Abstract
Mucociliary epithelium lining the upper and lower respiratory tract constitutes the first line of defense of the airway and lungs against inhaled pollutants and pathogens. The concerted beating of multiciliated cells drives mucociliary clearance. Abnormalities in both the structure and function of airway cilia have been implicated in obstructive lung diseases. Emerging evidence reveals a close correlation between lung diseases and environmental stimuli such as sulfur dioxide and tobacco particles. However, the underlying mechanism remains to be described. In this review, we emphasize the importance of airway cilia in mucociliary clearance and discuss how environmental pollutants affect the structure and function of airway cilia, thus shedding light on the function of airway cilia in preventing obstructive lung diseases and revealing the negative effects of environmental pollutants on human health.
Keywords: Airway epithelium, environmental pollutant, lung disease, mucociliary clearance
Introduction
The human airway is a dichotomous hollow tubular structure mainly lined by ciliated, brush, goblet, and basal cells.1 These cells form a continuous physical, secretory, and regulatory barrier of epithelium, which functions to protect the airway and lungs from inhaled pathogens and environmental pollutants.2 Basal cells are stem/progenitor cells that differentiate into ciliated cells and goblet cells in response to injury and repair.3 Goblet cells secrete the mucus and mucins that comprise the mucus gel layer, an important component of the mucociliary escalator.3 Airway ciliated cells dominate the epithelium and coordinate with the goblet cells to constitute the first line of defense.4 The concerted in‐plane beating of all ciliated cells propels the mucus layer forward, thus driving mucociliary clearance (MCC). Abnormalities in both the airway cilia structure and function lead to impaired mucociliary clearance.5 Cilia dysfunction has been implicated in a variety of lung diseases, such as cystic fibrosis, immotile cilia syndrome, bronchial asthma, and chronic obstructive pulmonary disease (COPD).6, 7, 8, 9, 10 For example, Yaghi and Dolovich have discussed the importance of airway epithelial cilia in the initiation or progression of obstructive lung diseases11; Price and Sisson have highlighted the redox modulation in airway ciliary function and diseases.12 Here, we review the structure and function of airway cilia, and focus on the influence of environmental pollutants on ciliary beating and their outcomes. This review will shed light on the function of airway cilia in preventing obstructive lung diseases and reveal the negative effects of environmental pollutants on human health.
Structure and function of airway cilia
Cilia are highly specialized hair‐like structures that protrude from the surface of epithelium. They are mainly composed of microtubule‐based axoneme, surrounded by a plasma membrane.13, 14, 15, 16 Cilia can be typically divided into primary nonmotile cilia and motile cilia, depending on the axoneme structure. The axoneme of primary cilia is arranged in a ring of nine peripheral doublet microtubules (termed a 9 + 0 axoneme), and the axoneme of a motile cilium has two single microtubules at the center of the nine peripheral doublet ring (termed a 9 + 2 axoneme).15, 17, 18, 19, 20, 21, 22 In addition, the peripheral doublets of motile cilia are attached by inner and outer dynein arms, which allow ciliary movement by ATP‐dependent conformational alterations. Aberrance in ciliary axoneme and dynein arm‐associated structures can result in impaired ciliary movement.
