Abstract
Cell junctions serve as a protective barrier for cells and provide an important channel for information transmission between cells and the surrounding environment. Viruses are parasites that invade and commandeer components of host cells in order to survive and replicate, and they have evolved various mechanisms to alter cell junctions to facilitate viral infection. In this review, we examined the current state of knowledge on the action of viruses on host cell junctions. The existing evidence suggests that targeting the molecules involved in the virus‐cell junction interaction can prevent the spread of viral diseases.
Keywords: Adherens junction, gap junction, infection, tight junction, virus
Introduction
Emerging and re‐emerging infectious diseases pose an increasing threat to global health.1, 2, 3 Clarifying the molecular mechanisms underlying viral infection can improve the detection, control, and treatment of viral diseases.4, 5, 6, 7, 8 Viruses are noncellular life forms composed of proteins and a DNA or RNA genome wrapped in a protective protein coat. As parasites, viruses infect an organism and self‐replicate using host cellular components.9, 10, 11 The first step in this process is invasion of target cells in the host tissue, which typically comprises a layer of epithelial cells connected via intercellular junctions. These junctions allow the transmission of information between cells and the surrounding environment and serve as a protective barrier against noxious stimuli.12 The attachment of a virus to the host cell membrane can alter or destroy junctional proteins, leading to cell infection.13, 14, 15, 16
Mammalian cell junctions are classified based on their function as tight junctions, anchoring junctions (adherens junctions, desmosomes, and hemidesmosomes), and communicating (gap) junctions.17, 18 Tight junctions are present in the gastrointestinal epithelium, bladder epithelium, brain capillary endothelium, and in testicular supporting cells, and form a branching network of sealing strands, each of which contains a row of transmembrane proteins that are inserted into the bilayers of the plasma membrane and are connected to other proteins through their extracellular domains.19, 20, 21 Tight junctions do not constitute a static barrier, and are highly dynamic structures whose components (eg, occludin) undergo continuous turnover.22 Anchoring junctions provide a mechanical connection between cells; they can be one of two types depending on their constituent cytoskeletal proteins.23, 24, 25, 26 Desmosomes and hemidesmosomes are linked to intracellular filaments, whereas adherens junctions are linked to actin.27 Adherens junctions serve as anchors that connect the actin cytoskeletons of adjacent cells via cadherin.28 These different types of anchoring junctions form an epithelial barrier that controls paracellular transport. Gap junctions enable communication between adjacent cells by allowing the movement of small molecules and ions in the cytoplasm in response to various signals. They also play an important role in regulating cell proliferation and differentiation during embryonic development.29, 30, 31, 32, 33
The major functions of cell junctions are to strengthen mechanical connections and permit the exchange of materials between cells to maintain physiological homeostasis. In this review, we summarize recent studies investigating the action of viruses on host cell junctions (Fig 1) and suggest that the molecules involved in this interaction are potential therapeutic targets for the treatment of viral diseases.
Figure 1.
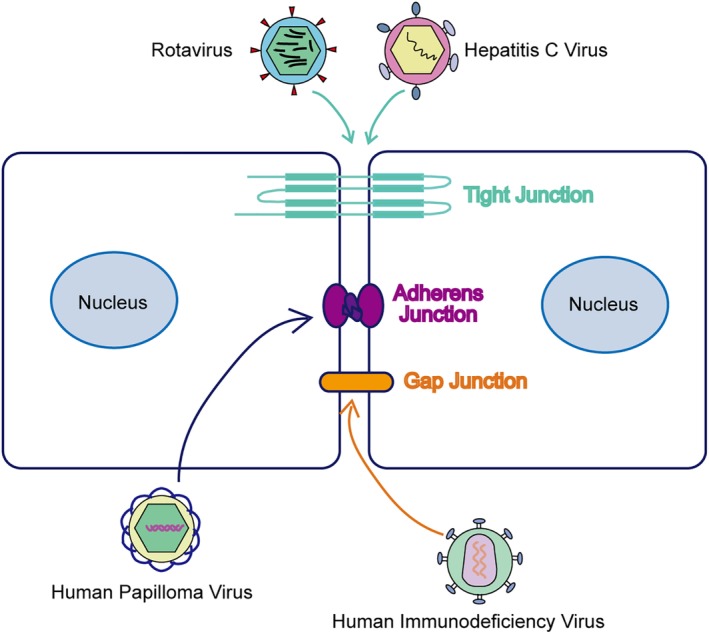
Different viruses invade host cells through specific cell junctions. Rotavirus and Hepatitis C virus disrupt the structure and function of tight junction. Human papilloma virus‐induced changes in the organization of adherens junction proteins. Human immunodeficiency virus spread damaged signals to the adjacent cells through gap junction.
