ABSTRACT
Background
While some individual foods and nutrients have been associated with knee osteoarthritis (KOA) progression, the association between dietary patterns and KOA progression has received little research attention.
Objective
The objective of this study was to determine whether dietary patterns, derived by principal components analysis (PCA), are associated with KOA progression.
Methods
In the Osteoarthritis Initiative (OAI), a prospective cohort with clinical centers in Maryland, Ohio, Pennsylvania, and Rhode Island, 2757 participants with existing KOA (mean age 62 y) and diet assessed at baseline were followed for ≤72 mo. Using PCA, Western and prudent dietary patterns were derived. Radiographic KOA progression was assessed using 2 separate measures, 1 full Kellgren–Lawrence (KL) grade increase and loss in joint space width (JSW). Symptomatic KOA progression was defined as an increase in or remaining in 1 of the 2 highest classification categories of the Western Ontario and McMaster Universities Arthritis Index (WOMAC).
Results
Adherence to Western and prudent dietary patterns was significantly associated with radiographic and symptomatic progression of KOA. With increasing Western pattern score, there was increased KL-worsening risk (compared with quartile 1, HR for quartile 4: 1.30; 95% CI: 1.05, 1.61; P-trend < 0.01) and increased odds of progression to higher WOMAC score (compared with quartile 1, OR for quartile 4: 1.39; 95% CI: 1.18, 1.63; P-trend < 0.01) but no significant change in JSW loss. With increasing prudent pattern score there was decreased KL-worsening risk (compared with quartile 1, HR for quartile 4: 0.79; 95% CI: 0.64, 0.98; P-trend = 0.02), decreased JSW loss (quartile 1: 0.46 mm; quartile 4: 0.38 mm; P-trend < 0.01), and decreased odds of higher WOMAC progression (compared with quartile 1, OR for quartile 4 0.73; 95% CI: 0.62, 0.86; P-trend < 0.01) in multivariable adjusted models.
Conclusions
Adherence to a Western dietary pattern was associated with increased radiographic and symptomatic KOA progression, while following a prudent pattern was associated with reduced progression. In general, for people already diagnosed with KOA, eating a diet rich in fruits, vegetables, fish, whole grains, and legumes may be related to decreased radiographic and symptomatic disease progression.
Keywords: Western diet, prudent diet, dietary patterns, symptomatic knee osteoarthritis progression, radiographic knee osteoarthritis progression
Introduction
Osteoarthritis affects approximately 10% of men and 18% of women worldwide (1). There is no cure with treatment mainly including pain relief or joint replacement, neither of which are without potentially undesirable side effects. Synovial inflammation, common in osteoarthritis (2, 3), may follow cartilage degradation and potentially drive progression of the disease (4). Dietary factors associated with obesity, inflammation, and other metabolic risk factors may also play a role in osteoarthritis pathogenesis. Previous studies have revealed that some individual foods and nutrients, such as soft drinks (5), milk (6), dietary fat (7), and strawberries (8), may be associated with progression of knee osteoarthritis (KOA), the most common form of osteoarthritis. Body mass index (BMI), one of the most consistent risk factors for KOA, may be an intermediate factor linking diet and KOA. A recent study has shown that with increasing fiber intake, there was a decreased risk of incident symptomatic KOA, for which BMI acted as a partial mediator (9).
However, overall dietary pattern analysis may be particularly useful and has been a recent direction for research in nutritional epidemiology (10). Diets are complex and composed of many foods that together might hasten or prevent disease; through the study of dietary patterns, in which multiple components of a diet are examined simultaneously, these interactions may be revealed for their harm or benefit (10–12). On a more practical note, people consume diets, rather than foods in isolation; thus, in order to make future dietary recommendations for those suffering from KOA easier to understand and follow, elucidating dietary patterns will be of great benefit and may serve to shape future clinical nutrition guidance in regard to this disease (13, 14). In a previous study, the Mediterranean diet was found to be associated with lower risks of the knee cartilage morphology, pain worsening, and symptomatic forms of KOA (15, 16).
In the Osteoarthritis Initiative (OAI), we investigated the association of the major dietary patterns derived by principal component analysis (PCA) with both radiographic and symptomatic progression of KOA. To our knowledge, these dietary patterns have not yet been studied in relation to KOA progression.
Subjects and Methods
The Osteoarthritis Initiative
The OAI is a longitudinal study (17), launched by the NIH, that included 4796 individuals aged 45–79 y in 2004. All participants had either established symptomatic KOA or significant risk factors for the development of KOA. Participants were followed annually by centrally trained radiologists or rheumatologists at each clinical center, where clinical data were collected in addition to imaging and biospecimen data and measures of physical performance (18). Uniformity of and adherence to established study procedures was ensured through site visits by the coordinating and imaging centers, and by an onsite quality assurance officer. About 97% of OAI participants had ≥1 follow-up visit, and visit adherence did not vary by baseline characteristics. This study was approved by the institutional review boards at all 4 clinical centers (Baltimore, MD; Columbus, OH; Pittsburgh, PA and Pawtucket, RI) and the coordinating center (San Francisco, CA), and informed written consent was obtained prior to each clinical visit (18).
We included individuals with mild to moderate KOA in at least 1 knee [defined as a Kellgren–Lawrence (KL) grade of 1, 2, or 3] at baseline based on an OAI central X-ray reading. Knees without osteoarthritis (defined as a KL grade of 0 at baseline) were excluded, as were those with severe radiographic osteoarthritis (defined as a KL grade of 4 at baseline), as they were less likely to experience further radiographic progression. We additionally excluded those with lateral joint space narrowing [defined as the grade for lateral compartment joint space narrowing (JSL) being greater than the grade for medial compartment joint space narrowing (JSM)]. We also excluded participants with unrealistic total daily calorie intake (<800 or >4200 kcal for men, <500 or >3500 kcal for women) (19, 20). Finally, 2757 participants (4332 knees) were included in the study population with a mean age of 62 y (Figure 1). Follow-up visits took place at 12, 24, 36, 48, 60, and 72 mo after baseline screening.
FIGURE 1.
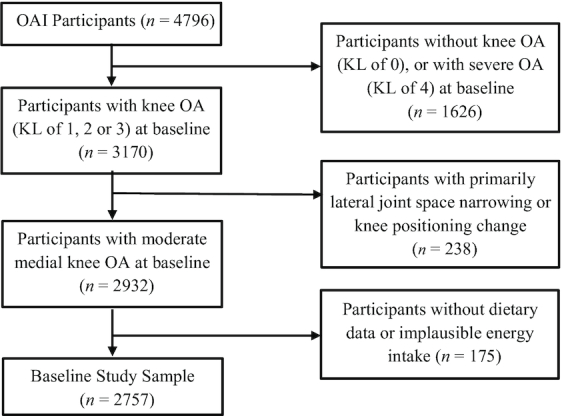
Study population and exclusions in the Osteoarthritis Initiative. After baseline, participants were lost to follow-up due to death, total knee replacement, or nonresponse: 129 in year 2, 139 in year 3, 221 in year 4, and 1377 in year 6. KL, Kellgren–Lawrence; OA, osteoarthritis; OAI, Osteoarthritis Initiative.