The airway cilia that line the pseudostratified epithelium of respiratory tract are motile with a 9 + 2 axoneme pattern, and each airway epithelial cell has more than 200 cilia on its surface. These cilia beat almost synchronously, thus driving continuously oral‐directed transport of mucus, termed mucociliary clearance (MCC). MCC is a complex and orderly cycle program,23 a critical event for fluid secretion and immune defense. The cilia start to move from the resting position by bending laterally and backward, which is called the recovery swing. When a cilium returns to the resting position, its tip points in the direction of propulsion; this stage ends and the resistance of mucus flow is minimized. The ciliary oscillations are coordinated by a heterogeneous wave pattern that couples the oscillating frequency of each cilium with the oscillating frequency of the adjacent cilia to promote the directional transport of the mucus.24 The wave swing is spread by the antirelaxation coordination but the mechanism of adjusting the synchronous swing of multiple cilia is not clear. In addition, the effectiveness of MCC is affected by a diversity of factors, including cilia numbers and their structure, humidity, temperature, age, pathogens, and environmental stimuli.25, 26, 27, 28, 29
Abnormal ciliary function and lung diseases
Given the importance of MCC in clearance of the inhaled particles and pathogens, inadequate MCC and the resulting decline of the host's lung defense functions can lead to the pathogenesis of various pulmonary diseases such as cystic fibrosis, COPD, and chronic bronchitis.30, 31 There are many factors that can lead to inadequate MCC, which include impaired fluid secretion, abnormal ciliary function, lack of cough, or the damage of epithelial cells lining the respiratory tract. Of these, abnormal ciliary function accounts for the majority.
Mounting evidence has revealed that airway cilia that are affected by environmental contaminants exhibit acquired structural or functional abnormalities accompanied by abnormalities in mucociliary clearance.3 For example, sulfur dioxide, sulfuric acid, nitrogen dioxide, and ozone all affect mucus cilia and respiratory function.32, 33, 34, 35, 36 In physiological conditions, inhaled particles and pathogens can be entrapped and then removed through MCC. However, excessive exposure to environmental pollutants can contribute to abnormal cilia structure and function, thus resulting in inadequate MCC and consequently leading to various lung diseases (Fig 1).
Figure 1.
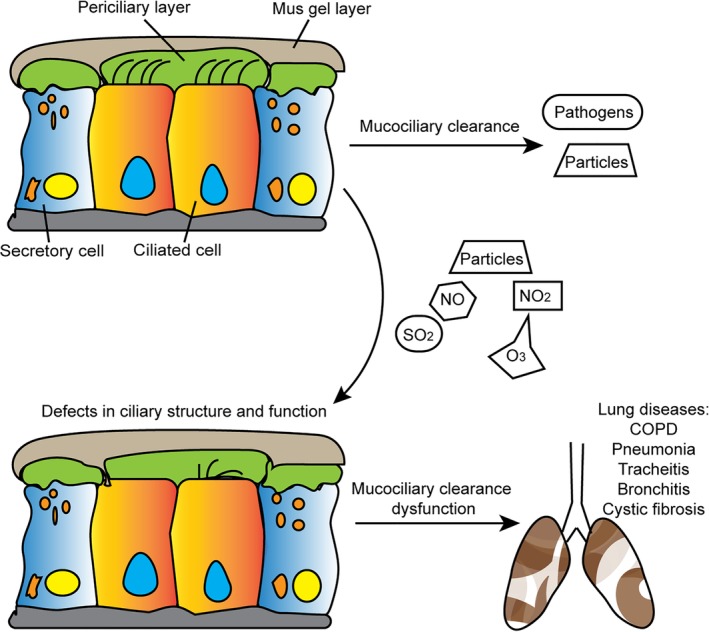
Secretory cells and ciliated epithelial cells constitute the first line of lung defense. Secretory cells produce gel‐forming mucins to entrap inhaled particles and ciliated epithelial cells transport them out of the lung through cilia beating. Reversely, some environmental pollutants, including particles, NO, NO2, SO2, and O3, can lead to impaired structure and function of airway cilia, thus resulting in inadequate MCC and consequently leading to various lung diseases.
