Abstract
Introduction
Diabetes mellitus is one of the most public health challenges of the twenty-first century. Globally, 382 million people had diabetes by the year 2013.
Purpose
The purpose of this study was to determine the prevalence of diabetes mellitus and identify its associated factors at public health institutions in Addis Ababa.
Patients and Methods
An institution-based cross-sectional study was carried out from June to July 2016. A total of 758 participants were selected using a multistage sampling technique. Data were collected with a structured interviewer-administered questionnaire; a WHO STEPwise approach of NCDs risk factors identification, and the collected data were checked for completeness immediately following data collection and the filled questionnaires were entered into Epi–Info 3.5.1, and then exported to SPSS 23 for further analysis. Descriptive statistics such as mean, percentages, standard deviation, and ranges were determined. To identify factors associated with diabetes mellitus, binary logistics regression was used.
Results
The overall prevalence of diabetes mellitus was 14.8%, with a sex-specific prevalence of 18.35% and 16.62% for males and females, respectively. Older age participants had higher risks of developing diabetes mellitus than younger age individuals. Alcohol drinkers had more risks of developing diabetes mellitus than non-alcohol drinkers. Participants with plasma HDL-C ≥40mg/dl were more likely to develop diabetes mellitus than those with <40mg/dl. Participants with a higher level of plasma triglyceride ≥130mg/dl were found to be more exposed to the risks of developing diabetes mellitus than study participants with a low level of triglycerides.
Conclusion
A higher prevalence of diabetes mellitus was observed in Addis Ababa public health institutions. Factors such as age, alcohol drinking, HDL, triglycerides, and vagarious physical activity were associated with diabetes mellitus. Concerned bodies need to work over the ever-increasing diabetes mellitus in Addis Ababa.
Keywords: diabetes mellitus, prevalence, associated factors, health facilities
Introduction
Globally, 4.6 million deaths are attributed to diabetes annually.1 Diabetes mellitus is one of the most public health challenges of the twenty-first century2 and estimated to affect one in ten adults of the global population by the year 2030.3 However, 382 million people had diabetes mellitus by the year 2013 and were expected to rise to 592 million by the year 2035, of which most were in developing countries.4
Diabetes is a complex group of diseases with multiple causal factors. Of these factors Obesity, physical inactivity, abnormality in glucose production, Beta Cell Dysfunction and genetic susceptibility were among the commonly stated.5 In addition, age, sex, alcohol intake, smoking, hypertension, family history, physical inactivity, and level of lipid profile were commonly identified factors causing diabetes.6–8 Diabetes can be classified based on its etiology and cause.9 but as to this study all types were incorporated as diabetes irrespective of their classification to determine the burden of diabetes in the study area.
Corpus of evidence suggested the magnitude of diabetes varies as with the differences in socio-demographic characteristics, study population, sample size, and took on different numerical values. The burden of pre-diabetes was higher in African region taking the ranges from 50% to 75%.10
Ethiopia is one of the top five countries with largest number of people affected by DM in sub-Saharan Africa.11 The prevalence of diabetes in Ethiopia was observed as lower as 1.3% as in an institutional study,12 and was as higher as 5% among 35 years and above old people,8 6.6% among females and 6.4% among men.13 Likewise, the prevalence of undiagnosed diabetes in Ethiopia was found at 5%.14
Diabetes mellitus has been becoming among the few major public health challenges to the health services and economic development of low and middle-income countries including Ethiopia. However, most of the studies conducted in Ethiopia on diabetes were single institution oriented, and hence this study would rather clearly show the burden of diabetes and its associated factors in the study area. Later, this study would help policymakers, researchers and the community at large to have a focus on diabetes mellitus prevention and control.
Materials and Methods
Participants and Study Design
The study received ethical approval from the Universal Medical College Research review and ethics committee, and then this research was conducted in Addis Ababa public health facilities from June–July 2016.
