Abstract
Context
Evidence suggests that heart rate (HR) is a prognostic factor for cardiovascular disease (CVD), for which persons with diabetes are at increased risk.
Objective
The objective of this article is to determine the association between HR and glycemic status in a nationally representative sample of US adults, and, among adults with diagnosed diabetes, the association between HR and hemoglobin A1c (HbA1c) level.
Design
A cross-sectional study was conducted.
Setting
The setting of this study is the National Health and Nutrition Examination Surveys, 2011 to 2016.
Participants
US general adult (age ≥ 20 years) population who had information on glycemic status based on self-report, HbA1c, and fasting plasma glucose (N = 8562).
Intervention
There was no intervention.
Main outcome measure
The main outcome measure of this study was mean HR (beats per minute).
Results
After adjustment for examination time, age, other demographic characteristics, health insurance, health behaviors, body mass index, CVD and kidney disease, and taking antihypertensive medications, mean HR was significantly higher for those with diagnosed (75 bpm), undiagnosed diabetes (75 bpm), and prediabetes (73 bpm) compared to those with normoglycemia (71 bpm, P < .05 for all); this association was robust both for men and women. Mean HR increased with increasing HbA1c level among individuals with diagnosed diabetes independent of other risk factors (HbA1c < 7.0% [< 53 mmol/mol], 73 bpm vs A1c ≥ 11.0% [≥ 97mmol/mol], 79 bpm, P < .001); this association was most pronounced for women.
Conclusions
Adjusted mean HR was higher among individuals with diabetes and increased glycemia, which may reflect underlying autonomic and/or myocardial dysfunction among those with diabetes.
Keywords: diagnosed diabetes, epidemiology, glycemia, heart rate, NHANES, undiagnosed diabetes
Heart rate (HR) reflects a combination of inputs from the autonomic, cardiorespiratory, and adrenal systems and is easily measured in clinical care (1, 2). Emerging evidence demonstrates the importance of resting HR as a prognostic factor for cardiovascular disease (CVD) and mortality in the general population and in various comorbid groups (3-5), as well as a predictor of increased morbidity and mortality among people with coronary artery disease (3, 6-9). In addition, HR may be also an indirect measure of autonomic nervous system dysfunction (1).
Given that people with diabetes are at an increased risk both for CVD and cardiovascular autonomic dysfunction, some studies have assessed the association between diabetes and HR. The Chicago Heart Association Detection Project in Industry, a prospective study that used Medicare billing records, found there was a 10% higher odds of having a diabetes-related claim per 12 beats per minute (bpm)-higher baseline HR (10). In addition, higher resting HR was associated with diabetes mortality and this association was only partly due to body mass index (BMI) (10). Among nondiabetic participants in the community-based Insulin Resistance Atherosclerosis Study, HR was correlated with fasting insulin and an inverse association was found between HR and insulin sensitivity (11). In an observational study of Japanese men with premetabolic syndrome (defined as obese plus one component of metabolic syndrome), elevated HR preceded the development of full metabolic syndrome, which was defined as obese and at least 2 additional risk factors (dyslipidemia, hypertension, or elevated fasting plasma glucose [FPG]) (12). A prospective observational study in a clinical population of adults with type 2 diabetes found that during a 5-year observation period, elevated HR (> 75 bpm) was an independent predictor of cardiovascular death (13). Finally, data from 2 large cardiovascular outcome trials that included a substantial cohort with diabetes reported that higher HR analyzed as either a categorical (> 70 vs ≤ 70 bpm) or continuous variable was independently associated with CVD events and all-cause death (1, 9).
To our knowledge, the association between HR and diabetes or glycemic status has not been assessed in a nationally representative sample of adults in the United States. Thus, using data from the large National Health and Nutrition Examination Surveys (NHANES), we aimed to determine whether HR is elevated in individuals with prediabetes, undiagnosed diabetes, and diagnosed diabetes and whether, among individuals with diabetes, HR varies according to glycemia as assessed by hemoglobin A1c (HbA1c), a measure of glucose control. This was explored overall and by sociodemographic characteristics, access to health care, comorbidities, and medication use.
Research Design and Methods
Data come from the NHANES, a cross-sectional, stratified, multistage probability cluster survey that is conducted among the noninstitutionalized US population. Participants are interviewed in their home for demographic and health information (response rate, 61%-73%). Following the in-home interview, participants are scheduled to visit a mobile examination center (MEC) to complete a physical examination, including a measure of heart rate, and laboratory measures following a standardized protocol (response rate, 59% to 70%) (available at https://wwwn.cdc.gov/nchs/nhanes/Default.aspx). Written informed consent was obtained from all participants and was approved by the National Center for Health Statistics Institutional Review Board.
Study participants
Participants were all adults age 20 years or older who participated in the 2011 to 2016 NHANES survey cycles (N = 8562). These individuals self-reported demographic characteristics (age, sex, race/ethnicity, education, household income, health insurance status) and health-related factors (history of diabetes, sleep duration, work or leisure time activity, CVD, smoking status, menopausal status, and hypertension). Sleep duration was reported as the number of hours the participant usually sleeps at night on weekdays or workdays. Participants were considered sedentary if they did not report any vigorous or moderate work (eg, as part of employment or chores) or leisure time activity (eg, sports, fitness, or recreational activities). Participants also self-reported prescription medications they had taken in the prior 30 days. They were also asked to bring their prescription bottles to the examination, where the information was recorded.
Examination and laboratory measures
Participants were assigned to either a morning, afternoon, or evening MEC session. During the MEC examination, trained examiners measured participants’ 30-second pulse rate after the participant sat resting quietly for 5 minutes according to the standardized NHANES protocol. Briefly, participants were positioned with the right palm upward and the examiner palpated the radial pulse on the lateral flexor surface of the wrist with the pads of the index and middle fingers. Using a digital stopwatch or wall clock, the 30-second pulse rate was recorded and then multiplied by 2 to record the 60-second pulse rate.
A phlebotomist obtained a blood sample from all participants during the MEC visit using a standardized protocol by which HbA1c was measured (14). A half sample was assigned to the morning MEC session, in which participants were asked to fast for 8 to less than 24 hours to obtain an fasting plasma glucose (FPG) measure. The blood samples were sent to a central laboratory for testing. Participants were considered to have diagnosed diabetes if they answered “yes” when asked whether a doctor or health-care professional had ever told them that they had diabetes. Among those who did not report a diagnosis of diabetes, undiagnosed diabetes was defined as HbA1c greater than or equal to 48 mmol/mol (≥ 6.5%) or FPG greater than or equal to 126 mg/dL (fasting 8-< 24 hours). Prediabetes was defined as no previous diagnosis of diabetes and HbA1c 39 to 46 mmol/mol (5.7%-6.4%) or FPG 100 to 125 mg/dL. Normoglycemia was defined as no previous diagnosis of diabetes and HbA1c less than 39 mmol/mol (< 5.7%) and FPG less than 100 mg/dL (15).
Height and weight were recorded by a trained interviewer to determine BMI (kg/m2), defined as normal less than 25.0 kg/m2, overweight 25.0 to 29.9 kg/m2, and obese 30 kg/m2 or greater. Waist circumference was also measured, with cut points at greater than 102 cm for men and greater than 88 cm for women indicating greater CVD risk (16).
