Abstract
Background
Our recent studies have indicated that miR‐153‐3p is downregulated in the esophageal squamous cell carcinoma (ESCC) cell lines and tissues. Upregulation of miR‐153‐3p was found to inhibit migration and invasion of ESCC cells. However, whether miR‐153‐3p regulates the cisplatin sensitivity in ESCC cells remains unclear. In this study, we explored whether and how miR‐153‐3p regulates the proliferation and confers cisplatin resistance in ESCC by targeting the Nrf‐2 protein.
Methods
Eca109 cell line was transfected with microRNA‐153‐3p mimics or Nrf‐2siRNA and cell proliferation and cisplatin resistance were studied. A dual‐luciferase reporter assay was performed on Eca109 cells cotransfected with the wild‐type/mutant 3′UTR sequences of Nrf‐2 and control or microRNA‐153‐3p mimics. We determined the correlation between microRNA‐153‐3p and Nrf‐2 expression in human ESCC samples and explored the effect of Nrf‐2 in the overall survival rate of ESCC patients.
Results
MiR‐153‐3p significantly suppressed cell proliferation and increased the sensitivity of Eca‐109 cells to cisplatin. MiR‐153‐3p showed a negative correlation with Nrf‐2 in human esophageal carcinoma tissues. MiR‐153‐3p suppressed the expression of Nrf‐2 via binding to its 3′‐UTR region. Furthermore, inhibition of Nrf‐2 also decreased cell proliferation and increased the sensitivity of Eca109 cells to cisplatin. High expression of Nrf‐2 in human ESCC samples was associated with poor overall survival of ESCC patients.
Conclusion
MiR‐153‐3p inhibits cell proliferation and confers cisplatin resistance by downregulating Nrf‐2 expression in Eca‐109 cells. Thus, miR‐153‐3p/Nrf‐2 may play an important role in conferring cisplatin resistance in ESCC. Nrf‐2 appears to be a promising therapeutic target for ESCC.
Keywords: Esophageal squamous cell carcinoma, microRNA‐153‐3p, nuclear factor erythroid 2‐related factor 2, superoxide dismutase
Introduction
Esophageal carcinoma is a common malignant tumor of the digestive tract and esophageal squamous cell carcinoma (ESCC) is the major histopathological subtype of esophageal carcinoma.1 Cisplatin is commonly used for the treatment of malignant tumors, such as esophageal carcinoma.2, 3 However, patients with ESCC typically have a poor five‐year survival rate, which is largely attributable to resistance to chemotherapeutic agents including cisplatin.4, 5 Several recent studies have shown that microRNAs (miRs) play a crucial role in the progression of cancer by serving as oncogenes or tumor suppressors. For example, miR‐133b has been shown to suppress ESCC cell proliferation and invasion by inhibiting the expression of TAGLN2.6 MiR‐219‐5p has been reported to inhibit cell cycle progression and cell proliferation in ESCC cell lines by downregulating the expression of CCNA2 (also known as CyclinA2).7 In addition to regulating the infiltration and metastasis of cancer cells, abnormal expression of miRs is reportedly responsible for the development of cisplatin resistance in cancer cells.8 MiR‐153 is considered to be a tumor suppressor. In our recent study, we demonstrated downregulation of miR‐153 in the ESCC cell and tissues. Upregulation of miR‐153 has been shown to inhibit the migration and invasion of ESCC cells, both in vitro and in vivo.2 Some studies have found that miR‐153‐3p can inhibit the proliferation and invasive growth of breast cancer and osteosarcoma cells.9, 10 These findings indicate that miR‐153‐3p can act as a tumor suppressor and may serve as a potential target for the treatment of malignant tumors. However, whether miR‐153‐3p regulates the proliferation of ESCC cells and confers sensitivity to cisplatin chemotherapy remains unclear.
Nuclear factor erythroid 2‐related factor 2 (Nrf‐2) is a key transcriptional regulator of antioxidant and detoxification enzymes. Aberrant expression of Nrf‐2 has been demonstrated in cancer cells, where it plays a crucial role in cell proliferation and resistance to anticancer drugs.11 For instance, Nrf‐2 has been shown to exert an antioxidant effect, protect against cellular DNA damage, and to mediate cancer cell proliferation and infiltration by regulating the expression of the antioxidant enzyme HO‐1.12 In a study by Kim et al. Nrf‐2 was shown to improve the sensitivity of lung cancer cell line A549 to cisplatin.13 In addition, miR‐153‐3p has been shown to regulate Nrf‐2 expression by controlling the redox homeostasis in SH‐SY5Y cells.14 In another study, inhibiting miR‐153‐3p was shown to protect against paraquat‐induced dopaminergic neurotoxicity via targeting Nrf‐2 in the central nervous system.15 These studies indicate that Nrf‐2 may be a potential target of miR‐153‐3p in ESCC, and may play a critical role in tumor cell proliferation and cisplatin resistance in ESCC.
In this study, we explored whether miR‐153‐3p regulated the proliferation of ESCC cells and conferred cisplatin resistance via targeting the Nrf‐2 protein. In addition, we also explored the underlying mechanisms. Our findings may provide a new approach for overcoming resistance of ESCC cells to cisplatin.
