Abstract
Purpose
miR-191 and miR-425 have been proved to be highly expressed in gastric carcinoma (GC). However, little research has been done on their clinical value in serum of patients with advanced GC. In addition, it is not clear whether they can be used as markers for the response and prognosis of GC patients treated with oxaliplatin combined with 5-fluorouracil and FOLFOX chemotherapy.
Patients and Methods
A total of 230 patients with advanced GC admitted to our hospital were selected as the study objects, all of whom received FOLFOX chemotherapy regimen. Another 100 cases of healthy subjects were included. QRT-PCR was employed to detect the serum expression of miR-191 and miR-425 in patients.
Results
Compared with the healthy subjects, the serum expressions of miR-191 and miR-425 in GC patients were significantly upregulated, which were correlated with differentiation degree and TNM staging, respectively. According to the ROC curve, the AUC of miR-191 and miR-425 for GC diagnosis was 0.937 and 0.901, respectively, while the AUC for differentiation degree diagnosis was 0.854 and 0.822, and that for TNM staging diagnosis was 0.860 and 0.829, respectively. The predictive AUC of miR-191 and miR-425 for chemosensitivity was 0.868 and 0.835, respectively, with a combined predictive AUC of 0.935. Low differentiation degree, high TNM staging, high miR-191 and high miR-425 expressions were independent risk factors for chemotherapy insensitivity. Differentiation degree, TNM staging, chemotherapy effect, miR-191 and miR-425 were independent influencing factors for the prognosis of GC patients.
Conclusion
Up-regulated expression of miR-191 and miR-425 in the serum of patients with advanced GC are effective biomarkers for the diagnosis, chemotherapy and prognosis evaluation of GC.
Keywords: miR-191, miR-425, FOLFOX, chemotherapy, prognosis, GC
Introduction
Gastric carcinoma (GC) ranks the fourth place in the incidence rate of all malignant tumors, just followed by lung cancer, colon cancer and breast cancer, whose mortality rate ranks the second among all cancers.1 The incidence and mortality of GC vary geographically. About half of the cases occur in East Asia, among which China accounts for about 42.6% of the world’s new cases and 45% of all GC-related deaths every year.2 Surgical resection can be performed in patients with early or locally advanced GC, with the 5-year survival rate of 90%.3 However, as the disease presents no obvious clinical symptoms and characteristics at the early stage, a majority of patients only to be found in the advances stages when diagnosed, resulting in poor diagnostic rate, with an overall 5-year survival rate of less than 30%.4 Chemotherapy becomes the prioritized surgical approach for advanced GC patients, but only serve the purpose of palliative treatment as GC is too difficult to cure.5 Clinically, oxaliplatin combined with 5-fluorouracil and folinic acid (FOLFOX) is an effective chemotherapy regimen for patients with advanced GC.6 However, GC is a kind of malignant tumor with strong heterogeneity, whose primary or acquired drug resistance prevent chemotherapy from completely destroying the tumor cells, while chemotherapy insensitivity is a common cause of tumor recurrence and metastasis.7 Therefore, the evaluation of the chemotherapy effect and survival rate of patients with advanced GC can help optimize the treatment strategy.
MiRNA is a class of endogenous non-coding small RNA, which can directly bind to the mRNA 3ʹ non-coding region of the target gene, thus directly degrading the mRNA or inhibiting the translation process.8 MiRNA can alter the occurrence and development of various malignant tumors by affecting their biological functions, including proliferation, migration, and invasion.9 It also plays a role in the diagnosis, severity judgment and prognosis of various malignant tumors including GC.10,11 MiR-191 and miR-425 are abnormally expressed in various cancers, such as lung cancer, liver cancer, GC, etc.12,13 MiR-191, a part of miR-191/miR-425 clusters, is upregulated in the blood of patients with various malignant tumors, which can be used as a non-invasive biomarker for tumor diagnosis and prognosis.14 Studies in recent years have shown that the overexpression of miR-191/miR-425 clusters in breast cancer cells can lead to changes in gene expression profiling, thereby fundamentally changing the occurrence and progression of breast cancer.15 In addition, Vaira16 revealed that miR-425-3p could predict the response of hepatocellular carcinoma to sorafenib treatment. However, the role of blood miR-191 and miR-425 in the diagnosis, FOLFOX chemotherapy, and prognosis of GC patients remains poorly understood.
The expression of serum miR-191 and miR-425 of GC patients was detected by qRT-PCR to explore their clinical value in GC patients and their relationship with chemotherapy response and prognosis.
Materials and Methods
General Information
A total of 230 patients with advanced GC admitted to Affiliated Cancer Hospital of Zhengzhou University from February 2011 to May 2014 were enrolled, including 156 males and 74 females, aged 36–78 years. The inclusion criteria were as follows: GC patients diagnosed histologically or pathologically without any previous radiotherapy or chemotherapy prior to this study, whose Eastern Cooperative Oncology Group (ECOG) score17 was no more than 2 points, and received at least two cycles of chemotherapy with an estimated survival time of no less than 12 weeks. The exclusion criteria were as follows: Patients with other malignant tumors. Patients with central nervous system metastasis. Patients with mental disorders who cannot cooperate in this study. Patients withdraw from the experiment or lost to follow-up. Another 100 healthy subjects from the same period were selected, including 60 males and 40 females, aged 35–76 years. The research program was approved by the Medical Ethics Committee of Affiliated Cancer Hospital of Zhengzhou University and the experiment was carried out in accordance with the Helsinki Declaration. Written informed consent forms were obtained from all patients in this study.
Chemotherapy
All GC patients received FOLFOX chemotherapy regimen.18 On the first day, oxaliplatin (130mg/m2) was given intravenously for 2 hrs, followed by leucovorin (200mg/m2) for 2 hrs, and then 5-fluorouracil (450mg/m2) for 22 hrs. Chemotherapy was repeated every 3 weeks, with 21 days as a cycle, and a total of 2 cycles were performed. In comply with the National Cancer Institute-Common Toxicity Criteria (NCI-CTC) of United States,19 the dosage of 5-fluorouracil was reduced by 15% in the event of grade 3–4 diarrhea, stomatitis or dermatitis, and the dosage of oxaliplatin was decreased by 15% in the case of persistent paresthesia and functional impairment during the chemotherapy cycle.
