Abstract
Background
Circular RNAs (circRNAs) participate in the development of human cancers by regulating multiple cell processes. CircRNA antisense to the cerebellar degeneration‐related protein 1 transcript (circCDR1as) expression is dysregulated in many cancers, including non‐small‐cell lung cancer (NSCLC). However, the mechanism by which circCDR1as mediates the development of NSCLC remains unknown.
Methods
A total of 30 paired cancer and normal tissues were collected from patients with NSCLC. The expression levels of circCDR1as, microRNA (miR)‐219a‐5p and Sex determining region Y‐box protein 5 (SOX5) were measured in tissues or cells by quantitative real‐time polymerase chain reaction or western blot. Cell viability, apoptosis, migration and invasion were detected by 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyl‐tetrazolium bromide, colony formation, flow cytometry and transwell assays, respectively. The target relationship between miR‐219a‐5p and circCDR1as or SOX5 was validated by dual‐luciferase reporter assay.
Results
CircCDR1as expression was elevated in NSCLC tissues and cells in comparison to the matched controls. Interference of circCDR1as led to obvious inhibition of cell viability, migration and invasion and increase of apoptosis in NSCLC cells. MiR‐219a‐5p acted as a target of circCDR1as and miR‐219a‐5p downregulation attenuated the regulatory effect of circCDR1as silencing on NSCLC progression. Moreover, miR‐219a‐5p targeted SOX5 to repress the progression of NSCLC in vitro. Besides, circCDR1as knockdown reduced the expression of SOX5 by increasing miR‐219a‐5p level.
Conclusion
Knockdown of circCDR1as inhibited the progression of NSCLC by decreasing cell viability, migration and invasion and increasing apoptosis by upregulating miR‐219a‐5p and downregulating SOX5.
Keywords: circCDR1as, miR‐219a‐5p, NSCLC, SOX5, viability
Introduction
Lung cancer is a global problem with high incidence and mortality.1 A total of 85% of lung cancer cases are diagnosed as non‐small‐cell lung cancer (NSCLC).2 In the past decades, significant advances have been achieved in the prevention, diagnosis and treatment of NSCLC.3, 4 However, the five‐year survival rate of patients remains low. Hence, determining new targets will be significant for the treatment of NSCLC.
Noncoding RNAs of several types play essential roles in cancer diagnosis and therapy.5 Among these types, circular RNA (circRNA) consists of a continuous closed loop.6 Several circRNAs have been reported to be aberrantly expressed and associated with the tumorigenesis of lung cancer.7 Furthermore, emerging evidence suggests that several circRNAs might contribute to the progression of NSCLC.8, 9, 10 Accruing evidence demonstrates that circRNA antisense to cerebellar degeneration‐related protein 1 transcript (circCDR1as) could promote the development of hepatocellular carcinoma, colorectal cancer and laryngeal squamous cell carcinoma and inhibit the progression of bladder cancer and ovarian cancer.11, 12, 13, 14, 15 Importantly, a previous study reveals that circCDR1as acts as an oncogene to facilitate the development of NSCLC.16 However, the mechanism underlying the participation of circCDR1as in the progression of NSCLC remains unclear.
MicroRNAs (miRNAs) are associated with the development, detection and treatment of NSCLC.17 Previous studies suggest that low expression of miR‐219a‐5p is required for the development of NSCLC.18, 19 Moreover, the sex determining region Y‐box protein (SOX) family is crucial for multiple processes in the progression of human cancers.20 SOX5 is a representative of the SOX family which could drive the malignancy of lung cancer, including NSCLC.21, 22 It has been documented that the competing endogenous RNA (ceRNA) network is a crucial mechanism underlying circRNA in human diseases by sponging miRNA to target mRNA.23 Interestingly, it has been predicted there were the same complementary sites of miR‐219a‐5p between circCDR1as and SOX5, indicating that circCDR1as might act as a ceRNA for miR‐219a‐5p to derepress SOX5. Here, we measured the expression of circCDR1as in NSCLC tissues and cells. Moreover, we assessed the function of circCDR1as on cell viability, apoptosis, migration and invasion and confirmed the ceRNA network of circCDR1as/miR‐219a‐5p/SOX5 in NSCLC cells.
Methods
Patients and tissues
A total of 30 NSCLC patients who did not receive chemotherapy or radiotherapy were recruited from the First Affiliated Hospital of Xi'an Medical University and all participants signed their informed consent for participation in the study. This study was approved by the Ethics Committee of the First Affiliated Hospital of Xi'an Medical University. The tumor tissues and adjacent normal tissues from patients were collected during surgery and stored at −80°C until RNA isolation.
Cell culture and transfection
Human lung cancer cell lines (A549, Calu‐3, CAEP and SK‐MES‐1) and human bronchial epithelioid cells (HBE) were purchased from BeNa Culture Collection (Beijing, China) and cultured in Dulbecco's Modified Eagle Medium (DMEM) (Solarbio, Beijing, China) containing 10% fetal bovine serum (Zhejiang Tianhang Biotechnology, Huzhou, China) and 1% penicillin‐streptomycin solution (Procell, Wuhan, China).
The overexpression vectors of circCDR1as and SOX5 were generated with pcDNA3.1 vector (pcDNA) (YouBio, Changsha, China). The pcDNA vector was used as a negative control. The short interfering RNA (siRNA) for circCDR1as (si‐circCDR1as#1, 5′‐GCAAUAUCCAGGGUUUCCGAU‐3′; si‐circCDR1as#2, 5′‐UGUCUGCAAUAUCCAGGGUUU‐3′), siRNA negative control (si‐NC, 5′‐UUCUCCGAACGUGUCACGU‐3′), miR‐219a‐5p mimic (miR‐219a‐5p, 5′‐UGAUUGUCCAAACGCAAUUCU‐3′), mimic negative control (miR‐NC, 5′‐UUCUCCGAACGUGUCACGUTT‐3′), miR‐219a‐5p inhibitor (anti‐miR‐219a‐5p, 5′‐AGAAUUGCGUUUGGACAAUCA‐3′) and inhibitor negative control (anti‐NC, 5′‐CAGUACUUUUGUGUAGUACAA‐3′) were generated by Fulengen (Guangzhou, China). A549 and Calu‐3 cells were transfected with these conducted oligonucleotides or vectors using Lipofectamine 3000 (Thermo Fisher, Wilmington, DE, USA) for 24 hours.
