Abstract
Background
Wearable activity trackers may facilitate walking for chronic pain management.
Objective
We assessed the acceptability of a commercially available tracker and three alternative modes of reporting daily steps among older adults in a low-income, urban community. We examined whether using the tracker (Fitbit ZipTM) was associated with improvements in functioning and activity.
Design
Randomized controlled pilot and feasibility trial.
Subjects
Fifty-one African American adults in Detroit, Michigan, aged 60 to 85 years, with chronic musculoskeletal pain (28 in the intervention group, 23 controls).
Methods
Participants completed telephone surveys at baseline and eight weeks. Intervention participants wore trackers for six weeks, alternately reporting daily step counts via text messages, automated telephone calls, and syncing (two weeks each). We used multimethods to assess satisfaction with trackers and reporting modalities. Adherence was indicated by the proportion of expected days on which valid step counts were reported. We assessed changes in pain interference, physical function, social participation, walking frequency, and walking duration.
Results
More than 90% of participants rated trackers as easy to use, but some had technical or dexterity-related difficulties. Text reporting yielded 79% reporting adherence vs 69% each for automated calls and syncing. Intervention participants did not show greater improvement in functioning or walking than controls.
Conclusions
With appropriate support, wearable activity trackers and mHealth reporting for chronic pain self-care are feasible for use by vulnerable older adults. Future research should test whether the effects of trackers on pain-related outcomes can be enhanced by incorporating behavior change strategies and training in evidence-based cognitive-behavioral techniques.
Keywords: mHealth, African Americans, Older Adults, Chronic Pain, Physical Activity
Introduction
At least one-third of older Americans experience chronic pain, and the prevalence is increasing steadily over time [1]. The 2016 National Pain Strategy [2] calls for expanding access to chronic pain self-management (CPSM) support to help people cope with persistent pain and reduce its impact on daily functioning. Walking, a nearly universally accessible type of physical activity, is a core self-management strategy that helps reduce chronic musculoskeletal pain and improve function [3].
Commercially available, wearable activity trackers are an increasingly popular way to encourage walking for chronic pain management [2]. Activity trackers differ from traditional pedometers in that they are linked to companion mobile apps and websites and can automatically record activity, display trends, and give tailored feedback [4]. These functions, in turn, can help people track progress in increasing activity, facilitate goal-setting, and enhance engagement in pain self-care [5].
Although most research on commercial activity trackers has been conducted among younger adults, accumulating evidence indicates that older adults are also able to use activity trackers for health management [4,6,7]. One limitation of existing research, however, is the dearth of evidence on tracker usability among socioeconomically disadvantaged older adults of color, who are less likely than white older adults to use technology for health-related purposes [8]. These vulnerable subgroups of the elderly may face barriers to using mobile health, or mHealth, tools, in the form of low technology literacy and access [9], and may be more likely to have functional and visual impairments that interfere with device use [10,11]. Despite these issues, these same groups of older adults might have the most to gain from using trackers [12], as they are disproportionately burdened by severe pain and pain-related disability [13–15], while having inferior access to high-quality pain treatment [13]. Addressing racial and socioeconomic disparities in pain and pain treatment has been designated a high priority in both the National Pain Strategy [2] and the Federal Pain Research Strategy [16] .
Study Aims
The overall purpose of the present pilot investigation was to assess the feasibility (including barriers) of using trackers and mHealth reporting tools in a sample of older African American adults with chronic pain living in an economically challenged urban community. This study was designed to lay the groundwork for further research involving mHealth tools for pain self-management in this population. Specifically, we evaluated the acceptability of the inexpensive (approximately $50), clip-on Fitbit Zip (referred to subsequently as “tracker”). We also measured adherence to, and satisfaction with, three alternative methods of reporting daily step counts: by text message, automated phone call, and syncing with an app.
Because the process of self-monitoring and the feedback provided by trackers may motivate increased physical activity even in the absence of other behavior change strategies [17–19], we also collected preliminary data regarding short-term pain-related outcomes associated with use of the tracker, comparing randomly assigned intervention and control groups. Information gleaned from the present trial will be used to inform the design of a multicomponent pain self-management intervention for this population that incorporates activity tracking and other mHealth tools.
Methods
Our study protocol was approved by the University of Michigan Institutional Review Board (HUM00133021).
Participants
Recruitment
The target sample size of 50 participants was determined by feasibility. Participants were recruited between November 2017 and April 2018 from flyers at community locations serving older adults in Detroit and a health research volunteer registry (Participant Resource Pool) of African American older adults. This registry is maintained by the Healthier Black Elders Center at the Wayne State University Institute of Gerontology and funded via a Resource Centers for Minority Aging Research grant from the National Institute on Aging. Registrants were recruited primarily at community health education and other outreach events [20].
People interested in the present study were screened over the telephone. Eligible individuals providing informed consent completed a baseline interview, after which they were randomized to the intervention group or control group in a 3:2 ratio, using a computer-generated block randomization scheme (sealedenvelope.com) with randomly selected block sizes of 5 and 10. A CONSORT study flow diagram is provided in the Supplementary Data.
Eligibility Criteria
All participants were 60 years or over; community-living; ambulatory with or without an assistive device; had a cell phone and Internet access at home or elsewhere; and reported chronic musculoskeletal pain (“Have you had pain in your muscles or joints on most days for at least three months?”), with pain intensity during the last week ≥4 (on a scale of 1 = no pain to 10 = worst imaginable pain, with 4 considered “moderate” pain intensity [21]) and at least one day in the last month when pain made it difficult to do usual activities. Potential participants were excluded if they reported serious acute illness or hospitalization in the last month, had surgery scheduled in the next month, or had significant cognitive limitations (a dementia diagnosis or self-reported memory difficulties that get in the way of daily activities).