Curative Effect Evaluation
According to RECIST1.1 solid tumor efficacy evaluation criteria,20 the diagnosis and corresponding clinical symptoms were as follows: Complete response (CR): The target lesion disappeared completely and remained for at least 2 weeks. Partial response (PR): A reduction of at least 30% in the sum of the maximum length and diameter of baseline lesions, without the occurrence of new lesions. Progressive disease (PD): The sum of the maximum length and diameter of baseline lesions increases by at least 20% or new lesions appear. Stable disease (SD): The sum of the maximum length and diameter of baseline lesions decreased but did not reach PR, or increased but did not reach PD, falling between PR and PD.
QRT-PCR Detection
An amount of 5mL of peripheral venous blood samples were taken from all study subjects and placed in vacuum blood collection vessels. The samples were then centrifuged at 1500g for 10min at 4°C, and 1.0mL of the obtained serum was collected and stored at -80°C for later use. Next, the total RNA was extracted using MagMAX mir Vana isolation kit (Shanghai Even bridge biotechnology Co., Ltd., A27828), whose RNA concentration and purity were detected by NanoDrop 1000 ultramicrospectrophotometer (NanoDrop, Wilmington, DE, USA), while RNA integrity identified by agarose gel electrophoresis. The RNA samples were retro-transcribed according to the instructions of TaqMan MicroRNA reverse transcription kit (Shanghai Even bridge biotechnology Co., Ltd., 4366596). PCR experiments were performed in triplicate using TaqMan Universal PCR Master Mix (Shanghai Runwell Technology Co., Ltd., AB-4324018) of ABI 7300 real-time fluorescence quantitative PCR system (Applied Biosystems, Foster City, CA, USA, 4318157). The amplification conditions of qPCR were as follows: 94°C: 5min, 94°C: 30s, 55°C: 30s, 72°C: 30s, totaling 40 cycles. Finally, the cyclic threshold (Ct) value was calculated by SDS 2.0.1 software, and the data were analyzed by 2−ΔΔct. The primer sequence was designed and synthesized by Guangzhou Ruibo Biotechnology Co., Ltd.
Primer sequence of MiR-191
Forward: 5ʹ-AAGGAATCTTTCTGCACTCAAGCAT-3ʹ,
Reverse: 5ʹ-ATGCTTGAGTGCAGAGATTCCCTT-3ʹ.
Primer sequence of MiR-425:
Forward: 5ʹ-ACACTCCAGCTGGGAATGACACGATCACTCC-3,
Reverse: 5ʹ-TGGTGTCGTGGAGTCG-3ʹ.
Primer sequence of U6:
Forward: 5ʹ-CTCGCTTCGGCAGCACA-3ʹ,
Reverse: 5ʹ-ACGCTTCACGAATTTGCGT-3ʹ.
Statistical Methods
The counting data were expressed by case/percentage n(%) and a chi-square test was adopted for comparison of counting data between groups. Data with normal distribution were presented as mean ± standard deviation (Meas±SD). A t-test was adopted for inter-group comparison, and a paired t test was employed for comparison between groups before and after chemotherapy. Receiver operating characteristic curve, also known as ROC, and area under the curve (AUC) were employed to evaluate the diagnostic value of miR-191 and miR-425 in GC. With miR-191 and miR-425 as independent variables, logistic regression model was established to fit the ROC curve of joint detection according to the probability value. Spearman rank correlation coefficient was applied for correlation analysis. Logistic single-factor and multiple-factor regression analysis was used to analyze the risk factors of the efficacy of radiotherapy and chemotherapy in GC patients. Kaplan-Meier survival curve was drawn to calculate the survival rate, and Log rank test was used for survival analysis. Multivariate Cox regression analysis was adopted to analyze the risk factors affecting the prognosis of GC patients. P<0.05 was considered to be statistically different. SPSS 22.0 software (Company, Chicago, Illinois, USA) was employed for statistical analysis.
Results
Clinical Value of Serum miR-191 and miR-425 in GC
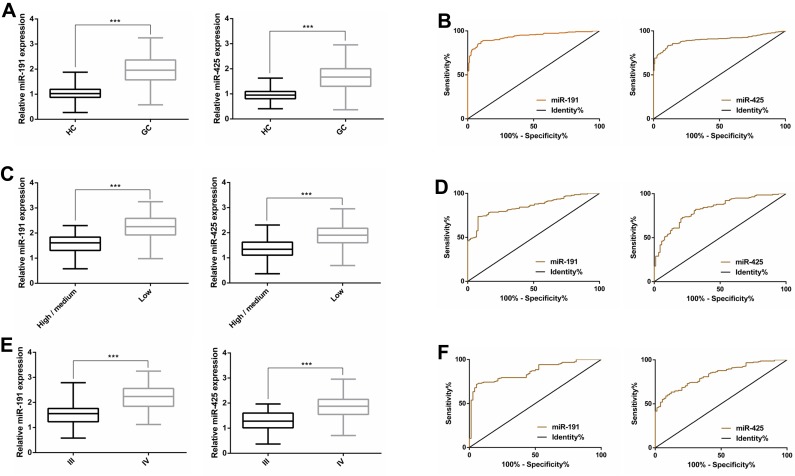
QRT-PCR showed that the serum miR-191 and miR-425 in GC patients were significantly up-regulated compared with those in healthy subjects (P<0.001). Further observation of the relationship between the clinical characteristics and the expressions of miR-191 and miR-425 indicated that miR-191 and miR-425 were related to the degree of differentiation and TNM staging, respectively (P<0.05). In addition, ROC curve analysis revealed that the AUC of serum miR-191 and miR-425 for GC diagnosis was 0.937 and 0.901, respectively, the AUC of serum miR-191 and miR-425 for differentiation degree diagnosis was 0.854 and 0.822, and the AUC of serum miR-191 and miR-425 for TNM staging diagnosis was 0.860 and 0.829, respectively (Figure 1, Tables 1 and 2).
