Abstract
Objective
Based on the albumin-bilirubin (ALBI) and platelet-albumin-bilirubin (PALBI) grade to assess the long-term outcomes of patients with large hepatocellular carcinoma (HCC) after transarterial chemoembolization combined with cryoablation (TACE-CRA).
Materials and Methods
We studied 86 patients with HCC nodules (up to 3 HCCs with maximum diameters of 4.1–12.0 cm) who subsequently underwent TACE-CRA from July 2007 to August 2018. The overall survival (OS) was compared between groups classified by ALBI and PALBI grade. Baseline characteristics were collected to identify the risk factors for determination of poor OS after TACE-CRA. The prognostic performances of CTP class, ALBI and PALBI grade were compared.
Results
After a median follow-up time of 33.8 months, 41 patients had died. The cumulative1-, 3- and 5-year OS rates were 74.5%, 38.0% and 29.3%, respectively. Stratified according to ALBI grade, the cumulative 3- and 5-year OS rates were 41.2% and 41.2% in grade 1, respectively, and 20.9% and 9.8% in grades 2–3, respectively (P < 0.001). Stratified according to PALBI grade, the cumulative 3- and 5-year OS rates were 41.2% and 37.5% in grade 1, respectively, and 36.3% and 21.2% in grades 2–3, respectively (P = 0.002). Multivariate analysis results showed that older age, and ALBI grade 2–3 were associated with overall mortality. ALBI grade demonstrated significantly greater area under the curve values than CTP class and PALBI in predicting 1-, 3- and 5-year OS.
Conclusion
ALBI grade offers accurate prediction of long-term outcome for patients with HCC (diameter > 4 cm) after TACE-CRA.
Keywords: hepatocellular carcinoma, transarterial chemoembolization, cryoablation, albumin-bilirubin, platelet-albumin-bilirubin
Introduction
Hepatocellular carcinoma (HCC) is a common malignancy, ranking sixth among the most common cancer and third among the leading causes of cancer-related mortality worldwide, with the morbidity and mortality is still increasing.1–3 Approximately more than 70% of HCC are associated with hepatitis B virus infection in China. Large- or giant-sized HCC lesions (more than 5 or 10 cm in the largest diameter of the nodule) are often checked out due to a lack of screening and routine medical examination. Barcelona Clinic Liver Cancer (BCLC) guideline suggest that patients with over 5 cm of HCC lesion are grouped into intermediate and advanced stage.4,5 Transarterial chemoembolization (TACE) has been recommended as a standard treatment for unresectable HCC in BCLC B stage, using various combinations of embolic agents and chemotherapeutic drugs. However, viable tumor cells often fail to be inactivated completely after TACE treatment and complete tumor necrosis rate only is 16.9%.6 Given TACE may occlude the main artery supplying the tumor and cause subsequent thermal ablation (TA) becomes more effective, reflecting minimized heat loss by convection. TACE combined with TA has been regarded as an important treatment strategy for patients with large HCC.7,8 Previous studies have reported that the combination of TACE and radiofrequency ablation (RFA) improves significantly median survival duration of patients with large HCC compared with TACE or RFA alone.9
Cryoablation (CRA) has become one of the most promising loco-regional treatment in HCC with various advantages, including lack of severe damage to large blood vessels, formation of a visual ice-ball activation of cryoimmunology in cancer and no association with severe pain.10 Percutaneous CRA for cancer cell by following mechanisms:1 intracellular ice formation;2 solute-solvent shifts that cause cell dehydration;3 rupture, and small-vessel obliteration with resulting hypoxia. A recent study has reported that, for the treatment of one or two HCC lesions > 4 cm, CRA was equally safe and effective, and resulted in a significantly lower local tumor progression when compared with RFA.11 If the diameter of the area frozen exceeds 6 cm, as a serious complication, cryoshock often occurred after patients with cirrhosis underwent CRA.12–14 The incidence of the phenomenon is rare and has garnered much attention from clinical researchers.
Similar to previous study related to TACE combined with RFA or microwave ablation (MWA) for patients with large HCC, Cui W has reported that TACE combined with cryoablation (TACE-CRA) may improve overall survival (OS) in patients who presented with large HCC compared with TACE only, especially, when tumor dameter > 10cm.15 However, TACE or CRA may cause liver function to worsen because noncancerous liver parenchyma is also damaged. Hepatic functional reserve has always been considered to be critical for survival outcomes of HCC patients. Therefore, the assessment of hepatic function after TACE-CRA is very important. Child-Pugh grade is the most widely used assessment method for hepatic function, but highly subjective factors, such as severity of ascites and degree of hepatic encephalopathy, may reduce assessment ability. Recently, as a simple and objective evaluation method, ALBI or PALBI grade has been applied widely to assess hepatic functional reserve, learning from the previous studies,16–18 we designed a plan, and the main aim was to assess the long-term outcomes of patients with large HCC (diameter > 4cm) after TACE-CRA based on the ALBI or PALBI grade.
Materials and Methods
Patients and Study Design
This study was conducted in accordance with the principles of the Declaration of Helsinki, and the study protocol was approved by the Ethics Committee of the Sun Yat-sen University Cancer Center (Guangzhou, China). Because of the retrospective nature of the study, patient consent for inclusion was waived. The medical records of all HCC patients between July 2007 and August 2018 were reviewed. For this cohort study, the primary diagnosis of patients with HCC was based on HBV-infected individuals with cirrhosis and contrast-enhanced MRI image combined with high serum alpha-fetoprotein (AFP) level. The final diagnosis was based on pathologic findings of needle biopsy samples before ablation. The inclusion criteria were as follows: (a) age from 18 to 75 years; (b) Eastern Cooperative Oncology Group (ECOG) performance status score of 0/1; (c) up to 3 HCCs and single nodule with maximum diameters of 4.1–12.0 cm, and (d) Child-Pugh classification A or B. The exclusion criteria were as follows: (a) serious medical comorbidities, including heart, lung and renal dysfunction; (b) severe coagulation disorders (i.e., prothrombin time >25 s, prothrombin activity < 40%, and platelet count < 50 cells×109/L); (c) active severe infection and (d) loss follow-up.
