Abstract
Animal models that closely resemble both human disease findings and their onset mechanism have contributed to the advancement of biomedical science. The Watanabe heritable hyperlipidemic (WHHL) rabbit and its advanced strains (the coronary atherosclerosis-prone and the myocardial infarction-prone WHHL rabbits) developed at Kobe University (Kobe, Japan), an animal model of human familial hypercholesterolemia, have greatly contributed to the elucidation of the pathophysiology of human lipoprotein metabolism, hypercholesterolemia, atherosclerosis, and coronary heart disease, as described below. 1) The main part of human lipoprotein metabolism has been elucidated, and the low-density lipoprotein (LDL) receptor pathway hypothesis derived from studies using fibroblasts was proven in vivo. 2) Oxidized LDL accumulates in the arterial wall, monocyte adhesion molecules are expressed on arterial endothelial cells, and monocyte-derived macrophages infiltrate the arterial intima, resulting in the formation and progression of atherosclerosis. 3) Coronary lesions differ from aortic lesions in lesion composition. 4) Factors involved in the development of atherosclerosis differ between the coronary arteries and aorta. 5) The rupture of coronary lesions requires secondary mechanical forces, such as spasm, in addition to vulnerable plaques. 6) Specific lipid molecules in the blood have been identified as markers of the progression of coronary lesions. At the end of the breeding of the WHHL rabbit family at Kobe University, this review summarizes the history of the development of the WHHL rabbit family and their contribution to biomedical science.
Keywords: Atherosclerosis, Coronary heart disease, Lipoprotein metabolism, Serum markers for coronary lesions, WHHL rabbit
Introduction
Animal models for human disease have been greatly contributed to the elucidation of the pathophysiology of human lipoprotein metabolism and atherosclerosis. In particular, the Watanabe heritable hyperlipidemic (WHHL) rabbit, an animal model of familial hypercholesterolemia1), and its advanced strains developed by selective breeding, the coronary atherosclerosis-prone WHHL rabbits (WHHL-CA; provisional name)2, 3) showing spontaneous coronary atherosclerosis, and the myocardial infarction-prone WHHL rabbits (WHHLMI) showing spontaneous coronary atherosclerosis and myocardial infarction4), have played an important role in advancing these research areas. These rabbit strains were developed at Kobe University (Kobe, Japan). In this review, WHHL rabbits, WHHL-CA rabbits, and WHHLMI rabbits are referred to as the WHHL rabbit family. Unlike mice and rats, rabbits are similar to humans in lipoprotein metabolism, atherosclerosis, and myocardial function (see the latter part of this review5) for details). Compared with human hypercholesterolemia and atherosclerosis, cholesterol-fed rabbits also have several difficulties. 1) The cause of hypercholesterolemia is due to excess fat-supplemented feed, and the major lipoproteins are not low-density lipoprotein (LDL) but remnant lipoproteins (β- very low-density lipoprotein [VLDL])6). 2) There are large individual differences in serum lipid levels due to individual differences in the response to cholesterol feeding. 3) Arterial lesions are characterized by excessive macrophage accumulation7), and there are large individual differences in the development of coronary lesions. 4) It is difficult to conduct long-term experiments due to fatty liver caused by excessive lipid loading8). However, the WHHL rabbit family resembles humans for the pathophysiology of lipoprotein metabolism and atherosclerotic lesions, and relatively small individual differences in serum lipid levels and the degree of atherosclerosis. Consequently, the WHHL rabbit family contributed remarkably to the elucidation of the pathophysiology of human lipoprotein metabolism, atherosclerosis, coronary heart disease, and the development of statins, inhibitors of cholesterol biosynthesis. In Kobe University, breeding of each WHHL rabbit strain was terminated each time after an advanced rabbit strain was developed, and only WHHLMI rabbits had been bred since 1999. However, breeding of the WHHLMI rabbit at Kobe University ended in June 2018. Currently, a small number of WHHLMI rabbits are bred at Saga University (Saga, Japan) for cryopreservation of WHHLMI rabbit embryos, and a few other institutions are trying to breed WHHLMI rabbits. Until the end of breeding of the WHHL family, 4,639 rabbits had been provided by Kobe University to many researchers around the world (Supplementary Table 1). At the end of the breeding of WHHLMI rabbits at Kobe University, the contribution of the WHHL rabbit family to these studies are summarized here. With regard to the history of the WHHL rabbit family, the first part summarizes the history of the development of the WHHL rabbit family and their contribution to the pathophysiology of human lipoprotein metabolism, atherosclerosis, and coronary heart disease, and the second part summarizes their contribution to the development of agents for improving lipoprotein metabolism and atherosclerosis5). In this review, the terms “WHHL rabbit”, “WHHL-CA rabbit”, and “WHHLMI rabbit” indicate the homozygotes, and the heterozygote is described as “heterozygous WHHL rabbit”, “heterozygous WHHL-CA rabbit”, or “heterozygous WHHLMI rabbit”.
Supplementary Table 1. The result about providing of WHHL rabbit family.
| Regions | Countries | Organizations | Provided rabbits |
|---|---|---|---|
| Asia | Hong Kong | 1 | 12 |
| Japan | 80 | 4,154 | |
| Taiwan | 1 | 8 | |
| Singapore | 1 | 4 | |
| South Korea | 1 | 34 | |
| Europe | Austria | 1 | 6 |
| Belgium | 2 | 32 | |
| Finland | 2 | 17 | |
| France | 1 | 6 | |
| Germany | 2 | 52 | |
| Italy | 1 | unknown | |
| Spain | 1 | unknown | |
| Sweden | 2 | 6 | |
| Switzerland | 1 | 4 | |
| The Netherland | 1 | 6 | |
| United Kingdom | 4 | 18 | |
| North America | Canada | 2 | 80 |
| USA | 21 | 184 | |
| Oceania | Australia | 3 | 16 |
| Total | 19 | 128 | 4,639 |
The WHHL rabbit family is a collected term for WHHL rabbits, coronary atherosclerosis-prone WHHL rabbits (WHHL-CA rabbits), and myocardial infarction-prone WHHL rabbits (WHHLMI rabbits).
The Outline of the Characteristics of the WHHL Rabbit Family
Fig. 1 illustrates the characteristics of the WHHL rabbit family. The proband of WHHL rabbit strain, a mutant rabbit with hypercholesterolemia, showed hyperlipidemia, aortic and coronary atherosclerosis, and xanthoma on the digital joints9, 10). The original WHHL rabbit showed the following features: hypercholesterolemia due to LDL receptor deficiency11, 12), low high-density lipoprotein cholesterol, atherosclerotic lesions on the aorta (Fig. 2) and various arteries except coronary lesions13–15), and other related diseases, such as xanthoma on tendons of the limb1, 16) osteonecrosis due to the abnormal accumulation of fat in osteocytes and marrow cells17), corneal opacities due to the infiltration of macrophages and epithelial cells18), sensorineural hearing loss due to inner ear cell damage19), and metabolic syndrome-like findings20, 21). In addition to the characteristics of WHHL rabbits, coronary atherosclerosis occurred in WHHL-CA rabbits2, 3), and in WHHLMI rabbits, unstable coronary lesions (Fig. 3)4, 22, 23), myocardial infarction (Fig. 3)4), acute coronary syndromes-like findings22, 24), calcific aortic valve sclerosis with decreased aortic valve area25), cutaneous xanthoma26), and overactive bladder27) were developed. However, the mean systolic blood pressure of WHHLMI rabbits measured at the auricular artery was approximately 100 mmHg28) and had similar levels with normal Japanese white rabbits. Coronary lesions of WHHLMI rabbits are initiated from fatty streaks, and then progress to fatty patches, atheroma, fibroatheroma, thin-capped fibroatheroma, and advanced complicated lesions with reduced cellular components, calcification, vasa vasorum, etc.4, 22, 29). These features of coronary lesions of WHHLMI rabbits resemble human atherosclerosis30). Regarding thrombus formation, the activities of vitamin K-dependent clotting factors and levels of the clotting factor VIII and fibrinogen were significantly higher in WHHL rabbits than in normal rabbits31). However, platelet aggregation induced by collagen, platelet-activating factor, or adenosine diphosphate was reduced in WHHL rabbits32). Consequently, thrombin and prothrombin times of WHHL rabbits were similar to normal rabbits33). In the WHHL rabbit family, despite the development of hypercholesterolemia and atherosclerosis, thrombus formation is not always enhanced. Thrombus was formed when coronary spasm disrupted coronary plaques or injured the endothelial cell layer22, 24). These studies suggest that scattering of tissue factor into the extracellular matrix due to the collapse of macrophage-derived foam cells rather than intracellular tissue factor may be involved in thrombus formation.
