Abstract
This study aims to evaluate the anti-inflammatory, cytotoxicity, and genotoxicity activities of Dissotis multiflora (Sm) Triana and Paullinia pinnata Linn used traditionally in Cameroon to treat infectious diseases. Phytochemical screening was carried out using the LC-MS procedure. The ferrous oxidation-xylenol orange (FOX) assay was used to determine the 15-lipoxygenase (15-LOX) inhibitory activity of the plant samples. The tetrazolium-based colorimetric (MTT) assay was performed using Vero cells. The Ames test was carried out using Salmonella typhimurium TA98 and TA100 tester strains. LC-MS chromatogram of D. multiflora led to the identification of four known compounds, namely, 5-(3,5-dinitrophenyl)-2H-tetrazol (2), 2,2'-{[2-(6-amino-9H-purine-9-yl)ethyl]imino}diethanol (14), 1,2,5-oxadiazolo [3,4-b]pyrazine, 5,6-di (3,5-dimethyl-1-piperidyl) (19), and nimbolinin D (20) while four compounds were also identified in P. pinnata known as 2-hydroxycarbamoyl-4-methyl-pentanoic acid (2), pheophorbide A (16), 1-[4-({2-[(1-methyl-1H-indol-5-yl)amino]-4-pyrimidinyl}oxy)-1-naphthyl]-3-[1-(4 methylphenyl)-3-(2-methyl-2-propanyl)-1H-pyrazol-5-yl]urea (17), and nimbolinin D (18). D. multiflora and P. pinnata inhibited 15-LOX activity in concentration-dependent manner. The LC50 (concentration that kills 50% of cells) values of the extracts ranged from 0.13 ± 00 to 1 ± 00 mg/mL for P. pinnata and D. multiflora, respectively. P. pinnata was cytotoxic at concentrations tested while D. multiflora was not. The selectivity index (SI) values ranged from 0.16 to 10.30 on Vero cell lines. No genotoxic effect was observed against both strains tested. These extracts are sources of compounds which can be used to control infectious diseases and associated inflammation. However, caution should be taken while using P. pinnata for medicinal purposes.
1. Background
Inflammation is the immune system's reaction to infection and injury involved in the pathogeneses of arthritis, cancer, stroke, neurodegenerative, and cardiovascular disease. This even removes offending factors and restores tissue structure and physiological function [1]. However, a massive production of proinflammatory molecules such as interleukin-6 (IL-6), tumour necrosis factor-α (TNF-α), and nitric oxide (NO) can modulate inflammation [2, 3].
Several studies have shown evidence of plant biological activities [4]. Since ancient times, plants are used for treatment of various diseases and are often assumed to be safe [5]. However, the lack of data on the toxicity of plants necessitates exhaustive evaluation of their safety given that some of them are a primary source of cytotoxic and genotoxic substances which can induce adverse effects [6–8]. Therefore, it is important to evaluate the efficacy and toxicity of natural products prior to potential use as antimicrobial agents [9].
In the search for effective safe natural antibacterial and anti-inflammatory compounds, the current study selected two Cameroonian medicinal plants based on their traditional use. Aqueous decoctions and powdered leaves from Dissotis multiflora (Sm) Triana from the Melastomataceae family are widely used in the Cameroon traditional medicine to treat infectious diseases and related conditions including diarrhea. In terms of biological activities, few studies have reported the presence of phytochemicals, antibacterial, antioxidant, and antidiarrheal activities of ethanolic leaf extract of D. multiflora [10, 11]. Paullinia pinnata Linn from the Sapindaceae family is used for the treatment of wounds and other skin diseases, typhoid, syphilis, gonorrhea, stomachache, waist pain, and diarrhea [12]. Modern pharmacology research has indicated that the different parts of P. pinnata possesses antioxidant, antidiarrheal, antityphoid, antibacterial, wound healing, cytoprotective, anti-inflammatory, and anxiolytic activities [13–19]. Phytochemical studies of P. pinnata revealed the presence of secondary metabolites while numerous phenolic compounds with biological activities have been isolated [20–24].
The widespread use of D. multiflora and P. pinnata for medicinal purposes to treat several infectious diseases motivated this study, knowing that there are no or few previous studies reporting their anti-inflammatory activity, cytotoxicity, and genotoxicity. Therefore, the present work was designed to investigate the 15-LOX inhibitory activity, cytotoxicity, and genotoxicity of D. multiflora and P. pinnata ethanolic extracts.
2. Materials and Methods
2.1. Plant Material and Extraction
The leaves of Dissotis multiflora (Sm) Triana (Melastomataceae) and Paullinia pinnata Linn (Sapindaceae) were collected in December 2013 in Nkoupa Matapit, West Cameroon. Plant identification and extraction were done as previously described [11, 19].