During the study period, 254 consecutive HCC patients underwent TACE-CRA. Among these patients, 86 patients (mean age, 52.4 years ± 10.4; range, 32–72 years) with 132 HCC nodules met the inclusion criteria and were included in this study. The clinicopathologic data, including age, gender, hepatitis B surface antigen; tumour size, tumour number, Barcelona Clinic Liver Cancer (BCLC) grade, Child-Turcotte-Pugh (CTP) grade, AFP, albumin, total bilirubin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), international normalized ratio (INR), platelet count, TACE sessions, CRA sessions, platelet-albumin-bilirubin (PALBI) grade and albumin-bilirubin (ALBI) grade were collected.
Equipment
The Allura Xper FD 20 (Philips Healthcare, Best, Amsterdam, the Netherlands) digital subtraction angiography (DSA) instrument was used for the TACE procedures. A 16-slice spiral computed tomography (CT) scanner (Brilliance CT BigBore; Phillip Medical Systems, the Netherlands) was used for cryoablation puncture guidance and image acquisition. The cryoablation was performed with the Cryo-HitTM (Galilmedical, Israel) using argon gas as a cryogen. Consumables included the puncture needle, the artery catheter sheath, the angiography catheter (Terumo, Tokyo, Japan), and the micro-catheter (Terumo, Tokyo, Japan), lipiodol (Lipiodol Ultrafluide; Guerbet, Aulnay-Sous-Bois, France), gelatin sponge particles (Alicon, Hangzhou, China), and chemotherapeutics including lobaplatin (Chang’an International Pharmaceutical, Hainan, China) and epirubicin (Shenzhen Main Luck Pharmaceuticals, Shenzhen, China).
TACE Procedure
TACE was carried out by using techniques we described previously by our three radiologists (J.H.H., Z.M.H., and J.Y.N.), who had 8–20 years of experience in TACE. The chemotherapeutic emulsion which consisted of 10–20 mL lipiodol, 30–50mg lobaplatin and 20–40mg of epirubicin was slowly injected for chemoembolization by using a 5-Fr Yashrio catheter or 2.7-Fr micro-catheter (Progreat; Terumo) until the blood flow slowed. Embolization using 150–350–560 mL of gel foam (Ailikang Medicine, Hangzhou, China) mixed with contrast medium was injected to reduce the residual blood flow if necessary until there was no longer any tumour staining after repeat angiography. The tumor-feeding artery was selected or super-selected whenever possible.
CRA Procedure
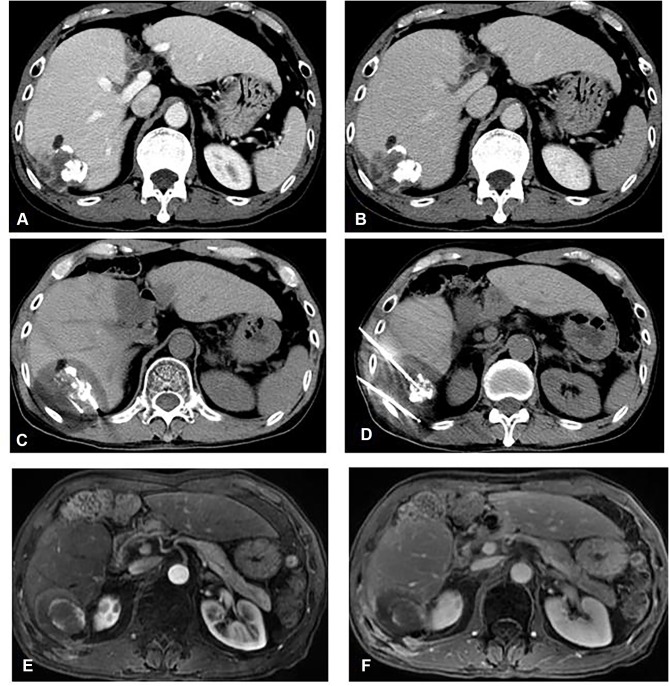
In the TACE-CRA cohort, single TACE procedure was performed in each patient as the first therapeutic step. Three to four weeks after the initial TACE, percutaneous CRA was performed under CT guidance. CRA sessions were administered at a rate of one CRA twice a week. The number of CRA sessions in each patient was mainly determined by the size of the targeted area. The tumour section in main tumour that indicated a defect in lipiodol uptake was the targeted area that was frozen. Before the ablation procedures, a therapy plan was made for each patient: generally, patients with <5 cm in diameter were treated with a cycle of one session, patients 5–10 cm in diameter underwent two sessions and patients with >10 cm underwent three sessions. The aim of the treatments was cytoreduction (to ablate the greatest amount of residual viable tumour to control growth). The residual viable tumour was assessed using contrast-enhanced CT or magnetic resonance imaging (MRI) done previously. We present one case of TACE-CRA for HCC from diagnosis, and treatment to post-ablation assessment (Figure 1).
Figure 1.
A 54-year-old female patient with hepatocellular carcinoma (HCC) (5.2 x 4.4 x 4.0 cm in diameter) located on S6 segment underwent TACE-CRA treatment. (A, B) CT axial scan shows the HCC nodule accept TACE before CRA perform and iodine oil deposits well and clearly shows with high-density areas; (C, D) CT axial scan shows two cryosurgical needles are inserted into the tumour by CT guided and an ablation area with lower density than peripheral liver parenchyma after CRA; (E, F) MRI axial scan in arterial and delay phase after CRA, an ablation area was showed after three months, illustrating no viable tumour.