Chronic kidney disease (CKD) was determined using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation, which estimates glomerular filtration rate from serum creatinine based on age, sex, and race (17). An estimated glomerular filtration rate of less than 60 mL/min per 1.73m2 was considered indicative of CKD.
Statistical analysis
Descriptive statistics (percentage, mean, SE) were used to assess the distribution of characteristics of the study population by glycemic status. Predictive margins regression was used to determine mean HR by glycemic status (normoglycemia [reference], prediabetes, undiagnosed diabetes, and diabetes) and HbA1c level (HbA1c < 53 mmol/mol [< 7.0%], [reference], HbA1c 53-< 75 mmol/mol [7.0%-< 9.0%], HbA1c 75-< 97 mmol/mol [9.0%-< 11.0%], and HbA1c ≥97 mmol/mol [≥ 11.0%]) among those with diagnosed diabetes; mean HR was also stratified by participant characteristics. Mean HR was adjusted for time of day of the examination because HR, on average, tends to be lower in the morning (18). Participants defined as having prediabetes or normoglycemia were assigned by design to the morning sample (to exclude existence of diabetes by FPG, for which there was a fasting blood draw in the morning only). Sampling weights were applied to account for the MEC session to which the participant was assigned.
In addition, predictive margins regression was used to account for demographic and health-related characteristics that may be associated with HR. Models by glycemic status were adjusted sequentially for: time of examination (model 1); model 1 and age (model 2); model 2 and sex, race/ethnicity, education, and poverty income ratio (value ≤ 1.00 indicates household income is at or below the poverty level) (model 3); model 3 and health insurance (model 4); model 4 and smoking status, duration of sleep, work or leisure time activity, BMI, waist circumference, menopausal status (women only), history of CVD, kidney disease, and antihypertensive medications, because these have been shown to affect HR (19) (model 5). Models by HbA1c level among those with diagnosed diabetes were additionally adjusted for oral antidiabetic medications, including glucagon-like peptide 1 receptor agonist, sulfonylureas, biguanides, α-glucosidase inhibitors, and thiazolidindiones (model 6). Models were determined for the total population and also stratified by sex. All statistical analyses used sample weights and accounted for nonresponse and the cluster design using SUDAAN (SUDAAN User’s Manual, Release 9.2, 2008; Research Triangle Institute).
Results
Participant characteristics
Participant characteristics are shown in Table 1. A higher proportion of adults with diagnosed diabetes were older, non-Hispanic black, had less than a high school education, had health insurance, sleep fewer than 7 hours per night, were more sedentary, and were more likely to be obese compared to those with normoglycemia. As expected, the prevalence of CVD, kidney disease, and use of antihypertensive medications and statins were significantly higher among those with diagnosed diabetes compared to those with normoglycemia. Similar associations persisted for those with undiagnosed or prediabetes compared to those with normoglycemia.
Table 1.
Characteristics of participants, NHANES 2011 to 2016
| Normoglycemia (Reference) (N = 3164) | Prediabetes (N = 2564) | Undiagnosed Diabetes (N = 564) | Diagnosed Diabetes (N = 2270) | |
|---|---|---|---|---|
| Age, y | ||||
| 20 to 44 | 62.8 (1.51) | 31.9 (1.27)d | 23.2 (2.69)d | 13.9 (0.71)d |
| 45 to 64 | 28.2 (1.31) | 42.1 (1.38)d | 46.4 (3.25)d | 46.5 (1.45)d |
| ≥ 65 | 9.0 (0.70) | 26.0 (1.15)d | 30.5 (2.75)d | 39.6 (1.24)d |
| Sex | ||||
| Men | 43.9 (0.92) | 52.6 (1.35)d | 56.8 (3.81)c | 51.3 (1.33)d |
| Women | 56.1 (0.92) | 47.4 (1.35)c | 42.2 (3.81)d | 48.7 (1.33)d |
| Race/Ethnicity | ||||
| Non-Hispanic white | 73.6 (2.35) | 70.8 (2.40)c | 61.4 (4.58)d | 66.2 (2.80)d |
| Non-Hispanic black | 11.0 (1.32) | 13.2 (1.40)c | 16.3 (2.57)c | 17.1 (1.86)d |
| All Hispanic | 15.4 (1.61) | 16.0 (1.66) | 22.2 (3.28)c | 17.7 (2.18) |
| Mexican American | 8.3 (1.08) | 9.6 (1.30) | 12.7 (2.29)c | 11.1 (1.91)c |
| Education | ||||
| < High school | 12.7 (1.26) | 18.6 (1.36)d | 24.2 (2.19)d | 23.2 (1.65)d |
| High school/GED | 18.0 (1.18) | 23.4 (1.57)d | 25.2 (2.42)c | 23.6 (1.41)d |
| ≥ High school | 69.3 (2.07) | 58.1 (1.94)d | 50.6 (3.28)d | 53.2 (1.78)d |
| Below poverty (PIR, ≤ 1.00) | 15.3 (1.27) | 15.3 (1.04) | 22.3 (3.03)c | 19.7 (1.57)c |
| Health insurance coverage | 81.7 (0.98) | 82.2 (1.34) | 81.6 (2.01) | 89.8 (0.70)d |
| Current smoker | 18.6 (1.40) | 21.4 (1.15) | 18.6 (2.54) | 15.4 (1.02) |
| Sleep < 7 h | 28.6 (1.41) | 29.9 (0.93) | 30.8 (2.87) | 34.9 (1.54)c |
| No work time activity | 55.5 (1.40) | 54.2 (1.28) | 61.1 (3.97) | 64.4 (1.60)d |
| No leisure time activity | 39.7 (1.61) | 52.3 (1.70)d | 65.4 (3.15)d | 61.3 (1.79)d |
| BMI, kg/m2 | ||||
| Mean | 27.5 (0.17) | 30.3 (0.23)d | 33.6 (0.53)d | 33.0 (0.23)d |
| < 25 | 38.1 (1.39) | 22.5 (1.34)d | 11.8 (1.62)d | 11.7 (0.98)d |
| 25 to < 30 | 33.5 (1.00) | 33.9 (1.15) | 21.5 (2.68)d | 26.7 (1.21)d |
| ≥ 30 | 28.3 (1.04) | 43.7 (1.54)d | 66.7 (3.26)d | 61.6 (1.56)d |
| High-risk waist circumference (> 88 cm for women; > 102 cm for men) (mean, SD) | 47.1 (1.50) | 65.3 (1.65)d | 81.7 (1.98)d | 82.2 (1.44)d |
| Hypertension | 20.9 (1.26) | 41.7 (1.37)d | 51.1 (3.87)d | 68.8 (1.47)d |
| History of CVD | 2.2 (0.37) | 7.8 (0.80)d | 10.1 (2.53)c | 18.3 (0.96)d |
| eGFRa (< 60 mL/min per 1.73 m2) | 2.5 (0.34) | 7.2 (0.65)d | 11.9 (1.68)d | 20.9 (1.00)d |
| Medicationsb | ||||
| ACE inhibitors/ARB | 7.8 (0.65) | 23.6 (1.42)d | 30.0 (2.85)d | 60.4 (1.56)d |
| Beta-blocker | 4.2 (0.47) | 14.4 (0.95)d | 21.0 (3.24)d | 39.9 (1.37)d |
| Diuretics | 6.0 (0.61) | 15.6 (1.03)d | 24.5 (2.70)d | 30.5 (0.97)d |
| CA-channel blocker | 3.7 (0.40) | 7.9 (0.61)d | 14.3 (1.88)d | 19.4 (1.13)d |
| Statins | 8.0 (0.71) | 23.9 (1.16)d | 25.7 (2.59)d | 55.9 (1.62)d |
Data are shown as percentage (SE).