Procedure
Both intervention and control group participants completed telephone surveys at baseline and eight-week follow-up (to account for orientation scheduling plus six-week intervention). Participants were offered a $10 gift card for the completion of each interview. Controls did not complete any other study activities.
Intervention participants attended an orientation session at which they were given a tracker. The model used—the Fitbit Zip—has acceptable validity [22], and a similar model was shown to be accurate in older adults with canes or walkers [23]. Participants were asked to wear the device every day, clipped anywhere close to their body, for six weeks during waking hours, except when showering, bathing, or swimming. We chose a commercially available tracker as they are less expensive and more easily obtained than research-grade models, increasing the relevance of study findings to eventual real-world implementation.
Intervention participants were instructed to report their daily step count data in three alternative ways for a two-week period each. The three reporting modes included two methods that did not require use of an app—that is, manually inputting step counts via daily interactive voice response (IVR) calls and short message service (SMS) text messages—as well as syncing the device with an app to automatically upload step data.
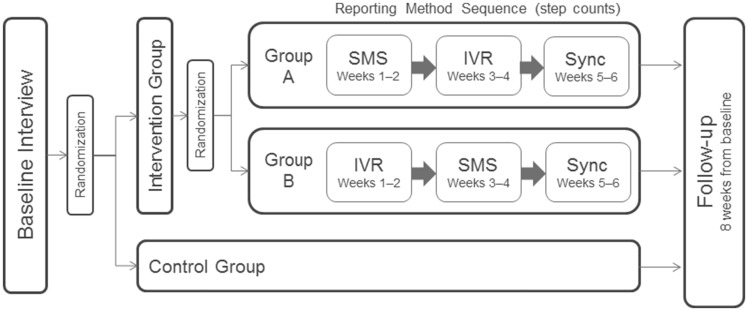
A random half of intervention participants received SMS messages each evening for the first two weeks before switching to IVR for two weeks; in the other half, this order was reversed to eliminate any order effects on adherence (Figure 1). All participants were asked to use the sync feature for the final two-week period.
Figure 1.
Study procedure for intervention and control groups. IVR = interactive voice response (automated phone calls); SMS = short message service (text messages).
Reporting via SMS and IVR
During the orientation session, participants received instruction on how the SMS text messages and IVR calls would work and selected a time near bedtime to receive the daily messages and calls. Both SMS and IVR prompts asked participants to input the number of steps displayed on the device, and a follow-up prompt asked them to indicate whether there were more than two hours that day when they did not wear their tracker. If there was no response to a text message within one hour, the system sent a second message. If the participant did not answer the initial IVR call, the system made two additional attempts 15 minutes and one hour later.
Sync
During the orientation session, staff assisted participants with downloading the Fitbit app onto their smartphone or tablet and syncing it to their tracker and to an account created and monitored by study staff. Having a smartphone or tablet was not required for participation; however, no participant used a laptop or desktop computer for syncing. Participants were asked to sync their trackers with the app every few days during the final two weeks of the study period, so that step counts would be uploaded automatically.
Participants were given a number they could call at any time to receive technical support from study staff and were invited to keep their tracker after the study period ended. Control group members were mailed a tracker to keep after the study ended.
Measures
Adherence. We evaluated overall and mode-specific adherence to step count reporting over the six-week period. This was indicated by the percentage of days with a valid step count—that is, a successfully logged numerical value—out of the 14 days that data were expected for a given mode (SMS, IVR, sync).
Satisfaction/Acceptability. In the follow-up survey, intervention group participants were asked a series of Likert scale items about their experience with the trackers and reporting modes. To complement and help explain quantitative findings, we also collected qualitative data to yield insight into challenges and facilitators for device and reporting method use; identify environmental and individual barriers to using devices; and obtain feedback on how program implementation might be improved for this particular population. Qualitative data were collected through open-ended questions posed to intervention group participants in the follow-up telephone survey. Additionally, six intervention group participants, selected to maximize diversity of perspectives (e.g., higher and lower adherence, self-reported ease or difficulty of technology use, with and without smartphone), were invited to complete a qualitative debriefing telephone interview about their experience in the program. Finally, study staff logged the reasons for all technical support calls with participants.
Outcomes. The PROMIS-29 Adult Profile [24] includes four-item subscales measuring Pain Interference (how much pain interferes with activities in four domains; 1 = not at all to 5 = very much), Physical Functioning (e.g., whether respondent can go up and down stairs at a normal pace; 1 = without any difficulty to 5 = unable to do), and Social Participation (trouble doing activities in four leisure/social domains; 1 = not at all to 5 = very much).
Participants were also asked about the frequency (1 = not at all to 5 = 5+ times/wk) and duration (1 = 10–30 minutes, 2 = 31–60 minutes, 3 = 60+ minutes) of their low-intensity walks (defined as walks lasting for at least 10 minutes that do not cause exertion) [25].
Descriptive variables for the purposes of sample characterization included age, gender, race/ethnicity, educational attainment, employment status, marital status, health literacy (“extremely” or “quite” confident in filling out medical forms on their own, which is a robust single-item indicator of health literacy [26]), comorbidities (participants were asked if they had ever been told by a doctor or other health professional that they had any of 16 common chronic health conditions, with open-ended options to add others), and pain intensity in the last seven days (1 = no pain to 10 = worst imaginable pain).
Data Analysis
All data were analyzed in IBM SPSS Statistics (version 24; Armonk, NY, USA). We calculated descriptive statistics on baseline demographic and health characteristics, comparing intervention and control groups using independent-sample t tests for continuous variables and chi-square tests for categorical variables, and on survey items related to satisfaction, acceptability, and adherence to step count reporting.