Methods
Survivin (Cat#2808) and cleaved caspase‐3 were purchased from Cell Signaling Technology (Danvers, MA, USA). CyclinD1 (ab134175) and Nrf‐2 was purchased from Abcam (Cambridge, MA, USA). β‐actin (Cat#AC026) was purchased from ABclonal (Wuhan, China). Peroxidase‐labeled anti‐rabbit IgG secondary antibody (Cat#074‐1506) and anti‐mouse IgG secondary antibody (Cat#074–1806) were purchased from KPL (MA, USA).
All culture media and reagents were purchased from Gibco (Thermo Fisher Scientific, Sunnyvale, CA, USA). miR‐153‐3p mimics, negative control mimics (NC mimics), Nrf‐2 short interfering RNA (Nrf‐2‐siRNA), and negative control siRNA (NC‐siRNA) were purchased from Gene Pharma, China.
Human tissue samples
A total of 25 fresh ESCC samples along with paired adjacent nontumor tissue specimens were obtained from patients with ESCC at the Fourth Hospital of Hebei Medical University from 2016 to 2017. All fresh tissue specimens were kept in liquid nitrogen and stored at −80°C until analysis. All patients with ESCC were diagnosed by an experienced pathologist and had received no anticancer treatment such as radiotherapy and chemotherapy prior to surgery. This study was approved by the Ethics Committee of the Fourth Hospital of Hebei Medical University. Written informed consent was obtained from all patients.
We obtained 60 paraffin‐embedded specimens of ESCC and 30 adjacent nontumor tissue specimens from the Department of Pathology, Second Hospital of Hebei Medical University, between January 2010 and July 2014.3 This study was approved by the Hebei Medical University Ethics Committee. Handling of data and specimens was done in compliance with the ethical and legal standards.
Diagnosis of ESCC was performed by two pathologists based on the criteria stipulated by the World Health Organization (WHO). Clinical data, histopathological data, and the overall survival information were available until death, or the end of the investigation.
Cell culture and transfection
Human Eca109 cells were obtained from the Resource Center of the Peking Union Medical College Hospital of China (Beijing, China). Eca109 cell line was maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin and grown in a humidified incubator with 5% CO2. Transfection of miR‐153‐p mimic, NC mimics, Nrf‐2 siRNA and NC siRNA were carried out using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. The sequences of miR‐153 mimics, Nrf‐2‐siRNA, and NC‐siRNA were as follows:
miR‐153 mimics: 5′‐UUGCAUAGUCACAAAAGUGAUC‐3′;
Nrf‐2‐siRNA: 5′‐GGUUGAGACUACCAUGGUUTT‐3′;
NC‐siRNA: 5′‐UUCUCCGAACGUGUCACGUTT‐3′.
Quantitative reverse transcription polymerase chain reaction (qRT‐PCR)
The expression levels of miR‐153‐3p and Nfr‐2 mRNA were determined using qRT‐PCR. Total RNA was isolated from EC109 cells and tissues using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. Total miRNA was isolated using miRNeasy kit (Qiagen GmbH, Hilden, Germany) following the manufacturer's protocol. RNA was converted into cDNA using a PrimeScript RT reagent kit (Takara, Dalian, China). Further, qPCR assay was conducted using a SYBR Premix Ex Taq kit (Takara). β‐actin was used as a housekeeping gene for Nrf‐2 mRNA, and U6 was selected as the loading control for miR‐153‐3p. The relative expression levels of miR‐153‐3p and Nrf‐2 mRNA were calculated using the 2‐ΔΔCt method.
Cell counting kit‐8 (CCK‐8) assay
A total of 2 × 104 cells/mL were seeded in 96‐well culture plates with five replicate wells per group. Cells transfected with NC‐siRNA or SOD‐2 siRNA were treated with or without different concentrations of cisplatin, according to the design of experiments. Cell survival was detected using the CCK‐8 kit (Dojindo, Shanghai, China) according to the manufacturer's protocol. Experiments were carried out at least three times and performed in triplicate.
Colony formation assay
After transfection, a total of 2 × 103 cells/mL were seeded into a six‐well plate. All cells were incubated at 37°C for 9–12 days when the colonies showed more than 50 cells. The cells were fixed with formalin for 30 minutes and stained with 1% crystal violet according to the protocol described in our previous publication.3 Colony formation number was subsequently calculated. Experiments were repeated at least three times.
Western blotting
RIPA lysis buffer was used to isolate total protein from ESCC cells. BCA protein assay kit (Pierce, Thermo Fisher Scientific, USA) was used to detect the concentration of protein according to the manufacturer's specifications. Protein (50 μg) was separated by 10% SDS polyacrylamide gel electrophoresis (SDS‐PAGE) and transferred to polyvinylidene fluoride (PVDF) nylon membranes. The membranes were subsequently blocked with 5% blocking solution for two hours at 37°C, followed by overnight incubation with primary antibodies against Nrf‐2, cyclin D1, survivin, cleaved caspase‐3, and β‐actin at 4°C. The membranes were then incubated with secondary antibodies for two hours at 25°C. Protein signals were acquired using a chemiluminesence (ECL) detection system. The resulting images were quantified using densitometric analysis software (BIO‐1D).
