Abstract
Leukemia stem cells (LSC) are more resistant to standard chemotherapy and their persistence during remission can cause relapse, which is still one of the major clinical challenges in the treatment of acute myeloid leukemia (AML). A better understanding of the mutational patterns and the prognostic impact of molecular markers associated with stemness could lead to better clinical management and improve patients’ outcomes. We applied a previously described 17-gene expression score comprising genes differently expressed between LSC and leukemic bulk blasts, for 934 adult patients with de novo AML, and studied associations of the 17-gene LSC score with clinical data and mutation status of 81 genes recurrently mutated in cancer and leukemia. We found that patients with a high 17-gene score were older and had more mutations. The 17-gene score was found to have a prognostic impact in both younger (aged <60 years) and older (aged ≥60 years) patients with AML. We also analyzed the 17-gene LSC score in the context of the 2017 European LeukemiaNet genetic-risk classification and found that for younger patients the score refined the classification, and identified patients currently classified in the European LeukemiaNet Favorable-risk category who had a worse outcome.
Introduction
Acute myeloid leukemia (AML) is a heterogeneous disease.1–3 Although many advances have been made in understanding the biology and treatment of AML, the long-term survival rates are still only ~40% for younger adults (aged <60 years), and ~10-15% for older patients (aged ≥60 years).1–3 One major clinical challenge impeding improved outcome is relapse following achievement of complete remission (CR). It is hypothesized that relapse occurs because of the persistence of leukemia stem cells (LSC) and subsequent outgrowth of the leukemia clone.4–8 Studies on the clinical relevance of LSC are still rare because no LSC-specific phenotype has been firmly established. Although the percentage of CD34+/CD38--expressing cells, which were initially assumed to include all LSC, was shown to affect prognosis,9,10 the use of more permissive immunodeficient mouse models revealed that LSC can also be found in the CD34+/CD38+ and CD34− compartments.4,7,9,11–15 Instead of using surface markers to identify and quantify the presence of LSC, in 2011, Eppert et al.4 described a LSC-related gene-expression signature comprising 44 genes that were deregulated in LSC. The derived stem cell-like signature was shown to associate with inferior outcome in adult patients with cytogenetically normal AML.4,16 Recently, Ng et al.7 used a similar approach to generate a LSC-derived gene-expression signature consisting of 17 genes that also associated with inferior outcome. However, it is still not fully determined whether this signature is associated with clinical characteristics and gene mutations. Moreover, the prognostic value of the 17-gene LSC score in the context of other, well-established risk classifications, for example the one by the European LeukemiaNet (ELN)1, has not, to our knowledge, been assessed.
We, therefore, derived the 17-gene score from a set of samples from adults with AML and determined associations between the signature and known prognosticators1,17,18 as well as mutational data of 81 cancer- and leukemia-associated genes.19 Moreover, we validated the prognostic impact of the 17-gene signature alone and in the context of the 2017 ELN genetic-risk classification.1
Methods
Patients and treatment
We investigated 934 adult patients with de novo AML (other than acute promyelocytic leukemia), for whom material for molecular analyses was available. Availability of material for analysis was the only criterion for inclusion in our study – we did not select AML patients based on their age, ELN risk group, specific clinical trial they were enrolled onto, etc. Because of differences in the treatment protocols between younger and older patients, we performed outcome analyses separately for these two groups of patients. Within each age group, patients were treated similarly, receiving a cytarabine/anthracycline-based induction on Cancer and Leukemia Group B (CALGB) trials.20–34 No patient received an allogeneic stem cell transplant in first CR. Details of CALGB treatment protocols are provided in the Online Supplementary Appendix and Online Supplementary Table S1. There were no significant differences in CR rates, disease-free survival (DFS) or overall survival (OS) for younger patients enrolled onto CALGB 8525, 9222, 9621, 10503, 10603 and 19808 treatment trials (Online Supplementary Table S2) nor were there any significant differences in CR rates, DFS or OS among older patients enrolled onto CALBG 9420, 9720, 10201 and 10502 trials (Online Supplementary Table S3). CALGB is now part of the Alliance for Clinical Trials in Oncology (Alliance). All patients were enrolled on CALGB 8461 (cytogenetic studies), CALGB 9665 (leukemia tissue bank) and CALGB 20202 (molecular studies) companion protocols. Patients provided written informed consent, and study protocols were in accordance with the Declaration of Helsinki and approved by Institutional Review Boards.
Transcriptome analyses and calculation of the 17-gene leukemia stem cell score
Pretreatment bone marrow and/or blood samples containing ≥20% leukemic blasts were obtained from all patients and mononuclear cells were enriched through Ficoll-Hypaque gradient centrifugation and cryopreserved until use. Total RNA was extracted from patients’ samples using the TRIzol method according to the manufacturer’s protocol and used for RNA-sequencing analyses (see also the Online Supplementary Appendix). RNA-sequencing libraries were prepared using the Illumina (San Diego, CA, USA) TruSeq Stranded Total RNA Sample Prep Kit with Ribo-Zero Gold (n. RS1222201) according to the manufacturer’s instructions. Sequencing was performed with Illumina HiSeq systems using the HiSeq version 3 sequencing reagents to an approximate cluster density of 800,000/mm2. Image analysis, base calling, error estimation, and quality thresholds were performed using HiSeq Controller software (version 2.2.38) and Real Time Analyzer software (version 1.18.64). Transcript abundance was quantified from the RNA-sequencing data using kallisto,35 with a reference transcriptome consisting of Homo sapiens GRCh38 protein-encoding and non-coding transcripts except rRNA; the strand-specific option of “first read reverse” was chosen. Abundance values are represented in transcripts per million.