Rotavirus (RV) and tight junctions
Rotavirus (RV) is the most common cause of severe vomiting and diarrhea in infants and young children.34 RV is an enterovirus with a wheel‐like structure that is assembled in the lumen of the endoplasmic reticulum (ER); subviral particles germinate until the cells are lysed, with the mature virus remaining in the ER. RV enters cells via receptor‐mediated calcium‐dependent endocytosis, which causes calcium ions (Ca2+) to move from the endocytic vesicle to the cytoplasm.35 Once the Ca2+ concentration in the endocytic vesicle decreases below a certain threshold, the coat proteins of endocytic vesicles are degraded and the virus enters the cytosol.36
The tight junction protein occludin is distributed in the margins of adjacent cells under normal conditions. However, this arrangement is perturbed upon RV infection.37 For instance, in infantile diarrhea caused by RV, the structure and function of tight junctions are disrupted, leading to changes in cell membrane permeability that enable RV to invade host cells.38 In Caco‐2 cells, the distribution of the tight junction proteins occludin and claudin‐1 was altered by incubation with RV.39 Meanwhile, MDCKII cells were infected with RV through the basal surface, suggesting that this area harbors RV receptors.40 The primary site of RV infection is along the edge of intestinal epithelial cells.41, 42 The binding of the RV coat protein viral protein 8 to receptors located on the intestinal cell surface leads to the destruction of tight junctions by activating the host cell RhoA/ROCK / MLC signaling pathway, which stimulates the translocation of viral receptors from the basolateral to the apical surface and further increases RV invasion.43, 44 RhoA and its downstream effector Rho kinase (ROCK) are key molecules that mediate destruction of tight junction when RV infection.45 Thus, disruption of tight junctions may play an important role in the pathogenesis diarrhea caused by RV.46
Hepatitis C virus (HCV) and tight junctions
Hepatitis C is an infectious disease caused by HCV that mainly affects the liver.47 HCV is a small, enveloped, positive‐strand RNA virus that spreads through tight junctions and infects liver cells. Cellular entry of HCV is accomplished by its binding to tight junction‐associated coreceptors on hepatocytes and subsequent endocytosis.48 The tight junction proteins occludin and claudin‐1 are the key molecules involved in this process. HCV was shown to readily infect and escape hepatocellular carcinoma (HCC) cells that were modified to express claudin‐1 and occludin.49 The GTPase protein dynamin II plays an important role in HCV internalization by forming a complex with occludin, which serves as a bridge between dynamin II and viral particles.50
The tetraspanin molecule CD81 and human scavenger receptor class B member 1 are HCV receptors that cooperate with tight junction proteins to facilitate HCV entry into liver cells.51 Viral particles first bind to glycosaminoglycans or low‐density lipoprotein receptor on the host cell.52 This is followed by interaction with claudin‐1 at tight junctions and CD81 and the lateral migration of the virus through the plasma membrane, which continues until the virus has recruited a sufficient number of receptors to initiate the signaling required for internalization.53 The combination of HCV and CD81 will promote the movement of the virus to tight junction associated proteins claudin‐1 and occludin‐1. Intracellular CD81 and claudin‐1 are colocalized on the plasma membrane and transported on the plasma membrane. CD81 and claudin‐1 containing vesicles fused to Rab5 expressing endosomes.54, 55 However, it remains to be determined whether claudin or occludin protein mediate endocytosis of the virus.56 Clarifying the precise role of tight junction proteins at different stages of HCV infection can inspire new strategies to prevent and treat hepatitis C.
Human papilloma virus (HPV) and adherens junctions
HPV is a spherical DNA virus that stimulates the proliferation of squamous epithelial cells of skin mucosa in humans.57, 58, 59, 60, 61 Infection of cervical epithelial cells with HPV leads to cervical cancer in women.62 This is a result of a loss of cell adhesion and polarity, allowing the invasion and migration of tumor cells.63, 64, 65 This metastatic transformation involves alterations in adherens junction proteins that undermine epithelial cell structure.66 During the epithelial‐to‐mesenchymal transition, epithelial cells lose polarity and their connection to adjacent cells and the basement membrane, which increases their migratory and invasive capacities.67 At the molecular level, this process involves the rearrangement of adherens junction proteins including β‐catenin, which links E‐cadherin to the actin cytoskeleton and is involved in cancer‐related signaling and inflammatory responses.68, 69, 70, 71 Thus, HPV‐induced changes in the organization of adherens junction proteins promotes infection.
