ABSTRACT
Background
Choline-related nutrients are dietary precursors of a gut microbial metabolite, trimethylamine-N-oxide, which has been linked to cardiometabolic diseases and related death. However, epidemiologic evidence on dietary choline and mortality remains limited, particularly among nonwhite populations.
Objectives
This study aimed to investigate the associations of choline-related nutrients with cardiometabolic and all-cause mortality among black and white Americans and Chinese adults.
Methods
Included were 49,858 blacks, 23,766 whites, and 134,001 Chinese, aged 40–79 y, who participated in 3 prospective cohorts and lived ≥1 y after enrollment. Cox regression models were used to estimate HRs and 95% CIs for cardiometabolic [e.g., ischemic heart disease (IHD), stroke, and diabetes] and all-cause deaths. To account for multiple testing, P values < 0.003 were considered significant.
Results
Mean choline intake among blacks, whites, and Chinese was 404.1 mg/d, 362.0 mg/d, and 296.8 mg/d, respectively. During a median follow-up of 11.7 y, 28,673 deaths were identified, including 11,141 cardiometabolic deaths. After comprehensive adjustments, including for overall diet quality and disease history, total choline intake was associated with increased cardiometabolic mortality among blacks and Chinese (HR for highest compared with lowest quintile: 1.26; 95% CI: 1.13, 1.40 and HR: 1.23; 95% CI: 1.11, 1.38, respectively; both P-trend < 0.001); among whites, the association was weaker (HR: 1.12; 95% CI: 0.95, 1.33; P-trend = 0.02). Total choline intake was also associated with diabetes and all-cause mortality in blacks (HR: 1.66; 95% CI: 1.26, 2.19 and HR: 1.20; 95% CI: 1.12, 1.29, respectively), with diabetes mortality in Chinese (HR: 2.24; 95% CI: 1.68, 2.97), and with IHD mortality in whites (HR: 1.31; 95% CI: 1.02, 1.69) (all P-trend < 0.001). The choline–mortality association was modified by alcohol consumption and appeared stronger among individuals with existing cardiometabolic disease. Betaine intake was associated with increased cardiometabolic mortality in Chinese only (HR: 1.16; 95% CI: 1.08, 1.25; P-trend < 0.001).
Conclusions
High choline intake was associated with increased cardiometabolic mortality in racially diverse populations.
Keywords: trimethylamine-N-oxide, choline, cardiometabolic mortality, all-cause mortality, multiethnic prospective cohort
Introduction
Emerging evidence implies a crucial role for the gut microbiota in human nutrition and cardiometabolic health (1–4). Trimethylamine-N-oxide (TMAO) is a gut microbial-derived metabolite of trimethylamine-containing nutrients such as choline and betaine (1, 3, 5). Recent reviews and meta-analyses have reported positive associations between elevated circulating TMAO and risks of cardiovascular disease (CVD), type 2 diabetes, and mortality, especially among patients with existing cardiometabolic conditions (2, 4, 6, 7).
Choline is an essential nutrient abundant in red meat, eggs, fish, and dairy, in the forms of phosphatidylcholine, free choline, glycerophosphocholine, sphingomyelin, and phosphocholine (8, 9). Betaine, a choline derivative, mostly comes from wheat products and spinach, and some comes from fish and meats (8–11). Although the recommended daily intake of choline is 550 mg for men and 425 mg for women in the United States (12), most population-based studies have reported a lower intake of ∼300 mg/d among Americans (8, 10, 13–17). Studies have also indicated that the food sources of dietary choline may vary by race/ethnicity: e.g., in a multiethnic study (11), African Americans consumed more poultry-derived choline, Japanese Americans and Native Hawaiians had high fish/shellfish-derived choline, and Latinos had more legume-derived choline. On the other hand, no significant racial/ethnic variation was observed for betaine intake (11).
Currently, epidemiologic evidence on the associations of choline-related nutrients with mortality and/or incident CVD remains inconsistent. Two large US cohort studies reported positive associations of choline intake with all-cause and CVD mortality (13), but a prospective study of Japanese adults reported null associations (18). For incident CVD, several cohort studies from the United States and Europe, mostly among whites, found nonsignificant positive associations of dietary choline and betaine with CVD risk (10, 17, 19), whereas a cohort study of blacks reported a positive association of betaine intake with ischemic heart disease (IHD) and an inverse association of choline intake with ischemic stroke (14). The inconsistent findings might result from racial/ethnic differences in dietary habits/choline food sources, choline-metabolizing genetic polymorphisms, and/or gut microbial production of TMAO (20–23). However, most previous studies were conducted in a single race/ethnicity (mainly whites), thus the potential racial/ethnic differences could not be appropriately addressed. In this study including 3 prospective cohorts, the Southern Community Cohort Study (SCCS), Shanghai Men's Health Study (SMHS), and Shanghai Women's Health Study (SWHS), we compared mean daily intakes and major food sources of choline-containing compounds and betaine among black and white Americans and Chinese adults. We also investigated the associations of each choline-related nutrient with cardiometabolic-specific (i.e., IHD, stroke, and diabetes) and all-cause mortality, and further evaluated whether the associations were modified by sociodemographic characteristics, lifestyle factors, and comorbidity status.
Methods
Study population
Detailed cohort profiles of the SCCS, SMHS, and SWHS have been described elsewhere (24–26). Briefly, between March 2002 and September 2009, the SCCS enrolled a total of 84,735 primarily low-income adults, aged 40–79 y, across 12 southeastern states in the United States. Two-thirds of the study participants were blacks. The SMHS and SWHS recruited 61,480 men and 74,940 women aged 40–74 y in Shanghai, China, between 2002 and 2006 and between 1996 and 2000, respectively. Along with written informed consent, each cohort conducted a baseline survey to collect information on sociodemographic factors, lifestyle, dietary habits, and medical history. All study participants were followed up to monitor their health and vital status (see Outcome ascertainment). These cohorts were approved by the Institutional Review Boards of Vanderbilt University, Meharry Medical College, and Shanghai Cancer Institute. The present study was approved by Scientific Committees of each cohort.
We excluded individuals who reported implausible total energy intakes (using the predefined study-specific cutoffs of <600 or >8000 kcal/d for the SCCS, <800 or >4200 kcal/d for the SMHS, and <500 or >3500 kcal/d for the SWHS) or who had invalid follow-up information. To reduce potential reverse causation, we further excluded the first year of observation including participants who died or were lost to follow-up within the first year after study enrollment. For the SCCS, participants who reported race/ethnicity other than black or white were excluded owing to the small sample size—race/ethnicity was self-identified in all 3 cohorts. After the exclusions, a total of 207,625 participants (49,858 blacks, 23,766 whites, and 134,001 Chinese) remained as the final analytic sample (Supplemental Figure 1).
Assessment of dietary intake
To capture habitual dietary intake at baseline, all 3 cohorts used validated FFQs comprised of foods commonly consumed in the study population. The SCCS FFQ asked about the consumption frequencies of 89 food items, which were assigned standard portion sizes using the race/ethnicity- and sex-specific portion sizes from the NHANES and the USDA Continuing Survey of Food Intakes (27–29). Total energy and nutrient intakes were calculated based on the USDA Food Composition Databases (30). The SMHS and SWHS FFQs asked about both the frequencies and quantities of consuming 81 and 77 food items, respectively. Intakes of total energy and nutrients were calculated based on the 2002 Chinese Food Composition Table (31). Total choline, choline-containing compounds (i.e., phosphatidylcholine, free choline, glycerophosphocholine, sphingomyelin, and phosphocholine), and betaine intakes were estimated using the USDA Database for the Choline Content of Common Foods for all 3 cohorts (9), given that there are no choline/betaine data in the Chinese Food Composition Tables. For the SMHS and SWHS, we also referred to the USDA National Nutrient Database for Standard Reference, as a complementary database (32). All food items in the SMHS and SWHS FFQs can be found with an exact or similar match in the USDA database. We have shown that the correlations are high between intakes of common nutrients derived from the USDA and Chinese databases (e.g., r > 0.90 for B vitamins and methionine), indicating the validity of using the USDA database in our Chinese population to calculate choline and betaine intakes (33, 34).
Outcome ascertainment
The primary outcome was deaths due to cardiometabolic diseases (i.e., any types of CVD and diabetes), which were further evaluated separately by deaths from IHD, stroke, and diabetes, as the secondary outcomes. The SCCS ascertained status, date, and underlying cause of death via annual linkage of the cohort to the National Death Index and Social Security Administration mortality files through 31 December, 2015. Both the SMHS and SWHS confirmed the vital status and underlying cause of death via annual linkage to the Shanghai Vital Statistics Registry and in-person follow-up surveys through 31 December, 2016. The underlying causes of death were defined using the International Classification of Diseases, 9th and 10th Revisions (total CVD, 390–459 and I00–I99; IHD, 410–414 and I20–I25; stroke, 430–438 and I60–I69; and diabetes, 249–250 and E10–E14, respectively).