ALBI and PALBI Grade for Hepatic Function
ALBI grade is a novel method, which was used for assessment of hepatic function. Compared with CTP classification, ALBI grade eliminated subjective variables, such as ascites and encephalopathy, which had characteristics including simple and objective. ALBI score was calculated before treatment using the appropriate clinical parameters and ALBI grade were defined as follows: (log 10 bilirubin[BI] [μ mol/L] × 0.66) + (albumin[AL] [g/L] ×-0.085), (grade 1, 2, and 3 = ≤ −2.60, > −2.60 to −1.39, and > −1.39, respectively). PALBI score was calculated by using the following formula: 2.02 × log BI – 0.37 · (log BI)2–0.04 · AL – 3.48 · log PC + 1.01 · (log PC)2, where PC is platelet count in 1000 per microliter. PALBI grades were defined as grade 1 (< −2.53), grade 2 (> −2.53 and ≤ −2.09), and grade 3 (score > −2.09).
Follow-Up and Endpoints
At one and three months after treatment and then roughly 3–6 months interval. Thereafter, the follow-up visit covered several evaluations, including routine physical examination; laboratory tests such as total bilirubin, serum albumin, prothrombin time and tumour marker levels, and contrast-enhanced image including CT, or MRI. Technique effectiveness was defined as the absence of contrast-enhancement on imaging in any area of the mass after one month. The end points of this study were death. OS was calculated from the date of first session of TACE-CRA treatment for HCC to the date of death or last date of follow-up (survival or loss). Major complications were defined as events which caused substantial morbidity and disability that increased the level of care, or led to hospital admission, or substantially prolonged the hospital stay.19
Statistical Analysis
Statistical analyses were performed using SPSS 21.0 (SPSS, Chicago, IL). Either Pearson χ2 analysis or Fisher exact tests were performed to compare the categorical variables, and Student’s t-test was applied to compare the continuous variables. The OS rate was assessed by the Kaplan–Meier method with the Log-rank test. A Cox proportional hazards model was used to identify the significant effects of risk factors on the survival. For all tests, a P value of less than 0.05 was considered to be statistically significant. Univariate and multivariable analyses of independent prognostic factors were evaluated by means of the forward stepwise Cox regression model. The performance of the model was evaluated by calibration and discrimination via the time-dependent area under the curve (tAUC). Homogeneity was compared in different serum markers, which was generated by means of the parametric survival analysis. The Akaike information criterion was performed in the Cox proportional hazards model, which offer an overall assessment in serum markers of HCC underwent TACE-CRA.
Results
Baseline Characteristics
According to the abovementioned formula, 86 patients with HCC (Diameter > 4cm) were divided into two subgroups according to ALBI grade. The characteristics of the patients and tumours based on ALBI grade are summarized in Table 1. In this study, there were 59 patients (11 females, 48 males; average age 52.0±9.7 years) in the ALBI grade 1 group and 27 patients (7 females, 20 males; average age 54.8±12.9 years) in the ALBI grade 2–3 group. There were comparable between the two groups (P = 0.261 and P = 0.441). In the ALBI grade 1 group, 26 patients had a single tumour; 33 patients had multiple tumours; and the median maximum tumour diameter was 4.7 cm (range, 4.2–9.5 cm). In the ALBI grade 2–3 group, 11 patients had a single tumour; 16 patients had multiple tumours; and the median maximum tumour diameter was 5.2 cm (range, 4.8–11.8 cm). There were comparable between the two groups (P = 0.772 and P = 0.238). Total bilirubin, ALT and AST level in ALBI grade 1 group, were lower than those in ALBI grade 2–3 group (P = 0.016, P < 0.001 and P < 0.001). And albumin level in ALBI grade 1 group was higher than those in ALBI grade 2–3 group (P < 0.001).
Table 1.
Baseline Characteristics Stratified by ALBI Grade
| Parameter | ALBI Grade 1 (n=59) | ALBI Grade 2/3 (n=27) | P value |
|---|---|---|---|
| Age (y)* | 52.0±9.7 | 54.8±12.9 | 0.261 |
| Gender | 0.441 | ||
| Male | 48 (81.4) | 20 (74.1) | |
| Female | 11 (18.6) | 7 (25.9) | |
| ECOG | 0.826 | ||
| 0 | 45 (76.7) | 20 (74.1) | |
| 1 | 14 (23.3) | 7 (25.9) | |
| Hepatitis B surface antigen | 0.878 | ||
| Positive | 45 (76.7) | 21(77.8) | |
| Negative | 14 (23.3) | 6(22.2) | |
| Tumor size (cm) | 0.238 | ||
| ≤7 | 50 (84.7) | 20 (74.1) | |
| >7 | 9 (15.3) | 7 (25.9) | |
| Tumor number | 0.772 | ||
| Single | 26 (44.1) | 11 (40.7) | |
| Multiple | 33 (55.9) | 16 (59.3) | |
| BCLC grade | 0.769 | ||
| A | 25 (42.4) | 10 (37.0) | |
| B | 28 (47.4) | 15 (55.6) | |
| C | 6 (10.2) | 2 (7.4) | |
| CTP grade | 0.004 | ||
| A | 58 (98.7) | 22 (81.5) | |
| B | 1 (1.3) | 5 (18.5) | |
| α-fetoprotein level (ng/mL) † | 1260.7 (2.0–12,536.9) | 1620.2 (1.4–21,263.9) | 0.668 |
| Albumin level (μmol/L) † | 42.2 (12.6–47.8) | 37.1 (13.9–54.1) | <0.001 |
| Total bilirubin level (μmol/L) † | 13.6 (4.3–44.9) | 34.5 (5.1–51.6) | 0.016 |
| ALT level (U/L) † | 34.0 (7.2–77.4) | 59.1 (8.9–234.6) | <0.001 |
| AST level (U/L) † | 32.4 (14.7–221.3) | 59.9 (14.2–387.0) | <0.001 |
| INR* | 1.1±0.2(0.87–1.38) | 1.1±0.1(0.89–1.49) | 0.522 |
| Platelet count (109) † | 178.3 (55–569) | 169.5 (50–238) | 0.489 |
| TACE session | 0.112 | ||
| ≤3 | 43(72.9) | 15(55.6) | |
| >3 | 16(27.1) | 12(44.4) | |
| CRA session | 0.134 | ||
| ≤3 | 53(89.8) | 21(77.8) | |
| >3 | 6(10.2) | 6(22.2) | |
| TACE-related CR | 0.615 | ||
| Yes | 4(6.8) | 2(7.4) | |
| No | 55(93.2) | 25(92.6) |
Notes: Unless otherwise indicated data are number of patients, with percentage in parentheses. *Data are means ± standard deviation. †Data are medians, with interquartile range in parentheses.