Fig. 1.
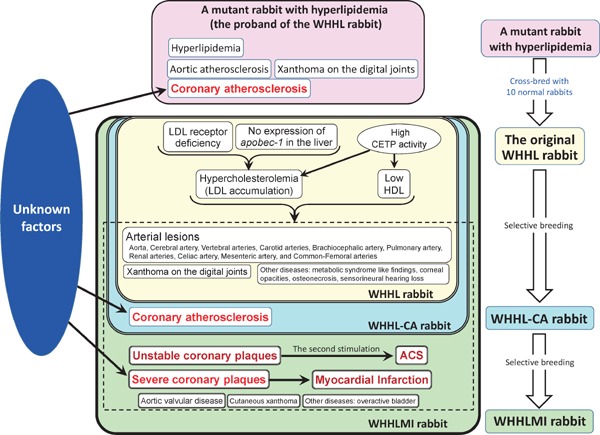
The characteristics of the WHHL rabbit family and the proband rabbit
The WHHL rabbit family is a collected term for WHHL rabbits, coronary atherosclerosis-prone WHHL rabbits (WHHL-CA rabbits), and myocardial infarction-prone WHHL rabbits (WHHLMI rabbits). The characteristics shown in the yellow square are specific to WHHL rabbits. The characteristics shown in the yellow and blue squares are specific to WHHL-CA rabbits. The characteristics shown in the yellow and green squares are those of WHHLMI rabbits. Black arrows indicate causes and the results.
Fig. 2.
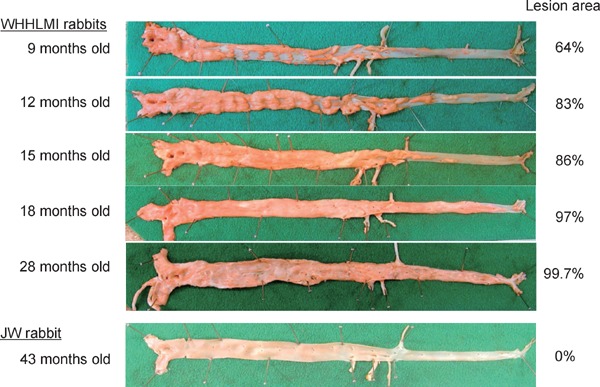
Aortic atherosclerosis of WHHLMI rabbits
Aortas were stained with Sudan staining. Lesion area was evaluated as the ratio of the lesion area to the surface lumen area.
Fig. 3.
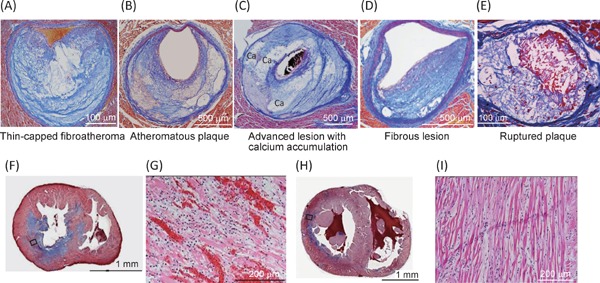
Photomicrographs of coronary lesions (A–E) and myocardial lesions (F–I) of WHHLMI rabbits
Panels A–D demonstrate spontaneously developed coronary lesions, and panel E demonstrated a ruptured coronary lesion after spasm provocation. Panels G and I are magnified photomicrographs of square in panel F and panel H, respectively. Panels A-F, and H show Azan staining, and panels G and H show HE staining. Panels A and E are modified from Shiomi M, et al.22), panels B–D are modified from Shiomi M, et al.23), and panels F-I are modified from Shiomi M, et al.4).
The History of the WHHL Rabbit Family
The history of the WHHL rabbit family is shown in Fig. 4.
Fig. 4.
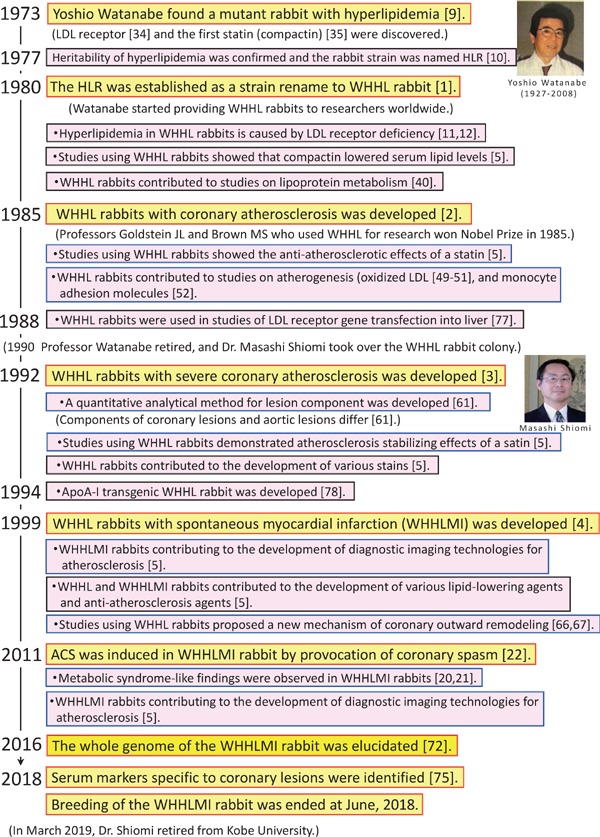
The history of the WHHL rabbit family
Discovery of a Mutant Rabbit Showing Hyperlipidemia and Establishment of the Original WHHL Rabbit Strain
In 1973, Yoshio Watanabe (1927–2008) at Kobe University accidentally found a male rabbit with hyperlipidemia. In normal chow feeding, the serum cholesterol level was 447 mg/dl at 8 months old without any abnormality in other serum biochemical parameters9). In this rabbit, atherosclerotic lesions were observed in the aorta and coronary arteries. He cross-bred the mutant rabbit with 10 female Japanese white rabbits to establish a new rabbit strain for spontaneous hyperlipidemia, and the offspring were bred with the mutant. Thereafter, he crossed between rabbits with hyperlipidemia and tried to establish a rabbit strain. However, reproductive ability is drastically depressed after three or more inbreeding generations in rabbits. He attempted to establish a rabbit strain with hyperlipidemia by crossing between above 10 lines to avoid inbreeding depression. After overcoming the crisis of inbreeding depression, he reported this strain as the hyperlipidemic rabbit (HLR) to a Japanese journal for laboratory animals10). From these breeding experiments, hyperlipidemia of the HLR was inherited in accordance with Mendel's laws. In 1973, the LDL receptor pathway34) and the first statin, compactin35), were also discovered. After seven years of cross-breeding, Watanabe established a rabbit strain of hyperlipidemia and submitted a paper to Atherosclerosis in 1979 1). In the review process, this rabbit strain was renamed the Watanabe heritable hyperlipidemic (WHHL) rabbit by the advice of the chief editor, Adams CWM. After publication, many researchers around the world requested him to provide the WHHL rabbit for their study. In response to their request, 4,639 WHHL rabbit families have been provided to 126 research institutes in 19 countries (1980–2018) (Supplementary Table 1), and more than 700 papers written in English have been published by July 2019. Research papers using the WHHL rabbit family are listed on the WHHL website (http://www.med.akita-u.ac.jp/∼doubutu/WHHL/w-index.html) with subject search function.