Statistical analyses
All dietary intakes were adjusted for total energy using the residual method (35). Mean intakes of choline, choline-containing compounds, and betaine (mg/d) were estimated in each race and sex group using the generalized linear model, adjusted for age and total energy intake. Partial correlation coefficients of choline-related nutrients were estimated, controlling for age, sex, and total energy intake. Baseline characteristics across total choline intake were compared using the chi-square test and the generalized linear model. Choline-related nutrients were classified into race- and sex-specific quintiles, considering substantial differences in the mean intakes across race and sex (Table 1). Cox regression models were used to estimate the HRs and 95% CIs for mortality associated with choline-related nutrients, using the lowest quintile as the reference. Follow-up time was counted from 1 y after the date of enrollment to date of death, end of follow-up, or loss to follow-up—whichever occurred first. The proportional hazard assumption was tested with goodness-of-fit tests using Schoenfeld residuals; no violation of the assumption was observed. Covariates included age (continuous), educational attainment (<12 y, high school graduation, some college, and ≥university degree), annual household income [low, lower-middle, upper-middle, and high; for the SCCS: <15,000 US dollars (USD), ≥15,000 to <25,000 USD, ≥25,000 to <50,000 USD, and ≥50,000 USD; for the SMHS: <6000 Chinese yuan (CNY), ≥6000 to <12,000 CNY, ≥12,000 to <24,000 CNY, and ≥24,000 CNY; and for the SWHS: <10,000 CNY, ≥10,000 to <20,000 CNY, ≥20,000 to <30,000 CNY, and ≥30,000 CNY], marital status (married and single/separated/divorced/widowed), smoking status (never, former, and current), smoking pack-years (continuous), alcohol consumption (none, ≤2 and >2 drinks/d in men, and ≤1 and >1 drink/d in women; 1 drink = 14 g ethanol), physical activity level (race- and sex-specific tertiles of total metabolic equivalent hours), BMI (in kg/m2; <18.5, 18.5–24.9, 25.0–29.9, 30.0–34.9, and ≥35.0 for blacks and whites and <18.5, 18.5–22.9, 23.0–27.49, and ≥27.5 for Chinese), healthy eating index developed to assess the overall diet quality and adherence to the dietary guidelines (race- and sex-specific quintiles) (29, 36), the Charlson comorbidity index using weighted comorbid conditions (0, 1, 2, 3, ≥4) (37), total energy (kcal/d; continuous), and menopausal status in women (pre and post). Given the strong association of refined carbohydrate intake with mortality and cardiometabolic diseases in the SMHS and SWHS (38, 39), refined carbohydrate intake (sex-specific quintiles) was further controlled as a potential confounder in those cohorts. Missing rates of the covariates were very low, mostly <1% of each variable—we imputed missing data using the cohort- and sex-specific median (for continuous variables) or mode (for categorical variables) values of nonmissing covariates. Linear trends across the quintiles were tested using a continuous variable with the median values in each quintile. Stratified analyses were conducted to evaluate the effect modifications of the associations by the aforementioned covariates. Interactions between choline intake and covariates were tested by the likelihood ratio test using the multiplicative interaction term. To confirm the robustness of our findings, we further conducted a series of sensitivity analyses, excluding the first 2 y of follow-up data, excluding participants with a history of CVD, not adjusting for healthy eating index, and treating cancer deaths as competing risk events. Because of multiple comparisons across 3 racial groups and 5 uncorrelated choline-containing compounds (phosphatidylcholine and sphingomyelin were highly correlated with total choline), associations with total cardiometabolic and all-cause mortality were considered significant when P values were <0.003 (0.05/15). A further correction was applied when cardiometabolic deaths were divided into IHD, stroke, and diabetes, and P values < 0.001 were considered significant. Because the numbers of comparisons were at least doubled in stratified analyses, P values for significance for interactions were taken to be half of those listed above, thus <0.0015. All analyses were performed using SAS Enterprise Guide version 7.1 (SAS Institute Inc.).
TABLE 1.
Daily intakes and major food sources of choline-related nutrients in blacks, whites, and Chinese1
| Blacks (n = 49,858) | Whites (n = 23,766) | Chinese (n = 134,001) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Men | Women | Food source, % | Men | Women | Food source, % | Men2 | Women2 | Food source, % | |
| Total choline, mg/d | 494.1 ± 102.1 | 345.1 ± 99.8 | Red meat (22.4) | 438.0 ± 99.8 | 307.6 ± 101.3 | Red meat (23.1) | 317.1 ± 94.1 | 279.9 ± 93.6 | Eggs (23.0) |
| Eggs (17.3) | Eggs (15.1) | Soy foods (20.2) | |||||||
| Vegetables (9.2) | Dairy (12.2) | Red meat (13.6) | |||||||
| Dairy (7.8) | Vegetables (8.8) | Vegetables (11.7) | |||||||
| Poultry (7.7) | Poultry (7.0) | Fish (11.3) | |||||||
| Phosphatidylcholine, mg/d | 284.9 ± 87.8 | 193.4 ± 85.8 | Red meat (29.2) | 237.1 ± 85.9 | 162.5 ± 87.1 | Red meat (32.7) | 177.5 ± 64.8 | 159.5 ± 64.5 | Eggs (39.0) |
| Eggs (28.0) | Eggs (25.8) | Soy foods (19.0) | |||||||
| Poultry (9.3) | Poultry (8.9) | Red meat (15.3) | |||||||
| Vegetables (7.4) | Vegetables (6.1) | Fish (11.3) | |||||||
| Processed meat (6.3) | Processed meat (4.7) | Vegetables (5.6) | |||||||
| Free choline, mg/d | 104.0 ± 25.2 | 74.2 ± 24.6 | Vegetables (16.7) | 99.6 ± 24.6 | 71.1 ± 25.0 | Vegetables (17.5) | 72.2 ± 25.0 | 62.2 ± 24.9 | Soy foods (34.3) |
| Other grains (10.8) | Dairy (12.8) | Vegetables (25.8) | |||||||
| Fruit (10.3) | Other grains (10.6) | Fruit (8.8) | |||||||
| Dairy (9.3) | Whole grain (7.8) | Rice (7.8) | |||||||
| Red meat (9.0) | Red meat (7.3) | Wheat (6.4) | |||||||
| Glycerophosphocholine, mg/d | 64.6 ± 18.8 | 46.7 ± 18.3 | Dairy (24.5) | 63.7 ± 18.4 | 45.2 ± 18.6 | Dairy (36.6) | 45.3 ± 15.0 | 37.8 ± 15.0 | Red meat (22.5) |
| Red meat (12.5) | Red meat (10.9) | Fish (19.0) | |||||||
| Other grains (12.0) | Other grains (10.7) | Milk (18.1) | |||||||
| Fish (10.8) | Fruit (6.5) | Rice (18.0) | |||||||
| Fruit (9.6) | Fish (6.2) | Vegetables (10.2) | |||||||
| Sphingomyelin, mg/d | 29.5 ± 9.5 | 21.0 ± 9.3 | Red meat (36.8) | 26.9 ± 9.3 | 19.3 ± 9.4 | Red meat (37.4) | 12.0 ± 4.7 | 10.0 ± 4.7 | Red meat (34.3) |
| Eggs (17.7) | Dairy (15.6) | Eggs (27.3) | |||||||
| Poultry (17.0) | Eggs (15.1) | Fish (18.9) | |||||||
| Dairy (9.8) | Poultry (14.9) | Poultry (11.7) | |||||||
| Processed meat (8.3) | Processed meat (6.0) | Milk (6.0) | |||||||
| Phosphocholine, mg/d | 13.3 ± 4.2 | 11.6 ± 4.1 | Vegetables (28.7) | 13.4 ± 4.1 | 11.0 ± 4.2 | Dairy (30.4) | 10.2 ± 5.0 | 10.2 ± 4.9 | Vegetables (31.6) |
| Dairy (19.7) | Vegetables (26.0) | Soy foods (24.2) | |||||||
| Red meat (11.3) | Red meat (10.1) | Milk (17.9) | |||||||
| Fruit (9.8) | Poultry (6.7) | Fruit (9.6) | |||||||
| Poultry (8.1) | Fruit (6.0) | Fish (6.3) | |||||||
| Betaine, mg/d | 214.7 ± 74.6 | 169.5 ± 72.9 | Vegetables (24.6) | 184.2 ± 73.0 | 135.8 ± 74.0 | Whole grains (25.2) | 79.1 ± 38.4 | 52.5 ± 38.2 | Wheat (62.3) |
| Whole grains (18.9) | Other grains (20.6) | Vegetables (12.7) | |||||||
| Other grains (17.5) | Red meat (15.3) | Fish (10.4) | |||||||
| Red meat (11.5) | Vegetables (13.6) | Rice (3.5) | |||||||
| Poultry (8.0) | Poultry (7.7) | Red meat (3.0) | |||||||
Values are least-square means ± SDs adjusted for age and total energy intake unless indicated otherwise. Adjusted for total energy by using the residual method, the overall mean ± SD intakes of dietary choline and betaine were 404.1 ± 120.5 mg/d and 187.4 ± 76.0 mg/d in blacks, 362.0 ± 121.1 mg/d and 155.8 ± 76.5 mg/d in whites, and 296.8 ± 93.1 mg/d and 64.6 ± 39.4 mg/d in Chinese, respectively.
The overall results of total choline intake were shown in a previous report (34).
Results
The mean dietary intakes of choline and betaine were 404.1 and 187.4 mg/d in blacks, 362.0 and 155.8 mg/d in whites, and 296.8 and 64.6 mg/d in Chinese, respectively (Table 1). Black men showed the highest mean intakes of total choline and betaine (494.1 and 214.7 mg/d, respectively), whereas Chinese women showed the lowest intakes (279.9 and 52.5 mg/d, respectively). Phosphatidylcholine and free choline accounted for >50% and >20% of total choline intake, respectively. The major food sources of total choline and phosphatidylcholine were red meat and eggs among blacks and whites but were eggs and soy foods among Chinese. For betaine intake, grains and vegetables were major food sources among all racial groups. No significant sex differences in major food sources were observed in each racial group (Supplemental Table 1). Total choline was strongly correlated with phosphatidylcholine and sphingomyelin (r = 0.92 and 0.84 in blacks, 0.89 and 0.83 in whites, and 0.93 and 0.78 in Chinese, respectively; P < 0.001; Supplemental Table 2), but not with other choline-containing compounds nor with betaine.
Compared with individuals who had a low choline intake, blacks and whites with a high intake had lower educational attainment, higher BMI, and more comorbidities (Table 2). However, Chinese with a high choline intake had higher levels of education and income but lower BMI than those with a low intake.
TABLE 2.