Diagnosed diabetes based on self-report; undiagnosed diabetes based on FPG equal to or greater than 126 mg/dL or HbA1c equal to or greater than 48 mmol/mol (≥ 6.5%); prediabetes based on FPG 100 to 125 mg/dL or HbA1c 39 to 46 mmol/mol (5.7%-6.4%); normal glucose based on FPG less than 100 mg/dL and HbA1c less than 39 mmol/mol (< 5.7%).
Participants defined as having prediabetes or normoglycemia, by definition (to exclude existence of diabetes by FPG), were assigned to the morning sample.
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; BMI, body mass index; CA, calcium; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; GED, General Educational Development; HbA1c, hemoglobin A1c; NHANES, National Health and Nutrition Examination Survey, PIR, poverty income ratio.
aeGFR was estimated based on the Chronic Kidney Disease–Epidemiology Collaboration equation (17).
bPrescription medication use is not mutually exclusive.
c P less than .05 vs normoglycemia.
d P less than .001 vs normoglycemia.
Mean heart rate by glycemic status
After adjusting for time of day of the examination, overall mean HR remained elevated in those with diagnosed diabetes, but was not statistically different compared to those with normoglycemia (73 bpm vs 72 bpm) (Table 2). After stratifying by age, however, mean HR was significantly higher for those with diagnosed diabetes compared to those with normoglycemia for all age groups (P < .05 for all). This significant difference persisted for men, Hispanics, and those with a high school education or greater; those without health insurance; those who were overweight; those engaged in work time activity; those with hypertension; those with no history of CVD or CKD; and those not using β-blockers or statins.
Table 2.
Mean heart rate (beat per minute, SE) adjusted for time of day of examination by glycemic status among adults age 20 years or older, NHANES 2011 to 2016
| Normoglycemia (Reference) (N = 3164) | Prediabetes (N = 2564) | Undiagnosed Diabetes (N = 564) | Diagnosed Diabetes (N = 2270) | |
|---|---|---|---|---|
| Overall | 72 (0.3) | 72 (0.4) | 74 (0.7)a | 73 (0.7) |
| Age, y | ||||
| 20 to 44 | 73 (0.3) | 74 (0.6)a | 78 (1.7)a | 80 (1.4)b |
| 45 to 64 | 70 (0.5) | 72 (0.6)a | 75 (1.3)a | 74 (0.8)a |
| ≥ 65 | 68 (0.7) | 70 (0.6)a | 71 (1.6) | 70 (0.7)a |
| Sex | ||||
| Men | 70 (0.3) | 71 (0.5)a | 73 (1.4)a | 72 (0.8)a |
| Women | 74 (0.4) | 74 (0.5) | 76 (1.3) | 74 (0.7) |
| Race/Ethnicity | ||||
| All Hispanic | 72 (0.5) | 72 (0.4) | 74 (1.2) | 74 (0.7)a |
| Mexican American | 72 (0.7) | 71 (0.5) | 74 (1.2) | 74 (1.0) |
| Non-Hispanic white | 72 (0.3) | 73 (0.5) | 74 (1.1)a | 73 (0.9) |
| Non-Hispanic black | 72 (0.5) | 71 (0.6) | 76 (1.5)a | 73 (0.8) |
| Education | ||||
| < High school | 72 (0.7) | 73 (0.6) | 74 (1.5) | 72 (0.7) |
| High school/GED | 72 (0.5) | 73 (0.7) | 72 (1.6) | 72 (1.2) |
| ≥ High school | 72 (0.3) | 72 (0.5) | 76 (1.0)b | 74 (1.0)a |
| PIR | ||||
| ≤ 1.00 | 74 (0.6) | 73 (0.6) | 74 (1.2) | 74 (0.8) |
| > 1.00 | 72 (0.3) | 72 (0.5) | 74 (0.9)a | 73 (0.7) |
| Health insurance coverage | ||||
| Yes | 72 (0.3) | 72 (0.4) | 75 (0.8)b | 73 (0.7) |
| No | 72 (0.6) | 72 (0.7) | 73 (1.2) | 75 (0.9)a |
| Smoking status | ||||
| Current | 74 (0.6) | 74 (0.7) | 73 (1.5) | 76 (2.0) |
| Former | 70 (0.6) | 71 (0.6) | 75 (1.8)a | 72 (0.9) |
| Never | 72 (0.4) | 72 (0.5) | 74 (1.2) | 72 (0.7) |
| Sleep, h | ||||
| < 7 | 71 (0.5) | 73 (0.8)a | 75 (1.3)a | 73 (0.9) |
| ≥ 7 | 72 (0.3) | 72 (0.4) | 74 (0.8)a | 73 (0.7) |
| Work time activity | ||||
| Yes | 71 (0.4) | 72 (0.5) | 74 (1.4) | 73 (0.9)a |
| No | 72 (0.4) | 73 (0.4) | 74 (0.9)a | 73 (0.8) |
| Leisure time activity | ||||
| Yes | 71 (0.3) | 72 (0.6) | 73 (0.9)a | 72 (0.9) |
| No | 74 (0.5) | 73 (0.5) | 74 (1.0) | 73 (0.8) |
| BMI, kg/m2 | ||||
| < 25.0 | 71 (0.4) | 71 (0.5) | 72 (1.9) | 72 (1.9) |
| 25.0–29.9 | 70 (0.4) | 71 (0.6) | 73 (2.2) | 73 (0.7)a |
| ≥ 30.0 | 74 (0.6) | 74 (0.6) | 75 (1.1) | 73 (0.7) |
| Waist circumference | ||||
| High risk (> 88 cm for women; > 102 cm for men) | 73 (0.4) | 74 (0.4) | 75 (0.8) | 73 (0.7) |
| Low risk (≤ 88 cm for women; ≤ 102 cm for men) | 71 (0.3) | 70 (0.5) | 72 (1.4) | 72 (1.5) |
| Hypertension, self-report | ||||
| Yes | 71 (0.6) | 72 (0.6) | 73 (1.1) | 73 (0.8)a |
| No | 72 (0.3) | 72 (0.4) | 75 (1.1)a | 73 (0.8) |
| History of CVD | ||||
| Yes | 69 (2.0) | 70 (1.0) | 67 (1.9) | 68 (1.0) |
| No | 72 (0.3) | 72 (0.4) | 75 (0.7)b | 74 (0.7)a |
| eGFR, mL/min per 1.73 m2 | ||||
| < 60 | 70 (1.2) | 69 (0.9) | 72 (2.1) | 71 (1.2) |
| ≥ 60 | 72 (0.3) | 72 (0.4) | 74 (0.7)a | 74 (0.8)a |
| ACE inhibitor or ARB | ||||
| Yes | 71 (1.1) | 71 (0.7) | 72 (1.4) | 73 (0.7) |
| No | 72 (0.3) | 73 (0.4)a | 75 (0.9)b | 73 (0.9) |
| β-Blockers | ||||
| Yes | 68 (1.4) | 68 (1.0) | 70 (2.4) | 68 (0.8) |
| No | 72 (0.3) | 73 (0.4)a | 76 (1.1)a | 75 (0.9)b |
| Diuretics | ||||
| Yes | 70 (1.1) | 72 (0.9) | 76 (1.8)a | 72 (0.9) |
| No | 72 (0.3) | 72 (0.4) | 74 (1.1) | 73 (0.8) |
| CA-channel blockers | ||||
| Yes | 70 (1.4) | 71 (1.2) | 73 (1.7) | 72 (1.3) |
| No | 72 (0.3) | 72 (0.4) | 75 (0.7)b | 73 (0.7) |
| Taking statin | ||||
| Yes | 69 (1.0) | 71 (0.7) | 70 (1.2) | 71 (0.8) |
| No | 72 (0.3) | 73 (0.4) | 76 (0.8)b | 75 (0.8)a |
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; BMI, body mass index; CA, calcium; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; GED, General Educational Development; HbA1c, hemoglobin A1c; NHANES, National Health and Nutrition Examination Survey, PIR, poverty income ratio.
