Abstract
Aim: The Geriatric Nutritional Risk Index (GNRI) was developed to assess the nutritional risk and is associated with mortality. However, there are limited reports on the relationship between the GNRI and overall survival (OS) in peripheral artery disease (PAD). Therefore, the purpose of this study was to examine the relationship between GNRI and OS and cardiovascular or limb events in patients with PAD.
Methods: A prospective cohort study was performed on 1,219 patients with PAD. The baseline GNRI was calculated from the serum albumin level and body mass index obtained at the first visit. The patients were divided into four groups according to the GNRI: G0 (> 98), G1 (92–98), G2 (82–91), and G3 (< 82). The endpoints were OS and freedom from major adverse cardiovascular events (MACE) and MACE plus limb events (MACLE).
Results: The median follow-up period was 73 months. There were 626 deaths (51.4%) during the follow-up. The rate of cardiovascular death was 51.3%. OS clearly depended on the GNRI (p < 0.01), with five-year OS rates of 80.8% for G0, 62.0% for G1, 40.0% for G2, and 23.3% for G3. In multivariate analyses, the GNRI, age, ankle–brachial pressure index (ABPI), critical limb ischemia, estimated glomerular filtration rate (eGFR), and C-reactive protein (CRP) were independent factors associated with OS, and GNRI, age, ABPI, coronary artery disease, diabetes mellitus, eGFR, and CRP were associated with MACE and MACLE (all p < 0.05). Statins were found to improve OS, MACE, and MACLE (p < 0.01).
Conclusions: GNRI is an independent predictor for OS, MACE, and MACLE in patients with PAD.
Keywords: Geriatric nutritional risk index, Malnutrition, All-cause mortality, Fate of leg, Peripheral arterial disease
See editorial vol. 27: 132–133
Introduction
Patients with peripheral artery disease (PAD) are complicated with diabetes mellitus (DM), chronic kidney disease (CKD), or other risk factors and may experience extensive and severe systemic atherosclerosis that causes mortality due to coronary artery disease (CAD) and ischemic stroke1–4). We previously reported that a low body mass index (BMI) is an independent predictor for all-cause mortality in patients with PAD3). The mortality rates for different levels of BMI follow a J-shaped curve, with cardiovascular mortality showing a tendency for a bimodal distribution with peaks at lower and higher BMI.
Malnutrition is common and is associated with increased mortality in cardiovascular disease (CVD), such as CAD and stroke5, 6), and nutritional indicators such as BMI, serum albumin, and total cholesterol are predictors of survival in patients with CVD5–7). The geriatric nutritional risk index (GNRI) is calculated from serum albumin and components of BMI and was developed as a screening tool to assess nutritional risk8). This index is a simple and established nutritional assessment in subjects who have CVD and those on hemodialysis, as well as other elderly patients8–11). However, long-term life expectancy, including cardiovascular events and the fate of the leg, according to the GNRI in diverse patients with PAD, has not been defined. Thus, the purpose of this study was to evaluate the clinical significance of GNRI for these events in patients with PAD.
Patients and Methods
Patients
The subjects were patients with PAD who were referred to the Cardiovascular Hospital of Central Japan (Kitakanto Cardiovascular Hospital) between January 1, 2001, and November 31, 2018. Prior to the start of the study, the patients received a full explanation of the examination methods and provided written informed consent. The study protocol was approved by the Medical Ethical Committee of the Cardiovascular Hospital of Central Japan. All patients had an ankle–brachial pressure index (ABPI) of < 0.90 at their first visit. The final diagnosis of PAD was based on clinical symptoms and iliac or femoropopliteal artery stenosis of ≥ 70% on angiography or ultrasound. The clinical stages of PAD were classified using the criteria of the Inter-Society Consensus for the Management of Peripheral Arterial Disease1).
Clinical and Laboratory Analysis
Clinical data were obtained by review of primary data for age, ABPI, BMI, DM, smoking history, and hypertension. Blood was collected during fasting in order to determine the albumin, creatinine, total cholesterol, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triglyceride, glucose, D-dimer, and C-reactive protein (CRP) levels. Hypertension was defined as blood pressure of ≥ 140/90 mmHg recorded at least twice or intake of antihypertensive agents. DM was defined as a fasting plasma glucose level of > 126 mg/dL for at least two measurements or a requirement for antidiabetic therapy12). The estimated glomerular filtration rate (eGFR) was obtained using the Modification of Diet in Renal Disease equation for creatinine, as modified by the Japanese Society of Nephrology: eGFR (mL/min/1.73 m2) = 194 × (serum creatinine)−1.094 × (age)−0.287 (× 0.739 if female)13). In patients on hemodialysis, the serum creatinine level just before the hemodialysis was used to obtain the eGFR. An electrocardiogram and echocardiography were recorded in all patients. CAD was defined as a treatment history or a positive sign in coronary angiography or stress/rest myocardial perfusion scintigraphy.
Assessment of GNRI
Baseline GNRI was calculated from serum albumin and BMI obtained at the first hospital visit, using the following formula8): GNRI = 14.89 × serum albumin (g/dL) + 41.7 × (present body weight/ideal body weight). If the patient's body weight exceeded the ideal body weight, the present body weight/ideal body weight was set to 1. The ideal body weight was defined as the value calculated from height and a BMI of 22 kg/m2, instead of the value calculated using the Lorentz formula in the original GNRI equation9, 14). The patients were divided into four groups according to the GNRI: G0 (no risk, > 98), G1 (low risk, 92–98), G2 (moderate risk, 82–91), and G3 (high risk, < 82)8, 15).