Figure 1.
Clinical value of serum miR-191 and miR-425 in GC. (A) The expression of miR-191and miR-425in serum of GC patients; (B) the ROC curves of miR-191and miR-425for GC diagnosis; (C) the expression of miR-191and miR-425 in differentiation degree; (D) the ROC curves of miR-191and miR-425for differentiation degree diagnosis; (E) the expression of miR-191 and miR-425 in TNM staging; (F) the ROC curves of miR-191 and miR-425 for TNM staging diagnosis.
Note: ***P<0.001.
Table 1.
ROC Parameters
| Parameters | AUC | 95%CI | Cut-Off | Specificity (%) | Sensitivity (%) |
|---|---|---|---|---|---|
| miR-191 | |||||
| GC | 0.937 | 0.914–0.960 | 1.352 | 90.00 | 87.83 |
| Differentiation degree | 0.854 | 0.806–0.902 | 1.994 | 92.13 | 73.76 |
| TNM staging | 0.860 | 0.812–0.908 | 1.964 | 94.59 | 71.79 |
| miR-425 | |||||
| GC | 0.901 | 0.869–0.932 | 1.200 | 89.13 | 83.48 |
| Differentiation degree | 0.822 | 0.768–0.876 | 1.652 | 78.65 | 73.05 |
| TNM staging | 0.829 | 0.777–0.880 | 1.720 | 87.84 | 63.46 |
Table 2.
Relationship Between miR-191, miR-425 and Clinicopathological Parameters of GC Patients (Meas±SD)
| Clinicopathological Parameters | n | miR-191 | t/F | P | miR-425 | t/F | P |
|---|---|---|---|---|---|---|---|
| Gender | 0.264 | 0.792 | 0.298 | 0.766 | |||
| Male | 156 | 1.980±0.563 | 1.675±0.498 | ||||
| Female | 74 | 1.959±0.545 | 1.654±0.487 | ||||
| Age (years) | 1.654 | 0.100 | 1.057 | 0.292 | |||
| <60 | 138 | 1.924±0.578 | 1.640±0.541 | ||||
| ≥60 | 92 | 2.047±0.516 | 1.710±0.412 | ||||
| Drinking | 0.730 | 0.466 | 0.734 | 0.464 | |||
| No | 128 | 1.997±0.568 | 1.689±0.502 | ||||
| Yes | 102 | 1.943±0.541 | 1.641±0.484 | ||||
| ECOG performance status | 1.340 | 0.182 | 1.563 | 0.119 | |||
| 0–1 | 148 | 1.937±0.534 | 1.630±0.481 | ||||
| 2 | 82 | 2.039±0.591 | 1.736±0.512 | ||||
| Differentiation degree | 10.980 | <0.001 | 9.651 | <0.001 | |||
| High+medium differentiation | 89 | 1.563±0.385 | 1.315±0.394 | ||||
| Low differentiation | 141 | 2.232±0.488 | 1.890±0.415 | ||||
| TNM staging | 10.660 | <0.001 | 9.462 | <0.001 | |||
| III | 74 | 1.509±0.388 | 1.288±0.384 | ||||
| IV | 156 | 2.194±0.483 | 1.848±0.435 | ||||
| Tumor size (cm) | 1.493 | 0.137 | 1.258 | 0.210 | |||
| <6 | 140 | 1.929±0.575 | 1.692±0.509 | ||||
| ≥6 | 90 | 2.041±0.520 | 1.631±0.469 | ||||
| Tumor site | 0.222 | 0.801 | 0.850 | 0.429 | |||
| Cardia, gastric fundus | 30 | 1.931±0.627 | 1.623±0.528 | ||||
| Corpus ventriculi | 105 | 1.961±0.559 | 1.714±0.500 | ||||
| Gastric antrum, pylorus | 95 | 2.000±0.534 | 1.631±0.476 | ||||
| CEA (ng/mL) | 1.030 | 0.304 | 1.323 | 0.187 | |||
| <5 | 155 | 1.947±0.567 | 1.638±0.528 | ||||
| ≥5 | 75 | 2.027±0.531 | 1.730±0.410 | ||||
| CA199 (kU/L) | 1.522 | 0.130 | 0.995 | 0.321 | |||
| <37 | 143 | 1.930±0.565 | 1.643±0.525 | ||||
| ≥37 | 87 | 2.045±0.537 | 1.709±0.437 |
Relationship Between Serum miR-191, miR-425 and Chemotherapy Sensitivity of GC Patients
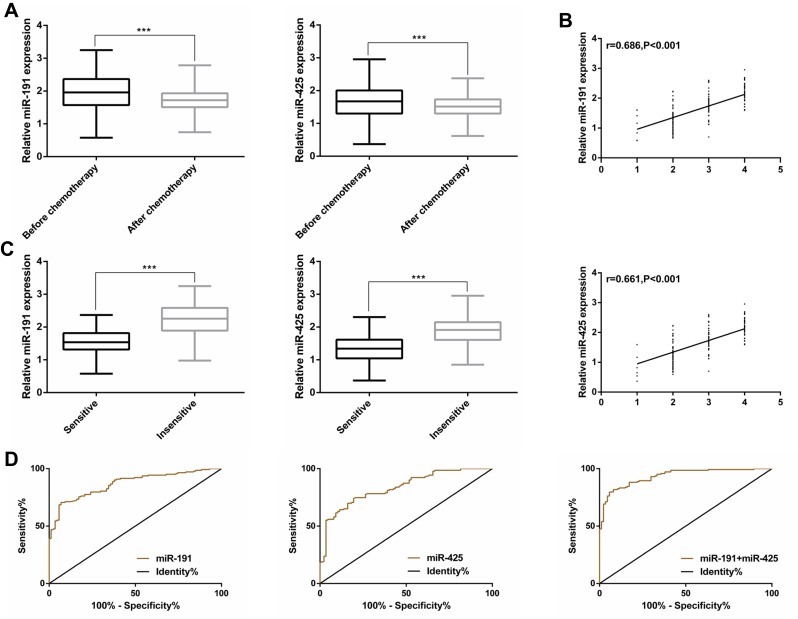
Of the 230 GC patients who received chemotherapy, the cases/(percentage) and corresponding diagnosis were as follows: 7/(3.05%): CR, 80/(34.78%): PR, 86/(37.39%): SD, and 57/(24.78%): PD. No clinical symptoms of sepsis or inflammatory diseases were observed during chemotherapy. Besides, qRT-PCR was employed to detect the expression of miR-191 and miR-425 in serum of GC patients before and after chemotherapy. It was found that both miR-191 and miR-425 were significantly down-regulated after chemotherapy (P<0.001). Based on the treatment effect, CR was set as 1, PR as 2, SD as 3, and PD as 4. Spearman correlation coefficient showed that miR-191 and miR-425 were both positively correlated with the chemotherapy effect (rmiR-19=0.686, P<0.001, rmiR-425=0.661, P<0.001). Serum miR-191 and miR-425 were significantly different between chemotherapy-sensitive and insensitive patients (P<0.001). In addition, the ROC curve indicated that the AUC of predicting chemosensitivity of miR-191 and miR-425 were 0.868 and 0.835, respectively, while that of miR-191/425 combined was 0.935 (Figure 2, Table 3).