Baseline characteristics by dietary choline intake in blacks, whites, and Chinese1
| Blacks (n = 49,858) | Whites (n = 23,766) | Chinese (n = 134,001) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q3 | Q5 | Q1 | Q3 | Q5 | Q1 | Q3 | Q5 | |
| Total choline intake, mg/d | 280.3 ± 61.9 | 394.9 ± 73.4 | 556.4 ± 126.5 | 250.0 ± 56.3 | 349.5 ± 62.3 | 491.7 ± 113.9 | 179.9 ± 33.0 | 290.5 ± 19.3 | 430.9 ± 72.5 |
| Phosphatidylcholine intake, mg/d | 136.7 ± 39.9 | 219.4 ± 55.2 | 351.4 ± 107.8 | 116.0 ± 34.6 | 180.9 ± 45.9 | 296.0 ± 100.9 | 93.0 ± 23.6 | 163.4 ± 23.3 | 254.0 ± 56.2 |
| Free choline intake, mg/d | 75.5 ± 24.7 | 87.2 ± 26.0 | 95.0 ± 36.3 | 70.1 ± 22.7 | 83.4 ± 22.8 | 91.5 ± 36.8 | 44.7 ± 12.1 | 65.3 ± 16.1 | 92.5 ± 29.4 |
| Glycerophosphocholine intake, mg/d | 45.7 ± 14.6 | 54.2 ± 17.6 | 61.1 ± 26.5 | 42.4 ± 15.2 | 53.3 ± 17.7 | 60.4 ± 28.4 | 29.8 ± 8.2 | 40.9 ± 10.7 | 53.9 ± 20.1 |
| Sphingomyelin intake, mg/d | 14.7 ± 5.1 | 23.5 ± 6.3 | 36.5 ± 11.4 | 13.8 ± 4.7 | 21.5 ± 5.6 | 32.4 ± 9.9 | 6.2 ± 2.4 | 10.7 ± 2.8 | 16.1 ± 5.0 |
| Phosphocholine intake, mg/d | 9.4 ± 3.4 | 12.5 ± 3.8 | 14.4 ± 4.9 | 9.3 ± 3.2 | 12.5 ± 3.7 | 13.9 ± 4.5 | 6.1 ± 2.7 | 10.1 ± 3.5 | 14.6 ± 5.5 |
| Betaine intake, mg/d | 153.2 ± 68.9 | 192.5 ± 76.9 | 210.1 ± 92.2 | 130.8 ± 59.2 | 161.0 ± 61.2 | 169.9 ± 72.5 | 52.8 ± 38.2 | 64.7 ± 36.3 | 77.1 ± 45.0 |
| Age, y | 51.0 ± 8.5 | 51.5 ± 8.5 | 51.9 ± 8.5 | 53.1 ± 9.0 | 54.1 ± 9.3 | 54.6 ± 8.8 | 55.1 ± 9.6 | 52.9 ± 9.3 | 52.3 ± 9.4 |
| Men, % | 40.8 | 40.8 | 40.8 | 38.9 | 38.9 | 38.9 | 45.5 | 45.5 | 45.5 |
| BMI, kg/m2 | 30.1 ± 7.4 | 30.6 ± 7.5 | 31.1 ± 7.8 | 29.0 ± 7.0 | 30.0 ± 7.4 | 31.0 ± 7.8 | 24.2 ± 3.5 | 23.8 ± 3.2 | 23.7 ± 3.2 |
| Menopause, % (only among women) | 62.9 | 65.6 | 66.8 | 74.9 | 76.2 | 78.7 | 61.5 | 46.5 | 42.4 |
| Charlson comorbidity index | 1.7 ± 1.4 | 1.8 ± 1.5 | 2.0 ± 1.5 | 2.0 ± 1.5 | 2.1 ± 1.6 | 2.2 ± 1.6 | 0.6 ± 0.9 | 0.5 ± 0.9 | 0.6 ± 1.0 |
| Educational attainment, % | |||||||||
| < High school graduation | 30.7 | 29.3 | 34.9 | 24.8 | 20.5 | 25.3 | 64.2 | 47.6 | 42.5 |
| High school graduation | 33.0 | 34.3 | 34.7 | 32.3 | 31.6 | 30.6 | 25.3 | 32.8 | 34.9 |
| Some college | 25.8 | 25.7 | 22.0 | 25.4 | 26.4 | 25.8 | 6.3 | 11.3 | 12.8 |
| ≥ University degree | 10.5 | 10.8 | 8.4 | 17.5 | 21.5 | 18.4 | 4.2 | 8.3 | 9.8 |
| Annual household income,2 % | |||||||||
| Low | 59.1 | 58.3 | 62.2 | 49.5 | 43.0 | 48.2 | 19.2 | 13.1 | 13.0 |
| Lower-middle | 22.3 | 22.4 | 21.8 | 18.4 | 19.3 | 18.2 | 44.7 | 39.9 | 36.6 |
| Upper-middle | 12.7 | 13.2 | 11.5 | 15.7 | 17.6 | 16.5 | 27.0 | 32.5 | 32.8 |
| High | 5.9 | 6.2 | 4.5 | 16.4 | 20.1 | 17.1 | 9.1 | 14.5 | 17.6 |
| Married, % | 72.4 | 70.0 | 70.5 | 53.3 | 50.8 | 52.0 | 90.9 | 93.8 | 92.7 |
| Smoking status, % | |||||||||
| Never | 38.0 | 38.2 | 37.0 | 32.9 | 34.6 | 32.5 | 64.7 | 67.8 | 66.5 |
| Former | 18.8 | 20.1 | 21.5 | 25.7 | 29.6 | 31.3 | 5.5 | 5.0 | 5.1 |
| Current | 43.1 | 41.7 | 41.4 | 41.4 | 35.8 | 36.2 | 29.9 | 27.2 | 28.4 |
| Smoking pack-years, among ever-smokers | 19.6 ± 18.7 | 18.1 ± 17.6 | 19.0 ± 18.8 | 32.8 ± 27.5 | 30.2 ± 26.0 | 34.0 ± 29.1 | 24.5 ± 16.6 | 23.5 ± 16.2 | 24.6 ± 16.6 |
| Alcohol consumption,3 % | |||||||||
| None | 46.1 | 45.8 | 46.4 | 50.7 | 49.1 | 51.0 | 88.3 | 85.1 | 76.3 |
| Moderate drinking | 32.2 | 35.4 | 36.6 | 34.9 | 39.7 | 36.5 | 7.0 | 9.8 | 13.0 |
| Heavy drinking | 21.8 | 18.8 | 17.0 | 14.4 | 11.2 | 12.6 | 4.7 | 5.1 | 10.6 |
| Total physical activity, MET-h/wk | 22.5 ± 18.8 | 23.4 ± 19.4 | 21.9 ± 18.8 | 21.5 ± 17.6 | 22.2 ± 18.1 | 20.9 ± 17.5 | 86.3 ± 47.6 | 85.4 ± 46.5 | 84.6 ± 47.4 |
| Healthy eating index | 54.9 ± 12.4 | 59.2 ± 11.4 | 57.5 ± 11.3 | 53.3 ± 13.1 | 59.3 ± 12.2 | 58.2 ± 12.0 | 32.5 ± 4.0 | 34.4 ± 4.3 | 32.1 ± 4.6 |
Values are mean ± SD of continuous variables or proportion (%) of categorical variables. Intakes were based on the race- and sex-specific Qs adjusted for total energy using the residual method. Differences across Qs were all statistically significant (P < 0.003) except for sex for all 3 racial groups and marital status for whites. CNY, Chinese yuan; MET, metabolic equivalent of task; Q, quintile; USD, US dollar.
Annual household income was defined as low, lower-middle, upper-middle, and high; for the Southern Community Cohort Study: <15,000 USD, ≥15,000 to <25,000 USD, ≥25,000 to <50,000 USD, and ≥50,000 USD; for the Shanghai Men's Health Study: <6000 CNY, ≥6000 to <12,000 CNY, ≥12,000 to <24,000 CNY, and ≥24,000 CNY; and for the Shanghai Women's Health Study: <10,000 CNY, ≥10,000 to <20,000 CNY, ≥20,000 to <30,000 CNY, and ≥30,000 CNY.
Heavy drinking was defined as alcohol consumption of >2 drinks/d in men or >1 drink/d in women; and moderate drinking was defined as alcohol consumption of >0 to ≤2 drinks/d in men or >0 to ≤1 drink/d in women.
During a median follow-up of 11.7 y (9.0 y for blacks and whites and 14.0 y for Chinese), we identified 28,673 deaths (8200 for blacks, 4009 for whites, and 16,464 for Chinese), including 11,141 deaths from cardiometabolic disease (3191 for blacks, 1292 for whites, and 6658 for Chinese). After adjusting for potential confounders, total choline intake was positively associated with cardiometabolic mortality among all racial groups, significantly so in blacks and Chinese (Table 3): HR (95% CI) for the highest compared with the lowest quintile = 1.26 (1.13, 1.40) in blacks, 1.23 (1.11, 1.38) in Chinese, and 1.12 (0.95, 1.33) in whites. All choline-related nutrients, except glycerophosphocholine and betaine, were associated with a 22–27% increased risk of cardiometabolic mortality among blacks, when comparing the highest with the lowest quintiles. Among whites, phosphatidylcholine was significantly associated with a 19% increased risk. Among Chinese, free choline, phosphocholine, and betaine were associated with a 13–26% increased risk, whereas glycerophosphocholine and sphingomyelin showed associations with decreased risk of cardiometabolic mortality.
TABLE 3.