Assessment of dietary intake
At baseline a validated 70-item Block Brief FFQ (21) was mailed to all participants. The completed FFQ was reviewed by study nurses for completion. The FFQ included questions about food and nutrient consumption using common units or portion sizes tailored for each food item, with consumption categorized as: never, a few times per year, once per month, 2–3 times per month, once per week, 3–4 times per week, 5–6 times per week, and every day. Participants provided information on average frequency of consumption for each food item during the previous year. The nutrients and total calorie intake were calculated based on USDA food composition data assessed by using NutritionQuest (www.nutritionquest.com).
Assessment of knee osteoarthritis progression
Progression of KOA was assessed by observation of the following radiographic and symptomatic changes over time: 1) 1 full KL-grade increase, 2) measured change in JSW, and 3) increase in Western Ontario and McMaster Universities Arthritis Index (WOMAC) categories or remaining in either of the 2 highest WOMAC categories. First, using a semiquantitative approach, we defined radiographic KOA progression as ≥1 full KL-grade increase for a specific knee. Adjudicated KL grades were measured at baseline and follow-up visits, done through longitudinal reading of serial knee X-rays for tibiofemoral radiographic KOA.
Second, we used a quantitative approach (millimeter accuracy) for a precise measure of JSW between adjacent bones of the knee (22, 23). Multiple JSWs were measured at fixed locations along the joint, recorded as JSW(x), with 0.025 intervals for x from 0.15 to 0.30. We picked medial JSW at x = 0.25, which has been proved to be the most responsive location to quantify KOA progression (24). Repeated measures of JSW change from baseline to 12, 24, 36, 48, and 72 mo were used as outcome variables. The maximum JSW loss was about 4 mm in our sample.
Third, symptomatic KOA progression was evaluated using validated WOMAC pain, functional disability, and total scores (25, 26). WOMAC items were assessed at 12, 24, 36, 48, 60, and 72 mo. Each item was scored from 0 to 4 (WOMAC scores of 0 and quartile thresholds of baseline nonzero scores), with higher scores representing greater levels of pain, stiffness, and functional disability. Subscores vary from 0 to 20 for pain, 0 to 8 for stiffness, 0 to 68 for functional disability, and 0 to 96 for total WOMAC score. Similar to Sharma et al. (27), we categorized WOMAC scores at each time point into 5 grades as follows: grade 1 (pain score = 0, functional disability score = 0 and total score = 0), grade 2 (pain score = 1, functional disability score = 1–4, or total score = 1–4); grade 3 (pain score = 2–3, functional disability score = 5–10, or total score = 5–12); grade 4 (pain score = 4–6, functional disability score = 11–20 or total score = 13–25); and grade 5 (pain score = 7–20, functional disability score = 21–68, or total score = 26–96). Symptomatic progression at each follow-up time point was defined as a dichotomous variable as follows: either 1) an increase into a higher WOMAC grade, or 2) remaining in 1 of the 2 highest grades (28).
Overall, there were 720 patients with radiographic KOA progression in at least 1 knee, and 2440 patients (3633 knees) with symptomatic KOA progression for >72 mo.
Covariates
Demographic and socioeconomic factors, including age, sex, race/ethnicity, marital status, education level, and annual income were ascertained at baseline. Self-reported race was categorized as African American, white, or “other” racial/ethnic group. Educational level was classified as high school or less, college, and above college.
In addition, several general clinical parameters were measured at baseline and during follow-up appointments that have been associated with radiographic progression or changes in symptoms as potential confounders. These were age, sex, race, baseline KL grade, assessed depression (defined as the CES-D 20 items scale >16) (29), BMI, weight change from baseline, physical activity, total energy intake, traumatic knee injury, knee surgery, income, education, smoking, and alcohol intake. Pain relief medication usage included nonsteroidal anti-inflammatory drugs (NSAIDs) and others, including narcotics, acetaminophen (Tylenol), S-adenosylmethionine (SAMe), methylsulfonylmethane (MSM), doxycycline (e.g., Vibra-Tabs, Doryx, Adoxa), and cyclooxygenase-2 (COX-2) inhibitors (Bextra, Celebrex).
Body weight and height were measured without shoes and in light clothing to guarantee accuracy. BMI (kg/m2) was categorized as <25.0, 25.0–29.9, or ≥30 according to WHO criteria (30). Weight changes were repeatedly measured from baseline to every follow-up visit. Physical activity was recorded by using the Physical Activity Scale for the Elderly (PASE), which is an established questionnaire for evaluating physical activity in elderly individuals. It has been validated in younger subjects as well (31, 32). The PASE score was included as a continuous variable in all analyses. We also accounted for radiographic changes of the beam angles and rim distance (from the tibial plateau to the tibial rim closest to the femoral condyle) between the follow-up visits and baseline, which is an indicator of knee-positioning consistency.
Statistical analysis
In descriptive analyses, all participant-level baseline characteristics were summarized as mean, median, standard deviations, and frequencies as appropriate. Basic chi-square tests, Mantel–Haenszel tests, and ANOVA were used to evaluate the differences according to Western and prudent dietary pattern scores.
To identify dietary patterns, FFQ items were aggregated into 25 food groups based on similarities in nutrient profile and culinary preference. PCA aggregated specific food groups based on their intercorrelation with one another. Briefly, each pattern or profile was a linear combination of all food groups weighted by their factor loadings. Each subject received a score for each dietary pattern, with a higher score indicating a higher adherence to this pattern. Based on the Scree test, we derived the following 2 dietary patterns: the prudent pattern, which was related to high intakes of vegetables, fruit, fish, whole grains, and legumes, and the Western pattern, which was characterized by high intakes of red and/or processed meats, refined grains, and french fries (Table 1). Detailed information can be found elsewhere (33). Since the derived patterns were statistically uncorrelated with each other, it is possible for an individual to have high (or low) scores for both Western and prudent dietary patterns simultaneously. Therefore, we created a combined score to evaluate overall dietary quality. This score was calculated as the difference between a participant's Western score and their prudent score, as each participant has a separate score for each dietary pattern. The combined score, from most healthy to most unhealthy, ranged from −6.0 to 6.0 (mean ± SD: 0 ± 1.41). Thus, a higher combined score represents a less healthy diet, and a lower score represents a more healthy diet. For example, if one had a high Western score and a low prudent score, subtracting the latter from the former would lead to a higher combined score, representative of a less healthy diet. If, however, one had a low Western and a high prudent score, then subtracting the latter from the former would lead to a lower combined score, representing a healthier diet overall. Then we applied the residual method to control for the total energy intake by using a linear regression model to calculate the residuals, with total energy intake as the independent and dietary pattern scores as the dependent variable. Then sex-specific quartile groups of each adjusted dietary pattern score were used in the multivariable models described below.
TABLE 1.
Factor-loading matrix for 2 major dietary patterns using the Block Brief FFQ in the OAI1
| Food or food group | Western2 | Prudent2 |
|---|---|---|
| French fries | 0.59 | — |
| Processed meats | 0.58 | — |
| Refined grains | 0.57 | — |
| Red meats | 0.55 | — |
| Poultry | 0.42 | — |
| Pizza | 0.41 | — |
| Margarine | 0.39 | — |
| Sugar-containing beverages | 0.39 | — |
| Eggs | 0.37 | — |
| Snacks | 0.36 | — |
| Desserts and sweets | 0.33 | — |
| Salad dressings | 0.33 | — |
| High-fat dairy products | 0.33 | — |
| Vegetables | — | 0.77 |
| Legumes | — | 0.67 |
| Fruit | — | 0.55 |
| Fish | — | 0.52 |
| Whole grains | — | 0.47 |
| Tomatoes | — | 0.47 |
| Potatoes other than french fries | 0.30 | 0.37 |
OAI, Osteoarthritis Initiative.