Qualitative data on satisfaction, barriers, and facilitators were compiled from open-ended survey responses and detailed notes made from recordings of postprogram interviews and technical support logs. These were reviewed and coded, and all meaningful statements were placed by two researchers (MRJ, VS) into conceptual categories under the broad a priori themes of challenges/disadvantages and facilitators/advantages. In place of specialized software for qualitative analysis, we used standard word processing and spreadsheet apps.
To obtain preliminary information about the association of tracker use with change in pain-related outcomes, we used the following procedure: Per the recommended scoring algorithm [27], items in each PROMIS-29 subscale of Pain Interference, Physical Function, and Social Participation were summed and converted to a subscale t score, a standardized score with a population average of 50 and a standard deviation of 10 [28]. Repeated-measures analysis of variance (ANOVA) was used to assess treatment effect: A time-by-treatment interaction term, indicating differential pre–post change by group, was the main predictor of interest. We used chi-square tests to evaluate differences in the proportion of participants in intervention vs control groups who achieved the minimally important difference—that is, the smallest clinically relevant difference—of ≥3 T-score points in the PROMIS Pain Interference measure [29] and in the proportion who increased the frequency and duration of their low-intensity walks from baseline to follow-up (vs decreased or stayed the same).
Results
Sample Characteristics
As shown in Table 1, the mean age of participants was 70.4 years; 88% of participants were female, and all participants were African American or African American plus another race. The sample was highly educated, with 86% having completed at least some college. About two-thirds of participants reported good or very good health; the remainder reported fair or poor health. The most common chronic conditions were hypertension (94%), arthritis (77%), and low back pain (71%). The mean pain intensity (SD) was 5.2 (1.8) at baseline. There were no significant differences between treatment groups on these variables.
Table 1.
Sample characteristics at baseline (N = 51)*
| All Participants (N = 51), No. (%) or M ± SD | Intervention Group (N = 28), No. (%) or M ± SD | Control Group (N = 23), No. (%) or M ± SD | P Value for Difference | |
|---|---|---|---|---|
| Demographics | ||||
| Age (range = 60–85), y | 70.4 ± 6.8 | 71.3 ± 7.1 | 69.3 ± 6.4 | 0.314 |
| Female | 45 (88) | 26 (93) | 19 (83) | 0.390 |
| African American† | 50 (98) | 28 (100) | 22 (96) | — |
| Married or partnered | 7 (12) | 2 (6) | 5 (21) | 0.119 |
| Some college or more | 44 (86) | 25 (89) | 19 (83) | 0.687 |
| Retired | 43 (84) | 25 (89) | 18 (78) | 0.442 |
| High health literacy‡ | 44 (86) | 24 (86) | 20 (87) | 0.769 |
| Health | ||||
| Self-rated health good/vs good | 33 (65) | 18 (64) | 15 (65) | 0.945 |
| Musculoskeletal pain conditions present | ||||
| Arthritis | 39 (77) | 23 (82) | 16 (70) | 0.392 |
| Low back pain | 36 (71) | 20 (71) | 16 (70) | 0.884 |
| Arthritis and low back pain | 27 (53) | 16 (57) | 11 (48) | 0.580 |
| Comorbidities present§ | ||||
| Hypertension | 48 (94) | 26 (93) | 22 (96) | 0.673 |
| Diabetes | 18 (35) | 10 (36) | 8 (35) | 0.945 |
| Chronic lung disease | 11 (22) | 6 (21) | 5 (22) | 0.979 |
| Heartburn/acid reflux | 19 (37) | 10 (36) | 9 (39) | 0.650 |
| Pain | ||||
| Pain intensity in last week (1= no pain to 10 = worst imaginable pain) | 5.2 ± 1.8 | 5.1 ± 1.3 | 5.4 ± 2.2 | 0.634 |
| Walking | ||||
| Frequency (at least 3 times/wk) | 21 (41) | 11 (39) | 10 (44) | 0.783 |
| Duration (at least 30 min per walk) | 15 (29) | 7 (25) | 8 (35) | 0.542 |
Participants with baseline and eight-week follow-up data. Six participants (five in the intervention group, one control) were lost to follow-up or withdrew.
Includes three participants who indicated they were African American plus another race (African American/white N = 2; African American/Asian American N = 1) and one who reported Hispanic/Latino ethnicity. One person did not indicate race.
“Extremely” or “quite” confident in filling out medical forms on their own (from Chew et al. [26]).
Also: heart attack (N = 5), blocked arteries (N = 8), migraines (N = 2), stroke (N = 1), osteoporosis (N = 6), asthma (N = 10), depression (N = 10).
A total of six participants (five in the intervention group) were lost to follow-up or withdrew. Compared with study completers, noncompleters had higher pain intensity (6.7 vs 5.2 on a 1–10 scale), were older (mean = 75 vs 70 years), and had more pain interference and difficulty with social participation and physical functioning. These differences were not statistically significant.
Feasibility/Acceptability Analyses
Adherence
Mean adherence to each reporting mode across participants was as follows: SMS = 79%, IVR = 68%, and sync = 68%. One participant learned after study enrollment that her cell phone plan did not allow her to text; this case was excluded from the SMS adherence calculations. Another person did not receive IVR calls on schedule due to a system error; this case was excluded from the IVR calculations. After observing a pattern in synced data whereby participants tended to have either very high or zero adherence (three participants were not able to successfully sync their devices at any point, and nine did not do so during the designated two-week period for syncing, compared with one person each with zero SMS or IVR reports), we also calculated the median adherence by mode: SMS = 93%, IVR = 75%, sync = 100%. Adherence scores were similar across age groups. Out of 554 occasions (i.e., person-days) with a valid response for IVR or SMS, 21% included at least two hours during waking hours when participants did not wear their tracker, as indicated in responses to the prompt asking about this.