Quantitative real‐time polymerase chain reaction (qRT‐PCR)
Tissues or cells were lysed in Trizol reagent (Solarbio) for the isolation of total RNA. For extraction of circRNA, the RNA was further treated by RNase R (Geneseed, Guangzhou, China). The complementary DNA (cDNA) was generated by reverse transcription using the First‐Strand cDNA Synthesis kit (Yeasen, Shanghai, China) and then used for qRT‐PCR assay after the mixture with SYBR (Thermo Fisher) and specific primers (Sangon, Shanghai, China). The primers were listed as: circCDR1as (Forward, 5′‐CCCAGTCTTCCATCAACTGG‐3′; Reverse, 5′‐ACCTTGACACAGGTGCCATC‐3′), SOX5 (Forward, 5′‐AGGTTTGGACTCACTTGACAGG‐3′; Reverse, 5′‐GTGAGGCTTGTTGGGAAAACTC‐3′) and miR‐219a‐5p (Forward, 5′‐TCTACAGTGCACGTGTCTCCAGT‐3′; Reverse, 5′‐CTCTCATTTGCTATATTCA‐3′). The glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) (Forward, 5′‐CAGCCTCAAGATCATCAGCA‐3′; Reverse, 5′‐GTCTTCTGGGTGGCAGTGAT‐3′) and U6 (Forward, 5′‐CTCGCTTCGGCAGCACA‐3′; Reverse, 5′‐AACGCTTCACGAATTTGCGT‐3′) were regarded as internal controls. The 2−ΔΔCt method was used to calculate the relative gene expression level.24
3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyl‐tetrazolium bromide (MTT) assay
After transfection, A549 and Calu‐3 cells were collected and adjusted to 3 × 104/mL. Cell suspensions (100 μL/well) were added to 96‐well plates and then cultured for 0, one, two and three days. At the indicated time point, cells were incubated with MTT solution (10 μL per well) (Beyotime, Shanghai, China) for another four hours. The medium was then removed and 100 μL dimethyl sulfoxide (Solarbio) was added to each well to dissolve the crystal, followed by measurement of optical density value at 490 nm through a microplate reader (Potenov, Beijing, China).
Colony formation assay
A549 and Calu‐3 cells (300 cells per well) were seeded into six‐well plates. After culture for 10 days, cells were fixed with methanol and stained with 1% crystal violet (Solarbio) and colony formation was calculated under a microscope (Olympus, Tokyo, Japan).
Flow cytometry
A549 and Calu‐3 cells (1 × 105 per well) were seeded into 24‐well plates and cultured for three days. Cells were then resuspended in binding buffer and stained in the dark with 5 μL Annexin V‐fluorescein isothiocyanate (FITC) and propidium iodide (PI) (Solarbio) for 10 minutes. Flow cytometry was used to assess the apoptotic cells through a flow cytometer (Countstar, Shanghai, China). The apoptotic rate was expressed as the percentage of cells at the upper right quadrant in the total number of cells.
Transwell assay
The 24‐well plates with transwell inserts (Corning, Corning, NY, USA) were used to test the abilities of migration and invasion. The inserts were precoated with Matrigel for the invasion assay, and the inserts that were not treated were used for migration assay. After transfection, 3 × 105 cells/mL cell suspensions of A549 and Calu‐3 were prepared in serum‐free DMEM and 100 μL cell suspensions were plated into the upper chambers. DMEM containing 10% serum was added to the lower chamber. After incubation for 24 hours, the migrated or invasive cells were fixed and stained with 0.1% crystal violet, followed by observation with three random fields under a 100× magnification microscope.
Dual‐luciferase reporter assay
The potential binding sites of miR‐219a‐5p and circCDR1as or SOX5 were predicted by starBase (http://starbase.sysu.edu.cn/index.php) or TargetScan (http://www.targetscan.org/vert_72/). The use and parameters of starBase and TargetScan were shown as previous reports.25, 26 The psiCHECK‐2 dual‐luciferase vectors (Promega, Madison, WI, USA) were used to generate the wild‐type luciferase reporter vectors (circCDR1as‐WT and SOX5‐WT) and corresponding mutant (circCDR1as‐MUT and SOX5‐MUT) by cloning the sequences of circCDR1as or 3′ untranslated regions (UTR) of SOX5 containing miR‐219a‐5p binding sites. For dual‐luciferase reporter assay, A549 and Calu‐3 cells were transfected with the constructed luciferase reporter vectors and miR‐219a‐5p or miR‐NC. At 24 hours post‐transfection, the luciferase activities of firefly and renilla were measured with a dual‐luciferase assay system (Promega). The firefly activity was normalized to renilla activity and the relative luciferase activity of the treatment group was normalized to that in the control group.
Western blot
The protein was prepared using a total protein extraction kit (Solarbio) and protein concentration was determined by bicinchoninic acid protein assays kit (Beyotime), followed by denaturation at 98°C for 10 minutes. Equal amounts of proteins were subsequently subjected to sodium dodecyl sulfate‐polyacrylamide gel electrophoresis and membrane transfer using nitrocellulose membranes (Millipore, Billerica, MA, USA). After the blockage of nonspecific binding sites using the western blocking buffer (Beyotime), the membranes were incubated with anti‐SOX5 (#PA5‐95266, 1:1000 dilution, Thermo Fisher) or anti‐GAPDH (#MA5‐15738, 1:5000 dilution, Thermo Fisher) and corresponding secondary antibody. The protein bands were visualized via enhanced chemiluminescence reagent (Beyotime). The gray values were analyzed with GAPDH as a loading control by QuantityOne software (Bio‐Rad, Hercules, CA, USA).
Statistical analysis
Data were analyzed by GraphPad Prism 7 software (GraphPad Inc., La Jolla, CA, USA) and presented as mean ± standard deviation. The experiments were performed at least three times. Student's t‐test or one‐way analysis of variance followed by Tukey's post hoc test was used to analyze the difference between two groups or multiple groups, respectively. The linear correlation between the levels of miR‐219a‐5p and circCDR1as or SOX5 in NSCLC tissues was analyzed by Spearman's correlation coefficient. The P‐value <0.05 was regarded as statistically significant.
Results
Abundance of circCDR1as upregulated in NSCLC tissues and cells
To explore the abnormally expressed circRNA in NSCLC, 30 paired tumor and adjacent normal tissues were harvested from NSCLC patients. As shown in Figure 1a, the expression of circCDR1as was significantly increased in NSCLC tissues compared with that in normal tissues. Moreover, the abundance of circCDR1as was also examined in NSCLC cell lines (A549, Calu‐3, CAEP and SK‐MES‐1). In comparison to HBE cells, the circRNA level was markedly enhanced in the four NSCLC cell lines, especially in A549 and Calu‐3 cells (Fig 1b). These results displayed high expression of circCDR1as in NSCLC.
Figure 1.
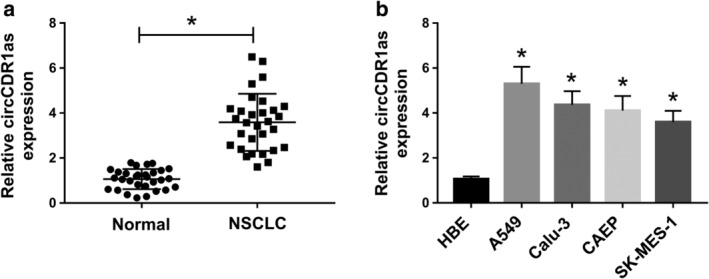
The expression of circCDR1as is enhanced in NSCLC tissues and cells. (a) The level of circCDR1as was measured in NSCLC tissues and normal tissues (n = 30) by qRT‐PCR. (b) The expression of circCDR1as was detected in NSCLC cell lines (A549, Calu‐3, CAEP and SK‐MES‐1) by qRT‐PCR. *P < 0.05.