Drug Administration and Treatments
All patients took at least one antiplatelet agent, and those with intermittent claudication initially received exercise rehabilitation and medication for more than three months. Oral aspirin, clopidogrel, or ticlopidine was administered, and an oral drug for the treatment of claudication (cilostazol, beraprost, or sarpogrelate) was added to exercise therapy. Drugs for other conditions were added as needed. Medications that continued for over three months or administered for more than three months before death were classified as drug treatment.
Data Analysis and Endpoints
Patients were followed up at one, three, and six months after treatment and evaluated at four- or six-month intervals at hospital visits. Vital signs and medication regimens were assessed using medical records and written questionnaires for quality of life completed at the Foot Care Club in our hospital or in response to an invitation letter3, 7, 16). An invitation to the meeting and a questionnaire were sent to each patient once a year. Through this club, we organized educational meetings to provide lectures on exercise programs, foot care, and new medical trends.
Myocardial infarction was defined as described previously16, 17). Ischemic stroke was defined as the presence of a new focal neurological deficit lasting 24 h or more. Brain magnetic resonance imaging/computed tomography was required to confirm stroke. Transient ischemic attack (TIA) was defined as the presence of a new focal neurological deficit lasting less than 24 h. Limb revascularization was performed in cases with worsening symptoms and progression or restenosis of the lesion. Restenosis after revascularization was defined as a decrease in ABPI of ≥ 0.15 and ≥ 50% stenosis on angiography or duplex ultrasonography16, 18). Major amputation was defined as “above-the-ankle amputation.”
The endpoints were overall survival (OS), freedom from major adverse cardiovascular events (MACE: all-cause death, nonfatal myocardial infarction, stroke, or TIA), and freedom from MACE plus limb events (MACLE: MACE, any repeat revascularization for a limb, and major amputation).
Statistical Analysis
Continuous variables were expressed as a median (interquartile range) and were compared by Wilcoxon's test. Categorical variables were expressed as a number (%) and were compared using the chi-squared test. The Kaplan–Meier method was used to determine the OS, MACE, and MACLE in the follow-up period. The hazard ratio (HR) and 95% confidence interval (CI) were calculated for individual factors in the Cox univariate analysis. Factors with p < 0.05 in this analysis were used in multivariate Cox regression models to determine factors associated with end-points. The C-index and Net Reclassification Index (NRI) were calculated to assess whether the accuracy of predicting these events would improve after adding the BMI, serum albumin level, or GNRI to a baseline model with established risk factors. Simple Pearson's correlations were calculated between the GNRI and risk factors other than the BMI and serum albumin level. Factors with p < 0.05 in the analysis were evaluated using stepwise forward linear multiple regression analysis to examine the relationships among the risk factors and the GNRI. Statistical analyses were carried out using IBM SPSS Statistics ver. 25.0 (IBM Corp., Armonk, NY, USA) and Microsoft R Open version 3.5.1 with additional packages, including Rcmdr, Epi, pROC, and PredictABEL (https://mran.revolutionanalytics.com/; Microsoft Corporation, Redmond, WA, USA). Individual differences were considered to be significant at p < 0.05.
Results
Patient Characteristics and Causes of Death
Follow-up was possible for 1,219 out of the 1,234 subjects. The median and mean ages of the 1,219 patients were 73 (67–79) and 72.6 ± 9.9 (range: 38 to 98) years, respectively. The median and mean follow-up periods were 58 (19–105) and 72.4 ± 60.1 months, respectively. There were 626 deaths (51.4%) during the follow-up due to cardiac or major vascular disease (n = 224, 35.8%), cerebrovascular disease (n = 97, 15.5%), malignancy (n = 125, 20.0%), pneumonia (n = 91, 14.5%), and other causes (n = 89, 14.2%). The prevalence of CVD-related deaths was 51.3% (n = 321).
The baseline characteristics and comorbidities of patients according to the GNRI are shown in Table 1. A total of 537 patients had GNRI of ≤ 98: G1, n = 308; G2, n = 164; and G3, n = 65. Patients with lower GNRI were older and had lower levels of ABPI, BMI, albumin, eGFR, total cholesterol, LDL-C, and triglyceride and higher levels of CRP and D-dimer. In patients with normal GNRI (> 98), the prevalence of critical limb ischemia (CLI), stroke, TIA, and hemodialysis was lower, and that for CAD and the revascularization rate were higher.
Table 1. Baseline clinical characteristics of patients with peripheral arterial disease with normal (>98) and low (≤ 98) geriatric nutritional risk indexes (GNRI).
