Abstract
Rheumatoid arthritis (RA) is a debilitating autoimmune disease characterized by chronic inflammation and progressive destruction of joint tissue. It is also characterized by aberrant blood phenotypes including anemia and suppressed lymphopoiesis that contribute to morbidity in RA patients. However, the impact of RA on hematopoietic stem cells (HSC) has not been fully elucidated. Using a collagen-induced mouse model of human RA, we identified systemic inflammation and myeloid overproduction associated with activation of a myeloid differentiation gene program in HSC. Surprisingly, despite ongoing inflammation, HSC from arthritic mice remain in a quiescent state associated with activation of a proliferation arrest gene program. Strikingly, we found that inflammatory cytokine blockade using the interleukin-1 receptor antagonist anakinra led to an attenuation of inflammatory arthritis and myeloid expansion in the bone marrow of arthritic mice. In addition, anakinra reduced expression of inflammation-driven myeloid lineage and proliferation arrest gene programs in HSC of arthritic mice. Altogether, our findings show that inflammatory cytokine blockade can contribute to normalization of hematopoiesis in the context of chronic autoimmune arthritis.
Introduction
Rheumatoid arthritis (RA) is a chronic, systemic inflammatory autoimmune disease affecting up to 1% of the population.1 RA is associated with significant morbidity and mortality, and substantial healthcare-related costs.2 While the pathogenesis of RA is most often associated with breaking of central tolerance and activation of autoimmune T and B lymphocytes, all types of blood cells contribute to the RA disease process. Platelets and myeloid cells, such as neutrophils and macrophages, have been shown to infiltrate the joint synovia, damaging tissue and presenting antigens that initiate autoimmunity.3 RA is also associated with co-morbid hematologic manifestations including chronic anemia, impaired production of naïve T cells, autoimmune cytopenias and leukocytosis during disease ‘flares’.4–7 In addition, RA is associated with elevated levels of pro-inflammatory cytokines including interleukin-1 (IL-1), tumor necrosis factor (TNF) and interferon (ifn)-y.4 Therapeutic blockade of these factors has been used with success to alleviate the symptoms of inflammatory arthritis in patients, underscoring the importance of pro-inflammatory cytokines in the pathogenesis of RA.5
Maintenance of the blood system requires a carefully orchestrated interaction between blood-forming hematopoietic stem cells (HSC) and cell-extrinsic signals provided by their microenvironment in the bone marrow (BM).6 Such signals regulate HSC quiescence and/or direct HSC differentiation into lineage-biased multipotent progenitors (MPP) and mature blood cells.7 Pro-inflammatory cytokines can activate ‘emergency’ gene programs in HSC associated with overproduction of myeloid- and platelet-biased MPP subsets that, in turn, overproduce myeloid cells and platelets at the expense of other lineages.8–10 Along these lines, hematopoietic defects in RA patients have been ascribed to pro-inflammatory cytokines.11 However, the underlying molecular mechanism(s) and impact of therapeutic intervention on HSC function have not been well characterized.
Here, we used the type II collagen-induced arthritis (CIA) mouse model of human RA to identify the impact of chronic autoimmune arthritis on HSC function. We found that CIA leads to expansion of myeloid progenitors in the BM and overproduction of myeloid cells. HSC from CIA mice activated a myeloid gene program, although they retained their quiescence and long-term repopulating function. Interestingly, HSC quiescence was associated with a proliferation arrest program, characterized by downregulation of cell cycle and mRNA translation genes, which may serve to limit spurious HSC activation. Notably, we showed that pharmacological cytokine blockade using the recombinant IL-1 receptor antagonist anakinra alleviated inflammatory arthritis and myeloid expansion in the blood and BM of mice with CIA. In addition, anakinra treatment reduced activation of inflammation-induced myeloid and proliferation arrest gene programs in HSC. Taken together, our findings suggest that anti-inflammatory therapies such as cytokine blockade can restore hematopoietic function in the context of chronic inflammatory diseases such as RA.
Methods
The methods are described in detail in the Online Supplement.
Mice and in vivo treatments
Male, 6- to 12-week old C57BL/6J (strain #000664) and B10.RIII (strain #000457) mice from The Jackson Laboratory (Bar Harbor, ME, USA) were maintained in a temperature- and light- controlled environment with irradiated chow and water ad libitum for all experiments. Arthritis was induced by intradermal injection of type II chicken (for C57BL/6J mice) or bovine (for B10.RIII mice) collagen (Sigma-Aldrich) emulsified in Complete Freund Adjuvant (CFA; Sigma-Aldrich).12 Anakinra (Swedish Orphan Biovitrum) was administered daily at a dose of 50 mg/kg via subcutaneous injection. All animal procedures were approved by the University of Colorado Denver Anschutz Medical Campus Institutional Animal Care and Use Committee (IACUC).
Flow cytometry and hematopoietic stem cell isolation
BM was flushed from femora and tibiae with Hank’s balanced salt solution (HBSS) without calcium or magnesium salts but containing 2% heat-inactivated fetal bovine serum (FBS). BM cells were depleted of red blood cells using ACK lysis except for the erythroblast analysis. Next, 1×107 cells were stained for hematopoietic stem and progenitor cells or 1×106 for mature BM cells and were analyzed on a BD FACSCelesta or LSRII instrument. For HSC isolation, posterior limb, anterior limb and pelvic bones were crushed in HBSS + 2% FBS, treated with ACK, placed on a Histopaque 1119 gradient and enriched in c-Kit cells using anti-c-Kit microbeads (Miltenyi) and separation on an AutoMACS Pro (Miltenyi). Cells were double-sorted to purity on a FACSAria IIu or FACSAria Fusion (Becton Dickenson) at 20 psi using a 100 μm nozzle.
Hematopoietic stem cell and bone marrow transplantation assays
Lethally irradiated (11 Gy, split dose 3 h apart) CD45.1+ Boy/J congenic recipient mice were transplanted with either 250 CD45.2+ donor HSC plus 5×105 CD45.1+ Sca-1 depleted cells, or 5×105 unfractionated BM cells, via the retro-orbital vein in a 100 μL volume of HBSS + 2% FBS. Recipient mice were maintained on autoclaved water containing Bactrim for 4 weeks following transplantation, and donor blood chimerism was assessed every 4 weeks up to 16 weeks via bleeding from the tail vein or retro-orbital sinus.
Gene expression analyses
RNA-sequencing analysis was performed on three biological replicate pools of 4×103 to 1×104 HSC isolated from groups of two mice per condition (control and CIA). DESeq2-1.12.3 within R-3.3.0 was used for data normalization and differential expression analysis with an adjusted P-value threshold of 0.05. Fluidigm analyses were performed using commercially designed DeltaGene primer sets on a Biomark instrument (Fluidigm). Relative gene expression was calculated using the ΔCt method. Data were normalized to Actb.
Cytokine analysis
Serum cytokine levels were determined using a Luminex 36- analyte ProCartaPlex cytokine array (Thermo Fisher) according to the manufacturer’s instructions.
Statistical analysis
Statistical analyses were performed using Prism 7 software (GraphPad). P-values were determined using a Mann-Whitney h-test or one-way analysis of variance. P-values ≤0.05 were considered statistically significant.