Contribution of WHHL Rabbits to Studies of Lipoprotein Metabolism
Table 1 summarizes lipoprotein metabolism elucidated in studies using WHHL rabbits. At the establishment of the WHHL rabbit strain, only hyperlipidemia, aortic atherosclerosis, and xanthomas on the digital joints were reported as the characteristics of the original WHHL rabbit strain (Fig. 1)1). To elucidate the cause of the hyperlipidemia, plasma lipoprotein profile and LDL receptor function were examined in collaboration with Sankyo Co. Ltd. (Tokyo, Japan). WHHL rabbits showed LDL accumulation in the plasma, delayed clearance of radiolabeled LDL from the plasma, and almost no LDL binding activity in fibroblasts11), similar to human familial hypercholesterolemia34). However, skin fibroblasts do not play a central role in lipoprotein metabolism in vivo. Goldstein JL and Brown MS, who proposed the LDL receptor pathway hypothesis from in vitro studies, also requested Watanabe to provide WHHL rabbits for in vivo studies of the LDL receptor pathway. Toru Kita from Goldstein's group demonstrated that the LDL binding activity of WHHL liver membrane fraction was about 13% of that of normal rabbits12), and the transformation from VLDL to LDL was delayed in WHHL plasma36). In addition, cholesterol biosynthesis in the liver was not increased in WHHL rabbits37). These results indicate that hypercholesterolemia in the WHHL rabbit family is due to reduced LDL uptake by the liver. Thereafter, the group of Goldstein and Brown demonstrated that 12 base pairs were deleted in the LDL binding domain in the WHHL LDL receptor protein38), and the maturation of the LDL receptor protein was delayed in WHHL fibroblasts39). In a study of LDL receptor protein maturation kinetics39), the kinetics of LDL receptor protein maturation in fibroblasts of heterozygous WHHL rabbits showed an intermediate pattern between normal and homozygotes although serum cholesterol levels of heterozygous WHHL rabbits are almost normal. Since rabbits are herbivorous and the cholesterol pool in the liver depends on its synthesis of cholesterol6), half the number of normal LDL receptors in the heterozygote may be sufficient to maintain the hepatic cholesterol pool. Based on these studies, Goldstein JL and Brown MS clarified lipoprotein metabolism in vivo40) and won the Nobel Prize in 1985. Furthermore, as similar to humans, since apoB-editing enzyme, which induces a stop codon into apoB-100 mRNA, is not expressed in the liver of rabbits41), lipoprotein particles secreted from the liver have apoB100, and the fractional catabolic rate of apoB-100-containing lipoproteins is very slow compared with apoB-48-containing lipoproteins because apoB-100-containing lipoproteins are not catabolized via remnant receptors. Therefore, the absence of apoB-editing enzyme expression in the rabbit liver is also associated with hypercholesterolemia in the WHHL rabbit family. While in mice and rats, since the apoB-editing enzyme is expressed in their liver42), serum levels of apoB-containing lipoproteins are very low. Thus, WHHL rabbits contributed greatly to the elucidation of human lipoprotein metabolism.
Table 1. Lipoprotein metabolism elucidated in studies using WHHL rabbits.
|
Contribution of WHHL Rabbits to Studies of Atherogenesis
Table 2 summarizes the pathogenesis of atherosclerotic lesions elucidated in studies using WHHL rabbits. After the elucidation of the major parts of lipoprotein metabolism, studies on atherogenesis were started using the aorta of WHHL rabbits. In histopathological observations, the WHHL aortas showed progressive atherosclerotic lesions, including fatty streaks, raised foam cell lesions, atheromatous plaques, and advanced lesions with cholesterol crystals43–46). Electron microscopy observations showed lipid-laden foam cells in the intima43–46). These foam cells were derived from smooth muscle cells and macrophages47). In the extracellular matrix, lipid vesicles were markedly accumulated among collagen fibers47), and cholesterol esters containing peroxidized fatty acids were detected in the atherosclerotic aorta48). In vitro studies demonstrated that macrophages can ingest chemically modified lipoproteins and transform to foam cells. Kita et al.49) and Carew et al.50) separately demonstrated that probucol, an antioxidant, suppresses atherosclerosis in WHHL aorta. The results indicate that oxidized LDL is a causative substance of atherosclerosis. Thereafter, an immunohistochemical study demonstrated that peroxidized LDL accumulated in WHHL aorta51). In addition, monocyte adhesion molecules are expressed on the arterial endothelial cells of WHHL aorta52). So, macrophages in atherosclerotic lesions are derived from circulating monocytes. Ross's famous working hypothesis about the development of atherosclerosis, “Response-to-Injury Hypothesis”, had been revised several times. In the first hypothesis, it was thought that smooth muscle cells migrated from the arterial tunica media and proliferated in intimal lesions by the stimulation of PDGF secreted by activated platelets, and atherosclerotic lesions were formed53, 54). However, after the observation of atherosclerotic lesions in WHHL rabbits by the Ross's group44–47), this working hypothesis was extensively revised in 1993 by Ross himself. In his revised hypothesis, injury of arterial endothelial cells was expanded to endothelial dysfunction, and the role of macrophages and immune responses was added to the hypothesis55). This has led to the current “Inflammatory Theory” of atherosclerosis56, 57). Thus, WHHL rabbits have contributed to the elucidation of the pathogenesis of human atherosclerosis. However, the structure of the arteries is different in humans and many animal species. In human normal arteries, an intimal layer is observed inside the internal elastic lamina, whereas there is only an endothelium layer in animals. In the deep area of arterial intima in humans, lipid vesicles were observed in the extracellular matrix before the development of atherosclerosis30). In the early stages of atherosclerosis, macrophages infiltrate into the arterial intima from the surface of the intima and the vasa vasorum in the deep intima. In WHHLMI rabbits, the vasa vasorum was observed with extracellular lipid deposits and macrophage accumulation in the deep area of advanced coronary lesions, in addition to the intimal surface29). Therefore, it should be noted that the pathogenesis of atherosclerosis may be partially different between humans and animals. In addition, macrophages in atherosclerotic lesions express VLDL receptors in humans and WHHLMI rabbits but not in mice58). VLDL particles were also detected in human atherosclerotic lesions59). Therefore, VLDL is also directly related to atherogenesis in humans and rabbits.