Flow cytometry assay
Cell apoptosis was detected using an Annexin V‐FITC apoptosis detection kit (BD Biosciences, CA, USA) according to the manufacturer's guidelines. EC109 cells were transfected with miR‐153 mimics, Nrf‐2‐siRNA, and their NC controls, and then treated with different concentrations of DDP for 24 hours. Further cells were stained with PI and Annexin, and maintained for 15 minutes in the dark. FlowJo 7.6 software was used to analyze the data, as described elsewhere.3 Both early apoptotic cells (Annexin V‐positive and PI‐negative) and late apoptotic cells (Annexin V‐positive and PI‐positive) were included while determining the apoptosis rate. Experiments were performed in triplicate.
Luciferase reporter assay
Luciferase reporter assay was performed to confirm whether miR‐153‐3p interacted with Nrf‐2 mRNA. Fragments of the Nrf‐2 3′‐ untranslated region (UTR) sequence were obtained from the Shanghai Heyuan Biological Co. Ltd. (Shanghai, China). Post 48 hours luciferase activity was determined using the dual‐luciferase reporter assay system (Promega, Madison, WI, USA) according to the manufacturer's guidelines.2
Immunohistochemistry
All samples were sectioned to 5 μm thickness and immunohistochemically stained as previously described.3 The samples were stained with primary antibodies for cyclin D1, survivin, and Nrf‐2 and incubated overnight at 4°C. Tissues were incubated with diluted secondary antibody for 45 minutes at 37°C. Visualization was achieved with peroxidase‐labeled streptavidin‐biotin and diaminobenzidine (DAB) staining. Positive cells were observed under a light microscope. The degree of immunostaining was scored independently by two pathologists, as described elsewhere.3
Statistical analysis
Statistical analysis was performed using the SPSS 21.0 software. Association between SOD‐2 expression and CyclinD1 as well as Survivin in human ESCC samples was assessed using the Chi‐squared test. Data from in vitro experiments are expressed as mean ± standard deviation (SD) and statistically analyzed using one‐way analysis of variance (ANOVA). P‐values < 0.05 were considered indicative of a statistically significant difference.
Results
miR‐153‐3p inhibits proliferation of Eca‐109 cells
To determine if miR‐153‐3p influenced ESCC cell proliferation, the Eca‐109 cell line was transfected with miR‐153‐3p mimic. Post transfection for 48 hours, the expression level of miR‐153‐3p in Eca109 cells was significantly increased compared to NC mimics control (Fig 1a). Upregulation of miR‐153‐3p significantly suppressed cell viability and colony formation by Eca‐109 cells, which suggested that miR‐153‐3p can suppress proliferation of ESCC cells (Fig 1b–d; P < 0.05). To confirm the effect of miR‐153‐3p on proliferation of ESCC cells, the expression levels of proliferation‐related proteins, Cyclin D1 and Survivin, were also measured by western blotting. The results showed that the expression levels of Cyclin D1 and Survivin were significantly decreased in cells with miR‐153‐3p mimics transfection (Fig 1e; P < 0.05). These results suggested that miR‐153‐3p suppressed the proliferation of human ESCC cells and acts as a tumor suppressor gene.
Figure 1.
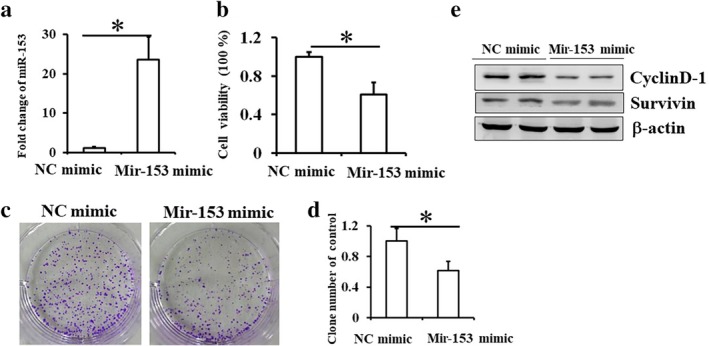
miR‐153‐3p inhibits proliferation of Eca‐109 cells. Eca‐109 cells were transfected with control or miR‐RNA‐153‐3p mimics for 48 hours. (a) Successful overexpression of miR‐RNA‐153‐3p was determined by RT‐PCR. (b) Cell proliferation was measured by CCK8 assay. (c) Colony formation was measured and shown in Eca109 cells transfected with control or miR‐RNA‐153‐3p mimics for 9–12 days, and (d) the number of the colonies was calculated. Mean (± standard deviation) values from three independent experiments are presented (*P < 0.05 vs. control). (e) Results of western blot showing the expressions of CyclinD1 and Survivin in Eca109 cells transfected with control or miR‐RNA‐153‐3p mimics.