Results
Aberrant blood system in mice with collagen-induced arthritis
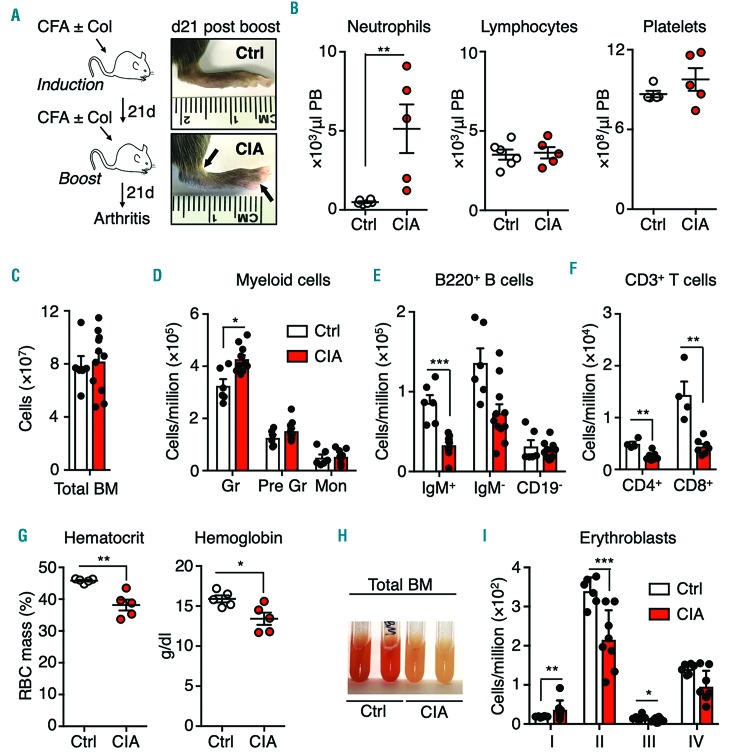
The CIA system is the most commonly studied model of RA, and faithfully recapitulates several disease features, including B- and T-cell-driven autoimmunity and chronic production of pathogenic cytokines.12 To assess the impact of RA on the blood system, C57BL/6 mice were injected intradermally at the base of the tail twice 21 days apart with an emulsion of CFA and type II collagen (Figure 1A). Non-arthritic control mice were injected with CFA alone on the same treatment schedule. After the second (boost) injection, mice rapidly developed polyarthritis characterized by swelling and erythema of the front and rear paws, metatarsals and/or ankles within a week of the boost injection (Figure 1A, Online Supplementary Figure S1A). Peripheral blood parameters in CIA mice 21 days after the boost injection showed a significant increase in neutrophils, while lymphocyte and platelet numbers were not significantly different (Figure 1B). While the overall number of BM cells in the femora and tibiae of CIA mice were unchanged, (Figure 1C), Mac-1+Gr1hi BM granulocytes were significantly increased in CIA mice while the numbers of Mac-1+Gr1intLy6C− immature granulocytes and Mac-1+Gr1intLy6C+ monocytes were unchanged (Figure 1D and Online Supplementary Figure S1B). On the other hand, the numbers of CD19+IgM+ immature B cells and CD19+ IgM− B cells were significantly decreased, as were the numbers of CD4+ and CD8+ T cells (Figure 1D-F and Online Supplementary Figure S1B). CIA mice were anemic, with significantly decreased hematocrit and hemoglobin levels (Figure 1G). CIA BM was also pale, with decreased basophilic erythroblasts (population II) (Figure 1H, I and Online Supplementary Figure S1B). Altogether, CIA mice exhibited myeloid expansion, consistent with previously published studies using the KRNxG7 genetic autoimmune arthritis model.13 Importantly our findings mirror features of human RA, such as leukocytosis, anemia and immunosenescence.14,15
Figure 1.
Altered blood system in mice with collagen-induced arthritis. (A) The strategy for producing collagen-induced arthritis (CIA) and representative images showing swelling and anklyosis in the hind paws of a CIA mouse and a control (Ctrl) mouse 21 days after disease induction. (B) Peripheral blood (PB) parameters, determined by a complete blood count, of Ctrl and CIA mice (n=5 per group). (C) Total bone marrow (BM) cellularity of hind legs, and (D-F) numbers of the indicated populations (see Online Supplementary Figure S1 for FACS gating and surface marker definitions) expressed as number per million BM cells (n=6 Ctrl and 11 CIA). (G) Red blood cell parameters in PB (n=5 per group). (H) Representative image of flushed BM. (I) Erythroblast number per million BM cells (n=6 Ctrl and 11 CIA). *P<0.05; **P<0.01 ***P<0.001, as determined by the Mann-Whitney U-test. The data were compiled from three independent experiments. CFA: complete Freund adjuvant; Gr: mature granulocytes; Pre-Gr: immature granulocytes; Mon: monocytes; RBC: red blood cells.
Collagen-induced arthritis leads to myeloid expansion and activation of a myeloid gene program in hematopoietic stem cells
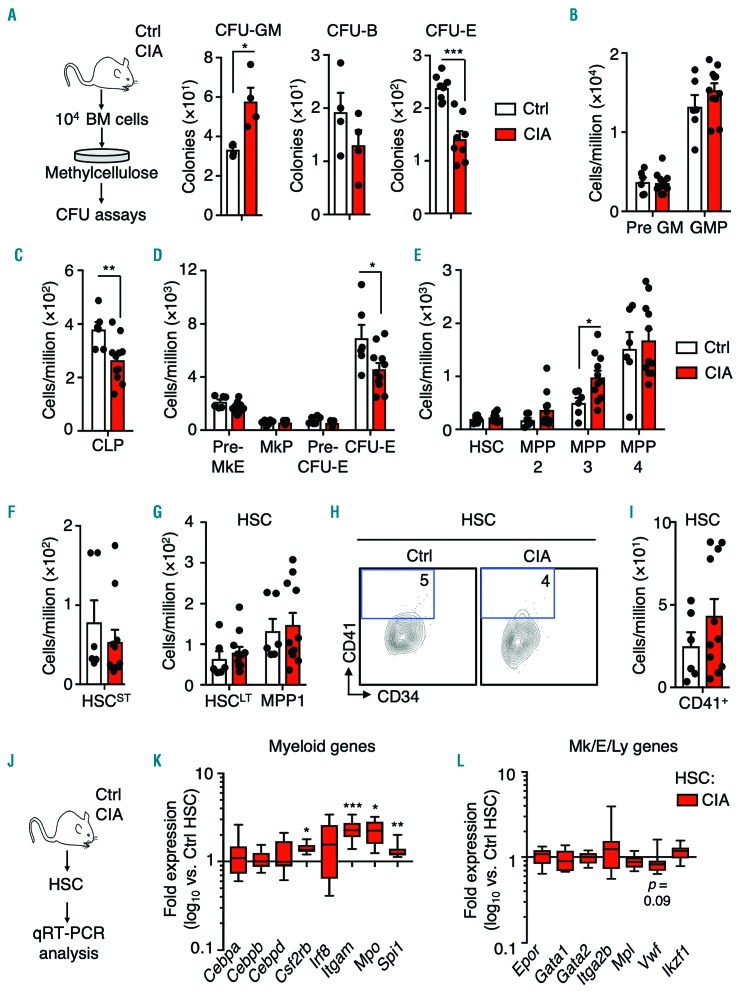
We next assessed BM lineage potential using methylcellulose-based colony-forming unit (CFU) assays (Figure 2A). Strikingly, the numbers of granulocyte-macrophage colonies (CFU-GM) (Figure 2A) were significantly increased, whereas erythroid colony formation was impaired (Figure 2A). CFU-GM were also significantly increased in the spleens of CIA mice alongside a significant increase in splenocyte numbers, indicative of extramedullary hematopoiesis16 (Online Supplementary Figure S2A-C). While granulocyte-macrophage progenitors (GMP; Lin−c-Kit+CD41−CD150−FcYR+) were unchanged in the BM, common lymphoid progenitors (CLP; Lin− Flk2+IL7R+c-KitintSca1int) and phenotypic CFU-E cells (Lin−c- Kit+CD41−FcYR−CD15O−CD105+) were significantly reduced (Figure 2B-D and Online Supplementary Figure S1C). Recently, distinctive lineage-biased MPP populations downstream of HSC were identified and termed MPP2, MPP3 and MPP4, (LSK Flk2−CD48+CD150+, LSK Flk2−CD48+CD150−, and LSK Flk2+, respectively); these populations exhibit megakaryocyte/erythroid, myeloid and lymphoid lineage priming, respectively.7 CIA mice exhibited expansion of myeloid-biased MPP3 in the BM (Figure 2E and Online Supplementary Figure S1C), consistent with myeloid overproduction. Within the phenotypic LSK Flk2−CD48−CD150+ compartment, hereafter referred to in the text as HSC, the distribution of CD34− long-term HSC (HSCLT) and metabolically-activated CD34+ MPP1 subsets was unchanged (Figure 2G and Online Supplementary Figure S1C). Despite some heterogeneity between individual mice, we also did not observe a significant increase in the abundance of CD41-expressing (CD41+) HSC, which rapidly differentiate into megakaryocytes in the context of inflammation (Figure 2H, I).17,18 Likewise, the numbers of short-term HSC (HSCST; LSK Flk2−CD48−CD150−) were unchanged (Figure 2F and Online Supplementary Figure S1C). Since remodeling of BM stroma occurs in RA patients and animal models,19 we analyzed stromal cell populations comprising the endosteal HSC niche6 and observed a significant decrease in bone-forming mesenchymal stromal cells (MSC), consistent with reduced bone-forming activity in a genetic mouse model of RA (Online Supplementary Figure S2D, E).19,20 Lastly, we confirmed activation of a myeloid lineage gene program in HSC from CIA mice using custom Fluidigm real-time quantitative polymerase chain reaction (qRT-PCR) assays (Figure 2J). Consistent with prior reports,21 expression of myeloid genes was increased in HSC from CIA mice, including the myeloid master regulator Spi1/Ph.1 and its target genes Itgam1 and Csf2rb, as well as Mpo (Figure 2K). On the other hand, other lineage determinant genes were minimally altered (Figure 2L). Altogether, these data identify aberrant activation of an ‘emergency’ myeloid differentiation pathway in CIA mice, characterized by BM remodeling, myeloid expansion and activation of a myeloid gene program in HSC.