Satisfaction with Trackers and Reporting Modes
In follow-up surveys, the vast majority of participants strongly agreed (61%) or agreed (36%) that the tracker was easy to use, and similar percentages strongly agreed (57%) or agreed (36%) that they would continue to track steps after the study ended (Table 2). In terms of preference for reporting mode, more participants (39%) indicated that they preferred reporting data by SMS compared with IVR (25%) or syncing (32%; one person [4%] selected “don’t know”) (not shown in Table). A post hoc analysis showed that the mean age of the participants who preferred IVR was 79.1 years, compared with 69.6 for SMS and 67.3 for syncing (P = 0.001). The majority of intervention participants (N = 18, or 64%) sought technical support from study staff (including tracker device, battery, IVR, SMS, or syncing) on one or more occasions during the intervention period.
Table 2.
Participant satisfaction with activity tracker
| Follow-up Survey Question (Please use the scale provided to answer how much you agree or disagree with the following statements.) | Responses (N = 28),No. (%) | |
|---|---|---|
| I found my [activity tracker] device easy to use. | Strongly agree | 17 (61) |
| Agree | 10 (36) | |
| Neither agree nor disagree | 1 (4) | |
| Disagree | 0 (0) | |
| Strongly disagree | 0 (0) | |
| I had technical difficulties with my [activity tracker] device itself. | Strongly agree | 1 (4) |
| Agree | 3 (11) | |
| Neither agree nor disagree | 2 (7) | |
| Disagree | 9 (32) | |
| Strongly disagree | 13 (46) | |
| I had trouble syncing my [activity tracker]. | Strongly agree | 5 (18) |
| Agree | 2 (7) | |
| Neither agree nor disagree | 4 (14) | |
| Disagree | 5 (18) | |
| Strongly disagree | 11 (39) | |
| Don’t know | 1 (4) | |
| I found reporting my step count every day to be burdensome. | Strongly agree | 3 (11) |
| Agree | 1 (4) | |
| Neither agree nor disagree | 5 (18) | |
| Disagree | 7 (25) | |
| Strongly disagree | 12 (43) | |
| I will continue to track my steps with the [activity tracker]. | Strongly agree | 16 (57) |
| Agree | 10 (36) | |
| Neither agree nor disagree | 2 (7) | |
| Disagree | 0 (0) | |
| Strongly disagree | 0 (0) | |
Qualitative analysis of open-ended survey items, debrief interviews, and technology support calls revealed varied perspectives on the usability of the tracker and each of the reporting technologies (Table 3).
Table 3.
Acceptability of activity tracker and reporting modes
| Activity tracker | Main challenges/disadvantages identified*: Device function: inaccurate step counts; screen response; battery replacement. Device application: difficulty attaching to clothing; remembering to use; potential for loss; unintentional removal from case |
| Main facilitators/advantages identified*: motivating; increased awareness of activity; easy to use/wear; orientation/training support | |
| SMS | Main challenges/disadvantages identified*: typing/accuracy difficulties; requires regular checking of text messages; phone service/data plan limitations |
| Main facilitators/advantages identified*: straightforward/easy; does not require being home to receive a call | |
| IVR | Main challenges/disadvantages identified*: missed calls/scheduling around call times/inability to call back; confusing prompts; unaccepted valid inputs; lack of ability to correct error; system glitches |
| Main facilitators/advantages identified*: easy; predictable; confirmation of successful step count input; hearing a person’s voice | |
| Automatic sync | Main challenges/disadvantages identified*: inaccurate/unsuccessful syncing; app installation; compatibility with device; didn’t always have mobile device nearby; lack of confidence in tech literacy |
| Main facilitators/advantages identified*: easy; automatic; no concerns re: missed calls or texts |
Qualitative data from open-ended items on follow-up survey (N = 28), postprogram qualitative interviews (N = 6), and staff communication logs.
Trackers
In open-ended responses and/or in-depth interviews, nearly all participants reported that the tracker was easy to use (N = 27, 96%), just as in the close-ended question, and many (N = 21, 75%) reported that the device was motivating (N = 19, 68%) and/or increased their awareness of their activity levels (N = 13, 46%). However, others reported varying degrees of difficulty. Challenges included trouble replacing the battery (N = 4, 14%), step counts that were perceived as inaccurate (N = 3, 11%), difficulty clipping the device onto clothing (N = 3, 11%), remembering to put the device on in the morning (N = 3, 11%), getting the screen to respond to touch (one person who lacked feeling in the tip of her fingers found this especially challenging; N = 2, 7%), having the device pop out of its rubber case (N = 2, 7%), locating the step count display (N = 1, 4%), or losing the device (there were two lost devices out of 30 distributed during the study period).
SMS
Participants who viewed the SMS reporting process positively described this method as straightforward and easy and valued that they did not have to worry about missing a call. Problems cited with SMS included difficulties typing on their phone (N = 3, 11%), not reading text messages regularly (N = 2, 7%), apparent system glitches (N = 2, 7%), or issues with their phone service or data plan (N = 2, 7%).
IVR
Some participants found reporting by IVR easy and appreciated the predictability of the calls and the fact that the system would confirm successful input of step count; in one case, a participant commented that “it’s good to hear a voice.” Those that found the IVR process less appealing reported such issues as missed calls or difficulty being available for the scheduled calls (N = 7, 25%), apparent system glitches (N = 5, 18%), problems with following prompts (N = 3, 11%), frustration with the inability to correct an error or return a missed call (N = 2, 7%), and trouble getting the system to recognize or accept their inputs (N = 1, 4%).
Sync
Some participants described the syncing process as easy and liked that they did not have to worry about missing a call or text. Others cited frustrations due to getting the device to sync properly (N = 6, 21%), feeling that they did not have good technological skills (N = 2, 7%), problems installing the app on their device (N = 2, 7%; one participant acquired a new phone during the study period, and another accidentally deleted the app on their device), compatibility with their mobile device (N = 1, 4%; the participant felt that the app was draining the phone battery), or not always having their device nearby (N = 1, 4%).