| Factor | All patients | G0 | G1–3 | p-value |
|---|---|---|---|---|
| n = 1219 | GNRI >98 | GNRI ≤ 98 | ||
| n = 682 (55.9%) | n = 537 (44.1%) | |||
| Age (year) | 73 (67–79) | 71 (65–77) | 76 (70–82) | < 0.001 |
| Gender (male) | 923 (75.7%) | 528 (77.4%) | 395 (73.6%) | 0.122 |
| Ankle brachial pressure index | 0.70 (0.50–0.81) | 0.71 (0.56–0.83) | 0.63 (0.43–0.80) | < 0.001 |
| Critical limb ischemia | 248 (20.3%) | 73 (10.7%) | 175 (32.6%) | < 0.001 |
| Body mass index (kg/m2) | 22.0 (19.8–24.3) | 23.4 (21.7–25.1) | 19.7 (18.0–21.7) | < 0.001 |
| Risk factors | ||||
| History of stroke or TIA | 224 (18.4%) | 109 (16.0%) | 115 (21.4%) | 0.017 |
| Coronary artery disease | 346 (35.8%) | 223 (40.1%) | 123 (30.0%) | 0.001 |
| Hypertension | 800 (65.6%) | 460 (67.4%) | 340 (63.3%) | 0.145 |
| Diabetes mellitus | 464 (38.1%) | 274 (40.2%) | 190 (35.4%) | 0.096 |
| Smoking | 874 (71.7%) | 502 (73.6%) | 372 (69.3%) | 0.096 |
| Hemodialysis | 134 (11.0%) | 47 (6.9%) | 87 (16.2%) | < 0.001 |
| Laboratory data | ||||
| Albumin (g/dL) | 4.0 (3.7–4.2) | 4.1 (4.0–4.5) | 3.7 (3.3–4.0) | < 0.001 |
| eGFR (mL/min/1.73 m2) | 55.0 (41.7–67.4) | 57.1 (46.1–68.7) | 50.7 (35.3–65.1) | < 0.001 |
| C-reactive protein (mg/dL) | 0.19 (0.08–0.51) | 0.15 (0.08–0.34) | 0.26 (0.10–0.86) | < 0.001 |
| Total cholesterol (mg/dL) | 185 (159–214) | 192 (167–219) | 176 (149–205) | < 0.001 |
| LDL-C (mg/dL) | 113 (91–135) | 118 (95–139) | 107 (83–130) | < 0.001 |
| HDL-C (mg/dL) | 48 (39–58) | 47 (39–57) | 48 (39–58) | 0.486 |
| Triglyceride (mg/dL) | 121 (83–169) | 139 (100–192) | 100 (71–140) | < 0.001 |
| D-dimer (µg/dL) | 0.9 (0.5–2.1) | 0.7 (0.5–1.5) | 1.3 (0.8–2.8) | < 0.001 |
| Drugs | ||||
| Statin | 635 (52.1%) | 371 (54.4%) | 264 (49.2%) | 0.069 |
| Aspirin | 670 (55.0%) | 385 (56.5%) | 285 (53.1%) | 0.239 |
| Thienopyridines | 556 (45.6%) | 325 (47.7%) | 231 (43.0%) | 0.107 |
| Cilostazol | 575 (47.2%) | 318 (46.6 %) | 257 (47.9%) | 0.669 |
| Beraprost | 424 (34.8%) | 227 (33.3%) | 197 (36.7%) | 0.226 |
| Sarpogrelate | 95 (7.8%) | 53 (7.8%) | 42 (7.8%) | 1.000 |
| ACE inhibitor | 128 (10.5%) | 79 (11.6%) | 49 (9.1%) | 0.188 |
| ARB | 360 (29.5%) | 213 (31.2%) | 147 (27.4%) | 0.146 |
| β-blocker | 194 (15.8%) | 112 (16.4%) | 80 (14.9%) | 0.477 |
| Ca antagonist | 613 (50.3%) | 358 (52.5%) | 255 (47.5%) | 0.083 |
| Warfarin | 139 (11.4%) | 77 (11.3%) | 62 (11.6%) | 0.928 |
| Direct oral anticoagulant | 25 (2.1%) | 15 (2.2%) | 10 (1.9%) | 0.839 |
| Revascularization | 695 (57.0%) | 419 (61.4%) | 276 (51.4%) | < 0.001 |
| Endovascular treatment | 524 (43.0%) | 315 (46.2%) | 209 (38.9%) | 0.011 |
| Bypass surgery | 171 (14.0%) | 104 (15.2%) | 67 (12.5%) | 0.021 |
TIA: transient ischemic attack, eGFR: estimated glomerular filtration rate, LDL-C: low density lipoprotein cholesterol, HDL-C: high density lipoprotein cholesterol, ACE: angiotensin-converting enzyme, ARB: angiotensin receptor blocker
All-Cause Mortality
The cumulative three-, five-, and ten-year OS rates in all patients were 78.8, 67.9, and 43.9%, respectively. These OS rates showed significant differences among GNRI levels (all p < 0.001) (Fig. 1). The cumulative five-year OS rates were 80.8% for G0, 62.0% for G1, 40.0% for G2, and 23.3% for G3. In univariate analyses, age, ABPI, GNRI, lower eGFR, higher CRP and D-dimer, history of stroke or TIA, and CLI were significantly associated with OS (Table 2). Revascularization or treatment with statins or aspirin improved mortality. In multivariate analyses, age, ABPI, GNRI, levels of eGFR and CRP, and prevalence of CLI were significantly related to OS, and statins also improved OS.
Fig. 1.
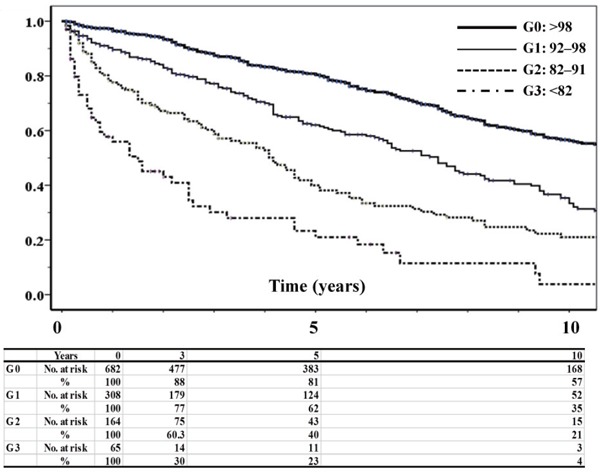
Kaplan–Meier estimates of OS according to the GNRI. There were significant differences among each GNRI level (all p < 0.001).