a P less than .05 vs normoglycemia.
b P less than .001 vs normoglycemia.
Among those with undiagnosed diabetes, after adjusting for time of examination, mean heart rate was significantly higher overall compared to those with normoglycemia (74 bpm vs 72 bpm, P = .002). Higher heart rate in those with undiagnosed diabetes was also found for those younger than age 65 years, men, non-Hispanic whites and blacks, those with a high school education or greater, those above the poverty income ratio index, those with health insurance, in former smokers, those without CVD, without hypertension, without CKD, and those not taking angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, β-blockers, CA-channel blockers, or statins. There were few differences in mean HR among those with prediabetes compared to those with normoglycemia.
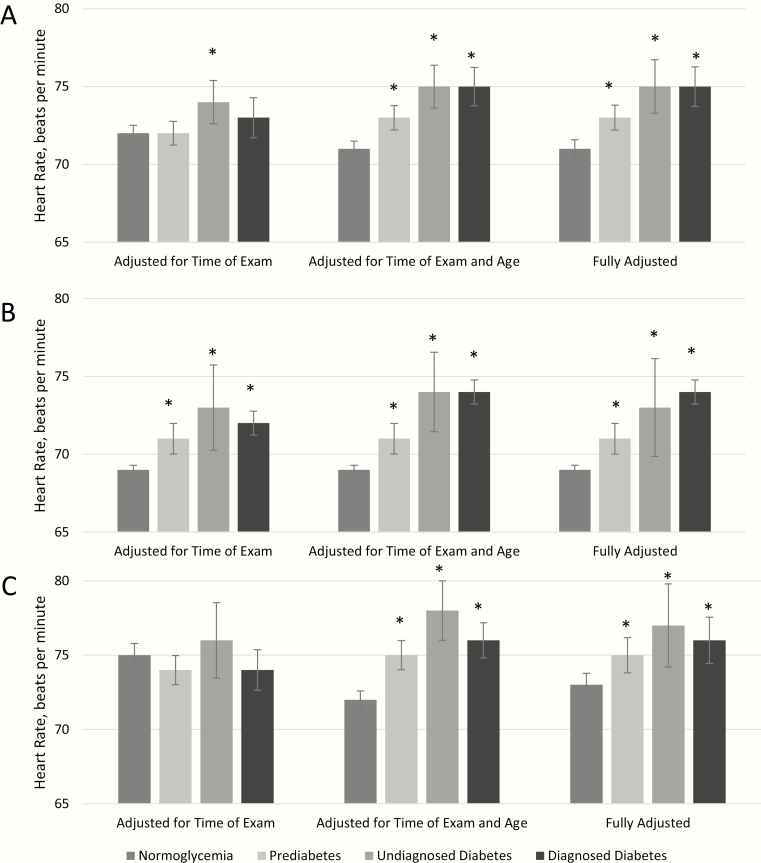
After adjusting simultaneously for time of examination, demographic characteristics, access to care, health behaviors, comorbidities, and antihypertensive medications, mean HR was significantly higher for those with prediabetes, undiagnosed diabetes, and diagnosed diabetes (73 bpm, 75 bpm, 75 bpm, respectively) compared to those with normoglycemia (71 bpm, P < .05 for all) (Table 3 and Fig. 1). The adjusted estimates were robust, with the greatest change in mean HR occurring after adjusting for age. This association remained when the results were stratified by sex; both for men and women mean HR was higher for those with prediabetes, undiagnosed diabetes, and diagnosed diabetes compared to those with normoglycemia after adjusting for age and after additional adjustment for all covariates.
Table 3.
Adjusted mean heart rate (beats per minute, SE) by glycemic status among adults age 20 years or older, NHANES 2011–2016
| Normoglycemia (Reference) | Prediabetes | Undiagnosed Diabetes | Diagnosed Diabetes | |
|---|---|---|---|---|
| Model 1 | 72 (0.3) | 72 (0.4) | 74 (0.7)a | 73 (0.7) |
| Model 2 | 71 (0.3) | 73 (0.4)b | 75 (0.7)b | 75 (0.6)b |
| Model 3 | 71 (0.3) | 73 (0.5)b | 76 (0.9)b | 75 (0.6)b |
| Model 4 | 71 (0.3) | 73 (0.4)b | 76 (0.9)b | 75 (0.6)b |
| Model 5 | 71 (0.3) | 73 (0.4)b | 75 (0.9)b | 75 (0.6)b |
Heart rate means are determined using predictive margins regression.
Abbreviation: NHANES, National Health and Nutrition Examination Survey.
a P less than .05 vs normoglycemia.
b P less than .001 vs normoglycemia.
Model 1: Adjusted for time of examination.
Model 2: Additionally adjusted for age.
Model 3: Additionally adjusted for sex, race/ethnicity, education, and poverty income ratio.
Model 4: Additionally adjusted for health insurance.
Model 5: Additionally adjusted for smoking, sleep, work, or leisure time activity, body mass index, waist circumference, menopausal status (women only), history of cardiovascular disease, kidney disease, and hypertensive medication.
Figure 1.
Mean heart rate (beats per minute) by level of glycemia in A, the total population, B, men, and C, women, National Health and Nutrition Examination Survey 2011 to 2016. Fully adjusted model includes time of examination, age, sex, race/ethnicity, education, poverty income ratio, health insurance, smoking, sleep, work, or leisure time activity, body mass index, waist circumference, menopausal status (women only), history of cardiovascular disease, kidney disease, and hypertensive medication. *P less than .05 vs normoglycemia.
Mean heart rate by hemoglobin A1c level in diagnosed diabetes
Table 4 shows mean HR among those with diagnosed diabetes by levels of HbA1c. After adjusting only for time of examination, mean HR increased with increasing HbA1c level among individuals with diagnosed diabetes, from 73 bpm in participants with HbA1c less than 53 mmol/mol (< 7.0%) to 78 bpm in individuals with HbA1c 75 to less than 97 mmol/mol (9.0%-< 11.0%) and 80 bpm in participants with HbA1c greater than or equal to 97 mmol/mol (≥ 11.0%) (P < .001 for both). HR generally increased and was significantly higher with increasing HbA1c across most stratum of demographic characteristics, health behaviors, comorbidities, and medication use.