Risk of cardiometabolic death by intakes of choline-related nutrients1
| Blacks (n = 49,858) | Whites (n = 23,766) | Chinese (n = 134,001) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Deaths, n | Median, mg/d | HR (95% CI)2 | Deaths, n | Median, mg/d | HR (95% CI)2 | Deaths, n | Median, mg/d | HR (95% CI)2,3 | |
| Total choline | |||||||||
| Q1 | 553 | 263.8 | 1 (ref.) | 244 | 236.9 | 1 (ref.) | 1874 | 185.7 | 1 (ref.) |
| Q2 | 590 | 310.1 | 1.09 (0.97, 1.22) | 210 | 277.9 | 0.85 (0.71, 1.02) | 1318 | 245.0 | 1.04 (0.96, 1.12) |
| Q3 | 617 | 348.6 | 1.12 (1.00, 1.26) | 241 | 311.9 | 0.94 (0.79, 1.13) | 1119 | 289.3 | 1.01 (0.92, 1.10) |
| Q4 | 674 | 397.1 | 1.18 (1.05, 1.32) | 265 | 353.2 | 1.01 (0.84, 1.20) | 1123 | 336.3 | 1.09 (0.98, 1.20) |
| Q5 | 757 | 531.7 | 1.26 (1.13, 1.40) | 332 | 461.7 | 1.12 (0.95, 1.33) | 1224 | 414.0 | 1.23 (1.11, 1.38) |
| P-trend4 | <0.001 | 0.02 | <0.001 | ||||||
| Phosphatidylcholine | |||||||||
| Q1 | 579 | 123.3 | 1 (ref.) | 233 | 102.8 | 1 (ref.) | 1859 | 92.9 | 1 (ref.) |
| Q2 | 579 | 161.4 | 1.03 (0.92, 1.16) | 216 | 134.0 | 0.94 (0.78, 1.14) | 1295 | 130.9 | 0.99 (0.92, 1.07) |
| Q3 | 625 | 194.1 | 1.10 (0.98, 1.23) | 239 | 161.5 | 1.00 (0.83, 1.20) | 1148 | 161.8 | 1.03 (0.95, 1.12) |
| Q4 | 621 | 236.2 | 1.07 (0.95, 1.20) | 268 | 197.8 | 1.06 (0.88, 1.26) | 1120 | 194.8 | 1.07 (0.98, 1.17) |
| Q5 | 787 | 353.7 | 1.27 (1.14, 1.41) | 336 | 301.2 | 1.19 (1.00, 1.41) | 1236 | 246.2 | 1.07 (0.97, 1.18) |
| P-trend4 | <0.001 | 0.008 | 0.09 | ||||||
| Free choline | |||||||||
| Q1 | 557 | 57.0 | 1 (ref.) | 267 | 54.1 | 1 (ref.) | 1653 | 39.8 | 1 (ref.) |
| Q2 | 602 | 66.3 | 1.06 (0.94, 1.20) | 259 | 63.9 | 0.90 (0.75, 1.07) | 1205 | 52.3 | 1.05 (0.97, 1.14) |
| Q3 | 644 | 74.3 | 1.14 (1.01, 1.29) | 253 | 72.0 | 0.89 (0.74, 1.07) | 1119 | 62.8 | 1.11 (1.02, 1.21) |
| Q4 | 713 | 84.6 | 1.28 (1.13, 1.45) | 263 | 81.4 | 0.92 (0.76, 1.11) | 1183 | 75.6 | 1.11 (1.01, 1.21) |
| Q5 | 675 | 114.7 | 1.26 (1.11, 1.43) | 250 | 105.4 | 0.89 (0.73, 1.08) | 1498 | 97.8 | 1.26 (1.15, 1.38) |
| P-trend4 | <0.001 | 0.69 | <0.001 | ||||||
| Glycerophosphocholine | |||||||||
| Q1 | 578 | 34.5 | 1 (ref.) | 286 | 31.1 | 1 (ref.) | 2132 | 25.0 | 1 (ref.) |
| Q2 | 608 | 40.6 | 1.05 (0.94, 1.18) | 266 | 38.6 | 0.94 (0.79, 1.11) | 1448 | 32.6 | 0.97 (0.90, 1.04) |
| Q3 | 623 | 46.1 | 1.03 (0.92, 1.16) | 248 | 45.1 | 0.85 (0.71, 1.01) | 1189 | 39.2 | 0.93 (0.86, 1.01) |
| Q4 | 670 | 53.7 | 1.09 (0.97, 1.22) | 258 | 53.7 | 0.86 (0.72, 1.02) | 1005 | 46.3 | 0.87 (0.79, 0.95) |
| Q5 | 712 | 75.9 | 1.11 (0.99, 1.25) | 234 | 77.0 | 0.83 (0.69, 1.00) | 884 | 58.9 | 0.87 (0.79, 0.97) |
| P-trend4 | 0.05 | 0.06 | 0.002 | ||||||
| Sphingomyelin | |||||||||
| Q1 | 577 | 12.8 | 1 (ref.) | 247 | 12.1 | 1 (ref.) | 2152 | 5.5 | 1 (ref.) |
| Q2 | 582 | 17.6 | 1.03 (0.92, 1.16) | 212 | 16.3 | 0.91 (0.75, 1.09) | 1399 | 8.3 | 0.94 (0.88, 1.01) |
| Q3 | 654 | 21.3 | 1.14 (1.02, 1.28) | 260 | 19.6 | 1.08 (0.91, 1.29) | 1184 | 10.4 | 0.92 (0.85, 1.00) |
| Q4 | 647 | 26.0 | 1.12 (1.00, 1.25) | 264 | 23.6 | 1.08 (0.91, 1.29) | 964 | 12.8 | 0.80 (0.73, 0.88) |
| Q5 | 731 | 38.1 | 1.23 (1.10, 1.37) | 309 | 33.8 | 1.15 (0.97, 1.37) | 959 | 16.8 | 0.88 (0.79, 0.98) |
| P-trend4 | <0.001 | 0.03 | 0.001 | ||||||
| Phosphocholine | |||||||||
| Q1 | 540 | 7.4 | 1 (ref.) | 259 | 7.3 | 1 (ref.) | 1740 | 4.7 | 1 (ref.) |
| Q2 | 594 | 9.8 | 1.05 (0.93, 1.19) | 258 | 9.6 | 0.99 (0.82, 1.18) | 1204 | 7.3 | 1.02 (0.94, 1.10) |
| Q3 | 638 | 11.6 | 1.11 (0.98, 1.25) | 245 | 11.3 | 0.91 (0.75, 1.10) | 1082 | 9.5 | 1.03 (0.94, 1.12) |
| Q4 | 675 | 13.9 | 1.13 (0.99, 1.28) | 266 | 13.4 | 0.98 (0.81, 1.20) | 1145 | 12.0 | 1.07 (0.98, 1.17) |
| Q5 | 744 | 17.8 | 1.22 (1.07, 1.40) | 264 | 17.5 | 1.00 (0.81, 1.23) | 1487 | 16.5 | 1.13 (1.03, 1.23) |
| P-trend4 | 0.002 | 0.82 | 0.003 | ||||||
| Betaine | |||||||||
| Q1 | 620 | 104.2 | 1 (ref.) | 285 | 86.7 | 1 (ref.) | 1700 | 27.7 | 1 (ref.) |
| Q2 | 641 | 137.1 | 1.05 (0.94, 1.18) | 239 | 111.6 | 0.85 (0.71, 1.01) | 1264 | 41.3 | 0.98 (0.91, 1.06) |
| Q3 | 626 | 165.2 | 1.03 (0.92, 1.16) | 263 | 133.6 | 0.93 (0.79, 1.11) | 1232 | 52.2 | 1.08 (1.00, 1.16) |
| Q4 | 652 | 205.9 | 1.04 (0.92, 1.16) | 257 | 166.3 | 0.85 (0.72, 1.02) | 1096 | 68.3 | 1.05 (0.97, 1.13) |
| Q5 | 652 | 287.0 | 0.99 (0.88, 1.12) | 248 | 240.6 | 0.83 (0.69, 0.99) | 1366 | 114.0 | 1.16 (1.08, 1.25) |
| P-trend4 | 0.73 | 0.08 | <0.001 | ||||||
Death from any kinds of cardiovascular disease and diabetes. Intakes were based on the race- and sex-specific Qs. Q, quintile.
Adjusted for age, educational attainment, annual household income, marital status, smoking status, smoking pack-years, alcohol consumption, physical activity level, obesity status, healthy eating index, Charlson comorbidity index, intake of total energy, and menopausal status in women.
Intake of refined carbohydrate was further adjusted for the Chinese population.
To adjust for multiple comparisons across the 3 racial groups and different choline-related nutrients, trend P values < 0.003 (0.05/15) were considered significant.
We further examined choline intakes in relation to cause-specific mortality due to IHD, stroke, and diabetes (Table 4). Blacks showed strong positive associations of total choline, phosphatidylcholine, free choline, and sphingomyelin with diabetes mortality—HRs for the highest compared with the lowest quintiles were 1.59–1.79 with all P-trend < 0.001—but no associations with IHD or stroke mortality. Among whites, the highest quintiles of total choline, phosphatidylcholine, and sphingomyelin were associated with 1.31- to 1.52-fold increased HRs for IHD mortality with all P-trend < 0.001, but there were no associations with diabetes or stroke mortality. Among Chinese, total choline, phosphatidylcholine, free choline, and phosphocholine were all associated with diabetes mortality: the HRs were 1.51–2.24; betaine was associated with IHD mortality with an HR of 1.24 (all P-trend < 0.001).
TABLE 4.
Risk of death from ischemic heart disease, stroke, and diabetes by intakes of choline-related nutrients1
| Ischemic heart disease | Stroke | Diabetes | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Blacks2 | Whites2 | Chinese2, 3 | Blacks2 | Whites2 | Chinese2, 3 | Blacks2 | Whites2 | Chinese2, 3 | |
| Total choline | |||||||||
| Q1 | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| Q2 | 1.12 (0.92, 1.37) | 0.73 (0.54, 0.99) | 1.02 (0.89, 1.18) | 1.04 (0.76, 1.43) | 1.09 (0.62, 1.89) | 1.05 (0.93, 1.18) | 1.03 (0.75, 1.41) | 0.71 (0.42, 1.18) | 1.23 (0.98, 1.54) |
| Q3 | 0.96 (0.78, 1.18) | 0.92 (0.69, 1.22) | 1.13 (0.97, 1.33) | 1.19 (0.87, 1.61) | 1.10 (0.63, 1.92) | 0.89 (0.77, 1.01) | 1.13 (0.83, 1.54) | 0.75 (0.45, 1.24) | 1.26 (0.98, 1.62) |
| Q4 | 1.05 (0.86, 1.28) | 1.15 (0.88, 1.50) | 1.14 (0.96, 1.36) | 1.22 (0.90, 1.65) | 0.99 (0.56, 1.75) | 0.99 (0.85, 1.15) | 1.29 (0.96, 1.73) | 0.95 (0.59, 1.53) | 1.50 (1.14, 1.97) |
| Q5 | 1.08 (0.89, 1.32) | 1.31 (1.02, 1.69) | 1.29 (1.06, 1.56) | 1.20 (0.89, 1.62) | 1.12 (0.65, 1.93) | 1.02 (0.87, 1.21) | 1.66 (1.26, 2.19) | 0.96 (0.61, 1.51) | 2.24 (1.68, 2.97) |