Interference of circCDR1as represses cell viability, migration and invasion and induces apoptosis in NSCLC cells
To investigate the role of circCDR1as in NSCLC development, the level of circRNA was knocked down in A549 and Calu‐3 cells using two siRNA sequences for circCDR1as, in which si‐circCDR1as#1 exhibited higher inhibitive efficacy (Fig 2a). Furthermore, the functional assays were performed in the two cell lines transfected with si‐NC or si‐circCDR1as#1. As displayed in Figure 2b,c, knockdown of circCDR1as obviously decreased the viability of A549 and Calu‐3 cells after being cultured for three days. Meanwhile, silencing of circCDR1as significantly inhibited the colony formation of A549 and Calu‐3 cells (Fig 2d). Moreover, the flow cytometry data indicated that a higher apoptotic rate was induced by silencing circCDR1as in A549 and Calu‐3 cells at three days (Fig 2e). In addition, transwell assay showed that downregulation of circCDR1as led to significant inhibition on the abilities of migration and invasion in A549 and Calu‐3 cells after incubation for 24 hours (Fig 2f,g). Collectively, knockdown of circCDR1as suppressed the progression of NSCLC in vitro.
Figure 2.
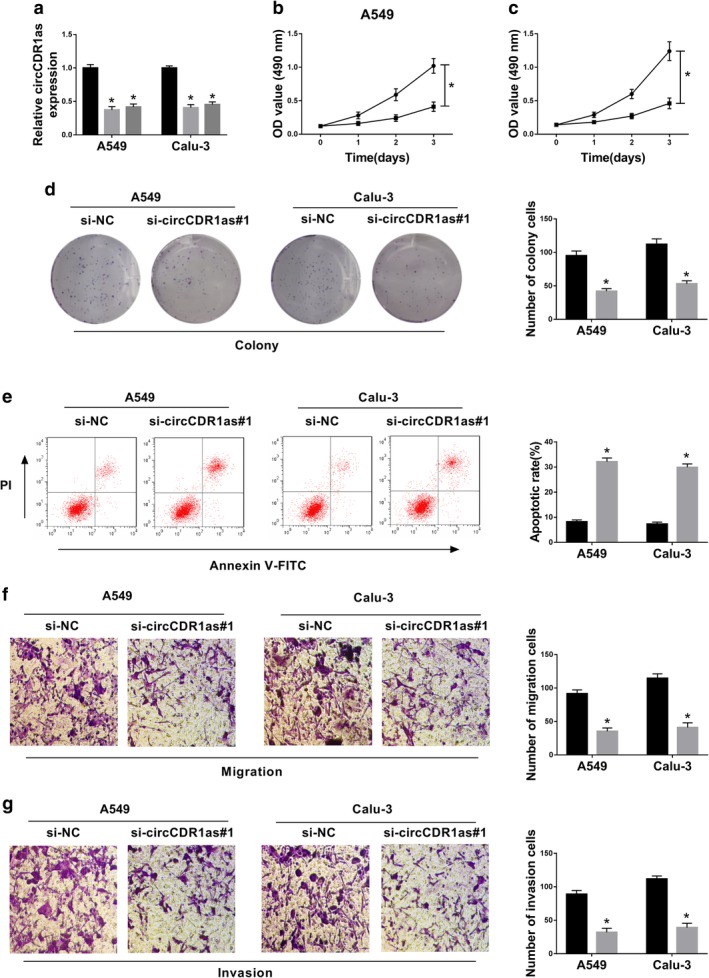
Knockdown of circCDR1as inhibits cell viability, migration and invasion and promotes apoptosis in NSCLC cells. (a) The expression of circCDR1as was examined in A549 and Calu‐3 cells transfected with si‐circCDR1as#1, #2 or si‐NC by qRT‐PCR. ( ) si‐NC, (
) si‐NC, ( ) si‐circCDR1as#1 and (
) si‐circCDR1as#1 and ( ) si‐circCDR1as#2. (b and c) The viability of A549 and Calu‐3 cells transfected with si‐circCDR1as#1 or si‐NC was assessed by MTT at 0, 1, 2 and 3 days. (
) si‐circCDR1as#2. (b and c) The viability of A549 and Calu‐3 cells transfected with si‐circCDR1as#1 or si‐NC was assessed by MTT at 0, 1, 2 and 3 days. ( ) si‐NC and (
) si‐NC and ( ) si‐circCDR1as#1. (d) The colony formation of A549 and Calu‐3 cells transfected with si‐circCDR1as#1 or si‐NC was measured. (
) si‐circCDR1as#1. (d) The colony formation of A549 and Calu‐3 cells transfected with si‐circCDR1as#1 or si‐NC was measured. ( ) si‐NC and (
) si‐NC and ( ) si‐circCDR1as#1. (e) The apoptotic rate of A549 and Calu‐3 cells transfected with si‐circCDR1as#1 or si‐NC was analyzed by flow cytometry at three days. (
) si‐circCDR1as#1. (e) The apoptotic rate of A549 and Calu‐3 cells transfected with si‐circCDR1as#1 or si‐NC was analyzed by flow cytometry at three days. ( ) si‐NC and (
) si‐NC and ( ) si‐circCDR1as#1. (f and g) Cell migration and invasion were measured in A549 and Calu‐3 cells transfected with si‐circCDR1as#1 or si‐NC by transwell analysis at 24 hours. (
) si‐circCDR1as#1. (f and g) Cell migration and invasion were measured in A549 and Calu‐3 cells transfected with si‐circCDR1as#1 or si‐NC by transwell analysis at 24 hours. ( ) si‐NC and (
) si‐NC and ( ) si‐circCDR1as#1. *P < 0.05.
) si‐circCDR1as#1. *P < 0.05.
CircCDR1as is a sponge for miR‐219a‐5p in NSCLC cells
Given that circRNA was able to serve as a sponge for miRNAs, the targets of circCDR1as were searched by starBase. The predicted binding sites of circCDR1as and miR‐219a‐5p are shown in Figure 3a. To confirm this prediction, luciferase reporter vectors circCDR1as‐WT and circCDR1as‐MUT were constructed and transfected into A549 and Calu‐3 cells. The luciferase activity was evidently reduced by overexpression of miR‐219a‐5p in circCDR1as‐WT group, while it was not changed in circCDR1as‐MUT group (Fig 3b,c). Moreover, the expression of miR‐219a‐5p was remarkably reduced by overexpression of circCDR1as and enhanced by knockdown of circCDR1as in A549 and Calu‐3 cells (Fig 3d,e). Additionally, the abundance of miR‐219a‐5p was markedly decreased in NSCLC tissues and cells when compared to the corresponding controls (Fig 3f,g). Besides, there was a negative correlation between the levels of miR‐219a‐5p and circCDR1as in NSCLC tissues (R2 = 0.6787, P < 0.0001) (Fig 3h). These findings suggested that miR‐219a‐5p was bound to circCDR1as in NSCLC cells.