2.2. Cell Culture
African green monkey (Vero) kidney cell lines obtained from the American Type Culture Collection (ATCC) were maintained at 37°C and 5% CO2 in a humidified environment in modified Eagle's medium (MEM) high glucose (4.5 g/L) containing L-glutamine (Lonza, Belgium) and supplemented with 5% foetal bovine serum (Capricorn Scientific GmbH, South America) and 1% gentamicin (Virbac, RSA).
2.3. LC-MS Procedure
LC-MS analysis of D. multiflora and P. pinnata extracts was carried out following a modified method of Abay et al. [25] as previously described by Gheorghe et al. [26]. An oven with reverse phase column C18 (30°C) was used.
2.4. Inhibition of 15-Lipoxygenase (15-LOX) Enzyme
The assay was performed spectrophotometrically based on the formation of the complex Fe3+/xylenol orange according to Pinto et al. [27] as previously described by Motlhatlego et al. [28].
2.5. Cytotoxicity Assay
Cytotoxicity of the extract was determined in the MTT [3-(4, 5-dimethylthiazol-2-yl)-2, 5 diphenyltetrazolium bromide] reduction assay against Vero cell lines according to Mosmann [29] as described by Omokhua et al. [30]. From the previously reported MIC values [10, 19] and lethal concentration 50 (LC50) values obtained in the current study, the selectivity index (SI) values were calculated using the following formula: SI = LC50/MIC.
2.6. Genototoxicity Assay
The genotoxicity evaluation of plant samples was done in the histidine-deficient growth medium using the Salmonella microsome assay according to Maron and Ames [31] as described by Omokhua et al. [30].
2.7. Statistical Analysis
Each experiment was performed in triplicates. Data are expressed as mean ± standard deviation. Microsoft Excel was used to enter and capture data from which graphs and tables were extracted.
3. Results and Discussion
3.1. LC-MS Analysis of Plant Extracts
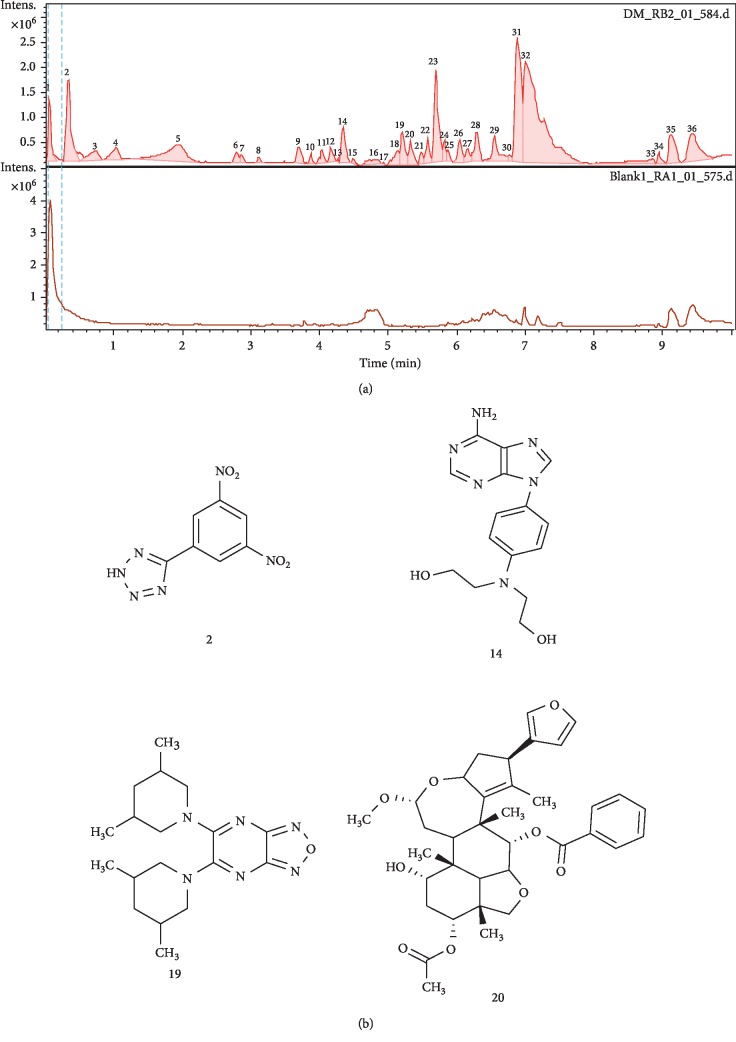
LC-MS of D. multiflora and P. pinnata revealed the presence of many compounds with known or unknown therapeutic potentials. The LC-MS chromatogram of D. multiflora (Figure 1(a)) showed 36 peaks from which four compounds were identified (Figure 1(b)), namely, the 5-(3,5-dinitrophenyl)-2H-tetrazole (2), pseudo-molecular ion peak at m/z 290 g/mol [M + H]+ corresponding to the molecular formula C7H4N6O4 and retention time of 0.4 min, 2,2′-{[2-(6-amino-9H-purine-9-yl)ethyl]imino}diethanol (14) molecular formula C15H18N6O2, molecular weight 314.34 g/mol, and retention time of 4.4 min, 1,2,5-oxadiazolo [3,4-b]pyrazine, 5,6-di(3,5-dimethyl-1-piperidyl) (19), pseudo-molecular ion peak at m/z 345.2397 [M + H]+ corresponding to the molecular formula C17H27N6O and retention time of 5.2 min, and nimbolinin D (20), molecular formula C36H44O9, molecular ion peak at m/z 621.3083 g/mol [M + H]+, and retention time of 5.7 min. 5-(3,5-dinitrophenyl)-2H-tetrazole derivatives were found to possess antimycobacterial, antibacterial, antifungal, and cytotoxic properties [32] while nimbolinin D possess anti-inflammatory activity, especially the inhibition of the production of nitric oxide [33]. The presence of bioactive constituents in the ethanolic leaf of D. multiflora gives scientific basis for the use of this plant which is underexploited and here screened for the first time for its chromatographic profile.