Table 2. Predicted risk factors for overall survival in Cox univariate and multivariate analyses.
| Factor | Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Age (year) | 1.065 | 1.055–1.075 | < 0.001 | 1.043 | 1.030–1.055 | < 0.001 |
| Sex (male) | 1.051 | 0.863–1.280 | 0.623 | |||
| Ankle brachial pressure index | 0.388 | 0.294–0.513 | < 0.001 | 0.644 | 0.420–0.985 | 0.043 |
| Critical limb ischemia | 2.708 | 2.268–3.233 | < 0.001 | 1.374 | 1.025–1.843 | 0.034 |
| History of stroke or TIA | 1.743 | 1.441–2.108 | < 0.001 | 1.126 | 0.867–1.463 | 0.374 |
| GNRI | 0.935 | 0.927–0.944 | < 0.001 | 0.961 | 0.948–0.974 | < 0.001 |
| eGFR (mL/min/1.73m2) | 0.984 | 0.980–0.988 | < 0.001 | 0.988 | 0.983–0.993 | < 0.001 |
| C-reactive protein (mg/dL) | 1.202 | 1.159–1.247 | < 0.001 | 1.169 | 1.109–1.233 | < 0.001 |
| D-dimer (µg/dL) | 1.019 | 1.011–1.027 | < 0.001 | 1.006 | 0.994–1.019 | 0.290 |
| Statin | 0.255 | 0.206–0.316 | < 0.001 | 0.351 | 0.267–0.462 | < 0.001 |
| Aspirin | 0.638 | 0.544–0.748 | < 0.001 | 0.862 | 0.693–1.071 | 0.181 |
| Revascularization | 0.640 | 0.545–0.751 | < 0.001 | 0.780 | 0.551–1.105 | 0.162 |
HR: hazard ratio, CI: confidence interval, GNRI: geriatric nutritional risk index, eGFR: estimated glomerular filtration rate.
MACE and MACLE
MACE and MACLE showed significant differences between the patients with normal and lower GNRI (Fig. 2). In multivariate analyses, age, ABPI, CAD, DM, GNRI, and levels of eGFR and CRP were independent factors associated with MACE (Table 3), and age, ABPI, CAD, DM, GNRI, and levels of eGFR and CRP were independent factors associated with MACLE (Table 4). Statins were found to reduce MACE and MACLE.
Fig. 2.
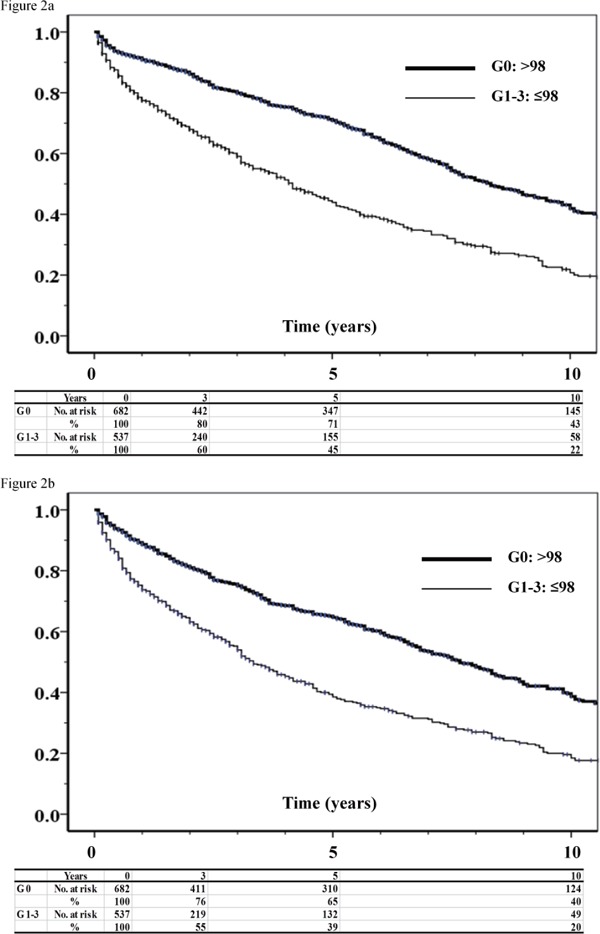
Kaplan–Meier estimates of freedom from MACE (a) and freedom from MACLE (b) in patients with normal and lower GNRI
There was a significant difference between groups G0 (GNRI > 98) and G1–G3 (GNRI ≤ 98) (p < 0.001).