Figure 2.
Relationship between serum miR-191, miR-425 and chemotherapy sensitivity of GC patients. (A) Expression of serum miR-191 and miR-425 before and after chemotherapy of GC patients; (B) MiR-191 and miR-425 were positively correlated with chemotherapy effect; (C) expression of serum miR-191 and miR-425 in chemotherapy-sensitive and insensitive patients. (D) The ROC curve of miR-191 miR-425 and miR-191/425 combined for predicting the sensitivity to chemotherapy.
Note: ***P<0.001.
Table 3.
ROC Parameters
| Parameters | AUC | 95%CI | Cut-Off | Specificity (%) | Sensitivity (%) |
|---|---|---|---|---|---|
| miR-191 | 0.868 | 0.823–0.913 | 2.029 | 93.10 | 70.63 |
| miR-425 | 0.835 | 0.784–0.887 | 1.644 | 80.46 | 74.13 |
| miR-191+miR-425 | 0.935 | 0.904–0.965 | 0.729 | 94.25 | 79.72 |
Relationship Between the Clinicopathological Parameters and the Sensitivity to Chemotherapy in GC Patients
According to the therapeutic effect of patients, CR and PR were defined as chemotherapy-sensitive (n=87), while SD and PD were defined as chemotherapy-insensitive (n=143). The correlation between between the clinicopathological parameters, miR-191 and miR-425 and the sensitivity to chemotherapy in GC patients was analyzed. Univariate analysis results showed that age, differentiation degree, TNM staging, tumor size, miR-191, and miR-425 were correlated with the sensitivity to chemotherapy (P<0.05). The median values of miR-191 (1.958) and miR-425 (1.667) were set as segmentation points, and the binary Logistic regression equation was employed to carry out multivariate logistic regression analysis of the factors with differences. The results demonstrated that the differentiation degree, TNM staging, miR-191 and miR-425 were independent risk factors for chemotherapy sensitivity in GC patients (P<0.05). (Tables 4–6)
Table 5.
Logistic Regression Analysis Assignment
| Factors | Variables | Assignments |
|---|---|---|
| Age (years) | X1 | <60=1, ≥60=2 |
| Differentiation degree | X2 | High+medium differentiation=1, low differentiation=2 |
| TNM staging | X3 | III=1, IV=2 |
| Tumor size (cm) | X4 | <6=1, ≥6=2 |
| miR-191 | X5 | <1.958=1, ≥1.958=2 |
| miR-425 | X6 | <1.667=1, ≥1.667=2 |
Table 4.
Relationship Between the Clinicopathological Parameters, miR-191 and miR-425 and the Sensitivity to Chemotherapy in GC Patients [n(%)]
| Factors | n | Sensitive (n=87) | Insensitive (n=143) | χ2 | P |
|---|---|---|---|---|---|
| Gender | 0.336 | 0.562 | |||
| Male | 156 | 61 (70.11) | 95 (66.43) | ||
| Female | 74 | 26 (29.89) | 48 (33.57) | ||
| Age (years) | 4.687 | 0.030 | |||
| <60 | 138 | 60 (68.97) | 78 (54.55) | ||
| ≥60 | 92 | 27 (31.03) | 65 (45.45) | ||
| Drinking | 3.246 | 0.072 | |||
| No | 128 | 55 (63.22) | 73 (51.05) | ||
| Yes | 102 | 32 (36.78) | 70 (48.95) | ||
| ECOG performance status | 2.029 | 0.154 | |||
| 0–1 | 148 | 61 (70.11) | 87 (60.84) | ||
| 2 | 82 | 26 (29.89) | 56 (39.16) | ||
| Differentiation degree | 8.324 | 0.004 | |||
| High+medium differentiation | 89 | 44 (50.57) | 45 (31.47) | ||
| Low differentiation | 141 | 43 (49.43) | 98 (68.53) | ||
| TNM staging | 12.220 | <0.001 | |||
| III | 74 | 40 (45.98) | 34 (23.78) | ||
| IV | 156 | 47 (54.02) | 109 (76.22) | ||
| Tumor size (cm) | 6.348 | 0.012 | |||
| <6 | 140 | 62 (71.26) | 78 (54.55) | ||
| ≥6 | 90 | 25 (28.74) | 65 (45.45) | ||
| Tumor site | 1.913 | 0.384 | |||
| Cardia, gastric fundus | 30 | 13 (14.94) | 17 (11.89) | ||
| Corpus ventriculi | 105 | 43 (49.43) | 62 (43.36) | ||
| Gastric antrum, pylorus | 95 | 31 (35.63) | 64 (44.76) | ||
| CEA (ng/mL) | 0.224 | 0.636 | |||
| <5 | 155 | 57 (65.52) | 98 (68.53) | ||
| ≥5 | 75 | 30 (34.48) | 45 (31.47) | ||
| CA199 (kU/L) | 0.00 | 0.980 | |||
| <37 | 143 | 54 (62.07) | 89 (62.24) | ||
| ≥37 | 87 | 33 (37.93) | 54 (37.76) | ||
| miR-191 | 11.550 | <0.001 | |||
| <1.958 | 115 | 56 (64.37) | 59 (41.26) | ||
| ≥1.958 | 115 | 31 (35.63) | 84 (58.74) | ||
| miR-425 | 8.153 | 0.004 | |||
| <1.667 | 115 | 54 (62.07) | 61 (42.66) | ||
| ≥1.667 | 115 | 33 (37.93) | 82 (57.34) |
Table 6.