Human immunodeficiency virus (HIV) and gap junctions
HIV is the causative agent of acquired immunodeficiency syndrome (AIDS).72, 73, 74 During infection, HIV targets the cytomembrane and penetrates the epithelial barrier by destroying cell junctions.75 The blood‐brain barrier (BBB) is a highly selective semi‐permeable boundary that separates circulating blood from extracellular fluids in the brain and central nervous system (CNS).76, 77 The pericytes and perivascular astrocytes that constitute the BBB differentially modulate neurovascular function in neuroAIDS pathogenesis.78 Gap junctions, which are composed of connexin proteins, are abundant in the cells of the BBB and mediate intercellular communication in the CNS.
Astrocytes are the most widely distributed cells in the mammalian brain and the largest type of glial cell. Adjacent astrocytes are separated by a narrow space containing interstitial fluid. HIV uses channels containing connexins including connexin 43 in astrocytes to spread toxic factors to and induce apoptosis in uninfected cells, even in the absence of active viral replication.79, 80, 81 Although the rate of viral replication in astrocytes is too low to be detected, disruption of connexin channels by HIV can exacerbate neurological pathophysiology.82 This opens the possibility of mitigating HIV‐associated neurological dysfunction by targeting gap junctions in astrocytes.
Pericytes are located below the brain microvascular endothelial cells, covering approximately 30% of the abluminal surface.83 Pericytes in the human brain express C‐X‐C chemokine receptor type (CXCR)4 and CCR5, which are the two major co‐receptors participating in the HIV‐1 infection process. CXCR4 and CXCR5 contribute to HIV‐induced CNS impairment when the BBB is compromised, which is associated with increased microvascular permeability.84 Thus, gap junctions in pericytes mediate HIV‐induced loss of BBB integrity. Additionally, although HIV infects only a small fraction of pericytes, the damage that it inflicts is amplified by gap junctions that spread viral factors to adjacent uninfected cells (Fig 2).
Figure 2.

HIV hijack gap junction to spread toxic factors and damaged signals to the adjacent uninfected cells. In pericytes, although HIV only infects a small number of cells, the damage is amplified by the spread of viral factors to adjacent uninfected cells via the gap junction channel. ( The purple cell) HIV infected cell, (The green cells
The purple cell) HIV infected cell, (The green cells ) Uninfected cell, (
) Uninfected cell, ( The yellow gap junctions) Normal gap junction, and (
The yellow gap junctions) Normal gap junction, and ( The purple gap junctions) HIV “Hijacked” gap junction.
The purple gap junctions) HIV “Hijacked” gap junction.
HIV does not primarily disrupt the cell junction of the BBB. It also has a disruptive effect on the cell junction of other tissues. HIV‐1 can damage the human retinal pigment epithelium (HRPE) barrier. HIV‐1 particles can induce cells to release proinflammatory cytokines IL‐6 and MCP‐1, which down‐regulate the expression of ZO‐1 and claudin‐1 in the HRPE barrier, leading to the destruction of HRPE cell junction and impaired cell monolayer integrity.85, 86, 87 HIV also disrupts cellular junctions in oral epithelial tissue. The long‐term interaction between HIV capsid protein and polarized oral epithelial cells destroys tight junctions and adherens junctions of epithelial cells through the mitogen‐activated protein kinase signaling pathway.88 HIV also infects intestinal epithelium, gastric epithelium and other tissues by affecting cell junctions. It is of great significance to study the key sites of cell junctions during HIV invasion.89, 90
Conclusions
Nonenveloped viruses initiate the infection cycle through binding of their capsid proteins to a viral receptor on the surface of target cells. This activates intracellular signaling pathways, which is often accompanied by the lateral translocation of the virus across the plasma membrane to cell junctions prior to their internalization via caveolar endocytosis. The interaction of the virus with specific cell junction proteins such as occludin, claudin, or connexin suggests the possibility that proteins at the precise point of cellular entry can be targeted by therapeutics. Blockers of these cell junction proteins can provide protection against viral infection. The mechanism by which cell junctions amplify viral invasion signals and dysfunctional signals increases the rates of viral amplification, so these cells with this particular mechanism should be detected and may be applied to the expansion and delivery of drug therapy signals.91, 92, 93, 94 On the other hand, as‐yet unidentified junctional proteins could contribute to the process of cell invasion by viruses. Elucidating the mechanisms by which viruses exploit host cell junctions to propagate can provide a basis for the development of effective strategies to treat viral infectious diseases.