Absolute values of <0.30 were excluded from the table for simplicity.
We defined the first full-grade increase of KL grade as the first endpoint for KOA progression. Separate Cox proportional hazards models were used to assess the associations of the 2 dietary patterns with KL progression. For each participant, the follow-up time was calculated from the baseline date to the date of the first increase of KL grade, death, or 72 mo, whichever came first. To deal with ties of failure times, we applied the discrete likelihood method in the models. Robust covariance estimates were used to account for the intracluster correlation between 2 knees in the same subject. We adjusted for demographic and socioeconomic factors, baseline KL grades, traumatic knee injury and knee surgery, usage of NSAIDs, and total energy intake. BMI (continuous variable) and time-varying weight changes from baseline were additionally adjusted for in separate models. Other pain relief medications were not included in the model since there is no evidence to date that pain relief medications are associated with structural (visible on radiograph) progression in KOA. For <1% of the study population with missing BMI and physical activity scale data, missing values were replaced by a sex-specific sample median for each variable. HRs and 95% CIs were calculated to evaluate the strength of associations. The median value for each quartile of dietary pattern intake was used as a continuous variable to test for significant linear trends.
For the second analysis, linear mixed models were used to test the prospective associations between dietary patterns and JSW changes after consideration of the hierarchical data structure (participant–knee–repeated JSW measures). We conducted 3-level random effects models with random within-patient knee and time points. The Akaike Information Criterion and Bayesian Information Criterion were used to evaluate the covariance structure. Unequal cluster sizes (number of knees, time points) were handled in the model under the assumption of missing at random. Models were additionally adjusted for follow-up time points, baseline JSW, and changes in rim and beam angles. Least square means and their SEs were calculated for each dietary quartile, and linear trends were estimated as in the previously described analysis. BMI and weight changes from baseline were adjusted for in separated models.
To assess the associations between dietary patterns and symptomatic KOA progression for the third analysis, we developed generalized linear mixed models for repeated binary responses after adjusting for the covariates above, follow-up time points, depression, and usage of pain relief medications, including NSAIDs as well as narcotics, acetaminophen (Tylenol), SAMe, MSM, doxycycline (e.g., Vibra-Tabs, Doryx, Adoxa), and COX-2 inhibitors (Bextra, Celebrex). ORs and 95% CIs were estimated. Additionally, we investigated effect modification for each outcome by baseline knee surgery status and sex.
Statistical significance was determined by a 2-sided α level of 0.05. Data analyses were performed using SAS 9.4 (SAS Institute). We used the STROBE cohort checklist when writing our report (34).
Results
Table 1 shows the factor loading matrix for the Western and prudent dietary patterns. Positive loading values are representative of a food group's positive correlation with a dietary pattern, and a negative value represents a negative correlation, with the magnitude of values indicating the degree to which each food contributes to the dietary pattern. (For simplicity, negative values are not shown due to small absolute values of <0.30). French fries, processed meats, refined grains, and red meat contribute heavily to the Western dietary pattern, while vegetables, legumes, fruit, fish, and whole grains contribute heavily to the prudent dietary pattern.
In Table 2, we show the baseline characteristics of the study sample according to increasing quartiles of the Western and prudent dietary pattern scores. The group of subjects in the highest quartile (quartile 4) of the Western dietary pattern score, who were consuming the least healthy diet, were younger, more were African American, and they were more likely to be depressed; more of them were current smokers, more were overweight or obese, and they consumed more calories per day. On the other hand, the subjects in the highest quartile (quartile 4) of the prudent dietary pattern score, who were consuming the healthiest diet, were older, more had higher education, and fewer were suffering from depression; they were less likely to be current smokers, overweight or obese, or NSAIDs users; and they consumed more total calories per day.
TABLE 2.
Baseline characteristics of participants with KOA according to quartiles of Western and prudent dietary pattern scores1
| Quartiles of Western dietary pattern | Quartiles of prudent dietary pattern | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Q1 | Q2 | Q3 | Q4 | Q1 | Q2 | Q3 | Q4 | |||
| Variable | (n = 2757) | (n = 689) | (n = 689) | (n = 690) | (n = 689) | P- value | (n = 689) | (n = 689) | (n = 690) | (n = 689) | P- value |
| Age, y | 62.1 ± 9.0 | 64.4 ± 8.7 | 63.2 ± 9.1 | 61.6 ± 8.8 | 59.1 ± 8.6 | <0.01 | 59.8 ± 8.8 | 62.5 ± 9.1 | 62.9 ± 9.0 | 63.2 ± 8.8 | <0.01 |
| Male | 40.5 | 40.5 | 40.5 | 40.4 | 40.5 | 1.00 | 40.5 | 40.5 | 40.4 | 40.5 | 1.00 |
| Race | |||||||||||
| White | 78.8 | 83.6 | 81.4 | 79 | 71.1 | <0.01 | 72.4 | 83.2 | 79.7 | 79.8 | <0.01 |
| African American | 18.2 | 12.3 | 15.5 | 18.1 | 26.7 | 25.5 | 13.8 | 16.4 | 17 | ||
| Other | 3.1 | 4.1 | 3 | 2.9 | 2.2 | 2 | 3 | 3.9 | 3.2 | ||
| Education | |||||||||||
| ≤High school | 16.7 | 11.5 | 13.8 | 17.1 | 24.5 | <0.01 | 23.9 | 15.4 | 14.3 | 13.2 | <0.01 |
| College | 46.1 | 44.1 | 48.5 | 45.7 | 46.3 | 48.2 | 46.6 | 44.6 | 45.1 | ||