Abbreviations: ALBI, albumin-bilirubin; ECOG, Eastern Cooperative Oncology Group; AST, aspartate aminotransferase; ALT, alanine aminotransferase; cm, centimeter; INR, international normalized ratio; BCLC, Barcelona Clinic Liver Cancer; CTP, Child-Turcotte-Pugh; TACE, transarterial chemoembolization; CRA, cryoablation; CR, complete response.
Factors Associated with OS
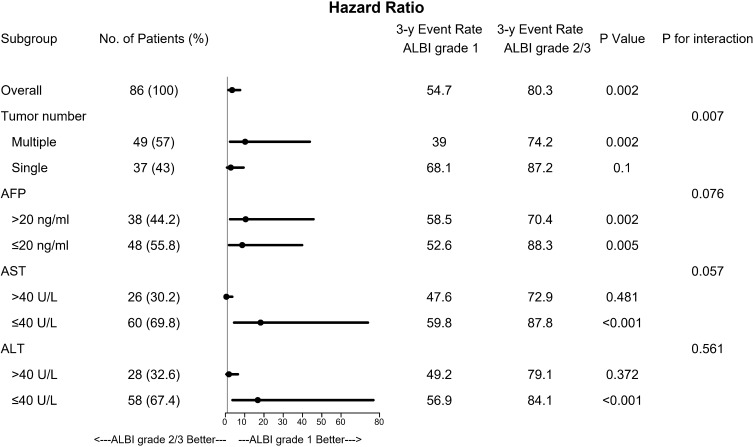
During the follow-up period, 45 patients were still alive at their last visit, after a median follow-up time of 33.8 months (range 13.3–118.2 months). The cumulative OS rates at 1-, 3-, and 5 years were 74.5%, 38.0% and 29.3%, respectively. Univariate analysis showed that older age (>65 years old), larger size of tumour (>7 cm in diameter), higher ALBI and PALBI grades were the independent risk factors associated with poor OS. Multivariate analysis showed that >65 years of age and ALBI 2–3 grades were the independent risk factors associated with poor OS (Table 2). We further compared the prognoses between these two groups by using subgroup analysis. As shown in Figure 2, the OS rates were lower in the ALBI grade 2–3 group than in the ALBI grades 1 group throughout all subgroups.
Table 2.
Factors Associated with Poor OS After TACE-CRA for HCC According to Univariate and Multivariate Analysis
| Factor | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age (years) | 1.932 (1.427, 5.336) | 0.007 | 2.382 (1.884, 7.823) | 0.002 |
| <65 | 45 | 45 | ||
| ≥65 | 41 | 41 | ||
| Gender | 1.478 (0.038, 2.362) | 0.246 | … | … |
| Male | 68 | 68 | ||
| Female | 18 | 18 | ||
| Hepatitis B surface antigen | 2.512 (0.368, 4.447) | 0.442 | … | … |
| Positive | 66 | 66 | ||
| Negative | 20 | 20 | ||
| Tumor size (cm) | 1.916 (1.562, 3.274) | 0.012 | … | … |
| <7 | 70 | 70 | ||
| ≥7 | 16 | 16 | ||
| Tumor number | 2.317 (0.835, 5.078) | 0.206 | … | … |
| Single | 37 | 37 | ||
| Multiple | 49 | 49 | ||
| α-fetoprotein level (ng/mL) | 1.884 (1.271, 2.793) | 0.445 | … | … |
| ≤20 | 44 | 44 | ||
| >20 | 42 | 42 | ||
| ALT level (U/L) | 2.872 (0.723, 4.382) | 0.568 | … | … |
| ≤40 | 40 | 40 | ||
| >40 | 46 | 46 | ||
| AST level (U/L) | 1.328 (0.273, 2.944) | 0.672 | … | … |
| ≤40 | 38 | 38 | ||
| >40 | 48 | 48 | ||
| Platelet count (/mm3) | 3.235 (0.556, 7.834) | 0.512 | … | … |
| ≤105 | 12 | 12 | … | |
| >105 | 74 | 74 | ||
| INR | 2.892 (1.457, 5.823) | 0.445 | … | … |
| >1.1 | 64 | 64 | ||
| ≤1.1 | 22 | 22 | ||
| CRA session | 1.345 (0.493, 3.665) | 0.563 | … | … |
| ≤3 | 48 | 48 | ||
| >3 | 12 | 12 | ||
| TACE session | 1.877 (0.632, 3.488) | 0.346 | … | … |
| ≤3 | 62 | 62 | ||
| >3 | 5 | 5 | ||
| Child–Pugh grade | 1.012 (0.332, 3.192) | 0.093 | … | … |
| A | 80 | 80 | ||
| B | 6 | 6 | ||
| ALBI grade | 3.398 (1.950, 6.058) | 0.001 | 3.398 (1.950, 6.058) | <0.001 |
| 1 | 59 | 59 | ||
| 2–3 | 27 | 27 | ||
| PALBI grade | 2.734 (1.251, 5.547) | 0.002 | … | … |
| 1 | 44 | 44 | ||
| 2–3 | 42 | 42 | ||
Note: Data in parentheses are 95% confidence intervals. Variables were analyzed by a univariate model of Cox Proportional Hazard Test; those with a P-value < 0.05 were showed here and were forwarded to the multivariate analysis.
