specific data collected via CART-WHEEL.org show that, when a relevant study is available, the recruitment of patients with that rare tumor increases promptly. This platform can help patients and patient groups connect with researchers, leading to greater research opportunities.
APPENDIX
TABLE A1.
Type of Rare Tumor Reported by Patients Who Consented to CART-WHEEL.org
Presented in part at the COSA 42nd Annual Scientific Meeting, Hobart, Australia, November 17-19, 2015.
Supported by grants for consumer participation from the Victorian Cancer Agency, the Royal Melbourne Hospital Foundation and the Picchi Brothers Foundation; and fellowships from the Stafford Fox Medical Research Foundation (C.L.S.); Cancer Council Victoria (Sir Edward Dunlop Fellowship in Cancer Research to C.L.S.); and the Victorian Cancer Agency (Clinical Fellowships CRF10-20 and CRF16014 to C.L.S.). This work was made possible through the Australian Cancer Research Foundation, the Victorian State Government Operational Infrastructure Support and Australian Government National Health and Medical Research Council Independent Research Institute Infrastructure Support Scheme.
AUTHOR CONTRIBUTIONS
Conception and design: Damien Kee, Susie Bae, Katherine M. Tucker, Ibrahim Tohidi-Esfahani, Jayesh Desai, Judith Trotman, Clare L. Scott
Administrative support: Susie Bae, Marita Black
Financial support: Judith Trotman, Clare L. Scott
Collection and assembly of data: Damien Kee, Clare Parker, Susie Bae, Ibrahim Tohidi-Esfahani, Marita Black, Rachel Delahunty, Sumitra Ananda, Heather E. Cunliffe, Peter Gibbs, Judith Trotman, Clare L. Scott
Data analysis and interpretation: Damien Kee, Clare Parker, Susie Bae, Michelle Harrison, Rachel Delahunty, Sumitra Ananda, Michael Friedlander, Heather E. Cunliffe, Peter Gibbs, Jayesh Desai, Judith Trotman, Clare L. Scott
Manuscript writing: All authors
Final approval of manuscript: All authors
Provision of study material or patients: Susie Bae, Michelle Harrison, Peter Gibbs, Jayesh Desai, Judith Trotman, Clare L. Scott
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/cci/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Katherine M. Tucker
Travel, Accommodations, Expenses: AstraZeneca
(OPTIONAL) Uncompensated Relationships: AstraZeneca
Michael Friedlander
Honoraria: AstraZeneca, MSD, Lilly, Takeda
Consulting or Advisory Role: AstraZeneca, MSD, AbbVie, Lilly, Takeda
Speakers' Bureau: AstraZeneca
Research Funding: BeiGene (Inst)
Peter Gibbs
Honoraria: Roche, Amgen, Merck, Sirtex Medical, SERVIER, MSD Oncology
Research Funding: Roche (Inst), Ventana Medical Systems (Inst), Amgen (Inst), Merck Serono (Inst)
Travel, Accommodations, Expenses: SERVIER
Jayesh Desai
Consulting or Advisory Role: Lilly, Eisai, BeiGene, Biocon, Amgen (Inst)
Research Funding: Roche (Inst), GlaxoSmithKline (Inst), Novartis (Inst), Bionomics (Inst), BeiGene (Inst), Lilly (Inst), Bristol-Myers Squibb (Inst), AstraZeneca (Inst), MedImmune (Inst)
Judith Trotman
Research Funding: BeiGene (Inst), Roche (Inst), Genentech (Inst), Pharmacyclics (Inst), Janssen-Cilag (Inst), Takeda (Inst), Celgene (Inst)
Travel, Accommodations, Expenses: Roche, Genentech
Clare L. Scott
Research Funding: Roche, Genentech, Clovis Oncology, Sierra Oncology, Eisai
Patents, Royalties, Other Intellectual Property: Royalty agreement for venetoclax (Inst)
Expert Testimony: AstraZeneca
Travel, Accommodations, Expenses: AstraZeneca
Other Relationship: Clovis Oncology
No other potential conflicts of interest were reported.