Table 2. Pathophysiology of atherosclerosis elucidated in studies using WHHL rabbit family.
|
Development of Coronary Atherosclerosis-prone WHHL Rabbits (the WHHL-CA Rabbit) and Myocardial Infarction-Prone WHHL Rabbits (the WHHLMI Rabbit)
Table 3 summarizes the characteristics of coronary lesions elucidated in studies using the WHHL rabbit family. In the proband mutant rabbit of WHHL rabbits, atherosclerosis was developed in the coronary arteries, as well as in the aorta9). However, in the original WHHL rabbit strain before 1980, the incidence of coronary atherosclerosis was very low2). The serum cholesterol levels were relatively lower in the proband mutant rabbit (216–450 mg/dl)9) than in the original WHHL rabbit strain (518 ± 129 mg/dl)1). The decreased incidence of coronary lesions may be due to cross-breeding with normal rabbits during the development of the original WHHL rabbit strain, suggesting that factor(s) other than serum cholesterol levels are associated with the development of coronary lesions (Fig. 1). After the establishment of the original WHHL rabbit strain, Watanabe started a project to increase the incidence of coronary lesions. He performed selective breeding for 5 years using rabbits with coronary lesions. After selective breeding, the incidence of coronary lesions was markedly increased. However, the degree of coronary stenosis was still mild3). After 7 years of the second selective breeding for increasing the degree of coronary stenosis, the degree of cross-sectional narrowing was increased more than two-fold compared with the original WHHL rabbits before the selective breeding3). This strain was provisionally named as the WHHL-CA rabbit. As a result, WHHL-CA rabbits can be used to study coronary atherosclerosis and the effects of statins on coronary atherosclerosis. However, the incidence of myocardial infarction was still very low. In human patients who died from myocardial infarction, many macrophages and/or T-cells were observed at the site of rupture or erosion of thrombosed coronary lesions60). In WHHL-CA rabbits, quantitative analyses of coronary lesions showed that coronary lesions were rich in collagen fibers, but aortic lesions were rich in macrophages and extracellular lipid deposits61). This analytical method was the first in the world and is widely used now. We added macrophage-rich coronary lesions to the selection criteria for the development of myocardial infarction and performed the third selective breeding. After 7 years of the third selective breeding, the cumulative incidence of fatal myocardial infarction increased to more than 90% at 30 months old4). Based on this result, the WHHL-CA rabbit that spontaneously develops myocardial infarction was renamed the WHHLMI rabbit. Myocardial lesions of WHHLMI rabbits were classified into subendocardial, intramural, and transmural infarctions (Fig. 3). Myocardial lesions of WHHLMI rabbits were myocardial fibrosis accompanied by eosinophilic myocardial cells, hyperemia, and infiltration of inflammatory cells. The electrocardiogram recorded just before sudden death showed the elevation of ST-segment and deep Q wave. The culprit coronary arteries exhibited more than 90% cross-sectional narrowing with vulnerable features (a necrotic core covered by a macrophage-infiltrated thin fibrous cap). These findings suggest that in WHHLMI rabbits, myocardial lesions progressed by repeated ischemic damage, probably due to tachyarrhythmia and died at the last ischemic attack. In young WHHLMI rabbits (10–15 months old) that suddenly died, no myocardial lesions were observed under a light microscope, although the coronary arteries were observed that were almost occluded by serial atherosclerotic lesions. In this case, fresh ischemic changes in the myocardial cells were detected using an electron microscope62). These young rabbits probably died by the first ischemic attack. There were no gender differences in the cumulative incidence of fatal myocardial infarction63). The incidence of myocardial infarction increased with the progression of coronary stenosis, although serum lipid levels were not associated with the incidence of myocardial infarction in WHHLMI rabbits. Therefore, the development of coronary lesions and/or myocardial infarction may be regulated by some genetic factor(s) other than serum cholesterol levels in WHHLMI rabbits. Similar to human coronary lesions30), various types of coronary lesions were observed in WHHLMI rabbits (Fig. 3), such as fatty streak, fibrous lesion, fibroatheroma, thin-capped fibroatheroma, and advanced complicated lesions with reduced cellular components, calcification, vasa vasorum, etc.22, 23, 29). However, physiological intimal thickening in normal arteries observed in humans30) was not observed in WHHLMI rabbits. WHHLMI rabbits promoted more studies of coronary lesions because severe coronary lesions always developed in WHHLMI rabbits compared with WHHL-CA rabbits. Quantitative analysis of the lesion components revealed differences between the aortic lesions and coronary lesions61). It has been clarified that the vasoconstrictor response was enhanced in the atherosclerotic coronary arteries64). This study suggests that the development of atherosclerotic lesions is likely to cause coronary artery spasm and has led to later studies on the provocation of acute coronary syndromes by spasm22, 65). It is well known that coronary arteries expand in response to the progression of atherosclerotic lesions, and this coronary artery enlargement is called compensatory remodeling. WHHLMI coronary arteries also expanded at the site of arterial lesions, but the coronary lumen area was nearly constant in the range of 10%–68% cross-sectional narrowing66). This coronary outward remodeling in WHHLMI rabbits was caused by attenuation of the tunica media by infiltration of macrophages into the tunica media, which expressed matrix metalloproteinase, and proliferation of smooth muscle cells on the adventitia side of the tunica media67). This coronary artery enlargement was not a mere physiological compensation. In addition, the degree of curvature of coronary arteries is related to the progression of lesions and the macrophage content in the lesions68). Thus, WHHL-CA rabbits and WHHLMI rabbits contribute to studies of coronary lesions that cannot be analyzed in studies of aortic lesions.
Table 3. Characteristics of coronary lesions elucidated in studies using WHHL rabbit family.
|
Provocation of Acute Coronary Syndromes in WHHLMI Rabbits
On coronary angiography performed just before the onset of myocardial infarction, 67% of patients had coronary lesions with diameter stenosis less than 50%69). Falk reported that occlusive thrombi following the rupture of the coronary plaque were associated with coronary events70). These observations indicate that in human ischemic heart events, coronary lesions prone to rupture were more frequent than coronary lesions with higher stenosis. Although most WHHLMI rabbits had coronary lesions with more than 90% cross-sectional narrowing, several WHLMI rabbits died from myocardial infarction had coronary lesions less than 70% cross-sectional narrowing63). Furthermore, no clear rupture of these lesions was observed in WHHLMI rabbits, although coronary lesions that appeared vulnerable were observed in WHHLMI rabbits4). These observations suggest that the second stimulation, such as mechanical force, plays an important role in the rupture of vulnerable lesions. We provoked coronary spasm by an intravenous injection of ergonovine during an intravenous infusion of norepinephrine22, 65). As a result, serum markers for myocardial injury (heart-type fatty acid-binding protein, cardiac troponin-I, and myoglobin) were markedly increased 4 h after spasm provocation, and fractional shortening of the left ventricle evaluated by echocardiogram was decreased at spasm provocation. In coronary lesions, a mild injury was observed in 83% of rabbits, and occlusive thrombus following lesion disruption (Fig. 3E) was observed in 9% of rabbits. In human acute coronary syndromes, no occlusive thrombus has been observed in the coronary arteries in about one-fifth of patients71). These results suggest that coronary spasm can be a cause of acute coronary syndromes. Infusion of angiotensin II using an osmotic pump caused coronary plaque erosion and rupture that were associated with thrombosis in WHHLMI rabbits24). In addition, a mild increase in blood pressure due to surgical treatment caused myocardial infarction along with myocardial hypertrophy28). Therefore, WHHLMI rabbits can be an animal model for acute coronary syndromes.
Arterial Lesions Developed in Other Arteries
In addition to coronary arteries and aortas, arterial lesions were observed in cerebral arteries, vertebral arteries, carotid arteries, brachiocephalic arteries, pulmonary arteries, celiac arteries, superior mesenteric arteries, renal arteries, common iliac arteries, and femoral arteries in the WHHL rabbit family fed with normal chow13, 15). However, lesion composition depends on the arteries. Fibrous lesions are frequently observed in cerebral arteries, vertebral arteries, renal arteries, and iliac-femoral arteries. Pulmonary lesions are rich in foam cells derived from macrophages. Various lesions, including atheroma, are observed in carotid arteries, brachiocephalic arteries, celiac arteries, and superior mesenteric arteries. However, arterial lesions were not observed in intracranial and intravisceral small arteries13, 15). Differences in lesion components in various arteries may depend on the differences in arterial structure, blood flow, blood pressure, and risk factors dependent on each arterial lesion.