The 17-gene LSC score was derived similarly to that in the publication by Ng et al.7 using RNA-sequencing data and the same weights that were published initially for a microarray platform.7 Briefly, the 17-gene LSC score was calculated as the weighted sum of the normalized expression values of the 17 genes included in the signature: 17-gene LSC score = (DNMT3B × 0.0874) + (ZBTB46 × −0.0347) + (NYNRIN × 0.00865) + (ARHGAP22 × −0.0138) + (LAPTM4B × 0.00582) + (MMRN1 × 0.0258) + (DPYSL3 × 0.0284) + (KIAA0125 × 0.0196) + (CDK6 × −0.0704) + (CPXM1 × −0.0258) + (SOCS2 × 0.0271) + (SMIM24 × −0.0226) + (EMP1 × 0.0146) + (NGFRAP1 × 0.0465) + (CD34 × 0.0338) + (AKR1C3 × −0.0402) + (GPR56 × 0.0501).7 The derived scores were used to divide patients into two groups using the median as the cutoff: a group with a high score (17-genehigh) and a group with a low score (17-genelow).
Cytogenetic and molecular analyses
Details of the cytogenetic and molecular analyses are provided in the Online Supplementary Appendix.
Results
Clinical and cytogenetic characteristics associated with the 17-gene leukemia stem cell score
Pretreatment characteristics of the 934 patients are shown in Table 1. For all patients, we determined the 17-gene LSC score, which indicates a stem cell-like gene-expression profile, and separated them into 17-genelow and 17-genehigh groups using the median. Comparison between patients with a 17-genelow and 17-genehigh score showed that the former were younger at diagnosis (median: 46 vs. 53 years; P<0.001) and had lower platelet counts (median: 50 vs. 63x109/L; P<0.001). Cytogenetically, there was no difference in the frequency of the presence of cytogenetically normal AML between the groups. Among cytogenetically abnormal patients, those with a 17-genelow score more frequently had core-binding factor AML (CBF-AML; P<0.001), including all patients with t(8;21)(q22;q22) and 88% with inv(16)(p13q22) or t(16;16)(p13;q22). On the other hand, the group with a 17-genehigh score included all patients with inv(3)(q21q26) or t(3;3)(q21;q26) and contained more patients with a complex karyotype than in the 17-genelow group (P<0.001). Most patients with a complex karyotype in the 17-genehigh group had a typical complex karyotype (i.e., complex karyotype with unbalanced chromosome abnormalities leading to loss of material from 5q, 7q and/or 17p), whereas an atypical complex karyotype (i.e., complex karyotype without 5q, 7q and/or 17p abnormalities)36 was found with a higher frequency among 17-genelow patients.
Table 1.
Comparison of pretreatment clinical and cytogenetic characteristics in 934 patients with acute myeloid leukemia according to low and high 17-gene leukemia stem cell scores.
Mutational landscape associated with the 17-gene leukemia stem cell score
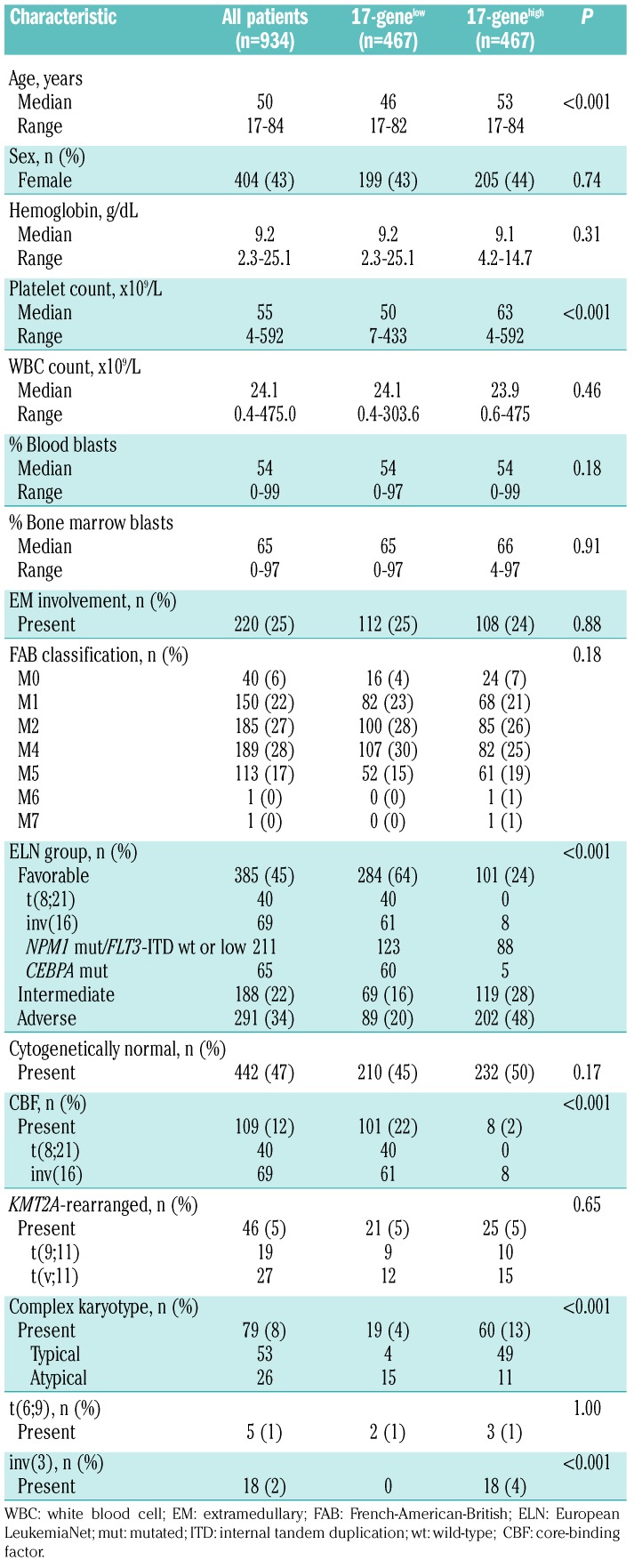
To obtain more detailed insights into the mutational patterns associated with the 17-gene LSC signature, we analyzed 81 cancer and leukemia-associated genes.19 We found that 77 genes were mutated in at least one patient (Online Supplementary Table S4). Patients with a 17-genelow score had fewer mutations compared with patients with a 17-genehigh score (median: 2 vs. 3; P<0.001). Moreover, 12 gene mutations occurred at significantly different frequencies between patients with 17-genelow and 17-genehigh scores (Figure 1). Biallelic CEBPA (P<0.001), GATA2 (P=0.008), and KIT (P<0.001) mutations were more frequent in the 17-genelow group of patients (Figure 1A). In contrast, patients with a 17-genehigh score more frequently harbored mutations in ASXL1 (P=0.001), DNMT3A (P<0.001), KMT2A (P=0.04), RUNX1 (P=0.002), SRSF2 (P=0.02), STAG2 (P=0.009), TET2 (P=0.008) and TP53 (P<0.001) genes. Additionally, FLT3-internal tandem duplications were more frequent in these patients than in those with a 17-genelow score (P<0.001) (Figure 1B and Online Supplementary Table S4), as previously described by Ng et al.7
Figure 1.