Environmental pollutants
Environmental pollutants are foreign products that change the normal composition and properties of the environment and are directly or indirectly harmful to humans and other organisms.37 They mainly consist of irritating gases and harmful particles, including sulfur dioxide, nitrogen dioxide, ozone, indoor air pollutants, and tobacco particles.38 Environmental pollutants can be divided into three classes: atmospheric pollutants, water pollutants, and industrial pollutants. These can be further divided according to the form of pollutant: gaseous pollutants, liquid pollutants, and solid pollutants; and according to the nature of the pollutant: chemical contaminants, physical pollutants, and biological contaminants.39 The harm of environmental pollutants to the human body is mainly reflected in respiratory mucosa damage and obstructive pulmonary disease because the respiratory tract is the first thing affected by environmental exposure.40 Several studies have shown that in environments with air pollution, the cilia in the human respiratory tract become shorter or are missing, which affects their ability to clear the respiratory tract. For example, when experimental animals were exposed to higher concentrations of ozone (4 ppm), it was observed that the vesicles of the ciliated membrane and the structure of the tracheal cilia were damaged.41 In addition, mucosal cilia clearance may be inhibited due to factors such as quantity of contaminant concentration and duration of exposure.
Sulfur dioxide
Sulfur dioxide is one of the major air pollutants in industrialized countries. The main outdoor source of sulfur dioxide is the combustion of sulfur‐containing minerals, mainly coal and petroleum, in the commercial industry.42 Sulfur dioxide is water soluble and easily inhaled into the respiratory tract where it forms sulfurous acid and sulfuric acid.43 These acids are strong irritants and have burning effects on the human respiratory tract. This leads to lung diseases such as bronchitis and asthma, accompanied by lung pain, cough, phlegm, and other adverse reactions.44 Van et al. reported that the ultrastructure of the airway epidermis of guinea pigs changed after 30 minutes of sulfur dioxide treatment.45 Abraham et al. noted that in healthy non‐smokers, MCC significantly accelerated after 2.5 hours of exposure to sulfur dioxide, which was caused by an increase in ciliary beat frequency. However, excessive sulfur dioxide caused a decrease in MCC.46 Like phytohormones, the effect of sulfur dioxide on cilia is dependent on the concentration; low concentration causes promotion and high concentration causes inhibition.
Nitrogen dioxide
Nitrogen dioxide is one of the most common air pollutants. It is mainly produced by various combustion processes, especially in industrial and urban areas.47 Nitrogen dioxide is very harmful to the human body. Human lung function is damaged with even a little exposure to nitrogen dioxide. If exposed to nitrogen dioxide for a long time, the chance of respiratory infections increases, as well as the risk of permanent organic lesions in the lungs.48 Helleday et al. studied a significant decrease in ciliary beat frequency in healthy people exposed to nitrogen dioxide and found that it may be important for MCC function.49 Blomberg et al. found that nitrogen dioxide had no significant effect on the MCC of the upper respiratory tract, from the tip of the nose to the midpoint of the trachea, but that it did reduce the MCC of the lower respiratory tract, including the lower half of the trachea and the lungs.50 Like sulfur dioxide, nitrogen dioxide affects the respiratory tract by affecting MCC.
Indoor air pollutants
Formaldehyde, acrolein, phenols, and ammonia, which are usually present in indoor air pollutants, have an effect on ciliary oscillations and structure, as well as mucous flow, which may be a cause of respiratory disease.51, 52, 53 Formaldehyde has the strongest effect, followed by acrolein.54, 55 After exposure to acrolein, the tip of the cilia is swollen, making the cilia function abnormally; formaldehyde and ammonia reduce the flow of the sputum, which causes functional damage to the MCC.56
Smoking
Smoking is a main cause of human disease. Tobacco particles, nicotine, and other chemicals in cigarettes cause serious damage to peoples’ heart and lung function and lead to coronary heart disease, COPD, cerebrovascular disease, and cancer.57 Smoking also reduces the number of cilia in the respiratory tract, affects the frequency of ciliary oscillations, and thus affects the airway epithelial MCC.58 For example, compared with healthy non‐smokers, smokers are deficient in cilia with abnormal structures and functions of ciliated cells. Long‐term smoking can lead to an increase in the number of abnormal cilia in the bronchi and may damage the tracheobronchial function.59 Examination of the nasal mucosa of children exposed to smoke showed a loss of cilia. Electron microscopic analysis of the ultrastructure of cilia showed that smokers had more ciliary abnormalities than non‐smokers, including composite cilia and giant cilia, as well as other abnormalities in the microtubules, axon 9 + 2 tissue, and the cilia localizations.60 Smoking is also one of the main causes of COPD.