Figure 3.
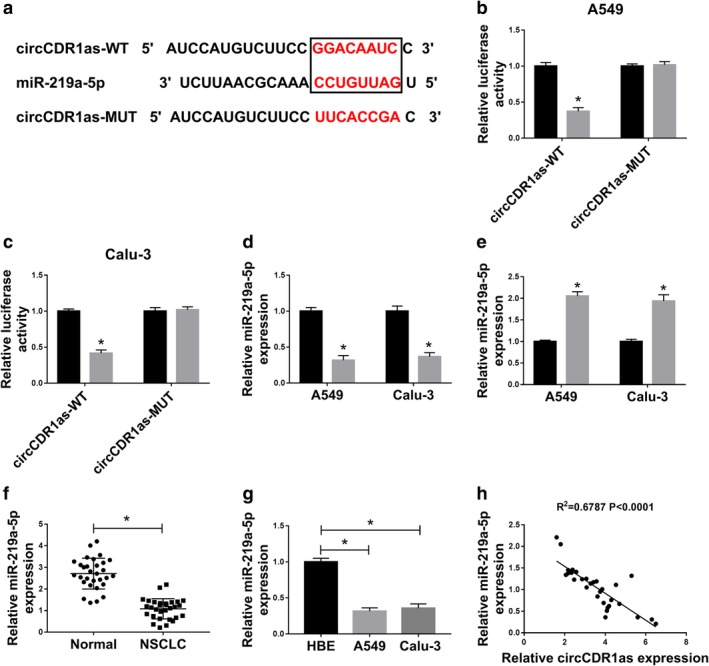
CircCDR1as is a decoy of miR‐219a‐5p in NSCLC cells. (a) The binding sites of circCDR1as and miR‐219a‐5p were predicted by starBase. (b and c) Luciferase activity was measured in A549 and Calu‐3 cells transfected with circCDR1as‐WT or circCDR1as‐MUT and miR‐NC or miR‐219a‐5p. ( ) miR‐NC and (
) miR‐NC and ( ) miR‐219a‐5p. (d and e) The expression of miR‐219a‐5p was detected in A549 and Calu‐3 cells transfected with pcDNA, circCDR1as, si‐NC or si‐circCDR1as#1 by qRT‐PCR. (d) (
) miR‐219a‐5p. (d and e) The expression of miR‐219a‐5p was detected in A549 and Calu‐3 cells transfected with pcDNA, circCDR1as, si‐NC or si‐circCDR1as#1 by qRT‐PCR. (d) ( ) pcDNA and (
) pcDNA and ( ) circCDR1as. (e) (
) circCDR1as. (e) ( ) si‐NC and (
) si‐NC and ( ) si‐circCDR1as#1. (f) The expression of miR‐219a‐5p was measured in NSCLC tissues and normal tissues (n = 30) by qRT‐PCR. (g) The level of miR‐219a‐5p was detected in A549 and Calu‐3 cells by qRT‐PCR. (h) The linear relationship between the levels of miR‐219a‐5p and circCDR1as in NSCLC tissues was analyzed. *P < 0.05.
) si‐circCDR1as#1. (f) The expression of miR‐219a‐5p was measured in NSCLC tissues and normal tissues (n = 30) by qRT‐PCR. (g) The level of miR‐219a‐5p was detected in A549 and Calu‐3 cells by qRT‐PCR. (h) The linear relationship between the levels of miR‐219a‐5p and circCDR1as in NSCLC tissues was analyzed. *P < 0.05.
Knockdown of miR‐219a‐5p attenuates the effect of circCDR1as silencing on processes of NSCLC cells
To explore whether miR‐219a‐5p was responsible for the regulation of NSCLC progression mediated by circCDR1as, A549 and Calu‐3 cells were transfected with si‐NC, si‐circCDR1as#1, si‐circCDR1as#1 and anti‐miR‐NC or anti‐miR‐219a‐5p. As shown in Figure 4a, the expression of miR‐219a‐5p upregulated by circCDR1as silencing was effectively decreased by introduction of miR‐219a‐5p inhibitor. Moreover, knockdown of miR‐219a‐5p protected the cell viability and colony formation from silencing circCDR1as‐mediated suppression in A549 and Calu‐3 cells (Fig 4b–d). Furthermore, deficiency of miR‐219a‐5p alleviated the apoptosis induced by circCDR1as interference in A549 and Calu‐3 cells (Fig 4e). Besides, downregulation of miR‐219a‐5p restored the abilities of migration and invasion inhibited by circCDR1as knockdown in the two cell lines (Fig 4f,g). Taken together, knockdown of circCDR1as inhibited NSCLC progression by increasing miR‐219a‐5p.
Figure 4.
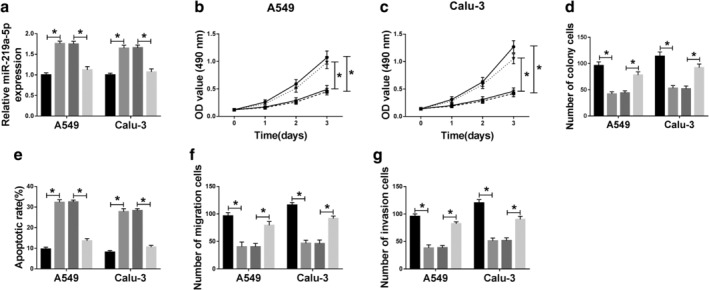
Deficiency of miR‐219a‐5p reverses the effect of circCDR1as knockdown on processes of NSCLC cells. (a) The expression of miR‐219a‐5p was measured in A549 and Calu‐3 cells transfected with si‐NC, si‐circCDR1as#1, si‐circCDR1as#1 and anti‐miR‐NC or anti‐miR‐219a‐5p by qRT‐PCR. ( ) si‐NC, (
) si‐NC, ( ) si‐circCDR1as#1, (
) si‐circCDR1as#1, ( ) si‐circCDR1as#1+anti‐miR‐NC and (
) si‐circCDR1as#1+anti‐miR‐NC and ( ) si‐circCDR1as#1+anti‐miR‐219a‐5p. (b and c) Cell viability, (d) colony formation, (e) apoptosis, (f and g) migration and invasion were detected in A549 and Calu‐3 cells transfected with si‐NC, si‐circCDR1as#1, si‐circCDR1as#1 and anti‐miR‐NC or anti‐miR‐219a‐5p by MTT, colony formation, flow cytometry and transwell assays, respectively. (b, c) (
) si‐circCDR1as#1+anti‐miR‐219a‐5p. (b and c) Cell viability, (d) colony formation, (e) apoptosis, (f and g) migration and invasion were detected in A549 and Calu‐3 cells transfected with si‐NC, si‐circCDR1as#1, si‐circCDR1as#1 and anti‐miR‐NC or anti‐miR‐219a‐5p by MTT, colony formation, flow cytometry and transwell assays, respectively. (b, c) ( ) si‐NC, (
) si‐NC, ( ) si‐circCDR1as#1, (
) si‐circCDR1as#1, ( ) si‐circCDR1as#1+anti‐miR‐NC and (
) si‐circCDR1as#1+anti‐miR‐NC and ( ) si‐circCDR1as#1+anti‐miR‐219a‐5p. (d–g) (
) si‐circCDR1as#1+anti‐miR‐219a‐5p. (d–g) ( ) si‐NC, (
) si‐NC, ( ) si‐circCDR1as#1, (
) si‐circCDR1as#1, ( ) si‐circCDR1as#1+anti‐miR‐NC and (
) si‐circCDR1as#1+anti‐miR‐NC and ( ) si‐circCDR1as#1+anti‐miR‐219a‐5p. *P < 0.05.