Differences in the frequencies of gene mutations between patients with low and those with high 17-gene leukemic stem cell scores. Mutations that had a significantly higher frequency in the (A) 17-genelow or (B) 17-genehigh group.
Outcome associated with the 17-gene leukemia stem cell score
All patients were assigned to the 17-genelow and 17-genehigh groups based on the median of the initial analysis of the entire cohort of patients. We kept this initial grouping for all additional sub-analyses that could have potentially reassigned some patients into different 17-gene score groups. This led to differences in the sizes of the 17-genelow and 17-genehigh score groups in younger and older patients.
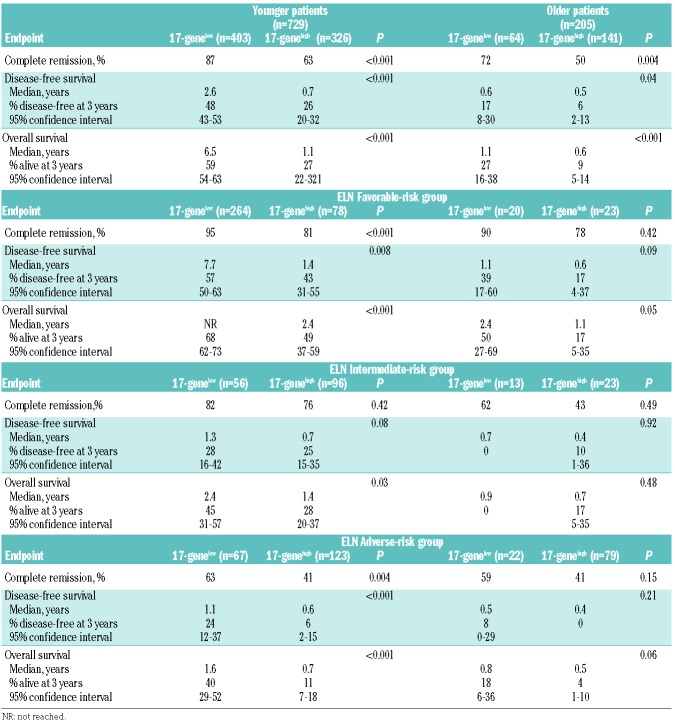
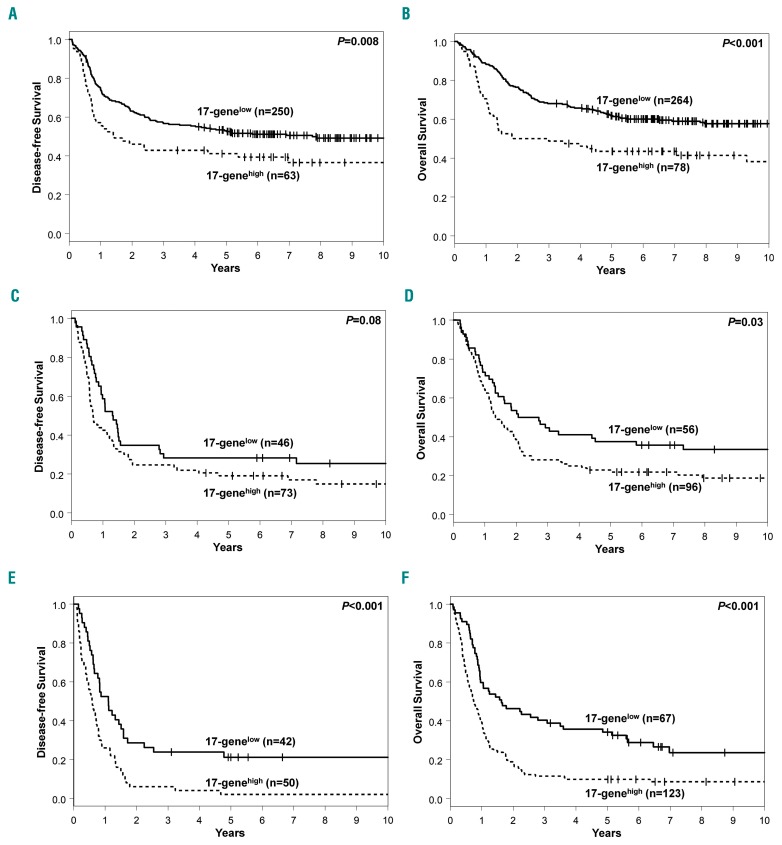
Similarly to Ng et al.,7 we found that the 17-gene LSC score was strongly associated with outcome in both the younger (Table 2; Figure 2A, B) and older (Table 2; Figure 2C, D) cohorts of patients. Among younger patients, those with a 17-genelow score had higher CR rates (P<0.001) (Table 2) and longer DFS (P<0.001) (Figure 2A) and OS (P<0.001) (Figure 2B). Similar results were found in older patients: CR rates (P=0.004) (Table 2), DFS (P=0.04), (Figure 2C) and OS (P<0.001) (Figure 2D).