Disclosure
The authors have no conflicts of interest to declare.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (31701209).
Contributor Information
Dan Dong, Email: 254412470@qq.com.
Wei Xie, Email: wxie@sdnu.edu.cn.
Min Liu, Email: minliu@sdnu.edu.cn.
References
- 1. Wang HM, Hou PL, Zhao GM, Yu L, Gao YW, He HB. Development and evaluation of serotype‐specific recombinase polymerase amplification combined with lateral flow dipstick assays for the diagnosis of foot‐and‐mouth disease virus serotype A, O and Asia1. BMC Vet Res 2018; 14: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. He CQ, Liu YX, Wang HM, Hou PL, He HB, Ding NZ. New genetic mechanism, origin and population dynamic of bovine ephemeral fever virus. Vet Microbiol 2016; 182: 50–6. [DOI] [PubMed] [Google Scholar]
- 3. Zhao GM, Hou PL, Huan YJ, He CQ, Wang HM, He HB. Development of a recombinase polymerase amplification combined with a lateral flow dipstick assay for rapid detection of the Mycoplasma bovis. BMC Vet Res 2018; 14: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dimitrov DS. Virus entry: Molecular mechanisms and biomedical applications. Nat Rev Microbiol 2004; 2: 109–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Du X, Zhou J. Application of biosensors to detection of epidemic diseases in animals. Res Vet Sci 2018; 118: 444–8. [DOI] [PubMed] [Google Scholar]
- 6. Zheng S, Wu X, Zhang L et al The occurrence of porcine circovirus 3 without clinical infection signs in Shandong Province. Transbound Emerg Dis 2017; 64: 1337–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang X, Yang J, Wang H et al Overexpression of Hdac6 extends reproductive lifespan in mice. Protein Cell 2017; 8: 360–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li H, Yang GW, Ma F et al Molecular characterization of a fish‐specific toll‐like receptor 22 (TLR22) gene from common carp (Cyprinus carpio L.): Evolutionary relationship and induced expression upon immune stimulants. Fish Shellfish Immunol 2017; 63: 74–86. [DOI] [PubMed] [Google Scholar]
- 9. Shan SJ, Qi CC, Zhu YY, Li H, An LG, Yang GW. Expression profile of carp IFN correlate with the up‐regulation of interferon regulatory factor‐1 (IRF‐1) in vivo and in vitro: The pivotal molecules in antiviral defense. Fish Shellfish Immunol 2016; 52: 94–102. [DOI] [PubMed] [Google Scholar]
- 10. Zhu YY, Qi CC, Shan SJ et al Characterization of common carp (Cyprinus carpio L.) interferon regulatory factor 5 (IRF5) and its expression in response to viral and bacterial challenges. BMC Vet Res 2016; 12: 127–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li T, Shan SJ, Wang L, Yang GW, Zhu JP. Identification of a fish‐specific NOD‐like receptor subfamily C (NLRC) gene from common carp (Cyprinus carpio L.): Characterization, ontogeny and expression analysis in response to immune stimulation. Fish Shellfish Immunol 2018; 82: 371–7. [DOI] [PubMed] [Google Scholar]
- 12. Arnold TR, Stephenson RE, Miller AL. Rho GTPases and actomyosin: Partners in regulating epithelial cell‐cell junction structure and function. Exp Cell Res 2017; 358: 20–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang D, Meng Q, Huo L et al Overexpression of Hdac6 enhances resistance to virus infection in embryonic stem cells and in mice. Protein Cell 2015; 6: 152–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hou PL, Zhao GM, He CQ, Wang HM, He HB. Biopanning of polypeptides binding to bovine ephemeral fever virus G(1) protein from phage display peptide library. BMC Vet Res 2018; 14: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li H, Li T, Guo YJ et al Molecular characterization and expression patterns of a non‐mammalian toll‐like receptor gene (TLR21) in larvae ontogeny of common carp (Cyprinus carpio L.) and upon immune stimulation. BMC Vet Res 2018; 14: 153–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhao GM, Wang HM, Hou PL, He CQ, He HB. Rapid visual detection of Mycobacterium avium subsp paratuberculosis by recombinase polymerase amplification combined with a lateral flow dipstick. J Vet Sci 2018; 19: 242–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Garcia MA, Nelson WJ, Chavez N. Cell‐Cell Junctions Organize Structural and Signaling Networks. Cold Spring Harb Perspect Biol 2018; 10: a029181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xie W, Yang Y, Gao S et al The tumor suppressor CYLD controls epithelial morphogenesis and homeostasis by regulating mitotic spindle behavior and adherens junction assembly. J Genet Genomics 2017; 44: 343–53. [DOI] [PubMed] [Google Scholar]