) si‐circCDR1as#1+anti‐miR‐219a‐5p. *P < 0.05.
SOX5 is a target of miR‐219a‐5p in NSCLC cells
As the function of miRNA is to mediate the targeted mRNAs, the targets of miR‐219a‐5p were explored by TargetScan. SOX5, an oncogene in NSCLC, is shown with the potential binding sites of miR‐219a‐5p (Fig 5a). To validate this association, dual‐luciferase reporter assay was performed in A549 and Calu‐3 cells. The results indicated that overexpression of miR‐219a‐5p resulted in obvious loss of luciferase activity in SOX5‐WT group, while it did not induce a significant effect on the activity in SOX5‐MUT group (Fig 5b,c). Moreover, the expression of SOX5 was significantly decreased by miR‐219a‐5p overexpression and increased by miR‐219a‐5p knockdown in A549 and Calu‐3 cells at mRNA and protein levels (Fig 5d–g). In addition, the mRNA and protein levels of SOX5 were significantly upregulated in NSCLC tissues and cells compared to their counterparts, respectively (Fig 5h–k). Meanwhile, an inverse relationship was observed in the levels of miR‐219a‐5p and SOX5 in NSCLC tissues (R2 = 0.6586, P < 0.0001) (Fig 5l). These data indicated that SOX5 served as a target of miR‐219a‐5p in NSCLC cells.
Figure 5.
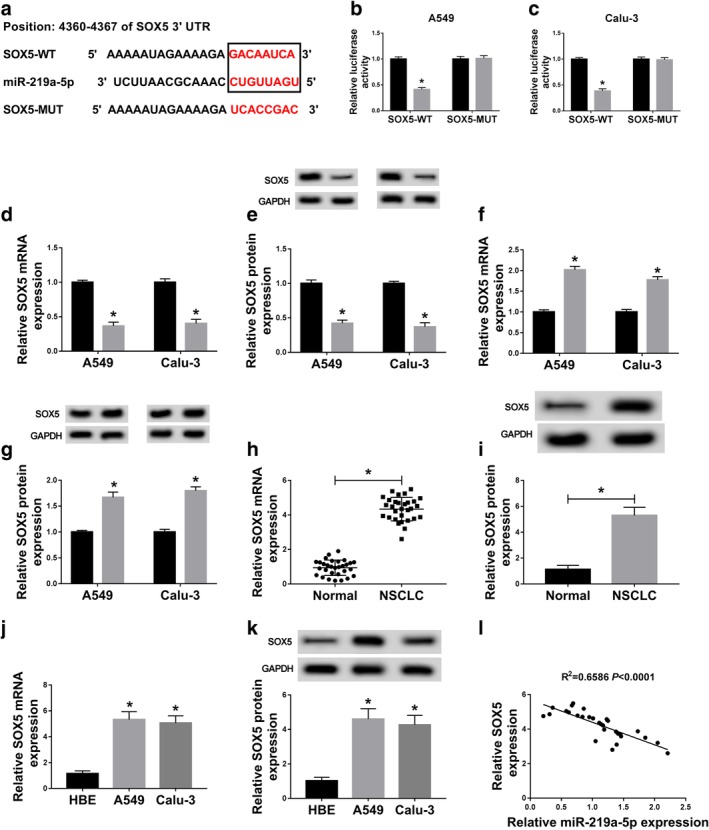
SOX5 is a target of miR‐219a‐5p in NSCLC cells. (a) The binding sites of miR‐219a‐5p and SOX5 were predicted by TargetScan. (b and c) Luciferase activity was detected in A549 and Calu‐3 cells transfected with SOX5‐WT or SOX5‐MUT and miR‐NC or miR‐219a‐5p. ( ) miR‐NC and (
) miR‐NC and ( ) miR‐219a‐5p. (d–g) The mRNA and protein levels of SOX5 were measured in A549 and Calu‐3 cells transfected with miR‐NC, miR‐219a‐5p, anti‐miR‐NC or anti‐miR‐219a‐5p by qRT‐PCR and western blot. (d, e) (
) miR‐219a‐5p. (d–g) The mRNA and protein levels of SOX5 were measured in A549 and Calu‐3 cells transfected with miR‐NC, miR‐219a‐5p, anti‐miR‐NC or anti‐miR‐219a‐5p by qRT‐PCR and western blot. (d, e) ( ) miR‐NC and (
) miR‐NC and ( ) miR‐219a‐5p. (f, g) (
) miR‐219a‐5p. (f, g) ( ) anti‐miR‐NC and (
) anti‐miR‐NC and ( ) anti‐miR‐219a‐5p. (h and i) The expression of SOX5 at mRNA and protein levels was examined in NSCLC tissues and normal tissues (n = 30). (j and k) The mRNA and protein levels of SOX5 were measured in A549 and Calu‐3 cells by qRT‐PCR and western blot. (l) The linear correlation between levels of miR‐219a‐5p and SOX5 in NSCLC tissues was analyzed. *P < 0.05.
) anti‐miR‐219a‐5p. (h and i) The expression of SOX5 at mRNA and protein levels was examined in NSCLC tissues and normal tissues (n = 30). (j and k) The mRNA and protein levels of SOX5 were measured in A549 and Calu‐3 cells by qRT‐PCR and western blot. (l) The linear correlation between levels of miR‐219a‐5p and SOX5 in NSCLC tissues was analyzed. *P < 0.05.