| >College | 37.1 | 44.4 | 37.7 | 37.2 | 29 | 27.7 | 38 | 41 | 41.7 | ||
| Missing | 0.04 | 0 | 0 | 0 | 0.1 | 0.1 | 0 | 0 | 0 | ||
| Family income | |||||||||||
| ≤25 k | 13.2 | 12.8 | 11.9 | 13.5 | 14.5 | 0.11 | 16 | 11.6 | 12.6 | 12.5 | 0.02 |
| 25-50 k | 25.6 | 24.2 | 25.7 | 24.5 | 28.2 | 27.3 | 23.5 | 28.7 | 23.1 | ||
| 50-100 k | 34.6 | 35.3 | 35.1 | 37.5 | 30.6 | 30.9 | 39.3 | 32.5 | 35.8 | ||
| >100 k | 20.5 | 20.5 | 21.5 | 20.6 | 19.6 | 20 | 18.9 | 21.2 | 22.1 | ||
| Missing | 6.0 | 7.3 | 5.8 | 3.9 | 7.1 | 5.8 | 6.7 | 5.1 | 6.5 | ||
| Depressed | 8.9 | 6.8 | 6.7 | 10.6 | 11.3 | <0.01 | 13.9 | 7.5 | 7.2 | 6.7 | <0.01 |
| Smoking status | |||||||||||
| Never | 53.4 | 56 | 52.4 | 54.2 | 50.8 | <0.01 | 52.7 | 54.3 | 53.8 | 52.7 | <0.01 |
| Current | 6.2 | 3.9 | 4.8 | 5.8 | 10.4 | 10.4 | 5.8 | 4.9 | 3.8 | ||
| Past | 40.4 | 40.1 | 42.8 | 40 | 38.8 | 36.9 | 39.9 | 41.3 | 43.5 | ||
| PASE | 159.0 ± 81.4 | 159.9 ± 83.4 | 157.8 ± 79.7 | 162.8 ± 82.4 | 155.6 ± 80.2 | 0.40 | 158.2 ± 81.3 | 152.5 ± 79.7 | 157.2 ± 79.0 | 168.1 ± 85.1 | <0.01 |
| BMI | |||||||||||
| <25 | 18.2 | 27.9 | 19.6 | 15.7 | 9.7 | <0.01 | 10.6 | 18.9 | 18.8 | 24.5 | <0.01 |
| 25–30 | 39.9 | 40.6 | 41.1 | 42.3 | 35.6 | 39 | 42.4 | 41.3 | 36.9 | ||
| ≥30 | 41.9 | 31.5 | 39.3 | 42 | 54.7 | 50.4 | 38.8 | 39.9 | 38.6 | ||
| KL grade (index knee) | |||||||||||
| 1 | 33.9 | 5.1 | 5.7 | 8.6 | 7.8 | 9.1 | 7.4 | 6.2 | 4.4 | ||
| 2 | 44.0 | 35.6 | 35 | 33.5 | 31.6 | 0.78 | 29.5 | 34.8 | 35.8 | 35.6 | 0.10 |
| 3 | 22.1 | 43.1 | 43.7 | 43.5 | 45.7 | 46.9 | 44 | 41 | 44.1 | ||
| Baseline JSW, mm | 5.4 ± 1.3 | 5.4 ± 1.3 | 5.4 ± 1.2 | 5.4 ± 1.2 | 5.4 ± 1.3 | 0.68 | 5.4 ± 1.3 | 5.4 ± 1.3 | 5.4 ± 1.2 | 5.5 ± 1.2 | 0.68 |
| NSAID use2 | 21.4 | 20.6 | 18.6 | 23.3 | 22.9 | 0.11 | 22.4 | 20.9 | 20.7 | 21.5 | 0.88 |
| Total calories, kcal/d | 1.4 ± 0.6 | 1.6 ± 0.5 | 1.4 ± 0.5 | 1.3 ± 0.5 | 1.5 ± 0.6 | <0.01 | 1.5 ± 0.6 | 1.3 ± 0.5 | 1.4 ± 0.5 | 1.6 ± 0.5 | <0.01 |
Values are means ± SEs or percentages unless otherwise indicated. Tests for significant difference across categories of dietary pattern intake included the chi-square test, Mantel–Haenszel test, and ANOVA. JSW, joint space width; KL, Kellgren–Lawrence Scale; NSAID, nonsteroidal anti-inflammatory drug; PASE, Physical Activity Scale for the Elderly; Q, quartile.
NSAID (including aspirin, ibuprofen, etc.) use for joint pain or arthritis in past 30 d.
We analyzed the association of the Western and prudent dietary patterns with 1) radiographic progression of KOA (Table 3), 2) loss of JSW (Table 4), and 3) progression of symptomatic KOA (Table 5). During a maximum of 72 mo of follow-up (median follow-up time of 48 mo), adherence to a Western pattern was associated with an increased risk of radiographic progression of KOA, defined as ≥1 full-grade change in the KL grade (compared with quartile 1, HR for quartile 4: 1.30; 95% CI: 1.05, 1.61; P-trend < 0.01), while adherence to a prudent dietary pattern was associated with a decreased risk of radiographic progression of KOA in the multivariable model (compared with quartile 1, HR for quartile 4: 0.79; 95% CI: 0.64, 0.98; P-trend = 0.02). While these associations were attenuated after additional adjustment for BMI and weight changes, a significant linear trend remained for the Western (compared with quartile 1, HR for quartile 4: 1.22; 95% CI: 0.98, 1.51; P-trend = 0.05) and prudent (compared with quartile 1, HR for quartile 4: 0.81; 95% CI: 0.66, 1.00; P-trend = 0.03) dietary patterns. Consistently, a higher combined score was associated with a significantly increased risk of radiographic progression of KOA in a dose–response fashion (compared with quartile 1, HR for quartile 4: 1.25; 95% CI: 1.01, 1.54, P-trend < 0.01), though this association was attenuated when we additionally adjusted for BMI and weight change (compared with quartile 1, HR for quartile 4: 1.19; 95% CI: 0.96, 1.47; P-trend = 0.03) (Table 3).
TABLE 3.
HRs and 95% CIs for radiographic progression of knee osteoarthritis according to quartiles of dietary pattern scores1
| Model 12, 3 | Model 23, 4 | |||||||
|---|---|---|---|---|---|---|---|---|
| Cases, n | Person-years | HR (95% CI) | P- value | P-trend | HR (95% CI) | P- value | P- trend | |
| Western5 | ||||||||
| Q1 | 159 | 2550 | 1.00 | Ref | <0.01 | 1.00 | Ref | 0.05 |
| Q2 | 174 | 2547 | 1.13 (0.91, 1.41) | 0.26 | 1.09 (0.88, 1.35) | 0.45 | ||
| Q3 | 199 | 2521 | 1.30 (1.05, 1.61) | 0.02 | 1.23 (0.99, 1.53) | 0.06 | ||
| Q4 | 188 | 2466 | 1.30 (1.05, 1.61) | 0.02 | 1.22 (0.98, 1.51) | 0.08 | ||
| Prudent5 | ||||||||
| Q1 | 203 | 2444 | 1.00 | Ref | 0.02 | 1.00 | Ref | 0.03 |
| Q2 | 172 | 2523 | 0.88 (0.71, 1.08) | 0.23 | 0.90 (0.73, 1.11) | 0.31 | ||
| Q3 | 181 | 2584 | 0.82 (0.67, 1.00) | 0.05 | 0.84 (0.69, 1.03) | 0.09 | ||
| Q4 | 164 | 2533 | 0.79 (0.64, 0.98) | 0.03 | 0.81 (0.66, 1.00) | 0.05 | ||
| Western − prudent5 | ||||||||
| Q1 | 167 | 2550 | 1.00 | Ref | <0.01 | 1.00 | Ref | 0.03 |
| Q2 | 165 | 2585 | 0.96 (0.77, 1.19) | 0.68 | 0.92 (0.74, 1.14) | 0.46 | ||
| Q3 | 194 | 2505 | 1.21 (0.98, 1.49) | 0.07 | 1.16 (0.94, 1.43) | 0.16 | ||
| Q4 | 194 | 2444 | 1.25 (1.01, 1.54) | 0.04 | 1.19 (0.96, 1.47) | 0.12 | ||
JSW, joint space width; KL, Kellgren–Lawrence; NSAID, nonsteroidal anti-inflammatory drug; PASE, Physical Activity Scale for the Elderly; Q, quartile.
Adjusted for age, sex, race (African American, white, other), baseline KL grades 1–3, injury/surgery (yes, no), baseline PASE score, NSAID use (yes, no), and total energy intake (kcal/d, continuous) using a Cox proportional hazards model.
Additional adjustment for income, education, smoking, and alcohol did not significantly alter results.
Additionally adjusted for BMI (<25.0, 25.0–29.9, and ≥30) and weight change from baseline (continuous).