Abbreviations: OS, overall survival; HCC, hepatocellular carcinoma; TACE, transarterial chemoembolization; CRA, cryoablation; ALT, alanine aminotransferase; AST, aspartate aminotransferase (AST); INR, international normalized ratio; ALBI, albumin–bilirubin; PALBI, platelet-albumin–bilirubin.
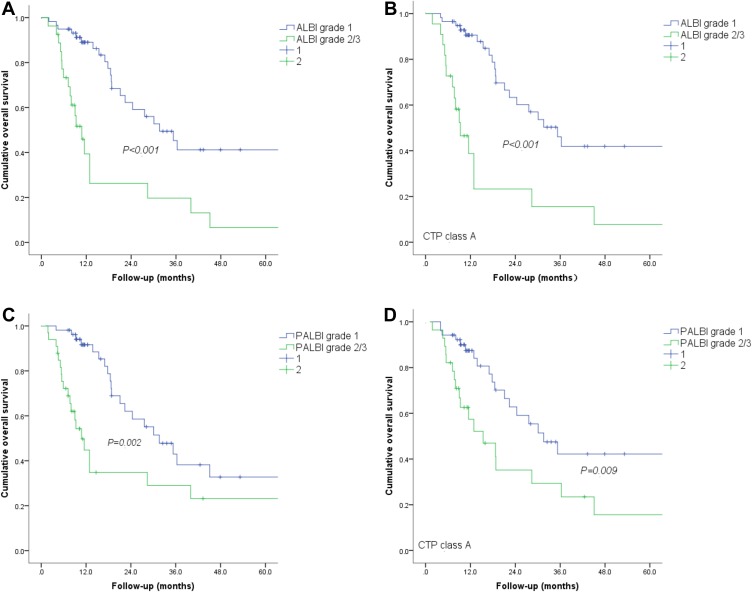
Figure 2.
Graphs show cumulative OS rates in patients with HCC after TACE-CRA. (A) Patients with ALBI grade 1 had higher OS rate than did those with ALBI grades 2 −3 (P < 0.001); (B) Graphs show that, of patients with CTP grade A, those with ALBI grade 1 had better OS rates than their counterparts (P < 0.001); (C) Patients with PALBI grade 1 had higher OS rate than did those with ALBI grades 2 −3 (P = 0.002); (D) Graphs show that, of patients with CTP grade A, those with PALBI grade 1 had better OS rates than their counterparts (P= 0.009).
Correlation of CTP Class with ALBI or PALBI Grade
1-, 3-, and 5-years OS rates of 90.2%, 41.2% and 41.2%, respectively, showed in ALBI grade 1 group. And 1-, 3- and 5-years OS rates of 25.3%, 20.9% and 9.8%, respectively, showed in ALBI grades 2–3 group (P = 0.001), (Figure 3A). For patients with CTP grade A, 56 (70.0%) were categorized as ALBI grade 1, 22 (27.5%) as ALBI grade 2, and two (2.5%) as ALBI grade 3. For CTP grade B patients, one (16.7%) was classified as ALBI grade 1, four (66.6%) as grade 2, and one (16.7%) as grade 3. CTP grade A patients with ALBI grade 2–3 had a significantly lower OS rate than those with grades 1 (P < 0.001), (Figure 3B). 1-, 3-, and 5-year OS rates of 93.8%, 41.2% and 37.5%, respectively, showed in PALBI grade 1 group. And 1-, 3- and 5-year OS rates of 42.6%, 36.3% and 21.2%, respectively, showed in PALBI grade 2–3 group (P = 0.002), (Figure 3C). Similarly, 59 (73.8%), 17 (21.2%), and 4 (5.0%) patients with CTP grade A were classified as PALBI grades 1, 2, and 3, respectively. 1 (16.7%), 4 (66.6%), and 1 (16.7%) patients with CTP grade B were categorized as PALBI grades 1, 2, and 3, respectively. Significant differences in OS rates were found between patients with CTP grade A and PALBI grade 1 and those who were CTP grade A and PALBI grades 2–3 (P = 0.009), (Figure 3D).
Figure 3.
Forest plot shows that patients with ALBI grade 1 had higher OS rates in all subgroup analyses.
Abbreviations: AFP, a-fetoprotein; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CI, confidence interval; HBsAg, hepatitis B surface antigen; HR, hazard ratio.