Figure 1.
(a) LC-MS spectrum of D. multiflora leaf extract. (b) Chemical structures of 4 compounds identified in the leaf extract of D. multiflora.
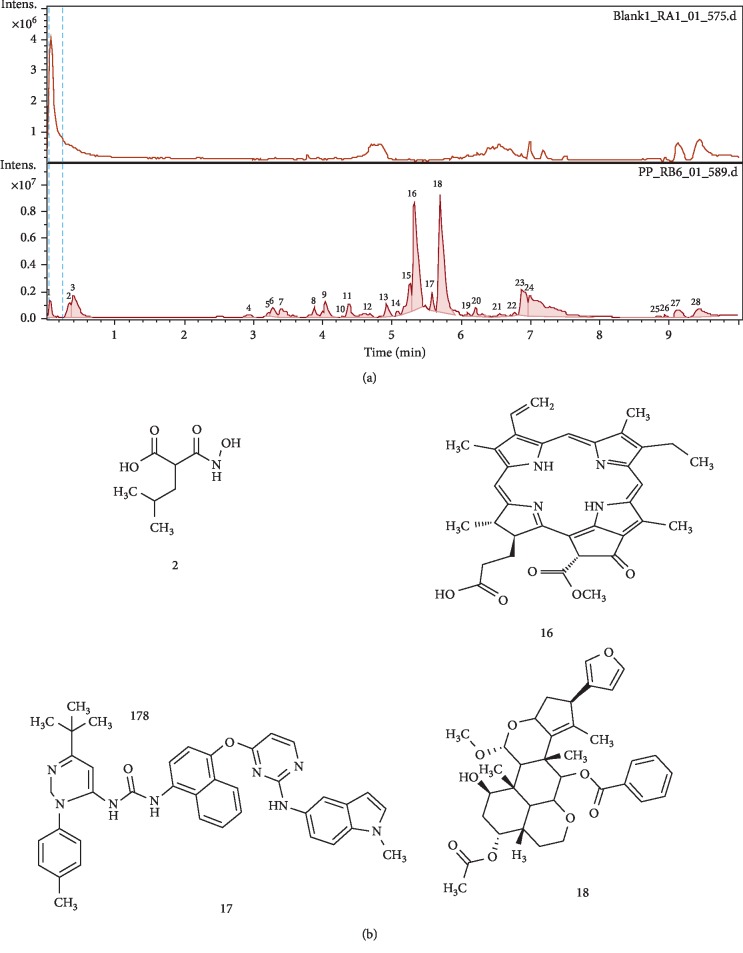
Concerning P. pinnata, the LC-MS chromatogram (Figure 2(a)) showed 28 peaks from which four compounds were identified (Figure 2(b)), namely, the 2-hydroxycarbamoyl-4-methyl-pentanoic acid (2), molecular formula C7H13NO4, molecular weight 184 g/mol, and retention time of 0.4 min, pheophorbide A (16), molecular formula C35H36N4O5, molecular ion peak at m/z 593.2763 g/mol [M + H]+, and retention time of 5.4 min, 1-[4-({2-[(1-methyl-1H-indol-5-yl)amino]-4-pyrimidinyl}oxy)-1-naphthyl]-3-[1-(4 methylphenyl)-3-(2-methyl-2-propanyl)-1H-pyrazol-5-yl]urea (17), molecular formula C37H36N8O2, molecular weight 637.3042 g/mol, and retention time of 5.6 min, and nimbolinin (18) D, molecular formula C36H44O9, molecular ion peak at m/z 621.3083 g/mol [M + H]+, and retention time of 5.7 min. Amongst the fourth identified compounds, nimbolinin and pheophorbide A are known compounds present in other plants used also in traditional medicine. Nimbolinin D exhibited anti-inflammatory activity [33] while pheophorbide A was cytostatic, induced interruption of G0/G1 phasis and U87 MG cells apoptosis in the absence of direct photoactivation [34]. It also induced significant antiproliferative effects in a number of human cancer cell lines [35]. The identification of new constituents in P. pinnata leaf extract adds to the existing data that emphasize the presence of numerous bioactive compounds isolated and characterized [20–22, 24].