- 19. Zihni C, Mills C, Matter K, Balda MS. Tight junctions: From simple barriers to multifunctional molecular gates. Nat Rev Mol Cell Biol 2016; 17: 564–80. [DOI] [PubMed] [Google Scholar]
- 20. Lou MF, Zhang XY, Fu RS, Wang DH. Effects of dietary fiber content on energetics in nonreproductive and reproductive Brandt's voles (Lasiopodomys brandtii). Can J Zool 2015; 93: 251–8. [Google Scholar]
- 21. Zhang F, Huang YH, Liu SZ et al Pseudomonas reactans, a bacterial strain isolated from the intestinal flora of Blattella germanica with anti‐beauveria bassiana activity. Environ Entomol 2013; 42: 453–9. [DOI] [PubMed] [Google Scholar]
- 22. Torres‐Flores JM, Arias CF. Tight junctions go viral! Viruses 2015; 7: 5145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. He X, Liu Z, He Q et al Identification of novel microtubule‐binding proteins by taxol‐mediated microtubule stabilization and mass spectrometry analysis. Thorac Cancer 2015; 6: 649–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Luo Y, Ran J, Xie S a. ASK1 controls spindle orientation and positioning by phosphorylating EB1 and stabilizing astral microtubules. Cell Discovery 2016; 2: 16033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hou L, Chen LJ, Wang JY et al Construction of stress responsive synthetic promoters and analysis of their activity in transgenic Arabidopsis thaliana . Plant Mol Biol Rep 2012; 30: 1496–506. [Google Scholar]
- 26. Xie SB, Zhou J. Harnessing plant biodiversity for the discovery of novel anticancer drugs targeting microtubules. Front Plant Sci 2017; 8: 720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li L, Gao Y, Chen H et al Cell polarity, cell adhesion, and spermatogenesis: Role of cytoskeletons. F1000Research 2017; 6: 1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brucher BL, Jamall IS. Cell‐cell communication in the tumor microenvironment, carcinogenesis, and anticancer treatment. Cell Physiol Biochem 2014; 34: 213–43. [DOI] [PubMed] [Google Scholar]
- 29. Ahir BK, Pratten MK. Structure and function of gap junction proteins: Role of gap junction proteins in embryonic heart development. Int J Dev Biol 2014; 58: 649–62. [DOI] [PubMed] [Google Scholar]
- 30. Meng XQ, Dai YY, Jing LD et al Subcellular localization of proline‐rich tyrosine kinase 2 during oocyte fertilization and early‐embryo development in mice. J Reprod Dev 2016; 62: 351–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sun S, Zhou J. Molecular mechanisms underlying stress response and adaptation. Thorac Cancer 2018; 9: 218–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yang Y, Mu T, Li T et al Effects of FSTL1 on the proliferation and motility of breast cancer cells and vascular endothelial cells. Thorac Cancer 2017; 8: 606–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li D, Sun X, Zhang L et al Histone deacetylase 6 and cytoplasmic linker protein 170 function together to regulate the motility of pancreatic cancer cells. Protein Cell 2014; 5: 214–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Suzuki H. Rotavirus replication: Gaps of knowledge on virus entry and morphogenesis. Tohoku J Exp Med 2019; 248: 285–96. [DOI] [PubMed] [Google Scholar]
- 35. Zhang L, Gao ZY, Yu L, Zhang B, Wang J, Zhou J. Nucleotide‐binding and oligomerization domain (NOD)‐like receptors in teleost fish: Current knowledge and future perspectives. J Fish Dis 2018; 41: 1317–30. [DOI] [PubMed] [Google Scholar]