Table 3. Risk factors for major adverse cardiovascular events (MACE) in Cox univariate and multivariate analyses.
| Factor | Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Age (year) | 1.045 | 1.036–1.054 | < 0.001 | 1.031 | 1.020–1.042 | < 0.001 |
| Sex (male) | 1.149 | 0.956–1.381 | 0.138 | |||
| Ankle brachial pressure index | 0.428 | 0.331–0.553 | < 0.001 | 0.642 | 0.437–0.943 | 0.024 |
| Critical limb ischemia | 2.337 | 1.973–2.769 | < 0.001 | 1.276 | 0.971–1.676 | 0.080 |
| History of stroke or TIA | 1.498 | 1.250–1.796 | < 0.001 | 0.988 | 0.775–1.260 | 0.923 |
| Coronary heart disease | 1.306 | 1.124–1.517 | < 0.001 | 1.542 | 1.252–1.898 | < 0.001 |
| Diabetes | 1.283 | 1.102–1.493 | 0.001 | 1.422 | 1.161–1.741 | 0.001 |
| GNRI | 0.950 | 0.941–0.958 | < 0.001 | 0.974 | 0.962–0.987 | < 0.001 |
| eGFR (mL/min/1.73 m2) | 0.987 | 0.984–0.991 | < 0.001 | 0.994 | 0.990–0.998 | 0.005 |
| C-reactive protein (mg/dL) | 1.161 | 1.121–1.202 | < 0.001 | 1.129 | 1.074–1.186 | < 0.001 |
| D-dimer (µg/dL) | 1.015 | 1.007–1.024 | < 0.001 | 1.004 | 0.991–1.018 | 0.525 |
| Statin | 0.330 | 0.276–0.394 | < 0.001 | 0.384 | 0.306–0.482 | < 0.001 |
| Aspirin | 0.858 | 0.741–0.993 | 0.040 | 0.974 | 0.793–1.196 | 0.802 |
| Revascularization | 0.725 | 0.624–0.842 | < 0.001 | 0.985 | 0.945–1.027 | 0.469 |
HR: hazard ratio, CI: confidence interval, GNRI: geriatric nutritional risk index, eGFR: estimated glomerular filtration rate
Table 4. Risk factors for major adverse cardiovascular and limb events (MACLE) in Cox univariate and multivariate analyses.
| Factor | Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Age (year) | 1.036 | 1.028–1.045 | < 0.001 | 1.019 | 1.009–1.030 | < 0.001 |
| Ankle brachial pressure index | 0.417 | 0.326–0.534 | < 0.001 | 0.598 | 0.417–0.858 | 0.005 |
| Critical limb ischemia | 2.186 | 1.841–2.588 | < 0.001 | 1.194 | 0.910–1.567 | 0.201 |
| History of stroke or TIA | 1.497 | 1.251–1.791 | < 0.001 | 1.069 | 0.843–1.357 | 0.581 |
| Coronary heart disease | 1.227 | 1.048–1.436 | 0.011 | 1.353 | 1.082–1.693 | 0.008 |
| Diabetes | 1.199 | 1.032–1.395 | 0.018 | 1.284 | 1.051–1.569 | 0.015 |
| GNRI | 0.954 | 0.946–0.962 | < 0.001 | 0.974 | 0.962–0.986 | < 0.001 |
| eGFR (mL/min/1.73 m2) | 0.989 | 0.986–0.992 | < 0.001 | 0.993 | 0.989–0.997 | < 0.001 |
| C-reactive protein (mg/dL) | 1.145 | 1.105–1.187 | < 0.001 | 1.101 | 1.047–1.157 | < 0.001 |
| D-dimer (µg/dL) | 1.013 | 1.004–1.021 | 0.004 | 1.001 | 0.987–1.015 | 0.910 |
| Statin | 0.329 | 0.277–0.391 | < 0.001 | 0.381 | 0.305–0.475 | < 0.001 |
| Aspirin | 0.852 | 0.737–0.986 | 0.031 | 0.929 | 0.764–1.130 | 0.461 |
| Revascularization | 0.848 | 0.731–0.984 | 0.030 | 0.876 | 0.687–1.118 | 0.288 |
HR: hazard ratio, CI: confidence interval, GNRI: geriatric nutritional risk index, eGFR: estimated glomerular filtration rate
GNRI as a Predictor of Life Expectancy and Cardiovascular or Limb Events
Differences of the C-index and NRI of the baseline models with or without GNRI, albumin level, or BMI for OS, MACE, and MACLE are shown in Table 5. GNRI was a significant risk factor improving the C-index for OS, MACE, and MACLE. NRI improved significantly after adding the GNRI to the baseline model, beyond the baseline model plus BMI or albumin level in OS, MACE, and MACLE.
Table 5. Discrimination of each predictive model for all-cause death, major adverse cardiovascular events (MACE), and MACE plus limb events (MACLE) using the C-index and net reclassification index (NRI).
| C-index (95%CI) | p-value | NRI (95%CI) | p-value | |
|---|---|---|---|---|
| All-cause death | ||||
| Established risk factors | 0.8118 (0.7871–0.8366) | Reference | Reference | |
| Established risk factors+BMI | 0.8161 (0.7917–0.8405) | 0.302 | 0.1940 (0.0822–0.3058) | 0.001 |
| Established risk factors+albumin | 0.8171 (0.7929–0.8414) | 0.181 | 0.2185 (0.1076–0.3294) | 0.000 |
| Established risk factors+GNRI | 0.8218 (0.7980–0.8456) | 0.042 | 0.2485 (0.1380–0.3591) | 0.000 |
| MACE | ||||
| Established risk factors | 0.8011 (0.7745–0.8278) | Reference | Reference | |
| Established risk factors+BMI | 0.8022 (0.7758–0.8285) | 0.698 | 0.0950 (−0.0194–0.2094) | 0.104 |
| Established risk factors+albumin | 0.8072 (0.7811–0.8334) | 0.073 | 0.1394 (0.0264–0.2525) | 0.016 |
| Established risk factors+GNRI | 0.8091 (0.7834–0.8348) | 0.043 | 0.1668 (0.0537–0.2799) | 0.004 |
| MACLE | ||||
| Established risk factors | 0.7854 (0.7584–0.8123) | Reference | Reference | |
| Established risk factors+BMI | 0.7893 (0.7628–0.8159) | 0.390 | 0.1344 (0.0195–0.2492) | 0.022 |
| Established risk factors+albumin | 0.7934 (0.7666–0.8201) | 0.110 | 0.1381 (0.0244–0.2518) | 0.017 |
| Established risk factors+GNRI | 0.7984 (0.7723–0.8245) | 0.034 | 0.1992 (0.0859–0.3121) | 0.001 |
Established risk factors included age, ankle brachial pressure index, critical limb ischemia, coronary heart disease, diabetes mellitus, estimated glomerular filtration rate, C-reactive protein, and use of statins.