Table 2.
Comparison of outcomes according to the 17-gene leukemic stem cell score in younger adults (aged <60 years) and older adults (aged ≥60 years) with acute myeloid leukemia.
Figure 2.
Differences in outcome between patients with low and those with high 17-gene leukemic stem cell scores. (A) Disease-free survival (DFS) and (B) overall survival (OS) of younger adult patients (aged <60 years) according to the 17-gene leukemia stem cell (LSC) score. (C) DFS and (D) OS of older patients (aged ≥60 years) according to the 17-gene LSC score.
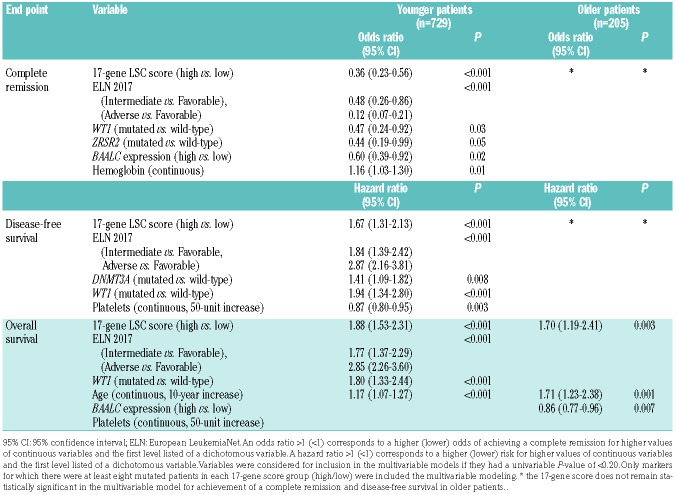
Next, we tested the prognostic impact of the 17-gene LSC score in multivariable analyses (Table 3). In younger patients, the 17-gene LSC score remained prognostically significant for all clinical endpoints, namely CR, DFS, and OS. In older patients, the score was prognostically significant only for OS, but it was not significant in the final models for achievement of a CR or DFS (Table 3).
Table 3.
Multivariable models for outcome evaluating the 17-gene leukemia stem cell score and known prognosticators in younger (aged <60 years) and older (aged ≥60 years) adults with acute myeloid leukemia.\
Prognostic impact of the 17-gene leukemia stem cell score in the context of the current European LeukemiaNet classification
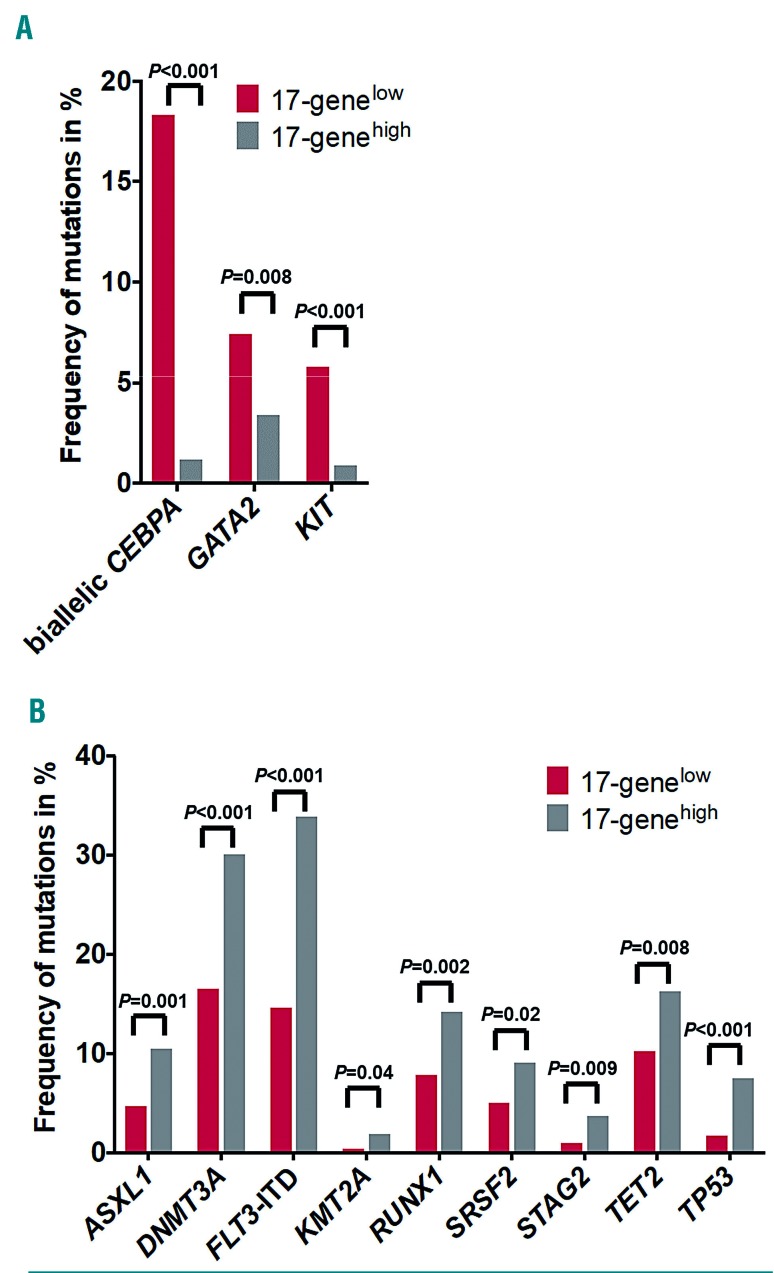
To test the prognostic value of the 17-gene LSC score in the context of the current 2017 ELN classification, we classified all patients according to the published guidelines into ELN Favorable-, Intermediate- and Adverse-risk groups.1 In both age cohorts, we found significant differences in the ELN risk-group distribution between patients with 17-genelow and 17-genehigh scores (P<0.001 for younger and P=0.009 for older patients). Among the younger patients, two-thirds with a 17-genelow score were classified as having Favorable-risk, whereas 14% and 17% were classified as having, respectively, Intermediate- and Adverse-risk. On the other hand, younger patients with a 17-genehigh score were most frequently classified in the Adverse-risk group (41%), followed by the Intermediate-(32%) and Favorable-risk (26%) groups. Among the older patients, the majority in both the 17-genelow and 17-genehigh score groups were classified in the Adverse-risk group (40% and 63%, respectively) (Online Supplementary Table S5), followed by Favorable- (36%) and Intermediate-(24%) risk groups in the 17-genelow group and by equal numbers for Favorable-risk (18%) and Intermediate-risk (18%) groups in the 17-genehigh group.
Next, we tested whether the 17-gene LSC score can be used to refine the prognostic impact of the ELN classification. Among younger patients, we found that the 17-gene LSC score could refine the ELN classification for the ELN Favorable- and Adverse-risk groups, but not for the Intermediate-risk group. Younger patients with a 17-genelow score in the ELN Favorable-risk group had higher CR rates (P<0.001) (Table 2) and longer DFS (P=0.008) (Figure 3A) and OS (P<0.001) (Figure 3B) than the 17-genehigh patients. Likewise, younger Adverse-risk patients with a 17-genelow score had higher CR rates (P=0.004) (Table 2), and longer DFS (P<0.001) (Figure 3E) and OS (P<0.001) (Figure 3F). On the other hand, among younger patients in the Intermediate-risk group, there was no significant difference in CR rates (Table 2) or DFS (P=0.08) (Figure 3C), but those with a 17-genelow score had longer OS (P=0.03) (Figure 3D).
Figure 3.