miR‐153‐3p enhances sensitivity to cisplatin
After transfection of NC mimics or miR‐153‐3p into Eca109 cells, the cells were treated with different concentrations of cisplatin (0, 1, 2, 4, 8, 16 and 32 μg/mL) for 24 hours. The viability of control Eca109 cells decreased with the concentration of cisplatin (from 4–32 μg/mL), while transfection with miR‐153‐3p mimics significantly increased the sensitivity of ESCC to cisplatin (from 1–32 μg/mL). However, irrespective of the concentration of cisplatin, the cell viability with miR‐153‐3p mimic was significantly lower than that with NC mimic (Fig 2a). The IC50 of cisplatin in Eca‐109 cells with miR‐153‐3p mimics was lower than that of the control cells (Fig 2b). Thus, miR‐153‐3p increased the chemosensitivity of ESCC. We found that low concentration of cisplatin (2 μg/mL) did not induce apoptosis in control cells, while it induced apoptosis of cells transfected with miR‐153‐3p mimic (Fig 2c,d; P < 0.05). Low concentration of cisplatin also significantly increased the expression of cleaved caspase‐3 in Eca‐109 cells transfected with miR‐153‐3p mimics as compared to cells transfected with NC mimics (Fig 2e). Thus, our results indicate that upregulation of miR‐153‐3p significantly increased the sensitivity of ESCC to cisplatin, which suggests that loss of mir‐153‐3p leads to cisplatin resistance in ESCC.
Figure 2.
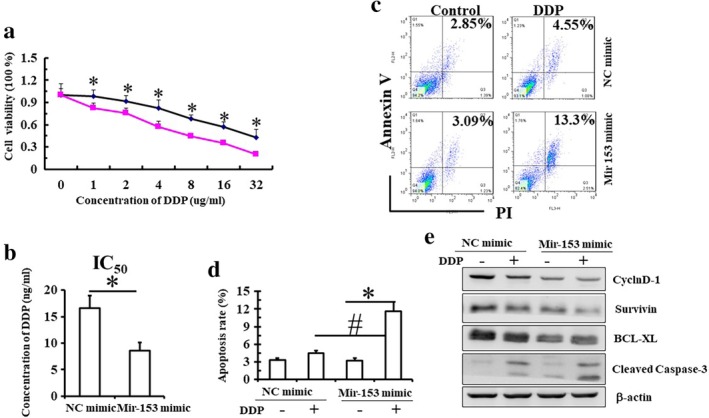
miR‐153‐3p enhances the sensitivity to cisplatin. (a) Eca109 cells transfected with control or miR‐RNA‐153‐3p mimics were treated with different concentrations of cisplatin (0–32 μg/mL) for 24 hours, and the cell viability was measured by CCK8 assay. ( ) NC mimic, (
) NC mimic, ( ) Mir153 mimic. (b) IC50 of cisplatin in cells is shown. (c,d) Eca109 cells transfected with control or miR‐RNA‐153‐3p mimics were treated with 2 μg/mL cisplatin for 24 hours, and cell apoptosis was detected by FCM with double staining by Annexin and PI. Mean (± standard deviation) values from three independent experiments are shown (*P < 0.05 vs. control; #P < 0.05, control cells treated with cisplatin vs. microRNA‐153‐3p mimics cells treated with cisplatin). (e) Results of western blot showing the expressions of CyclinD1 and Survivin in cells.
) Mir153 mimic. (b) IC50 of cisplatin in cells is shown. (c,d) Eca109 cells transfected with control or miR‐RNA‐153‐3p mimics were treated with 2 μg/mL cisplatin for 24 hours, and cell apoptosis was detected by FCM with double staining by Annexin and PI. Mean (± standard deviation) values from three independent experiments are shown (*P < 0.05 vs. control; #P < 0.05, control cells treated with cisplatin vs. microRNA‐153‐3p mimics cells treated with cisplatin). (e) Results of western blot showing the expressions of CyclinD1 and Survivin in cells.
miR‐153‐3p inhibits cell proliferation via downregulating Nrf‐2 expression
To explore whether miR‐153‐3p regulates cell proliferation via targeting Nrf‐2 protein, we measured the correlation between miR‐153‐3p and Nrf‐2 in 25 pairs of fresh ESCC and adjacent normal esophageal epithelial samples. The expression level of miR‐153‐3p in ESCC tissues showed a negative correlation with the expression level of Nrf‐2 mRNA (Fig 3a). We found that upregulation of miR‐153‐3p due to transfection with mimics inhibited the expression of Nrf‐2 in Eca109 cells in vitro (Fig 3b). To verify whether miR‐153‐3p targets Nrf‐2 directly, we performed the luciferase reporter assay and found that miR‐153‐3p could directly bind to Nrf‐2 mRNA at the 3′‐UTR region and inhibit its expression (Fig 3c). Subsequently, we mutated the Nrf2 mRNA 3′‐UTR which was the binding site of miR‐153‐3p and found that the relative luciferase activity did not downregulate it. These results confirmed that miR‐153‐3p downregulated Nrf‐2 expression by directly binding to Nrf2 mRNA 3′‐UTR region.
Figure 3.