| P-trend4 | 0.63 | <0.001 | 0.01 | 0.22 | 0.68 | 0.93 | <0.001 | 0.72 | <0.001 |
| Phosphatidylcholine | |||||||||
| Q1 | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| Q2 | 1.06 (0.87, 1.29) | 1.00 (0.74, 1.34) | 1.02 (0.89, 1.17) | 0.79 (0.57, 1.09) | 0.81 (0.45, 1.46) | 0.98 (0.87, 1.09) | 1.21 (0.89, 1.65) | 0.91 (0.54, 1.53) | 1.02 (0.82, 1.27) |
| Q3 | 1.01 (0.82, 1.23) | 1.01 (0.75, 1.35) | 1.12 (0.96, 1.30) | 1.16 (0.86, 1.55) | 1.15 (0.67, 1.98) | 0.94 (0.83, 1.06) | 1.13 (0.83, 1.55) | 1.01 (0.61, 1.65) | 1.12 (0.89, 1.43) |
| Q4 | 0.91 (0.74, 1.12) | 1.27 (0.96, 1.67) | 1.12 (0.96, 1.31) | 1.08 (0.80, 1.46) | 1.17 (0.69, 2.00) | 0.97 (0.85, 1.11) | 1.34 (0.99, 1.80) | 0.96 (0.58, 1.58) | 1.34 (1.06, 1.71) |
| Q5 | 1.11 (0.91, 1.35) | 1.52 (1.17, 1.98) | 1.18 (0.99, 1.39) | 1.16 (0.87, 1.56) | 0.96 (0.55, 1.66) | 0.90 (0.78, 1.04) | 1.67 (1.26, 2.22) | 1.10 (0.69, 1.75) | 1.51 (1.17, 1.94) |
| P-trend4 | 0.74 | <0.001 | 0.05 | 0.12 | 0.82 | 0.20 | <0.001 | 0.66 | <0.001 |
| Free choline | |||||||||
| Q1 | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| Q2 | 1.08 (0.88, 1.34) | 0.86 (0.66, 1.12) | 0.96 (0.83, 1.11) | 1.08 (0.80, 1.47) | 0.81 (0.45, 1.47) | 1.07 (0.95, 1.20) | 1.09 (0.80, 1.49) | 0.88 (0.55, 1.43) | 1.35 (1.07, 1.69) |
| Q3 | 1.14 (0.92, 1.42) | 0.92 (0.70, 1.21) | 0.99 (0.85, 1.16) | 0.96 (0.70, 1.33) | 1.37 (0.79, 2.39) | 1.13 (0.99, 1.28) | 1.33 (0.97, 1.82) | 0.65 (0.38, 1.12) | 1.52 (1.20, 1.93) |
| Q4 | 1.34 (1.07, 1.66) | 0.90 (0.67, 1.19) | 1.11 (0.95, 1.30) | 1.03 (0.74, 1.43) | 1.14 (0.63, 2.06) | 1.06 (0.93, 1.21) | 1.64 (1.20, 2.24) | 1.03 (0.63, 1.70) | 1.54 (1.21, 1.96) |
| Q5 | 1.26 (1.00, 1.58) | 0.94 (0.70, 1.26) | 1.21 (1.03, 1.42) | 1.08 (0.77, 1.52) | 1.05 (0.56, 1.97) | 1.25 (1.09, 1.43) | 1.59 (1.14, 2.22) | 0.89 (0.52, 1.52) | 1.67 (1.31, 2.14) |
| P-trend4 | 0.03 | 0.89 | 0.003 | 0.84 | 0.51 | 0.003 | <0.001 | 0.99 | <0.001 |
| Glycerophosphocholine | |||||||||
| Q1 | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| Q2 | 1.03 (0.85, 1.26) | 1.07 (0.84, 1.37) | 1.10 (0.97, 1.24) | 0.83 (0.61, 1.13) | 1.10 (0.63, 1.92) | 0.88 (0.79, 0.98) | 1.12 (0.83, 1.52) | 0.58 (0.35, 0.96) | 1.16 (0.95, 1.41) |
| Q3 | 0.99 (0.81, 1.21) | 0.78 (0.60, 1.03) | 0.99 (0.86, 1.15) | 0.79 (0.58, 1.07) | 1.08 (0.61, 1.89) | 0.86 (0.77, 0.97) | 1.27 (0.94, 1.71) | 0.89 (0.57, 1.39) | 1.13 (0.90, 1.42) |
| Q4 | 0.97 (0.79, 1.19) | 0.68 (0.51, 0.89) | 1.03 (0.88, 1.20) | 0.95 (0.70, 1.27) | 1.16 (0.66, 2.01) | 0.70 (0.61, 0.81) | 1.24 (0.92, 1.67) | 0.86 (0.55, 1.35) | 1.19 (0.93, 1.53) |
| Q5 | 0.96 (0.78, 1.18) | 0.89 (0.68, 1.17) | 0.95 (0.80, 1.13) | 0.99 (0.74, 1.34) | 1.00 (0.56, 1.81) | 0.73 (0.63, 0.86) | 1.41 (1.05, 1.90) | 0.61 (0.36, 1.02) | 1.44 (1.11, 1.87) |
| P-trend4 | 0.68 | 0.11 | 0.39 | 0.58 | 0.84 | <0.001 | 0.02 | 0.21 | 0.004 |
| Sphingomyelin | |||||||||
| Q1 | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| Q2 | 0.82 (0.67, 1.00) | 0.86 (0.64, 1.15) | 1.07 (0.95, 1.22) | 1.04 (0.77, 1.41) | 0.68 (0.38, 1.21) | 0.85 (0.77, 0.95) | 1.40 (1.03, 1.92) | 0.71 (0.42, 1.20) | 1.05 (0.86, 1.29) |
| Q3 | 0.99 (0.82, 1.20) | 1.20 (0.91, 1.58) | 1.00 (0.86, 1.16) | 1.14 (0.84, 1.54) | 1.15 (0.70, 1.91) | 0.84 (0.74, 0.95) | 1.47 (1.08, 2.01) | 0.99 (0.62, 1.59) | 1.18 (0.95, 1.48) |
| Q4 | 0.88 (0.72, 1.08) | 1.11 (0.84, 1.46) | 0.97 (0.83, 1.14) | 1.27 (0.95, 1.71) | 0.91 (0.53, 1.57) | 0.65 (0.56, 0.75) | 1.43 (1.05, 1.94) | 1.11 (0.70, 1.75) | 1.02 (0.79, 1.32) |
| Q5 | 0.98 (0.81, 1.20) | 1.48 (1.14, 1.91) | 1.01 (0.83, 1.22) | 1.09 (0.80, 1.48) | 0.91 (0.53, 1.56) | 0.73 (0.62, 0.86) | 1.79 (1.33, 2.41) | 0.80 (0.50, 1.29) | 1.20 (0.91, 1.60) |
| P-trend4 | 0.99 | <0.001 | 0.72 | 0.37 | 0.99 | <0.001 | <0.001 | 0.70 | 0.16 |
| Phosphocholine | |||||||||
| Q1 | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| Q2 | 1.16 (0.94, 1.44) | 1.28 (0.98, 1.69) | 0.93 (0.81, 1.07) | 1.06 (0.78, 1.45) | 1.11 (0.65, 1.90) | 1.03 (0.92, 1.16) | 0.94 (0.67, 1.31) | 0.67 (0.40, 1.13) | 1.33 (1.06, 1.66) |
| Q3 | 1.18 (0.94, 1.47) | 1.15 (0.86, 1.54) | 0.95 (0.81, 1.11) | 1.01 (0.73, 1.41) | 0.71 (0.39, 1.32) | 1.02 (0.90, 1.16) | 1.08 (0.78, 1.50) | 0.80 (0.48, 1.32) | 1.39 (1.09, 1.77) |
| Q4 | 1.10 (0.87, 1.38) | 1.17 (0.86, 1.59) | 1.09 (0.94, 1.28) | 0.87 (0.71, 1.24) | 0.90 (0.49, 1.67) | 0.96 (0.84, 1.10) | 1.36 (0.98, 1.90) | 0.84 (0.50, 1.40) | 1.51 (1.19, 1.92) |
| Q5 | 1.24 (0.97, 1.58) | 1.22 (0.89, 1.68) | 1.13 (0.97, 1.31) | 1.04 (0.72, 1.50) | 1.00 (0.52, 1.90) | 1.04 (0.91, 1.18) | 1.38 (0.98, 1.95) | 0.67 (0.38, 1.18) | 1.65 (1.31, 2.07) |
| P-trend4 | 0.18 | 0.52 | 0.01 | 0.90 | 0.97 | 0.75 | 0.008 | 0.40 | <0.001 |
| Betaine | |||||||||
| Q1 | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| Q2 | 1.07 (0.88, 1.31) | 0.95 (0.73, 1.22) | 1.10 (0.97, 1.26) | 1.04 (0.77, 1.42) | 0.45 (0.25, 0.83) | 0.90 (0.81, 1.01) | 1.01 (0.75, 1.35) | 0.75 (0.46, 1.21) | 0.98 (0.80, 1.21) |
| Q3 | 1.09 (0.89, 1.33) | 0.80 (0.61, 1.05) | 1.16 (1.01, 1.32) | 1.29 (0.96, 1.74) | 0.79 (0.47, 1.32) | 1.01 (0.90, 1.13) | 1.05 (0.78, 1.41) | 0.98 (0.62, 1.54) | 1.15 (0.94, 1.41) |
| Q4 | 1.13 (0.92, 1.39) | 0.81 (0.62, 1.06) | 1.20 (1.05, 1.38) | 1.09 (0.80, 1.48) | 0.78 (0.47, 1.31) | 0.91 (0.81, 1.03) | 1.05 (0.78, 1.41) | 0.78 (0.49, 1.26) | 1.07 (0.87, 1.33) |
| Q5 | 1.07 (0.87, 1.32) | 0.93 (0.71, 1.22) | 1.24 (1.09, 1.42) | 0.89 (0.64, 1.23) | 0.79 (0.46, 1.36) | 1.12 (1.01, 1.25) | 1.36 (1.02, 1.81) | 0.60 (0.36, 1.01) | 1.05 (0.85, 1.29) |
| P-trend4 | 0.60 | 0.44 | <0.001 | 0.41 | 0.91 | 0.03 | 0.01 | 0.10 | 0.60 |
Values are HRs (95% CIs). Intakes were based on the race- and sex-specific Qs. The numbers of deaths from ischemic heart disease were 993 among blacks, 557 among whites, and 2152 among Chinese; those from stroke were 441 among blacks, 131 among whites, and 2919 among Chinese; and those from diabetes were 507 among blacks, 172 among whites, and 897 among Chinese. Q, quintile.
Adjusted for age, educational attainment, annual household income, marital status, smoking status, smoking pack-years, alcohol consumption, physical activity level, obesity status, healthy eating index, Charlson comorbidity index, intake of total energy, and menopausal status in women.
Intake of refined carbohydrate was further adjusted for the Chinese population.
To adjust for multiple comparisons across the 3 racial groups, 3 major causes of cardiometabolic deaths, and different choline-related nutrients, P-trend values < 0.001 (0.05/45) were considered significant.
Similar patterns, but with associations of attenuated magnitudes, were found for all-cause mortality (Table 5). Among blacks, the HR (95% CI) for the highest compared with the lowest quintile was 1.20 (1.12, 1.29) (P-trend < 0.001). Among Chinese, a positive association was suggested, but the P-trend value failed to reach statistical significance after accounting for multiple comparisons (HR: 1.08; 95% CI: 1.00, 1.15 for the highest compared with the lowest quintile; P-trend = 0.04). However, no associations of total choline with all-cause mortality were found among whites.
TABLE 5.