Outcomes
Baseline PROMIS Pain Interference t scores were 58.7 (intervention group) and 57.0 (control group). This indicates a level of Pain Interference approximately one standard deviation higher than the mean for the general population. Physical Function (33.2 vs 32.7) and Social Participation (38.1 vs 37.0) t scores indicated that the study sample on average scored more than one standard deviation below the mean, indicating worse functioning.
We did not find evidence of an intervention effect on outcomes, as all time-by-treatment interactions were nonsignificant (Table 4). The established “minimally important difference” of 3 points for the PROMIS Pain Interference subscale [29] was achieved by virtually identical proportions of intervention participants (26%) and controls (25%).
Table 4.
Baseline and follow-up means and effect sizes for pain and functioning outcomes, intervention and control groups
| Intervention Group (N = 28) |
Control Group (N = 23) |
Treatment Effect Size, Partial Eta Squared* | P Value for Treatment Effect* | |||
|---|---|---|---|---|---|---|
| M ± SD |
M ± SD |
|||||
| Baseline | Follow-up | Baseline | Follow-up | |||
| PROMIS 4-item Pain Interference, t score† | 58.7 ± 6.1 | 57.9 ± 6.4 | 57.0 ± 8.4 | 57.0 ± 8.0 | 0.003 | 0.726 |
| PROMIS 4-item Physical Function difficulty, t score† | 33.2 ± 4.3 | 31.9 ± 4.2 | 32.7 ± 6.2 | 32.0 ± 4.7 | 0.006 | 0.600 |
| PROMIS 4-item Social Participation, t score† | 38.1 ± 7.3 | 38.4 ± 7.3 | 37.0 ± 6.4 | 38.0 ± 8.0 | 0.001 | 0.810 |
Based on repeated-measures analysis of variance.
T scores are normed such that a score of 50 represents the mean or average for the reference population and 1 standard deviation = 10 t-score points. For Pain Interference, a higher score means worse symptomatology (possible range = 42–76). For Physical Function (range = 23–57) and Social Participation (range = 29–64), lower scores represent worse functioning.
A greater proportion of participants in the intervention group (46%, 95% CI = 28–64%) than the control group (32%, 95% CI = 13–51%) increased self-reported walking frequency from baseline (P = 0.387). Nearly equal percentages in both groups increased self-reported walking duration (26% vs 24%) (not shown in Table).
We calculated, on a post hoc basis, the average and median step count for synced data (mean [SD] = 3,004 [2,048], median = 2,603) for the purposes of characterizing the activity level of this sample, under the assumption that the synced data were the most accurate. As another way of trying to maximize accuracy, we then calculated average and median IVR and SMS-reported step counts excluding days where participants reported that there were at least two hours during which they failed to wear the device (IVR mean [SD] = 2,957 [2,474], median = 2,195; SMS mean [SD] = 3,308 [1,842], median = 2,812). Finally, because of potential implications for planning future interventions, we assessed seasonal differences in step counts: (January–March mean [SD] = 2,152 [1,036], median = 2,267; April–July mean [SD] = 3,857 [2,468], median = 3,311).
Discussion
Wearable activity trackers are a potentially useful mobile health tool for chronic pain self-management. Yet older adults from vulnerable populations may face obstacles to using these devices that should be considered in intervention design. We found that a clip-on activity tracker was generally well received in this sample of 51 older African American adults with chronic musculoskeletal pain living in an economically disadvantaged urban community. Each participant wore the device for six weeks and tested three different modes of reporting daily step data for two weeks each: automated phone calls, SMS text messages, and syncing the device with an app.
Across modes, participants were able to report a valid step count for at least two-thirds of person-days over a six-week period. Compared with IVR or SMS reporting, a greater number of people—about one-third of the sample—did not sync at all during the designated period. Qualitative data revealed that failure to sync may have occurred for a variety of reasons, including device battery depletion, app–device connectivity, and user error. Slightly more participants preferred reporting by SMS than the other two modes. Technology-related challenges with the trackers and with reporting steps were not uncommon; most were user-end problems and were successfully addressed by study staff. Dexterity-related problems were reported by a few participants. Finally, we did not find evidence in this small pilot study that tracking steps for six weeks without additional pain psychoeducation or behavior change support improved pain-related outcomes or significantly increased walking over the study period. Nonetheless, qualitative feedback suggested that, for three-quarters of participants, wearing a tracker was motivating and/or increased awareness of their activity level.
Feasibility/Acceptability
Our study adds to others documenting the feasibility of using activity trackers in specific subgroups of older adults [4,6]. To our knowledge, no prior comparable study has focused specifically on older adults of color in a resource-challenged setting, where access to technology may be more limited and comorbidity burden is high [30]. More than 90% of intervention group participants agreed that the tracker was easy to use and that they planned to keep tracking their steps after the study ended.
The proportion of person-days on which valid data were reported—more than two-thirds—represents a lower bound on how frequently people wore the devices, as there may have been days that they wore the device but did not successfully report data by IVR or SMS. On one-fifth of person-days with valid manual step count reports, participants reported that there were at least two hours when they did not wear their tracker, resulting in an undercount of steps on those days. The potential for undercounting steps due to inconsistent wearing should be considered when tracking steps for goal-achievement purposes or as an outcome in research.
Our qualitative findings regarding participants’ views about the tracker were similar to those in an acceptability study by Mercer et al. [4], who tested various trackers in a sample of adults age 50+ with chronic illness. In both cases, some participants reported that the devices improved their awareness of their activity levels and helped with goal setting. Though participants in both studies required some instructional support for device operation, they were willing and able to learn, with one participant in our study describing the process as easier than anticipated.