Figure 2.
(a) LC-MS spectrum of P. pinnata leaf extract. (b) Chemical structures of 4 compounds identified in the leaf extract of P. pinnata.
3.2. Inhibition of 15-Lipoxygenase (15-LOX) Enzyme
The 15-LOX enzyme intervenes in the synthesis of leukotrienes from arachidonic acid. It has been proven that the bioactive leukotrienes are mediators of numerous proinflammatory and allergic reactions. Therefore, the 15-LOX inhibition activity is the most important one, even in the control of inflammatory conditions [36]. The 15-LOX inhibitory activity of ethanolic extracts from D. multiflora and P. pinnata was evaluated at different concentrations (6.25, 12.5, 25, 50, and 100 μg/mL) and compared with quercetin (Figure 3). Extracts exhibited 15-LOX inhibitory effects in a concentration-dependant manner with inhibition percentage varying between 4 and 45.85% for the extracts of both plants. Phenol was found in both extracts and may be responsible of the 15-LOX inhibitory activity [10, 37].
Figure 3.
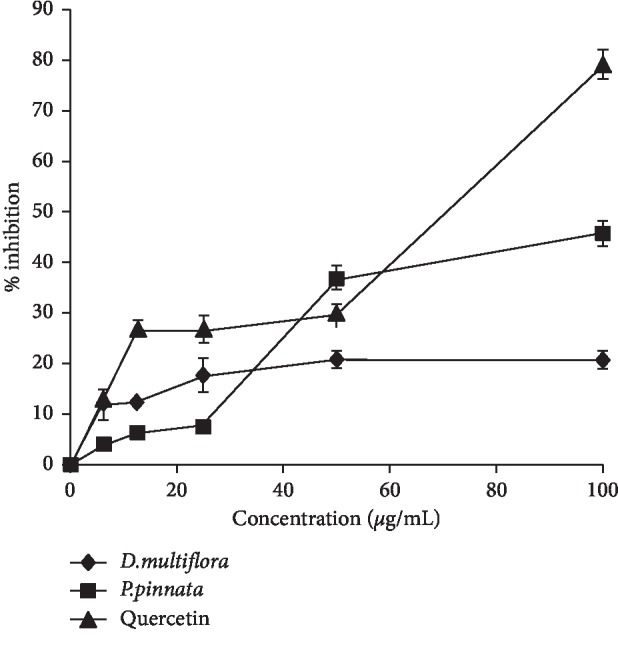
Inhibitory activity of the ethanolic extracts and fractions from D. multiflora and P. pinnata on 15-lipoxygenase. Data are expressed as mean ± standard deviation of three replicates.
3.3. Cytotoxicity Assay
The cytotoxicity test was performed against Vero monkey kidney cells to ensure the safe use of D. multiflora and P. pinnata. The LC50 values of the extracts ranged between 0.13 ± 00 and 1 ± 00 mg/mL for crude extracts from P. pinnata and D. multiflora, respectively (Table 1). It has been shown that LC50 values of 20 μg/mL and below were considered to be toxic [38]. In this sense, all extracts are nontoxic. In a previous study, we determined the antibacterial activity of the ethanolic leaf extracts of D. multiflora and P. pinnata against Salmonella typhi, Shigella flexneri, Proteus mirabilis, Klebsiella pneumoniae, Bacillus cereus, and Escherichia coli [10, 19]. The SI values of the extracts were calculated by dividing the cytotoxicity LC50 (in mg/mL) by MIC (mg/mL). Results showed that the SI values ranged between 1.28 and 10.30 for D. multiflora while varying from 0.16 to 0.66 for P. pinnata (Table 1). According to Mongalo et al. [39], SI values above 1 refer to less toxic and below 1 to be toxic. Hence, D. multiflora with SI values above 1 showed no preliminary indication of toxicity while P. pinnata with SI values lower than 1 may be devoid of antibacterial activity. To the best of our knowledge, the present study reports for the first time the cytotoxicity of D. multiflora. In an in vivo study, D. multiflora was reported to be devoid of subacute toxicity at doses lower than 200 mg/kg. The cytotoxic potential of the crude plant extract of Dissotis rotundifolia, a plant of the same genera, was reported to be highly toxic to the MRC-5 cell line [40] while Abere et al. [41] reported that doses lower than 500 mg/kg were nontoxic by subacute toxicity. P. pinnata ethanolic leaf extracts were less active against the bacterial strains. Hence, the worse selectivity indices at concentrations tested. The in vivo toxicity test has shown that P. pinnata extract was not toxic at doses up to 200 mg/kg [24, 42]. One should observe that the results of in vitro toxicity evaluation differ substantially from that observed in vivo. This may be due to pharmacokinetic and pharmacodynamical considerations [43].