Western pattern: Q1 is more healthy, Q4 is less healthy; prudent pattern: Q1 is less healthy, Q4 is more healthy; Western − prudent pattern: Q1 is more healthy, Q4 is less healthy.
TABLE 4.
Loss of JSW according to quartiles of dietary pattern scores1
| Model 12, 3 | Model 23,4 | ||||||
|---|---|---|---|---|---|---|---|
| n | Q | ΔJSW, mm | P- value | P-trend | ΔJSW, mm | P- value | P-trend |
| Western5 | |||||||
| 689 | Q1 | 0.39 ± 0.03 | Ref | 0.12 | 0.40 ± 0.03 | Ref | 0.42 |
| 689 | Q2 | 0.40 ± 0.03 | 0.92 | 0.39 ± 0.03 | 0.74 | ||
| 690 | Q3 | 0.43 ± 0.03 | 0.18 | 0.42 ± 0.03 | 0.51 | ||
| 689 | Q4 | 0.42 ± 0.03 | 0.29 | 0.42 ± 0.03 | 0.53 | ||
| Prudent5 | |||||||
| 689 | Q1 | 0.46 ± 0.03 | Ref | <0.01 | 0.45 ± 0.03 | Ref | <0.01 |
| 689 | Q2 | 0.42 ± 0.03 | 0.14 | 0.42 ± 0.03 | 0.25 | ||
| 690 | Q3 | 0.39 ± 0.03 | 0.01 | 0.39 ± 0.03 | 0.03 | ||
| 689 | Q4 | 0.38 ± 0.03 | <0.01 | 0.38 ± 0.03 | <0.01 | ||
| Western − prudent5 | |||||||
| 689 | Q1 | 0.39 ± 0.03 | Ref | <0.01 | 0.39 ± 0.03 | Ref | 0.04 |
| 689 | Q2 | 0.38 ± 0.03 | 0.74 | 0.38 ± 0.03 | 0.54 | ||
| 690 | Q3 | 0.43 ± 0.03 | 0.08 | 0.43 ± 0.03 | 0.15 | ||
| 689 | Q4 | 0.44 ± 0.03 | 0.04 | 0.43 ± 0.03 | 0.12 | ||
Values are means ± SEs unless otherwise indicated. JSW, joint space width; KL, Kellgren–Lawrence; NSAID, nonsteroidal anti-inflammatory drug; PASE, Physical Activity Scale for the Elderly; Q, quartile; Ref, reference.
Adjusted for age, sex, race (African American, white, other), baseline KL grade 1–3, injury/surgery (yes, no), NSAID use (yes, no), changes of rim and beam angle, baseline JSW (mm, continuous), baseline PASE score and total energy intake (kcal/d, continuous) using a linear mixed model.
Additional adjustment for income, education, smoking, and alcohol did not significantly alter results.
Additionally adjusted for BMI (<25.0, 25.0–29.9, and ≥30 kg/m2) and weight change from baseline (continuous).
Western pattern: Q1 is more healthy, Q4 is less healthy; Prudent pattern: Q1 is less healthy, Q4 is more healthy; Western − prudent pattern: Q1 is more healthy, Q4 is less healthy.
TABLE 5.
ORs and 95% CIs for symptomatic progression of knee osteoarthritis according to quartiles of dietary pattern scores1
| Model 12, 4 | Model 23, 4 | |||||||
|---|---|---|---|---|---|---|---|---|
| Cases | Person-years | OR (95% CI) | P-value | P-trend | OR (95% CI) | P-value | P-trend | |
| Western5 | ||||||||
| Q1 | 594 | 1156 | 1.00 | Ref | <0.01 | 1.00 | Ref | <0.01 |
| Q2 | 609 | 1130 | 1.08 (0.92, 1.27) | 0.32 | 1.06 (0.89, 1.25) | 0.51 | ||
| Q3 | 608 | 1105 | 1.20 (1.02, 1.41) | 0.03 | 1.15 (0.97, 1.37) | 0.11 | ||
| Q4 | 629 | 1071 | 1.39 (1.18, 1.63) | <0.01 | 1.26 (1.06, 1.50) | <0.01 | ||
| Prudent5 | ||||||||
| Q1 | 618 | 1077 | 1.00 | Ref | <0.01 | 1.00 | Ref | <0.01 |
| Q2 | 611 | 1094 | 0.90 (0.77, 1.06) | 0.20 | 0.90 (0.76, 1.07) | 0.22 | ||
| Q3 | 614 | 1141 | 0.92 (0.78, 1.07) | 0.27 | 0.90 (0.76, 1.06) | 0.21 | ||
| Q4 | 597 | 1150 | 0.73 (0.62, 0.86) | <0.01 | 0.71 (0.60, 0.84) | <0.01 | ||
| Western − prudent5 | ||||||||
| Q1 | 591 | 1128 | 1.00 | Ref | <0.01 | 1.00 | Ref | <0.01 |
| Q2 | 617 | 1176 | 1.19 (1.01, 1.39) | 0.03 | 1.19 (1.01, 1.41) | 0.04 | ||
| Q3 | 613 | 1114 | 1.24 (1.06, 1.46) | <0.01 | 1.22 (1.03, 1.44) | 0.02 | ||
| Q4 | 619 | 1044 | 1.40 (1.19, 1.64) | <0.01 | 1.29 (1.08, 1.53) | <0.01 | ||
COX-2, cyclooxygenase-2; JSW, joint space width; KL, Kellgren–Lawrence; MSM, methylsulfonylmethane; NSAID, nonsteroidal anti-inflammatory drug; PASE, Physical Activity Scale for the Elderly.
Adjusted for age, sex, race (African American, white, other), baseline KL grade 1–3, injury/surgery (yes, no), depression (yes, no), narcotics for pain (yes, no), NSAID use (yes, no), SAMe (yes, no), MSM (yes, no), doxycycline (e.g., Vibra-Tabs, Doryx, Adoxa) (yes, no), COX-2 inhibitors (Bextra, Celebrex) (yes, no), and baseline PASE score and total energy intake (kcal/day, continuous) using a generalized linear mixed model.
Additionally adjusted for BMI (<25.0, 25.0–29.9, and ≥30 kg/m2), and weight change from baseline (continuous)
Additional adjustment for income, education, smoking, and alcohol did not significantly alter results.
Western pattern: Q1 is more healthy, Q4 is less healthy; Prudent pattern: Q1 is less healthy, Q4 is more healthy; Western − prudent pattern: Q1 is more healthy, Q4 is less healthy.
In the analysis of JSW loss (Table 4) as the second measure of radiographic progression of KOA, increased adherence to a Western dietary pattern was not associated with an increased JSW loss (quartile 1 loss of JSW: 0.39 mm; quartile 4 loss of JSW: 0.42 mm; P-trend = 0.12), and this lack of association remained after adjusting for BMI and weight change from baseline (quartile 1 loss of JSW: 0.40 mm; quartile 4 loss of JSW: 0.42 mm; P-trend = 0.42). Increased adherence to a prudent dietary pattern was significantly associated with decreased JSW loss (quartile 1 loss of JSW: 0.46 mm; quartile 4 loss of JSW: 0.38 mm; P-trend < 0.01). This association remained after additional adjustment for BMI and weight change from baseline (quartile 1 loss of JSW: 0.45 mm; quartile 4 loss of JSW: 0.38 mm; P-trend < 0.01). In addition, a higher combined score was associated with more JSW loss (quartile 1 loss of JSW = 0.39 mm; quartile 4 loss of JSW: 0.44 mm; P-trend < 0.01). After adjustment for BMI and change in weight from baseline, this association remained (quartile 1 loss of JSW: 0.39 mm; quartile 4 loss of JSW: 0.43 mm; P-trend = 0.04).