Risk of death from all causes by intakes of choline-related nutrients1
| Blacks (n = 49,858) | Whites (n = 23,766) | Chinese (n = 134,001) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Deaths, n | Median, mg/d | HR (95% CI)2 | Deaths, n | Median, mg/d | HR (95% CI)2 | Deaths, n | Median, mg/d | HR (95% CI)2, 3 | |
| Total choline | |||||||||
| Q1 | 1477 | 263.8 | 1 (ref.) | 794 | 236.9 | 1 (ref.) | 4282 | 185.7 | 1 (ref.) |
| Q2 | 1542 | 310.1 | 1.10 (1.02, 1.18) | 706 | 277.9 | 0.92 (0.83, 1.01) | 3241 | 245.0 | 1.02 (0.97, 1.07) |
| Q3 | 1616 | 348.6 | 1.13 (1.05, 1.21) | 798 | 311.9 | 1.04 (0.94, 1.15) | 2964 | 289.3 | 1.02 (0.96, 1.08) |
| Q4 | 1737 | 397.1 | 1.19 (1.11, 1.27) | 820 | 353.2 | 1.03 (0.93, 1.13) | 2918 | 336.3 | 1.03 (0.97, 1.10) |
| Q5 | 1828 | 531.7 | 1.20 (1.12, 1.29) | 891 | 461.7 | 1.04 (0.94, 1.15) | 3059 | 414.0 | 1.08 (1.00, 1.15) |
| P-trend4 | <0.001 | 0.08 | 0.04 | ||||||
| Phosphatidylcholine | |||||||||
| Q1 | 1544 | 123.3 | 1 (ref.) | 772 | 102.8 | 1 (ref.) | 4279 | 92.9 | 1 (ref.) |
| Q2 | 1509 | 161.4 | 1.04 (0.97, 1.12) | 711 | 134.0 | 0.96 (0.87, 1.07) | 3175 | 130.9 | 0.97 (0.93, 1.02) |
| Q3 | 1611 | 194.1 | 1.10 (1.02, 1.18) | 773 | 161.5 | 1.04 (0.94, 1.15) | 2956 | 161.8 | 1.01 (0.96, 1.07) |
| Q4 | 1675 | 236.2 | 1.13 (1.06, 1.21) | 852 | 197.8 | 1.09 (0.99, 1.20) | 2939 | 194.8 | 1.04 (0.98, 1.10) |
| Q5 | 1861 | 353.7 | 1.20 (1.12, 1.29) | 901 | 301.2 | 1.07 (0.97, 1.18) | 3115 | 246.2 | 1.01 (0.95, 1.07) |
| P-trend4 | <0.001 | 0.02 | 0.37 | ||||||
| Free choline | |||||||||
| Q1 | 1458 | 57.0 | 1 (ref.) | 800 | 54.1 | 1 (ref.) | 3968 | 39.8 | 1 (ref.) |
| Q2 | 1578 | 66.3 | 1.07 (1.00, 1.15) | 842 | 63.9 | 1.02 (0.92, 1.13) | 3041 | 52.3 | 0.99 (0.94, 1.04) |
| Q3 | 1686 | 74.3 | 1.15 (1.07, 1.24) | 793 | 72.0 | 1.00 (0.90, 1.11) | 2876 | 62.8 | 1.01 (0.96, 1.07) |
| Q4 | 1749 | 84.6 | 1.20 (1.12, 1.30) | 797 | 81.4 | 1.00 (0.90, 1.12) | 3048 | 75.6 | 1.01 (0.96, 1.07) |
| Q5 | 1729 | 114.7 | 1.18 (1.09, 1.28) | 777 | 105.4 | 0.95 (0.85, 1.06) | 3531 | 97.8 | 1.06 (1.00, 1.12) |
| P-trend4 | <0.001 | 0.48 | 0.02 | ||||||
| Glycerophosphocholine | |||||||||
| Q1 | 1593 | 34.5 | 1 (ref.) | 840 | 31.1 | 1 (ref.) | 4835 | 25.0 | 1 (ref.) |
| Q2 | 1548 | 40.6 | 0.99 (0.92, 1.06) | 801 | 38.6 | 1.00 (0.91, 1.10) | 3471 | 32.6 | 0.94 (0.90, 0.99) |
| Q3 | 1638 | 46.1 | 1.00 (0.93, 1.08) | 819 | 45.1 | 1.01 (0.92, 1.12) | 3009 | 39.2 | 0.91 (0.86, 0.96) |
| Q4 | 1689 | 53.7 | 1.03 (0.96, 1.10) | 791 | 53.7 | 0.97 (0.87, 1.07) | 2676 | 46.3 | 0.86 (0.81, 0.91) |
| Q5 | 1732 | 75.9 | 1.01 (0.94, 1.09) | 758 | 77.0 | 0.96 (0.87, 1.07) | 2473 | 58.9 | 0.87 (0.81, 0.92) |
| P-trend4 | 0.40 | 0.43 | <0.001 | ||||||
| Sphingomyelin | |||||||||
| Q1 | 1551 | 12.8 | 1 (ref.) | 811 | 12.1 | 1 (ref.) | 4755 | 5.5 | 1 (ref.) |
| Q2 | 1608 | 17.6 | 1.08 (1.01, 1.16) | 756 | 16.3 | 1.01 (0.92, 1.12) | 3375 | 8.3 | 0.96 (0.91, 1.00) |
| Q3 | 1628 | 21.3 | 1.10 (1.03, 1.18) | 791 | 19.6 | 1.06 (0.96, 1.17) | 2977 | 10.4 | 0.94 (0.89, 0.99) |
| Q4 | 1681 | 26.0 | 1.13 (1.06, 1.21) | 813 | 23.6 | 1.09 (0.98, 1.20) | 2753 | 12.8 | 0.92 (0.87, 0.97) |
| Q5 | 1732 | 38.1 | 1.15 (1.07, 1.24) | 838 | 33.8 | 1.05 (0.95, 1.16) | 2604 | 16.8 | 0.92 (0.86, 0.98) |
| P-trend4 | <0.001 | 0.18 | 0.01 | ||||||
| Phosphocholine | |||||||||
| Q1 | 1548 | 7.4 | 1 (ref.) | 886 | 7.3 | 1 (ref.) | 4063 | 4.7 | 1 (ref.) |
| Q2 | 1577 | 9.8 | 1.05 (0.98, 1.13) | 804 | 9.6 | 0.99 (0.90, 1.09) | 3097 | 7.3 | 1.01 (0.97, 1.07) |
| Q3 | 1691 | 11.6 | 1.13 (1.05, 1.22) | 816 | 11.3 | 1.01 (0.91, 1.12) | 2850 | 9.5 | 1.01 (0.95, 1.06) |
| Q4 | 1692 | 13.9 | 1.14 (1.05, 1.23) | 778 | 13.4 | 0.99 (0.88, 1.10) | 2875 | 12.0 | 1.00 (0.94, 1.06) |
| Q5 | 1692 | 17.8 | 1.16 (1.06, 1.26) | 725 | 17.5 | 0.96 (0.86, 1.09) | 3579 | 16.5 | 1.05 (1.00, 1.11) |
| P-trend4 | <0.001 | 0.62 | 0.08 | ||||||
| Betaine | |||||||||
| Q1 | 1694 | 104.2 | 1 (ref.) | 893 | 86.7 | 1 (ref.) | 4059 | 27.7 | 1 (ref.) |
| Q2 | 1634 | 137.1 | 1.03 (0.96, 1.10) | 790 | 111.6 | 0.94 (0.86, 1.04) | 3230 | 41.3 | 0.98 (0.94, 1.03) |
| Q3 | 1599 | 165.2 | 1.01 (0.95, 1.09) | 785 | 133.6 | 0.95 (0.86, 1.05) | 2997 | 52.2 | 0.99 (0.94, 1.04) |
| Q4 | 1656 | 205.9 | 1.03 (0.96, 1.11) | 734 | 166.3 | 0.86 (0.78, 0.95) | 2812 | 68.3 | 0.99 (0.94, 1.04) |
| Q5 | 1617 | 287.0 | 0.99 (0.92, 1.06) | 807 | 240.6 | 0.95 (0.86, 1.05) | 3366 | 114.0 | 1.07 (1.02, 1.13) |
| P-trend4 | 0.75 | 0.19 | <0.001 | ||||||
Intakes were based on the race- and sex-specific Qs. Q, quintile.
Adjusted for age, educational attainment, annual household income, marital status, smoking status, smoking pack-years, alcohol consumption, physical activity level, obesity status, healthy eating index, Charlson comorbidity index, intake of total energy, and menopausal status in women.
Intake of refined carbohydrate was further adjusted for the Chinese population.
To adjust for multiple comparisons across the 3 racial groups and different choline-related nutrients, P-trend values < 0.003 (0.05/15) were considered significant.
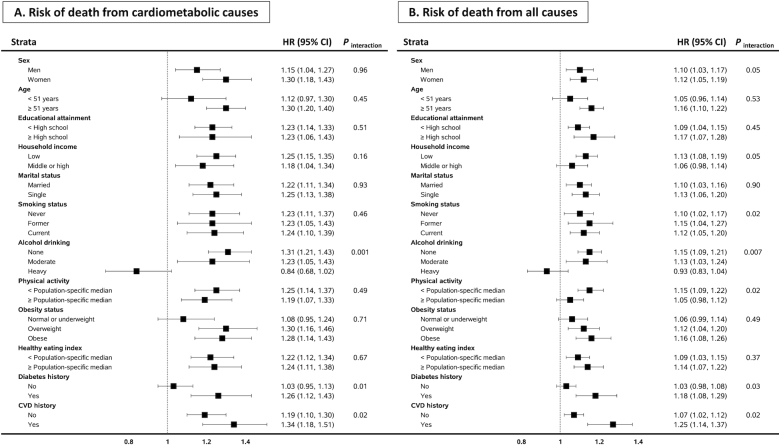
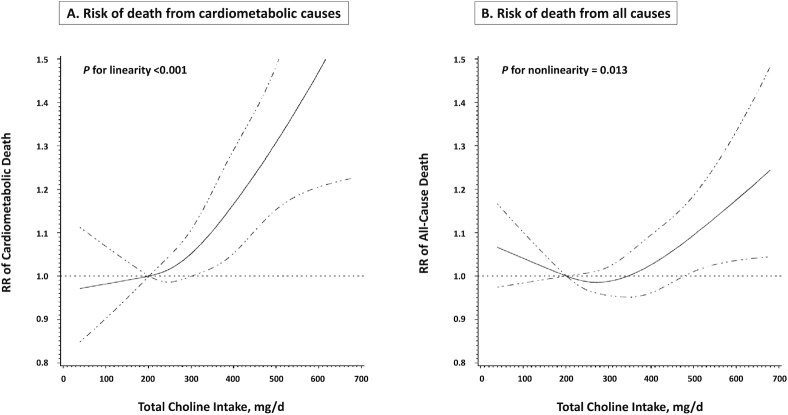
We further conducted stratified analyses by sociodemographic factors, lifestyle, and comorbidity status (Figure 1); the analyses used a pooled data set of the 3 racial groups, given that there was no significant heterogeneity across races in the total choline–cardiometabolic mortality association, and the pooled analysis increased the statistical power. After accounting for multiple comparisons, we found that the associations of total choline with cardiometabolic and all-cause mortality were only significant if the participants were not heavy drinkers (P-interaction = 0.001 and 0.007, respectively). Furthermore, the choline–mortality associations were likely to be stronger among individuals with a history of diabetes or CVD, but P-interaction did not meet the multiple comparison cutoff for significance. Potential nonlinear associations were assessed via restricted cubic splines among total study populations: results suggested a linear association of total choline intake with cardiometabolic mortality and a nonlinear association with all-cause mortality (Figure 2).