Table 4.
Mean heart rate (beats per minute, SE) adjusted for time of day of examination by hemoglobin A1c level among adults with diagnosed diabetes age 20 years or older, NHANES 2011 to 2016
| Diagnosed Diabetes | ||||
|---|---|---|---|---|
| A1c <53 mmol/mol (<7.0%) (N = 1029) (Reference) | A1c 53-<75 mmol/mol (7.0% -<9.0%) (N = 730) | A1c 65-<97 mmol/mol (9.0% - <11.0%) (N = 217) | A1c ≥97 mmol/mol (≥11.0%) (N = 147) | |
| Overall | 73 (0.7) | 74 (0.6) | 78 (1.1)b | 80 (1.4)b |
| Age, y | ||||
| 20 to 44 | 78 (1.3) | 80 (1.8) | 85 (2.6)a | 82 (2.1) |
| 45 to 64 | 73 (0.9) | 76 (0.7)a | 77 (1.5)a | 80 (2.1)a |
| ≥ 65 | 71 (0.9) | 70 (0.9) | 73 (2.4) | 78 (1.6)b |
| Sex | ||||
| Men | 73 (0.8) | 73 (0.6) | 77 (1.8)a | 77 (1.9)a |
| Women | 73 (0.8) | 76 (1.2)a | 79 (1.4)a | 84 (1.7)b |
| Race/Ethnicity | ||||
| All Hispanic | 72 (0.7) | 75 (0.8)a | 82 (1.8)b | 80 (1.9)b |
| Mexican American | 72 (1.1) | 75 (1.2)a | 82 (2.0)b | 79 (2.3)a |
| Non-Hispanic white | 74 (0.9) | 74 (0.9) | 78 (1.7)a | 78 (3.7) |
| Non-Hispanic black | 72 (0.8) | 75 (1.0)a | 77 (2.9) | 82 (1.5)b |
| Education | ||||
| < High school | 72 (0.7) | 75 (1.0)a | 78 (1.6)a | 83 (1.8)b |
| High school/GED | 73 (1.2) | 71 (1.3) | 79 (1.5)a | 75 (2.7) |
| ≥ High school | 73 (1.0) | 76 (0.9) | 77 (1.7)a | 82 (1.8)b |
| PIR | ||||
| ≤ 1.00 | 75 (0.9) | 76 (1.2) | 79 (1.7)a | 81 (2.8) |
| > 1.00 | 72 (0.7) | 74 (0.7)a | 78 (1.2)b | 81 (1.6)b |
| Health insurance coverage | ||||
| Yes | 73 (0.7) | 75 (0.6) | 77 (1.1)b | 79 (1.6)b |
| No | 75 (1.3) | 73 (1.2) | 82 (2.3)a | 84 (1.8)a |
| Smoking status | ||||
| Current | 79 (2.4) | 77 (1.8) | 79 (2.8) | 79 (1.8) |
| Former | 72 (1.1) | 73 (1.5) | 77 (2.1)a | 76 (3.0) |
| Never | 72 (0.8) | 74 (0.8) | 78 (1.7)a | 83 (1.9)b |
| Sleep, h | ||||
| < 7 | 71 (0.9) | 75 (0.9)a | 78 (1.5)b | 79 (1.2)b |
| ≥ 7 | 73 (0.8) | 74 (1.0) | 78 (1.2)b | 81 (1.8)b |
| Work time activity | ||||
| Yes | 74 (0.7) | 73 (0.9) | 80 (2.3)a | 77 (1.9) |
| No | 72 (0.9) | 75 (0.8)a | 76 (1.34)a | 82 (1.7)b |
| Leisure time activity | ||||
| Yes | 72 (0.8) | 75 (1.1) | 78 (2.3)a | 80 (2.3)b |
| No | 73 (0.8) | 74 (0.6) | 78 (1.2)a | 81 (1.6)b |
| BMI, kg/m2 | ||||
| < 25.0 | 73 (2.3) | 73 (1.1) | 76 (4.4) | 80 (3.2) |
| 25.0–29.9 | 73 (0.9) | 73 (1.2) | 80 (1.7)b | 75 (2.9) |
| ≥ 30.0 | 73 (0.8) | 75 (0.8) | 78 (1.5)a | 83 (1.1)b |
| Waist circumference | ||||
| High risk (> 88 cm for women; >102 cm for men) | 73 (0.7) | 75 (0.8) | 78 (1.2)a | 82 (1.6)b |
| Low risk (≤ 88 cm for women; ≤ 102 cm for men) | 72 (1.8) | 72 (1.1) | 80 (3.4) | 77 (2.9) |
| Hypertension, self-report | ||||
| Yes | 73 (0.9) | 75 (0.8) | 77 (1.4)a | 80 (1.9)a |
| No | 72 (0.8) | 74 (1.1) | 79 (1.6)b | 81 (1.7)b |
| History of CVD | ||||
| Yes | 67 (1.1) | 69 (1.0) | 72 (3.1) | 75 (4.5) |
| No | 74 (0.7) | 76 (0.6) | 79 (1.0)b | 81 (1.2)b |
| eGFR, mL/min per 1.73 m2 | ||||
| < 60 | 70 (1.3) | 72 (1.5) | 74 (2.0) | 83 (2.8)b |
| ≥ 60 | 74 (0.8) | 75 (0.7) | 78 (1.23)b | 80 (1.7)a |
| ACE inhibitor or ARB | ||||
| Yes | 73 (0.9) | 74 (0.8) | 76 (1.4) | 81 (2.3)a |
| No | 72 (0.8) | 74 (1.0) | 80 (1.5)b | 80 (1.4)b |
| β-blockers | ||||
| Yes | 68 (0.7) | 68 (1.2) | 73 (2.4)a | 79 (4.5)a |
| No | 75 (0.8) | 77 (0.7)a | 79 (1.1)b | 81 (1.1)b |
| Diuretics | ||||
| Yes | 73 (1.2) | 74 (1.2) | 76 (1.7) | 79 (2.5)a |
| No | 73 (0.7) | 74 (0.6)a | 78 (1.3)b | 81 (1.7)b |
| CA-channel blockers | ||||
| Yes | 73 (1.7) | 73 (1.0) | 78 (2.7) | 80 (4.8) |
| No | 73 (0.7) | 75 (0.8) | 78 (1.1)b | 80 (1.1)b |
| Taking statin | ||||
| Yes | 72 (1.0) | 73 (0.7) | 73 (1.8) | 78 (2.6)a |
| No | 74 (0.8) | 76 (1.0)a | 82 (1.2)b | 82 (1.6)b |
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; BMI, body mass index; CA, calcium; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; GED, General Educational Development; HbA1c, hemoglobin A1c; NHANES, National Health and Nutrition Examination Survey, PIR, poverty income ratio.
a P less than .05 vs HbA1c less than 7.0%.
b P less than .001 vs HbA1c less than 7.0%.
