Abstract
Background
This retrospective study compared the efficacy and side effect profile between postoperative adjuvant radiotherapy and chemoradiotherapy in stage II or stage III thoracic esophageal squamous cell carcinoma (TESCC) patients who underwent curative (R0) esophagectomy.
Methods
A total of 272 TESCC patients who underwent radical esophagectomy from 2007 to 2016 were included in this retrospective analysis. All cases were pathologically confirmed with stage II or III disease and 148 patients received postoperative chemoradiotherapy (CRT), while the remaining 124 patients received postoperative radiotherapy (RT) alone.
Results
In CRT and RT groups, the three‐year overall survival rates were 51.3 versus 31.5% (P < 0.01) and the median overall survival (OS) was 39 months (95% CI, 31.6 to 46.3 months) and 30 months (95% CI, 21.0 to 38.9 months), respectively (P = 0.213). Three‐year disease‐free survival rates (DFS) were 30.5% versus 15.9% (P = 0.008), while the median DFS times were 26 months (95% CI, 17.7 to 34.3 months) and 19 months (95% CI, 16.4 to 21.6 months), respectively (P = 0.156). Univariate and multivariate analyses showed AJCC (American Joint Committee on Cancer seventh edition) stage and N stage were independent prognostic factors for overall survival, while the N stage was an independent prognostic factor for disease‐free survival.
Conclusions
Postoperative chemoradiotherapy led to one‐ and three‐year overall survival benefits along with an obvious increase in treatment side effects for stage II to III TESCC patients, with no further improvement in five‐year survival. However, the chemoradiotherapy benefits mainly favor stage III,number of resected lymph nodes less than 15, younger (less than 60 years old) and smoking patients.
Keywords: Adjuvant therapy, postoperative chemoradiotherapy, postoperative radiotherapy, thoracic esophageal squamous cell carcinoma
Introduction
Concurrent chemoradiotherapy before dissection has been recommended by the latest National Comprehensive Cancer Network (NCCN) guidelines, whilst observation and follow‐up have been recommended for esophageal squamous cell carcinoma (ESCC) patients, instead of postoperative therapies. There is no high‐quality evidence to evaluate the effect of post‐operative treatment approaches and adjuvant treatments are the optimal choices with the purpose of reducing local recurrence and distant metastasis for radical ESCC patients in China. Moreover, it is still controversial whether adjuvant therapy should be applied after radical surgery because the conclusions of existing research on adjuvant therapy of ESCC are inconsistent.1, 2, 3, 4, 5, 6, 7 As an important regional treatment, radiotherapy can eliminate the residual tumor cells around the tumor beds and local lymphatic drainage areas, thereby reducing the local recurrence rate.8 However, subclinical metastases outside the radiotherapy area may be a source of future recurrence and metastasis.9 As a proven method, chemotherapy may theoretically reduce the rate of metastasis.9 To some extent, postoperative adjuvant chemoradiotherapy has its theoretical advantages. Therefore, adjuvant therapy has been extensively and intensively researched by scholars worldwide. At present, numerous worldwide studies have confirmed the satisfying curative effect of postoperative adjuvant radiotherapy or chemoradiotherapy in ESCC.9, 10, 11, 12, 13, 14, 15, 16, 17 Although both adjuvant radiotherapy and chemoradiotherapy show better survival in contrast with surgery alone, the survival benefit for postoperative CRT compared to RT remains controversial.9, 18, 19, 20, 21
Currently, it is necessary to clarify the benefits of CRT and the role of RT in the cohort of patients with TESCC. In this retrospective study, we varied the design and selection criteria considered in earlier studies. The present study attempts to assess the survival benefit of CRT by comparing with RT alone. We discovered factors contributing to poor prognosis in patients with stage II and III TESCC after tumor resection.
Methods
Patients’ selection
The medical records of all patients with TESCC who had undergone radical esophagectomy at the West China Hospital of Sichuan University between January 2007 and December 2016 were retrospectively reviewed. Patients were included in the present study if they met all the following criteria: (i) Patients had undergone radical esophagectomy with a systematic mediastinal lymphadenectomy and were pathologically confirmed with stage II/III thoracic ESCC (American Joint Committee on Cancer seventh edition); (ii) patients who received adjuvant chemoradiotherapy or postoperative radiotherapy alone; (iii) Eastern Cooperative Oncology Group (ECOG) performance status <2; (iv) normal liver, kidney, and bone marrow functions demonstrated by blood tests; Cardiopulmonary functions were approximately normal, and patients were supposed to be able to tolerate chemoradiotherapy; and (v) patients aged 18–80 years old. Patients were excluded from the study for the following reasons. (i) The pathological type of esophageal cancer was not pure squamous cell carcinoma or with cancer diagnosed at another site; (ii) patients with positive operative margins, defined as the microscopic positive margin of the International Union Against Cancer (UICC) criteria; (iii) patients had received preoperative chemotherapy or radiotherapy and patients who received postoperative chemotherapy only; (iv) patients who had died within 30 days of operative complications and (v) patients with any concurrent disease such as serious diabetes, uncontrolled hypertension, or serious chronic obstructive pulmonary disease.
A total of 272 patients who satisfied the inclusion criteria were finally included in the study. Of these patients, 148 cases received postoperative chemoradiotherapy (CRT group), while 124 patients received postoperative radiotherapy alone (RT group).