Comparison of ALBI Grade, PALBI Grade and CTP Class in Predicting OS
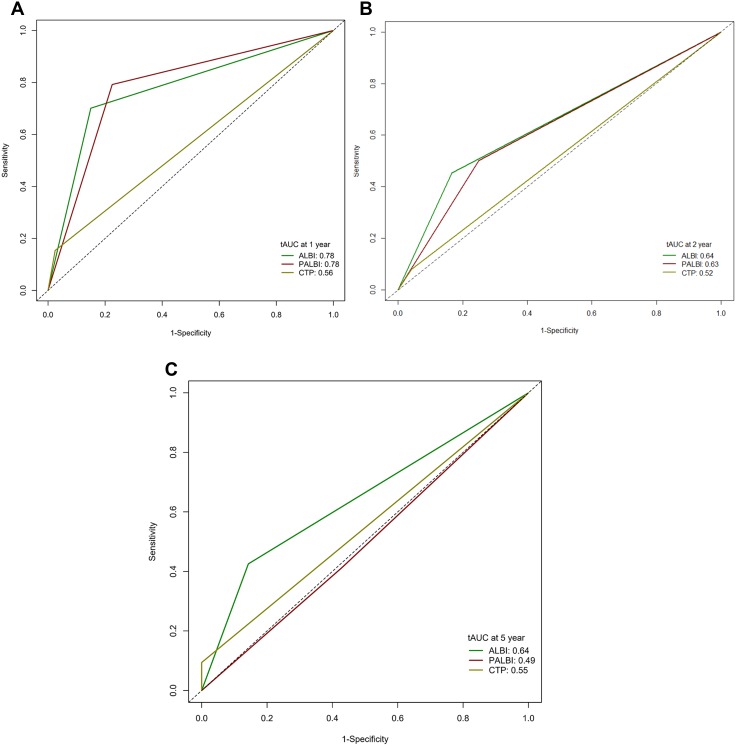
The discriminatory capabilities of PALBI grade, ALBI grade and CTP class were quantified using AUC values. ALBI grade had higher AUC values than PALBI grade or CTP class in predicting 1-, 2- and 5-year OS (Figure 4). The 1-, 2- and 5-year AUC values for ALBI grade were 0.78 (95% CI: 0.72–0.86), 0.64 (95% CI: 0.58–0.69) and 0.64 (95% CI: 0.55–0.70), respectively. The 1-, 2- and 5-year AUC values for PALBI grade were 0.78 (95% CI: 0.67–0.84), 0.63 (95% CI: 0.55–0.71) and 0.49 (95% CI: 0.42–0.54), respectively. The 1-, 2- and 5-year AUC values for CTP class were 0.56 (95% CI: 0.44–0.63), 0.52 (95% CI: 0.47–0.59) and 0.55 (95% CI: 0.50–0.61), respectively.
Figure 4.
Receiver operating characteristic curves and corresponding AUC for 1-(A), 2-(B) and 5 year (C) OS.
Abbreviations: CTP, Child-Turcotte-Pugh; ALBI, albumin-bilirubin; APLBI, platelet-albumin bilirubin; AUC, area under the curve.
Prognostic Performance of Serum Markers for Associations with OS After TACE-CRA
The prognostic performance of ALBI and PALBI grade, CTP class, AFP, AST, ALT, albumin, platelet counts, bilirubin, and BCLC grade were evaluated by Akaike information criterion methods and homogeneity. Table 3 shows that ALBI had the highest homogeneity (likelihood ratio χ2) and lowest Akaike information criterion value compared with the other markers.
Table 3.
Assessment Accuracy of Biomarkers and Clinical Grade for Overall Survival After TACE-CRA
| Biomarkers and Clinical Grade | Discriminatory Ability | Homogeneity | Akaike Information Criterion |
|---|---|---|---|
| ALBI grade | 21.237 | 50.291 | 2002.341 |
| PALBI | 19.432 | 48.234 | 2210.233 |
| CTP grade | 8.326 | 22.917 | 2108.912 |
| Barcelona Clinic Liver Cancer grade | 5.283 | 14.129 | 2356.785 |
| a-fetoprotein level | 10.273 | 32.343 | 2321.532 |
| Prothrombin time international normalized ratio | 3.298 | 21.293 | 2429.204 |
| Platelet count | 6.391 | 8.824 | 2419.783 |
| Albumin level | 8.293 | 3.019 | 2709.681 |
| Bilirubin level | 7.783 | 3.291 | 2807.817 |
Complications
None of TACE-CRA-related deaths occurred. Hepatic dysfunction was the most common complication, including manifestations of ascites or icterus and higher biochemical test results during the follow-up period. Among all patients, major complications related to TACE-CRA procedures were observed in 3 (3.5%), including liver abscess (n=1), and severe ascites (n=2). Minor complications were observed in 12 (14.0%) patients, including fever, mild abdominal pain, nausea, vomiting, local superficial partial-thickness frostbite and thrombocytopenia. All minor complications were transient and resolved within one week.
Discussion
To patients with large HCC, reasonable treatment strategies have always been difficult and challenging for interventionalists. TACE can block the supplying artery of the tumour easily, but not remove the whole tumour completely. Therefore, TACE combined with local ablation has been feasible and effective treatment in large HCC, especially, those with more than 5 cm in diameter. Several combination therapies have been applied in clinical practice, such as TACE combined with RFA, MWA or ethanol injection (EI).20–22 However, there are still few reports in this report related to TACE-CRA in treatment in patient with large HCCs. CRA offers two important potential advantages compared with local ablation based on heat such as MWA or RFA as following: firstly, a larger ice-ball was generated from simultaneous multiple cryoprobes; secondly, CRA can be identified clearly when undergoing intraprocedural CT result from the clear boundary and shape of the ice-ball.11,23 Rong et al showed that 866 HCC patients who met Milan criteria underwent CRA and their cumulative LTP and OS rate were 24.2% and 59.5% at 5 years, respectively. Older age, multiple lesions, and HCC family history were found to be independent, significant negative predictors to the post-CRA of OS.24 In our study, we observed the survival outcome after TACE-CRA in patients with HCC (Diameter > 4cm) based on ALBI or PALBI grade.