CI: confidence interval, BMI: body mass index, GNRI: geriatric nutrition risk index
Correlations among GNRI and Other Risk Factors
Correlations among GNRI and other risk factors were analyzed with stepwise forward multiple regression analysis, besides BMI and albumin level. These correlations are shown in Table 6. GNRI had significant correlations with CLI, age, total cholesterol, ABPI, triglyceride, sex (male), CRP, eGFR, and hypertension.
Table 6. Correlations between geriatric nutritional risk index and other risk factors in stepwise forward multiple regression analysis.
| Factor | β | B | 95% CI | p-value |
|---|---|---|---|---|
| Critical limb ischemia | −0.232 | −4.760 | −6.037 to −3.482 | < 0.001 |
| Age (year) | −0.183 | −0.161 | −0.209 to −0.113 | < 0.001 |
| Total Cholesterol (mg/dL) | 0.161 | 0.28 | 0.016 to 0.041 | < 0.001 |
| Ankle brachial pressure index index | 0.115 | 3.558 | 1.826 to 5.289 | < 0.001 |
| Triglyceride (mg/dL) | 0.100 | 0.10 | 0.005 to 0.016 | 0.001 |
| Sex (male) | 0.100 | 1.705 | 0.619 to 2.792 | 0.001 |
| C-reactive protein (mg/dL) | −0.098 | −0.452 | −0.691 to −0.213 | 0.001 |
| eGFR (mL/min/1.73 m2) | 0.083 | 0.028 | 0.009 to 0.046 | 0.005 |
| Hypertension | 0.073 | 1.074 | 0.101 to 2.046 | 0.013 |
R2 = 0.270, F for change in R2 = 6.258, P < 0.001
β: standardized coefficient, B: non- standardized coefficient, CI: confidence interval for B, eGFR: estimated glomerular filtration rate
Factors with p < 0.05 in Pearson correlation analysis: age, sex, critical limb ischemia, ankle brachial pressure index, history of stroke or transient ischemic attack, coronary artery disease, diabetes mellitus, hypertension, smoking, eGFR, C-reactive protein, total cholesterol, HDL-cholesterol, LDL-cholesterol, triglyceride, D-dimer
Discussion
All-Cause Mortality
This is the first report on the relationships between GNRI and all-cause mortality in diverse patients with PAD. Yokoyama et al. reported that GNRI is an independent predictor of MACE and MACLE in patients with PAD following endovascular therapy19). However, they did not report a relationship between GNRI and all-cause mortality. GNRI is an independent predictor for OS, and mortality is strongly correlated with ABPI, CLI, eGFR, CRP level, and GNRI. CLI and low ABPI are related to a higher five-year risk of a cardiovascular event3, 20), based on severe systemic atherosclerosis. Several studies suggested that CKD is a prognostic indicator of CVD21, 22), and cardiovascular mortality is 10–30 times higher in patients treated with hemodialysis than in the general population21). Malnutrition is also an independent risk factor for mortality, with several reports showing a correlation between malnutrition and mortality in patients with CVD5, 6, 23). GNRI was developed as a simplified tool to assess the nutritional risk8) and was also found to be an independent predictor of OS in patients with PAD in the current study. These consistent results suggest that patients with severe PAD and lower GNRI or lower eGFR have severe systemic atherosclerosis that leads to higher mortality.
MACE and MACLE
Lower ABPI, CAD, DM, lower eGFR, and lower GNRI are independent factors associated with MACE and MACLE in multivariate analyses. The prevalence of CLI and lower ABPI are related to a higher rate of cardiovascular events due to severe systemic atherosclerosis3, 20). We reported that DM is an independent risk factor for the progression of Fontaine stages24) and MACE and MACLE in patients with PAD7, 25). In this study, a lower GNRI was also found to be an independent predictor for MACE or MACLE in diverse patients with PAD. CVDs are a major cause of MACE or MACLE, and malnutrition reflected by a lower GNRI might be responsible for MACE and MACLE. A recent study also showed that underweight patients with CLI have an extremely poor prognosis26), and GNRI was found to be a reliable predictive marker for amputation-free survival in patients with CLI15). Therefore, our results suggest that screening for GNRI is important for nutritional management of patients with PAD.
GNRI as a Risk Predictor
Several nutritional indicators, including BMI, serum albumin, and total cholesterol, have been reported to predict survival in patients with CVD5–7). Comparisons of the validity of several nutritional tools suggested that GNRI is as useful as others for the assessment of nutritional risk9, 19). GNRI is also an established nutritional assessment tool for subjects with CVD and those on hemodialysis8–11, 14). Our study suggests that GNRI with dual evaluation of BMI and serum albumin may improve the diagnostic accuracy by overcoming the limitations of each parameter alone and may accurately predict OS, MACE, and MACLE in patients with PAD.