Differences in outcome between younger adult patients (aged <60 years) with low and those with high 17-gene leukemic stem cell scores in the context of the current European LeukemiaNet 2017 classification. (A) Disease-free survival (DFS) and (B) overall survival (OS) of younger patients within the European LeukemiaNet (ELN) Favorable-risk group according to the 17-gene leukemia stem cell (LSC) score. (C) DFS and (D) OS of younger patients within the ELN Intermediate-risk group according to the 17-gene LSC score. (E) DFS and (F) OS of younger patients within the ELN Adverse-risk group according to the 17-gene LSC score.
Among older patients, the 17-gene score had almost no impact on outcome after classifying the patients according to the ELN recommendations. We found that only older patients in the Favorable-risk group with a 17-genelow score had longer OS than those with a 17-genehigh score (P=0.05) (Online Supplementary Figure S1, Table 2). The 17-gene score showed no prognostic impact in the Adverse- and Intermediate-risk groups.
Discussion
The prognosis of AML patients is still poor, and relapse after achieving a CR is a major clinical challenge.1–4,7 It is thought that leukemia relapse is caused by the persistence of LSC.4–7 A better understanding of LSC and their prognostic impact in AML is necessary in order to improve patients’ outcomes. However, the lack of a well-established phenotype has thus far impeded studies on the clinical relevance of LSC frequency. Ng et al.7 recently developed a gene-expression signature, consisting of 17 genes that were found to be deregulated in LSC, to quantify the presence of LSC. They showed that the 17-gene LSC signature has a prognostic impact. In our study, we not only validated these data in an independent set of 934 adult patients with de novo AML, but also analyzed the prognostic impact of the 17-gene LSC score in the context of the current ELN classification. Moreover, we describe a detailed mutational landscape associated with the 17-gene LSC score.
Whereas the 17-gene score was initially derived using microarray data, Ng et al.7 showed in a relatively small set of patients (n=169) that RNA-sequencing data can also be used to derive the score. We validated this finding in a larger set of 934 AML patients with RNA-sequencing data using the same published weights of the score as Ng et al.,7 and demonstrated the robustness of the 17-gene score. We assigned the score to each patient and classified them into a 17-genehigh or 17-genelow LSC group for all further analyses, using the median as the cut point.
Clinically, we found that patients with a 17-genelow score were younger and had lower platelet counts at diagnosis. Similar differences in age were also described by Ng et al.7 Next, we compared cytogenetic findings between the groups of patients with 17-genelow and 17-genehigh scores and although we did not find any difference in the incidence of cytogenetically normal AML, there was a different distribution of the specific cytogenetic abnormalities between the groups. Patients with CBF-AML were much more frequently classified in the 17-genelow score group, especially those with a t(8;21)(q22;q22) who were never found to have a 17-genehigh score. As previously reported, patients with CBF-AML had a relatively favorable outcome compared with patients belonging to other cytogenetic subgroups.37–41 On the other hand, patients in the 17-genehigh score group more frequently carried cytogenetic abnormalities associated with adverse outcome, such as a complex karyotype, especially a typical complex karyotype,36 and inv(3)(q21q26) or t(3;3)(q21;q26).1,17,36,40–42 Of note, patients with inv(3) or t(3;3) were classified exclusively in the 17-genehigh score group.