Surgical procedure
In our study, all the patients underwent left‐ or right‐sided thoracotomy for esophagectomy followed by two‐ or three‐field lymph node dissection and mediastinal lymphadenectomy dissection for curative intent, while perigastric lymph node resection was carried out in patients whose tumors were located in the middle or lower thorax, and an intrathoracic supra‐aortic esophagogastric anastomosis was then performed.
Postoperative therapies
Adjuvant therapies were started three to four weeks after the operation. Radiotherapy was given with a 6‐MV‐X‐Ray linear accelerator. A total dose of 40–50 Gy (1.8–2 Gy/fraction/day, five fractions a week) was delivered to patients. Radiation methods include three‐dimensional conformal radiotherapy (3D‐CRT) and intensity modulated radiation therapy (IMRT). The clinical target volume (CTV) for treatment generally encompassed the mediastinum (in terms of the anatomic landmarks of a perioperative CT scan). The planning target volume (PTV) was determined as the CTV plus 0.8 cm margins.
There were 148 patients who received concurrent or sequential chemoradiotherapy. Platinum‐based chemotherapies were administrated with a median of four cycles (range 2–6) and a combination of cisplatin (25 mg/m2 intravenously on days 1–3) plus paclitaxel (135–175 mg/m2 intravenously on day 1) or cisplatin (25 mg/m2 intravenously on days 1–3) plus 5‐fluorouracil (5‐FU) (500 mg/m2 intravenously on days 1–5), repeated every 21 days.
Follow‐up
Follow‐ups were once every three months within the first two years, once every half year from the third to fifth year and once every year thereafter. Patients were instructed to carry out follow up evaluations including physical examination, blood test, esophagogram, chest CT scan, and abdominal CT scan or ultrasound, endoscopy, bone scanning, and/or cerebral MRI was performed if clinically indicated.
Definitions and statistical analysis
The long‐term outcome was determined from medical records and follow‐up information. OS was defined as the time from operation to death (or the last follow‐up visit), and DFS was defined as the time from operation to first disease failure, including locoregional recurrence, distant metastasis and combined recurrence (or death from any cause). Diagnoses of locoregional recurrence were based on regrowth of cancer within the area of the previous resection, including local anastomotic sites and local nodal clearance. Recurrence beyond those sites was considered distant progression. The diseases with simultaneous locoregional and distant recurrences were named combined recurrence.
All statistical analyses were performed using IBM SPSS version 20.0 (IBM Corporation, Armonk, NY, USA). Survival analysis was performed using the Kaplan‐Meier method, and the log‐rank test was used to detect survival differences between the two groups. Categorical variables were compared by using the chi‐square test. Multivariate analysis was carried out by the method of Cox regression. Statistical significance was defined as P < 0.05.
Results
Patients’ characteristics
Patients’ characteristics are presented in Table 1. There was a total of 272 patients, 124 cases (45.6%) received postoperative adjuvant radiotherapy alone, while 148 (54.4%) received postoperative chemoradiotherapy. Because of potential bias in the clinician's treatment selection, the percentage of patients with positive lymph nodes in CRT group was higher than in the RT group (P = 0.015).
Table 1.
The clinical characteristics of the patients
| No. of patients (%) | |||||
|---|---|---|---|---|---|
| Characteristic | Overall | CRT | RT | χ2 | P‐value |
| Gender | 0.211 | 0.646 | |||
| Male | 225 (82.7) | 121 (81.8) | 104 (83.9 | ||
| Female | 47 (17.3) | 27 (18.2) | 20 (16.1) | ||
| Age (years) | 3.584 | 0.167 | |||
| <60 years | 173 (63.6) | 101 (68.2) | 72 (58.1) | ||
| 60 ≤ years < 70 years | 79 (29.0) | 39 (26.4.) | 40 (32.3) | ||
| ≥70 years | 20 (7.4) | 8 (5.4) | 12 (9.6) | ||
| Drinking | 2.252 | 0.133 | |||
| Yes | 171 (62.9) | 99 (66.9) | 72 (58.1) | ||
| No | 101 (37.1) | 49 (33.1) | 52 (41.9) | ||
| Smoking | 1.395 | 0.238 | |||
| Yes | 196 (72.1) | 111 (75.0) | 85 (68.5) | ||
| No | 76 (27.9) | 37 (25.0) | 39 (31.5) | ||
| Tumor location | 1.462 | 0.481 | |||
| Upper | 27 (9.9) | 12 (8.1) | 15 (12.1) | ||
| Middle | 146 (53.7) | 83 (56.1) | 63 (50.1) | ||
| Lower | 99 (36.4) | 53 (35.8) | 46 (37.8) | ||
| Depth of invasion | 1.645 | 0.649 | |||
| pT1 | 9 (3.3) | 5 (3.4) | 4 (3.2) | ||
| pT2 | 37 (13.6) | 22 (14.9) | 15 (12.1) | ||
| pT3 | 167 (61.4) | 93 (62.8) | 74 (59.7) | ||
| pT4 | 59 (21.7) | 28 (18.9) | 31 (25.0) | ||
| LN involved | 5.941 | 0.015 | |||
| pN0 | 87 (32.0) | 38 (25.7) | 49 (39.5) | ||
| pN1‐3 | 185 (68.0) | 110 (74.3) | 75 (60.5) | ||
| No. of resected nodes | 0.658 | 0.417 | |||
| ≥15 | 184 (67.6) | 97 (65.5) | 87 (70.2) | ||
| <15 | 88 (32.4) | 51 (34.5) | 37 (29.8) | ||
| Differentiation | 1.846 | 0.397 | |||
| G1 | 5 (1.8) | 3 (2.0) | 2 (1.6) | ||
| G2 | 113 (41.5) | 56 (37.8) | 57 (46.0) | ||
| G3 | 154 (56.7) | 89 (60.2) | 65 (52.4) | ||
| Pathological stage | 0.063 | 0.802 | |||
| II | 90 (33.1) | 48 (32.4) | 42 (33.9) | ||
| III | 182 (66.9) | 100 (67.6) | 82 (66.1) | ||
| Vascular cancer embolus | 0.226 | 0.635 | |||
| Yes | 26 (9.6) | 13 (8.8) | 13 (10.5) | ||
| No | 246 (90.4) | 135 (91.2) | 111(89.5) | ||
| Chemotherapy regimen | |||||
| PF | 89 (60.1) | 0 | |||
| TP | 59 (39.9) | 0 | |||
| Chemotherapy cycles | |||||
| 2–3 | 77 (52.0) | 0 | |||
| 4–5 | 63 (42.6) | 0 | |||
| 6 | 8 (5.4) | 0 | |||
CRT, postoperative chemoradiotherapy; G, histopathological grading; LN, lymph nodes; No., number; PF, cisplatin plus 5‐fluorouracil; RT, postoperative radiotherapy; TP, paclitaxel plus cisplatin.