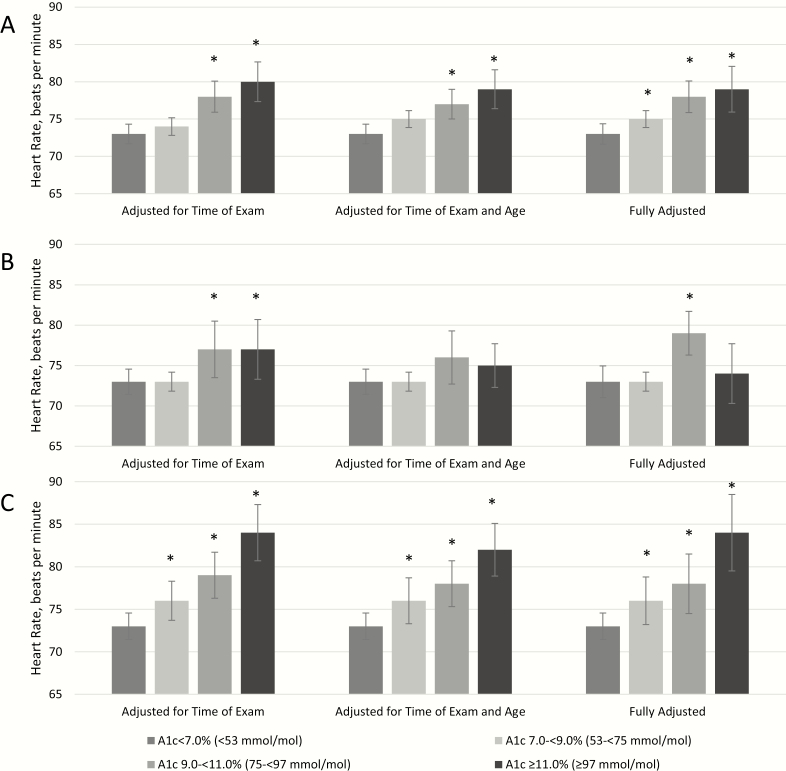
With simultaneous adjustment for time of examination, age and other demographic characteristics, access to care, comorbidities, health behaviors, antihypertensive medications, and oral antidiabetic medications, mean HR among those with diagnosed diabetes significantly increased with increasing levels of HbA1c (Table 5 and Fig. 2). In the fully adjusted model, mean HR was significantly higher for those with HbA1c 53 to less than 75 mmol/mol (7.0%-< 9.0%), 75 to less than 97 mmol/mol (9.0%-< 11.0%), and 97 mmol/mol or greater (≥ 11.0%) (75 bpm, 78 bpm, and 79 bpm, respectively) compared to those with HbA1c less than 53 mmol/mol (< 7.0%) (73 bpm, P < .05 for all). These results were less consistent for men but were robust for women. Among women, in the fully adjusted model mean HR was significantly higher for those with HbA1c greater than or equal to 53 mmol/mol (≥ 7.0%) compared to those with HbA1c less than 53 mmol/mol (< 7.0%) (P < .05 for all).
Table 5.
Adjusted mean heart rate (beats per minute, SE) by hemoglobin A1c level among adults with diagnosed diabetes age 20 years or older with diabetes, NHANES 2011 to 2016
| Diagnosed Diabetes | ||||
|---|---|---|---|---|
| HbA 1c < 53 mmol/mol(< 7.0%) (N = 1029)(Reference) | HbA 1c 53 to < 75 mmol/mol(7.0%-< 9.0%)(N = 730) | HbA 1c 65 to < 97 mmol/mol(9.0%-< 11.0%)(N = 217) | HbA 1c ≥ 97 mmol/mol(≥ 11.0%)(N = 147) | |
| Model 1 | 73 (0.7) | 74 (0.6) | 78 (1.1)b | 80 (1.4)b |
| Model 2 | 73 (0.7) | 75 (0.6) | 77 (1.0)b | 79 (1.3)b |
| Model 3 | 73 (0.6) | 75 (0.7)a | 78 (1.0)b | 78 (1.6)a |
| Model 4 | 73 (0.6) | 75 (0.7)a | 78 (1.0)b | 78 (1.6)a |
| Model 5 | 73 (0.8) | 75 (0.6)a | 78 (1.0)b | 79 (1.6)a |
| Model 6 | 73 (0.7) | 75 (0.6)a | 78 (1.2)b | 79 (1.6)b |
Heart rate means are determined using predictive margins regression.
HbA1c less than 7.0% is the reference.
Abbreviations: HbA1c, hemoglobin A1c; NHANES, National Health and Nutrition Examination Survey.
a P less than .05 vs HbA1c less than 7.0%.
b P less than .001 vs HbA1c less than 7.0%.
Model 1: Adjusted for time of examination.
Model 2: Additionally adjusted for age.
Model 3: Additionally adjusted for sex, race/ethnicity, education, and poverty income ratio.
Model 4: Additionally adjusted for health insurance.
Model 5: Additionally adjusted smoking, sleep, work, or leisure time activity, body mass index, waist circumference, menopausal status (women only), history of cardiovascular disease, kidney disease, and for hypertensive medication.
Model 6: Additionally adjusted for oral antidiabetic medications (glucagon-like peptide 1 receptor agonist, sulfonylureas, biguanides, α-glucosidase inhibitors, thiazolidindiones).
Figure 2.
Mean heart rate by hemoglobin A1c level among adults with diagnosed diabetes in A, the total diagnosed diabetes population, B, men with diagnosed diabetes, and C, women with diagnosed diabetes, National Health and Nutrition Examination Survey 2011 to 2016. Fully adjusted model includes time of examination, age, sex, race/ethnicity, education, poverty income ratio, health insurance, smoking, sleep, work, or leisure time activity, body mass index, waist circumference, menopausal status (women only), history of cardiovascular disease, kidney disease, hypertensive medication, and oral antidiabetic medications. *P less than .05 vs less than 53 mmol/mol (HbA1c < 7.0%).
Discussion
In summary, these data demonstrate a significantly higher HR in participants both with diagnosed and undiagnosed diabetes compared to those with normoglycemia. These differences were averaging at about 4 bpm, after adjusting for other factors known to affect HR such as age, other demographic characteristics, comorbidities, antihypertensive medications, and health-care access. The magnitude of this difference in HR is significant, particularly in the context of this being a nationally representative study (20, 21). Recent analyses from the Multi-Ethnic Study of Atherosclerosis cohort of more than 5000 participants demonstrated that, for 1 bpm increase in resting HR, there was a 4% greater adjusted relative risk for incident heart failure (22). The highest observed difference in HR was among those with diagnosed diabetes and HbA1c 97 mmol/mol or greater (≥ 11.0%) compared to those with HbA1c less than 53 mmol/mol (< 7.0%), at approximately 6 bpm, which supports the notion that the difference between the nondiabetic and diabetic populations is a function of the degree of hyperglycemia in the latter. This could explain why prior studies show higher HR in populations with diabetes compared to those without diabetes. Importantly, the association between HR and degree of glycemia in this study suggests an underlying pathological basis driving the elevation in HR. However, the association between HR and HbA1c was strongest among women.
Only a few studies have assessed the association between levels of glycemia and HR in individuals with diabetes. The most comprehensive data were reported among people with type 1 diabetes enrolled in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC). In this cohort, intensive treatment (goal of HbA1c < 42 mmol/mol [< 6.0%]), compared to conventional treatment, resulted in a significantly lower resting HR for up to 10 years post–random assignment during the observational EDIC study (23) when HbA1c converged in the original treatment groups. Similarly, in the present study, we found that HR increased with increasing HbA1c among those with diagnosed diabetes, the majority of whom have type 2 diabetes. Furthermore, a cross-sectional study of 5125 Korean adults showed that participants with higher resting HR (> 80 bpm) had almost 3 times higher odds of type 2 diabetes, as measured by FPG, compared to those with a lower resting HR (< 69 bpm) and this association was independent of age, sex, BMI, smoking, drinking, and family history of diabetes (24), thus, in concert with the findings reported herein.