All participants were provided written informed consent. The study areas were selected from public health institutions in Addis Ababa. The source population was all people living in Addis Ababa who were above 18 years old by the data collection period and who used health services in public health facilities. Study participants were 18 and above years old people who used health services at selected public health facilities from June–July 2016. A multistage sampling technique was used to select participants. The first stage was the selection of sub-cities:- of the ten 10 sub-cities in Addis Ababa, 3 of them were selected by simple random sampling technique (lottery method); namely Arada, Yeka and Kirkos Sub-city. The second stage was the selection of institutions from identified sub-cities:- accordingly one hospital and ten Health centers were selected. They were Yekatit 12 Hospital Medical College, Semen Health center, Afincho Ber Health Center, Woreda 06 Health center, Kotebe Health Center, Woreda 08 Health Center, Woreda 04 Health Center, Hiwot Amba Health Center, Efoyta Health Center, and Gotera Health Center. They were selected by lottery method from their respective sub-cities.
The participants were selected by simple random sampling technique. As this was a sub-study with hypertension, the sample size was determined with proportion of hypertension which was 37.7%,15 due to the fact that prevalence of hypertension gave a large sample size than diabetes, the proportion giving the largest sample was taken at 758, after considering design effect of 2% and 10% non-response rate (Table 1).
Table 1.
Sampling Procedure and Desired Sample Size from Each Respective Public Health Institutions in Addis Ababa Ethiopia, July 2016 (n=758)
| Selected Health Facilities by Sub-City | Sample Size (Inclusive of 10% Non-Response Rate) |
|---|---|
| Arada Sub-city | |
| Yekatit 12 Hospital Medical College | 69 |
| Semen Health Center | 68 |
| Beata Health Center | 69 |
| AfinchoBer Health Center | 69 |
| Yeka Sub-city | |
| Woreda 06 Health Center | 69 |
| Kotobe Health Center | 69 |
| Woreda 08 Health Center | 69 |
| Woreda 04 Health Center | 69 |
| Kirkos Sub-city | |
| Hiwot Amba Health Center | 69 |
| Efoyita Health Center | 69 |
| Gotera Health Center | 69 |
| Total sample size | 758 |
Data Collection
Data were collected with a structured and pre-tested interviewer-administered questionnaire. The pre-test was done in Zewditu Memorial Hospital on 40 patients two weeks before the actual data collection period. Data were collected through questionnaires derived from the WHO STEPwise approach for surveillance of non-communicable disease (NCDs) risk factors.16 The questionnaire contained socio-demographic characteristics and an individual’s behavioral characteristics. The three steps that were followed during data collection were an interview-based questionnaire, physiological measures, and biochemical measures.
Variables
The dependent variable was diabetes mellitus, while independent variables were socio-demographics (age, sex, educational level, marital status, monthly income, employment status, and family history), individual behavioral characteristics (physical activity, alcohol intake), and biochemical variables (HDL, triglycerides, and cholesterol) and BMI.
Operational Definitions
Current smoker is someone who smoked greater than 100 cigarettes in their lifetime and had smoked in the last 28 days.17 Regular drinkers are those who drink for ≥10 days a month for the last six months. Current alcohol drinkers are those who reported consuming alcohol within the last month. Overweight – BMI of 25.0–29.9. Diabetes Mellitus – Fasting plasma glucose 126mg/dl (7.0mmol) or higher. High total cholesterol level – serum cholesterol ≥200mg/dl. Physical inactivity – less than 10 mins activity at stretch, during leisure, work or transport. High triglyceride – serum concentration of triglyceride ≥ 130mg/dl.
Statistical Analysis
Collected data were checked for completeness. Immediately following data collection, filled questionnaires were entered into Epi–Info version 3.5.1, and then exported to SPSS version 23 for further analysis. Descriptive statistics such as mean, percentages, standard deviation, and ranges were done. To identify factors associated with diabetes mellitus, binary logistics regression was done. Univariable logistic regression was done to see independent effects of variables on diabetes mellitus, while those variables with p<0.2 were taken to multivariable logistic regression analysis to control confounding variables at p<0.05.