Genome Analyses of WHHLMI Rabbits and Identification of Serum Markers Specific for Coronary Atherosclerosis
In 2016, the whole genome of WHHLMI rabbits was analyzed and compared with those of Japanese white rabbits and New Zealand white rabbits72). Although the authors described “genome information of WHHL rabbits” in the paper, they used “WHHLMI rabbits” in their study. In phylogenic tree analyses, three rabbit groups were different among each group. The genetic diversity of WHHLMI rabbits was low compared with other groups due to selective breeding for myocardial infarction. Deleterious mutation was observed in 25 genes on 15 chromosomes in WHHLMI rabbits, including genes that may be involved in inflammation or the development and progression of atherosclerosis, such as ALDH2, VWF, DOCK4, OLR1, CRHR2, and HCK. Analyzed WHHLMI rabbits had a large variation in the severity of coronary plaques (data not shown), although every WHHLMI rabbit has the same mutation at the same loci. Therefore, these mutations were not associated with the progression of coronary lesions and the development of myocardial infarction. In addition, the gene abnormality in LDLR was a 12 base pair deletion in a LDL binding domain in a previous study38), but in this analysis it was an 11 base pair deletion. Rabbit genome information is now available from the National Center for Biotechnology Information database at http://www.ncbi.nlm.nih.gov/sra, and a comprehensive database containing both rabbit genome and transcriptome information has been comprehensively constructed by the Chinese Academy of Sciences73) at http://www.picb.ac.cn/RabGTD/. Fan et al.74) identified 29.8 million single nucleotide polymorphisms and 1.6 million small indels in the 30 genomes in WHHLMI rabbits in the above rabbit genome analyses, suggesting the difficulty in the detection of the gene(s) associated with the progression of coronary lesions and the development of myocardial infarction in WHHLMI rabbits.
We attempted to identify serum markers for the progression of coronary lesions and/or the development of myocardial infarction in WHHLMI rabbits75). Using WHHLMI rabbits has several advantages in studies of identification of serum markers for coronary lesions; 1) it is easy to keep constant several factors, such as dietary habits, environmental conditions, non-target diseases, and social stress; 2) there is no influence of artificial factors that occur when atherosclerotic lesions are induced artificially; 3) coronary lesions and/or myocardial infarction are developed spontaneously. Our previous studies suggested that factors associated with coronary lesions differ from those of aortic lesions68, 75). Because there were gender differences in the metabolome analysis of 59 metabolites and lipidome analysis of 313 lipid molecules, we analyzed data for females with large coronary artery disease deviations75). In female WHHLMI rabbits, molecules selected as serum markers for coronary lesions were lysophosphatidylcholine (LPC) 22:4 and diacylglycerol 18:0–18:0 at 4 months old, LPC 20:4 (sn-2), ceramide d18:1–18:2, citric acid plus isocitric acid, pyroglutamic acid at 8 months old, and phosphatidylethanolamine plasminogen 16:1p-22:2 at 16 months old. These markers were coronary lesion-specific markers independent of aortic lesions and conventional markers such as serum cholesterol levels. Although further studies will be required to extrapolate these data to humans, these serum markers may be useful to detect patients who will develop cardiovascular disease in the near future.
Genetically Modified WHHL Rabbits and Gene Therapy
Genetically modified animals are essential for elucidating the pathogenesis of diseases and for developing gene therapy technology. Improvement in gene-editing technology has made it possible to create genetically modified animals in various animal species. However, there is a difference in the characteristic phenotype of lipoproteins and atherosclerosis between mice and rabbits after gene transfection76), and the characteristics of genetically modified mice do not always reflect human pathophysiological conditions76). The first gene transfer to WHHL rabbits was the transfer of the LDL receptor gene into the liver in 1988 77), and 32 studies were reported. The first transgenic WHHL rabbit was developed in 1993 78), and 9 studies were reported. Fan et al.79) tabulated genetically modified rabbits (including knockout rabbits) related to lipid metabolism and atherosclerosis which were developed by 2018. Some of the gene-modified rabbits were cross-bred with the WHHL rabbit family to transfer a target gene to the WHHL rabbit family, and the influence of these genes on atherogenesis was examined80, 81). Studies using transgenic WHHL rabbits revealed that Lp(a) enhanced atherosclerosis80) and overexpression of LPL improved hypercholesterolemia and obesity, but enhanced atherosclerosis81), probably due to an increase in the expression of LPL on vascular macrophages. Gene transfer into WHHLMI rabbits and the development of genetically modified WHHLMI rabbits will be useful for identifying genes involved in the progression or suppression of atherosclerosis and elucidating the pathophysiological conditions. In 2014, Yang et al.82) developed LDL receptor knockout rabbits using the gene-editing method, but they did not refer to coronary lesions. In our experience, LDL receptor deficiency in WHHL rabbits is responsible for hypercholesterolemia and aortic atherosclerosis, but the progression of coronary atherosclerosis is regulated by unknown factors75). There was a great variation in the severity of coronary lesions in the homozygous offspring obtained by cross-breeding the homozygous WHHLMI rabbits with the heterozygotes (unpublished results), despite similar levels of serum lipid levels and aortic lesions. Although the development of the LDLR-knockout rabbits is excellent, it is worrying whether severe coronary lesions always develop.
Conclusion
Since the breeding and providing of WHHL rabbit family have been completed, this review summarized the history of the development and research contribution of the WHHL rabbit family. The WHHL rabbit family has greatly contributed to the elucidation of lipoprotein metabolism and the pathogenesis of atherosclerosis and coronary heart disease. Studies using the WHHL rabbit family show that animal models for human disease corresponding well to human pathological conditions can greatly contribute to the progression of biomedical science. Finally, information on the WHHL rabbit family and a list of research articles using the WHHL rabbit family can be found on the WHHL website (http://www.med.akita-u.ac.jp/∼doubutu/WHHL/WHHL-home.html).
Acknowledgments
This author is grateful to the many pharmaceutical companies listed below for supporting the breeding of WHHL rabbit family; Sankyo Co., Ltd, Japan; Daiichi-Sankyo Co., Ltd, Japan; Takeda Pharmaceutical Company, Japan; Nippon Shinyaku Co. Ltd., Japan; Bayer Yakuhin, Ltd., Japan; Novartis Pharma K.K., Japan; Otsuka Pharmaceutical Co. Ltd., Japan; Banyu Pharmaceutical Co., Ltd., Japan, Shionogi & Co., Ltd, Japan; Taisho Pharmaceutical Co., Ltd., Japan; Kissei Pharmaceutical Co., Ltd., Japan; Ono Pharmaceutical Co., Ltd., Japan; Fujirebio Inc., Japan; Asahi Kasei Pharmaceutical Co., Ltd., Japan; Mitsubishi Tanabe Pharma Co., Ltd., Japan; and Warner Lambert Japan Co., Ltd., Japan. This author is grateful to the researchers who used WHHL rabbit family effectively for their studies and expresses sincere appreciation for the continuous efforts of the staff of the Institute for Experimental Animals, Kobe University Graduate School of Medicine (Kobe, Japan) in maintaining the WHHL rabbit family.
Conflicts of Interests
There is no conflict of interest associated with this manuscript within the past 36 months.