Table 1.
Cytotoxic effects and selectivity index values of ethanolic leaf extract of D. multiflora and P. pinnata on Vero cells.
| Plant | LC50 (mg/mL) | Selectivity index (SI) | |||||
|---|---|---|---|---|---|---|---|
| S. typhi | S. flex | K. pneu | B. cer | E. coli | P. mirab | ||
| D. multiflora | 1.00 ± 0.00 | 10.30 | 5.12 | 5.12 | 1.28 | 2.56 | 2.56 |
| P. pinnata | 0.13 ± 0.06 | 0.16 | 0.66 | 0.33 | 0.16 | 0.16 | 0.33 |
| Doxorubicin | 5.92 ± 1.21 | — | — | — | — | — | — |
S. typhi: Salmonella typhi; S. flex: Shigella flexneri; K. pneu: K. pneumoniae; B. cer: Bacillus cereus; E. coli: Escherichia coli; P. mirab: Proteus mirabilis. Doxorubicin hydrochloride in μg/mL was used as positive control. Minimum inhibitory concentration values of extracts (mg/mL) from previous works; LC50 = Lowest concentration of extract which is lethal to 50% of the cells.
3.4. Genotoxicity Assay
The genotoxicity test was performed in order to determine ranges of plant extracts capable of producing genetic damage with resultant gene mutations without metabolic activation. From the Ames test, we observed that all the extracts tested at various concentrations had no number of revertant colonies of S. typhimurium strains TA98 and TA100 equal to or greater than twice those of the negative control (Table 2). Therefore, both plant extracts did not induce gene mutations [31]. In all cases, the values fall within normal limits and in accordance with the literature [44].
Table 2.
Number of revertant colonies of Salmonella typhimurium TA98 and TA100 tester strains induced by ethanolic extracts of D. multiflora and P. pinnata.
| Plant | Concentration (mg/mL) | His + revertants plate | |
|---|---|---|---|
| TA98 | TA100 | ||
| D. multiflora | 5 | 23.00 ± 0.57 (1.131) | 135.00 ± 1.00 (1.298) |
| 0.5 | 21.66 ± 2.66 (1.065) | 120.66 ± 2.33 (1.160) | |
| 0.005 | 14.33 ± 0.33 (0.704) | 133.00 ± 2.00 (1.278) | |
|
| |||
| P. pinnata | 5 | 19.00 ± 0.00 (0.934) | 142.33 ± 2.33 (1.368) |
| 0.5 | 14.33 ± 0.33 (0.704) | 139.33 ± 2.33 (1.339) | |
| 0.005 | 7.33 ± 0.33 (0.360) | 116.00 ± 0.00 (1.115) | |
|
| |||
| 4 NQO | 128.00 ± 0.66 (2 µg/mL) | 389.33 ± 1.33 (1 µg/mL) | |
|
| |||
| 10% DMSO | 22.00 ± 0.57 (1.082) | 98.12 ± 0.33 (0.919) | |
|
| |||
| Water | 20.33 ± 0.88 (1) | 101.00 ± 3.00 (1) | |
The positive control used in this study was 4-nitroquinoline 1-oxide (4-NQO) (Sigma) at concentrations of 2 and 1 μg/mL for S. typhimurium TA98 and TA100, respectively. His: histidine; DMSO: dimethylsuphoxide (negative control); H2O: water (negative control). All cultures were made in triplicate (except the solvent control where five replicates were made). The results are expressed as a mean number of revertants ± standard deviation and mutagenic index values (in parentheses).
4. Conclusions
D. multiflora and P. pinnata extracts had 15-LOX inhibitory effects. The LC-MS led to the identification of compounds with known biological activities in both extracts. No cytotoxic effect was observed with D. multiflora and P. pinnata on the tested cell lines. However, P. pinnata is devoid of useful antibacterial activity justifying the worse selectivity indices. Our study indicated the potential nongenotoxic effect of both plant samples tested. However, study including a metabolic activation step is necessary to approve this finding. The information on 15-LOX inhibitory effects, cytotoxicity, and genotoxicity of these plant samples motivates further research that aims at isolating and determining biological properties of safe compounds from both plants.
Acknowledgments
The authors are thankful to the African-German Network of Excellence in Science (AGNES), the German Federal Ministry of Education and Research (BMBF), and the Alexander von Humboldt Foundation (AvH) through the programme AGNES Mobility Grant (Intra-Africa) for Junior Researchers for financial support. The authors are very grateful to Prof. Jacobus Nicolaas Eloff, Prof. Lyndy McGaw, and the Phytomedicine Programme, University of Pretoria, South Africa, for providing facilities for this study. They also thank the laboratory of Chemistry, Higher Teachers' Training College, University of Yaounde I, Cameroon for assistance in LC-MS analysis.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
AAD conducted the practical work and prepared the draft of the manuscript, SVD performed LC-MS analysis, and MAN and EFX designed the study. All authors read and approved the final manuscript.