Multivariate Logistic Regression Analysis
| Factors | β | S.E | Wals | OR (95% CI) | P |
|---|---|---|---|---|---|
| Age (years) | 0.180 | 0.286 | 0.399 | 1.198 (0.684–2.097) | 0.527 |
| Differentiation degree | 1.450 | 0.681 | 4.534 | 4.265 (1.122–16.209) | 0.033 |
| TNM staging | 2.121 | 0.786 | 7.283 | 8.339 (1787–38.911) | 0.007 |
| Tumor size (cm) | 1.159 | 0.630 | 3.380 | 3.185 (0.996–10.955) | 0.066 |
| miR-191 | 1.851 | 0.768 | 5.809 | 6.369 (1.413–28.702) | 0.016 |
| miR-425 | 1.509 | 0.591 | 6.515 | 4.521 (1.419–14.398) | 0.011 |
Relationship Between Clinicopathological Parameters, miR-191 and miR-425 and Prognosis of GC Patients
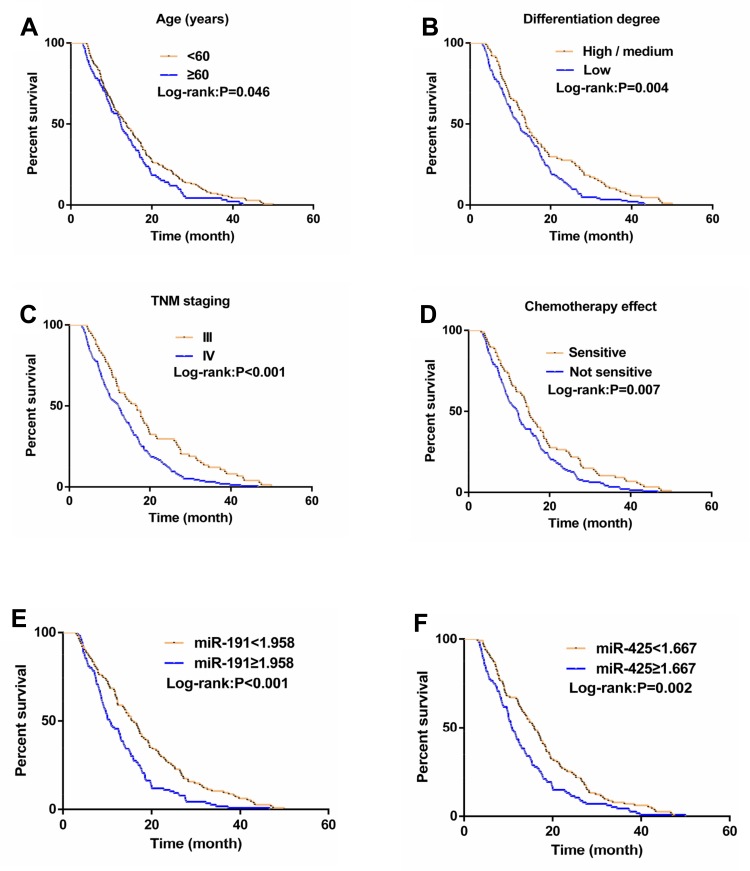
The median follow-up time of 230 GC patients was 12.9 months (ranging from 3.0 to 50.0 months), with a median survival time was 13.0 months. Univariate analysis demonstrated that age, differentiation degree, TNM staging, chemotherapy effect, miR-191, and miR-425 were associated with median survival time (P<0.05). The median survival time of patients with miR-191 <1.958 was significantly longer than that of patients with miR-191 ≥1.958 (P<0.01), while that of patients with miR-425 <1.667 was also significantly longer than that of patients with miR-425 ≥1.667 (P<0.01). Further multivariate Cox regression analysis demonstrated that differentiation degree, TNM staging, chemotherapy effect, miR-191, and miR-425 were independent prognostic factors for GC patients (P<0.05). (Figure 3, Tables 7 and 8).
Figure 3.
Relationship between clinicopathological parameters, miR-191 and miR-425 and prognosis in GC patients. The overall survival curve was plotted according to age (A), differentiation (B), TNM staging (C), chemotherapy effect (D), miR-191 (E), and miR-425 (F).
Table 7.