The biological mechanism behind the observed associations between HR and glycemia or HbA1c is largely unknown; however, data from previous studies suggest that autonomic dysfunction could be an explanation (1, 25). Higher HR is a marker of lower parasympathetic tone or higher sympathetic activity (25-27), and higher sympathetic tone can cause insulin resistance by adrenergic stimulation (28). Conversely, insulin resistance and hyperinsulinemia can cause sympathetic overactivity leading to cardiac autonomic dysfunction (29). Although the association between HR and glycemic status was weak in univariate analysis stratified by BMI, the association became significant for those who were overweight or obese after further adjustment for age and other factors (data not shown). This is not inconsistent with prior reports showing that a higher HR predicted the development of obesity, insulin resistance, and diabetes, possibly because of differences in sympathetic activation and deconditioning (30), further supporting our findings.
In univariate analyses adjusted for time of examination, we found that HR was significantly higher for those with undiagnosed diabetes compared to those with normoglycemia, but HR was not significantly higher for those with diagnosed diabetes compared to those with normoglycemia. Because those with undiagnosed diabetes are not aware of their disease, it is likely that other comorbidities may be less aggressively monitored and treated compared to those with diagnosed diabetes, which may explain the higher HR in the former. Given that HR is an important prognostic factor for CVD and related complications, particularly among those with diabetes, this underscores the need for an increased awareness in these patients.
In the present study, we found no difference in HR by diabetes status among women when the analysis was adjusted for time of examination only. However, after adjusting for age, and then also further adjusting for demographic characteristics, health behaviors, health status, and menopausal status, HR was higher for women with diabetes compared to those without. Thus, among women, age may be an important factor for average HR. In addition, among women with diabetes, we found that HR increased significantly with increasing HbA1c levels, but a clear increase with HbA1c was not found with men (test for HbA1c and sex interaction, P = .023) Differences in HR among women were most dramatic between those with controlled (HbA1c < 7.0%) vs uncontrolled (HbA1c ≥ 9.0%) diagnosed diabetes. Finally, women had slightly higher HRs than men, which has been documented in previous studies (31, 32).
We found that among individuals with diagnosed diabetes and those with undiagnosed diabetes, HR was lower for those with vs without a history of CVD. This lower HR was likely driven by use of β-blockers, because the common use of β-blockers in those with a history of CVD is known to have direct effects on lowering HR (19). Indeed, we found that, among those not taking β-blockers, HR was higher for those with diabetes compared to those without, but HR was similar by glycemic status among those taking β-blockers.
Several longitudinal studies have shown that higher resting HR is associated with cardiovascular outcomes and mortality in the general population. CVD is a major cause of mortality among individuals with diabetes (33). Data from the ONTARGET and TRANSCEND trials, which included 31 531 patients with stable heart disease and/or diabetes who were followed for a median of 5 years, reported that higher HR was independently associated with significant increases in CVD events and all-cause death (9). Follow-up data of 6743 participants from the NHANES III, found that higher resting HR was associated both with cardiovascular and noncardiovascular mortality (34). Finally, a systematic meta-analysis using data from 3 prospective cohorts (Cardiovascular Health Study, Health ABC study, and Kuopio Ischemic Heart Disease Study) found that baseline HR was positively correlated with fasting glucose (21). Data from the Multi-Ethnic Study of Atherosclerosis, a cohort of adults age 45 to 84 years from 6 US communities, also found that higher resting HR was a predictor of heart failure (22). Given these study results, HR may be an important attribute to consider in the clinical care of patients with diabetes who are at high risk of CVD.
There are important strengths of this study. This study used a nationally representative sample of US adults; thus, the results are generalizable to the total US noninstitutionalized population. The NHANES provides laboratory information using standardized protocols on FPG and HbA1c that allowed us to examine HR in diagnosed and undiagnosed diabetes, prediabetes, and normoglycemia as well as by HbA1c among those with diagnosed diabetes. In addition, other potential factors related to diabetes and HR from the examination and questionnaire were assessed and controlled for in multiple linear regression. However, the cross-sectional study design of NHANES, which prevents any conclusions on causality and the direction of the association between HR and glycemia, are a limitation. Although the vast majority of diabetes in our study was type 2 diabetes (35), we were unable to distinguish between type 1 and type 2 diabetes, although there is no evidence of differences in HR based on diabetes type, after adjusting for confounders. To preserve sample size, we did not use a 2-hour oral glucose tolerance test to define levels of glycemia, which may have captured more participants with dysglycemia. Finally, we did not have data on medication adherence to assess its association with HR.
In summary, in a nationally representative sample of US adults, mean HR was higher among individuals with diabetes and prediabetes compared to those without after adjustment for multiple confounders. In addition, higher HR was independently associated with higher HbA1c levels among those with diagnosed diabetes, and most notably among women. These findings may reflect subclinical autonomic and/or myocardial dysfunction among those with diabetes or uncontrolled glucose levels. Given that higher resting HR has been associated with all-cause mortality and cardiovascular mortality, HR is an easily measured attribute and its accurate assessment should remain a priority in routine clinical care. Future research should assess the basis for the elevation of HR in the hyperglycemic state and how this might affect mortality.
Acknowledgments
Financial Support: This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (Grant GS-10F-0381L) and the National Institutes of Health (Grants R01DK107956 and U01 DK119083 to R.B.P.).
Glossary
Abbreviations
- BMI
Body mass index
- CKD
Chronic Kidney Disease
- CVD
cardiovascular disease
- HbA1c
hemoglobin A1c
- FPG
Fasting plasma glucose
- HR
heart rate
- MEC
mobile examination center
- NHANES
National Health and Nutrition Examination Surveys
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability: NHANES data are available publicly at https://wwwn.cdc.gov/nchs/nhanes/Default.aspx.