FIGURE 1.
Risk of cardiometabolic and all-cause mortality by total choline intake: stratified analyses. HRs (95% CIs) represent the highest compared with the lowest quintile (race- and sex-specific) of total choline intake. Estimates were adjusted for age, educational attainment, annual household income, marital status, smoking status, smoking pack-years, alcohol consumption, physical activity level, obesity status, healthy eating index, Charlson comorbidity index, intake of total energy, and menopausal status in women; and were stratified by cohort. Intake of refined carbohydrate was further adjusted for the Chinese population. Because the numbers of comparisons were at least doubled in stratified analyses, P values for significance for interactions were taken to be half of the corrected P values across the 3 racial groups and different choline-related nutrients, thus all <0.0015. CVD, cardiovascular disease.
FIGURE 2.
Dose–response relation of total choline intake with cardiometabolic and all-cause mortality. HRs (solid line) and 95% CIs (dashed line) were adjusted for race, sex, age, educational attainment, annual household income, marital status, smoking status, smoking pack-years, alcohol consumption, physical activity level, obesity status, healthy eating index, Charlson comorbidity index, intake of total energy, and menopausal status in women; and were stratified by cohort. Intake of refined carbohydrate was further adjusted for the Chinese population. To minimize potential effects of extreme values, participants with the top 1% of total choline intake were excluded from the analysis. The 10th percentile was set as the reference, and 4 knot positions were fitted at the 5th, 25th, 75th, and 95th percentiles.
Results from the sensitivity analyses indicated that overall patterns of the choline–mortality associations were similar even after excluding further follow-up data and prevalent cases of CVD or after applying different adjustment methods and competing risk analyses (Supplemental Tables 3–6).
Discussion
In this prospective analysis, including >200,000 men and women from racially diverse populations, we found that high intakes of total choline and certain choline-containing compounds were associated with increased risk of cardiometabolic mortality, especially diabetes and IHD mortality. Overall, the choline–cardiometabolic mortality associations appeared to be more evident among blacks and Chinese than among whites. In addition, the choline–cardiometabolic mortality association was modified by alcohol drinking status and seemed more pronounced among individuals with a history of diabetes or CVD.
Choline is an essential nutrient for normal functions of cell membranes and muscle, cholinergic neurotransmission, lipid transport, and one-carbon metabolism (40). Choline deficiency can lead to fatty liver disease, hemorrhagic kidney necrosis, muscle damage, and organ dysfunction (40–42). Despite its vital role in the human body, choline can also serve as a dietary precursor of TMAO via the gut microbial metabolism (2), a potential risk factor for CVD and related deaths. To date, epidemiological findings regarding choline intake and cardiometabolic disease and/or mortality have been mixed. In line with our findings, the Nurses’ Health Study and Health Professionals Follow-up Study observed significant positive associations of dietary phosphatidylcholine with CVD and all-cause mortality (13)—the corresponding HRs (95% CIs) were 1.26 (1.15, 1.39) and 1.11 (1.06, 1.17), respectively. However, the Atherosclerosis Risk in Communities Study and the European Prospective Investigation into Cancer and Nutrition study, composed of mostly white populations, found no association of dietary choline intake with incident CVD (10, 19). On the contrary, the Jackson Heart Study, which consisted of 3924 blacks, reported an inverse association of choline intake with incident stroke and a positive association of betaine intake with IHD risk (14). Relevant evidence from Asian populations has been very limited: a prospective study conducted in Japan found no association between choline intake and mortality (18). Overall, a recent meta-analysis including these prospective studies, comprising a total of 184,010 participants with 18,076 incident CVD events and 5343 CVD deaths, suggested no significant associations of dietary choline or betaine intake with CVD risk or mortality (43).
In the present study, we found significant positive associations between choline intake and certain cardiometabolic mortality (i.e., IHD and diabetes) among blacks, whites, and Chinese, after adjusting for many disease risk factors and overall diet quality. Although there was no significant heterogeneity across races in the association, the magnitude and pattern of the associations between choline-related nutrients and cardiometabolic mortality appeared different across races. The most consistent and pronounced associations were observed among blacks for total choline and choline-containing compounds with both cardiometabolic and all-cause mortality. The associations were generally weaker among whites, with the primary observed association between phosphatidylcholine/sphingomyelin and IHD mortality. Among Chinese, the association of total choline with diabetes mortality was the strongest, whereas betaine intake also showed significant positive associations with cardiometabolic and all-cause mortality. Distinct from other choline-related nutrients, sphingomyelin and glycerophosphocholine were inversely associated with stroke and all-cause mortality among Chinese, but not among blacks or whites. Epidemiologic and/or biological evidence to date on the role of choline-containing compounds in human cardiometabolic health remains limited. One possible explanation for the variation we observed is that the choline–mortality association might be modified by different habitual diets, food sources of choline, and comorbidity status, which can modulate the gut microbial ability for producing TMAO (20, 21). As shown in Table 1, although red meat and eggs are the primary dietary sources of choline, other major food sources vary across racial groups: poultry and processed meat in blacks, dairy and poultry in whites, soy foods and fish in Chinese. We also found that comorbidity status, e.g., the prevalence of diabetes, varied substantially by race. In addition, genetic variants related to choline metabolism and function may alter the dietary requirement for choline across populations. Previous studies indicated that genetic variants in choline kinase-α, choline dehydrogenase, phosphatidylethanolamine N-methyltransferase, solute carrier 44A1, and flavin monooxygenase isoform 3 genes were associated with differences in choline dynamics (22, 23). Allele frequencies of those genes significantly differ between European Americans, African descendants, Asian Americans, and Mexican Americans (22), suggesting possible population-specific associations. Future studies on the role of gene–diet–microbiome interactions in choline metabolism and CVD pathogenesis will help elucidate the underlying mechanisms.
Our findings suggest that a history of diabetes or CVD strengthened the choline–mortality association, consistent with results from previous studies (1, 5, 13). Emerging evidence has indicated that higher circulating concentrations of TMAO and its precursors such as choline and betaine were associated with increased risks of CVD morbidity and mortality, especially among individuals with existing cardiometabolic conditions (2, 4, 6, 7). Of note, patients with diabetes, CVD, or chronic kidney disease have shown a higher TMAO concentration than general populations (44–47). It is possible that cardiometabolic diseases may enhance TMAO concentrations, which may be further increased with a high-choline diet. Interestingly, we observed a trend of inverse associations of choline intake with both cardiometabolic and all-cause mortality among heavy drinkers (>2 drinks/d for men and >1 drink/d for women). Considering that choline is an important factor maintaining liver function and the vast majority of choline metabolism occurs in the liver (48), a high choline intake may help recover liver damage from excessive alcohol drinking and, in turn, lead to a reduced risk of death among heavy drinkers. We had no data on liver function biomarkers in our cohorts, but besides cardiometabolic and all-cause mortality, we also observed a potential inverse association of total choline intake with deaths from liver diseases among heavy drinkers (data not shown). Future studies investigating the health effects of choline-related nutrients need to take into consideration participants’ cardiometabolic disease status and alcohol drinking status.
This large, population-based, prospective investigation including diverse populations and detailed information on diet, lifestyle, and medical history allowed us to explore the choline–mortality associations by race and by a range of cardiometabolic risk factors, with comprehensive adjustment for potential confounders. Despite the methodological strengths, we note several limitations of our current study. First, although we used validated FFQs showing high accuracy and reproducibility for major choline/betaine-containing foods (e.g., red meat, eggs, fish, and wheat products) among the southeastern US populations (27) and urban Chinese populations (49, 50), the validity of choline-related nutrient intakes has not been directly assessed in our study populations. Measurement errors in diet assessment might affect the results, but the errors should be nondifferential in our prospective design. Second, owing to a single dietary assessment at baseline, we could not consider dietary changes over time, which might attenuate the overall associations (51). Third, despite our comprehensive adjustments for potential confounders, we cannot rule out residual confounding or unmeasured confounders.
In summary, high choline intake is associated with increased risk of cardiometabolic mortality, especially for diabetes and IHD mortality and among blacks, Chinese, non-/moderate-drinkers, and individuals with a history of diabetes or CVD. Replacing major food sources of choline (i.e., red meat and eggs) with plant-based foods (e.g., vegetables, nuts, and legumes) may reduce total choline intake. Whether the observed choline–cardiometabolic mortality association may be mediated by gut microbial production of TMAO warrants future studies.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—DY, LPL, and X-OS: designed the study; LPL, X-OS, WJB, Y-BX, MDS, HL, Y-TG, and WZ: provided essential reagents or essential materials; DY and JJY: performed statistical analysis and drafted the manuscript; DY: had primary responsibility for the final content; and all authors: read and approved the final manuscript. The authors report no conflicts of interest.
Notes
Supported by Vanderbilt University Medical Center Faculty Research Scholars Program and by National Heart, Lung, and Blood Institute grant R21 HL140375. The Southern Community Cohort Study (SCCS) is supported by National Cancer Institute (NCI) grant U01 CA202979. SCCS data collection was performed by the Survey and Biospecimen Shared Resource which is supported in part by Vanderbilt-Ingram Cancer Center grant P30 CA68485. The Shanghai Women's Health Study is supported by NCI grant UM1 CA182910 (to WZ) and the Shanghai Men's Health Study is supported by NCI grant UM1 CA173640 (to X-OS).
Supplemental Tables 1–6 and Supplemental Figure 1 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Data described in the article, code book, and analytic code will be made available upon request pending application and approval.
Abbreviations used: CNY, Chinese yuan; CVD, cardiovascular disease; IHD, ischemic heart disease; SCCS, Southern Community Cohort Study; SMHS, Shanghai Men's Health Study; SWHS, Shanghai Women's Health Study; TMAO, trimethylamine-N-oxide; USD, US dollar.