Result
Socio-Demographic Characteristics
A total of 758 participants involved in this study, giving a response rate of 100%. More than half, 58.3%, and 57.3% of participants were female and married participants, respectively. Participants’ age ranges from 19 to 96 years with a mean and standard deviation of 43 ± 14SD. Thirty-five percent of participants’ educational level was College level and above. Thirty-four percent of participants were unemployed (Table 2).
Table 2.
Socio-Demographic Characteristics of Participants at Selected Public Health Institutions in Addis Ababa Ethiopia, July 2016 (n=758)
| Variables | Category | Frequency (n) | Percent (%) |
|---|---|---|---|
| Sex | Male | 316 | 41.7 |
| Female | 442 | 58.3 | |
| Age in years | 19–25 | 79 | 10.4 |
| 26–32 | 87 | 11.5 | |
| 33–39 | 145 | 19.1 | |
| 40–46 | 167 | 22.0 | |
| 47–53 | 130 | 17.2 | |
| 54–60 | 65 | 8.6 | |
| ˃60 | 85 | 11.2 | |
| Marital status | Single | 198 | 26.1 |
| Married | 434 | 57.3 | |
| Widowed | 87 | 11.5 | |
| Divorced | 39 | 5.1 | |
| Educational status | Illiterate | 116 | 15.3 |
| Primary | 163 | 21.5 | |
| Secondary | 211 | 27.8 | |
| College and above | 268 | 35.4 | |
| Occupation | Governmental employee | 208 | 27.4 |
| NGO employee | 44 | 5.8 | |
| Private | 225 | 29.7 | |
| Unemployed | 263 | 34.7 | |
| Daily labor | 18 | 2.4 |
Lifestyle Characteristics of Study Participants
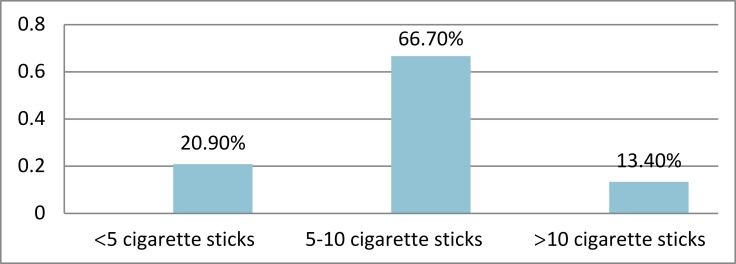
About 6% of participants in this study were smokers. Sixty-seven percent of smokers smoked 5–10 sticks per day (Figure 1). Thirty-four percent of participants were current alcohol drinkers, of which 18.8% of participants drank alcohol 5–7 days a week. Sixty percent of participants engaged in moderate physical activity 40% of which spent 4–6 hrs on physical activity per week. Ninety-seven percent of participants had the walking experience (Tables 3 and 4).
Figure 1.
Cigarette smoked to participants per day at selected public health institutions in Addis Ababa, Ethiopia July 2016 (n=758).
Table 3.
Life Style of Participants at Selected Public Health Institutions in Addis Ababa Ethiopia, July 2016 (n=758)
| Variables | Category | Frequency(n) | Percent (%) |
|---|---|---|---|
| Vagarious physical activity | Yes | 145 | 19.1 |
| No | 613 | 80.9 | |
| Average hours spent for Vagarious physical | 1–3 hrs | 26 | 17.9 |
| Activity per week | 4–6 hrs | 67 | 46.2 |
| 7 or more hours | 52 | 35.9 | |
| Moderate physical activity | Yes | 450 | 59.4 |
| No | 308 | 40.6 | |
| Average hours spent for moderate physical | 1–3 hrs | 103 | 22.9 |
| Activity per week | 4–6 hrs | 209 | 46.4 |
| 7 or more hours | 138 | 30.7 | |
| Walking experience | Yes | 738 | 97.4 |
| No | 20 | 2.6 | |
| Average hours spent for walking per week | 1–3 hrs | 216 | 29.3 |
| 4–6 hrs | 336 | 45.5 | |
| 7 or more hours | 186 | 25.2 |
Table 4.