References
- 1.Ricciotti E., FitzGerald G. A. Prostaglandins and inflammation. Arteriosclerosis, Thrombosis, and Vascular Biology. 2011;31(5):986–1000. doi: 10.1161/atvbaha.110.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ravipati A. S., Zhang L., Koyyalamudi S. R., et al. Antioxidant and anti-inflammatory activities of selected Chinese medicinal plants and their relation with antioxidant content. BMC Complementary and Alternative Medicine. 2012;12(1):p. 173. doi: 10.1186/1472-6882-12-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dzoyem J., Donfack A., Tane P., McGaw L., Eloff J. Inhibition of nitric oxide production in LPS-stimulated RAW 264.7 macrophages and 15-LOX activity by Anthraquinones from Pentas Schimperi. Planta Medica. 2016;82(14):1246–1251. doi: 10.1055/s-0042-104417. [DOI] [PubMed] [Google Scholar]
- 4.Kuete V., Fokou F. W., Karaosmanoğlu O., Beng V. P., Sivas H. Cytotoxicity of the methanol extracts of Elephantopus mollis, Kalanchoe crenata and 4 other Cameroonian medicinal plants towards human carcinoma cells. BMC Complementary and Alternative Medicine. 2017;17(1):p. 280. doi: 10.1186/s12906-017-1793-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaabane F., Boubaker J., Loussaif A., et al. Antioxidant, genotoxic and antigenotoxic activities of Daphne gnidium leaf extracts. BMC Complementary and Alternative Medicine. 2012;12(1):p. 153. doi: 10.1186/1472-6882-12-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shafaei A., Esmailli K., Farsi E., Aisha A. F., Majid A. M., Ismail Z. Genotoxicity, acute and subchronic toxicity studies of nano liposomes of Orthosiphon stamineus ethanolic extract in Sprague Dawley rats. BMC Complementary and Alternative Medicine. 2015;15(1):p. 360. doi: 10.1186/s12906-015-0885-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Popat A., Shear N. H., Malkiewicz I., et al. The toxicity of Callilepis laureola, a South African traditional herbal medicine. Clinical Biochemistry. 2001;34(3):229–236. doi: 10.1016/s0009-9120(01)00219-3. [DOI] [PubMed] [Google Scholar]
- 8.Mattana C. M., Cangiano M. A., Alcaráz L. E., et al. Evaluation of cytotoxicity and genotoxicity of Acacia aroma leaf extracts. The Scientific World Journal. 2014;2014:6. doi: 10.1155/2014/380850.380850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okem A., Finnie J. F., Van Staden J. Pharmacological, genotoxic and phytochemical properties of selected South African medicinal plants used in treating stomach-related ailments. Journal of Ethnopharmacology. 2012;139(3):712–720. doi: 10.1016/j.jep.2011.11.034. [DOI] [PubMed] [Google Scholar]
- 10.Afagnigni A. D., Nyegue M. A., Ndoye F. C., Voundi O. S., Fonkoua M. C., Etoa F. X. Antibacterial and antioxidant activities of ethanolic leaves extracts of Dissotis multiflora Triana (Melastomataceae) International Journal of Pharmaceutical Sciences and Drug Research. 2016;8(1):50–56. doi: 10.25004/ijpsdr.2016.080108. [DOI] [Google Scholar]
- 11.Afagnigni A. D., Nyegue M. A., Ndoye F. C., et al. Antidiarrheal activity of Dissotis multiflora (Sm) Triana (Melastomataceae) leaf extract in Wistar rats and sub-acute toxicity evaluation. Evidence-Based Complement Altern Med. 2017;2017:9. doi: 10.1155/2017/4038371.4038371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Annan K., Houghton P. J., Govindarajan R. In vitro and in vivo wound healing actions of Paullinia pinnata. Planta Medica. 2007;73(9):p. 969. doi: 10.1055/s-2007-987243. [DOI] [Google Scholar]
- 13.Jimoh F. O., Sofidiya M. O., Afolayan A. J. Antioxidant properties of the methanol extracts from the leaves of Paullinia pinnata. Journal of Medicinal Food. 2007;10(4):707–711. doi: 10.1089/jmf.2006.253. [DOI] [PubMed] [Google Scholar]
- 14.Annan K., Gbedema S. Y., Adu F. Antibacterial and radical scavenging activity of fatty acids from Paullinia pinnata Linn. Pharmacognosy Magazine. 2009;4:119–123. [Google Scholar]
- 15.Ior L. D., Uguru M. O., Olotu P. N., Ohemu T. L., Ukpe A. A. Evaluation of analgesic and anti-inflammatory activities and phytochemical screening of the leaves extract of Paullinia pinnata (Sapindaceae) Journal of Chemical and Pharmaceutical Research. 2011;3(4):351–356. [Google Scholar]