The majority of participants did not experience any notable problems with the device. Among those who did experience difficulties, several mentioned problems related to the clip-on nature of this model (e.g., hard to manipulate clip), suggesting that for some older adults, a wrist-worn tracker may be preferable. A number of people cited the in-person orientation session as essential in showing them how to use the device. Overall, findings confirm the importance of one-on-one instruction in device use, including clear written materials that participants can refer back to and the availability of tech support for individualized problems with the devices.
Data Reporting Modes
The rich activity data collected by trackers are useful not only for self-monitoring but also for health care providers who wish to track patient progress in increasing activity to manage chronic pain [5]. We tested three alternative modes of daily step count reporting: SMS, IVR, and automatic syncing. Slightly more participants preferred reporting steps via SMS than via the other two methods, and this mode also yielded the highest average adherence in the two-week reporting period. However, some participants did experience difficulties with SMS, such as being unaccustomed to checking for texts and having the manual dexterity needed to type on the phone.
Prior studies have demonstrated the feasibility of using daily IVR calls for collecting pedometer data as part of a cognitive-behavioral intervention for chronic low back pain [5]. IVR calls are considered a more universally accessible mHealth tool than text messaging or apps and are more easily used by individuals with low dexterity or technological literacy [31]. This is likely why, in our sample, the average age of the subgroup of participants preferring IVR was more than 10 years greater than that for the other two modes. Qualitative data suggested, however, that some participants felt that the calls were more disruptive than text messages, and some expressed frustration with the inability to correct inputting errors or report step counts after missing a call.
Among participants who were able to sync (all but three were able to sync data for some part of the study period), this automated method yielded the highest percentage of days with valid step counts and was preferred by one-third of participants. Syncing is likely to be the most accurate means of reporting steps, as it avoids issues of social desirability or user error. Yet, seven people reported that they had a hard time syncing their device. In our study sample, 82% of participants had their own smartphone (not shown). All remaining participants had access to a tablet or a smart device through family members or others in the household. Smartphone penetration is rapidly increasing among older adults, a trend that began among more affluent subgroups [9] but over time is happening more broadly. We observed a range of participant familiarity with the features on their smartphones, but virtually all were willing to learn how to use them to accomplish study tasks.
Outcomes
In this small study, which was not powered to detect statistical significance, we did not find evidence that using a tracker for a six-week period resulted in short-term improvements in pain interference, physical functioning, or social participation. Increased daily walking is associated with improved functioning among people with chronic musculoskeletal pain [3]; therefore, these outcomes may have been positively impacted if wearing the tracker motivated participants to increase walking. Evidence is mixed about whether such an increase in activity occurred. Nearly half of intervention participants, compared with only about one-third of control group participants, increased self-reported walking frequency (as reported on baseline and follow-up surveys). This difference was not significant, though we note that this measure of walking behavior has only low to moderate reliability and therefore may not have been sensitive to change [25]. About two-thirds of participants reported that wearing the tracker positively affected their daily walking, and qualitative comments from participants elaborated on this idea (“Sometimes you just need a little something to motivate you to get you on the right track. The first thing I do each morning is put the Fitbit on. My back is feeling better; I have more energy.”). As a post hoc analysis, we created spaghetti plots for intervention group participants representing their tracker-recorded steps over time, but no pattern was evident that suggested that participants tended to increase their steps over the study period. It is possible that people were motivated to walk more after receiving the device than they had done previously. However, in the absence of structured, incremental goal-setting, most participants did not steadily increase their steps over time, and overall increases in activity related to tracking were modest.
Mean and median step counts for this sample, calculated across different reporting modes and including vs excluding days with self-reported incomplete wear, ranged from 2,000 to 3,000 per day—far below the threshold of >6,000 steps/d that has been recommended to avoid functional limitations associated with knee osteoarthritis [32]. It is therefore likely that for most participants, daily activity remained below the level needed to have an impact on pain-related outcomes. The fact that average step counts were, on average, almost twice as high during spring and summer compared with winter months implies that seasonal effects should be considered in designing interventions for this population, for example, by helping participants to identify accessible indoor activity options.
Doubts have been raised about the effectiveness of activity trackers to motivate sustained improvements in physical activity in the absence of other behavior change techniques [12,18,33]. Sullivan and Lachman [19] note that people from vulnerable populations in particular are likely to require additional types of behavior change support that trackers do not provide, such as action planning and addressing environmental barriers. Our findings also suggest that in order to improve pain-related outcomes, additional pain management content may be needed.
Limitations
The strengths of this small study include the use of multimethods to assess feasibility and acceptability and a randomized design that, compared with a single-group design, offers a stronger degree of preliminary evidence regarding the impact of using a tracker on pain outcomes in our priority population. We were able to enroll 57 participants in a five-month period, and our retention rate over the two-month study period was high at 89% (51 out of 57), suggesting the feasibility of conducting a larger trial using similar methods. There are also a number of limitations. First, intervention participants were asked to wear a tracker for only six weeks, which may have been too brief a period to have an impact on functional outcomes. Second, we chose the inexpensive Fitbit Zip instead of a research-grade accelerometer in order to test a tracking strategy that would be scalable and practical for real-world use. However, even the approximately $50 price of the Zip or an equivalent model may be out of reach for many older adults or community-based service providers. Due to the limited scope of the present study, we did not assess the cost-related feasibility of using trackers at either the individual or organizational level. Because this is a critical aspect of scalability, we plan to incorporate measures of affordability into the next phase of our research. Future studies could also test the feasibility of using inexpensive or free smartphone tracking apps that do not require additional hardware [12].