Symptomatic KOA progression (Table 5) increased significantly with increasing Western pattern quartiles in a dose–response fashion (compared with quartile 1, quartile 4 OR: 1.39; 95% CI: 1.18, 1.63; P-trend < 0.01), and this association remained after adjustment for BMI and weight change from baseline (compared with quartile 1, quartile 4 OR: 1.26; 95% CI: 1.06, 1.50; P-trend < 0.01). Symptomatic KOA progression generally decreased with a higher prudent dietary pattern score (compared with quartile 1, quartile 4 OR: 0.73; 95% CI: 0.62, 0.86; P-trend < 0.01), and after adjustment for BMI and weight change, this association remained (compared with quartile 1, quartile 4 OR: 0.71; 95% CI: 0.60, 0.84; P-trend < 0.01). Consistent with a Western pattern, we observed a strong dose–response relationship between the combined score and symptomatic KOA progression (compared with quartile 1, quartile 4 OR: 1.40; 95% CI: 1.19, 1.64; P-trend < 0.01). This association remained after adjusting for BMI and weight change from baseline (compared with quartile 1, quartile 4 OR: 1.29; 95% CI: 1.08, 1.53; P-trend < 0.01).
We investigated effect modification for each outcome by baseline knee surgery status and sex. The results were not significant (P values ranged from 0.06 to 0.96).
Discussion
In this longitudinal cohort study of participants with KOA, increasing adherence to a Western dietary pattern was associated with increased radiographic and symptomatic KOA progression, while adherence to a prudent dietary pattern was associated with decreased radiographic and symptomatic KOA progression.
Western and healthy dietary patterns derived using PCA have previously been studied in relation to KOA severity in a cross-sectional study of 231 Iranian women (35), in whom neither a Western nor a healthy dietary pattern was associated with KOA severity, though a traditional Iranian dietary pattern that includes a high intake of red and organ meats, eggs, nuts, legumes, potatoes and onions, olives and olive oil, and legumes, was associated with more severe KOA in postmenopausal women (35).
The association between Mediterranean dietary pattern and KOA has been examined previously in the OAI cohort (15). Participants who were in the highest quintile of Mediterranean diet consumption were less likely to experience incident KOA accompanied by symptoms including a worsening of knee pain (15). Further, in a study of 99 participants diagnosed with osteoarthritis who were randomly assigned to a Mediterranean or control diet for 16 weeks, those consuming the Mediterranean diet had a significant improvement in knee flexion and decrease in the inflammatory biomarker IL-1α (36).
Characteristics of osteoarthritis progression include cartilage degradation, subchondral bone changes (e.g., bone marrow lesions and cysts), and frequently the additional presence of osteophytes, with inflammation playing a role (2, 3). BMI, as a general measure of body adiposity and an important risk factor for osteoarthritis incidence (37, 38) and progression (38–40), may be both a confounder and mediator in studies of diet–disease relationships (9). After adjusting for BMI and weight changes, the associations of 2 dietary patterns with radiographic and symptomatic progression became attenuated, but were generally retained, indicating potential independent findings beyond BMI.
Synovial inflammation may also be associated with disease progression (4), and it is this inflammation that may provide additional insight into a possible link between the Western and prudent dietary patterns and osteoarthritis. French fries and red and processed meats, with their high–saturated fat content (41), and sugar-sweetened beverages (42) have been linked with increased inflammation, while fruits, vegetables (43, 44), and whole grains (45) have been associated with lowered inflammation. Overall, the Western diet has been associated with increased markers of inflammation that are also associated with osteoarthritis, such as IL-6 (46, 47) and C-reactive protein (47, 48), and a healthy dietary pattern has been associated with decreased concentrations of these same inflammatory markers (47, 43, 49–51). These changes in inflammatory mediators may explain the respectively harmful and beneficial roles of these dietary patterns in regard to KOA.
Strengths of this study include its prospective design and repeated, computer-based measures of digitized radiographs (22) obtained using a validated method (23). We examined a comprehensive relationship of major dietary patterns derived using PCA with radiographic progression of KOA defined by both semiquantitative KL grade increase and quantitative JSW loss, and symptomatic progression measured by WOMAC category changes over up to 72 mo. Additionally, we innovatively created a combined score of Western and prudent dietary patterns to evaluate overall dietary quality. This pattern uniquely described consumption of a Western diet eliminating the benefit of the prudent dietary pattern, allowing us to isolate the association between a more strictly Western diet among those in the highest quartile of consumption and KOA progression. Further, when conducting our analyses, we were able to include depression as a confounder in our models, which may be associated with food choices as well as potentially being associated with psychological and pain sensitization that may influence KOA phenotype, though these variables may not influence radiographic disease progression.
In terms of limitations, while dietary information was only obtained at baseline, and measurement error could have been minimized if repeated dietary measures could have been averaged (13), the Block Brief FFQ was administered prior to assessment of radiographic and symptomatic KOA progression, and such measurement error would have biased results toward the null (13). Second, though we adjusted for many factors associated with KOA progression and dietary intake, there is a possibility of residual confounding. Third, in studying disease progression, there was the potential for collider bias. By stratifying on having diagnosed KOA, in order to study KOA progression, we conditioned on a collider (incident KOA) that opened an association between diet and KOA progression through other variables (that might be known or unknown) (52, 53). However, we were able to adjust for the most well-known variables associated with incident KOA, and thus our positive findings are less likely to be interpreted as collider bias. Fourth, OR was used to measure the association in the analysis for symptomatic progression; however, OR may not approximate the rate ratios when responses are not rare. Further, the baseline diet was measured before OA progression; thus, our findings may be less likely to be interpreted as due to reverse causation. Another limitation of our study is that it included mostly white participants; thus, our findings may not be generalizable to those with other backgrounds, particularly considering that dietary patterns may vary by ethnic group. In addition, PCA is a data-driven approach to dietary pattern formulation which only represents dietary patterns of those in the OAI cohort. Our combined dietary pattern in the OAI cohort may not be generalizable to a specific recommendation of a healthy diet. However, our derived patterns were consistent with dietary patterns from other cohorts. Both the Western and prudent dietary patterns based on the PCA approach have been validated (10) in other cohorts and contained food items similar to those represented in the OAI cohort. Studying dietary patterns in general also has some limitations, despite its many benefits, in that any individual foods offering the greatest benefit or harm in terms of KOA progression may be overlooked in dietary pattern analysis, for example, the magnitude of their benefit may be diluted, or the food components related to a certain dietary pattern may not all influence disease outcomes (13).
In conclusion, following a Western dietary pattern was associated with increased radiographic and symptomatic KOA progression, while following a prudent dietary pattern was associated with decreased radiographic and symptomatic KOA progression in a longitudinal cohort of men and women. Reducing consumption of an unhealthy Western diet high in french fries, red and processed meats, poultry, refined grains, sugar-containing beverages and foods, and pizza, while also increasing consumption of a healthy prudent diet high in fruits, vegetables, legumes, fish, and whole grains, may slow KOA progression after KOA incidence. Further longitudinal studies should be replicated in other populations.