Univariate Analysis of Prognostic Factors in Patients with GC
| Factors | n | Median Survival Time (Month) | χ2 | P |
|---|---|---|---|---|
| Gender | 0.182 | 0.670 | ||
| Male | 156 | 12.6 | ||
| Female | 74 | 14.0 | ||
| Age (years) | 3.988 | 0.046 | ||
| <60 | 138 | 13.8 | ||
| ≥60 | 92 | 12.4 | ||
| Drinking | 1.424 | 0.233 | ||
| No | 128 | 13.1 | ||
| Yes | 102 | 12.9 | ||
| ECOG performance status | 2.052 | 0.152 | ||
| 0–1 | 148 | 13.0 | ||
| 2 | 82 | 12.8 | ||
| Differentiation degree | 8.169 | 0.004 | ||
| High+medium differentiation | 89 | 14.0 | ||
| Low differentiation | 141 | 12.3 | ||
| TNM staging | 13.690 | <0.001 | ||
| III | 74 | 16.9 | ||
| IV | 156 | 12.3 | ||
| Tumor size (cm) | 0.926 | 0.336 | ||
| <6 | 140 | 13.7 | ||
| ≥6 | 90 | 11.4 | ||
| Tumor site | 3.966 | 0.138 | ||
| Cardia, gastric fundus | 30 | 12.9 | ||
| Corpus ventriculi | 105 | 13.0 | ||
| Gastric antrum, pylorus | 95 | 12.6 | ||
| CEA (ng/mL) | 2.839 | 0.092 | ||
| <5 | 155 | 14.0 | ||
| ≥5 | 75 | 10.9 | ||
| CA199 (kU/L) | 1.004 | 0.316 | ||
| <37 | 143 | 14.1 | ||
| ≥37 | 87 | 12.3 | ||
| Chemotherapy effect | 7.201 | 0.007 | ||
| Sensitive | 87 | 14.8 | ||
| Insensitivity | 143 | 12.1 | ||
| miR-191 | 15.450 | <0.001 | ||
| <1.958 | 115 | 15.8 | ||
| ≥1.958 | 115 | 10.7 | ||
| miR-425 | 9.253 | 0.002 | ||
| <1.667 | 115 | 15.9 | ||
| ≥1.667 | 115 | 10.9 |
Table 8.
Multivariate Cox Regression Analysis of Prognosis in Patients with GC
| Factors | HR (95% CI) | P |
|---|---|---|
| Age (years) | 1.483 (0.781–2.768) | 0.224 |
| Differentiation degree | 2.142 (1.161–3.957) | 0.015 |
| TNM staging | 3.786 (2.324–6.167) | <0.001 |
| Chemotherapeutic effect | 2.138 (1.167–3.982) | 0.017 |
| miR-191 | 3.517 (1.978–6.267) | <0.001 |
| miR-425 | 2.367 (1.175–4.683) | 0.013 |
Discussion
GC patients are usually in an advanced stage when diagnosed, at which time platinum compounds are the main chemotherapy regimen in clinical practice.21 However, some patients present primary or secondary drug resistance in chemotherapy application, leading to poor therapeutic effect and seriously affecting prognosis.22 Therefore, the differentiation of patients with poor prognosis after chemotherapy can help optimize the treatment for patients with advanced GC.
Many studies have confirmed that abnormal miRNA expression in blood is closely related to the severity and prognosis of various malignant tumors.23,24 MiR-191 and miR-425 are believed to be highly expressed in GC.25 As reported by Shi,26 the up-regulated expression of miR-191 in GC cells and tissues can promote the growth of GC cells by inhibiting n-deacetylase/n-sulfone-based transferase 1 (NDST1). It has also been reported that the up-regulated expression of miR-425 in human GC cells can promote invasion and metastasis.27 Therefore, it can be concluded that miR-191 and miR-425 play an essential role in the occurrence and development of GC. However, previous studies focus on GC tissues or cells, in which tumor tissues need to be acquired by means of surgical resection or puncture, which is relatively traumatic, while serum miR detection stands out for its small trauma.28 In the present study, the expression levels of miR-191 and miR-425 in serum of GC patients were significantly up-regulated compared with normal people, and further ROC curve was drawn to find that the AUC of miR-191 and miR-425 in diagnosing GCwas 0.937 and 0.901, respectively, with good diagnostic value. Previous studies have shown that the expression of miR-191/425 clusters in GC tissues and serum increased significantly, and the AUC values of serum miR-191 and miR-425 for GC diagnosis were 0.849 and 0.548, respectively.29 This may be due to the fact that only advanced GC patients were included in this study, resulting in differences in diagnostic efficacy. In addition, by analyzing the relationship between the two and the clinical pathological parameters of GC patients, it was found that miR-191 and miR-425 were related to the differentiation degree and TNM staging, respectively, and had differential diagnostic value for these pathological parameters, which indicated that miR-191 and miR-425 could be used as markers for the evaluation of GC.
FOLFOX is supposed to be an effective palliative treatment for advanced GC patients.30 It is well established that markers can be used to predict the efficacy of GC chemotherapy. For example, in the study of Oh,31 vascular endothelial growth factor (VEGF) gene polymorphism can distinguish the response rate of FOLFOX chemotherapy in advanced GC patients (22.2% vs 32.3%), and the increased expression is a prognostic factor affecting progression-free survival. In addition, it has been observed that serum miR-19a is significantly up-regulated in serum during the drug-resistant phase of colorectal cancer, which can be used as a predictive marker of drug resistance in FOLFOX chemotherapy regimen.32 In this study, the expressions of miR-191 and miR-425 in serum of GC patients after chemotherapy were detected, and it was found both expressions were significantly down-regulated after chemotherapy, which indicated that the FOLFOX chemotherapy could inhibit the expression of both; however, the mechanism remains unclear. Next, the relationship between miR-191, miR-425 and the chemotherapy efficacy was analyzed, and the results exhibited that serum miR-191 and miR-425 were positively correlated with the chemotherapy efficacy, respectively, before chemotherapy. Moreover, ROC curve revealed that the AUC of predicting chemosensitivity of miR-191 and miR-425 were 0.868 and 0.835, respectively, while that of combined prediction of miR-191 and miR-425 was 0.935, suggesting that the combined detection of the two has a high predictive value for chemosensitivity. What is more, logistic regression analysis revealed that patients with low differentiation, TNM staging, high miR-191 and miR-425 expression in GC were at increased risk of chemotherapy insensitivity. Previous studies have supported that miR-191 can be acted as a candidate target gene for the treatment of hepatocellular carcinoma.33 According to Zhang,34 miR-425 can regulate the chemical resistance of colorectal cancer cells by regulating programmed cell death 10 (PDCD10). Combined with this study, miR-191 and miR-425 may play a role in the chemotherapy of various tumors, but the drug resistance mechanism of both in GC chemotherapy remains a subject of investigation. Further observation of the relationship between miR-191, miR-425 and the prognosis of GC patients demonstrated that the median survival time of low miR-191 (<1.958) and miR-425 (<1.667) was significantly prolonged, and the differentiation degree, TNM staging, chemotherapy effect, miR-191 and miR-425 were independent prognostic factors for GC patients. Although previous studies have confirmed that differentiation degree and TNM staging can affect the prognosis of GC patients after chemotherapy,35,36 here it is the first time that micR-191 and miR-425 are verified to be the influencing factors of chemotherapy and prognosis in GC patients.