Concluding remarks
Environmental pollutants have been implicated in several lung diseases, such as obstructive pulmonary diseases and bronchitis. Airway cilia are essential for MCC and protect the lungs from diseases caused by environmental pollutants. Emerging evidence reveals that environmental pollutants impair the physiological roles of airway cilia. However, the molecular mechanisms of how environmental pollutants affect the structure and function of airway cilia remain largely unknown. Recent studies reveal that some redox regulatory proteins, including protein phosphatase 2A, protein kinase A, protein kinase C, soluble guanylyl cyclase, and dynein ATPases, are enriched in cilia and play critical roles in regulating airway cilia.12 It is postulated that oxidants in environmental contaminants lead to redox imbalance and impair airway cilia. More research regarding redox signaling in cilia regulation will likely uncover the molecular mechanism underlying lung diseases induced by environmental pollutants.
In addition, with a more in‐depth understanding of airway ciliogenesis and the development of genome editing, it is feasible to cure lung diseases caused by cilia deficiency. In addition, future studies will explore the details of environmental pollutants, including the type of pollutants, maximum exposure dose, and time that can lead to airway cilia defects and dysfunctions of MCC. These studies may help to increase awareness of the damage environmental pollutants cause to airway cilia and provide a basis for establishing environmental protection laws.
Disclosure
The authors declare no conflict of interest regarding the publication of this article.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (31701216), and Natural Science Foundation of Shandong Province (ZR2017MC008).
Contributor Information
Songbo Xie, Email: xiesongbo@sdnu.edu.cn.
Min Liu, Email: minliu@sdnu.edu.cn.
References
- 1. Funk MC, Bera AN, Menchen T et al Cyclin O (Ccno) functions during deuterosome‐mediated centriole amplification of multiciliated cells. EMBO J 2015; 34: 1078–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hild M, Jaffe AB. Production of 3‐D airway organoids from primary human airway basal cells and their use in high‐throughput screening. Curr Protoc Stem Cell Biol 2016; 37: Ie.9.1–Ie.9.15. [DOI] [PubMed] [Google Scholar]
- 3. Tilley AE, Walters MS, Shaykhiev R, Crystal RG. Cilia dysfunction in lung disease. Annu Rev Physiol 2015; 77: 379–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Turner J, Roger J, Fitau J et al Goblet cells are derived from a FOXJ1‐expressing progenitor in a human airway epithelium. Am J Respir Cell Mol Biol 2011; 44: 276–84. [DOI] [PubMed] [Google Scholar]
- 5. Bennett WD. Effect of beta‐adrenergic agonists on mucociliary clearance. J Allergy Clin Immunol 2002; 110: S291–7. [DOI] [PubMed] [Google Scholar]
- 6. Rubin BK. Immotile cilia syndrome (primary ciliary dyskinesia) and inflammatory lung disease. Clin Chest Med 1988; 9: 657–68. [PubMed] [Google Scholar]
- 7. Xie W, Yang Y, Gao S et al The tumor suppressor CYLD controls epithelial morphogenesis and homeostasis by regulating mitotic spindle behavior and adherens junction assembly. J Genet Genomics 2017; 44: 343–53. [DOI] [PubMed] [Google Scholar]