Overall survival and disease‐free survival
Overall survival rates for the entire population of 272 patients were 88.24% at the first year, 42.28% at the third year, and 21.69% at the fifth year, respectively, with a median OS of 35.0 months (95% CI 28.5–41.5). The three‐year OS rate was 51.4% versus 31.5 (P < 0.001) for group CRT and RT, respectively (Table 2). The median OS was 39.0 months (95% CI 31.6–46.3) in the group CRT versus 30.0 months (95% CI 21.0–38.9) in the group RT (P = 0.213; HR, 0.69) (Fig 1).
Table 2.
The OS rate and DFS rate of CRT group and RT group
| CRT group (n = 148) | RT group (n = 124) | χ2 | P‐value | ||
|---|---|---|---|---|---|
| OS rate | One year | 136 (91.9) | 104 (83.9) | 4.182 | 0.041 |
| Three years | 76 (51.4) | 39 (31.5) | 10.948 | 0.001 | |
| Five years | 37 (25.0) | 22 (17.7) | 2.092 | 0.148 | |
| DFS rate | One year | 104 (70.2) | 78 (62.8) | 1.654 | 0.198 |
| Three years | 45 (30.5) | 20 (15.9) | 7.561 | 0.06 | |
| Five years | 26 (17.6) | 12 (9.3) | 3.495 | 0.62 | |
Figure 1.
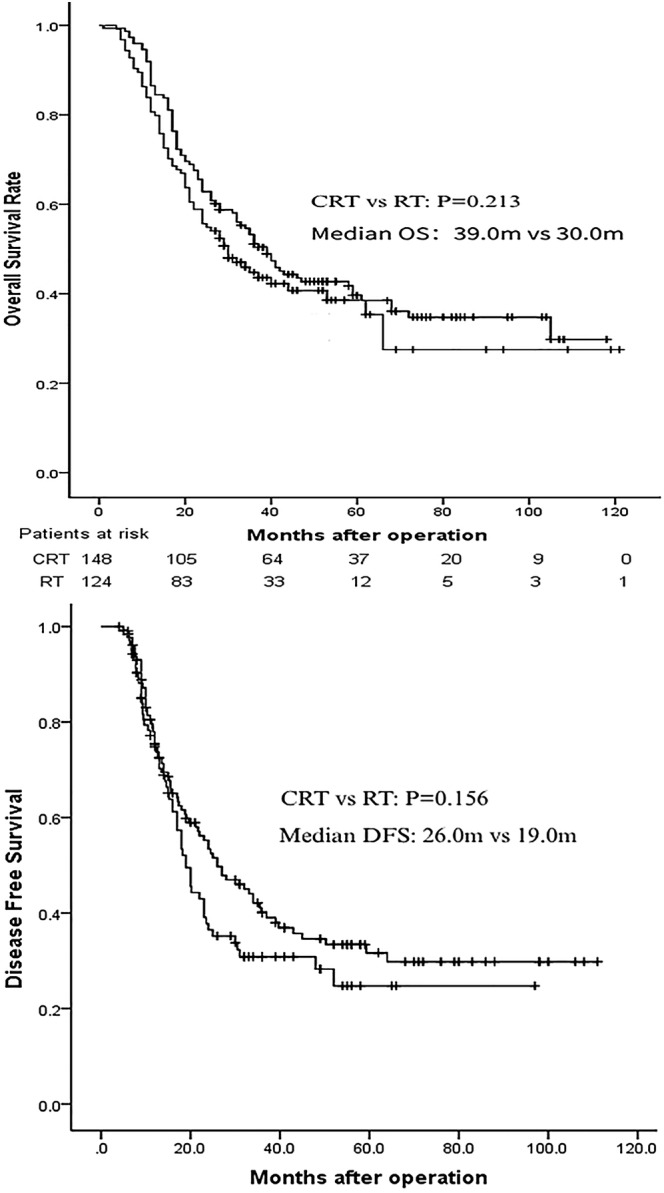
Effects of different postoperative adjuvant therapies on OS and DFS in all 272 patients. CRT group, patients who received postoperative adjuvant chemoradiotherapy; RT group, patients who received postoperative adjuvant radiotherapy alone.