Taken together, this study confirmed that miR-191 and miR-425 were upregulated in serum of patients with advanced GC, which are expected to be effective biomarkers for GC diagnosis, chemotherapy and prognosis evaluation. However, there are still shortcomings in the present study. To begin with, in vitro experiments are absent, and we failed to observed drug resistance mechanism of miR-191 and miR-425 in GC cells. And secondly, miR-191 and miR-425 need to be combined with traditional biomarkers of gastric cancer such as carbohydrate antigen 724 and pepsinogen in the clinical practice of pancreatic cancer. Nevertheless, these deficiencies will be addressed in follow-up studies.
Acknowledgment
Supported by the Project of tackling key problems of Medical Science and Technology in Henan Province No. 2018020504.
Disclosure
The author reports no conflicts of interest in this work.
References
- 1.Konno-Shimizu M, Yamamichi N, Inada K, et al. Cathepsin E is a marker of gastric differentiation and signet-ring cell carcinoma of stomach: a novel suggestion on gastric tumorigenesis. PLoS One. 2013;8(2):e56766. doi: 10.1371/journal.pone.0056766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang FH, Shen L, Li J, et al. The Chinese Society of Clinical Oncology (CSCO): clinical guidelines for the diagnosis and treatment of gastric cancer. Cancer Commun (Lond). 2019;39(1):10. doi: 10.1186/s40880-019-0349-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shinohara T, Satoh S, Kanaya S, et al. Laparoscopic versus open D2 gastrectomy for advanced gastric cancer: a retrospective cohort study. Surg Endosc. 2013;27(1):286–294. doi: 10.1007/s00464-012-2442-x [DOI] [PubMed] [Google Scholar]
- 4.Jin C, Shi W, Wang F, et al. Long non-coding RNA HULC as a novel serum biomarker for diagnosis and prognosis prediction of gastric cancer. Oncotarget. 2016;7(32):51763–51772. doi: 10.18632/oncotarget.v7i32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fazio N, Biffi R, Maibach R, et al. Preoperative versus postoperative docetaxel-cisplatin-fluorouracil (TCF) chemotherapy in locally advanced resectable gastric carcinoma: 10-year follow-up of the SAKK 43/99 Phase III trial. Ann Oncol. 2016;27(4):668–673. doi: 10.1093/annonc/mdv620 [DOI] [PubMed] [Google Scholar]
- 6.Shah MA, Cho JY, Tan IB, et al. A randomized Phase II study of FOLFOX with or without the MET inhibitor onartuzumab in advanced adenocarcinoma of the stomach and gastroesophageal junction. Oncologist. 2016;21(9):1085–1090. doi: 10.1634/theoncologist.2016-0038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng P, Chen L, Yuan X, et al. Exosomal transfer of tumor-associated macrophage-derived miR-21 confers cisplatin resistance in gastric cancer cells. J Exp Clin Cancer Res. 2017;36(1):53. doi: 10.1186/s13046-017-0528-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vidal AF, Cruz AM, Magalhaes L, et al. hsa-miR-29c and hsa-miR-135b differential expression as potential biomarker of gastric carcinogenesis. World J Gastroenterol. 2016;22(6):2060–2070. doi: 10.3748/wjg.v22.i6.2060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lloyd KA, Moore AR, Parsons BN, et al. Gastrin-induced miR-222 promotes gastric tumor development by suppressing p27kip1. Oncotarget. 2016;7(29):45462–45478. doi: 10.18632/oncotarget.9990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Imaoka H, Toiyama Y, Okigami M, et al. Circulating microRNA-203 predicts metastases, early recurrence, and poor prognosis in human gastric cancer. Gastric Cancer. 2016;19(3):744–753. doi: 10.1007/s10120-015-0521-0 [DOI] [PubMed] [Google Scholar]
- 11.Kosaka N, Iguchi H, Ochiya T. Circulating microRNA in body fluid: a new potential biomarker for cancer diagnosis and prognosis. Cancer Sci. 2010;101(10):2087–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao J, Qiao CR, Ding Z, et al. A novel pathway in NSCLC cells: miR191, targeting NFIA, is induced by chronic hypoxia, and promotes cell proliferation and migration. Mol Med Rep. 2017;15(3):1319–1325. doi: 10.3892/mmr.2017.6100 [DOI] [PubMed] [Google Scholar]
- 13.Yuwen D, Ma Y, Wang D, et al. Prognostic role of circulating exosomal miR-425-3p for the response of NSCLC to platinum-based chemotherapy. Cancer Epidemiol Biomarkers Prev. 2019;28(1):163–173. doi: 10.1158/1055-9965.EPI-18-0569 [DOI] [PubMed] [Google Scholar]
- 14.Nagpal N, Kulshreshtha R. miR-191: an emerging player in disease biology. Front Genet. 2014;5:99. doi: 10.3389/fgene.2014.00099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Di Leva G, Piovan C, Gasparini P, et al. Estrogen mediated-activation of miR-191/425 cluster modulates tumorigenicity of breast cancer cells depending on estrogen receptor status. PLoS Genet. 2013;9(3):e1003311. doi: 10.1371/journal.pgen.1003311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vaira V, Roncalli M, Carnaghi C, et al. MicroRNA-425-3p predicts response to sorafenib therapy in patients with hepatocellular carcinoma. Liver Int. 2015;35(3):1077–1086. doi: 10.1111/liv.2015.35.issue-3 [DOI] [PubMed] [Google Scholar]