- 16.Lasisi A. A., Ayinde B. W., Adeleye A. O., Onocha P. A., Oladosu I. A., Idowu P. A. New triterpene isovanniloyl and antibacterial activity of constituents from the roots of Paullinia pinnata Linn (Sapindaceae) Journal of Saudi Chemical Society. 2015;19(2):117–122. doi: 10.1016/j.jscs.2011.12.012. [DOI] [Google Scholar]
- 17.Aliyu M., Anuka J. A., Yaro A. H., Magaji M. G. Evaluation of the anxiolytic effect of methanolic leaves extract of Paullinia pinnata lin in mice. British Journal of Pharmaceutical Research. 2014;4(13):1638–1646. doi: 10.9734/bjpr/2014/10990. [DOI] [Google Scholar]
- 18.Lunga P., Tamokou J. D., Fodouop S. P., Kuiate J.-R., Tchoumboue J., Gatsing D. Antityphoid and radical scavenging properties of the methanol extracts and compounds from the aerial part of Paullinia pinnata. SpringerPlus. 2014;3(1):p. 302. doi: 10.1186/2193-1801-3-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Afagnigni A. D., Nyegue M. A., Ndoye F. C., Voundi O. S., Fonkoua M. C., Etoa F. X. In vitro assessment of antibacterial and antioxidant activities of ethanolic leaves extracts of Paullinia pinnata Linn (Sapindaceae) World Journal of Pharmaceutical Sciences. 2016b;4(5):173–182. [Google Scholar]
- 20.Annan K., Houghton P. J. Antibacterial activity of Paullinia pinnata extracts. Proceedings of the Abstract of the Ninth International Congress; 2004; Canterbury, UK. International Society of Ethnobiology; [Google Scholar]
- 21.Miemanang R. S., Krohn K., Hussain H., Dongo E. Paullinoside A and paullinomide A: a new cerebroside and a new ceramide from leaves of Paullinia pinnata. Zeitschrift für Naturforschung B. 2006;61(9):1123–1127. doi: 10.1515/znb-2006-0910. [DOI] [Google Scholar]
- 22.Dongo E., Hussain H., Miemanang R. S., Tazoo D., Schulz B., Krohn K. Chemical constituents of Klainedoxa gabonenses and Paullinia pinnata. Records of Natural Products. 2009;3(3):165–169. [Google Scholar]
- 23.Yusuf A. Z., Zakir A., Shemau Z., Abdullahi M., Halima S. A. Phytochemical analysis of the methanol leaves extract of Paullinia pinnata linn. Journal of Pharmacognosy and Phytotherapy. 2014;6(2):10–16. doi: 10.5897/jpp2013.0299. [DOI] [Google Scholar]
- 24.Gatsing D., Lunga P. K., Nkodo J. M. M., Tamokou J. D., Kuiate J. R., Tchoumboue J. Post-treatment evaluation of the side effects of methanol leaf extract from Paullinia pinnata Linn, an antityphoid plant. Pharmacologia. 2015;6(7):264–272. doi: 10.5567/pharmacologia.2015.264.272. [DOI] [Google Scholar]
- 25.Abay G., Altun M., Koldaş S., Tüfekçi A., Demirtas I. Determination of antiproliferative activities of volatile contents and HPLC profiles of dicranum scoparium (dicranaceae, bryophyta) Combinatorial Chemistry & High Throughput Screening. 2015;18(5):453–463. doi: 10.2174/1386207318666150305112504. [DOI] [PubMed] [Google Scholar]
- 26.Gheorghe A., Van Nuijs A., Pecceu B., et al. Analysis of cocaine and its principal metabolites in waste and surface water using solid-phase extraction and liquid chromatography-ion trap tandem mass spectrometry. Analytical and Bioanalytical Chemistry. 2008;391(4):1309–1319. doi: 10.1007/s00216-007-1754-5. [DOI] [PubMed] [Google Scholar]
- 27.Pinto M., Tejeda A., Duque A. L., Macías P. Determination of lipoxygenase activity in plant extracts using a modified ferrous oxidation-xylenol orange assay. Journal of Agricultural and Food Chemistry. 2007;55(15):5956–5959. doi: 10.1021/jf070537x. [DOI] [PubMed] [Google Scholar]
- 28.Motlhatlego K. E., Mfotie Njoya E., Abdalla M. A., Eloff J. N., McGaw L. J. The potential use of leaf extracts of two Newtonia (Fabaceae) species to treat diarrhoea. South African Journal of Botany. 2018;116:25–33. doi: 10.1016/j.sajb.2018.02.395. [DOI] [Google Scholar]