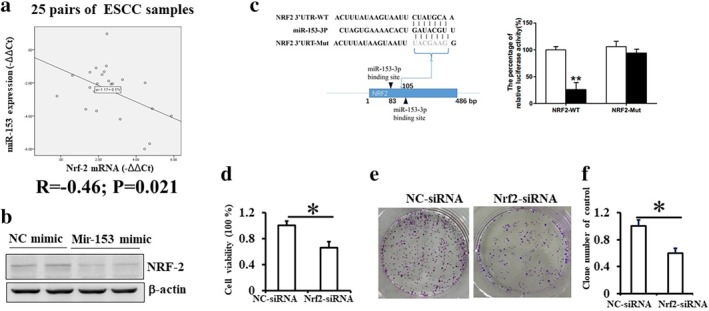
miR‐153‐3p inhibits cell proliferation via downregulating Nrf‐2 expression. (a) A total of 25 ESCC samples and matched paratumoral tissues were obtained from patients who underwent surgical resection. Real‐time PCR results showed an inverse relationship between the expressions of microRNA‐153‐3p and Nrf‐2 mRNA. (b) Results of western blot showing the expression of Nrf‐2 in Eca109 cells transfected with control or microRNA‐153‐3p mimics. (c) Predicted microRNA‐153‐3p target sequence in 3′UTR of Nrf‐2. A dual‐luciferase reporter system analysis was performed on Eca109 cells cotransfected with the wild‐type/mutant 3′UTR sequences of Nrf‐2 and control or microRNA‐153 mimics. Data presented as mean ± standard deviation. ( ), mimics control, (
), mimics control, ( ) miR‐153‐3p mimics. (d) Cell viability in Eca109 cells transfected with control or Nrf‐2 siRNA as measured by CCK8 assay. (e) Colony formation was measured in Eca109 cells transfected with control or Nrf‐2 siRNA for 9–12 days, and (f) the number of colonies was calculated. Mean (± standard deviation) values from three independent experiments are shown (*P < 0.05).
) miR‐153‐3p mimics. (d) Cell viability in Eca109 cells transfected with control or Nrf‐2 siRNA as measured by CCK8 assay. (e) Colony formation was measured in Eca109 cells transfected with control or Nrf‐2 siRNA for 9–12 days, and (f) the number of colonies was calculated. Mean (± standard deviation) values from three independent experiments are shown (*P < 0.05).
Furthermore, we explored the role of Nrf‐2 in the proliferation of Eca109 cells. We found that the viability of Eca109 cells was significantly suppressed by Nrf‐2 siRNA (Fig 3d). Inhibition of Nrf‐2 also decreased colony formation by Eca109 cells (Fig 3e,f). These findings suggested that Nrf‐2 may play a critical role in proliferation of ESCC cells. Thus, miR‐153‐3p inhibited cell proliferation by downregulating Nrf‐2 expression.
Inhibition of Nrf‐2 promotes the sensitivity of EC109 cells to cisplatin
Since inhibition of Nrf‐2 contributed to proliferation of Eca109 cells, we further investigated whether Nrf‐2 induced cisplatin resistance. The viability of control Eca109 cells decreased over a range of cisplatin concentrations (from 4–32 μg/mL), while inhibition of Nrf‐2 significantly increased the chemosensitivity of ESCC to cisplatin (from 1–32 μg/mL) (Fig 4a). Under different concentrations of cisplatin treatment, Nrf‐2 siRNA significantly enhanced the death of Eca109 cells compared to NC siRNA (Fig 4a). The IC50 of cisplatin in Eca109 cells transfected with Nrf‐2‐siRNA was notably lower than that of Eca109 cells transfected with NC‐siRNA (Fig 4b). Furthermore, we also found that low concentration of cisplatin (2 μg/mL) promoted apoptosis of cells transfected with Nrf‐2 siRNA, compared to the control cells (Fig 4c,d; P < 0.05). Therefore, our results suggested that inhibition of Nrf‐2 significantly increased the sensitivity of ESCC cells to cisplatin.
Figure 4.

Inhibition of Nrf‐2 promotes the sensitivity of EC109 cells to cisplatin. (a) Eca109 cells transfected with control or Nrf‐2 siRNA were treated with different concentrations of cisplatin (0–32 μg/mL) for 24 hours, and the cell viability was measured by CCK8 assay. IC50 values of cisplatin in cells are shown (b). Eca109 cells transfected with control or Nrf‐2 siRNA were treated with 2 μg/mL cisplatin for 24 hours, and cell apoptosis was detected by FCM with double staining with by Annexin and PI (c, d). Mean (±standard deviation) values from three independent experiments are shown (*P < 0.05 vs. control; #P < 0.05, control cells treated with cisplatin compared to Nrf‐2 siRNA cells treated with cisplatin). ( ) NC siRNA, (
) NC siRNA, ( ) NRF‐2 siRNA.
) NRF‐2 siRNA.
Increased expression of Nrf‐2 in human esophageal squamous cell carcinoma
A total of 60 samples of ESCC were collected and the expressions of Nrf‐2, CyclinD1, and Survivin were determined by immunohistochemistry (Fig 5a). Of the 60 ESCC samples, 44 (73.3%) samples showed higher expression of Nrf‐2 as compared to that in normal esophageal epithelial tissues (Fig 5b). We analyzed the correlation between Nrf‐2, CyclinD1, and Survivin in ESCC samples. Positive Nrf‐2 expression showed a strong correlation with the expressions of CyclinD1 and Survivin (Fig 5c). Kaplan‐Meier analysis indicated that higher expressions of Nrf‐2, CyclinD1, and Survivin were associated with poor overall survival of patients with ESCC (Fig 5d). The results indicated that increased expression of Nrf‐2 may be related to poor prognosis in human ESCC since Nrf‐2 contributed to cell proliferation and cisplatin resistance in ESCC.
Figure 5.