- 17.Liu M, Hu G, Wang Y, et al. Comparison of FOLFOX and DOF regimens as first-line treatment in East Asian patients with advanced gastric cancer. Onco Targets Ther. 2018;11:375–381. doi: 10.2147/OTT [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ni XF, Wu CP, Jiang JT. Serum VEGFR-3 and survival of advanced gastric cancer patients treated with FOLFOX. World J Gastroenterol. 2010;16(17):2163–2169. doi: 10.3748/wjg.v16.i17.2163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hadoux J, Malka D, Planchard D, et al. Post-first-line FOLFOX chemotherapy for grade 3 neuroendocrine carcinoma. Endocr Relat Cancer. 2015;22(3):289–298. doi: 10.1530/ERC-15-0075 [DOI] [PubMed] [Google Scholar]
- 20.Fu X, Zhang Y, Yang J, et al. Efficacy and safety of trastuzumab as maintenance or palliative therapy in advanced HER2-positive gastric cancer. Onco Targets Ther. 2018;11:6091–6100. doi: 10.2147/OTT [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen QN, Wei CC, Wang ZX, Sun M. Long non-coding RNAs in anti-cancer drug resistance. Oncotarget. 2017;8(1):1925–1936. doi: 10.18632/oncotarget.12461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi WJ, Gao JB. Molecular mechanisms of chemoresistance in gastric cancer. World J Gastrointest Oncol. 2016;8(9):673–681. doi: 10.4251/wjgo.v8.i9.673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sierzega M, Kaczor M, Kolodziejczyk P, Kulig J, Sanak M, Richter P. Evaluation of serum microRNA biomarkers for gastric cancer based on blood and tissue pools profiling: the importance of miR-21 and miR-331. Br J Cancer. 2017;117(2):266–273. doi: 10.1038/bjc.2017.190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ries J, Baran C, Wehrhan F, et al. The altered expression levels of miR-186, miR-494 and miR-3651 in OSCC tissue vary from those of the whole blood of OSCC patients. Cancer Biomark. 2019;24(1):19–30. doi: 10.3233/CBM-180032 [DOI] [PubMed] [Google Scholar]
- 25.Ueda T, Volinia S, Okumura H, et al. Relation between microRNA expression and progression and prognosis of gastric cancer: a microRNA expression analysis. Lancet Oncol. 2010;11(2):136–146. doi: 10.1016/S1470-2045(09)70343-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi X, Su S, Long J, Mei B, Chen Y. MicroRNA-191 targets N-deacetylase/N-sulfotransferase 1 and promotes cell growth in human gastric carcinoma cell line MGC803. Acta Biochim Biophys Sin (Shanghai). 2011;43(11):849–856. doi: 10.1093/abbs/gmr084 [DOI] [PubMed] [Google Scholar]
- 27.Zhang Z, Li Y, Fan L, et al. microRNA-425-5p is upregulated in human gastric cancer and contributes to invasion and metastasis in vitro and in vivo. Exp Ther Med. 2015;9(5):1617–1622. doi: 10.3892/etm.2015.2318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zeng X, Xiang J, Wu M, et al. Circulating miR-17, miR-20a, miR-29c, and miR-223 combined as non-invasive biomarkers in nasopharyngeal carcinoma. PLoS One. 2012;7(10):e46367. doi: 10.1371/journal.pone.0046367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peng WZ, Ma R, Wang F, Yu J, Liu ZB. Role of miR-191/425 cluster in tumorigenesis and diagnosis of gastric cancer. Int J Mol Sci. 2014;15(3):4031–4048. doi: 10.3390/ijms15034031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jeong SH, Han JH, Kim JH, et al. Bax predicts outcome in gastric cancer patients treated with 5-fluorouracil, leucovorin, and oxaliplatin palliative chemotherapy. Dig Dis Sci. 2011;56(1):131–138. doi: 10.1007/s10620-010-1280-8 [DOI] [PubMed] [Google Scholar]
- 31.Oh SY, Kwon HC, Kim SH, et al. The relationship of vascular endothelial growth factor gene polymorphisms and clinical outcome in advanced gastric cancer patients treated with FOLFOX: VEGF polymorphism in gastric cancer. BMC Cancer. 2013;13:43. doi: 10.1186/1471-2407-13-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Q, Xia HW, Ge XJ, Zhang YC, Tang QL, Bi F. Serum miR-19a predicts resistance to FOLFOX chemotherapy in advanced colorectal cancer cases. Asian Pac J Cancer Prev. 2013;14(12):7421–7426. doi: 10.7314/APJCP.2013.14.12.7421 [DOI] [PubMed] [Google Scholar]
- 33.Elyakim E, Sitbon E, Faerman A, et al. hsa-miR-191 is a candidate oncogene target for hepatocellular carcinoma therapy. Cancer Res. 2010;70(20):8077–8087. doi: 10.1158/0008-5472.CAN-10-1313 [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y, Hu X, Miao X, et al. MicroRNA-425-5p regulates chemoresistance in colorectal cancer cells via regulation of programmed cell death 10. J Cell Mol Med. 2016;20(2):360–369. doi: 10.1111/jcmm.12742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cho IR, Park JC, Park CH, et al. Pre-treatment neutrophil to lymphocyte ratio as a prognostic marker to predict chemotherapeutic response and survival outcomes in metastatic advanced gastric cancer. Gastric Cancer. 2014;17(4):703–710. doi: 10.1007/s10120-013-0330-2 [DOI] [PubMed] [Google Scholar]
- 36.Liu K, Qian T, Tang L, Wang J, Yang H, Ren J. Decreased expression of microRNA let-7i and its association with chemotherapeutic response in human gastric cancer. World J Surg Oncol. 2012;10:225. doi: 10.1186/1477-7819-10-225 [DOI] [PMC free article] [PubMed] [Google Scholar]