- 36. Salgado EN, Garcia Rodriguez B, Narayanaswamy N, Krishnan Y, Harrison SC. Visualization of calcium ion loss from rotavirus during cell entry. J Virol 2018; 92: e01327–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Silva‐Ayala D, Lopez T, Gutierrez M, Perrimon N, Lopez S, Arias CF. Genome‐wide RNAi screen reveals a role for the ESCRT complex in rotavirus cell entry. Proc Natl Acad Sci U S A 2013; 110: 10270–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Catto‐Smith AG, Emselle S, Bishop RF. Changes in macromolecular transport appear early in Caco‐2 cells infected with a human rotavirus. Scand J Gastroenterol 2008; 43: 314–22. [DOI] [PubMed] [Google Scholar]
- 39. Beau I, Cotte‐Laffitte J, Amsellem R, Servin AL. A protein kinase A‐dependent mechanism by which rotavirus affects the distribution and mRNA level of the functional tight junction‐associated protein, occludin, in human differentiated intestinal Caco‐2 cells. J Virol 2007; 81: 8579–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Soliman M, Cho EH, Park JG et al Rotavirus‐induced early activation of the RhoA/ROCK/MLC signaling pathway mediates the disruption of tight junctions in polarized MDCK cells. Sci Rep 2018; 8: 13931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yang HT, Zou SS, Zhai LJ et al Pathogen invasion changes the intestinal microbiota composition and induces innate immune responses in the zebrafish intestine. Fish Shellfish Immunol 2017; 71: 35–42. [DOI] [PubMed] [Google Scholar]
- 42. Zhang FM, Liu DZ, Wang L et al Characterization of IgM‐binding protein: A pIgR‐like molecule expressed by intestinal epithelial cells in the common carp (Cyprinus carpio L.). Vet Immunol Immunopathol 2015; 167: 30–5. [DOI] [PubMed] [Google Scholar]
- 43. Nava P, Lopez S, Arias CF, Islas S, Gonzalez‐Mariscal L. The rotavirus surface protein VP8 modulates the gate and fence function of tight junctions in epithelial cells. J Cell Sci 2004; 117: 5509–19. [DOI] [PubMed] [Google Scholar]
- 44. Lopez S, Arias CF. Multistep entry of rotavirus into cells: A Versaillesque dance. Trends Microbiol 2004; 12: 271–8. [DOI] [PubMed] [Google Scholar]
- 45. Zihni C, Balda MS, Matter K. Signalling at tight junctions during epithelial differentiation and microbial pathogenesis. J Cell Sci 2014; 127: 3401–13. [DOI] [PubMed] [Google Scholar]
- 46. Hou PL, Zhao GM, Wang HM, He CQ, He HB. Rapid detection of bovine viral diarrhea virus using recombinase polymerase amplification combined with lateral flow dipstick assays in bulk milk. Vet Arh 2018; 88: 627–42. [Google Scholar]
- 47. Scheel TK, Rice CM. Understanding the hepatitis C virus life cycle paves the way for highly effective therapies. Nat Med 2013; 19: 837–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhu YZ, Qian XJ, Zhao P, Qi ZT. How hepatitis C virus invades hepatocytes: The mystery of viral entry. World J Gastroenterol 2014; 20: 3457–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Farquhar MJ, Hu K, Harris HJ et al Hepatitis C virus induces CD81 and claudin‐1 endocytosis. J Virol 2012; 86: 4305–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Liu S, Kuo W, Yang W et al The second extracellular loop dictates Occludin‐mediated HCV entry. Virology 2010; 407: 160–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Morikawa K, Zhao Z, Date T et al The roles of CD81 and glycosaminoglycans in the adsorption and uptake of infectious HCV particles. J Med Virol 2007; 79: 714–23. [DOI] [PubMed] [Google Scholar]
- 52. Barth H, Schnober EK, Zhang F et al Viral and cellular determinants of the hepatitis C virus envelope‐heparan sulfate interaction. J Virol 2006; 80: 10579–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Shan SJ, Liu RR, Jiang L et al Carp toll‐like receptor 8 (Tlr8): An intracellular Tlr that recruits TIRAP as adaptor and activates AP‐1 pathway in immune response. Fish Shellfish Immunol 2018; 82: 41–9. [DOI] [PubMed] [Google Scholar]