The median DFS of the whole cohort of 272 patients was 23.0 months (95% CI 19.4–26.6). DFS rates for the whole group were 66.8% at the first year, 23.9% at the third year, and 13.9% at the fifth year, respectively. The one year, three‐, and five‐year DFS rates were 70.2% versus 62.8%, 30.5% versus 15.9 and 17.6% versus 9.3% for group CRT and CT, respectively. The median DFS of the CRT group and RT group in our study was 26.0 months (95% CI 17.7–34.3) and 19.0 months (95% CI 16.4–21.6), respectively (P = 0.156; HR, 0.69) (Fig 1).
Subgroup statistical analysis showed that in patients who were younger than 60 years old and whose resected lymph nodes by surgeons during the operation were less than 15, postoperative CRT was much more effective than RT at improving OS and DFS. There was an obvious difference in OS and DFS between the CRT and RT group in patients younger than 60 years old (median OS: 41.0 vs. 29.0 months; P = 0.04; median DFS: 32.0 vs. 20.0 months; P = 0.02, respectively) and whose resected lymph nodes by surgeons during the operation were less than 15 (median OS: 41.0 vs. 20.0 months; P = 0.006; median DFS: 37.0 vs. 14.0 months; P = 0.006, respectively)(Fig 2).
Figure 2.
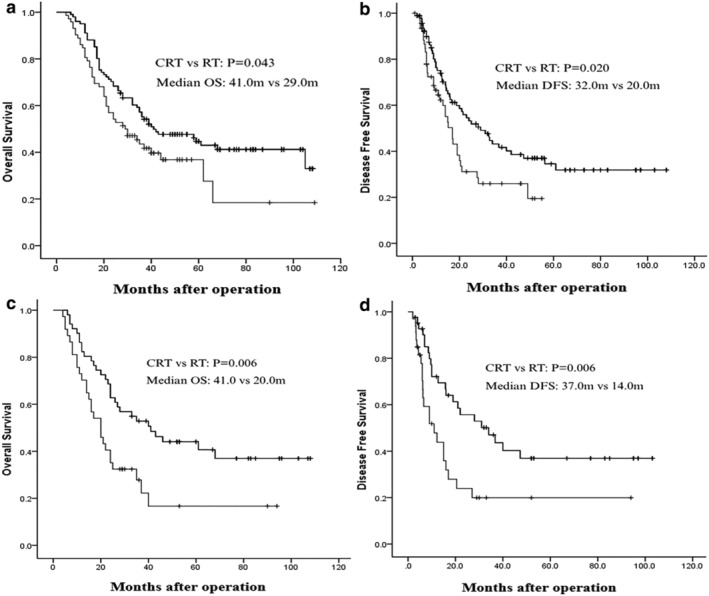
(a) Overall survival for patients younger than 60 years old and (c) patients with number of resected lymph nodes less than 15; (b) Disease‐free survival for patients younger than 60 years old and (d) patients with number of resected lymph nodes less than 15.
Pattern of failure
The failure patterns of all patients in the two groups are detailed in Table 3. There were no significant differences between the two groups for recurrence and metastasis rate, with an overall recurrence and metastasis rate of 75.6% and 79.0% for group CRT and RT, respectively (P = 0.51).
Table 3.
Failure patterns of patients between two groups
| Failure pattern | CRT group (n = 148) | RT group (n = 124) | P‐value |
|---|---|---|---|
| Supraclavicular | 36 (24.3) | 27 (21.8) | 0.62 |
| Mediastinum | 18 (12.2) | 12 (9.7) | 0.51 |
| Abdominal cavity | 9 (6.1) | 7 (5.6) | 0.88 |
| Tumor bed | 6 (4.1) | 8 (6.5) | 0.37 |
| Distant organ metastasis | 28 (18.9) | 26 (20.9) | 0.67 |
| Mixed | 15 (10.1) | 18 (14.5) | 0.27 |
| Overall | 112(75.6) | 98(79.0) | 0.51 |
Univariate and multivariate analyses of prognostic factors
The extent of lymph node involvement, number of positive lymph nodes and AJCC stage were significantly associated with both OS and DFS in a univariate analysis, while the lower postoperative stage, without lymph node involvement (N‐) and lower numbers of positive lymph nodes were significantly associated with improved survival (Table 4).
Table 4.