Figure 2.
Myeloid expansion in the bone marrow of mice with collagen-induced arthritis. (A) Experimental design and numbers of colony-forming units (CFU)-granulocyte-macrophage (GM), CFU-burst (CFU-B) (n=4 per group) and CFU-erythroid (CFU-E) (n=8/group) in unfractionated bone marrow cells from control mice (Ctrl) and mice with collagen-induced arthritis (CIA). (B-I) Numbers of the indicated populations (see Online Supplementary Figure S1 for FACS gating and surface marker definitions) expressed as number per million cells. (H) Representative FACS plots showing the gating strategy for identifying the CD41+ fraction of hematopoietic stem cells (HSC) (n=6 Ctrl and 11 CIA). The data were compiled from three independent experiments. (J) Experimental design and (K, L) Fluidigm gene expression analysis of HSC from Ctrl and CIA mice showing (K) myeloid and (L) megakaryocyte (Mk), erythroid (E) and lymphoid (Ly) lineage genes. The data are presented as log10 fold expression in CIA HSC versus Ctrl HSC. Ct values were normalized to Actb (n=8-16 per group). Data are compiled from two independent experiments. *P<0.05; **P<0.01 ***P<0.001, as determined by the Mann-Whitney U-test. BM: bone marrow; Pre GM: precursor granulocyte-macrophage; GMP: granulocyte-macrophage progenitors, CLP; common lymphoid progenitors; Pre MkE: precursor megakaryocyte/erythroid; MkP: megakaryocyte progenitors; MPP: multipotent progenitors; qRT-PCR: real-time quantitative polymerase chain reaction.
Impact of collagen-induced arthritis on long-term reconstitution of hematopoietic stem cells
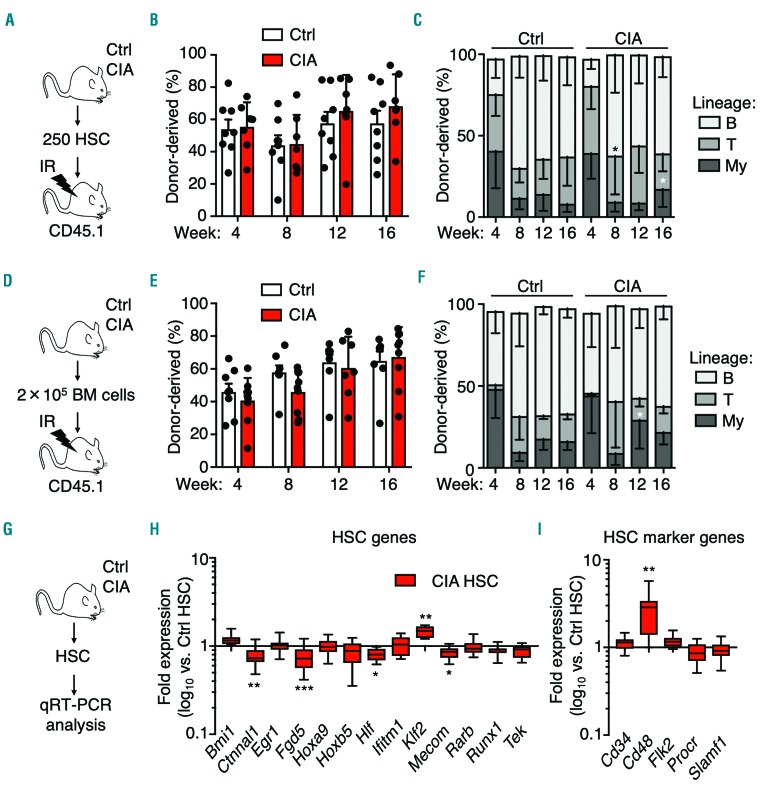
We and others previously showed that chronic inflammation impairs HSC long-term reconstitution capacity.8 To interrogate long-term HSC potential, we transplanted purified CD45.2+ HSC from control and CIA donor mice into lethally-irradiated (11 Gy) CD45.1+ recipient mice (Figure 3A and Online Supplementary Figure S3A). Strikingly, overall reconstitution capacity of HSC from CIA mice 16 weeks after transplantation was not significantly different from that of controls (Figure 3B), consistent with short-term CFU assays on purified HSC (Online Supplementary Figure S3B). Interestingly myeloid lineage output at week 16 was significantly increased (Figure 3C), with a significantly increased proportion of CIA donor-derived phenotypic MPP3, consistent with a myeloid biased phenotype (Online Supplementary Figure S3C, D). In parallel, we assessed HSC function independently of surface markers via transplantation of unfractionated BM cells from control and CIA mice (Figure 3D). We observed no defect in reconstitution, save for a slight myeloid bias (Figure 3E-F) and a decreased frequency of CIA donor-derived phenotypic HSC, likely related to a decreased frequency of HSC in the BM of CIA mice (Online Supplementary Figures S1D and S3E, F). These results suggest that long-term HSC potential is not compromised, although a degree of myeloid bias is present, consistent with prior published results in the KRNxG7 arthritis model.21 Likewise, expression of genes associated with HSC identity and self-renewal was largely unchanged (Figure 3G-I) except for an increase in Cd48 expression, suggesting that HSC from CIA mice may be primed toward differentiation into MPP (Figure 3I). However, at the protein level CD48 and other key surface markers associated with lineage bias or differentiation, such CD150, were unchanged,22 suggesting that such MPP priming could be restricted primarily to the mRNA level (Online Supplementary Figure S4A, B). In addition, reactive oxygen species, which accompany differentiation and can impair HSC function if chronically elevated,23,24 were unchanged in HSC from CIA mice (Online Supplementary Figure S4C). Taken together, these data indicate that, apart from myeloid priming, the functional and molecular properties of HSC are largely unperturbed in CIA mice.
Figure 3.
Hematopoietic stem cells from mice with collagen-induced arthritis retain reconstituting capacity. (A-C) Long-term engraftment of purified hematopoietic stem cells (HSC) isolated from control mice (Ctrl) and mice with collagen-induced arthritis (CIA). (A) Experimental design. (B) Donor chimerism and (C) lineage distribution in peripheral blood of recipient mice over time (n=8 Ctrl and 7 CIA recipient mice). (D-F) Long-term engraftment of unfractionated bone marrow isolated from Ctrl and CIA mice. (D) Experimental design. (E) Donor chimerism and (F) lineage distribution in peripheral blood of recipient mice over time (n=10 Ctrl and 9 CIA recipient mice). The data are representative of one of two independent experiments. (G) Experimental design and (H-I) Fluidigm gene expression analysis of HSC from Ctrl and CIA mice showing (H) HSC genes and (I) HSC surface marker genes. The data are presented as log10 fold expression in CIA HSC versus Ctrl HSC. Ct values were normalized to Actb (n=8-16 per group). *P<0.05, as determined by the Mann-Whitney U-test. The data were compiled from two independent experiments.