- 8. Luo Y, Li D, Ran J et al End‐binding protein 1 stimulates paclitaxel sensitivity in breast cancer by promoting its actions toward microtubule assembly and stability. Protein Cell 2014; 5: 469–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen M, Xie S. Therapeutic targeting of cellular stress responses in cancer. Thorac Cancer 2018; 9: 1575–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ran J, Zhou J. Targeted inhibition of histone deacetylase 6 in inflammatory diseases. Thorac Cancer 2019; 10: 405–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yaghi A, Dolovich MB. Airway epithelial cell cilia and obstructive lung disease. Cells 2016; 5: E40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Price ME, Sisson JH. Redox regulation of motile cilia in airway disease. Redox Biol 2019; 27: 101146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Abou Alaiwi WA, Lo ST, Nauli SM. Primary cilia: Highly sophisticated biological sensors. Sensors 2009; 9: 7003–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yang Y, Huijie H, Xiaofan W et al Mixed‐lineage leukemia protein 2 suppresses ciliary assembly by the modulation of actin dynamics and vesicle transport. Cell Discov 2019; 5: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yang Y, Ran J, Liu M et al CYLD mediates ciliogenesis in multiple organs by deubiquitinating Cep70 and inactivating HDAC6. Cell Res 2014; 24: 1342–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yu F, Guo S, Li T et al Ciliary defects caused by dysregulation of O‐GlcNAc modification are associated with diabetic complications. Cell Res 2019; 29: 171–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. He X, Liu Z, He Q et al Identification of novel microtubule‐binding proteins by taxol‐mediated microtubule stabilization and mass spectrometry analysis. Thorac Cancer 2015; 6: 649–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xie SB, Zhou J. Harnessing plant biodiversity for the discovery of novel anticancer drugs targeting microtubules. Front Plant Sci 2017; 8: 720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sui N, Tian S, Wang W et al Overexpression of glycerol‐3‐phosphate acyltransferase from suaeda salsa improves salt tolerance in arabidopsis. Front Plant Sci 2017; 8: 1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang SS, Wang F, Tan SJ, Wang MX, Sui N, Zhang XS. Transcript profiles of maize embryo sacs and preliminary identification of genes involved in the embryo sac‐pollen tube interaction. Front Plant Sci 2014; 5: 702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhou JJ, Liu Q, Zhang F et al Overexpression of OsPIL15, a phytochromeinteracting factor‐ like protein gene, represses etiolated seedling growth in rice. J Integr Plant Biol 2014; 56: 373–87. [DOI] [PubMed] [Google Scholar]
- 22. Jiang CY, Tholen D, Xu JM et al Increased expression of mitochondria‐localized carbonic anhydrase activity resulted in an increased biomass accumulation in Arabidopsis thaliana . J Plant Biol 2014; 57: 366–74. [Google Scholar]
- 23. Sleigh MA, Blake JR, Liron N. The propulsion of mucus by cilia. Am Rev Respir Dis 1988; 137: 726–41. [DOI] [PubMed] [Google Scholar]
- 24. Silverman H, Lynn JW, Dietz TH. Particle capture by the gills of Dreissena polymorpha: Structure and function of latero‐frontal cirri. Biol Bull 1996; 191: 42–54. [DOI] [PubMed] [Google Scholar]
- 25. IEEE international symposium on biomedical imaging. IEEE Pulse 2017; 8: 65. [DOI] [PubMed] [Google Scholar]
- 26. Chen M, Li Y, Liu Z et al Exopolysaccharides from a Codonopsis pilosula endophyte activate macrophages and inhibit cancer cell proliferation and migration. Thorac Cancer 2018; 9: 630–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu M, Ran J, Zhou J. Non‐canonical functions of the mitotic kinesin Eg5. Thorac Cancer 2018; 9: 904–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sun S, Zhou J. Molecular mechanisms underlying stress response and adaptation. Thorac Cancer 2018; 9: 218–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yang Y, Mu T, Li T et al Effects of FSTL1 on the proliferation and motility of breast cancer cells and vascular endothelial cells. Thorac Cancer 2017; 8: 606–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lundback B, Gulsvik A, Albers M et al Epidemiological aspects and early detection of chronic obstructive airway diseases in the elderly. Eur Respir J Suppl 2003; 40: 3s–9s. [DOI] [PubMed] [Google Scholar]