Univariate and multivariate analyses of OS and DFS
| OS | DFS | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UVA | MVA | UVA | MVA | ||||||||||
| Variable | Median OS | 95% CI | P‐value | HR | 95% CI | P‐value | Median DFS | 95% CI | P‐value | HR | 95% CI | P‐value | |
| Treatment method | CRT | 39.0 | 31.6–46.4 | 0.213 | 0.679 | 0.491–0.939 | 0.019* | 26.0 | 17.74–34.26 | 0.156 | 0.687 | 0.485–0.973 | 0.034* |
| RT | 30.0 | 21.0–38.9 | –– | –– | –– | –– | 19.0 | 16.35–21.65 | –– | –– | –– | –– | |
| AJCC stage | II | 66.0 | 58.8–73.1 | 0.000* | 0.524 | 0.342–0.801 | 0.003* | 34.0 | 11.72–56.28 | 0.001* | –– | –– | –– |
| III | 26.0 | 20.8–31.2 | –– | –– | –– | –– | 18.0 | 14.81–21.20 | –– | –– | –– | –– | |
| Tumor location (thoracic esophageal) | Upper | 68.0 | 40.9–95.1 | 0.149 | –– | –– | –– | 34.0 | 0.682–67.32 | 0.258 | –– | –– | –– |
| Middle | 32.0 | 23.2–40.8 | –– | –– | –– | –– | 19.0 | 13.97–24.03 | –– | –– | –– | –– | |
| Lower | 36.0 | 25.6–46.4 | –– | –– | –– | –– | 23.0 | 16.75–29.25 | –– | –– | –– | –– | |
| Lymph nodes involved | Yes | 30.0 | 23.5–36.5 | 0.044* | –– | –– | –– | 19.0 | 15.01–22.99 | 0.012* | –– | –– | –– |
| No | 53.0 | 35.0–70.9 | –– | –– | –– | –– | 32.0 | 8.78–55.22 | –– | –– | –– | –– | |
| Drinking | Yes | 29.0 | 21.7–36.3 | 0.021* | 1.335 | 0.949–1.878 | 0.097 | 20.0 | 15.88–24.12 | 0.067 | –– | –– | –– |
| No | 58.0 | 36.8–79.2 | –– | –– | –– | –– | 30.0 | 20.41–40.60 | –– | –– | –– | –– | |
| Gender | Male | 35.0 | 28.1–41.9 | 0.270 | –– | –– | –– | 23.0 | 18.98–27.02 | 0.47 | –– | –– | –– |
| Female | 40.0 | 30.1–46.3 | –– | –– | –– | –– | 23.5 | 11.48–35.52 | –– | –– | –– | –– | |
| Smoking | Yes | 34.0 | 27.2–40.8 | 0.260 | –– | –– | –– | 20.0 | 16.35–23.65 | 0.115 | –– | –– | –– |
| No | 41.0 | 13.1–68.9 | –– | –– | –– | –– | 27.0 | 18.92–35.08 | –– | –– | –– | –– | |
| Age (years) | <60y | 37.0 | 29.4–44.6 | 0.425 | –– | –– | –– | 23.5 | 17.23–29.77 | 0.649 | –– | –– | –– |
| 60 ≤ y < 70 | 31.0 | 15.4–46.7 | –– | –– | –– | –– | 21.2 | 14.96–27.44 | –– | –– | –– | –– | |
| ≥70y | 26.0 | 14.3–37.7 | –– | –– | –– | –– | 16.0 | 2.62–29.38 | –– | –– | –– | –– | |
| Differentiation | G1 | 105.0 | 0.000–237.6 | 0.843 | –– | –– | –– | 28.3 | 20.44–35.06 | 0.586 | –– | –– | –– |
| G2 | 37.0 | 27.0–46.9 | –– | –– | –– | –– | 24.0 | 20.90–27.10 | –– | –– | –– | –– | |
| G3 | 32.0 | 25.0–38.9 | –– | –– | –– | –– | 20.2 | 14.79–25.61 | –– | –– | –– | –– | |
| Depth of invasion | T1 | –– | –– | 0.165 | –– | –– | –– | –– | –– | 0.136 | 0.148 | 0.035–0.628 | 0.010* |
| T2 versus T4 | T2 | 46.0 | 14.7–77.3 | –– | –– | –– | –– | 24.0 | 14.28–33.72 | –– | 0.576 | 0.315–1.053 | 0.073 |
| T3 versus T4 | T3 | 33.0 | 24.6–41.4 | –– | –– | –– | –– | 23.0 | 18.26–27.74 | –– | 0.690 | 0.452–1.051 | 0.084 |
| T4 | 30.0 | 20.2–39.8 | –– | –– | –– | –– | 17.0 | 11.71–22.29 | –– | –– | –– | –– | |
| No. of positivelymph nodes N1 versus N3 N2 versus N3 | N0 | 53.0 | 35.0–70.9 | 0.000* | 0.353 | 0.160–0.780 | 0.010* | 32.0 | 12.50–51.50 | 0.032* | 0.249 | 0.113–0.546 | 0.001* |
| N1 | 35.0 | 25.8–44.2 | –– | 0.347 | 0.169–0.712 | 0.004* | 18.8 | 13.57–24.04 | –– | 0.463 | 0.220–0.977 | 0.043* | |
| N2 | 26.0 | 19.8–32.2 | –– | 0.418 | 0.195–0.896 | 0.025* | 19.7 | 13.77–25.63 | –– | 0.407 | 0.178–0.933 | 0.034* | |
| N3 | 12.0 | 10.9–13.0 | –– | –– | –– | –– | 11.2 | 10.62–11.78 | –– | –– | –– | –– | |
| No. of resected lymph nodes | ≥15 | 39.0 | 29.1–48.9 | 0.120 | 0.645 | 0.464–0.897 | 0.009* | 23.0 | 18.46–27.54 | 0.643 | –– | –– | –– |
| <15 | 25.0 | 16.1–33.9 | –– | –– | –– | –– | 20.0 | 13.57–26.43 | –– | –– | –– | –– | |
| Vessel carcinoma embolus | Yes | 29.0 | 13.1–44.9 | 0.370 | –– | –– | –– | 20.0 | 10.66–29.34 | 0.363 | –– | –– | –– |
| No | 36.0 | 28.6–43.4 | –– | –– | –– | –– | 23.0 | 18.90–27.10 | –– | –– | –– | –– | |
| Chemotherapy regimens | PF | 36.0 | 24.5–47.5 | 0.199 | 1.565 | 0.983–2.492 | 0.059 | 24.0 | 17.25–30.75 | 0.499 | –– | –– | –– |
| TP | 40.0 | 30.6–49.7 | –– | –– | –– | –– | 33.1 | 22.14–44.10 | –– | –– | –– | –– | |
| Chemotherapy cycles | 2–3 | 36.0 | 28.8–43.2 | 0.320 | –– | –– | –– | 26.0 | 16.60–35.39 | 0.234 | –– | –– | –– |
| 4–5 | 43.0 | 0.000–90.8 | –– | –– | –– | –– | 39.7 | 16.64–62.76 | –– | –– | –– | –– | |
| 6 | 27.0 | 7.6–46.4 | –– | –– | –– | –– | 17.4 | 15.24–19.56 | –– | –– | –– | ‐ | |
Significant difference.