References
- 1). Watanabe Y: Serial inbreeding of rabbits with hereditary hyperlipidemia (WHHL-rabbit). Atherosclerosis, 1980; 36: 261-268 [DOI] [PubMed] [Google Scholar]
- 2). Watanabe Y, Ito T, Shiomi M: The effect of selective breeding on the development of coronary atherosclerosis in WHHL rabbits, an animal model for familial hypercholesterolemia. Atherosclerosis, 1985; 56: 71-79 [DOI] [PubMed] [Google Scholar]
- 3). Shiomi M, Ito T, Shiraishi M, Watanabe Y: Inheritability of atherosclerosis and the role of lipoproteins as risk factors in the development of atherosclerosis in WHHL rabbits: Risk factors related to coronary atherosclerosis are different from those related to aortic atherosclerosis. Atherosclerosis, 1992; 96: 43-52 [DOI] [PubMed] [Google Scholar]
- 4). Shiomi M, Ito T, Yamada S, Kawashima S, Fan J: Development of an animal model for spontaneous myocardial infarction (WHHLMI rabbit). Arterioscler Thromb Vasc Biol, 2003; 23: 1239-1244 [DOI] [PubMed] [Google Scholar]
- 5). Shiomi M: The history of the WHHL rabbit, an animal model of familial hypercholesterolemia (II). - Contribution to the development and validation of the therapeutics for hypercholesterolemia and atherosclerosis -. J Atheroscler Thromb (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6). Mahley RW, Innerarity TL, Brown MS, Ho YK, Goldstein JL: Cholesteryl ester synthesis in macrophages: stimulation by beta-very low density lipoproteins from cholesterol-fed animals of several species. J Lipid Res, 1980; 21: 970-980 [PubMed] [Google Scholar]
- 7). Fan J, Kitajima S, Watanabe T, Xu J, Zhang J, Liu E, Chen YE: Rabbit models for the study of human atherosclerosis: From pathophysiological mechanisms to translational medicine. Pharmacol Ther, 2015; 146: 104-119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8). Kritchevsky D: Role of cholesterol vehicle in experimental atherosclerosis. Am J Clin Nutr, 1970; 23: 1105-1110 [DOI] [PubMed] [Google Scholar]
- 9). Watanabe Y: Studies on characteristics of spontaneously hyperlipemic rabbits and development of the strains with such property. Bull Azabu Vet Coll, 1977; 2: 99-124 (in Japanese) [Google Scholar]
- 10). Watanabe Y, Ito T, Kondo T: Breeding of a rabbit strain of hyperlipidemia and characteristic of these strain. Exp Anim, 1977; 26: 35-42 (in Japanese) [PubMed] [Google Scholar]
- 11). Tanzawa K, Shimada Y, Kuroda M, Tsujita Y, Arai M, Watanabe Y: WHHL-rabbit: A low density lipoprotein receptor-deficient animal model for familial hypercholesterolemia. FEBS Lett, 1980; 118: 81-84 [DOI] [PubMed] [Google Scholar]
- 12). Kita T, Brown MS, Watanabe Y, Goldstein JL: Deficiency of low density lipoprotein receptors in liver and adrenal gland of the WHHL rabbit, an animal model of familial hypercholesterolemia. Proc Natl Acad Sci USA, 1981; 78: 2268-2272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13). Ito T, Shiomi M: Cerebral atherosclerosis occurs spontaneously in homozygous WHHL rabbits. Atherosclerosis, 2001; 156: 57-66 [DOI] [PubMed] [Google Scholar]
- 14). Kamimura T, Suzuki S, Miyahara K, Shiomi M: Arteriosclerosis in the influx and intravisceral arteries of the liver, kidney and lung of WHHL rabbits. Exp Anim, 2001; 50: 423-426 [DOI] [PubMed] [Google Scholar]
- 15). Nakagawa T, Kikumori A, Kimura N, Shiomi M: Distribution of atherosclerotic lesions in various arteries of WHHLMI rabbits, an animal model of familial hypercholesterolemia. Exp Anim, 2019; 68: 293-300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16). Nakano A, Kinoshita M, Okuda R, Yasuda T, Abe M, Shiomi M: Pathogenesis of tedious xanthoma: histopathological study of the extremities of Watanabe heritable hyperlipidemic rabbits. J Orthop Sci, 2006; 11: 75-80 [DOI] [PubMed] [Google Scholar]
- 17). Kawai K, Maruno H, Watanabe Y, Hirohata K: Fat necrosis of osteocytes as a casusative factor in idiopathic osteonecrosis in heritable hyperlipidemic rabbits. Clin Orthop, 1980; 153: 273-282 [PubMed] [Google Scholar]
- 18). Kouchi M, Ueda Y, Horie H, Tanaka K: Ocular lesions in Watanabe heritable hyperlipidemic rabbits. Vet Ophthalmol, 2006; 9: 145-148 [DOI] [PubMed] [Google Scholar]
- 19). Takashima S: Pathological changes in the cochlea of heritable hyperlipidemic (WHHL) rabbits. Practica Oto-Rhino- and Laryngologica, 1985; 78: 1-17 (in Japanese) [Google Scholar]
- 20). Zhang B, Saku K, Hirata K, Liu R, Tateishi K, Yamamoto K, Arakawa K: Insulin resistance observed in WHHL rabbits. Atherosclerosis, 1991; 91: 277-278 [DOI] [PubMed] [Google Scholar]
- 21). Shiomi M, Kobayashi T, Kuniyoshi N, Yamada S, Ito T: Myocardial infarction-prone Watanabe heritable hyperlipidemic rabbits with mesenteric fat accumulation are a novel animal model for metabolic syndrome. Pathobiology, 2012; 79: 329-338 [DOI] [PubMed] [Google Scholar]
- 22). Shiomi M, Ishida T, Kobayashi T, Nitta N, Sonoda A, Yamada S, Koike T, Kuniyoshi N, Murata K, Hirata KI, Ito T, Libby P: Vasospasm of Atherosclerotic Coronary Arteries Precipitates Acute Ischemic Myocardial Damage in Myocardial Infarction-Prone Strain of the Watanabe Heritable Hyperlipidemic Rabbits. Arterioscler Thromb Vasc Biol, 2013; 33: 2518-2523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23). Shiomi M, Fan J: Unstable coronary plaques and cardiac events in myocardial infarction-prone Watanabe heritable hyperlipidemic rabbits: questions and quandaries. Curr Opin Lipidol, 2008; 19: 631-636 [DOI] [PubMed] [Google Scholar]
- 24). Li S, Wang YN, Niimi M, Ning B, Chen Y, Kang D, Waqar AB, Wang Z, Yu Q, Liu E, Zhang J, Shiomi M, Chen YE, Fan J: Angiotensin II Destabilizes Coronary Plaques in Watanabe Heritable Hyperlipidemic Rabbits. Arterioscler Thromb Vasc Biol, 2016; 36: 810-816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25). Hara T, Tsukada N, Okano M, Ishida T, Hirata KI, Shiomi M: Progression of calcific aortic valve sclerosis in WHHLMI rabbits. Atherosclerosis, 2018; 273: 8-14 [DOI] [PubMed] [Google Scholar]
- 26). Ikeda M, Kodama H, Nohara N: Process of foam cell formation in diet-induced hypercholesterolemic rabbit and the Watanabe heritable hyperlipidemic rabbit. J Dermatol, 1987; 14: 305-312 [DOI] [PubMed] [Google Scholar]
- 27). Yoshida M, Masunaga K, Nagata T, Satoji Y, Shiomi M: The effects of chronic hyperlipidemia on bladder function in myocardial infarction-prone Watanabe heritable hyperlipidemic (WHHLMI) rabbits. Neurorol Urodyn, 2010; 29: 1350-1354 [DOI] [PubMed] [Google Scholar]