Next, we looked for differences in the mutational patterns of 81 cancer- and leukemia-associated genes19 between 17-genelow and 17-genehigh score patients. Patients with a 17-genelow score had a lower median number of mutations and only three genes, namely, GATA2, CEBPA and KIT, were found to be mutated more frequently in this group. GATA2 mutations and biallelic CEBPA mutations are known to co-occur,43 and the higher incidence of KIT mutations in 17-genelow patients can be at least in part explained by the elevated frequency of CBF-AML in this group, since KIT mutations are associated with CBF-AML.37 Whereas both biallelic CEBPA mutations and GATA2 mutations, which occurred frequently in the 17-genelow score group, are associated with a favorable outcome, mutations associated with adverse outcome, such as those in the RUNX1, ASXL1, and TP53 genes,1,2,36,44–50 were more frequently found in patients with a 17-genehigh score.
We were also interested in characterizing further the prognostic significance of the 17-gene LSC score established by Ng et al.7 We not only validated its prognostic impact in a larger independent cohort of patients, but also asked the question whether the 17-gene LSC score could refine the well-established 2017 ELN classification.1 This is especially of interest because it appears that some patients classified as ELN Favorable-risk still have a poor outcome. These patients might benefit from other treatment options.1 When we classified the patients according to the ELN guidelines, we found significant differences in the distribution of patients with 17-genelow and 17-genehigh scores among specific ELN risk groups. In younger patients, the majority of 17-genelow score patients were classified as having Favorable-risk, whereas most patients in the 17-genehigh score group were in the Adverse-risk group.
With regard to clinical outcome, we found that the 17-gene LSC score is capable of refining the ELN classification in younger patients. In the Favorable-risk group, application of the 17-gene LSC score led to the identification of approximately 20% of patients with a 17-genehigh score who had a worse outcome than patients with a 17-genelow score. Prospective studies are needed to test whether these 17-genehigh score patients might benefit from different induction. A similar ability of the 17-gene LSC score to identify patients with different outcomes was shown for the Adverse-risk group, despite the fact that the outcome of patients in this group is in general poor. The usefulness of the 17-gene LSC score in the ELN Intermediate-risk group seems to be limited, with patients with a 17-genelow score having a better OS but not better CR rates or DFS. Likewise, the 17-gene LSC score could not improve the ELN classification in older AML patients, who are known to have a generally poor prognosis.1–3
In summary, we found that the 17-gene LSC score is associated with distinct clinical and molecular features. Moreover, we not only validated the prognostic impact of the 17-gene LSC score but also showed for the first time that the score can refine the current 2017 ELN classification, at least in younger patients. This is important because the 17-gene LSC score is associated with well-established prognostic markers that are included in the ELN guidelines. Prospective studies are needed to determine best treatment options for patients currently classified as having Favorable-risk who are identified to have a worse prognosis by the use of the 17-gene LSC score.
Acknowledgments
The authors would like to thank the patients who consented to participate in these clinical trials and the families who supported them. Our thanks also to Donna Bucci and Christopher Manring, the CALGB/Alliance Leukemia Tissue Bank at The Ohio State University Comprehensive Cancer Center, Columbus, OH, USA for sample processing and storage services and Lisa J. Sterling and Christine Finks for data management.
Footnotes
Funding
This work was supported in part by grants from the National Cancer Institute (Bethesda, MA, USA) [UG1CA233338, U10CA180821, U10CA180882 (to the Alliance), P30CA016058, P50CA140158, U10CA180850, U10CA180861, U10CA180866, U10CA180867, and U24CA196171]; the Leukemia Clinical Research Foundation; R35 CA198183 and the Warren D. Brown Foundation; and by an allocation of computing resources from The Ohio Supercomputer Center.