CI, confidence interval; CRT, chemoradiotherapy; HR, hazard ratio; MVA, multivariate analysis; PF, cisplatin plus 5‐fluorouracil; RT, radiotherapy; TP, paclitaxel plus cisplatin; UVA, univariate analysis.
In multivariate analysis, postoperative chemoradiotherapy and the number of positive lymph nodes were significantly associated with improved OS and DFS. A comprehensive univariate and multivariate analysis showed that the number of positive lymph nodes and AJCC stage were independent prognostic factors for OS, and that the number of positive lymph nodes were independent prognostic factors for DFS (Table 4).
Toxicity
The most common side effects in the study were gastrointestinal reactions and myelosuppression. The other side effects included radiation‐induced pneumonia, radiation‐induced esophagitis, radiation skin damaged, diarrhea, poor appetite, dizziness, and so on. The adverse reactions were evaluated according to the Common Terminology Criteria for Adverse Events (CTCAE 3.0). Myelosuppression and gastrointestinal reactions were experienced significantly more frequently by patients in Group CRT than those in Group RT. However, there were no significant differences in the incidence of other adverse reactions. Grade 1–2 myelosuppression and grade 1–2 gastrointestinal reactions between CRT and RT group were 70.9% versus 37.9% (P < 0.001), 40.5% versus 8.1% (P < 0.001), while the grade 3–4 myelosuppression and grade 3–4 gastrointestinal reactions were 21.0% versus 7.3% (P = 0.002) and 10.1% versus 3.2% (P = 0.026), respectively. There were no treatment‐related toxic deaths for the whole cohort.
Discussion
Although was no difference between the patients who received postoperative CRT and the adjuvant radiotherapy alone group in both the median OS and DFS, the three‐year OS and DFS rates were obviously improved, which favored the group who received CRT, which is partially consistent with previous studies.13, 14 In this study, the differences of three‐year OS and DFS rate were statistically significant between the CRT and RT groups, but the survival analysis curve did not show statistically significant differences; the inconsistency of this conclusion may relate to the nature of the study, which includes a higher incidence of lymph node metastasis in the CRT group. However, the disease‐free survival curves for patients who received chemoradiotherapy and for those who received radiotherapy shows a tendency of divergence from the follow‐up time of approximately 16 months. This suggests that the potential improvement in DFS may be achieved by a therapeutic regimen of plus chemotherapy to radiotherapy, and ultimately, to improvement in OS if the sample size is large enough. As to the one‐year overall survival rates, the statistical difference was relatively mild and the difference of one‐year DFS rate was not obvious while it became obvious in the third year. This may have been related to the timeliness of adjuvant therapy and postoperative tumor bed blood circulation disorders, which resulted from the operation damage that had not been completely rebuilt, thus leading to the increased proportion of hypoxic tumor cells.22, 23 The increase in the proportion of hypoxic tumor cells was associated with decreased sensitivity to radiotherapy and reduced local delivery of chemotherapeutic drugs.24, 25, 26, 27
In this study, there were no differences in the overall recurrence and metastasis rates between the CRT and RT groups, which is opposite to the previous study. Chen et al. and other scholars showed that adjuvant chemoradiotherapy could reduce the overall recurrence rate, distant metastasis rate, and mixed metastasis rate compared with adjuvant radiotherapy alone.9 In our study, although the univariate analysis showed that adding chemotherapy based on radiotherapy could not reduce the recurrence and metastasis rates of TESCC patients, the multivariate analysis showed that compared with adjuvant radiotherapy alone, the risks of recurrence and metastasis could be reduced by 31.3% when extra chemotherapy was added. The possible reasons are expected to encourage further research.
Univariate and multivariate analysis in this study showed that postoperative AJCC stage and number of positive lymph nodes (N stage) were independent prognostic factors for OS, while N stage was an independent prognostic factor for DFS. In other words, the prognosis of patients diagnosed as stage II was better than those at stage III. One retrospective analysis which involved 1715 esophageal squamous‐cell carcinoma patients who were treated after surgery, showed that the five‐year survival rates of patients with stage I, IIA, IIB and III were 83.8%, 70.8%, 52.1% and 41.1%, respectively, which is consistent with our results.28 Another study also showed that the one‐, three‐, five‐year survival rates and median OS in patients with stage I + II was significantly longer than those with stage III, while the prognosis of patients with 0–1 positive lymph node involvement was better than patients with the number of positive lymph nodes greater than two.21 It means that the more positive lymph nodes pathologically confirmed after surgery means that it is easier for a patient to relapse an metastasis, and finally lead to shorter DFS and OS time, which is in accordance with the present results of ours study.