Previous studies reported only 5-year OS rates (20.8–47.5%) of patients with single large HCC underwent TACE combined with RFA or MWA.25–27 Moreover, results of one recent 5-year outcome study of TACE-CRA for HCC performed in single medical center showed that 3- and 5-year RFS rates were lower than 20%, respectively. And 3 years and 5 years of OS rates were 38.0% and 29.3%, respectively, after TACE-CRA, which appear to be better survival outcome compared with most reports.28 When we considered the differences in patient sample, viral etiology, liver functional reserve, and BCLC grade among studies, it also was appropriate explanation. In addition, older age, and poor liver functional reserves (i.e., higher ALBI grade) were associated with poorer OS after TACE-CRA, findings consistent with results from previous studies. Similar to RFA for early-stage HCC in other studies, age was one independent prognostic factor for OS.29 More than 50% of the patients in our cohort were >65 years of age, and elderly patients are expected to have a shorter lifespan than younger patients.
Generally, the CTP score is derived from five parameters including serum albumin, bilirubin levels, extent of ascites, degree of hepatic encephalopathy, and coagulation profile.30 The CTP classification currently is applied to assess the hepatic function and prognoses of patients with liver cirrhosis. There are some defects of the CTP class in the assessment of prognosis of HCC patient. Firstly, the CTP score is calculated by arbitrary cutoff values result from equal weighting of five parameters. Secondly, because of the subjective clinical assessment of ascites and hepatic encephalopathy, it is difficult for different observers to score consistently. Thirdly, the CTP score was designed originally for patients with cirrhosis, but approximately 20% of HCCs result from livers without cirrhosis. Accordingly, the ALBI score consists of both serum albumin and bilirubin levels, can give an objective and evidence-based tool for assessment of liver function of HCC patients with better prognostic performance. Comparison with the ALBI and PALBI grade, the cutoff values for bilirubin levels in the CTP score are required to be modified for cholestatic diseases such as primary biliary cholangitis.
There were several major findings in our study. Firstly, TACE-CRA in treatment of HCC (>4 cm in diameter) in our cohort, resulting in long-term survival of up to 5 years with an OS rate of 29.3%, which higher than those in patients with large HCC undergoing TACE-RFA in previous studies. Secondly, the ALBI and PALBI grade exhibited reliable discriminative ability for assessment of OS after TACE-CRA, which suggests that this simple marker could provide an important reference for the treatment of patients with HCC (diameter > 4 cm) and assessment of their outcomes. Finally, we compared the prognostic performances of CTP class ALBI grade, and PALBI grade. ALBI grade demonstrated significantly greater area under the curve values than PALBI grade or CTP class in predicting OS.
There were several limitations in our study. First, this is a single-centre retrospective study with a relatively small sample. Limited sample size might have reduced statistical power in comparative analysis so that some associations were not detected. Second, all of the patients in our cohort were treated at the single medical centre. Therefore, referral bias could not be completely avoided. A multi-centre prospective study of the application is needed to validate the prognostic accuracy. Finally, the included patients had large HCCs with a maximum diameter of 4.1–12.0 cm; therefore, our findings need to be validated by investigating patients with HCCs >12.0 cm in diameter. The following studies should be validated to our results by using these recommended methods.
Conclusion
In conclusion, the 5-year OS after TACE-CRA based on ALBI grade 1 was acceptable, as an effective treatment modality for large HCC. ALBI grade was objective and better prognostic tool than CTP classification in predicting OS of patients with large HCCs (diameter > 4cm) after TACE-CRA.
Acknowledgment
Zhimei Huang and Mengxuan Zuo are co-first authors. I confirm the patient data were deidentified in my revised manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138 [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer J Int Du Cancer. 2015;136(5):E359–386. doi: 10.1002/ijc.29210 [DOI] [PubMed] [Google Scholar]
- 3.Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301–1314. doi: 10.1016/S0140-6736(18)30010-2 [DOI] [PubMed] [Google Scholar]
- 4.Yamakado K, Hirota S. Sub-classification of intermediate-stage (Barcelona clinic liver cancer stage-B) hepatocellular carcinomas. World J Gastroenterol. 2015;21:10604–10608. doi: 10.3748/wjg.v21.i37.10604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kothary N, Takehana C, Mueller K, et al. Watershed hepatocellular carcinomas: the risk of incomplete response following transhepatic arterial chemoembolization. J Vasc Interv Radiol. 2015;26:1122–1129. doi: 10.1016/j.jvir.2015.04.030 [DOI] [PubMed] [Google Scholar]
- 7.Biederman DM, Titano JJ, Bishay VL, et al. Radiation segmentectomy versus TACE combined with microwave ablation for unresectable solitary hepatocellular carcinoma up to 3 cm: a propensity score matching study. Radiology. 2017;283:895–905. doi: 10.1148/radiol.2016160718 [DOI] [PubMed] [Google Scholar]
- 8.Hu H, Chen GF, Yuan W, Wang JH, Zhai B. Microwave ablation with chemoembolization for large hepatocellular carcinoma in patients with cirrhosis. Int J Hyperthermia. 2018;34:1351–1358. doi: 10.1080/02656736.2018.1462536 [DOI] [PubMed] [Google Scholar]