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/105/3/721
References
- 1.Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood.2017;129(4):424–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Döhner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med.2015;373(12):1136–1152. [DOI] [PubMed] [Google Scholar]
- 3.Estey EH. Acute myeloid leukemia: 2012 update on diagnosis, risk stratification, and management. Am J Hematol.2012;87(1):89–99. [DOI] [PubMed] [Google Scholar]
- 4.Eppert K, Takenaka K, Lechman ER, et al. Stem cell gene expression programs influence clinical outcome in human leukemia. Nat Med.2011;17(9):1086–1093. [DOI] [PubMed] [Google Scholar]
- 5.Dick JE. Stem cell concepts renew cancer research. Blood.2008;112(13):4793–4807. [DOI] [PubMed] [Google Scholar]
- 6.Dorrance AM, Neviani P, Ferenchak GJ, et al. Targeting leukemia stem cells in vivo with antagomiR-126 nanoparticles in acute myeloid leukemia. Leukemia.2015;29(11): 2143–2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ng SWK, Mitchell A, Kennedy JA, et al. A 17-gene stemness score for rapid determination of risk in acute leukaemia. Nature.2016;540(7633):433–437. [DOI] [PubMed] [Google Scholar]
- 8.Sagar J, Chaib B, Sales K, Winslet M, Seifalian A. Role of stem cells in cancer therapy and cancer stem cells: a review. Cancer Cell Int.2007;7:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jentzsch M, Bill M, Nicolet D, et al. Prognostic impact of the CD34+/CD38- cell burden in patients with acute myeloid leukemia receiving allogeneic stem cell transplantation. Am J Hematol.2017;92(4): 388–396. [DOI] [PubMed] [Google Scholar]
- 10.Wang L, Gao L, Xu S, et al. FISH+CD34+CD38- cells detected in newly diagnosed acute myeloid leukemia patients can predict the clinical outcome. J Hematol Oncol.2013;6(1):85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heidel FH, Mar BG, Armstrong SA. Self-renewal related signaling in myeloid leukemia stem cells. Int J Hematol.2011;94(2):109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guan Y, Gerhard B, Hogge DE. Detection, isolation, and stimulation of quiescent primitive leukemic progenitor cells from patients with acute myeloid leukemia (AML). Blood.2003;101(8):3142–3149. [DOI] [PubMed] [Google Scholar]
- 13.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med.1997;3(7):730–737. [DOI] [PubMed] [Google Scholar]
- 14.Sarry J-E, Murphy K, Perry R, et al. Human acute myelogenous leukemia stem cells are rare and heterogeneous when assayed in NOD/SCID/IL2Rγc-deficient mice. J Clin Invest.2011;121(1):384–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raffel S, Falcone M, Kneisel N, et al. BCAT1 restricts αKG levels in AML stem cells leading to IDHmut-like DNA hypermethylation. Nature.2017;551(7680):384–388. [DOI] [PubMed] [Google Scholar]
- 16.Metzeler KH, Maharry K, Kohlschmidt J, et al. A stem cell-like gene expression signature associates with inferior outcomes and a distinct microRNA expression profile in adults with primary cytogenetically normal acute myeloid leukemia. Leukemia.2013;27(10): 2023–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grimwade D, Mrózek K. Diagnostic and prognostic value of cytogenetics in acute myeloid leukemia. Hematol Oncol Clin North Am.2011;25(6):1135–1161. [DOI] [PubMed] [Google Scholar]
- 18.Eisfeld AK, Kohlschmidt J, Mrózek K, et al. Mutation patterns identify adult patients with de novo acute myeloid leukemia aged 60 years or older who respond favorably to standard chemotherapy: an analysis of Alliance studies. Leukemia.2018;32(6): 1338–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eisfeld A-K, Mrózek K, Kohlschmidt J, et al. The mutational oncoprint of recurrent cyto-genetic abnormalities in adult patients with de novo acute myeloid leukemia. Leukemia.2017;31(10):2211–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mayer RJ, Davis RB, Schiffer CA, et al. Intensive postremission chemotherapy in adults with acute myeloid leukemia. N Engl J Med.1994;331(14):896–903. [DOI] [PubMed] [Google Scholar]
- 21.Schiffer CA, Davis RB, Schulman P, et al. Intensive post remission therapy of acute myeloid leukemia (AML) with cytoxan/etoposide (CY/VP16) and diaza-quone/mitoxantrone (AZQ/MITO). Blood.1991;78(suppl):460 (abstract 1829). [Google Scholar]
- 22.Moore JO, Dodge RK, Amrein PC, et al. Granulocyte-colony stimulating factor (fil-grastim) accelerates granulocyte recovery after intensive postremission chemotherapy for acute myeloid leukemia with aziridinyl benzoquinone and mitoxantrone: Cancer and Leukemia Group B study 9022. Blood.1997;89(3):780–788. [PubMed] [Google Scholar]
- 23.Cassileth PA, Harrington DP, Appelbaum FR, et al. Chemotherapy compared with autologous or allogeneic bone marrow transplantation in the management of acute myeloid leukemia in first remission. N Engl J Med.1998;339(23):1649–1656. [DOI] [PubMed] [Google Scholar]
- 24.Moore JO, George SL, Dodge RK, et al. Sequential multiagent chemotherapy is not superior to high-dose cytarabine alone as postremission intensification therapy for acute myeloid leukemia in adults under 60 years of age: Cancer and Leukemia Group B study 9222. Blood.2005;105(9):3420–3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kolitz JE, George SL, Dodge RK, et al. Dose escalation studies of cytarabine, daunoru-bicin, and etoposide with and without mul-tidrug resistance modulation with PSC-833 in untreated adults with acute myeloid leukemia younger than 60 years: final induction results of Cancer and Leukemia Group B study 9621. J Clin Oncol.2004;22(21): 4290–4301. [DOI] [PubMed] [Google Scholar]
- 26.Marcucci G, Moser B, Blum W, et al. A phase III randomized trial of intensive induction and consolidation chemotherapy ± oblimersen, a pro-apoptotic Bcl-2 anti-sense oligonucleotide in untreated acute myeloid leukemia patients >60 years old. J Clin Oncol.2007;25(suppl):360s (abstract 7012). [Google Scholar]