Behavioral Characteristics of Participants at Selected Public Health Institutions in Addis Ababa Ethiopia, July 2016 (n=758)
| Variables | Category | Frequency (n) | Percent (%) |
|---|---|---|---|
| History of smoking | Yes | 45 | 5.9 |
| No | 713 | 94.1 | |
| Exposure to smoking in the home | Yes | 83 | 10.9 |
| No | 675 | 89.1 | |
| Exposure to smoking outside the | Yes | 184 | 24.3 |
| Home | No | 574 | 75.7 |
| Alcohol drinking experience | Yes | 255 | 33.6 |
| No | 503 | 66.4 | |
| Frequency of alcohol drinking | 5–7days/week | 48 | 18.8 |
| 1–4 days/week | 73 | 28.6 | |
| 1–3 days/month | 83 | 32.5 | |
| Less than once/month | 51 | 20.1 | |
| Amount of alcohol consumed at single occasions | 1–3 standard of alcohol | 125 | 49.0 |
| 4–5 standard of alcohol | 113 | 44.3 | |
| ˃5 standard of alcohol | 17 | 6.7 | |
| Larger amount of alcohol consumed at single occasions | 1–4 standard of alcohol | 86 | 33.7 |
| 5–10 standard of alcohol | 157 | 61.6 | |
| ˃10 standard of alcohol | 12 | 4.7 | |
| Frequency of consuming equal more | ≤2 times/month | 143 | 56.1 |
| Than 3 standard of alcohol | 3–4 times/month | 91 | 35.7 |
| ≥5 times/month | 21 | 8.2 |
Physical and Biochemical Markers
Physical measurements of participants showed that more than half (53.2%) of participants had a BMI measurement of ≤24.9. Forty-two percent of participants had a total cholesterol level ≥200mg/dl, at the same time 33.9% and 41% of participants had a plasma triglyceride level of ≥130 mg/dl and HDL-C <40mg/dl, respectively,(Table 5).
Table 5.
Anthropometric and Biomedical Measurements of Participants at Selected Public Health Institutions in Addis Ababa, Ethiopia, July 2016 (n=758)
| Variables | Category | Frequency (n) | Percent (%) |
|---|---|---|---|
| BMI | ≤ 24.9 | 402 | 53.0 |
| 25–29.9 | 274 | 36.2 | |
| ≥ 30 | 82 | 10.8 | |
| Total cholesterol | ˂200mg/dL | 441 | 58.2 |
| ≥200mg/dL | 317 | 41.8 | |
| Triglyceride | ˂130mg/dL | 446 | 58.8 |
| ≥130mg/dL | 312 | 41.2 | |
| HDL | ˂40 mg/dL | 257 | 33.9 |
| ≥40 mg/dL | 501 | 66.1 |
Prevalence of Diabetes Mellitus
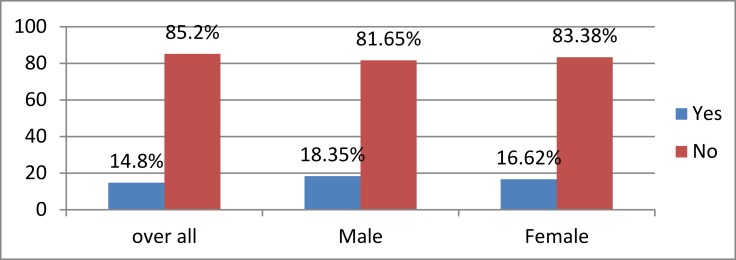
The prevalence of diabetes mellitus was 14.8%, where sex-specific prevalence was 18.35% and 16.62% for males and females, respectively, (Figure 2).
Figure 2.
Prevalence of diabetes mellitus at selected public health institutions of Addis Ababa, Ethiopia July 2016 (n=758).
Factors Affecting Diabetes Mellitus
Age, occupation, history of smoking, history of alcohol consumption, lipid profile, BMI, and physical activity independently showed significant association with diabetes mellitus at p<0.2. While control for the effects of selected variables was made, age, alcohol consumption, LDL-level, triglyceride, BMI, and vagarious physical exercise showed statistically significant association with diabetes mellitus at a p<0.05.