Subgroup analysis showed that patients aged younger than 60 years old, who smoked, with resected lymph nodes less than 15 and well‐differentiated tumors were more likely to benefit from adjuvant chemoradiotherapy compared with postoperative radiotherapy alone. The study by van Nistelrooij et al. showed that patients aged younger than 50 years were more likely to survive longer than 50 years older after esophagectomy, median OS was 33 months versus 23 months, and the five‐year survival rate was 40.5% versus 31% (P = 0.001), respectively.29 The results of the present study revealed that patients who received adjuvant CRT who were younger than 60 years old had a longer survival time compared to those older than 60, which was similar to the conclusion of the study by van Nistelrooij et al. (P = 0.04).29 This may be associated with better organ function, stronger immunity, fewer complicated diseases, greater tolerance to radiotherapy and chemotherapy of young patients, and higher compliance and response to treatment. At present, the NCCN guidelines recommend that the number of lymph nodes is more than 15 in the radical resection of esophageal cancer. The risk of recurrence and metastasis will be greatly increased if the number of resected lymph nodes is less than 15. Adjuvant radiotherapy and chemotherapy may have a synergistic effect in eliminating cancer cells of local residual and distant micrometastasis.9 Those reports, as well as our analysis, suggested that patients with both older age and less than 15 resected lymph nodes were at higher risk of disease progression, and of diminished survival. Therefore, more potent treatment modalities such as additional chemotherapy should be used on these patients.
As for side effects, the incidence of gastrointestinal reactions and myelosuppression in the group CRT was significantly higher than those in the group RT, but most were alleviated after symptomatic treatment. Other treatment‐related side effects were similar between the two groups, and most were grade 1–2; there were no treatment‐related deaths and patients were able to tolerate treatment. This is consistent with the findings of Chen et al. and Chi et al. that CRT can significantly increase the incidence of toxic and side effects compared with RT.9, 20
In the present analysis, stage II to III ESCC patients were benefited from the postoperative chemoradiotherapy for the one‐ and three‐year overall survival, while the benefits were disappeared for five‐year survival. However, in our study, chemoradiotherapy mainly benefitted stage III patients, number of resected lymph nodes less than 15, younger (less than 60 years old) and smoking patients. Since this was a single centre retrospective study, larger and prospective randomized clinical trials are warranted to confirm these findings.
Disclosure
No authors report any conflict of interest.
Acknowledgments
This work was partly supported by the Science and Technology Department of Sichuan Provincial, China (grant number 2017SZ0057).
Bingwen Zou and Yan Tu contributed equally to this work.
References
- 1. Zhang SS, Yang H, Xie X et al Adjuvant chemotherapy versus surgery alone for esophageal squamous cell carcinoma: A meta‐analysis of randomized controlled trials and nonrandomized studies. Dis Esophagus 2014; 27 (6): 574–84. [DOI] [PubMed] [Google Scholar]
- 2. Li L, Zhao L, Lin B et al Adjuvant therapeutic modalities following three‐field lymph node dissection for stage II/III esophageal squamous cell carcinoma. J Cancer 2017; 8 (11): 2051–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ténière P, Hay JM, Fingerhut A, Fagniez PL. Postoperative radiation therapy does not increase survival after curative resection for squamous cell carcinoma of the middle and lower esophagus as shown by a multicenter controlled trial. French University Association for Surgical Research. Surg Gynecol Obstet 1991; 173 (2): 123–30. [PubMed] [Google Scholar]
- 4. Zieren HU, Müller JM, Jacobi CA, Pichlmaier H, Müller RP, Staar S. Adjuvant postoperative radiation therapy after curative resection of squamous cell carcinoma of the thoracic esophagus: A prospective randomized study. World J Surg 1995; 19 (3): 444–9. [DOI] [PubMed] [Google Scholar]
- 5. Zhang J, Zhang YW, Chen ZW et al Adjuvant chemotherapy of cisplatin, 5‐fluorouracil and leucovorin for complete resectable esophageal cancer: A case‐matched cohort study in East China. Dis Esophagus 2008; 21 (3): 207–13. [DOI] [PubMed] [Google Scholar]
- 6. Lee J, Lee KE, Im YH et al Adjuvant chemotherapy with 5‐fluorouracil and cisplatin in lymph node‐positive thoracic esophageal squamous cell carcinoma. Ann Thorac Surg 2005; 80 (4): 1170–5. [DOI] [PubMed] [Google Scholar]
- 7. Tachibana M, Yoshimura H, Kinugasa S et al Postoperative chemotherapy vs chemoradiotherapy for thoracic esophageal cancer: A prospective randomized clinical trial. Eur J Surg Oncol 2003; 29 (7): 580–7. [DOI] [PubMed] [Google Scholar]