Third, because none of our participants chose to sync via a desktop or laptop computer, we have little information about the feasibility of this method in our priority population. Furthermore, we did not compare acceptability or adherence between participants who personally owned their smart device and the approximately one-fifth of participants who used a shared or borrowed device; the needs of this latter group should be carefully considered in future studies. Fourth, we did not collect qualitative data on the extent to which participants noticed or were motivated by the feedback provided by either the app or the device itself (e.g., smiley face on display after being active). Fifth, we do not know whether control group participants were using their own activity trackers, which would have made the groups more similar and diminished the apparent effect of the intervention.
Last, participants had high levels of education and health literacy, and our findings may not generalize to older adults with low health literacy. Yet because of historical and ongoing race-based discrimination, educational achievement is likely to bring diminished returns to African Americans compared with whites in terms of economic and health benefits [34,35]. Therefore, education can be a misleading indicator of individual socioeconomic status in this group [36]. Moreover, our participants reside in a city (Detroit, Michigan) that is highly impacted by spatial racism, with a high poverty rate, limited access to health-promoting resources, and adverse physical environmental conditions [37,38]. Our study responds to the need to conduct disparities-relevant pain research by providing novel information about the feasibility of using technology as a pain self-management tool in a marginalized older population.
Conclusions
This study provides novel evidence that, with attention to specific needs and tailored support, wearable activity trackers and other mHealth tools are feasible for use by older African American adults in an economically disadvantaged community. Such tools have potential for use in chronic pain self-management interventions or as recommended by health care providers, when appropriate. Our findings further demonstrate that using smartphone features, including tracker apps, as a tool for chronic pain self-management is increasingly practical even among marginalized subgroups of older adults. However, to maximize accessibility among the current cohort of older adults, having non-app-based options for tracking and other intervention tasks is advisable. Our findings that SMS reporting was linked to greater adherence and satisfaction as compared with IVR will inform our own future work and demonstrates that text messaging is a viable reporting option. Future studies of technology-enabled interventions for chronic pain management may benefit from more detailed analysis of how feasibility and acceptability vary among different age strata of older adults. There are also a number of practical takeaways that suggest best practices for implementing technology-enabled interventions with this population, for example, the importance of initial individualized in-person instruction, comprehensive written instructions, and ongoing availability of staff technical support.
Lastly, we found limited evidence that wearing a tracker may have motivated some people to increase activity in the short term, but we did not find strong evidence of a trend toward improved pain-related outcomes. Therefore, as a next step, we will test how the effects of activity trackers on pain-related outcomes can be enhanced by incorporating behavior change strategies and skills training in other cognitive-behavioral pain management techniques.
Supplementary Material
Acknowledgments
We would like to thank study staff Rebecca Courser, Cainnear Hogan, Max Geisendorfer, Sean Newman, Julia Castellano, and Jarvia Meggett; Anna Kratz for her help with data analysis; and the staff of the St. Patrick Senior Center, Detroit, Michigan.
Funding sources: This study was supported by a grant from the National Institutes of Health (P30 AG015281) and the Michigan Center for Urban African American Aging Research, by grants from the National Institute on Aging (K01 AG050706-01A1 to MRJ), and by the UM OAIC Pepper Center 2017 Pilot Grant (Janevic, PI). John Piette is a VA Senior Research Career Scientist and is funded by the Michigan Center for Diabetes Translational Research (NIH Grant P30DK092926).
Conflicts of interest: None of the authors has any conflicts of interest to disclose.
References
- 1. Zimmer Z, Zajacova A.. Persistent, consistent, and extensive: The trend of increasing pain prevalence in older Americans. J Gerontol B Psychol Psi Soc Psi. 2018; (doi:10.1093/geronb/gbx162). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Interagency Pain Research Coordinating Committee. National Pain Strategy. US Department of Health and Human Services; 2016. https://www.iprcc.nih.gov/National-Pain-Strategy/Overview. (accessed August 15, 2019).
- 3. O'Connor SR, Tully MA, Ryan B, et al. Walking exercise for chronic musculoskeletal pain: Systematic review and meta-analysis. Arch Phys Medic Rehabil 2015;96(4):724–34e3. [DOI] [PubMed] [Google Scholar]
- 4. Mercer K, Giangregorio L, Schneider E, Chilana P, Li M, Grindrod K.. Acceptance of commercially available wearable activity trackers among adults aged over 50 and with chronic illness: A mixed-methods evaluation. JMIR mHealth uHealth 2016;4(1):e7.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Heapy AA, Higgins DM, LaChappelle KM, et al. Cooperative pain education and self-management (COPES): study design and protocol of a randomized non-inferiority trial of an interactive voice response-based self-management intervention for chronic low back pain. BMC Musculoskelet Disord 2016;17(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McMahon SK, Lewis B.. Older adults' experiences using a commercially available monitor to self-track their physical activity. JMIR mHealth and uHealth 2016;4(2):e35.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lyons EJ, Swartz MC, Lewis ZH, Martinez E, Jennings K.. Feasibility and acceptability of a wearable technology physical activity intervention with telephone counseling for mid-aged and older adults: A randomized controlled pilot trial. JMIR mHealth uHealth 2017;5(3):e28.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mitchell UA, Chebli PG, Ruggiero L, Muramatsu N.. The digital divide in health-related technology use: The significance of race/ethnicity. Gerontologist 2019;59(1):6–14. [DOI] [PubMed] [Google Scholar]
- 9.Pew Research Center. Tech Adoption Climbs Among Older Adults. Washington, DC: Pew Research Center; 2017.