A proliferation arrest gene program is associated with quiescence in hematopoietic stem cells from mice with collagen-induced arthritis
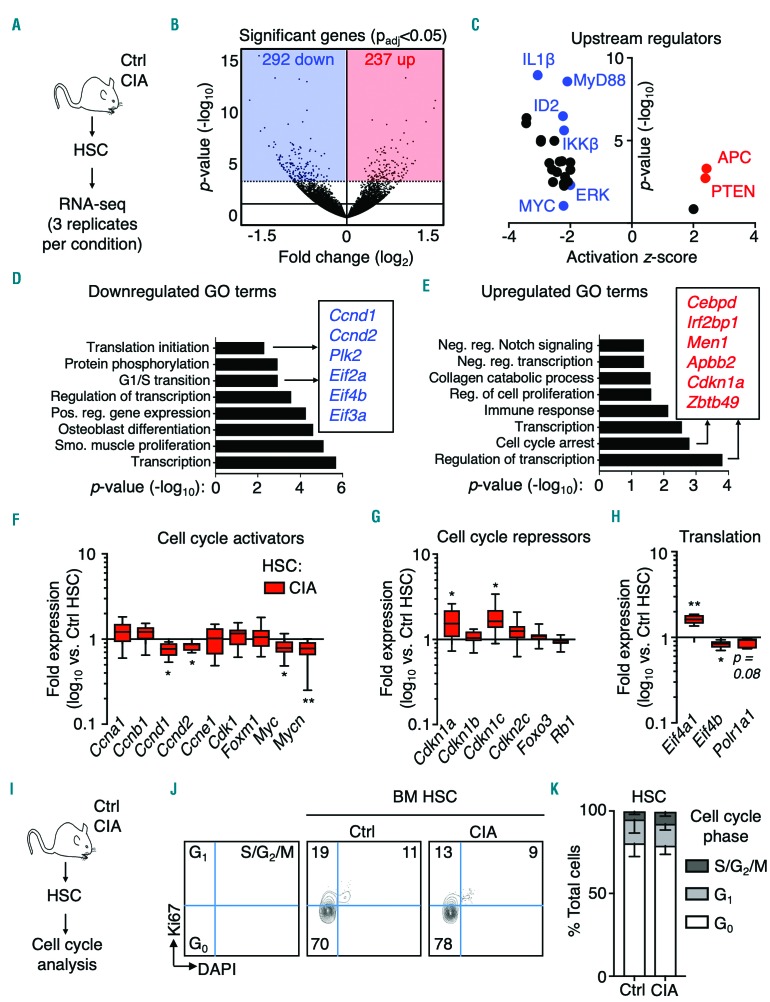
To gain additional insight into the impact of CIA on HSC molecular regulation, we performed RNA-sequencing analysis on HSC isolated from control and CIA mice (Figure 4A). Differential expression analysis identified 292 significantly downregulated genes and 237 upregulated genes based on an adjusted P-value (Padj) of >0.05 (Figure 4B and Online Supplementary Table S1). To uncover potential mechanisms regulating HSC function in CIA mice, we used the Upstream Regulator analysis function in Ingenuity Pathway Analysis (IPA) software. Strikingly, few regulatory pathways were significantly activated in HSC from CIA mice, most notably APC and PTEN, which both suppress HSC activity and enforce quiescence (Figure 4C and Online Supplementary Table S2).25 On the other hand, pathways activated in response to inflammatory and mitogenic cues were downregulated, including ERK, MYC and il-1/nf-kb (Figure 4C and Online Supplementary Table S2).26 In parallel, gene ontology (GO) analysis27 identified enrichment of downregulated genes involved in protein translation initiation, G1/S cell cycle transition, positive regulation of gene expression and transcription (Figure 4D and Online Supplementary Table S3). Conversely, significantly upregulated genes in HSC from CIA mice were enriched for cell cycle arrest, negative regulation of transcription, and regulation of cell proliferation categories (Figure 4E and Online Supplementary Table S4). Reinforcing these findings, gene set enrichment analysis identified significant (false discovery rate<0.1) enrichment of downregulated genes related to mRNA translation initiation, elongation and termination in HSC from CIA mice (Online Supplementary Table S5), whereas only two gene sets were significantly enriched, both related to mitochondrial ribosome function (Online Supplementary Table S6). The GEO accession number for the RNA-sequencing data reported in this paper is GSE129511.
Figure 4.
Activation of a proliferation arrest gene program in hematopoietic stem cells from mice with collagen-induced arthritis. (A) Experimental design. (B) Volcano plot showing significantly differentially expressed genes (shaded areas) based on Padj <0.05 (n=3 per group); hematopoietic stem cell (HSC) pools were sorted from three independent sets of mice. (C) Significantly differentially activated upstream regulators as determined by Ingenuity Pathway Analysis. (D, E) Gene ontology (GO) analysis of significantly differentially (D) downregulated and (E) upregulated gene sets. (F-H) Fluidigm gene expression analysis of HSC from control mice (Ctrl) and mice with collagen-induced arthritis showing (F) cell cycle activator genes; (G) cell cycle repression genes; (H) mRNA translation genes. The data are presented as log10 fold expression in CIA HSC versus Ctrl HSC. Ct values were normalized to Actb (n=8-16 per group). The data were compiled from two independent experiments. (I) Experimental design. (J) Representative FACS plots and (K) cell cycle distribution of HSC in Ctrl and CIA mice (n=8 Ctrl and 7 CIA mice). The data were compiled from two independent experiments. See also Online Supplementary Tables S1-S6. *P<0.05; **P<0.01 ***P<0.001, as determined by the Mann-Whitney U-test.
Using Fluidigm qRT-PCR to validate our RNA-sequencing analyses, we found decreased expression of Myc, Mycn, Ccnd1 and Ccnd2, which are all required for HSC cell cycle entry (Figure 4F). Conversely, cyclin-dependent kinase inhibitors (CKI) Cdkn1a (p21) and Cdkn1c (p57), which enforce HSC quiescence, were significantly upregulated in HSC from CIA mice (Figure 4G). In addition, Eif4b, which is required for Eif4a activity during mRNA translation, was decreased (Figure 4H). Collectively, these data identify a global downregulation of HSC activation pathways despite chronic inflammation.
To identify the functional impact, we analyzed the cell cycle distribution of HSC in control and CIA mice (Figure 4I). Strikingly, HSC from CIA mice retained a quiescent cell cycle phenotype (Figure 4J-K). Likewise, the cell cycle distribution of phenotypic MPP or Lin−cKit+Sca-1−CD34+ myeloid progenitors (MyPro) was unaltered (Online Supplementary Figure S5E). This suggests that myeloid expansion in CIA mice likely arises from preferential differentiation into myeloid-biased progenitors from HSC and/or MPP, rather than from increased proliferative activity. Thus, despite ongoing inflammatory arthritis, HSC retain a quiescent phenotype associated with a proliferation arrest gene program that could serve to prevent cell cycle entry during chronic inflammatory stress.
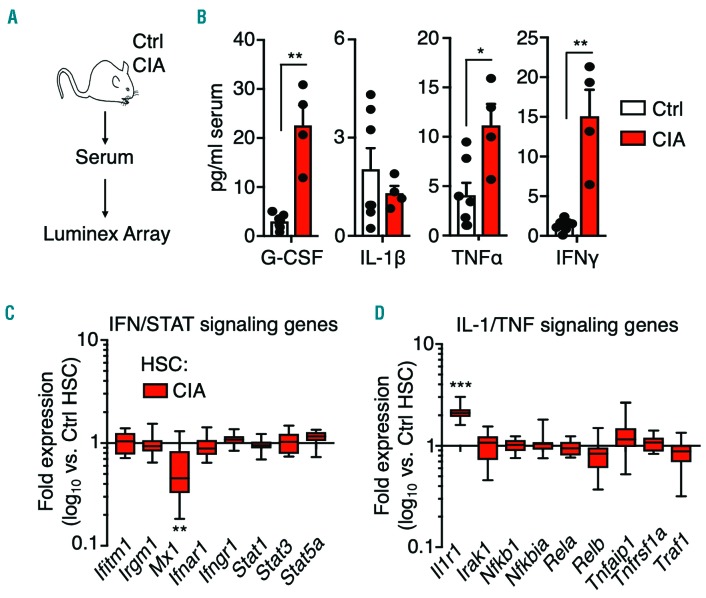
Systemic pro-inflammatory cytokine production in mice with collagen-induced arthritis
RA-associated cytokines, such as IL-1, TNF, IFN-γ, and myeloid growth factors, such as granulocyte-colony stimulating factor (G-CSF), can activate myeloid gene programs in hematopoietic stem and progenitor cells, leading to altered blood lineage output.8,10 We therefore used a Luminex-based 36-plex array to analyze cytokine levels in the serum of control and CIA mice (Figure 5A, Online Supplementary Figure S6). Several cytokines were increased in the serum of CIA mice, including TNF, G-CSF and IFN-γ. On the other hand, IL-1β, which is often produced locally at the joint synovia in RA patients,28 was not detected (Figure 5B). Using qRT-PCR, we found IFN target genes unchanged in HSC from CIA mice, consistent with unchanged Sca-1 surface expression (Figure 5C, Online Supplementary Figure S4A, B).16 In contrast, IL-1 receptor (Il1r1), which is a target of multiple cytokines including IL-1 itself, was increased in HSC from CIA mice (Figure 5D). These data suggest that systemic production of pro-inflammatory cytokines is a feature of CIA mice, similar to human RA patients.