Addition of miR‐219a‐5p inhibits cell viability, migration and invasion and facilitates apoptosis by decreasing SOX5 in NSCLC cells
To explore whether SOX5 was required for miR‐219a‐5p‐mediated regulatory role in NSCLC progression, A549 and Calu‐3 cells were transfected with miR‐NC, miR‐219a‐5p, miR‐219a‐5p and pcDNA or SOX5. As shown in Figures 6a,b, the mRNA and protein levels of SOX5 were significantly decreased by miR‐219a‐5p overexpression in A549 and Calu‐3 cells, which was restored by introduction of SOX5 overexpression vector. Furthermore, the MTT assay showed that overexpression of miR‐219a‐5p notably decreased the viability at three days in the two cell lines, which was attenuated by restoration of SOX5 (Fig 6c,d). The colony formation assay showed that miR‐219a‐5p overexpression significantly decreased the colony abilities of A549 and Calu‐3 cells, which was restored by upregulation of SOX5 (Fig 6e). In addition, addition of miR‐219a‐5p significantly induced cell apoptosis in A549 and Calu‐3 cells, and this effect was weakened by introduction of SOX5 (Fig 6f). Besides, the migratory and invasive abilities of A549 and Calu‐3 cells were remarkably repressed by overexpression of miR‐219a‐5p, and upregulation of SOX5 abated this effect (Fig 6g,h). These results displayed that miR‐219a‐5p inhibited NSCLC progression by decreasing SOX5 in vitro.
Figure 6.
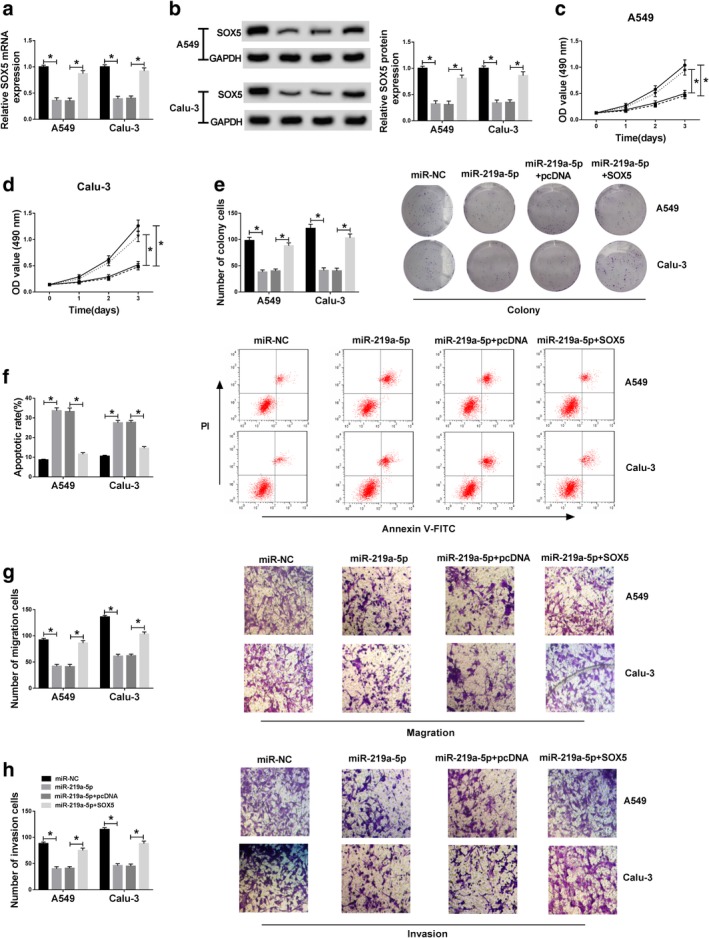
Overexpression of miR‐219a‐5p suppresses cell viability, migration and invasion and induces apoptosis by targeting SOX5 in NSCLC cells. The mRNA and protein levels of (a and b) SOX5, (c and d) cell viability, (e) colony formation, (f) apoptosis, (g) migration and (h) invasion were detected in A549 and Calu‐3 cells transfected with miR‐NC, miR‐219a‐5p, miR‐219a‐5p and pcDNA or SOX5 by qRT‐PCR, western blot, MTT, colony formation, flow cytometry and transwell assays, respectively. *P < 0.05. (a, b, e–h) ( ) miR‐NC, (
) miR‐NC, ( ) miR‐219a‐5p, (
) miR‐219a‐5p, ( ) miR‐219a‐5p+pcDNA and (
) miR‐219a‐5p+pcDNA and ( ) miR‐219a‐5p+SOX5. (c, d) (
) miR‐219a‐5p+SOX5. (c, d) ( ) miR‐NC, (
) miR‐NC, ( ) miR‐219a‐5p, (
) miR‐219a‐5p, ( ) miR‐219a‐5p+pcDNA and (
) miR‐219a‐5p+pcDNA and ( ) miR‐219a‐5p+SOX5.
) miR‐219a‐5p+SOX5.
Silencing circCDR1as reduces SOX5 expression by regulating miR‐219a‐5p in NSCLC cells
In order to further explore how and whether circCDR1as could regulate SOX5, A549 and Calu‐3 cells were transfected with si‐NC, si‐circCDR1as#1, si‐circCDR1as#1 and anti‐miR‐NC or anti‐miR‐219a‐5p. As shown in Figure 7a, the expression of SOX5 mRNA was significantly decreased by knockdown of circCDR1as in A549 and Calu‐3 cells, which was restored by miR‐219a‐5p exhaustion. Similarly, the protein level of SOX5 displayed the same trends in the two cell lines (Fig 7b). Together, circCDR1as positively regulated SOX5 expression by competitively binding miR‐219a‐5p.
Figure 7.
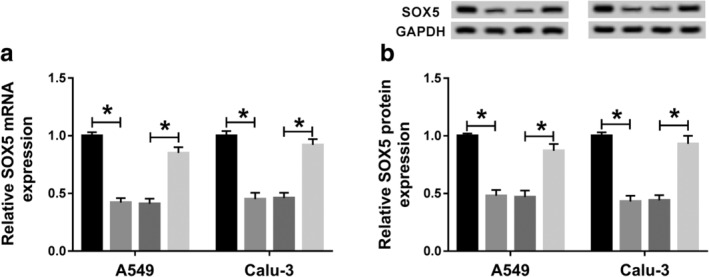
Knockdown of circCDR1as decreases SOX5 expression by regulating miR‐219a‐5p in NSCLC cells. The (a) mRNA and (b) protein levels of SOX5 were measured in A549 and Calu‐3 cells transfected with si‐NC, si‐circCDR1as#1, si‐circCDR1as#1 and anti‐miR‐NC or anti‐miR‐219a‐5p by qRT‐PCR and western blot. *P < 0.05. ( ) si‐NC, (
) si‐NC, ( ) si‐circCDR1as#1, (
) si‐circCDR1as#1, ( ) si‐circCDR1as#1+anti‐miR‐NC and (
) si‐circCDR1as#1+anti‐miR‐NC and ( ) si‐circCDR1as#1+anti‐miR‐219a‐5p.
) si‐circCDR1as#1+anti‐miR‐219a‐5p.