- 10. Warner DF, Brown TH.. Understanding how race/ethnicity and gender define age-trajectories of disability: An intersectionality approach. Soc Sci Med 2011;72(8):1236–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zambelli-Weiner A, Crews JE, Friedman DS.. Disparities in adult vision health in the United States. Am J Ophthalmol 2012;154(6):S23–30e1. [DOI] [PubMed] [Google Scholar]
- 12. Patel MS, Asch DA, Volpp KG.. Wearable devices as facilitators, not drivers, of health behavior change. JAMA 2015;313(5):459–60. [DOI] [PubMed] [Google Scholar]
- 13. Meghani SH, Polomano RC, Tait RC, Vallerand AH, Anderson KO, Gallagher RM.. Advancing a national agenda to eliminate disparities in pain care: Directions for health policy, education, practice, and research. Pain Med 2012;13(1):5–28. [DOI] [PubMed] [Google Scholar]
- 14. Janevic MR, McLaughlin SJ, Heapy AA, Thacker C, Piette JD.. Racial and socioeconomic disparities in disabling chronic pain: Findings from the health and retirement study. J Pain 2017;18(12):1459.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grol-Prokopczyk H. Sociodemographic disparities in chronic pain, based on 12-year longitudinal data. Pain 2017;158(2):313–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Interagency Pain Research Coordinating Committee. Federal Pain Research Strategy. IPRCC; 2017. https://www.iprcc.nih.gov/Federal-Pain-Research-Strategy/Overview, (accessed August 15, 2019).
- 17. Gualtieri L, Rosenbluth S, Phillips J.. Can a free wearable activity tracker change behavior? The impact of trackers on adults in a physician-led wellness group. JMIR Res Protoc 2016;5(4):e237.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cadmus-Bertram LA, Marcus BH, Patterson RE, Parker BA, Morey BL.. Randomized trial of a Fitbit-based physical activity intervention for women. Am J Prev Med 2015;49(3):414–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sullivan AN, Lachman ME.. Behavior change with fitness technology in sedentary adults: A review of the evidence for increasing physical activity. Front Public Health 2017;4:289.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chadiha LA, Washington OG, Lichtenberg PA, Green CR, Daniels KL, Jackson JS.. Building a registry of research volunteers among older urban African Americans: Recruitment processes and outcomes from a community-based partnership. Gerontologist 2011;51(Suppl 1):S106–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Von Korff M, Scher AI, Helmick C, et al. United States national pain strategy for population research: Concepts, definitions, and pilot data. J Pain 2016;17(10):1068. [DOI] [PubMed] [Google Scholar]
- 22. Tully MA, McBride C, Heron L, Hunter RF.. The validation of Fitbit Zip™ physical activity monitor as a measure of free-living physical activity. BMC Res Notes 2014;7(1):952.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Floegel TA, Florez-Pregonero A, Hekler EB, Buman MP.. Validation of consumer-based hip and wrist activity monitors in older adults with varied ambulatory abilities. J Gerontol A Biol Sci Med Sci 2017;72(2):229–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Deyo RA, Dworkin SF, Amtmann D, et al. Report of the NIH Task Force on Research Standards for Chronic Low Back Pain. J Pain 2014;15(6):569–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dipietro L, Caspersen CJ, Ostfeld AM, Nadel ER.. A survey for assessing physical activity among older adults. Med Sci Sports Exerc 1993;25(5):628–42. [PubMed] [Google Scholar]
- 26. Chew LD, Griffin JM, Partin MR, et al. Validation of screening questions for limited health literacy in a large VA outpatient population. J Gen Intern Med 2008;23(5):561–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.PROMIS Adult Profile Scoring Manual. Available at: http://www.healthmeasures.net/promis-scoring-manuals (accessed May 22, 2019).
- 28. Hays RD, Spritzer KL, Schalet BD, Cella D.. PROMIS((R))-29 v2.0 profile physical and mental health summary scores. Qual Life Res 2018;27(7):1885–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen CX, Kroenke K, Stump TE, et al. Estimating minimally important differences for the PROMIS Pain Interference scales: Results from 3 randomized clinical trials. Pain 2018;159(4):775–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Farmer MM, Ferraro KF.. Are racial disparities in health conditional on socioeconomic status? Soc Sci Med 2005;60(1):191–204. [DOI] [PubMed] [Google Scholar]
- 31. Piette JD, Rosland AM, Marinec NS, Striplin D, Bernstein SJ, Silveira MJ.. Engagement with automated patient monitoring and self-management support calls: Experience with a thousand chronically ill patients. Med Care 2013;51(3):216–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. White DK, Tudor‐Locke C, Zhang Y, et al. Daily walking and the risk of incident functional limitation in knee osteoarthritis: An observational study. Arthritis Care Res 2014;66(9):1328-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Phillips SM, Cadmus-Bertram L, Rosenberg D, Buman MP, Lynch BM.. Wearable technology and physical activity in chronic disease: Opportunities and challenges. Am J Prev Med 2018;54(1):144–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Assari S. Health disparities due to diminished return among black Americans: Public policy solutions. Soc Issues Policy Rev 2018;12(1):112–45. [Google Scholar]
- 35. Everett BG, Rehkopf DH, Rogers RG.. The nonlinear relationship between education and mortality: An examination of cohort, race/ethnic, and gender differences. Popul Res Policy Rev 2013;32(6):893–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Manly JJ. Deconstructing race and ethnicity: Implications for measurement of health outcomes. Med Care 2006;44(11 Suppl 3):S10–6. [DOI] [PubMed] [Google Scholar]
- 37.Authority Health. The Impact of Spatial Racism on Health. Authority Health; 2016. https://detroitmi.gov/sites/detroitmi.localhost/files/2019-04/4pm_April11_DHD_report.pdf (accessed August 15, 2019).
- 38.Detroit Health Department. 2018 Community Health Assessment. Detroit Health Department; 2018. https://detroitmi.gov/sites/detroitmi.localhost/files/2019-04/4pm_April11_DHD_report.pdf. (accessed August 15, 2019).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.