- 54. Harris HJ, Davis C, Mullins JG et al Claudin association with CD81 defines hepatitis C virus entry. J Biol Chem 2010; 285: 21092–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Harris HJ, Farquhar MJ, Mee CJ et al CD81 and claudin 1 coreceptor association: Role in hepatitis C virus entry. J Virol 2008; 82: 5007–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Feneant L, Levy S, Cocquerel L. CD81 and hepatitis C virus (HCV) infection. Viruses 2014; 6: 535–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yang GW, Guo HY, Li H et al Molecular characterization of LEAP‐2 cDNA in common carp (Cyprinus carpio L.) and the differential expression upon a Vibrio anguillarum stimulus; indications for a significant immune role in skin. Fish Shellfish Immunol 2014; 37: 22–9. [DOI] [PubMed] [Google Scholar]
- 58. Rombout J, Yang GW, Kiron V. Adaptive immune responses at mucosal surfaces of teleost fish. Fish Shellfish Immunol 2014; 40: 634–43. [DOI] [PubMed] [Google Scholar]
- 59. Huang YH, Wang XJ, Zhang F et al The Identification of a Bacterial Strain BGI‐1 isolated from the intestinal flora of Blattella germanica, and its anti‐entomopathogenic fungi activity. J Econ Entomol 2013; 106: 43–9. [DOI] [PubMed] [Google Scholar]
- 60. Liu M, Ran J, Zhou J. Non‐canonical functions of the mitotic kinesin Eg5. Thorac Cancer 2018; 9: 904–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Liu GF, Liu XM, Shahzad K, You W, Loor JJ, Wan FC. Bioinformatics analyses of bovine adipose tissue transcriptome from lilu beef cattle at different stages of growth. Pak J Zool 2018; 50: 1847–55. [Google Scholar]
- 62. Wardak S. Human Papillomavirus (HPV) and cervical cancer. Medycyna doswiadczalna i mikrobiologia 2016; 68: 73–84. [PubMed] [Google Scholar]
- 63. Sun GJ, Pan J, Liu KC, Wang SF, Wang X, Wang XM. Molecular cloning and expression analysis of P‐selectin glycoprotein ligand‐1 from zebrafish (Danio rerio). Fish Physiol Biochem 2012; 38: 555–64. [DOI] [PubMed] [Google Scholar]
- 64. Wang F, Yang HJ, He HB et al Study on the hemolysin phenotype and the genetype distribution of staphyloccocus aureus caused bovine mastitis in shandong dairy farms. Int J Appl Res Vet Med 2011; 9: 416–21. [Google Scholar]
- 65. Cui LL, Yang GW, Pan J, Zhang C. Tumor necrosis factor alpha knockout increases fertility of mice. Theriogenol 2011; 75: 867–76. [DOI] [PubMed] [Google Scholar]
- 66. Herfs M, Hubert P, Moutschen M, Delvenne P. Mucosal junctions: Open doors to HPV and HIV infections? Trends Microbiol 2011; 19: 114–20. [DOI] [PubMed] [Google Scholar]
- 67. Cunniffe C, Ryan F, Lambkin H, Brankin B. Expression of tight and adherens junction proteins in cervical neoplasia. Br J Biomed Sci 2012; 69: 147–53. [PubMed] [Google Scholar]
- 68. Heuser S, Hufbauer M, Marx B et al The levels of epithelial anchor proteins beta‐catenin and zona occludens‐1 are altered by E7 of human papillomaviruses 5 and 8. Journal General Virology 2016; 97: 463–72. [DOI] [PubMed] [Google Scholar]
- 69. Liu TT, Liu S, Ma L et al Oogenesis, vitellogenin‐mediated ovarian degeneration and immune response in the annual fish Nothobranchius guentheri . Fish Shellfish Immunol 2017; 66: 86–92. [DOI] [PubMed] [Google Scholar]
- 70. Li T, Li H, Peng SQ, Zhang FM, An LG, Yang GW. Molecular characterization and expression pattern of X box‐binding protein‐1 (XBP1) in common carp (Cyprinus carpio L.): Indications for a role of XBP1 in antibacterial and antiviral immunity. Fish Shellfish Immunol 2017; 67: 667–74. [DOI] [PubMed] [Google Scholar]
- 71. Shan SJ, Liu DZ, Wang L et al Identification and expression analysis of irak1 gene in common carp Cyprinus carpio L.: Indications for a role of antibacterial and antiviral immunity. J Fish Biol 2015; 87: 241–55. [DOI] [PubMed] [Google Scholar]
- 72. Zhang L, Qin J, Li Y et al Modulation of the stability and activities of HIV‐1 Tat by its ubiquitination and carboxyl‐terminal region. Cell Biosci 2014; 4: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Huo L, Li D, Sun X et al Regulation of Tat acetylation and transactivation activity by the microtubule‐associated deacetylase HDAC6. J Biol Chem 2011; 286: 9280–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Huo L, Li D, Sun L et al Tat acetylation regulates its actions on microtubule dynamics and apoptosis in T lymphocytes. J Pathol 2011; 223: 28–36. [DOI] [PubMed] [Google Scholar]