References
- 1. Pop-Busui R, Boulton AJ, Feldman EL, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(1):136–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pop-Busui R, Boulton AJ, Sosenko JM. Chapter 23, Peripheral and autonomic neuropathy in diabetes. In: Cowie CC, Casagrande SS, Menke A, et al. , eds. Diabetes in America. 3rd ed. Bethesda, MD: National Institutes of Health, NIH Pub No 17–1468; 2018:23-1–23-20. [Google Scholar]
- 3. Fox K, Borer JS, Camm AJ, et al. ; Heart Rate Working Group Resting heart rate in cardiovascular disease. J Am Coll Cardiol. 2007;50(9):823–830. [DOI] [PubMed] [Google Scholar]
- 4. Gillum RF, Makuc DM, Feldman JJ. Pulse rate, coronary heart disease, and death: the NHANES I Epidemiologic Follow-up Study. Am Heart J. 1991;121(1 Pt 1):172–177. [DOI] [PubMed] [Google Scholar]
- 5. Kannel WB, Kannel C, Paffenbarger RS Jr, Cupples LA. Heart rate and cardiovascular mortality: the Framingham Study. Am Heart J. 1987;113(6):1489–1494. [DOI] [PubMed] [Google Scholar]
- 6. Diaz A, Bourassa MG, Guertin MC, Tardif JC. Long-term prognostic value of resting heart rate in patients with suspected or proven coronary artery disease. Eur Heart J. 2005;26(10):967–974. [DOI] [PubMed] [Google Scholar]
- 7. Fox K, Ford I, Steg PG, Tendera M, Robertson M, Ferrari R; BEAUTIFUL Investigators Heart rate as a prognostic risk factor in patients with coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): a subgroup analysis of a randomised controlled trial. Lancet. 2008;372(9641):817–821. [DOI] [PubMed] [Google Scholar]
- 8. Jensen MT, Marott JL, Jensen GB. Elevated resting heart rate is associated with greater risk of cardiovascular and all-cause mortality in current and former smokers. Int J Cardiol. 2011;151(2):148–154. [DOI] [PubMed] [Google Scholar]
- 9. Lonn EM, Rambihar S, Gao P, et al. Heart rate is associated with increased risk of major cardiovascular events, cardiovascular and all-cause death in patients with stable chronic cardiovascular disease: an analysis of ONTARGET/TRANSCEND. Clin Res Cardiol. 2014;103(2):149–159. [DOI] [PubMed] [Google Scholar]
- 10. Carnethon MR, Yan L, Greenland P, et al. Resting heart rate in middle age and diabetes development in older age. Diabetes Care. 2008;31(2):335–339. [DOI] [PubMed] [Google Scholar]
- 11. Festa A, D’Agostino R Jr, Hales CN, Mykkänen L, Haffner SM. Heart rate in relation to insulin sensitivity and insulin secretion in nondiabetic subjects. Diabetes Care. 2000;23(5):624–628. [DOI] [PubMed] [Google Scholar]
- 12. Tomiyama H, Yamada J, Koji Y, et al. Heart rate elevation precedes the development of metabolic syndrome in Japanese men: a prospective study. Hypertens Res. 2007;30(5):417–426. [DOI] [PubMed] [Google Scholar]
- 13. Linnemann B, Janka HU. Prolonged QTc interval and elevated heart rate identify the type 2 diabetic patient at high risk for cardiovascular death. The Bremen Diabetes Study. Exp Clin Endocrinol Diabetes. 2003;111(4):215–222. [DOI] [PubMed] [Google Scholar]
- 14. Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey Laboratory Protocol. In: U.S. Department of Health and Human Services, ed. Hyattsville MD; 2011. [Google Scholar]
- 15. Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988-2012. JAMA. 2015;314(10):1021–1029. [DOI] [PubMed] [Google Scholar]
- 16. National Heart Lung and Blood Institute. Aim for a healthy weight: assessing your weight and health risk. In. Bethesda, MD: NHLBI. www.nhlbi.nih.gov/health/educational/lost_wt/index.htm. Accessed 2019. [Google Scholar]
- 17. Levey AS, Stevens LA, Schmid CH, et al. ; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gillum RF. The epidemiology of resting heart rate in a national sample of men and women: associations with hypertension, coronary heart disease, blood pressure, and other cardiovascular risk factors. Am Heart J. 1988;116(1 Pt 1):163–174. [DOI] [PubMed] [Google Scholar]
- 19. Reule S, Drawz PE. Heart rate and blood pressure: any possible implications for management of hypertension? Curr Hypertens Rep. 2012;14(6):478–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Böhm M, Schumacher H, Teo KK, et al. Achieved diastolic blood pressure and pulse pressure at target systolic blood pressure (120-140 mmHg) and cardiovascular outcomes in high-risk patients: results from ONTARGET and TRANSCEND trials. Eur Heart J. 2018;39(33):3105–3114. [DOI] [PubMed] [Google Scholar]
- 21. Khan H, Kunutsor S, Kalogeropoulos AP, et al. Resting heart rate and risk of incident heart failure: three prospective cohort studies and a systematic meta-analysis. J Am Heart Assoc. 2015;4(1):e001364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Opdahl A, Ambale Venkatesh B, Fernandes VRS, et al. Resting heart rate as predictor for left ventricular dysfunction and heart failure: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol. 2014;63(12):1182–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Paterson AD, Rutledge BN, Cleary PA, Lachin JM, Crow RS; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group The effect of intensive diabetes treatment on resting heart rate in type 1 diabetes: the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study. Diabetes Care. 2007;30(8):2107–2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim DI, Yang HI, Park JH, et al. The association between resting heart rate and type 2 diabetes and hypertension in Korean adults. Heart. 2016;102(21):1757–1762. [DOI] [PubMed] [Google Scholar]
- 25. Pop-Busui R. What do we know and we do not know about cardiovascular autonomic neuropathy in diabetes. J Cardiovasc Transl Res. 2012;5(4):463–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Spallone V, Ziegler D, Freeman R, et al. ; Toronto Consensus Panel on Diabetic Neuropathy Cardiovascular autonomic neuropathy in diabetes: clinical impact, assessment, diagnosis, and management. Diabetes Metab Res Rev. 2011;27(7):639–653. [DOI] [PubMed] [Google Scholar]
- 27. Watanabe K, Sekiya M, Tsuruoka T, et al. Relationship between insulin resistance and cardiac sympathetic nervous function in essential hypertension. J Hypertens. 1999;17(8):1161–1168. [DOI] [PubMed] [Google Scholar]
- 28. Deibert DC, DeFronzo RA. Epinephrine-induced insulin resistance in man. J Clin Invest. 1980;65(3):717–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bellavere F, Cacciatori V, Moghetti P, et al. Acute effect of insulin on autonomic regulation of the cardiovascular system: a study by heart rate spectral analysis. Diabet Med. 1996;13(8):709–714. [DOI] [PubMed] [Google Scholar]
- 30. Shigetoh Y, Adachi H, Yamagishi S, et al. Higher heart rate may predispose to obesity and diabetes mellitus: 20-year prospective study in a general population. Am J Hypertens. 2009;22(2):151–155. [DOI] [PubMed] [Google Scholar]
- 31. Ho JE, Larson MG, Ghorbani A, et al. Long-term cardiovascular risks associated with an elevated heart rate: the Framingham Heart Study. J Am Heart Assoc. 2014;3(3):e000668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Koenig J, Thayer JF. Sex differences in healthy human heart rate variability: a meta-analysis. Neurosci Biobehav Rev. 2016;64:288–310. [DOI] [PubMed] [Google Scholar]
- 33. Barrett-Connor E, Wingard D, Wong N, Goldberg R.. Chapter 18: Heart disease and diabetes. In: Cowie CC, Casagrande SS, Menke A, et al. , eds. Diabetes in America. 3rd ed. Bethesda, MD: National Institutes of Health, NIH Pub No 17–1468; 2018:18-1–18-30. [Google Scholar]
- 34. Alhalabi L, Singleton MJ, Oseni AO, Shah AJ, Zhang ZM, Soliman EZ. Relation of higher resting heart rate to risk of cardiovascular versus noncardiovascular death. Am J Cardiol. 2017;119(7):1003–1007. [DOI] [PubMed] [Google Scholar]
- 35. Centers for Disease Control and Prevention. National Diabetes Fact Sheet: National Estimates and General Information on Diabetes and Prediabetes in the United States, 2016. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2016. [Google Scholar]