Figure 5.
Systemic inflammation in mice with collagen-induced arthritis. (A) Experimental design. (B) Cytokine levels in serum from control mice (Ctrl) and mice with collagen-induced arthritis (CIA) (n=7 Ctrl and 4 CIA). Serum sample data were compiled from two independent experiments. (C,D) Gene expression analysis of hematopoietic stem cells (HSC) from Ctrl and CIA mice showing (C) IFN/STAT signaling genes and (D) IL-1/TNF signaling genes. The data are presented as log10 fold expression in CIA HSC versus Ctrl HSC. (n=14-16 per group) *P<0.05; **P<0.01, as determined by the Mann-Whitney U-test.
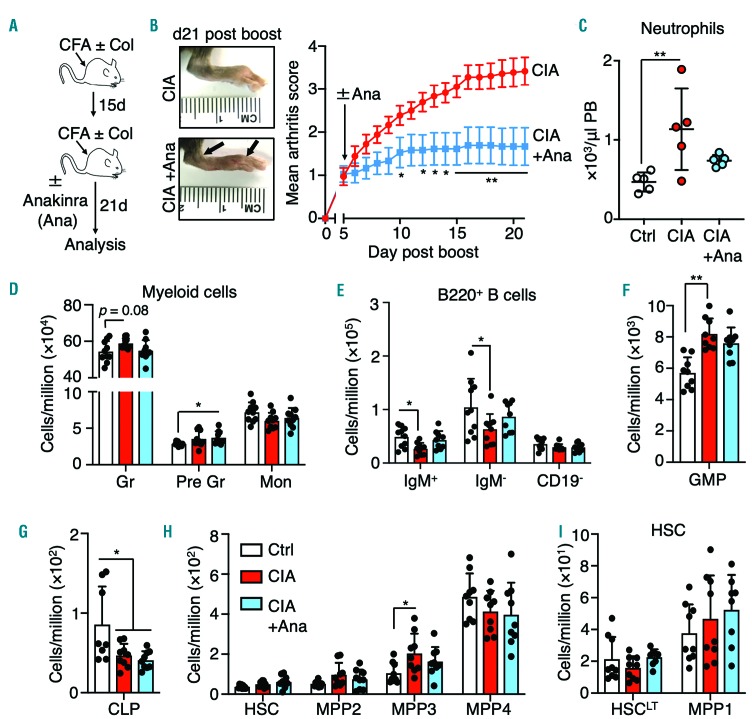
Impact of cytokine blockade on myeloid expansion in mice with collagen-induced arthritis
Cytokine blockade therapy, particularly against IL-1 and TNF, is efficacious in reducing inflammatory arthritis. It can also normalize blood parameters in RA patients,29 although its impact on hematopoiesis is not well described. We used anakinra, a recombinant form of human IL-1 receptor antagonist (IL-1Ra) that is approved for treatment of RA in human patients and is considered a paradigm for cytokine blockade therapy.30 We induced CIA in C57BL/10.RIII (B10.RIII) mice (Figure 6A), a C57BL/6-related strain that develops a severe and highly penetrant autoimmune arthritis following CIA induction.31 These mice are thus ideal for testing therapeutic interventions. Consistent with prior mouse studies, anakinra treatment significantly reduced arthritis severity based on clinical scoring of paw swelling (Figure 6B).32 Strikingly, anakinra normalized peripheral blood neutrophil and, to a lesser extent, red blood cell counts (Figure 6C, Online Supplementary Figure S7A). Anakinra treatment also normalized granulocyte and B-cell numbers in the BM, with a slight but statistical increase in immature granulocytes in anakinra-treated mice, perhaps reflecting slowed differentiation of these cells into mature granulocytes (Figure 6D, E). While the numbers of common lymphoid progenitors were not restored, anakinra treatment modestly reduced GMP and MPP3 expansion in CIA mice to a point below statistical significance relative to controls, suggesting reduced activation of myeloid differentiation pathways (Figure 6F-H). On the other hand, HSCLT and MPP1 numbers were unchanged in all conditions (Figure 6I). Altogether, these data indicate that pro-inflammatory cytokine blockade can at least partially alleviate myeloid expansion and neutrophilia associated with CIA.
Figure 6.
Cytokine blockade reduces myeloid expansion in mice with collagen-induced arthritis. (A) Induction of collagen-induced arthritis (CIA) and anakinra (Ana) treatment strategy in B10.RIII mice. (B) Representative images showing swelling and anklyosis in the hind paws of mice 17 days after disease induction (top left, CIA mice not treated with Ana; bottom left, CIA mice treated with Ana) and impact of Ana treatment on arthritis score (right) (n=9 per group). (C) Peripheral blood neutrophil count and (D-I) number of the indicated bone marrow populations expressed as number per million BM cells of control, CIA and CIA+Ana mice (n=9 per group). The data were compiled from two independent experiments. *P<0.05; **P<0.01 ***P<0.001, as determined by one-way analysis of variance or the Mann-Whitney U-test. CFA: complete Freund adjuvant; Col: collagen; Ctrl: control; PB: peripheral blood; Gr: mature granulocytes; Pre Gr: immature granulocytes; Mon: monocytes, GMP: granulocyte-macrophage progenitors, CLP; common lymphoid progenitors; MPP: multipotent progenitors.
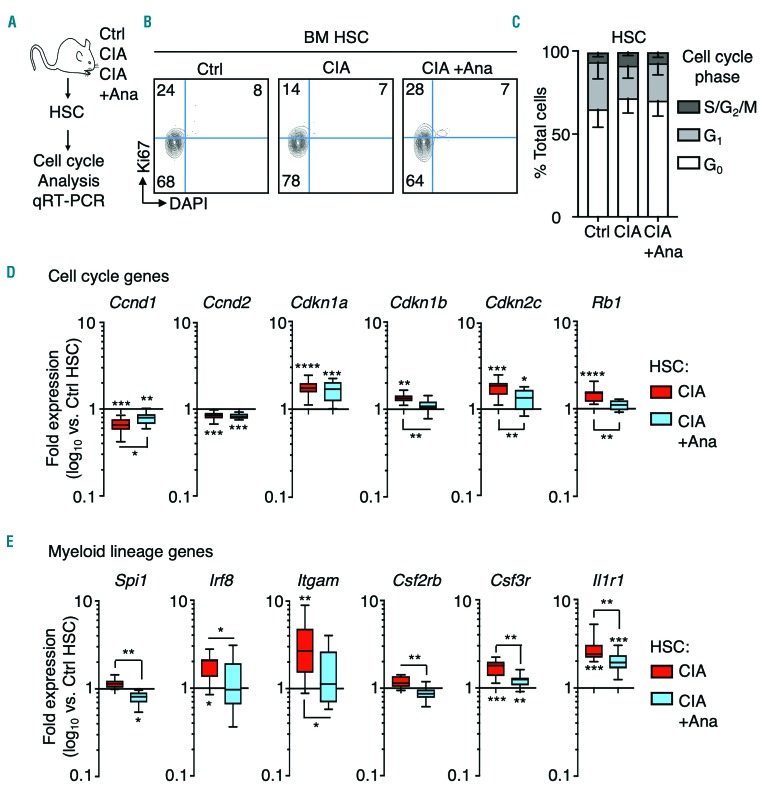
Cytokine blockade reverses inflammation-driven gene programs in hematopoietic stem cells from mice with collagen-induced arthritis
Given the impact of anakinra treatment on hematopoiesis, we next assessed the effect of anakinra on cell cycle activity in HSC, MPP and MyPro in CIA mice. We found their cell cycle distribution remained unchanged (Figure 7A-C), indicating that anakinra treatment does not alter cell cycle distribution in these populations. Nonetheless, anakinra treatment partially normalized expression of Ccnd1 and almost completely normalized expression of the cell cycle inhibitors Cdkn1b, Cdkn2c and Rb1 (Figure 7D). Strikingly, anakinra also significantly decreased expression of aberrantly activated myeloid lineage genes in HSC from CIA mice, including PU.1 and targets such as Csfr2b, Itgam, as well as Il1r1 and the myeloid transcription factor Irf8 (Figure 7E), consistent with reduced myeloid expansion in anakinra-treated CIA mice. Taken together, these data indicate that inflammation-driven myeloid and proliferation arrest gene programs in HSC are at least partially reversible, and can be alleviated by cytokine blockade therapy.