Discussion
NSCLC represents approximately 85% of lung cancer cases and patients with NSCLC at advanced stage have poor outcomes.27 Dysregulated circRNAs may be associated with the diagnosis and therapy of cancers.28 Several reports have demonstrated that circCDR1as could function as an oncogenic circRNA or tumor suppressor in different cancers because of the change of tumor microenvironment.11, 12, 13, 14, 15 Zhang et al. reported that circCDR1as expression was abnormal in NSCLC.16 However, the mechanism of circCDR1as in NSCLC development remains elusive. In the present study, we validate the suppressive effect of circCDR1as knockdown on NSCLC development in vitro. Moreover, our research first revealed the ceRNA network of circCDR1as/miR‐219a‐5p/SOX5 in NSCLC cells.
CircRNAs play pivotal roles in the tumorigenesis of lung cancer.7 By detecting the expression of circRNA in patient tissues and cell lines, we found that circCDR1as expression was enhanced in NSCLC, indicating that high expression of circCDR1as could contribute to the malignancy of NSCLC. Tumor growth and metastasis are the two key regulators of lung cancer malignancy.29 To investigate the potential value of this circRNA in NSCLC, loss‐of‐function experiments were performed to confirm the anti‐proliferation and anti‐metastasis roles of circCDR1as silencing in NSCLC, which was similar to the previous report by Zhang et al.16 Moreover, epithelial‐to‐mesenchymal transition has been regarded as an important mechanism involved in the regulation of development and metastasis of lung cancer.30, 31 Hence, whether circCDR1as can promote cell growth, migration and invasion by inducing epithelial‐to‐mesenchymal transition should be explored in the future.
CircRNAs may serve as ceRNAs or sponges for miRNAs to participate in the development of cancers.32 Several reports have documented that circCDR1as could act as a ceRNA to regulate the processes of tumor cells.33, 34 In the present study, we confirmed circCDR1as as a sponge of miR‐219a‐5p by dual‐luciferase reporter assay. Moreover, we found that miR‐219a‐5p expression declined in NSCLC and its overexpression repressed NSCLC development in vitro, which was also in agreement with previous studies.18, 19 This indicated the anti‐cancer role of miR‐219a‐5p in NSCLC. In addition, our research data revealed that the suppressive effect of circCDR1as knockdown on NSCLC development was attenuated by miR‐219a‐5p inhibitor, revealing the possibility that circCDR1as might contribute to the development of NSCLC by sponging miR‐219a‐5p.
To further elucidate the ceRNA network in this study, the targets of miR‐219a‐5p were analyzed. Previous studies have confirmed multiple targets of miR‐219a‐5p in different conditions, such as high mobility group protein A2 (HMGA2), fibroblast growth factor 9 (FGF9) and eyes absent homolog 2 (EYA2).18, 19, 35 In this study, we first confirmed the target association between miR‐219a‐5p and SOX5 in NSCLC cells. The SOX family plays an important role in the development and metastasis of human cancers.36 In our study, we found that SOX5 expression was increased in NSCLC tissues and cells, indicating that high expression of SOX5 might contribute to the development of NSCLC, which is in agreement with the study by Li et al.37 SOX5 exhibited the proproliferation and prometastasis roles in multiple cancers, including prostate, breast and gastric cancers.38, 39, 40 Similarly, we also found that high expression of SOX5 could promote cell viability, migration and invasion, which is also consistent with previous studies.22, 37 Meanwhile, the rescue experiments revealed that SOX5 reversed the therapeutic effect of miR‐219a‐5p on NSCLC development, indicating that miR‐219a‐5p suppressed NSCLC progression by targeting SOX5. Furthermore, our data uncovered that circCDR1as could positively regulate SOX5 expression by sponging miR‐219a‐5p in NSCLC cells. Previous studies have suggested that SOX5 is responsible for Wnt signaling which plays an essential role in the development of NSCLC.41, 42 However, whether circCDR1as could regulate the Wnt pathway in NSCLC by targeting SOX5 requires further study. Furthermore, the cell culture condition in vitro could not absolutely mimic the tumor microenvironment in vivo. Hence, preclinical analysis should be carried out in future by using a mouse model for lung cancer.
In conclusion, this research showed high expression of circCDR1as in NSCLC tissues and cells. Moreover, silencing of circCDR1as suppressed the progression of NSCLC via inhibiting cell viability, migration and invasion and promoting apoptosis, possibly by upregulating miR‐219a‐5p and decreasing SOX5. This may elucidate a new mechanism for understanding NSCLC malignancy in the future.
Disclosure
The authors declare that there are no competing interests associated with the manuscript.
Contributor Information
Yaming Li, Email: liyaming029@163.com.
Jinzhao Zhang, Email: zhangjinzhao2019@163.com.
References
- 1. Hirsch FR, Scagliotti GV, Mulshine JL et al Lung cancer: Current therapies and new targeted treatments. Lancet 2017; 389 (10066): 299–311. [DOI] [PubMed] [Google Scholar]
- 2. Herbst RS, Morgensztern D, Boshoff C. The biology and management of non‐small cell lung cancer. Nature 2018; 553 (7689): 446–54. [DOI] [PubMed] [Google Scholar]
- 3. Balata H, Fong KM, Hendriks LE et al Prevention and early detection for NSCLC: Advances in thoracic oncology 2018. J Thorac Oncol 2019; 14 (9): 1513–27. [DOI] [PubMed] [Google Scholar]
- 4. Reck M, Rabe KF. Precision diagnosis and treatment for advanced non‐small‐cell lung cancer. N Engl J Med 2017; 377 (9): 849–61. [DOI] [PubMed] [Google Scholar]
- 5. Beermann J, Piccoli MT, Viereck J, Thum T. Non‐coding RNAs in development and disease: Background, mechanisms, and therapeutic approaches. Physiol Rev 2016; 96 (4): 1297–325. [DOI] [PubMed] [Google Scholar]
- 6. Kristensen LS, Andersen MS, Stagsted LVW, Ebbesen KK, Hansen TB, Kjems J. The biogenesis, biology and characterization of circular RNAs. Nat Rev Genet 2019; 20 (11): 675–91. [DOI] [PubMed] [Google Scholar]
- 7. Braicu C, Zimta AA, Harangus A et al The function of non‐coding RNAs in lung cancer tumorigenesis. Cancers (Basel) 2019; 11 (5): E605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chi Y, Luo Q, Song Y et al Circular RNA circPIP5K1A promotes non‐small cell lung cancer proliferation and metastasis through miR‐600/HIF‐1alpha regulation. J Cell Biochem 2019; 120 (11): 19019–30. [DOI] [PubMed] [Google Scholar]