- 75. Veenstra M, Leon‐Rivera R, Li M, Gama L, Clements JE, Berman JW. Mechanisms of CNS viral seeding by HIV(+) CD14(+) CD16(+) monocytes: Establishment and reseeding of viral reservoirs contributing to HIV‐associated neurocognitive disorders. MBio 2017; 8: e01280–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Saylor D, Dickens AM, Sacktor N et al HIV‐associated neurocognitive disorder ‐ Pathogenesis and prospects for treatment. Nat Rev Neurol 2016; 12: 309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Liu M, Xie SB, Zhou J. Use of animal models for the imaging and quantification of angiogenesis. Exp Anim 2018; 67: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Sami Saribas A, Cicalese S, Ahooyi TM, Khalili K, Amini S, Sariyer IK. HIV‐1 Nef is released in extracellular vesicles derived from astrocytes: Evidence for Nef‐mediated neurotoxicity. Cell Death Dis 2017; 8: e2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Eugenin EA, Clements JE, Zink MC, Berman JW. Human immunodeficiency virus infection of human astrocytes disrupts blood‐brain barrier integrity by a gap junction‐dependent mechanism. J Neurosci 2011; 31: 9456–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Chen M, Li Y, Liu Z et al Exopolysaccharides from a Codonopsis pilosula endophyte activate macrophages and inhibit cancer cell proliferation and migration. Thorac Cancer 2018; 9: 630–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Luo Y, Li D, Ran J et al End‐binding protein 1 stimulates paclitaxel sensitivity in breast cancer by promoting its actions toward microtubule assembly and stability. Protein Cell 2014; 5: 469–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Malik S, Theis M, Eugenin EA. Connexin43 containing gap junction channels facilitate HIV bystander toxicity: Implications in NeuroHIV. Front Mol Neurosci 2017; 10: 404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Hill J, Rom S, Ramirez SH, Persidsky Y. Emerging roles of pericytes in the regulation of the neurovascular unit in health and disease. J Neuroimmune Pharmacol 2014; 9: 591–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Cho HJ, Kuo AM, Bertrand L, Toborek M. HIV alters gap junction‐mediated intercellular communication in human brain pericytes. Front Mol Neurosci 2017; 10: 410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Tan S, Duan H, Xun T et al HIV‐1 impairs human retinal pigment epithelial barrier function: Possible association with the pathogenesis of HIV‐associated retinopathy. Lab Invest 2014; 94: 777–87. [DOI] [PubMed] [Google Scholar]
- 86. Runkle EA, Mu D. Tight junction proteins: From barrier to tumorigenesis. Cancer Lett 2013; 337: 41–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ran J, Zhou J. Targeted inhibition of histone deacetylase 6 in inflammatory diseases. Thorac Cancer 2019; 10: 405–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Sufiawati I, Tugizov SM. HIV‐associated disruption of tight and adherens junctions of oral epithelial cells facilitates HSV‐1 infection and spread. PLOS One 2014; 9: e88803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Liu R, Huang L, Li J et al HIV Infection in gastric epithelial cells. J Infect Dis 2013; 208: 1221–30. [DOI] [PubMed] [Google Scholar]
- 90. Epple HJ, Zeitz M. HIV infection and the intestinal mucosal barrier. Ann N Y Acad Sci 2012; 1258: 19–24. [DOI] [PubMed] [Google Scholar]
- 91. Zheng S, Wu X, Shi J et al Rapid specific and visible detection of porcine circovirus type 3 using loop‐mediated isothermal amplification (LAMP). Transbound Emerg Dis 2018; 65: 597–601. [DOI] [PubMed] [Google Scholar]
- 92. Zheng S, Shi J, Wu X et al Presence of Torque teno sus virus 1 and 2 in porcine circovirus 3‐positive pigs. Transbound Emerg Dis 2018; 65: 327–30. [DOI] [PubMed] [Google Scholar]
- 93. Chen M, Xie S. Therapeutic targeting of cellular stress responses in cancer. Thorac Cancer 2018; 9: 1575–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Yang Y, Ran J, Liu M et al CYLD mediates ciliogenesis in multiple organs by deubiquitinating Cep70 and inactivating HDAC6. Cell Res 2014; 24: 1342–53. [DOI] [PMC free article] [PubMed] [Google Scholar]