- 8. Kim KH, Chang JS, Cha JH et al Optimal adjuvant treatment for curatively resected thoracic esophageal squamous cell carcinoma: A radiotherapy perspective. Cancer Res Treat 2017; 49 (1): 168–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen J, Pan J, Liu J et al Postoperative radiation therapy with or without concurrent chemotherapy for node‐positive thoracic esophageal squamous cell carcinoma. Int J Radiat Oncol Biol Phys 2013; 86 (4): 671–7. [DOI] [PubMed] [Google Scholar]
- 10. Chen J, Pan J, Zheng X et al Number and location of positive nodes, postoperative radiotherapy, and survival after esophagectomy with three‐field lymph node dissection for thoracic esophageal squamous cell carcinoma. Int J Radiat Oncol Biol Phys 2012; 82 (1): 475–82. [DOI] [PubMed] [Google Scholar]
- 11. Schreiber D, Rineer J, Vongtama D et al Impact of postoperative radiation after esophagectomy for esophageal cancer. J Thorac Oncol 2010; 5 (2): 244–50. [DOI] [PubMed] [Google Scholar]
- 12. Chen G, Wang Z, Liu XY, Liu FY. Adjuvant radiotherapy after modified Ivor‐Lewis esophagectomy: Can it prevent lymph node recurrence of the mid‐thoracic esophageal carcinoma? Ann Thorac Surg 2009; 87 (6): 1697–702. [DOI] [PubMed] [Google Scholar]
- 13. Lv J, Cao XF, Zhu B, Ji L, Tao L, Wang DD. Long‐term efficacy of perioperative chemoradiotherapy on esophageal squamous cell carcinoma. World J Gastroenterol 2010; 16 (13): 1649–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen HS, Wu SC, Hsu PK, Huang CS, Liu CC, Wu YC. The prognostic impact of preoperative and postoperative chemoradiation in clinical stage II and III esophageal squamous cell carcinomas: A population based study in Taiwan. Medicine (Baltimore) 2015; 94 (25): e1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rice TW, Adelstein DJ, Chidel MA et al Benefit of postoperative adjuvant chemoradiotherapy in locoregionally advanced esophageal carcinoma. J Thorac Cardiovasc Surg 2003; 126 (5): 1590–6. [DOI] [PubMed] [Google Scholar]
- 16. Hsu PK, Huang CS, Wang BY, Wu YC, Hsu WH. Survival benefits of postoperative chemoradiation for lymph node‐positive esophageal squamous cell carcinoma. Ann Thorac Surg 2014; 97 (5): 1734–41. [DOI] [PubMed] [Google Scholar]
- 17. Zou B, Pang J, Liu Y et al Postoperative chemoradiotherapy improves survival in patients with stage II‐III esophageal squamous cell carcinoma: An analysis of clinical outcomes. Thorac Cancer 2016; 7 (5): 515–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Saito T, Shigemitsu Y, Kinoshita T et al Cisplatin, vindesine, pepleomycin and concurrent radiation therapy following esophagectomy with lymph adenectomy for patients with an esophageal carcinoma. Oncology 1993; 50 (4): 293–7. [DOI] [PubMed] [Google Scholar]
- 19. Liu HC, Hung SK, Huang CJ et al Esophagectomy for locally advanced esophageal cancer, followed by chemoradiotherapy and adjuvant chemotherapy. World J Gastroenterol 2005; 11 (34): 5367–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fuzhi J, Weiguo Zhu, Quan Zhang, et al. A controlled clinical research of postoperative radiotherapy and concurrent chemoradiotherapy for lymph node positive esophageal cancer. Chinese J Clin Res 2015; 11 (28): 1429–32. [Google Scholar]
- 21. Yuxiang W, Shuchai Z, Ren L, Juan L, Rong Q. Clinical efficacy comparison of radiotherapy and chemoradiotherapy after radical surgery of esophageal cancer. Cancer Res Prev Treat 2005; 3 (32): 171–4. [Google Scholar]
- 22. Qi W, Zixiang W, Tianwei Z et al Long‐term outcomes of 530 esophageal squamous cell carcinoma patients with minimally invasive Ivor Lewis esophagectomy. J Surg Oncol 2018; 117 (5): 957–69. [DOI] [PubMed] [Google Scholar]
- 23. Huang PM, Hsu FM, Lin CC et al Do we need to add postoperative radiotherapy in patients undergoing trimodality therapy for esophageal squamous cell carcinoma with positive lymph nodes disease? Dig Surg 2018; 35 (2): 104–10. [DOI] [PubMed] [Google Scholar]
- 24. Jiang Y, Verbiest T, Devery AM et al Hypoxia potentiates the radiation‐sensitizing effect of olaparib in human non‐small cell lung cancer xenografts by contextual synthetic lethality. Int J Radiat Oncol Biol Phys 2016; 95 (2): 772–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Inoue M, Mukai M, Hamanaka Y, Tatsuta M, Hiraoka M, Kizaka‐Kondoh S. Targeting hypoxic cancer cells with a protein prodrug is effective in experimental malignant ascites. Int J Oncol 2004; 25 (3): 713–20. [PubMed] [Google Scholar]
- 26. Mihaylova VT, Bindra RS, Yuan J et al Decreased expression of the DNA mismatch repair gene Mlh1 under hypoxic stress in mammalian cells. Mol Cell Biol 2003; 23 (9): 3265–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Svensson H, Moller TR. Developments in radiotherapy. Acta Oncol 2003; 42 (5–6): 430–42. [DOI] [PubMed] [Google Scholar]
- 28. Chen J, Zhu J, Pan J et al Postoperative radiotherapy improved survival of poor prognostic squamous cell carcinoma esophagus. Ann Thorac Surg 2010; 90 (2): 435–42. [DOI] [PubMed] [Google Scholar]
- 29. van Nistelrooij AM, van Lanschot JJ, Tilanus HW, Wijnhoven BP. Influence of young age on outcome after esophagectomy for cancer. World J Surg 2012; 36 (11): 2612–21. [DOI] [PubMed] [Google Scholar]