ACKNOWLEDGEMENTS
The OAI is a public–private partnership comprising 5 contracts (N01-AR-2-2258, N01-AR-2-2259, N01-AR-2-2260, N01-AR-2-2261, and N01-AR-2-2262) funded by the NIH, a branch of the Department of Health and Human Services, and conducted by the Osteoarthritis Initiative (OAI) Study Investigators. Private funding partners include Pfizer, Inc.; Novartis Pharmaceuticals Corporation; Merck Research Laboratories; and GlaxoSmithKline. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health.
The authors’ responsibilities were as follows—CE, TMA, JD, BL: designed the study; CX, NM: analyzed the data; NM, CX, BL: wrote the manuscript; and all authors: read and approved the final manuscript. The authors report no conflicts of interest.
Notes
CX and NEM are co-first authors
Supported by the NIH National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01 AR074447 A1), a Brigham Research Institute (BRI) grant (agreement number: 2018A008050), and a and contract HHSN268201000020C (reference number: BAA-NHLBI-AR-10-06—National Heart, Lung and Blood Institute). The study sponsor was not involved in the study design, data analysis, or interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Abbreviations used: JSW, Joint space width; KL, Kellgren-Lawrence; KOA, Knee osteoarthritis; OAI, Osteoarthritis Initiative; PCA, Principal Component Analysis; WOMAC, Western Ontario & McMaster Universities Arthritis Index.
References
- 1. Glyn-Jones S, Palmer AJ, Agricola R, Price AJ, Vincent TL, Weinans H, Carr AJ. Osteoarthritis. Lancet. 2015;386(9991):376–87. [DOI] [PubMed] [Google Scholar]
- 2. Ishidou Y, Matsuyama K, Sakuma D, Setoguchi T, Nagano S, Kawamura I, Maeda S, Komiya S. Osteoarthritis of the hip joint in elderly patients is most commonly atrophic, with low parameters of acetabular dysplasia and possible involvement of osteoporosis. Arch Osteoporos. 2017;12(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hunter DJ. Osteoarthritis: time for us all to shift the needle. Rheumatology (Oxford). 2018;57(suppl_4):iv1–2. [DOI] [PubMed] [Google Scholar]
- 4. Berenbaum F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthritis Cartilage. 2013;21(1):16–21. [DOI] [PubMed] [Google Scholar]
- 5. Lu B, Ahmad O, Zhang FF, Driban JB, Duryea J, Lapane KL, McAlindon T, Eaton CB. Soft drink intake and progression of radiographic knee osteoarthritis: data from the osteoarthritis initiative. BMJ Open. 2013;3(7):e002993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lu B, Driban JB, Duryea J, McAlindon T, Lapane KL, Eaton CB. Milk consumption and progression of medial tibiofemoral knee osteoarthritis: data from the Osteoarthritis Initiative. Arthritis Care Res (Hoboken). 2014;66(6):802–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lu B, Driban JB, Xu C, Lapane KL, McAlindon TE, Eaton CB. Dietary fat intake and radiographic progression of knee osteoarthritis: data from the osteoarthritis initiative. Arthritis Care Res (Hoboken). 2017;69(3):368–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schell J, Scofield RH, Barrett JR, Kurien BT, Betts N, Lyons TJ, Zhao YD, Basu A. Strawberries improve pain and inflammation in obese adults with radiographic evidence of knee osteoarthritis. Nutrients. 2017;9(9):E949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dai Z, Jafarzadeh SR, Niu J, Felson DT, Jacques PF, Li S, Zhang Y. Body mass index mediates the association between dietary fiber and symptomatic knee osteoarthritis in the osteoarthritis initiative and the Framingham Osteoarthritis Study. J Nutr. 2018;148(12):1961–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hu FB, Rimm E, Smith-Warner SA, Feskanich D, Stampfer MJ, Ascherio A, Sampson L, Willett WC. Reproducibility and validity of dietary patterns assessed with a food-frequency questionnaire. Am J Clin Nutr. 1999;69(2):243–9. [DOI] [PubMed] [Google Scholar]
- 11. Hu FB. Obesity Epidemiology. Oxford, New York: Oxford University Press; 2008. [Google Scholar]
- 12. Fung TT, Meyer HE, Willett WC, Feskanich D. Association between diet quality scores and risk of hip fracture in postmenopausal women and men aged 50 years and older. J Acad Nutr Diet. 2018;118(12):2269–79. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Willett W. Nutritional Epidemiology. 3rd ed New York, Oxford: Oxford University Press; 2013. [Google Scholar]
- 14. Satija A, Yu E, Willett WC, Hu FB. Understanding nutritional epidemiology and its role in policy. Adv Nutr. 2015;6(1):5–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Veronese N, Koyanagi A, Stubbs B, Cooper C, Guglielmi G, Rizzoli R, Punzi L, Rogoli D, Caruso MG, Rotolo O, et al.. Mediterranean diet and knee osteoarthritis outcomes: a longitudinal cohort study. Clin Nutr. 2018;38(6):2735–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Veronese N, La Tegola L, Crepaldi G, Maggi S, Rogoli D, Guglielmi G. The association between the Mediterranean diet and magnetic resonance parameters for knee osteoarthritis: data from the Osteoarthritis Initiative. Clin Rheumatol. 2018;37(8):2187–93. [DOI] [PubMed] [Google Scholar]
- 17. Eckstein F, Kwoh CK, Link TM, investigators OAI . Imaging research results from the Osteoarthritis Initiative (OAI): a review and lessons learned 10 years after start of enrolment. Ann Rheum Dis. 2014;73(7):1289–300. [DOI] [PubMed] [Google Scholar]
- 18. Nevitt MC FD, Lester G. The Osteoarthritis Initiative: protocol for the cohort study. In: Appendices OSPa, ed., San Francisco: University of California; 2006. https://oai.epi-ucsf.org/datarelease/docs/StudyDesignProtocol.pdf.Accessed 27 July, 2019. [Google Scholar]
- 19. Lajous M, Willett WC, Robins J, Young JG, Rimm E, Mozaffarian D, Hernan MA. Changes in fish consumption in midlife and the risk of coronary heart disease in men and women. Am J Epidemiol. 2013;178(3):382–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Eshak ES, Iso H, Date C, Yamagishi K, Kikuchi S, Watanabe Y, Wada Y, Tamakoshi A, Group JS. Rice intake is associated with reduced risk of mortality from cardiovascular disease in Japanese men but not women. J Nutr. 2011;141(4):595–602. [DOI] [PubMed] [Google Scholar]
- 21. Potischman N, Carroll RJ, Iturria SJ, Mittl B, Curtin J, Thompson FE, Brinton LA. Comparison of the 60- and 100-item NCI-block questionnaires with validation data. Nutr CancerNutr. 1999;34(1):70–5. [DOI] [PubMed] [Google Scholar]
- 22. Duryea J, Zaim S, Genant HK. New radiographic-based surrogate outcome measures for osteoarthritis of the knee. Osteoarthritis Cartilage. 2003;11(2):102–10. [DOI] [PubMed] [Google Scholar]
- 23. Sharp JT, Angwin J, Boers M, Duryea J, von Ingersleben G, Hall JR, Kauffman JA, Landewe R, Langs G, Lukas C, et al.. Computer based methods for measurement of joint space width: update of an ongoing OMERACT project. J Rheumatol. 2007;34(4):874–83. [PubMed] [Google Scholar]