The odds of developing diabetes among 54–60 years old was four times as high as 19–25 years old (AOR: 3.39, 95% CI: 1.173–9.808, P<0.05). And the odds of developing diabetes among people with plasma triglyceride levels of ≥130mg/dl was two times as high as those with plasma triglyceride levels of ≤130mg/dl (AOR:2.3, 95% CI:1.126–3.53, p<0.05). The odds of developing diabetes in people who did not involve in vagarious physical activity as twice as those who involved in vagarious physical activity (AOR:2.0, 95% CI: 1.78–8.449, P<0.05). (Table 6).
Table 6.
Factors Associated with Diabetes Mellitus at Selected Public Health Institutions in Addis Ababa, Ethiopia, July 2016 (n=758)
| Variables | Diabetes Mellitus | COR (95% CI) | AOR (95% CI) | |
|---|---|---|---|---|
| Yes | No | |||
| No (%) | No (%) | |||
| Age in years | ||||
| 19–25 | 7(0.9) | 72(9.5) | 1 | 1 |
| 26–32 | 5(0.7) | 82(10.8) | 0.63(0.191, 2.063) | 0.29(0.079, 1.049) |
| 33–39 | 14(1.8) | 131(10.8) | 1.1(0.424, 2.847) | 0.62(0.220, 1.750) |
| 40–46 | 21(2.8) | 146(19.3) | 1.48(0.601, 3.642) | 0.63(0.223, 1.765) |
| 47–53 | 23(3.0) | 107(14.1) | 2.21(0.901, 5.423) | 1.05(0.382, 2.872) |
| 54–60 | 26(3.4) | 39(5.1) | 6.86(2.73, 17.225)* | 3.39(1.173, 9.808)** |
| ˃60 | 16(2.1) | 69(9.1) | 2.39(0.925, 6.152) | 1.47(0.465, 4.664) |
| Occupation | ||||
| Governmental employee | 25(3.3) | 183(24.1) | 1 | 1 |
| Private | 39(5.1) | 186(24.5) | 0.13(0.017, 0.969) | 1.004(0.539, 1.873) |
| Daily labor | 8(0.9) | 54(7.2) | 1.17(0.722, 1.893) | 0.70(0.348, 1.412) |
| Unemployed | 40(5.3) | 223(29.4) | 3.55(0.298, 9.698)* | 8.41(0.226, 31.790) |
| Smoking | ||||
| Yes | 12(1.6) | 33(4.4) | 1 | 1 |
| No | 100(13.2) | 613(80.9) | 0.45(0.224, 0.898)* | 0.53(0.212, 1.345) |
| Alcohol drinking | ||||
| Yes | 49(6.5) | 206(27.2) | 1 | 1 |
| No | 63(8.3) | 440(58.0) | 0.60(0.400, 0.906)* | 0.49(0.276, 0.861)** |
| HDL level | ||||
| ˂40mg/dl | 56(7.4) | 201(26.5) | 1 | 1 |
| ≥40mg/dl | 56(7.4) | 445 (58.7) | 0.45(0.301, 0.678)* | 0.44(0.266, 0.739)** |
| Triglyceride level | ||||
| ˂150mg/dl | 29(3.8) | 283 (37.3) | 1 | 1 |
| ≥150mg/dl | 83(10.9) | 363 (47.9) | 2.23(1.422, 3.501)* | 1.94(1.126, 3.531)** |
| Total cholesterol level | ||||
| ˂200mg/dl | 61(8.0) | 380(50.1) | 1 | 1 |
| ≥200mg/dl | 51(6.7) | 266(35.1) | 1.19(0.798, 1.788) | 1.04(0.619, 1.749) |
| BMI | ||||
| Normal | 42(5.5) | 360 (47.5) | 1 | 1 |
| Overweight | 57(7.5) | 217(28.6) | 2.25(1.461, 3.470)* | 2.19(1.319, 3.637)** |
| Obesity | 13 (1.7) | 69(9.1) | 1.64(0.835, 3.215) | 1.72(0.788, 3.734) |
| Vagarious activity | ||||
| Yes | 12(1.6) | 133(17.3) | 1 | 1 |
| No | 100(13.2) | 513(67.7) | 2.16(1.152, 4.050)* | 3.88(1.781, 8.449)** |
| Moderate activity | ||||
| Yes | 59(7.8) | 391(51.6) | 1 | 1 |
| No | 53(7.0) | 255(33.6) | 1.38(0.920, 2.061) | 1.37(0.867, 2.164) |
Note: *=p<0.2 **=p<0.05 =significance level.