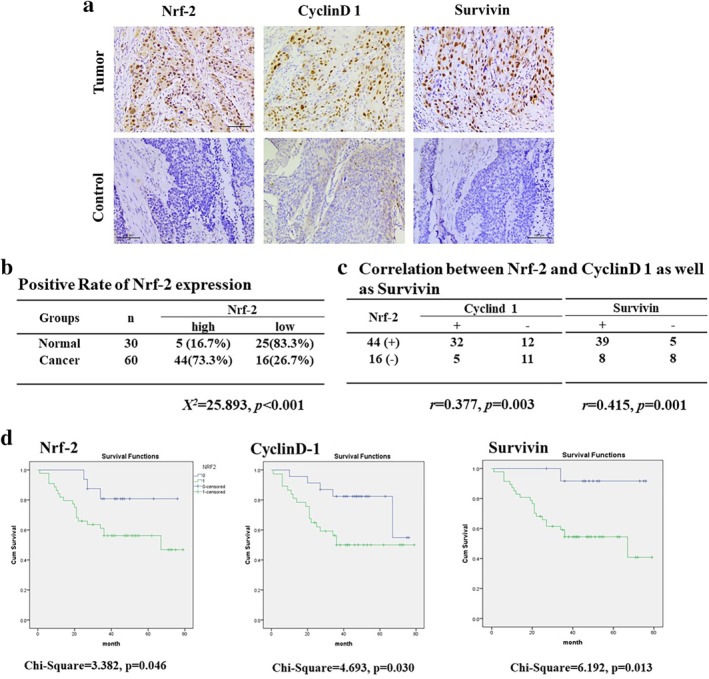
Expressions of Nrf‐2, CyclinD1, and Survivin in ESCC. (a, b) The expressions of Nrf‐2, CyclinD1, and Survivin in ESCC samples and paratumoral noncancerous tissues were detected by IHC. (c) Correlation between Nrf‐2 and CyclinD1 and Survivin is shown. (d) Kaplan‐Meier analysis was used to plot the overall survival curves of 60 patients with of ESCC with different expression of Nrf‐2, CyclinD1, and Survivin. ( ) 0, (
) 0, ( ) 1, (
) 1, ( ) 0‐censored, (
) 0‐censored, ( ) 1‐censored.
) 1‐censored.
Discussion
According to the guidelines of the National Comprehensive Cancer Network (NCCN),16 cisplatin, 5‐fluorouracil, and other chemotherapeutic drugs are recommended for treatment of patients with advanced esophageal carcinoma, who failed to receive prior surgery. Cisplatin, a DNA‐damaging agent, is widely used as a chemotherapeutic drug for the treatment of various human malignancies, including esophageal cancer. However, an increasing number of studies have shown tumor cell resistance to cisplatin, both in vivo and in vitro.17, 18 This is one of the major factors contributing to treatment failure in ESCC patients.19, 20 MicroRNAs (miRNAs) are endogenous, small non‐coding RNAs that play an essential role in the regulation of gene expression and tumor progression; in addition, these have been linked to chemotherapy resistance.21 Many studies have suggested that miR‐153 is a tumor suppressor miRNA.22, 23, 24, 25 miR‐153‐3p has been reported to suppress the proliferation and invasion of melanoma cells by targeting SNAI1.24 MiR‐153 was shown to downregulate Snail expression by directly targeting the 3‐untranslated region of SNAI1. miR‐153‐3p inhibited proliferation, migration, and invasion of acute lymphoblastic leukemia cells by suppressing inhibitor of growth protein 2 expression.25 In addition to regulating tumor cell infiltration and metastasis, miR‐153‐3p has also been shown to regulate chemosensitivity of cancer cells. MiR‐153‐3p has been reported to overcome the resistance of lung cancer cells to gefitinib by downregulating ABCE1.26 Our recent studies have shown that MiR‐153‐3p inhibits the migration and invasion of ESCC cells whereas miR‐153 antagomir promotes migration and invasion of normal esophageal epithelial cells.3 Therefore, whether miR‐153‐3p regulates the proliferation of ESCC cells and confers sensitivity to cisplatin chemotherapy is not clear. In addition, the underlying mechanisms have not yet been elucidated.
In the present study, we found that miR‐153‐3p suppressed the proliferation of human ESCC cells and Eca109 cells. Upregulation of miR‐153‐3p significantly increased the sensitivity of ESCC cells to cisplatin; this suggests that miR‐153‐3p also plays a critical role in overcoming cisplatin resistance in ESCC.
In a previous study, miR‐153 was shown to downregulate the expression of Nrf‐2 in breast cancer cell lines.27 According to another study, miR‐153 inhibition can protect neurons against OGD/R‐induced injury via regulating Nrf‐2/Ho‐1 signaling.28 These studies indicate that Nrf‐2 is a target gene of miR‐153. Nrf‐2, a crucial transcription factor for regulation of antioxidant genes, can suppress reactive oxygen species (ROS)‐mediated cell damage via upregulation of antioxidant enzymes; in addition, it can affect the proliferation and apoptosis of tumor cells, thereby regulating the sensitivity of tumor cells to chemotherapy.29 Fetoni et al. found that Nrf‐2 played an important role in the resistance of head and neck squamous cell carcinoma cells to cisplatin.30 In the present study, we observed a negative correlation between the expressions of miR‐153‐3p and Nrf‐2 in human esophageal carcinoma tissues. Furthermore, miR‐153‐3p suppressed the expression of Nrf‐2 via binding to its 3′‐UTR region in Eca109 cells. The results suggested that miR‐153‐3p inhibited the proliferation of Eca‐109 cells probably by downregulating the expression of Nrf‐2.