Figure 7.
Cytokine blockade attenuates inflammation-induced hematopoietic stem cell gene programs. (A) Experimental design. (B) Representative FACS plots and (C) cell cycle distribution of hematopoietic stem cells (HSC) from control mice (Ctrl), mice with collagen-induced arthritis (CIA) and CIA mice treated with anakinra (+Ana) (n=5 per group). (D) Fluidigm analysis of proliferation arrest gene programs in HSC from Ctrl, CIA and CIA +Ana mice. The data are presented as log10 fold expression versus Ctrl HSC (n=12-16 per group). (E) Fluidigm analysis of myeloid gene expression in HSC from Ctrl, CIA and CIA +Ana mice. The data are presented as log10 fold expression versus Ctrl HSC. Ct values were normalized to Actb (n=12-16 per group). The data are presented as log10 fold expression versus Ctrl HSC (n=12-16 per group). The data were compiled from two independent experiments. *P<0.05; **P<0.01 ***P<0.001, ****P<0.0001 as determined by one-way analysis of variance or the Mann-Whitney U-test. BM: bone marrow.
Discussion
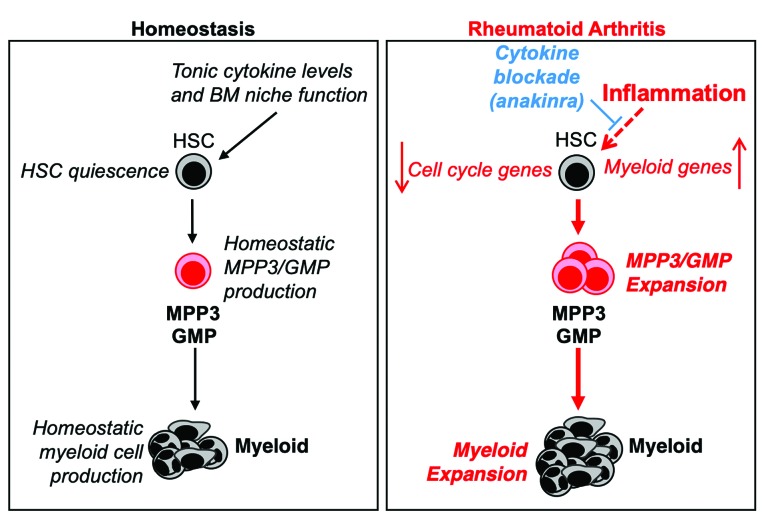
Human RA is associated with deregulations in the blood system that can contribute to disease pathogenesis and patient morbidity.14,15,33 Here, we used the CIA mouse model of human RA to better understand the impact of disease and therapeutic intervention on the hematopoietic system. We found that CIA induced a profoundly myeloid-skewed hematopoietic hierarchy characterized by selective expansion of myeloid progenitors and mature myeloid cells and activation of myeloid lineage genes in HSC. Despite chronic inflammation, HSC from CIA mice retained their long-term potential and maintained a quiescent cell cycle state associated with the induction of a proliferation arrest gene program. Strikingly, we found that cytokine blockade therapy was able to attenuate these effects (Figure 8).
Figure 8.
Model of inflammation-driven hematopoietic alterations in mice with collagen-induced arthritis. Under homeostatic conditions, hematopoietic stem cells (HSC) are largely quiescent but occasionally enter the cell cycle and give rise to lineage-biased multipotent progenitor (MPP) subsets, leading to a balanced lineage output and nominal HSC self-renewal capacity. In mice with collagen-induced arthritis (CIA), chronic inflammation leads to expansion of myeloid-biased MPP3 and granulocyte-macrophage progenitors (GMP), resulting in increased myeloid cell production. Concurrently, inflammation induces a myeloid lineage gene program in HSC that may bias HSC toward further overproduction of MPP3. Despite ongoing inflammation, HSC are maintained in a quiescent state characterized by repression of cell cycle and mRNA translation genes alongside induction of cell cycle inhibitor genes. Notably, pro-inflammatory cytokine blockade, here using anakinra, attenuates myeloid expansion and altered gene expression in HSC. These data indicate that chronic inflammation drives aberrant hematopoiesis in rheumatoid arthritis, and this phenotype can be attenuated by cytokine-blocking therapy.
Myeloid expansion, chronic anemia of inflammation and immunosenescence are well-documented hematopoietic phenotypes in human RA patients, although the precise causes remain elusive and may be multifactorial.14,15,33 Strikingly, myeloid expansion in CIA mice closely resembles that in other models of chronic inflammation including those induced by lipopolysaccharide, IL-1, IFN and pathogen infection.8,10,34 Pro-inflammatory signals can directly activate myeloid transcription factors including Spi1/PU.1 and C/EBP family members, which ‘override’ competing lineage programs and drive expansion of myeloid-biased progenitors.35–37 In line with this, we observed increased expression of Spi1/PU.1 and its target genes in HSC from CIA mice. In addition, transplantation of purified CIA HSC revealed an increased proportion of donor-derived myeloid cells and phenotypic MPP3, suggesting that BM inflammation primes HSC to differentiate preferentially into myeloid-lineage progenitors. Given that PU.1 activation in HSC and myeloid expansion are features of both our CIA and chronic IL-1 models, they are likely stereotypical responses to ongoing inflammation rather than a disease-specific mechanism, wherein PU.1 could serve as a central ‘node’ that activates a myeloid gene program in HSC following a variety of inflammatory insults. Newly developed pharmacological inhibitors of PU.138 could thus provide therapeutic benefit by blocking this ‘node’ independently of cytokines in an inflammatory disease.
In the literature, inflammation is often associated with increased HSC proliferation, typically in response to acute challenges.8,10 On the other hand, we previously found that HSC can maintain quiescence in the context of ongoing chronic type I IFN signaling.16 Likewise, here we showed that HSC quiescence, phenotypic pool size and long-term repopulating capacity are maintained in CIA mice. Quiescence protects HSC by preventing excessive apoptosis, differentiation, or replicative ‘aging’ associated with proliferation, and HSC lacking quiescence maintenance genes, such as Ckdn1a and Cdkn1c, become exhausted in response to stress.25 Notably, we found that HSC maintained a quiescent state despite ongoing BM remodeling, including decreased phenotypic endosteal MSC. MSC are required for HSC maintenance and quiescence,6 and there is evidence that MSC function may be degraded in the context of RA.13,33 Our data suggest there are mechanism(s) that may limit HSC proliferation in response to ‘emergency’ inflammatory signals and/or BM niche remodeling. Indeed, HSC from CIA mice activated a proliferation arrest gene program characterized by downregulation of genes including Myc and Ccnd1/2, alongside upregulation of Cdkn1a, Cdkn1b and Cdkn1c. Likewise, MYC and CCND2 are downregulated in CD34+ hematopoietic stem and progenitor cells from human RA patients.39 Such a program could protect HSC pool integrity by increasing the threshold necessary for HSC cell cycle entry, thereby compensating for impaired BM niche function and/or elevated levels of pro-inflammatory cytokines. The functional significance of this gene program could be further addressed by determining whether HSC from CIA mice fail to proliferate in response to a concurrent inflammatory stimulus. Future studies could also address the functional significance of disease-induced changes in key BM niche populations such as MSC in hematopoiesis.