- 31. Whitsett JA. Airway epithelial differentiation and mucociliary clearance. Ann Am Thorac Soc 2018; 15: S143–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Houtmeyers E, Gosselink R, Gayan‐Ramirez G, Decramer M. Regulation of mucociliary clearance in health and disease. Eur Respir J 1999; 13: 1177–88. [DOI] [PubMed] [Google Scholar]
- 33. Zhao S, Jiang Y, Zhao Y et al Csaein Kinase1‐like protein2 regulates Actin filament stability and stomatal closure via phosphorylation of Actin depolymerizing factor. Plant Cell 2016; 28: 1422–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kong XQ, Gao XH, Sun W, An J, Zhao YX, Zhang H. Cloning and functional characterization of a cation‐chloride cotransporter gene OsCCC1. Plant Mol Biol 2011; 75: 567–78. [DOI] [PubMed] [Google Scholar]
- 35. Li SP, van Os GM, Ren S et al Expression and functional analyses of EXO70 genes in Arabidopsis implicate their roles in regulating cell type‐specific exocytosis. Plant Physiol 2010; 154: 1819–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bai B, Zhao J, Li Y et al OsBBX14 delays heading date by repressing florigen gene expression under long and short‐day conditions in rice. Plant Sci 2016; 247: 25–34. [DOI] [PubMed] [Google Scholar]
- 37. Nakai S, Okuda T, Nishijima W, Okada M. Production of mono‐ and di‐carboxylated polyethylene glycols as a factor obstacle to the successful ozonation‐assisted biodegradation of ethoxylated compounds. Chemosphere 2015; 136: 153–9. [DOI] [PubMed] [Google Scholar]
- 38. Kleinman MT, Bailey RM, Chang YT et al Exposures of human volunteers to a controlled atmospheric mixture of ozone, sulfur dioxide and sulfuric acid. Am Ind Hyg Assoc J 1981; 42: 61–9. [DOI] [PubMed] [Google Scholar]
- 39. Lacal J, García‐Fontana C, Muñoz‐Martínez F, Ramos JL, Krell T. Sensing of environmental signals: Classification of chemoreceptors according to the size of their ligand binding regions. Environ Microbiol 2010; 12: 2873–84. [DOI] [PubMed] [Google Scholar]
- 40. Gorska K, Paplińska‐Goryca M, Nejman‐Gryz P, Goryca K, Krenke R. Eosinophilic and neutrophilic sirway inflammation in the phenotyping of mild‐to‐moderate asthma and chronic obstructive pulmonary disease. COPD 2017; 14: 181–9. [DOI] [PubMed] [Google Scholar]
- 41. Finlay BJ, Esteban GF, Clarke KJ, Olmo JL. Biodiversity of terrestrial protozoa appears homogeneous across local and global spatial scales. Protist 2001; 152: 355–66. [DOI] [PubMed] [Google Scholar]
- 42. Lv C, Wang X, Pang N et al The impact of airborne particulate matter on pediatric hospital admissions for pneumonia among children in Jinan, China: A case‐crossover study. J Air Waste Manag Assoc 2017; 67: 669–76. [DOI] [PubMed] [Google Scholar]
- 43. Tseng CY, Huang YC, Su SY et al Cell type specificity of female lung cancer associated with sulfur dioxide from air pollutants in Taiwan: An ecological study. BMC Public Health 2012; 12: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sun M, Yu H, Zhang K et al Determination of gaseous sulfur dioxide and its derivatives via fluorescence enhancement based on cyanine dye functionalized carbon nanodots. Anal Chem 2014; 86: 9381–5. [DOI] [PubMed] [Google Scholar]