In a previous study, curcumin was shown to attenuate all stages of tumor progression and exhibited a chemosensitization effect on head and neck squamous cell carcinoma cells to cisplatin via modulating the expressions of STAT3 and Nrf‐2.30 Moreover, Nrf‐2 was found to mediate the chemosensitivity of lung cancer cell line A549 to cisplatin.13 However, whether Nrf‐2 contributes to cisplatin resistance in ESCC is still unknown. In this study, we found that inhibition of Nrf‐2 also decreased cell proliferation and colony formation by Eca109 cells. Furthermore, inhibition of Nrf‐2 significantly increased the sensitivity of ESCC cells to cisplatin, which suggests that Nrf‐2 may play a critical role in cisplatin resistance in ESCC. The results further confirm that miR‐153‐3p inhibits proliferation of Eca‐109 cells and confers cisplatin resistance probably by downregulating Nrf‐2 expression. Since Nrf‐2 contributes to cell proliferation and cisplatin resistance in ESCC, we also collected human ESCC samples and analyzed the expression of Nrf‐2 in ESCC samples; in addition, we assessed its effect on the overall survival of patients with ESCC. We found high expression levels of Nrf‐2 in human ESCC samples, which was associated with poor overall survival of ESCC patients. The results indicate that increased expression of Nrf‐2 may be related to poor prognosis in human ESCC.
In summary, our data demonstrated that miR‐153‐3p acts as a tumor suppressor by inhibiting the proliferation of EC109 cells and enhances their sensitivity to cisplatin. MiR‐153‐3p inhibits cell proliferation and reverses cisplatin resistance probably by downregulating Nrf‐2 expression in Eca‐109 cells. We found high expression of Nrf‐2 in human ESCC samples, which was associated with poor overall survival in ESCC patients. Thus, miR‐153‐3p/Nrf‐2 may have a potent effect on cisplatin resistance in ESCC. Nrf‐2 represents a promising therapeutic target in the context of ESCC.
Disclosure
The authors declare that they have no conflict of interest.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (31570894; 81670939; 81672706), the Foundation of Hebei Educational Committee, China (SLRC2017045; key program, ZD2015010), and the Natural Science Foundation for Distinguished Young Scholars of Hebei Province, China (H2018206120, H.S; H2017206332, J.Z). We thank Shelly M Xie, an international student in School of International Education, Hebei Medical University, for assistance with editing the language.
Contributor Information
Xianghong Zhang, Email: zhangxianghong2008@163.com.
Haitao Shen, Email: haitaoshen78@hotmail.com.
References
- 1. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013; 63: 11–30. [DOI] [PubMed] [Google Scholar]
- 2. Zuo J, Wang D, Shen H, Liu F, Han J, Zhang X. MicroRNA‐153 inhibits tumor progression in esophageal squamous cell carcinoma by targeting SNAI1. Tumour Biol 2016. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 3. Zuo J, Zhao M, Liu B et al TNF‐α‐mediated upregulation of SOD‐2 contributes to cell proliferation and cisplatin resistance in esophageal squamous cell carcinoma. Oncol Rep 2019; 42: 1497–506. [DOI] [PubMed] [Google Scholar]
- 4. Messager M, Warlaumont M, Renaud F et al Recent improvements in the management of esophageal anastomotic leak after surgery for cancer. Eur J Surg Oncol 2017; 43 (2): 258–69. [DOI] [PubMed] [Google Scholar]
- 5. Zeng H, Zheng R, Guo Y et al Cancer survival in China, 2003‐2005: A population‐based study. Int J Cancer 2015; 136 (8): 1921–30. [DOI] [PubMed] [Google Scholar]
- 6. Tang Y, Liu JH, Shi ZX, Li Z, Liu HT, Lu P. [MicroRNA‐133b suppresses cell proliferation and invasion of esophageal squamous cell carcinoma via downregulating TAGLN2 expression]. Zhonghua Zhong Liu Za Zhi 2019; 41: 91–6. [DOI] [PubMed] [Google Scholar]
- 7. Ma Q. MiR‐219‐5p suppresses cell proliferation and cell cycle progression in esophageal squamous cell carcinoma by targeting CCNA2. Cell Mol Biol Lett 2019; 24: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhao Y, Ma K, Yang S et al MicroRNA‐125a‐5p enhances the sensitivity of esophageal squamous cell carcinoma cells to cisplatin by suppressing the activation of the STAT3 signaling pathway. Int J Oncol 2018; 53: 644–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Anaya‐Ruiz M, Cebada J, Delgado‐Lopez G, Sanchez‐Vazquez ML. miR‐153 silencing induces apoptosis in the MDA‐MB‐231 breast cancer cell line. Asian Pac J Cancer Prev 2013; 14: 2983–6. [PubMed] [Google Scholar]
- 10. Niu G, Li B, Sun L, An C. MicroRNA‐153 inhibits osteosarcoma cells proliferation and invasion by targeting TGF‐β2. PLOS One 2015; 10: e0119225. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11. Homma S, Ishii Y, Morishima Y et al Nrf2 enhances cell proliferation and resistance to anticancer drugs in human lung cancer. Clin Cancer Res 2009; 15: 3423–32. [DOI] [PubMed] [Google Scholar]