- 27.Attar EC, Johnson JL, Amrein PC, et al. Bortezomib added to daunorubicin and cytarabine during induction therapy and to intermediate-dose cytarabine for consolidation in patients with previously untreated acute myeloid leukemia age 60 to 75 years: CALGB (Alliance) study 10502. J Clin Oncol.2013;31(7):923–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blum W, Sanford BL, Klisovic R, et al. Maintenance therapy with decitabine in younger adults with acute myeloid leukemia in first remission: a phase 2 Cancer and Leukemia Group B study (CALGB 10503). Leukemia.2017;31(1):34–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stone RM, Berg DT, George SL, et al. Granulocyte-macrophage colony-stimulating factor after initial chemotherapy for elderly patients with primary acute myeloge-nous leukemia. N Engl J Med.1995;332(25):1671–1677. [DOI] [PubMed] [Google Scholar]
- 30.Kolitz JE, George SL, Marcucci G, et al. P-glycoprotein inhibition using valspodar (PSC-833) does not improve outcomes for patients under age 60 years with newly diagnosed acute myeloid leukemia: Cancer and Leukemia Group B study 19808. Blood.2010;116(9):1413–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee EJ, George SL, Caligiuri M, et al. Parallel phase I studies of daunorubicin given with cytarabine and etoposide with or without the multidrug resistance modulator PSC-833 in previously untreated patients 60 years of age or older with acute myeloid leukemia: results of Cancer and Leukemia Group B study 9420. J Clin Oncol.1999;17(9):2831–2839. [DOI] [PubMed] [Google Scholar]
- 32.Baer MR, George SL, Caligiuri MA, et al. Low-dose interleukin-2 immunotherapy does not improve outcome of patients age 60 years and older with acute myeloid leukemia in first complete remission: Cancer and Leukemia Group B study 9720. J Clin Oncol.2008;26(30):4934–4939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uy GL, Mandrekar SJ, Laumann K, et al. A phase 2 study incorporating sorafenib into the chemotherapy for older adults with FLT3-mutated acute myeloid leukemia: CALGB 11001. Blood Adv. 2017;1(5):331–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roboz GJ, Mandrekar SJ, Desai P, et al. A randomized trial of 10 days of decitabine alone or with bortezomib in previously untreated older patients with acute myeloid leukemia: CALGB 11002 (Alliance). Blood Adv. 2018;2(24):3608–3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bray NL, Pimentel H, Melsted P, Pachter L. Near-optimal probabilistic RNA-seq quantification. Nat Biotechnol.2016;34(5):525–527. [DOI] [PubMed] [Google Scholar]
- 36.Mrózek K, Eisfeld A-K, Kohlschmidt J, et al. Complex karyotype in de novo acute myeloid leukemia: typical and atypical subtypes differ molecularly and clinically. Leukemia.2019;33(7):1620–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paschka P, Marcucci G, Ruppert AS, et al. Adverse prognostic significance of KIT mutations in adult acute myeloid leukemia with inv(16) and t(8;21): a Cancer and Leukemia Group B study. J Clin Oncol.2006;24(24):3904–3911. [DOI] [PubMed] [Google Scholar]
- 38.Bloomfield CD, Lawrence D, Byrd JC, et al. Frequency of prolonged remission duration after high-dose cytarabine intensification in acute myeloid leukemia varies by cytoge-netic subtype. Cancer Res.1998;58(18): 4173–4179. [PubMed] [Google Scholar]
- 39.Byrd JC, Dodge RK, Carroll A, et al. Patients with t(8;21)(q22;q22) and acute myeloid leukemia have superior failure-free and overall survival when repetitive cycles of high-dose cytarabine are administered. J Clin Oncol.1999;17(12):3767–3775. [DOI] [PubMed] [Google Scholar]
- 40.Byrd JC, Mrózek K, Dodge RK, et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461). Blood.2002;100(13):4325–4336. [DOI] [PubMed] [Google Scholar]
- 41.Grimwade D, Hills RK, Moorman AV, et al. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council Trials. Blood.2010;116(3):354–365. [DOI] [PubMed] [Google Scholar]
- 42.Lugthart S, Gröschel S, Beverloo HB, et al. Clinical, molecular and prognostic significance of WHO type inv(3)(q21;q26.2)/t(3;3)(q21;q26.2) and various other 3q abnormalities in acute myeloid leukemia. J Clin Oncol.2010;28(24):3890–3898. [DOI] [PubMed] [Google Scholar]
- 43.Fasan A, Eder C, Haferlach C, et al. GATA2 mutations are frequent in intermediate-risk karyotype AML with biallelic CEBPA mutations and are associated with favorable prognosis. Leukemia.2013;27(2):482–485. [DOI] [PubMed] [Google Scholar]
- 44.Gaidzik VI, Bullinger L, Schlenk RF, et al. RUNX1 mutations in acute myeloid leukemia: results from a comprehensive genetic and clinical analysis from the AML study group. J Clin Oncol.2011;29(10): 1364–1372. [DOI] [PubMed] [Google Scholar]
- 45.Mendler JH, Maharry K, Radmacher MD, et al. RUNX1 mutations are associated with poor outcome in younger and older patients with cytogenetically normal acute myeloid leukemia and with distinct gene and microRNA expression signatures. J Clin Oncol.2012;30(25):3109–3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Metzeler KH, Becker H, Maharry K, et al. ASXL1 mutations identify a high-risk subgroup of older patients with primary cytoge-netically normal AML within the ELN favorable genetic category. Blood.2011;118(26): 6920–6929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pratcorona M, Abbas S, Sanders MA, et al. Acquired mutations in ASXL1 in acute myeloid leukemia: prevalence and prognostic value. Haematologica.2012;97(3):388–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schnittger S, Eder C, Jeromin S, et al. ASXL1 exon 12 mutations are frequent in AML with intermediate risk karyotype and are independently associated with an adverse outcome. Leukemia.2013;27(1):82–91. [DOI] [PubMed] [Google Scholar]
- 49.Haferlach C, Dicker F, Herholz H, Schnittger S, Kern W, Haferlach T. Mutations of the TP53 gene in acute myeloid leukemia are strongly associated with a complex aberrant karyotype. Leukemia.2008;22(8):1539–1541. [DOI] [PubMed] [Google Scholar]
- 50.Bowen D, Groves MJ, Burnett AK, et al. TP53 gene mutation is frequent in patients with acute myeloid leukemia and complex karyotype, and is associated with very poor prognosis. Leukemia.2009;23(1):203–206. [DOI] [PubMed] [Google Scholar]