Other than BMI and albumin level, GNRI has significant correlations with CLI, age, total cholesterol, ABPI, triglyceride, CRP, eGFR, and hypertension. These results showed that GNRI has correlations with the severity of PAD, inflammation, and CKD in addition to nutritional factors. The present study lacked data regarding serial changes in the nutritional status, so we could not determine any effect of this on the clinical outcome in patients with PAD. Nutritional intervention is usually difficult to perform strictly. In addition, the effectiveness of nutritional therapy for patients with PAD remains unclear. We showed that malnutrition and inflammation are common and are a risk factor for poor clinical outcomes in patients with PAD, suggesting the nutritional intervention to be a novel therapeutic target in the management of patients with PAD.
Limitations
The limitations of this study were as follows. First, the sample sizes were relatively small and the study was performed at a single facility. Second, the prescription rate of statins was relatively low according to the guidelines at the time of treatment; this rate increased over time. Third, the life expectancy of patients with PAD was poor and the numbers in G3 and G2 were not enough to show full-length Kaplan–Meier curves. Therefore, we showed Kaplan–Meier estimates of OS, MACE, and MACLE according to the GNRI only for 10 years. Fourth, changes in lifestyle, including the nutritional status, during follow-up were not accounted for in this study. Therefore, we were unable to clarify the changes in BMI and serum albumin during the follow-up period. These issues require examination in further studies on life expectancy and fate of the leg in patients with PAD.
Conclusion
GNRI was found to be an independent predictor of OS, MACE, and MACLE in patients with PAD. This suggests that a simple and practical assessment of GNRI might be useful for predicting long-term outcomes in these patients.
Acknowledgments
We would like to thank the vascular laboratory staff and clinical research coordinators of the Foot Care Club at our hospital.
Conflict of Interest
The authors declare no conflict of interest.
Grant Support
None.
References
- 1). Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG: Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg, 2007; 45 Suppl S: S5-67 [DOI] [PubMed] [Google Scholar]
- 2). Banerjee A, Fowkes FG, Rothwell PM: Associations between peripheral artery disease and ischemic stroke: implications for primary and secondary prevention. Stroke, 2010; 41: 2102-2107 [DOI] [PubMed] [Google Scholar]
- 3). Kumakura H, Kanai H, Aizaki M, Mitsui K, Araki Y, Kasama S, Iwasaki T, Ichikawa S: The influence of the obesity paradox and chronic kidney disease on long-term survival in a Japanese cohort with peripheral arterial disease. J Vasc Surg, 2010; 52: 110-117 [DOI] [PubMed] [Google Scholar]
- 4). Murphy TP, Dhangana R, Pencina MJ, D'Agostino RB, Sr.: Ankle-brachial index and cardiovascular risk prediction: an analysis of 11,594 individuals with 10-year follow-up. Atherosclerosis, 2012; 220: 160-167 [DOI] [PubMed] [Google Scholar]
- 5). Anker SD, Ponikowski P, Varney S, Chua TP, Clark AL, Webb-Peploe KM, Harrington D, Kox WJ, Poole-Wilson PA, Coats AJ: Wasting as independent risk factor for mortality in chronic heart failure. Lancet, 1997; 349: 1050-1053 [DOI] [PubMed] [Google Scholar]
- 6). Lee MY, Lin KD, Chang YH, Hsiao PJ, Shin SJ: Albuminuria is the stronger risk factor for peripheral arterial disease than eGFR decline in a type 2 diabetic Taiwanese population. Kidney Blood Press Res, 2010; 33: 352-359 [DOI] [PubMed] [Google Scholar]
- 7). Kumakura H, Kanai H, Hojo Y, Iwasaki T, Ichikawa S: Long-term survival and fate of the leg in de novo intermittent claudication. Eur Heart J Qual Care Clin Outcomes, 2017; 3: 208-215 [DOI] [PubMed] [Google Scholar]
- 8). Bouillanne O, Morineau G, Dupont C, Coulombel I, Vincent JP, Nicolis I, Benazeth S, Cynober L, Aussel C: Geriatric Nutritional Risk Index: a new index for evaluating at-risk elderly medical patients. Am J Clin Nutr, 2005; 82: 777-783 [DOI] [PubMed] [Google Scholar]
- 9). Yamada K, Furuya R, Takita T, Maruyama Y, Yamaguchi Y, Ohkawa S, Kumagai H: Simplified nutritional screening tools for patients on maintenance hemodialysis. Am J Clin Nutr, 2008; 87: 106-113 [DOI] [PubMed] [Google Scholar]
- 10). Cereda E, Pedrolli C: The use of the Geriatric Nutritional Risk Index (GNRI) as a simplified nutritional screening tool. Am J Clin Nutr, 2008; 87: 1966-1967; author reply 1967 [DOI] [PubMed] [Google Scholar]