REFERENCES
- 1.Gatta G, van der Zwan JM, Casali PG, et al. Rare cancers are not so rare: The rare cancer burden in Europe. Eur J Cancer. 2011;47:2493–2511. doi: 10.1016/j.ejca.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 2.Gaddipati H, Liu K, Pariser A, et al. Rare cancer trial design: Lessons from FDA approvals. Clin Cancer Res. 2012;18:5172–5178. doi: 10.1158/1078-0432.CCR-12-1135. [DOI] [PubMed] [Google Scholar]
- 3.Dockser Marcus A. To make progress in rare cancers, patients must lead the way. J Clin Oncol. 2009;27:2575–2577. doi: 10.1200/JCO.2009.21.9212. [DOI] [PubMed] [Google Scholar]
- 4.Blay J-Y, Coindre J-M, Ducimetière F, et al. The value of research collaborations and consortia in rare cancers. Lancet Oncol. 2016;17:e62–e69. doi: 10.1016/S1470-2045(15)00388-5. [DOI] [PubMed] [Google Scholar]
- 5. Nunn JS, Scott CL, Stubbs, JW, Cherry SF: Involving the public in rare cancer care and research: shaping the future for rare and uncommon cancers, in Raghavan D, Ahluwalia MS, Blanke CD, et al (eds): Textbook of Uncommon Cancer (ed 5). West Sussex, United Kingdom, Wiley-Blackwell, 2017, pp12-18. [Google Scholar]
- 6.Hubbard G, Kidd L, Donaghy E. Involving people affected by cancer in research: A review of literature. Eur J Cancer Care (Engl) 2008;17:233–244. doi: 10.1111/j.1365-2354.2007.00842.x. [DOI] [PubMed] [Google Scholar]
- 7.Bryant J, Sanson-Fisher R, Fradgley E, et al. A consumer register: An acceptable and cost-effective alternative for accessing patient populations. BMC Med Res Methodol. 2016;16:134. doi: 10.1186/s12874-016-0238-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Callard F, Rose D, Wykes T. Close to the bench as well as at the bedside: Involving service users in all phases of translational research. Health Expect. 2012;15:389–400. doi: 10.1111/j.1369-7625.2011.00681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arain M, Pyne S, Thornton N, et al. Consumer involvement in cancer research: Example from a cancer network. Health Expect. 2015;18:1530–1542. doi: 10.1111/hex.12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ziebland S, Chapple A, Dumelow C, et al. How the internet affects patients’ experience of cancer: A qualitative study. BMJ. 2004;328:564. doi: 10.1136/bmj.328.7439.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bae S, Friedlander M, Scott CL. CART-WHEEL.org can facilitate research into rare gynecological tumors. Int J Gynecol Cancer. 2011;21:1517–1519. doi: 10.1097/IGC.0b013e3182255152. [DOI] [PubMed] [Google Scholar]
- 12.Merriel RB, Gibbs P, O’Brien TJ, et al. BioGrid Australia facilitates collaborative medical and bioinformatics research across hospitals and medical research institutes by linking data from diverse disease and data types. Hum Mutat. 2011;32:517–525. doi: 10.1002/humu.21437. [DOI] [PubMed] [Google Scholar]
- 13.Javaid MK, Forestier-Zhang L, Watts L, et al. The RUDY study platform: A novel approach to patient driven research in rare musculoskeletal diseases. Orphanet J Rare Dis. 2016;11:150. doi: 10.1186/s13023-016-0528-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 15.Access data https://www.biogrid.org.au/page/69/access-data BioGrid Australia.
- 16.Tohidi-Esfahani I, Warden A, deNardis P, et al. Whimsical (Waldenström’s Macroglobulinemia Study Involving CART-WHEEL): Empowering patients internationally to contribute patient-derived data for observational research. Blood. 2017;130:1502. [Google Scholar]
- 17.Barker HE, Scott CL. Preclinical rare cancer research to inform clinical trial design. Nat Rev Cancer. 2019;19:481–482. doi: 10.1038/s41568-019-0172-2. [DOI] [PubMed] [Google Scholar]
- 18.National Cancer Institute Establish a network for direct patient engagement. https://www.cancer.gov/research/key-initiatives/moonshot-cancer-initiative/implementation/patient-engagement
- 19. Canadian Partnership Against Cancer: The 2016 cancer system performance report. https://www.systemperformance.ca/report/2016-cancer-system-performance-report/.
- 20.Sinha G. United Kingdom becomes the cancer clinical trials recruitment capital of the world. J Natl Cancer Inst. 2007;99:420–422. doi: 10.1093/jnci/djk140. [DOI] [PubMed] [Google Scholar]
- 21.Lau K, Massad M, Pollak C, et al. Clinical patterns and outcome in epithelioid hemangioendothelioma with or without pulmonary involvement: Insights from an internet registry in the study of a rare cancer. Chest. 2011;140:1312–1318. doi: 10.1378/chest.11-0039. [DOI] [PubMed] [Google Scholar]
- 22.Rosenbaum E, Jadeja B, Xu B, et al. Prognostic stratification of clinical and molecular epithelioid hemangioendothelioma subsets. Mod Pathol. doi: 10.1038/s41379-019-0368-8. [epub ahead of print on September 19, 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]