Discussion
The prevalence of diabetes mellitus in this study was 14.8%, which was higher than the studies in North West Ethiopia (5.1%),8 in central Ethiopia (6.5%),13 East Shoa of Ethiopia (5%),14 and Northern Ethiopia (1.3%).12
The finding from this study was also higher than findings from a community-based study in Southern Ethiopia (1.9%),18 institution-based study in Addis Ababa (2.6%),19 and Dessie town, Northeast Ethiopia (6.8%),20 and Mizan Aman Town, Southwest Ethiopia, (6.5%).21
The prevalence of undiagnosed diabetes in Bahir Dar city, northwest Ethiopia was 10.2%,22 which was slightly lower than the findings of the current study whilst the current study was consistent with the study in Spain (13.8%),6 and Tanzania (12%).7
A community-based study in Sudan revealed the prevalence of diabetes at 18.6%,23 which was higher than the findings of the current study. In contrary, the current finding was higher than the hospital-based study in southwestern Uganda (2.5%),24 and suburban population of Northwest Nigeria (4.3%).25 In this study, the prevalence of diabetes in male (18.35%) was higher than in females (16.62%) and supported by the study in Nigeria where the prevalence was 4.5% in males and 4.0% in females.25
In this study, age, alcohol consumption, LDL-level, triglyceride, BMI, and vagarious physical exercise were factors statistically associated with development of diabetes. Family history of diabetes, physical activity, renal problems and pancreatic diseases were identified factors associated with diabetes.23 But in another study, family history, smoking, hypertension and alcohol drinking were identified factors associated with diabetes.24 As consistent with the current study, age and obesity were identified factors for the development of diabetes.18
Age and family history of diabetes were identified factors associated with development of diabetes.19 Ever checked blood glucose level (AOR=1.91, 95% CI 1.03 to 3.51), family history of DM (AOR=2.5, 95% CI 1.21 to 5.18), do not know the symptoms of diabetes (AOR=2.06, 95% CI 1.08 to 3.89), body mass index (BMI) >25 kg/m2 (AOR=1.98, 95% CI 1.09 to 3.60) were factors associated with diabetes.22 Positive family history of diabetes (AOR: 20.24, 95% CI 4.74–86.43), systolic hypertension (AOR: 4.61, 95% CI 1.09–19.50), smoking habit (AOR: 12.12, 95% CI 2.30–63.73), and hypercholesterolemia (AOR: 8.97, 95% CI 2.05–39.23) were significantly associated with diabetes.20 Waist circumference smoking habits, hypertension, total cholesterol level, and body mass index were factors significantly associated with diabetes.21
Family history of diabetes, older age, and physical inactivity were significantly associated with diabetes among urban population while alcohol consumption was inversely associated with diabetes mellitus in a rural population.8 But, in this study, age, alcohol consumption, LDL-level, triglyceride, BMI, and vagarious physical exercise had shown statistically significant association with diabetes mellitus. Non-statistically significant increased occurrence of DM was observed with increasing age. Independent analysis showed that diabetes mellitus had a statistically significant association with Body Mass Index (BMI), alcohol consumption, history of hypertension and high triglyceride level at p<0.05.14
Conclusion and Recommendations
The overall prevalence of diabetes mellitus was 14.8%, where sex-specific prevalence was 18.35% and 16.62% in the case of males and female participants, respectively.