- 12. Cherry AD, Suliman HB, Bartz RR, Piantadosi CA. Peroxisome proliferator‐activated receptor γ co‐activator 1‐α as a critical co‐activator of the murine hepatic oxidative stress response and mitochondrial biogenesis in Staphylococcus aureus sepsis. J Biol Chem 2014; 289: 41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim HR, Kim S, Kim EJ et al Suppression of Nrf2‐driven heme oxygenase‐1 enhances the chemosensitivity of lung cancer A549 cells toward cisplatin. Lung Cancer 2008; 60: 47–56. [DOI] [PubMed] [Google Scholar]
- 14. Narasimhan M, Patel D, Vedpathak D, Rathinam M, Henderson G, Mahimainathan L. Identification of novel microRNAs in post‐transcriptional control of Nrf2 expression and redox homeostasis in neuronal, SH‐SY5Y cells. PLOS One 2012; 7: e51111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Narasimhan M, Riar AK, Rathinam ML, Vedpathak D, Henderson G, Mahimainathan L. Hydrogen peroxide responsive miR153 targets Nrf2/ARE cytoprotection in paraquat induced dopaminergic neurotoxicity. Toxicol Lett 2014; 228: 179–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu T, Li R, Zhao H et al eIF4E promotes tumorigenesis and modulates chemosensitivity to cisplatin in esophageal squamous cell carcinoma. Oncotarget 2016; 7 (41): 66851–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kuroda Y, Yamashiro K, Miyake M et al Factors associated with recurrence of age‐related macular degeneration after anti‐vascular endothelial growth factor treatment: A retrospective cohort study. Ophthalmology 2015; 122 (11): 2303–10. [DOI] [PubMed] [Google Scholar]
- 18. Berardi R, Brunelli A, Pagliaretta S et al Impact of VEGF, VEGFR, PDGFR, HIF and ERCC1 gene polymorphisms on thymic malignancies outcome after thymectomy. Oncotarget 2015; 6 (22): 19305–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen Y, Zhu SM, Xu XL, Zhao AN, Hu JL. Expression levels of HER2 and MRP1 are not prognostic factors of long‐term survival in 829 patients with esophageal squamous cell carcinoma. Oncol Lett 2016; 11: 745–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Modrek AS, Bayin NS, Placantonakis DG. Brain stem cells as the cell of origin in glioma. World J Stem Cells 2014; 6: 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rasmussen MH, Lyskjær I, Jersie‐Christensen RR et al miR‐625‐3p regulates oxaliplatin resistance by targeting MAP2K6‐p38 signalling in human colorectal adenocarcinoma cells. Nat Commun 2016; 7: 1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu H, Ren G, Zhu L, Liu X, He X. The upregulation of miRNA‐146a inhibited biological behaviors of ESCC through inhibition of IRS2. Tumour Biol 2016; 37: 4641–7. [DOI] [PubMed] [Google Scholar]
- 23. Jiang L, Zhao Z, Zheng L, Xue L, Zhan Q, Song Y. Downregulation of miR‐503 promotes ESCC cell proliferation, migration, and invasion by targeting cyclin D1. Genomics Proteomics Bioinformatics 2017; 15: 208–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zeng HF, Yan S, Wu SF. MicroRNA‐153‐3p suppress cell proliferation and invasion by targeting SNAI1 in melanoma. Biochem Biophys Res Commun 2017; 487: 140–5. [DOI] [PubMed] [Google Scholar]
- 25. Jiang J, Liu Y, Zhao Y, Tian F, Wang G. miR‐153‐3p suppresses inhibitor of growth protein 2 expression to function as tumor suppressor in acute lymphoblastic leukemia. Technol Cancer Res Treat 2019; 18: 1533033819852990. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26. Wang L, Lv X, Fu X, Su L, Yang T, Xu P. MiR‐153 inhibits the resistance of lung cancer to gefitinib via modulating expression of ABCE1. Cancer Biomark 2019; 25: 361‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang B, Teng Y, Liu Q. MicroRNA‐153 regulates NRF2 expression and is associated with breast carcinogenesis. Clin Lab 2016; 62: 39–47. [DOI] [PubMed] [Google Scholar]
- 28. Ji Q, Gao J, Zheng Y et al Inhibition of microRNA‐153 protects neurons against ischemia/reperfusion injury in an oxygen‐glucose deprivation and reoxygenation cellular model by regulating Nrf2/HO‐1 signaling. J Biochem Mol Toxicol 2017; 31: e21905. [DOI] [PubMed] [Google Scholar]
- 29. Wang XJ, Sun Z, Villeneuve NF. Nrf2 enhances resistance of cancer cells to chemotherapeutic drugs, the dark side of Nrf2. Carcinogenesis 2008; 29: 1235–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fetoni AR, Paciello F, Mezzogori D et al Molecular targets for anticancer redox chemotherapy and cisplatin‐induced ototoxicity: The role of curcumin on pSTAT3 and Nrf‐2 signalling. Br J Cancer 2015; 113: 1434–44. [DOI] [PMC free article] [PubMed] [Google Scholar]