Interestingly, these results are distinct from the increased HSC cell cycle activity and HSC depletion observed in a model of chronic M. avium infection.40 The difference could be due to ongoing consumption of mature immune cells (particularly granulocytes) during the active infection that drives continuous HSC proliferation. Notably, serum levels of IFN-γ in mice infected with M. avium are also over 20fold higher than those in our mice with CIA, which may be sufficient to activate HSC and overwhelm quiescenceenforcing mechanisms. While we found elevated serum IFN-γ levels in CIA mice, these did not translate into activation of IFN target genes in HSC, suggesting that either the IFN-γ level was not sufficiently high to activate HSC, or or HSC had become refractory to such signals. Altogether, our data suggest that chronic inflammation could actually induce protection of the HSC pool (at least up to a point) by activating stress response mechanisms that maintain quiescence. Such a model is in line with an emerging body of work showing that pro-inflammatory signaling can play a positive role in hematopoiesis.8 This model could explain the rarity of BM failure in RA patients despite some increased risk of myelodysplastic syndrome,41 as well as the rarity of graft failure in RA patients undergoing autologous BM transplantation.42
While concurrent activation of myeloid differentiation and proliferation arrest gene programs in HSC seems paradoxical, cell cycle arrest is crucial for myeloid differentiation by promoting accumulation of factors needed for differentiation.43 Myeloid transcription factors, including PU.1, promote cell cycle arrest via induction of cell cycle inhibitors such as Cdkn1a while repressing cell cycle activators like Ccnd1.43,44 Thus, induction of myeloid gene programs could serve a dual purpose during chronic inflammation: protecting the HSC pool from excess proliferation while promoting myeloid differentiation of actively cycling hematopoietic progenitor cells. In this way, HSC may participate minimally in day-to-day blood system maintenance45 during chronic inflammation, with occasional HSC divisions nonetheless leading to preferential myeloid progenitor production. On the other hand, telomere attrition has been identified in CD34+ hematopoietic stem and progenitor cells from human RA patients.46 RA is typified by years of ongoing disease, including ‘flares’ and therapies which could induce premature replicative ‘aging’ in the HSC compartment.11 Further analyses should clarify the extent to which HSC are affected by telomere attrition and whether such effects are due to increased proliferation versus impaired telomere maintenance. Divisional tracing approaches, such as H2B-GFP labeling47 in mouse models including the CIA model, could therefore provide valuable insights into the long-term impact of inflammatory disease on HSC proliferation.
Cytokine blockade therapies, including anakinra, have been used for over a decade to treat chronic inflammatory disorders, such as RA.5 Anakinra can correct aberrant blood parameters in RA patients.29 However, the impact of cytokine blockade on hematopoiesis in the BM has not been closely studied. Here, we show that anakinra treatment reduces myeloid progenitor expansion in mice with CIA, characterized by partial reduction of MPP3 and GMP. It should be noted that MPP3 is not a transient population; clonal analyses and transplantation assays have shown that MPP3 can persist in mice and continue to produce GMP for weeks if not months. Hence, it is possible that the impact of cytokine blockade on hematopoiesis is gradual, with further reduction in MPP3 and GMP numbers as these populations turn over and are not replaced due to reduced activity of ‘emergency’ differentiation pathways in HSC. Indeed, anakinra reduced activation of myeloid and proliferation arrest gene programs activated in HSC from CIA mice. This suggests that HSC molecular responses to inflammation are reversible and may be regulated in proportion to the level of inflammatory signaling and/or BM remodeling in the individual.
Our data are agnostic as to whether anakinra acts directly on HSC, or orthogonally by reducing inflammation at the joints, which in turn translates into less systemic inflammation. Indeed, we did not detect increased IL-1 levels in the serum of CIA mice, consistent with studies in human patients indicating that IL-1 production is often localized to the joint synovia.28 On the other hand, we found increased Il1r1 expression in HSC from CIA mice, suggesting that HSC could be sensitized to tonic IL-1 production in the BM, even if IL-1 levels themselves do not increase. Along these lines, anakinra treatment reduces expression of IL1R1 and other IL-1 target genes in blood cells from breast cancer patients.48 Hence, anakinra could also contribute to restored HSC function by breaking a feedback loop between IL-1 and Il1r1 expression in HSC from CIA mice. Future studies could determine the extent to which anakinra reduces the effects of inflammation on HSC via direct versus indirect mechanisms such as reduction in downstream cytokine production. Notably, other cytokine blocking drugs, such as the TNF inhibitor etanercept, also alleviate the symptoms of inflammatory arthritis in RA patients.49 It is therefore possible that blockade of other cytokines elicits a similar restoration of hematopoiesis. In addition, blockade of multiple inflammatory cytokines may further normalize hematopoiesis in RA and other diseases, although such interventions could also increase the risk of infection or cytopenia in patients.50 Taken together, our findings show that pro-inflammatory cytokine blocking treatment can reverse inflammatory-induced changes in hematopoiesis and HSC gene regulation, with the degree of impact likely related to the duration of treatment and/or the extent to which inflammatory arthritis is alleviated in the patient. Our data thus provide further rationale for the use of anticytokine therapies to redirect HSC fate and restore normal hematopoiesis in patients with RA and, potentially, other chronic inflammatory diseases.
Acknowledgments
The authors would like to thank Phillip Bendele, Shannon Spiegel, Kyle Rothermel and Connor Ohlsen for expert technical assistance. This work was supported by K01 DK098315, R01 DK119394 (to EMP), the Cleo Meador and George Ryland Scott Chair of Medicine in Hematology (to EMP), the Boettcher Foundation Webb-Waring Early Career Investigator Award (to EMP and KAK), F31 HL138754 (to JLR), an NIH Institutional National Service Award 2T32 AI07449 (to CJF), a National Science Foundation Predoctoral Research Fellowship (to TSM), R01 AI098417 (to JRH), and R01 AR51749 (to VMH). This work was also supported in part by the hniversity of Colorado Cancer Center Flow Cytometry Shared Resource, funded by NCI grant P30 CA046934.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/105/3/585
References
- 1.Myasoedova E, Crowson CS, Kremers HM, Therneau TM, Gabriel SE. Is the incidence of rheumatoid arthritis rising?: results from Olmsted County, Minnesota, 1955-2007. Arthritis Rheum. 2010;62 (6):1576–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Birnbaum H, Pike C, Kaufman R, Marynchenko M, Kidolezi Y, Cifaldi M. Societal cost of rheumatoid arthritis patients in the US. Curr Med Res Opin. 2010;26 (1):77–90. [DOI] [PubMed] [Google Scholar]
- 3.Papadaki G, Kambas K, Choulaki C, et al. Neutrophil extracellular traps exacerbate Th1-mediated autoimmune responses in rheumatoid arthritis by promoting DC maturation. Eur J Immunol. 2016;46 (11):2542–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Firestein GS, Malnnes IB. Immunopathogenesis of rheumatoid arthritis. Immunity. 2017;46 (2):183–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dinarello CA. Anti-inflammatory agents: present and future. Cell. 2010;140 (6):935–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505 (7483):327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pietras EM, Reynaud D, Kang Y-A, et al. Functionally distinct subsets of lineage-biased multipotent progenitors control blood production in normal and regenerative conditions. Cell Stem Cell. 2015;17 (1):35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pietras EM. Inflammation: a key regulator of hematopoietic stem cell fate in health and disease. Blood. 2017;130 (15):1693–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manz MG, Boettcher S. Emergency granulopoiesis. Nat Rev Immunol. 2014;14 (5):302–314. [DOI] [PubMed] [Google Scholar]