Older ages, alcohol drinking, having plasma HDL ≥ 40mg/dl, and triglyceride ≥130mg/dl were factors associated with increased risks of diabetes mellitus. Cessation of alcohol drinking, control of HDL-C and triglyceride level and vagarious physical activities were recommended measures for prevention and control of diabetes mellitus among population of Ethiopia.
Limitation of the Study
As the current study was a sub-study as part of (hypertension study), the sample size was calculated unusually with the other health problems. The study focused only on selected factors, factors such as stress and obstructive sleep apnea syndrome were not included.
Acknowledgments
The authors would like to thank Universal Medical College for facilitation of the study. The authors would also thank study participants for their willingness in taking part to the study. Finally, the researchers would like to express their gratitude to data collectors. The abstract of this paper was presented at Society for Academic Primary Care, Conference name SAPC ASM 2019 – Exeter as a poster presentation with interim findings. The posters Abstract was published in Poster Abstracts of https://sapc.ac.uk/conference/2019/abstract/prevalence-of-diabetes-mellitus-and-associated-factors-public-health
Abbreviations
DM, Diabetes Mellitus; NCDs, Non-Communicable Diseases; OSAS, Obstructive Sleep apnea syndrome; WHO, World Health Organization.
Ethics Approval and Consent to Participate
The study protocol was performed in accordance with the ethical principle. Ethical approval was obtained from ethics review board of Universal Medical College. The ethics approval was given in accordance with the Declaration of Helsinki. The data collectors obtained written informed consent from all participants.
Data Sharing Statement
A finding of this study was generated from data collected and analyzed on the basis of stated methods and materials hence all data were already available in the manuscript.
Consent for Publication
Consent for publication of the manuscript was not applicable due to the fact that there were no participant’s individual data videos or images.
Author Contributions
Both authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
References
- 1.International Diabetes Federation. Global Diabetes Plan 2011-2021. Available from: https://idf.org/our-activities/advocacy-awareness/resources-and-tools/129-global-diabetes-plan-2011-2021.html. Accessed March 6, 2012. [PubMed] [Google Scholar]
- 2.Zimmet P, Alberti KG, Magliano DJ, Bennett PH. Diabetes mellitus statistics on prevalence and mortality: facts and fallacies. Nat Rev Endocrinol. 2016;12(10):616. doi: 10.1038/nrendo.2016.105 [DOI] [PubMed] [Google Scholar]
- 3.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047–1053. doi: 10.2337/diacare.27.5.1047 [DOI] [PubMed] [Google Scholar]
- 4.Guariguata L, DR W, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103(2):137–149. doi: 10.1016/j.diabres.2013.11.002 [DOI] [PubMed] [Google Scholar]
- 5.Grant RW, Moore AF, Florez JC. Genetic architecture of type 2 diabetes: recent progress and clinical implications. Diabetes Care. 2009;32(6):1107–1114. doi: 10.2337/dc08-2171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soriguer F, Goday A, Bosch-Comas A, et al. Prevalence of diabetes mellitus and impaired glucose regulation in Spain: the Di@ bet. Es Study Diabetologia. 2012;55(1):88–93. doi: 10.1007/s00125-011-2336-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruhembe CC, Mosha TC, Nyaruhucha CM. Prevalence and awareness of type 2 diabetes mellitus among adult population in Mwanza city, Tanzania. Tanzan J Health Res. 2014;16:2. doi: 10.4314/thrb.v16i2.4 [DOI] [PubMed] [Google Scholar]
- 8.Abebe SM, Berhane Y, Worku A, Assefa A. Diabetes mellitus in North West Ethiopia: a community based study. BMC Public Health. 2014;14(1):97. doi: 10.1186/1471-2458-14-97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson JL, Loscalzo J. Harrison’s Principles of Internal Medicine. New York: Mcgraw-hill; 2012. [Google Scholar]