- 11). Kawamiya T, Suzuki S, Ishii H, Hirayama K, Harada K, Shibata Y, Tatami Y, Harata S, Kawashima K, Kunimura A, Takayama Y, Shimbo Y, Osugi N, Yamamoto D, Ota T, Kono C, Murohara T: Correlations between geriatric nutritional risk index and peripheral artery disease in elderly coronary artery disease patients. Geriatr Gerontol Int, 2017; 17: 1057-1062 [DOI] [PubMed] [Google Scholar]
- 12). Expert Committee on the Diagnosis and Classification of Diabetes Mellitus : Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care, 2003; 26 Suppl 1: S5-20 [DOI] [PubMed] [Google Scholar]
- 13). Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A: Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis, 2009; 53: 982-992 [DOI] [PubMed] [Google Scholar]
- 14). Takahashi H, Ito Y, Ishii H, Aoyama T, Kamoi D, Kasuga H, Yasuda K, Maruyama S, Matsuo S, Murohara T, Yuzawa Y: Geriatric nutritional risk index accurately predicts cardiovascular mortality in incident hemodialysis patients. J Cardiol, 2014; 64: 32-36 [DOI] [PubMed] [Google Scholar]
- 15). Luo H, Yang H, Huang B, Yuan D, Zhu J, Zhao J: Geriatric Nutritional Risk Index (GNRI) Independently Predicts Amputation Inchronic Criticallimb Ischemia (CLI). PLoS One, 2016; 11: e0152111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16). Kumakura H, Kanai H, Araki Y, Hojo Y, Iwasaki T, Ichikawa S: 15-Year Patency and Life Expectancy After Primary Stenting Guided by Intravascular Ultrasound for Iliac Artery Lesions in Peripheral Arterial Disease. JACC Cardiovasc Interv, 2015; 8: 1893-1901 [DOI] [PubMed] [Google Scholar]
- 17). Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, Katus HA, Lindahl B, Morrow DA, Clemmensen PM, Johanson P, Hod H, Underwood R, Bax JJ, Bonow RO, Pinto F, Gibbons RJ, Fox KA, Atar D, Newby LK, Galvani M, Hamm CW, Uretsky BF, Steg PG, Wijns W, Bassand JP, Menasche P, Ravkilde J, Ohman EM, Antman EM, Wallentin LC, Armstrong PW, Simoons ML, Januzzi JL, Nieminen MS, Gheorghiade M, Filippatos G, Luepker RV, Fortmann SP, Rosamond WD, Levy D, Wood D, Smith SC, Hu D, Lopez-Sendon JL, Robertson RM, Weaver D, Tendera M, Bove AA, Parkhomenko AN, Vasilieva EJ, Mendis S: Third universal definition of myocardial infarction. Circulation, 2012; 126: 2020-2035 [DOI] [PubMed] [Google Scholar]
- 18). Ranke C, Creutzig A, Alexander K: Duplex scanning of the peripheral arteries: correlation of the peak velocity ratio with angiographic diameter reduction. Ultrasound Med Biol, 1992; 18: 433-440 [DOI] [PubMed] [Google Scholar]
- 19). Yokoyama M, Watanabe T, Otaki Y, Watanabe K, Toshima T, Sugai T, Takahashi T, Kinoshita D, Tamura H, Nishiyama S, Takahashi H, Arimoto T, Shishido T, Yamauchi S, Yamanaka T, Miyamoto T, Kubota I: Impact of Objective Malnutrition Status on the Clinical Outcomes in Patients With Peripheral Artery Disease Following Endovascular Therapy. Circ J, 2018; 82: 847-856 [DOI] [PubMed] [Google Scholar]
- 20). Mehler PS, Coll JR, Estacio R, Esler A, Schrier RW, Hiatt WR: Intensive blood pressure control reduces the risk of cardiovascular events in patients with peripheral arterial disease and type 2 diabetes. Circulation, 2003; 107: 753-756 [DOI] [PubMed] [Google Scholar]
- 21). Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW: Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation, 2003; 108: 2154-2169 [DOI] [PubMed] [Google Scholar]
- 22). Hojo Y, Kumakura H, Kanai H, Iwasaki T, Ichikawa S, Kurabayashi M: Lipoprotein(a) is a risk factor for aortic and mitral valvular stenosis in peripheral arterial disease. Eur Heart J Cardiovasc Imaging, 2016; 17: 492-497 [DOI] [PubMed] [Google Scholar]
- 23). Davenport DL, Xenos ES, Hosokawa P, Radford J, Henderson WG, Endean ED: The influence of body mass index obesity status on vascular surgery 30-day morbidity and mortality. J Vasc Surg, 2009; 49: 140-147 [DOI] [PubMed] [Google Scholar]
- 24). Kumakura H, Kanai H, Araki Y, Kasama S, Sumino H, Ito T, Iwasaki T, Takayama Y, Ichikawa S, Fujita K, Nakashima K, Minami K: Sex-related differences in Japanese patients with peripheral arterial disease. Atherosclerosis, 2011; 219: 846-850 [DOI] [PubMed] [Google Scholar]
- 25). Kumakura H, Kanai H, Matsuo Y, Iwasaki T, Ichikawa S: Asymptomatic Cerebral Infarction is a Predictor of Long-Term Survival and Vascular or Limb Events in Peripheral Arterial Disease. Eur Heart J Qual Care Clin Outcomes, 2019; 5: 43-50 [DOI] [PubMed] [Google Scholar]
- 26). Murata N, Soga Y, Iida O, Yamauchi Y, Hirano K, Kawasaki D, Fujihara M, Tomoi Y: Complex relationship of body mass index with mortality in patients with critical limb ischemia undergoing endovascular treatment. Eur J Vasc Endovasc Surg, 2015; 49: 297-305 [DOI] [PubMed] [Google Scholar]