- 10.King KY, Goodell MA. Inflammatory modulation of HSCs: viewing the HSC as a foundation for the immune response. Nat Rev Immunol. 2011;11 (10):685–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colmegna I, Weyand CM. Haematopoietic stem and progenitor cells in rheumatoid arthritis. Rheumatology (Oxford). 2011;50 (2):252–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brand DD, Latham KA, Rosloniec EF. Collagen-induced arthritis. Nat Protoc. 2007;2 (5):1269–1275. [DOI] [PubMed] [Google Scholar]
- 13.Ma YD, Park C, Zhao H, et al. Defects in osteoblast function but no changes in longterm repopulating potential of hematopoietic stem cells in a mouse chronic inflammatory arthritis model. Blood. 2009;114 (20):4402–4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Papadaki HA, Kritikos HD, Valatas V, Boumpas DT, Eliopoulos GD. Anemia of chronic disease in rheumatoid arthritis is associated with increased apoptosis of bone marrow erythroid cells: improvement following anti-tumor necrosis factor-alpha antibody therapy. Blood. 2002;100 (2):474–482. [DOI] [PubMed] [Google Scholar]
- 15.Koetz K, Bryl E, Spickschen K, O’Fallon WM, Goronzy JJ, Weyand CM. T cell homeostasis in patients with rheumatoid arthritis. Proc Natl Acad Sci U S A. 2000; 97 (16):9203–9208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pietras EM, Lakshminarasimhan R, Techner J-M, et al. Re-entry into quiescence protects hematopoietic stem cells from the killing effect of chronic exposure to type I interferons. J Exp Med. 2014;211 (2):245–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haas S, Hansson J, Klimmeck D, et al. Inflammation-induced emergency megakaryopoiesis driven by hematopoietic stem cell-like megakaryocyte progenitors. Cell Stem Cell. 2015;17 (4):422–434. [DOI] [PubMed] [Google Scholar]
- 18.Gekas C, Graf T. CD41 expression marks myeloid-biased adult hematopoietic stem cells and increases with age. Blood. 2013;121 (22):4463–4472. [DOI] [PubMed] [Google Scholar]
- 19.Shaw AT, Gravallese EM. Mediators of inflammation and bone remodeling in rheumatic disease. Semin Cell Dev Biol. 2016; 49:2–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schepers K, Campbell TB, Passegué E. Normal and leukemic stem cell niches: insights and therapeutic opportunities. Cell Stem Cell. 2015;16 (3):254–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oduro KA, Liu F, Tan Q, Kim CK, et al. Myeloid skewing in murine autoimmune arthritis occurs in hematopoietic stem and primitive progenitor cells. Blood. 2012;120 (11):2203–2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Challen GA, Boles NC, Chambers SM, Goodell MA. Distinct hematopoietic stem cell subtypes are differentially regulated by TGF-1. Cell Stem Cell. 2010;6 (3):265–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen C, Liu Y, Liu Y, Zheng P. The axis of mTOR-mitochondria-ROS and stemness of the hematopoietic stem cells. Cell Cycle. 2009;8 (8):1158–1160. [DOI] [PubMed] [Google Scholar]
- 24.Ludin A, Gur-Cohen S, Golan K, et al. Reactive oxygen species regulate hematopoietic stem cell self-renewal, migration and development, as well as their bone marrow microenvironment. Antioxid Redox Signal. 2014;21 (11):1605–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pietras EM, Warr MR, Passegué E. Cell cycle regulation in hematopoietic stem cells. J Cell Biol. 2011;195 (5):709–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilson A, Murphy MJ, Oskarsson T, et al. c-Myc controls the balance between hematopoietic stem cell self-renewal and differentiation. Genes Dev. 2004;18 (22): 2747–2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4 (1):44–57. [DOI] [PubMed] [Google Scholar]
- 28.Rooney M, Symons JA, Duff GW. Interleukin 1 beta in synovial fluid is related to local disease activity in rheumatoid arthritis. Rheumatol Int. 1990;10 (5):217–219. [DOI] [PubMed] [Google Scholar]
- 29.Karanikolas G, Charalambopoulos D, Vaiopoulos G, et al. Adjunctive anakinra in patients with active rheumatoid arthritis despite methotrexate, or leflunomide, or cyclosporin-A monotherapy: a 48-week, comparative, prospective study. Rheumatology (Oxford). 2008;47 (9):1384–1388. [DOI] [PubMed] [Google Scholar]
- 30.Joosten LA, Helsen MM, Saxne T, van De Loo FA, Heinegard D, van Den Berg WB. IL-1 alpha beta blockade prevents cartilage and bone destruction in murine type II collagen-induced arthritis, whereas TNF-alpha blockade only ameliorates joint inflammation. J Immunol. 1999;163 (9):5049–5055. [PubMed] [Google Scholar]
- 31.Myers LK, Miyahara H, Terato K, Seyer JM, Stuart JM, Kang AH. Collagen-induced arthritis in B10.RIII mice (H-2r): identification of an arthritogenic T-cell determinant. Immunology. 1995;84(4):509–513. [PMC free article] [PubMed] [Google Scholar]
- 32.van Den Berg WB, Joosten LA, van De Loo FA. TNF alpha and IL-1 beta are separate targets in chronic arthritis. Clin Exp Rheumatol. 1999;17 (6 Suppl 18):S105–114. [PubMed] [Google Scholar]
- 33.Papadaki HA, Marsh JCW, Eliopoulos GD. Bone marrow stem cells and stromal cells in autoimmune cytopenias. Leuk Lymphoma. 2002;43 (4):753–760. [DOI] [PubMed] [Google Scholar]
- 34.Takizawa H, Fritsch K, Kovtonyuk LV, et al. Pathogen-induced TLR4-TRIF innate immune signaling in hematopoietic stem cells promotes proliferation but reduces competitive fitness. Cell Stem Cell. 2017;21 (2):225–225. [DOI] [PubMed] [Google Scholar]
- 35.Pietras EM, Mirantes-Barbeito C, Fong S, et al. Chronic interleukin-1 exposure drives haematopoietic stem cells towards precocious myeloid differentiation at the expense of self-renewal. Nat Cell Biol. 2016;18 (6): 607–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hirai H, Zhang P, Dayaram T, et al. C/EBPbeta is required for “emergency” granulopoiesis. Nat Immunol. 2006;7 (7):732–739. [DOI] [PubMed] [Google Scholar]
- 37.Libregts SF, Gutiérrez L, de Bruin AM, et al. Chronic IFN-production in mice induces anemia by reducing erythrocyte life span and inhibiting erythropoiesis through an IRF-1/PU.1 axis. Blood. 2011;118 (9):2578–2588. [DOI] [PubMed] [Google Scholar]
- 38.Antony-Debré I, Paul A, Leite J, et al. Pharmacological inhibition of the transcription factor PU.1 in leukemia. J Clin Invest. 2017;127 (12):4297–4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Colmegna I, Pryshchep S, Oishi H, Goronzy JJ, Weyand CM. Dampened ERK signaling in hematopoietic progenitor cells in rheumatoid arthritis. Clin Immunol. 2012;143 (1):73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matatall KA, Jeong M, Chen S, et al. Chronic infection depletes hematopoietic stem cells through stress-induced terminal differentiation. Cell Rep. 2016;17 (10):2584–2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wolach O, Stone R. Autoimmunity and inflammation in myelodysplastic syndromes. Acta Haematol. 2016;136 (2):108–117. [DOI] [PubMed] [Google Scholar]
- 42.Bingham SJ, Snowden J, McGonagle D, et al. Autologous stem cell transplantation for rheumatoid arthritis–interim report of 6 patients. J Rheumatol Suppl. 2001;64:21–24. [PubMed] [Google Scholar]
- 43.Kueh HY, Champhekar A, Champhekhar A, Nutt SL, Elowitz MB, Rothenberg EV. Positive feedback between PU.1 and the cell cycle controls myeloid differentiation. Science. 2013;341 (6146):670–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Staber PB, Zhang P, Ye M, Welner RS, et al. Sustained PU.1 levels balance cell-cycle regulators to prevent exhaustion of adult hematopoietic stem cells. Mol Cell. 2013;49 (5):934–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun J, Ramos A, Chapman B, Johnnidis JB, Le L, Ho Y-J, et al. Clonal dynamics of native haematopoiesis. Nature. 2014;514 (7522): 322–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Colmegna I, Diaz-Borjon A, Fujii H, Schaefer L, Goronzy JJ, Weyand CM. Defective proliferative capacity and accelerated telomeric loss of hematopoietic progenitor cells in rheumatoid arthritis. Arthritis Rheum. 2008;58 (4):990–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Foudi A, Hochedlinger K, Van Buren D, et al. Analysis of histone 2B-GFP retention reveals slowly cycling hematopoietic stem cells. Nat Biotechnol. 2008;27 (1):84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu T-C, Xu K, Martinek J, et al. IL1 receptor antagonist controls transcriptional signature of inflammation in patients with metastatic breast cancer. Cancer Res. 2018;78 (18):5243–5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brennan FM, Mcinnes IB. Evidence that cytokines play a role in rheumatoid arthritis. J Clin Invest. 2008;118(11):3537–3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ridker PM, MacFadyen JG, Thuren T, et al. Effect of interleukin-1 inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390 (10105):1833–1842. [DOI] [PubMed] [Google Scholar]