- 24. Duryea J, Neumann G, Niu J, Totterman S, Tamez J, Dabrowski C, Le Graverand MP, Luchi M, Beals CR, Hunter DJ. Comparison of radiographic joint space width with magnetic resonance imaging cartilage morphometry: analysis of longitudinal data from the Osteoarthritis Initiative. Arthritis Care Res (Hoboken). 2010;62(7):932–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15(12):1833–40. [PubMed] [Google Scholar]
- 26. McConnell S, Kolopack P, Davis AM. The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC): a review of its utility and measurement properties. Arthritis Rheum. 2001;45(5):453–61. [DOI] [PubMed] [Google Scholar]
- 27. Sharma L, Cahue S, Song J, Hayes K, Pai YC, Dunlop D. Physical functioning over three years in knee osteoarthritis: role of psychosocial, local mechanical, and neuromuscular factors. Arthritis Rheum. 2003;48(12):3359–70. [DOI] [PubMed] [Google Scholar]
- 28. Holla JF, Steultjens MP, Roorda LD, Heymans MW, Ten Wolde S, Dekker J. Prognostic factors for the two-year course of activity limitations in early osteoarthritis of the hip and/or knee. Arthritis Care Res (Hoboken). 2010;62(10):1415–25. [DOI] [PubMed] [Google Scholar]
- 29. Sawyer Radloff L. The CES-D scale: a self-reported depression scale for research in the general population. APM. 1977;1(3)385–401. [Google Scholar]
- 30. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i–xii., 1–253. [PubMed] [Google Scholar]
- 31. Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46(2):153–62. [DOI] [PubMed] [Google Scholar]
- 32. Johansen KL, Painter P, Kent-Braun JA, Ng AV, Carey S, Da Silva M, Chertow GM. Validation of questionnaires to estimate physical activity and functioning in end-stage renal disease. Kidney Int. 2001;59(3):1121–7. [DOI] [PubMed] [Google Scholar]
- 33. Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol. 2002;13(1):3–9. [DOI] [PubMed] [Google Scholar]
- 34. von Elm E, Hajizadehoghaz M, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573–7. [DOI] [PubMed] [Google Scholar]
- 35. Abdollahi AH, Mohseni R, Aliakbar S, Veisy Z, Yekaninejad MS, Maghbooli A, Mirzaei K. Association between major dietary patterns and grades of knee osteoarthritis in women. Asian J Clin Nutr. 2018;10(1):16–24. [Google Scholar]
- 36. Dyer J, Davison G, Marcora SM, Mauger AR. Effect of a Mediterranean type diet on inflammatory and cartilage degradation biomarkers in patients with osteoarthritis. J Nutr Health Aging. 2017;21(5):562–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zheng H, Chen C. Body mass index and risk of knee osteoarthritis: systematic review and meta-analysis of prospective studies. BMJ Open. 2015;5(12):e007568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cooper C, Snow S, McAlindon TE, Kellingray S, Stuart B, Coggon D, Dieppe PA. Risk factors for the incidence and progression of radiographic knee osteoarthritis. Arthritis Rheum. 2000;43(5):995–1000. [DOI] [PubMed] [Google Scholar]
- 39. Reijman M, Pols HA, Bergink AP, Hazes JM, Belo JN, Lievense AM, Bierma-Zeinstra SM. Body mass index associated with onset and progression of osteoarthritis of the knee but not of the hip: the Rotterdam Study. Ann Rheum Dis. 2007;66(2):158–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Felson DT, Goggins J, Niu J, Zhang Y, Hunter DJ. The effect of body weight on progression of knee osteoarthritis is dependent on alignment. Arthritis Rheum. 2004;50(12):3904–9. [DOI] [PubMed] [Google Scholar]
- 41. Ruiz-Nunez B, Dijck-Brouwer DA, Muskiet FA. The relation of saturated fatty acids with low-grade inflammation and cardiovascular disease. J Nutr Biochem. 2016;36:1–20. [DOI] [PubMed] [Google Scholar]
- 42. Aeberli I, Gerber PA, Hochuli M, Kohler S, Haile SR, Gouni-Berthold I, Berthold HK, Spinas GA, Berneis K. Low to moderate sugar-sweetened beverage consumption impairs glucose and lipid metabolism and promotes inflammation in healthy young men: a randomized controlled trial. Am J Clin Nutr. 2011;94(2):479–85. [DOI] [PubMed] [Google Scholar]
- 43. Giugliano D, Ceriello A, Esposito K. The effects of diet on inflammation: emphasis on the metabolic syndrome. J Am Coll Cardiol. 2006;48(4):677–85. [DOI] [PubMed] [Google Scholar]
- 44. Jahns L, Conrad Z, Johnson LK, Whigham LD, Wu D, Claycombe-Larson KJ. A diet high in carotenoid-rich vegetables and fruits favorably impacts inflammation status by increasing plasma concentrations of IFN-alpha2 and decreasing MIP-1beta and TNF-alpha in healthy individuals during a controlled feeding trial. Nutr ResNutr. Res. 2018;52:98–104. [DOI] [PubMed] [Google Scholar]
- 45. Xu Y, Wan Q, Feng J, Du L, Li K, Zhou Y. Whole grain diet reduces systemic inflammation: A meta-analysis of 9 randomized trials. Medicine (Baltimore). 2018;97(43):e12995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Larsson S, Englund M, Struglics A, Lohmander LS. Interleukin-6 and tumor necrosis factor alpha in synovial fluid are associated with progression of radiographic knee osteoarthritis in subjects with previous meniscectomy. Osteoarthritis Cartilage. 2015;23(11):1906–14. [DOI] [PubMed] [Google Scholar]
- 47. Galland L. Diet and inflammation. Nutr Clin Pract. 2010;25(6):634–40. [DOI] [PubMed] [Google Scholar]
- 48. Perruccio AV, Chandran V, Power JD, Kapoor M, Mahomed NN, Gandhi R. Systemic inflammation and painful joint burden in osteoarthritis: a matter of sex?. Osteoarthritis Cartilage. 2017;25(1):53–9. [DOI] [PubMed] [Google Scholar]
- 49. Gao X, Bermudez OI, Tucker KL. Plasma C-reactive protein and homocysteine concentrations are related to frequent fruit and vegetable intake in Hispanic and non-Hispanic white elders. J Nutr. 2004;134(4):913–8. [DOI] [PubMed] [Google Scholar]
- 50. Brighenti F, Valtuena S, Pellegrini N, Ardigo D, Del Rio D, Salvatore S, Piatti P, Serafini M, Zavaroni I. Total antioxidant capacity of the diet is inversely and independently related to plasma concentration of high-sensitivity C-reactive protein in adult Italian subjects. Br J Nutr. 2005;93(5):619–25. [DOI] [PubMed] [Google Scholar]
- 51. Smidowicz A, Regula J. Effect of nutritional status and dietary patterns on human serum C-reactive protein and interleukin-6 concentrations. Adv Nutr. 2015;6(6):738–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Paternoster L, Tilling K, Davey Smith G. Genetic epidemiology and Mendelian randomization for informing disease therapeutics: conceptual and methodological challenges. PLos Genet. 2017;13(10):e1006944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hernan MA, Hernandez-Diaz S, Robins JM. A structural approach to selection bias. Epidemiology. 2004;15(5):615–25. [DOI] [PubMed] [Google Scholar]