- 9. Xue YB, Ding MQ, Xue L, Luo JH. CircAGFG1 sponges miR‐203 to promote EMT and metastasis of non‐small‐cell lung cancer by upregulating ZNF281 expression. Thorac Cancer 2019; 10 (8): 1692–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang H, Wang X, Hu B et al Circular RNA ZFR accelerates non‐small cell lung cancer progression by acting as a miR‐101‐3p sponge to enhance CUL4B expression. Artif Cells Nanomed Biotechnol 2019; 47 (1): 3410–6. [DOI] [PubMed] [Google Scholar]
- 11. Yu L, Gong X, Sun L et al The circular RNA cdr1as act as an oncogene in hepatocellular carcinoma through targeting miR‐7 expression. PLOS One 2016; 11 (7): e0158347. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12. Tang W, Ji M, He G et al Silencing CDR1as inhibits colorectal cancer progression through regulating microRNA‐7. Onco Targets Ther 2017; 10: 2045–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang J, Hu H, Zhao Y et al CDR1as is overexpressed in laryngeal squamous cell carcinoma to promote the tumour's progression via miR‐7 signals. Cell Prolif 2018; 51 (6): e12521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li P, Yang X, Yuan W et al CircRNA‐cdr1as exerts anti‐oncogenic functions in bladder cancer by sponging microRNA‐135a. Cell Physiol Biochem 2018; 46 (4): 1606–16. [DOI] [PubMed] [Google Scholar]
- 15. Chen H, Mao M, Jiang J et al Circular RNA CDR1as acts as a sponge of miR‐135b‐5p to suppress ovarian cancer progression. Onco Targets Ther 2019; 12: 3869–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang X, Yang D, Wei Y. Overexpressed CDR1as functions as an oncogene to promote the tumor progression via miR‐7 in non‐small‐cell lung cancer. Onco Targets Ther 2018; 11: 3979–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Han Y, Li H. miRNAs as biomarkers and for the early detection of non‐small cell lung cancer (NSCLC). J Thorac Dis 2018; 10 (5): 3119–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sun X, Xu M, Liu H et al MicroRNA‐219 is downregulated in non‐small cell lung cancer and inhibits cell growth and metastasis by targeting HMGA2. Mol Med Rep 2017; 16 (3): 3557–64. [DOI] [PubMed] [Google Scholar]
- 19. Rao C, Miao X, Zhao G et al MiR‐219a‐5p enhances cisplatin sensitivity of human non‐small cell lung cancer by targeting FGF9. Biomed Pharmacother 2019; 114: 108662. [DOI] [PubMed] [Google Scholar]
- 20. Liang Z, Xu J, Gu C. Novel role of the SRY‐related high‐mobility‐group box D gene in cancer. Semin Cancer Biol 2019. 10.1016/j.semcancer.2019.07.011. [DOI] [PubMed] [Google Scholar]
- 21. Chen X, Zheng Q, Li W et al SOX5 induces lung adenocarcinoma angiogenesis by inducing the expression of VEGF through STAT3 signaling. Onco Targets Ther 2018; 11: 5733–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zou H, Wang S, Wang S et al SOX5 interacts with YAP1 to drive malignant potential of non‐small cell lung cancer cells. Am J Cancer Res 2018; 8 (5): 866–78. [PMC free article] [PubMed] [Google Scholar]
- 23. Lei K, Bai H, Wei Z et al The mechanism and function of circular RNAs in human diseases. Exp Cell Res 2018; 368 (2): 147–58. [DOI] [PubMed] [Google Scholar]
- 24. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real‐time quantitative PCR and the 2(‐Delta Delta C(T)) Method. Methods 2001; 25 (4): 402–8. [DOI] [PubMed] [Google Scholar]
- 25. Zhang XQ, Yang JH. Discovering circRNA‐microRNA interactions from CLIP‐Seq data. Methods Mol Biol 1724; 2018: 193–207. [DOI] [PubMed] [Google Scholar]
- 26. Riffo‐Campos ÁL, Riquelme I, Brebi‐Mieville P. Tools for sequence‐based miRNA target prediction: What to choose? Int J Mol Sci 2016; 17 (12): E1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gridelli C, Rossi A, Carbone DP et al Non‐small‐cell lung cancer. Nat Rev Dis Primers 2015; 1: 15009. [DOI] [PubMed] [Google Scholar]
- 28. Zhang M, Xin Y. Circular RNAs: A new frontier for cancer diagnosis and therapy. J Hematol Oncol 2018; 11 (1): 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Altorki NK, Markowitz GJ, Gao D et al The lung microenvironment: An important regulator of tumour growth and metastasis. Nat Rev Cancer 2019; 19 (1): 9–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Legras A, Pecuchet N, Imbeaud S et al Epithelial‐to‐mesenchymal transition and microRNAs in lung cancer. Cancers (Basel) 2017; 9 (8): E101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mittal V. Epithelial mesenchymal transition in tumor metastasis. Annu Rev Pathol 2018; 13: 395–412. [DOI] [PubMed] [Google Scholar]
- 32. Zhong Y, Du Y, Yang X et al Circular RNAs function as ceRNAs to regulate and control human cancer progression. Mol Cancer 2018; 17 (1): 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yuan W, Zhou R, Wang J et al Circular RNA Cdr1as sensitizes bladder cancer to cisplatin by upregulating APAF1 expression through miR‐1270 inhibition. Mol Oncol 2019; 13 (7): 1559–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yang W, Yang X, Wang X et al Silencing CDR1as enhances the sensitivity of breast cancer cells to drug resistance by acting as a miR‐7 sponge to down‐regulate REGgamma. J Cell Mol Med 2019; 23 (8): 4921–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhu X, Chen L, Lin J. miR‐219a‐5p represses migration and invasion of osteosarcoma cells via targeting EYA2. Artif Cells Nanomed Biotechnol 2018; 46 (Suppl. 3): S1004–S10. [DOI] [PubMed] [Google Scholar]
- 36. Grimm D, Bauer J, Wise P et al The role of SOX family members in solid tumours and metastasis. Semin Cancer Biol 2019. 10.1016/j.semcancer.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 37. Li G, Wang K, Wang J et al miR‐497‐5p inhibits tumor cell growth and invasion by targeting SOX5 in non‐small‐cell lung cancer. J Cell Biochem 2019; 120 (6): 10587–95. [DOI] [PubMed] [Google Scholar]
- 38. Yang B, Zhang W, Sun D et al Downregulation of miR‐139‐5p promotes prostate cancer progression through regulation of SOX5. Biomed Pharmacother 2019; 109: 2128–35. [DOI] [PubMed] [Google Scholar]
- 39. Sun C, Ban Y, Wang K, Sun Y, Zhao Z. SOX5 promotes breast cancer proliferation and invasion by transactivation of EZH2. Oncol Lett 2019; 17 (3): 2754–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ding S, Zhang Y. MicroRNA539 inhibits the proliferation and migration of gastric cancer cells by targeting SRYbox 5 gene. Mol Med Rep 2019; 20 (3): 2533–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li A, Hooli B, Mullin K et al Silencing of the Drosophila ortholog of SOX5 leads to abnormal neuronal development and behavioral impairment. Hum Mol Genet 2017; 26 (8): 1472–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Song Z, Wang H, Zhang S. Negative regulators of Wnt signaling in non‐small cell lung cancer: Theoretical basis and therapeutic potency. Biomed Pharmacother 2019; 118: 109336. [DOI] [PubMed] [Google Scholar]