- 9.Ako S, Nakamura S, Nouso K, et al. Transcatheter arterial chemoembolization to reduce size of hepatocellular carcinoma before radiofrequency ablation. Acta Med Okayama. 2018;72:47–52. doi: 10.18926/AMO/55662 [DOI] [PubMed] [Google Scholar]
- 10.Wang C, Wang H, Yang W, et al. Multicenter randomized controlled trial of percutaneous cryoablation versus radiofrequency ablation in hepatocellular carcinoma. Hepatology. 2015;61:1579–1590. doi: 10.1002/hep.27548 [DOI] [PubMed] [Google Scholar]
- 11.Littrup PJ, Aoun HD, Adam B, Krycia M, Prus M, Shields A. Percutaneous cryoablation of hepatic tumors: long-term experience of a large U.S. series. Abdom Radiol (NY). 2016;41(4):767–780. doi: 10.1007/s00261-016-0687-x [DOI] [PubMed] [Google Scholar]
- 12.Wong WS, Patel SC, Cruz FS, et al. Cryosurgery as a treatment for advanced stage hepatocellular carcinoma: results, complications, and alcohol ablation. Cancer. 1998;82:1268–1278. doi: [DOI] [PubMed] [Google Scholar]
- 13.Niu LZ, Li JL, Xu KC. Percutaneous cryoablation for liver cancer. J Clin Transl Hepatol. 2014;2:182–188. doi: 10.14218/JCTH.2014.00017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cui W, Fan W, Huang K, et al. Large hepatocellular carcinomas: treatment with transarterial chemoembolization alone or in combination with percutaneous cryoablation. Int J Hyperthermia. 2018;35(1):239–245. doi: 10.1080/02656736.2018.1493235 [DOI] [PubMed] [Google Scholar]
- 15.Lo GH. Is cryoablation really more effective than radiofrequency ablation in hepatocellular carcinoma. Hepatology. 2016;63(6):2061. doi: 10.1002/hep.28005 [DOI] [PubMed] [Google Scholar]
- 16.Ye L, Liang R, Zhang J, et al. Postoperative albumin-bilirubin grade and albumin-bilirubin change predict the outcomes of hepatocellular carcinoma after hepatectomy. Ann Transl Med. 2019;7(16):367. doi: 10.21037/atm [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ni JY, Fang ZT, An C, et al. Comparison of albumin-bilirubin grade, platelet-albumin-bilirubin grade and Child-Turcotte-Pugh class for prediction of survival in patients with large hepatocellular carcinoma after transarterial chemoembolization combined with microwave ablation. Int J Hyperther. 2019;36:840–852. doi: 10.1080/02656736.2019.1646927 [DOI] [PubMed] [Google Scholar]
- 18.Honmyo N, Kobayashi T, Hamaoka M, et al. Comparison of new prognostic systems for patients with resectable hepatocellular carcinoma: albumin-bilirubin grade and albumin-indocyanine green evaluation grade. Hepatol Res. 2019;49:1218–1226. doi: 10.1111/hepr.v49.10 [DOI] [PubMed] [Google Scholar]
- 19.Sacks D, McClenny TE, Cardella JF, Lewis CA. Society of interventional radiology clinical practice guidelines. J Vasc Intervent Radiol. 2003;14(9 Pt 2):S199–202. doi: 10.1097/01.RVI.0000094584.83406.3e [DOI] [PubMed] [Google Scholar]
- 20.Yuan P, Zhang Z, Kuai J. Analysis on efficacy and safety of TACE in combination with RFA and MWA in the treatment of middle and large primary hepatic carcinoma. J Buon. 2019;24(1):163–170. [PubMed] [Google Scholar]
- 21.Shimose S, Tanaka M, Iwamoto H, et al. Prognostic impact of transcatheter arterial chemoembolization (TACE) combined with radiofrequency ablation in patients with unresectable hepatocellular carcinoma: comparison with TACE alone using decision-tree analysis after propensity score matching. Hepatol Res. 2019;49(8):919–928. doi: 10.1111/hepr.v49.8 [DOI] [PubMed] [Google Scholar]
- 22.Fu Y, Zhao X, Yun Q, et al. Transarterial chemoembolization (TACE) plus percutaneous ethanol injection (PEI) for the treatment of unresectable hepatocellular carcinoma: a meta-analysis of randomized controlled trials. Int J Clin Exp Med. 2015;8(7):10388–10400. [PMC free article] [PubMed] [Google Scholar]
- 23.Hu KQ. Advances in clinical application of cryoablation therapy for hepatocellular carcinoma and metastatic liver tumor. J Clin Gastroenterol. 2014;48(10):830–836. doi: 10.1097/MCG.0000000000000201 [DOI] [PubMed] [Google Scholar]
- 24.Rong G, Bai W, Dong Z, et al. Long-term outcomes of percutaneous cryoablation for patients with hepatocellular carcinoma within Milan criteria. PLoS One. 2015;10(4):e0123065. doi: 10.1371/journal.pone.0123065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yan JY, Zhang JL, Wang MQ, et al. Combined transcatheter arterial chemoembolization and radiofrequency ablation in single-session for solitary hepatocellular carcinoma larger than 7 cm. Asia Pac J Clin Oncol. 2018;14(4):300–309. doi: 10.1111/ajco.12817 [DOI] [PubMed] [Google Scholar]
- 26.Yin X, Zhang L, Wang YH, et al. Transcatheter arterial chemoembolization combined with radiofrequency ablation delays tumor progression and prolongs overall survival in patients with intermediate (BCLC B) hepatocellular carcinoma. BMC Cancer. 2014;14:849. doi: 10.1186/1471-2407-14-849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zuo TY, FY L, MQ W, XX C. Transcatheter arterial chemoembolization combined with simultaneous computed tomography-guided radiofrequency ablation for large hepatocellular carcinomas. Chin Med J (Engl). 2017;130(22):2666–2673. doi: 10.4103/0366-6999.218002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang KB, Fan WZ, Zhang YY, Wang Y, Cui W, Li JP. Transarterial chemoembolization combined with cryoablation for unresectable large hepatocellular carcinoma: a controlled study. Zhonghua Yi Xue Za Zhi. 2016;96(37):2978–2982. doi: 10.3760/cma.j.issn.0376-2491.2016.37.006 [DOI] [PubMed] [Google Scholar]
- 29.Kao WY, Chiou YY, Hung HH, et al. Younger hepatocellular carcinoma patients have better prognosis after percutaneous radiofrequency ablation therapy. J Clin Gastroenterol. 2012;46(1):62–70. doi: 10.1097/MCG.0b013e31822b36cc [DOI] [PubMed] [Google Scholar]
- 30.Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014;383(9930):1749–1761. doi: 10.1016/S0140-6736(14)60121-5 [DOI] [PubMed] [Google Scholar]