- 45. Van Keymeulen A, Mascre G, Youseff KK et al Epidermal progenitors give rise to Merkel cells during embryonic development and adult homeostasis. J Cell Biol 2009; 187: 91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Abraham WM, Oliver W Jr, Welker MJ et al Differences in airway reactivity in normal and allergic sheep after exposure to sulfur dioxide. J Appl Physiol Respir Environ Exerc Physiol 1981; 51: 1651–6. [DOI] [PubMed] [Google Scholar]
- 47. Yu J, Wu J, Zhang Y et al Concurrent highly pathogenic porcine reproductive and respiratory syndrome virus infection accelerates Haemophilus parasuis infection in conventional pigs. Vet Microbiol 2012; 158: 316–21. [DOI] [PubMed] [Google Scholar]
- 48. Rose RM, Pinkston P, Skornik WA. Altered susceptibility to viral respiratory infection during short‐term exposure to nitrogen dioxide. Res Rep Health Eff Inst 1989; 24: 1–24. [PubMed] [Google Scholar]
- 49. Helleday R, Huberman D, Blomberg A, Stjernberg N, Sandström T. Nitrogen dioxide exposure impairs the frequency of the mucociliary activity in healthy subjects. Eur Respir J 1995; 8: 1664–8. [DOI] [PubMed] [Google Scholar]
- 50. Blomberg A, Krishna MT, Helleday R et al Persistent airway inflammation but accommodated antioxidant and lung function responses after repeated daily exposure to nitrogen dioxide. Am J Respir Crit Care Med 1999; 159: 536–43. [DOI] [PubMed] [Google Scholar]
- 51. Zhao C, Qiu J, Agarwal G et al Genome‐wide discovery of microsatellite markers from diploid progenitor species, arachis duranensis and A. ipaensis, and their application in cultivated peanut (A. hypogaea). Front Plant Sci 2017; 8: 1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Song J, Shi W, Liu R et al The role of the seed coat in adaptation of dimorphic seeds of the euhalophyte Suaeda salsa to salinity. Plant Spec Biol 2017; 32: 107–14. [Google Scholar]
- 53. Liu F, Yang Y, Gao J et al A comparative transcriptome analysis of a wild purple potato and its red mutant provides insight into the mechanism of anthocyanin transformation. PLOS One 2018; 13: e0191406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gilbert NL, Guay M, Miller JD, Judek S, Chan CC, Dales RE. Levels and determinants of formaldehyde, acetaldehyde, and acrolein in residential indoor air in Prince Edward Island, Canada. Environ Res 2005; 99: 11–7. [DOI] [PubMed] [Google Scholar]
- 55. Xie W, Zhou J. Aberrant regulation of autophagy in mammalian diseases. Biol Lett 2018; 14: 20170540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bohanon HR, Piadé JJ, Schorp MK, Saint‐Jalm Y. An international survey of indoor air quality, ventilation, and smoking activity in restaurants: A pilot study. J Expo Anal Environ Epidemiol 2003; 13: 378–92. [DOI] [PubMed] [Google Scholar]
- 57. Silverstein BS, Feld S, Kozlowski LT. The availability of low‐nicotine cigarettes as a cause of cigarette smoking among teenage females. J Health Soc Behav 1980; 21: 383–8. [PubMed] [Google Scholar]
- 58. Smallman LA, Gregory J. Ultrastructural abnormalities of cilia in the human respiratory tract. Hum Pathol 1986; 17: 848–55. [DOI] [PubMed] [Google Scholar]
- 59. Parrilla E, Armengot M, Mata M et al A ciliary motility index for activity measurement in cell cultures with respiratory syncytial virus. Am J Rhinol Allergy 2019; 33: 121–8. [DOI] [PubMed] [Google Scholar]
- 60. Rossman CM, Lee RM, Forrest JB, Newhouse MT. Nasal cilia in normal man, primary ciliary dyskinesia and other respiratory diseases: Analysis of motility and ultrastructure. Eur J Respir Dis Suppl 1983; 127: 64–70. [PubMed] [Google Scholar]