- 28). Ning B, Chen Y, Waqar AB, Yan H, Shiomi M, Zhang J, Chen YE, Wang Y, Itabe H, Liang J, Fan J: Hypertension enhances advanced atherosclerosis and induces cardiac death in Watanabe heritable hyperlipidemic rabbits. Am J Pathol, 2018; 188: 2936-2947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29). Yamada S, Koike T, Nakagawa T, Kuniyoshi N, Ying Y, Itabe H, Yamashita A, Asada Y, Shiomi M: Morphological features of coronary plaques in WHHLMI rabbits (Oryctolagus cuniculus), an animal model for familial hypercholesterolemia. Exp Anim, 2017; 66: 145-157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30). Virmani R, Kolodgie FD, Burke AP, Fab A, Schwarts SM: Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol, 2000; 20: 1262-1275 [DOI] [PubMed] [Google Scholar]
- 31). Mori Y, Wada H, Nagano Y, Deguchi K, Kita T, Shirakawa S: Hypercoagulable state in the Watanabe heritable hyperlipidemic rabbit, an animal model for the progression of atherosclerosis: effect of probucol on coagulation. Thromb Haemostasis, 1989; 61: 140-143 [PubMed] [Google Scholar]
- 32). Noddo F, Zatta A, Fiorito C, Prosdocimi M, Giorgio Weber G: Hematologic and biochemical profiles of selectively bred WHHL rabbits. Lab Anim Sci, 1993; 43: 319-323 [PubMed] [Google Scholar]
- 33). Bianciardi G, Tanganelli P, Palummo N, Zatta A, Prosdocimi M, Weber G: Platelet function abnormalities in 6–12 month-old Watanabe heritable hyperlipidemic (WHHL) rabbits. Thromb Res, 1991; 64(1): 109-115 [DOI] [PubMed] [Google Scholar]
- 34). Goldstein JL, Brown MS: Binding and degradation of low density lipoproteins by cultured human fibroblasts, comparison of cells from a normal subject and from a patient with homozygous familial hypercholesterolemia. J Biol Chem, 1974; 249: 5153-5162 [PubMed] [Google Scholar]
- 35). Endo A, Kuroda M, Tsujita Y: ML-236A, ML-236B, and ML-236C, new inhibitors of cholesterogenesis produced by Penicillium citrinium. J Antibiot (Tokyo), 1976; 29: 1346-1348 [DOI] [PubMed] [Google Scholar]
- 36). Kita T, Brown MS, Bilheimer DW, Goldstein JL: Delayed clearance of very low density and intermediate density lipoproteins with enhanced conversion to low density lipoprotein in WHHL rabbits. Proc Natl Acad Sci USA, 1982; 79: 5693-5697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37). Dietschy JM, Kita T, Suckling KE, Goldstein JL, Brown MS: Cholesterol synthesis in vivo and in vitro in the WHHL rabbit, an animal with defective low density lipoprotein receptors. J Lipid Res, 1983; 24: 469-480 [PubMed] [Google Scholar]
- 38). Yamamoto T, Bishop RW, Brown MS, Goldstein JL, Russell DW: Deletion in cysteine-rich region of LDL receptor impedes transport to cell surface in WHHL rabbit. Science, 1986; 232: 1230-1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39). Schneider WJ, Brown MS, Goldstein JL: Kinetic defects in the processing of the low density lipoprotein receptor in fibroblasts from WHHL rabbits and a family with familial hypercholesterolemia. Mol Biol Med, 1983; 1: 353-367 [PubMed] [Google Scholar]
- 40). Goldstein JL, Kita T, Brown MS: Defective lipoprotein receptors and atherosclerosis: Lessons from an animal counterpart of familial hypercholesterolemia. N Engl J Med, 1983; 309: 288-296 [DOI] [PubMed] [Google Scholar]
- 41). Kozarsky KF, Bonen DK, Giannoni F, Funahashi T, Wilson JM, Davidson NO: Hepatic expression of the catalytic subunit of the apolipoprotein B mRNA editing enzyme (apobec-1) ameliorates hypercholesterolemia in LDL receptor-deficient rabbits. Hum Gene Ther, 1996; 7: 943-957 [DOI] [PubMed] [Google Scholar]
- 42). Nakamuta M, Oka K, Krushkal J, Kobayashi K, Yamamoto M, Li WH, Chan L: Alternative mRNA splicing and differential promoter utilization determine tissue-specific expression of the apolipoprotein B mRNA-editing protein (Apobec1) gene in mice. Structure and evolution of Apobec1 and related nucleoside/nucleotide deaminases. J Biol Chem, 1995; 270: 13042-13056 [DOI] [PubMed] [Google Scholar]
- 43). Buja LM, Kita T, Goldstein JL, Watanabe Y, Brown MS: Cellular pathology of progressive atherosclerosis in the WHHL rabbit, an animal model of familial hypercholesterolemia. Arteriosclerosis, 1983; 3: 87-101 [DOI] [PubMed] [Google Scholar]
- 44). Rosenfeld ME, Tsukada T, Gown AM, Ross R: Fatty streak initiation in Watanabe heritable hyperlipidemic and comparably hypercholesterolemic fat-fed rabbits. Arteriosclerosis, 1987; 7: 9-23 [DOI] [PubMed] [Google Scholar]
- 45). Rosenfeld ME, Tsukada T, Chait A, Bierman EL, Gown AM, Ross R: Fatty streak expansion and maturation in Watanabe heritable hyperlipidemic and comparably hypercholesterolemic fat-fed rabbits. Arteriosclerosis, 1987; 7: 24-34 [DOI] [PubMed] [Google Scholar]
- 46). Tsukada T, Rosenfeld M, Ross R, Gown AM: Immunocytochemical analysis of cellular components in atherosclerotic lesions: Use of monoclonal antibodies with the Watanabe and fat-fed rabbit. Arteriosclerosis, 1986; 6: 601-613 [DOI] [PubMed] [Google Scholar]
- 47). Amanuma K, Kanaseki T, Ikeuchi Y, Ohkuma S, Takano T: Studies on fine structure and location of lipids in quick-freeze replicas of atherosclerotic aorta of WHHL rabbits. Virchows Arch A, 1986; 410: 231-238 [DOI] [PubMed] [Google Scholar]
- 48). Mowri H, Chinen k, Ohkuma S, Takano T: Peroxidized lipid isolated by HPLC from atherosclerotic aorta. Biochem Int, 1986; 12: 347-352 [PubMed] [Google Scholar]
- 49). Kita T, Nagano Y, Yokode M, Ishii K, Kume N, Ooshima A, Yoshida H, Kawai C: Probucol prevents the progression of atherosclerosis in Watanabe heritable hyperlipidemic rabbit, an animal model for familial hypercholesterolemia. Proc Natl Acad Sci USA, 1987; 84: 5928-5931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50). Carew TE, Schwenke DC, Steinberg D: Antiatherogenic effect of probucol unrelated to its hypercholesterolemic effect: Evidence that antioxidants in vivo can selectively inhibit low density lipoprotein degradation in macrophage-rich fatty streaks and slow the progression of atherosclerosis in the Watanabe heritable hyperlipidemic rabbits. Proc Natl Acad Sci USA, 1987; 84: 7725-7729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51). Haberland ME, Fong D, Cheng L: Malondialdehydealtered protein occurs in atheroma of Watanabe heritable hyperlipidemic rabbits. Science, 1988; 241: 215-218 [DOI] [PubMed] [Google Scholar]
- 52). Cybulsky MI, Gimbrone MA: Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science, 1991; 251: 788-791 [DOI] [PubMed] [Google Scholar]
- 53). Ross R, Glomset JA: The pathogenesis of atherosclerosis. N Engl J Med, 1976; 295: 369-377 [DOI] [PubMed] [Google Scholar]
- 54). Ross R, Glomset JA: The pathogenesis of atherosclerosis. N Engl J Med, 1976; 295: 420-425 [DOI] [PubMed] [Google Scholar]
- 55). Ross R: The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature, 1993; 236: 801-809 [DOI] [PubMed] [Google Scholar]
- 56). Ross R: Atherosclerosis-an inflammatory disease. N Engl J Med, 1999; 340: 115-126 [DOI] [PubMed] [Google Scholar]