- 29.Mosmann T. Rapid calorimetric assay for cellular growth and survival: application to proliferation and cytotoxic assays. Journal of Immunological Methods. 1983;65(1-2):263–271. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 30.Omokhua G. T., Abdalla M. A., Van Staden J., McGaw L. J. A comprehensive study of the potential phytomedicinal use and toxicity of invasive Tithonia species in South Africa. BMC Complementary and Alternative Medicine. 2018;18:p. 272. doi: 10.1186/s12906-018-2336-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maron D. M., Ames B. N. Revised methods for the Salmonella mutagenicity test. Mutation Research/Environmental Mutagenesis and Related Subjects. 1983;113(3-4):173–215. doi: 10.1016/0165-1161(83)90010-9. [DOI] [PubMed] [Google Scholar]
- 32.Němeček J., Sychra P., Macháček M., et al. Structure-activity relationship studies on 3,5-dinitrophenyl tetrazoles as antitubercular agents. European Journal of Medicinal Chemistry. 2017;130:419–432. doi: 10.1016/j.ejmech.2017.02.058. [DOI] [PubMed] [Google Scholar]
- 33.Akihisa T., Nishimoto Y., Ogihara E., Matsumoto M., Zhang J., Abe M. Nitric oxide production-inhibitory activity of limonoids from Azadirachta indica and Melia azedarach. Chemistry & Biodiversity. 2017;14(6) doi: 10.1002/cbdv.201600468.e1600468 [DOI] [PubMed] [Google Scholar]
- 34.Cho M., Park G.-M., Kim S.-N., Amna T., Lee S., Shin W.-S. Glioblastoma-specific anticancer activity of pheophorbide a from the edible red seaweed Grateloupia elliptica. Journal of Microbiology and Biotechnology. 2014;24(3):346–353. doi: 10.4014/jmb.1308.08090. [DOI] [PubMed] [Google Scholar]
- 35.Tang P. M.-K., Chan J. Y.-W., Au S. W.-N., et al. Pheophorbide a, an active compound isolated fromScutellaria barbata, possesses photodynamic activities by inducing apoptosis in human hepatocellular carcinoma. Cancer Biology & Therapy. 2006;5(9):1111–1116. doi: 10.4161/cbt.5.9.2950. [DOI] [PubMed] [Google Scholar]
- 36.Schneider I., Bucar F. Lipoxygenase inhibitors from natural plant sources: part 1—medicinal plants with inhibitory activity on arachidonate 5-lipoxygenase and 5 lipoxygenase/cycloxygenase. Phytotherapy Research. 2005;19(2):81–102. doi: 10.1002/ptr.1603. [DOI] [PubMed] [Google Scholar]
- 37.Wangensteen H., Samuelsen A. B., Malterud K. E. Antioxidant activity in extracts from coriander. Food Chemistry. 2004;88(2):293–297. doi: 10.1016/j.foodchem.2004.01.047. [DOI] [Google Scholar]
- 38.Zirihi G. N., Mambu L., Guédé-Guina F., Bodo B., Grellier P. In vitro antiplasmodial activity and cytotoxicity of 33 West African plants used for treatment of malaria. Journal of Ethnopharmacology. 2005;98(3):281–285. doi: 10.1016/j.jep.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 39.Mongalo N. I., McGaw L. J., Finnie J. F., Van Staden J. Pharmacological properties of extracts from six South African medicinal plants used to treat sexually transmitted infections (STIs) and related infections. South African Journal of Botany. 2017;112:290–295. doi: 10.1016/j.sajb.2017.05.031. [DOI] [Google Scholar]
- 40.Nguta J. M., Appiah-Opong R., Nyarko A. K., et al. Antimycobacterial and cytotoxic activity of selected medicinal plant extracts. Journal of Ethnopharmacology. 2016;182:10–15. doi: 10.1016/j.jep.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abere T. A., Okoto P. E., Agoreyo F. O. Antidiarrheal and toxicological evaluation of the leaf extract of Dissotis rotundifolia Triana (Melastomataceae) BMC Complementary and Alternative Medicine. 2010;10:p. 71. doi: 10.1186/1472-6882-10-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adeyemo S. O., Makinde J. M. Acute and sub-acute toxicity studies of the methanol extract of the leaves of Paullinia pinnata Linn in Wistar mice and rats. African Journal of Medicine and Medical Sciences. 2013;42(1):81–90. [PubMed] [Google Scholar]
- 43.Kuete V. Potential of Cameroonian plants and derived products against microbial infections: a review. Planta Medica. 2010;76(14):1479–1491. doi: 10.1055/s-0030-1250027. [DOI] [PubMed] [Google Scholar]
- 44.Elgorashi E., Taylor J. L., Maes A., Van staden J., De kimpe N., Verschaeve L. Screening of medicinal plants used in South African traditional medicine for genotoxic effects. Toxicology Letters. 2003;143(2):195–207. doi: 10.1016/s0378-4274(03)00176-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.