- 10.Peer N, Kengne A-P, Motala AA, Mbanya JC. Diabetes in the Africa Region: an update. Diabetes Res Clin Pract. 2014;103(2):197–205. doi: 10.1016/j.diabres.2013.11.006 [DOI] [PubMed] [Google Scholar]
- 11.Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94(3):311–321. doi: 10.1016/j.diabres.2011.10.029 [DOI] [PubMed] [Google Scholar]
- 12.Ambachew Y, Kahsay S, Tesfay R, Tesfahun L, Amare H, Gebreegzihabiher G. Prevalence of diabetes mellitus among patients visiting medical outpatient department of Ayder Referral Hospital, Mekelle, Ethiopia: A three years pooled data. IJPSR. 2015;6(2):435–439. [Google Scholar]
- 13.Nshisso LD, Reese A, Gelaye B, Lemma S, Berhane Y, Williams MA. Prevalence of hypertension and diabetes among Ethiopian adults. Diabetes Metab Synd. 2012;6(1):36–41. doi: 10.1016/j.dsx.2012.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Megerssa Y, Gebre M, Birru S, Goshu A, Tesfaye D. Prevalence of undiagnosed diabetes mellitus and its risk factors in selected institutions at Bishoftu Town, East Shoa, Ethiopia. J Diabetes Metab 8. 2013. doi: 10.4172/2155-6156.: [DOI] [Google Scholar]
- 15.Guchiye B Prevalence and Associated Factors of Hypertension among workers of Steel factories, Akaki, Addis Ababa 2014.
- 16.Bonita R, Winkelmann R, Douglas KA, de Courten M. The WHO stepwise approach to surveillance (STEPS) of non-communicable disease risk factors In: Global Behavioral Risk Factor Surveillance. Springer; 2003:9–22. [Google Scholar]
- 17.Teo KK, Ounpuu S, Hawken S, et al. Tobacco use and risk of myocardial infarction in 52 countries in the INTERHEART study: a case-control study. The Lancet. 2006;368(9536):647–658. doi: 10.1016/S0140-6736(06)69249-0 [DOI] [PubMed] [Google Scholar]
- 18.Zekewos A, Loha E, Egeno T, Wubshet K, Merga Z. Prevalence of diabetes mellitus and associated factors in Southern Ethiopia: a community based study. Ethiop J Health Sci. 2018;28:451–460. doi: 10.4314/ejhs.v28i4.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woldesemayat B, Amare H, Ataro Z, et al. Prevalence of diabetes mellitus and associated risk factors among adults attending at feres meda health centre, Addis Ababa, Ethiopia, 2017. J Biomed Mater Res. 2019;7(1):8–15. doi: 10.11648/j.ijbmr.20190701.12 [DOI] [Google Scholar]
- 20.Endris T, Worede A, Asmelash D. Prevalence of diabetes mellitus, prediabetes and its associated factors in Dessie Town, Northeast Ethiopia: a community-based study. Diabet Metab Synd Ob. 2019;12:2799–2809. doi: 10.2147/DMSO.S225854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aynalem SB, Zeleke AJ. Prevalence of diabetes mellitus and its risk factors among individuals aged 15 years and above in Mizan-Aman town, Southwest Ethiopia, 2016: a cross sectional study. Int J Endocrinol. 2018;2018:1–7. doi: 10.1155/2018/9317987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bantie GM, Wondaye AA, Arike EB, et al. Prevalence of undiagnosed diabetes mellitus and associated factors among adult residents of Bahir Dar city, Northwest Ethiopia: a community-based cross-sectional study. BMJ Open. 2019;9(10):e030158. doi: 10.1136/bmjopen-2019-030158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abdalla E, Ahmed R. Epidemiology of diabetes among adults in Jabra area “block 14” in Khartoum state – Sudan: community based study. Int J Community Med Public Health. 2017;4:1863. doi: 10.18203/2394-6040.ijcmph20172146 [DOI] [Google Scholar]
- 24.Dickson K Prevalence of diabetes and its associated risk factors in south-western Uganda.2019.
- 25.Sabir A, Balarabe S, Sani A, et al. Prevalence of diabetes mellitus and its risk factors among the suburban population of Northwest Nigeria. Sahel Med J. 2017;20:168. doi: 10.4103/smj.smj_47_16 [DOI] [Google Scholar]