- 57). Libby P: Inflammation in atherosclerosis. Nature, 2002; 420: 868-874 [DOI] [PubMed] [Google Scholar]
- 58). Takahashi S, Ito T, Zenimaru Y, Suzuki J, Miyamori I, Takahashi M, Takahashi M, Ishida T, Ishida T, Hirata K, Yamamoto TT, Iwasaki T, Hattori H, Shiomi M: Species differences of macrophage very low-density-lipoprotein (VLDL) receptor protein expression. Biochem Biophys Res Commun, 2011; 407: 656-662 [DOI] [PubMed] [Google Scholar]
- 59). Rapp JH, Lespine A, Hamilton RL, Colyvas N, Chaumeton AH, Tweedie-Hardman J, Kotite L, Kunitake ST, Havel RJ, Kane JP: Triglyceride-rich lipoproteins isolated by selected-affinity anti-apolipoprotein B immunosorption from human atherosclerotic plaque. Arterioscler Thromb, 1994; 14: 1767-1774 [DOI] [PubMed] [Google Scholar]
- 60). van der Wal AC, Becker AE, van der Loos CM, Das PK: Site of intimal rupture or erosion of thrombosed coronary atherosclerotic plaques is characterized by an inflammatory process irrespective of the dominant plaque morphology. Circulation, 1994; 89: 36-44 [DOI] [PubMed] [Google Scholar]
- 61). Shiomi M, Ito T, Tsukada T, Yata T, Ueda M: Cell compositions of coronary and aortic atherosclerotic lesions in WHHL rabbits differ: an immunohistochemical study. Arterioscler Thromb, 1994; 14: 931-937 [DOI] [PubMed] [Google Scholar]
- 62). Shiomi M, Fan J, Kawashima S: Relation of vulnerable plaque to development of myocardial infarction / acute coronary syndromes — Lessons from an animal model of spontaneous myocardial infarction. Commentary to the International Atherosclerosis Society, IAS Website (http://www.athero.org/comm-index.asp), Sep 5, 2003 [Google Scholar]
- 63). Ito T, Yamada S, Shiomi M: Progression of coronary atherosclerosis relates to the onset of myocardial infarction in an animal model of spontaneous myocardial infarction (WHHLMI rabbits). Exp Anim, 2004; 53: 339-346 [DOI] [PubMed] [Google Scholar]
- 64). Ishida T, Kawashima S, Hirata K, Sakoda T, Shimokawa Y, Miwa Y, Inoue N, Ueyama T, Shiomi M, Akita H, Yokoyama M: Serotonin-induced hypercontraction through 5-hydroxytryptamine 1B receptors in atherosclerotic rabbit coronary arteries. Circulation, 2001; 103: 1289-1295 [DOI] [PubMed] [Google Scholar]
- 65). Koike T, Tamura S, Yu Y, Kuniyoshi N, Shiomi M: High susceptibility of atherosclerotic coronary arteries to the onset of vasospasm and angina pectoris-like symptoms due to coronary spasm in WHHLMI rabbits. Exp Anim, 2016; 65: 419-426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66). Shiomi M, Ito T, Hasegawa M, Yoshida K, Gould KL: Novel insights into coronary lumen preservation during progression of coronary atherosclerosis in coronary atherosclerosis-prone rabbits. Coronary Artery Disease, 2004; 15: 419-426 [DOI] [PubMed] [Google Scholar]
- 67). Shiomi M, Yamada S, Matsukawa A, Itabe H, Ito T: Invasion of atheromatous plaques into tunica media causes coronary outward remodeling in WHHLMI rabbits. Atherosclerosis, 2008; 198: 287-293 [DOI] [PubMed] [Google Scholar]
- 68). Nagasaka R, Koike T, Tsukada N, Tamura S, Shiomi M: The coronary artery running pattern is one of the causes of individual differences in the progression of coronary atherosclerosis in WHHLMI rabbits, an animal model for coronary atherosclerosis. J Atheroscler Thromb, 2018; 25: 393-404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69). Falk E, Shah PK, Fuster V: Coronary plaque disruption. Circulation, 1995; 92: 657-671 [DOI] [PubMed] [Google Scholar]
- 70). Falk E: Plaque rupture with severe pre-existing stenosis precipitating coronary thrombosis: Characteristics of coronary atherosclerotic plaques underlying fatal occlusive thrombi. Br Heart J, 1983; 50: 127-1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71). Crea F, Libby P: Acute coronary syndromes: The way forward from mechanisms to precision treatment. Circulation, 2017; 136: 1155-1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72). Wang Z, Zhang J, Li H, Li J, Niimi M, Ding G, Chen H, Xu J, Zhang H, Xu Z, Dai Y, Gui T, Li S, Liu Z, Wu S, Cao M, Zhou L, Lu X, Wang J, Yang J, Fu Y, Yang D, Song J, Zhu T, Li S, Ning B, Wang Z, Koike T, Shiomi M, Liu E, Chen L, Fan J, Chen YE, Li Y: Hyperlipidemia-associated gene variations and expression patterns revealed by whole-genome and transcriptome sequencing of rabbit models. Sci Rep, 2016; 6: 26942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73). Zhou L, Xiao Q, Bi J, Wang Z, Li Y: Rabgtd: A comprehensive database of rabbit genome and transcriptome. Database, 2018, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74). Fan J, Chen Y, Yan H, Liu B, Wang Y, Zhang J, Chen YE, Liu E, Liang J: Genomic and Transcriptomic Analysis of Hypercholesterolemic Rabbits: Progress and Perspectives. Int J Mol Sci, 2018; 19: E3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75). Shiomi M, Takeda H, Irino Y, Kimura N, Yamada S, Kuniyoshi N, Kikumori A, Yu Y, Koike T, Yoshida M, Izumi Y, Shinohara M, Bamba T, Ishida T: Identification of novel serum markers for the progression of coronary atherosclerosis in WHHLMI rabbits, an animal model of familial hypercholesterolemia. Atherosclerosis, 2019; 284: 18-23 [DOI] [PubMed] [Google Scholar]
- 76). Fan J, Watanabe T: Transgenic rabbits as therapeutic protein bioreactors and human disease models. Pharmacol Ther, 2003; 99: 261-282 [DOI] [PubMed] [Google Scholar]
- 77). Wilson JM, Johnston DE, Jefferson DM, Mulligan RC: Correction of the genetic defect in hepatocytes from the Watanabe heritable hyperlipidemic rabbit. Proc Natl Acad Sci USA, 1988; 85: 4421-4425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78). Brousseau ME, Hoeg JM: Transgenic rabbits as models for atherosclerosis research. J Lipid Res, 1999; 40: 365-375 [PubMed] [Google Scholar]
- 79). Fan J, Chen Y, Yan H, Niimi M, Wang Y, Liang J: Principles and applications of rabbit models for atherosclerosis research. J Atheroscler Thromb, 2018; 25: 213-220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80). Fan J, Challah M, Shimoyamada H, Shiomi M, Marcovina S, Watanabe T: Defects of the LDL receptor in WHHL transgenic rabbits lead to a marked accumulation of plasma lipoprotein(a). J Lipid Res, 2000; 41: 1004-1012 [PubMed] [Google Scholar]
- 81). Koike T, Liang J, Wang X, Ichikawa T, Shiomi M, Liu G, Sun H, Watanabe T, Yamada N, Fan J: Overexpression of lipoprotein lipase in transgenic Watanabe heritable hyperlipidemic rabbits improves hyperlipidemia and obesity. J Biol Chem, 2004; 279: 7521-7529 [DOI] [PubMed] [Google Scholar]
- 82). Yang D, Xu J, Zhu T, Fan J, Lai L, Zhang J, Chen YE: Effective gene targeting in rabbits using RNA-guided Cas9 nuclease. J Mol Cell Biol, 2014; 6: 97-99 [DOI] [PMC free article] [PubMed] [Google Scholar]