References
- 1. Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung Y-M et al.. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zeisel SH, Warrier M. Trimethylamine N-oxide, the microbiome, and heart and kidney disease. Annu Rev Nutr. 2017;37:157–81. [DOI] [PubMed] [Google Scholar]
- 3. Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L et al.. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Heianza Y, Ma W, Manson JE, Rexrode KM, Qi L. Gut microbiota metabolites and risk of major adverse cardiovascular disease events and death: a systematic review and meta-analysis of prospective studies. J Am Heart Assoc. 2017;6(7):e004947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tang WHW, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368:1575–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Svingen GFT, Schartum-Hansen H, Pedersen ER, Ueland PM, Tell GS, Mellgren G, Njølstad PR, Seifert R, Strand E, Karlsson T et al.. Prospective associations of systemic and urinary choline metabolites with incident type 2 diabetes. Clin Chem. 2016;62:755–65. [DOI] [PubMed] [Google Scholar]
- 7. Shan Z, Sun T, Huang H, Chen S, Chen L, Luo C, Yang W, Yang X, Yao P, Cheng J et al.. Association between microbiota-dependent metabolite trimethylamine-N-oxide and type 2 diabetes. Am J Clin Nutr. 2017;106:888–94. [DOI] [PubMed] [Google Scholar]
- 8. Cho E, Zeisel SH, Jacques P, Selhub J, Dougherty L, Colditz GA, Willett WC. Dietary choline and betaine assessed by food-frequency questionnaire in relation to plasma total homocysteine concentration in the Framingham Offspring Study. Am J Clin Nutr. 2006;83:905–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Patterson KY, Bhagwat SA, Williams JR, Howe JC, Holden JM. USDA database for the choline content of common foods. Release two. [Internet] Beltsville, MD: Agricultural Research Service, USDA; January2008; [cited 12 July, 2018]. Available from: https://www.ars.usda.gov/ARSUserFiles/80400525/Data/Choline/Choln02.pdf. [Google Scholar]
- 10. Bidulescu A, Chambless LE, Siega-Riz AM, Zeisel SH, Heiss G. Usual choline and betaine dietary intake and incident coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) study. BMC Cardiovasc Disord. 2007;7:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yonemori KM, Lim U, Koga KR, Wilkens LR, Au D, Boushey CJ, Le Marchand L, Kolonel LN, Murphy SP. Dietary choline and betaine intakes vary in an adult multiethnic population. J Nutr. 2013;143:894–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and Choline. Dietary Reference Intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. [Internet] Washington (DC): National Academies Press (US); 1998; [cited 14 November, 2018]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK114310/. [PubMed] [Google Scholar]
- 13. Zheng Y, Li Y, Rimm EB, Hu FB, Albert CM, Rexrode KM, Manson JE, Qi L. Dietary phosphatidylcholine and risk of all-cause and cardiovascular-specific mortality among US women and men. Am J Clin Nutr. 2016;104:173–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Millard HR, Musani SK, Dibaba DT, Talegawkar SA, Taylor HA, Tucker KL, Bidulescu A. Dietary choline and betaine; associations with subclinical markers of cardiovascular disease risk and incidence of CVD, coronary heart disease and stroke: the Jackson Heart Study. Eur J Nutr. 2018;57:51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xu X, Gammon MD, Zeisel SH, Bradshaw PT, Wetmur JG, Teitelbaum SL, Neugut AI, Santella RM, Chen J. High intakes of choline and betaine reduce breast cancer mortality in a population-based study. FASEB J. 2009;23:4022–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chiuve SE, Giovannucci EL, Hankinson SE, Zeisel SH, Dougherty LW, Willett WC, Rimm EB. The association between betaine and choline intakes and the plasma concentrations of homocysteine in women. Am J Clin Nutr. 2007;86:1073–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bertoia ML, Pai JK, Cooke JP, Joosten MM, Mittleman MA, Rimm EB, Mukamal KJ. Plasma homocysteine, dietary B vitamins, betaine, and choline and risk of peripheral artery disease. Atherosclerosis. 2014;235:94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nagata C, Wada K, Tamura T, Konishi K, Kawachi T, Tsuji M, Nakamura K. Choline and betaine intakes are not associated with cardiovascular disease mortality risk in Japanese men and women. J Nutr. 2015;145:1787–92. [DOI] [PubMed] [Google Scholar]
- 19. Dalmeijer GW, Olthof MR, Verhoef P, Bots ML, van der Schouw YT. Prospective study on dietary intakes of folate, betaine, and choline and cardiovascular disease risk in women. Eur J Clin Nutr. 2008;62:386–94. [DOI] [PubMed] [Google Scholar]
- 20. Aron-Wisnewsky J, Clément K. The gut microbiome, diet, and links to cardiometabolic and chronic disorders. Nat Rev Nephrol. 2016;12:169–81. [DOI] [PubMed] [Google Scholar]
- 21. Doré J, Blottière H. The influence of diet on the gut microbiota and its consequences for health. Curr Opin Biotechnol. 2015;32:195–9. [DOI] [PubMed] [Google Scholar]
- 22. da Costa K-A, Corbin KD, Niculescu MD, Galanko JA, Zeisel SH. Identification of new genetic polymorphisms that alter the dietary requirement for choline and vary in their distribution across ethnic and racial groups. FASEB J. 2014;28:2970–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ganz AB, Cohen VV, Swersky CC, Stover J, Vitiello GA, Lovesky J, Chuang JC, Shields K, Fomin VG, Lopez YS et al.. Genetic variation in choline-metabolizing enzymes alters choline metabolism in young women consuming choline intakes meeting current recommendations. Int J Mol Sci. 2017;18(2):252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Signorello LB, Hargreaves MK, Blot WJ. The Southern Community Cohort Study: investigating health disparities. J Health Care Poor Underserved. 2010;21:26–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shu X-O, Li H, Yang G, Gao J, Cai H, Takata Y, Zheng W, Xiang Y-B. Cohort profile: the Shanghai Men's Health Study. Int J Epidemiol. 2015;44:810–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zheng W, Chow W-H, Yang G, Jin F, Rothman N, Blair A, Li H-L, Wen W, Ji B-T, Li Q et al.. The Shanghai Women's Health Study: rationale, study design, and baseline characteristics. Am J Epidemiol. 2005;162:1123–31. [DOI] [PubMed] [Google Scholar]
- 27. Buchowski MS, Schlundt DG, Hargreaves MK, Hankin JH, Signorello LB, Blot WJ. Development of a culturally sensitive food frequency questionnaire for use in the Southern Community Cohort Study. Cell Mol Biol (Noisy-le-grand). 2003;49:1295–304. [PubMed] [Google Scholar]
- 28. Schlundt DG, Buchowski MS, Hargreaves MK, Hankin JH, Signorello LB, Blot WJ. Separate estimates of portion size were not essential for energy and nutrient estimation: results from the Southern Community Cohort food-frequency questionnaire pilot study. Public Health Nutr. 2007;10:245–51. [DOI] [PubMed] [Google Scholar]
- 29. Yu D, Sonderman J, Buchowski MS, McLaughlin JK, Shu X-O, Steinwandel M, Signorello LB, Zhang X, Hargreaves MK, Blot WJ et al.. Healthy eating and risks of total and cause-specific death among low-income populations of African-Americans and other adults in the southeastern United States: a prospective cohort study. PLoS Med. 2015;12:e1001830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Signorello LB, Munro HM, Buchowski MS, Schlundt DG, Cohen SS, Hargreaves MK, Blot WJ. Estimating nutrient intake from a food frequency questionnaire: incorporating the elements of race and geographic region. Am J Epidemiol. 2009;170:104–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yang Y, Wang G, Pan X,editors. China food composition tables. Beijing: Beijing Medical University Press; 2002. [Google Scholar]
- 32. US Department of Agriculture, Agricultural Research Service. USDA National Nutrient Database for standard reference, release 25. [Internet] Beltsville, MD: Methods and Application of Food Composition Laboratory, Agricultural Research Service, USDA; 2012; [cited 12 July, 2018]. Available from: http://www.ars.usda.gov/nea/bhnrc/mafcl. [Google Scholar]
- 33. Shrubsole MJ, Shu XO, Li H-L, Cai H, Yang G, Gao Y-T, Gao J, Zheng W. Dietary B vitamin and methionine intakes and breast cancer risk among Chinese women. Am J Epidemiol. 2011;173:1171–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yu D, Shu X-O, Xiang Y-B, Li H, Yang G, Gao Y-T, Zheng W, Zhang X. Higher dietary choline intake is associated with lower risk of nonalcoholic fatty liver in normal-weight Chinese women. J Nutr. 2014;144:2034–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65:1220S–8S.; discussion 1229S–31S. [DOI] [PubMed] [Google Scholar]
- 36. Yu D, Zhang X, Xiang Y-B, Yang G, Li H, Gao Y-T, Zheng W, Shu X-O. Adherence to dietary guidelines and mortality: a report from prospective cohort studies of 134,000 Chinese adults in urban Shanghai. Am J Clin Nutr. 2014;100:693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. [DOI] [PubMed] [Google Scholar]
- 38. Yu D, Shu X-O, Li H, Xiang Y-B, Yang G, Gao Y-T, Zheng W, Zhang X. Dietary carbohydrates, refined grains, glycemic load, and risk of coronary heart disease in Chinese adults. Am J Epidemiol. 2013;178:1542–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yu D, Zhang X, Shu X-O, Cai H, Li H, Ding D, Hong Z, Xiang Y-B, Gao Y-T, Zheng W et al.. Dietary glycemic index, glycemic load, and refined carbohydrates are associated with risk of stroke: a prospective cohort study in urban Chinese women. Am J Clin Nutr. 2016;104:1345–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zeisel SH. Choline: critical role during fetal development and dietary requirements in adults. Annu Rev Nutr. 2006;26:229–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fischer LM, daCosta KA, Kwock L, Stewart PW, Lu T-S, Stabler SP, Allen RH, Zeisel SH. Sex and menopausal status influence human dietary requirements for the nutrient choline. Am J Clin Nutr. 2007;85:1275–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zeisel SH, da Costa K-A. Choline: an essential nutrient for public health. Nutr Rev. 2009;67:615–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Meyer KA, Shea JW. Dietary choline and betaine and risk of CVD: a systematic review and meta-analysis of prospective studies. Nutrients. 2017;9(7):711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dambrova M, Latkovskis G, Kuka J, Strele I, Konrade I, Grinberga S, Hartmane D, Pugovics O, Erglis A, Liepinsh E. Diabetes is associated with higher trimethylamine N-oxide plasma levels. Exp Clin Endocrinol Diabetes. 2016;124:251–6. [DOI] [PubMed] [Google Scholar]
- 45. Mente A, Chalcraft K, Ak H, Davis AD, Lonn E, Miller R, Potter MA, Yusuf S, Anand SS, McQueen MJ. The relationship between trimethylamine-N-oxide and prevalent cardiovascular disease in a multiethnic population living in Canada. Can J Cardiol. 2015;31:1189–94. [DOI] [PubMed] [Google Scholar]
- 46. Tang WHW, Wang Z, Li XS, Fan Y, Li DS, Wu Y, Hazen SL. Increased trimethylamine N-oxide portends high mortality risk independent of glycemic control in patients with type 2 diabetes mellitus. Clin Chem. 2017;63:297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kim RB, Morse BL, Djurdjev O, Tang M, Muirhead N, Barrett B, Holmes DT, Madore F, Clase CM, Rigatto C et al.. Advanced chronic kidney disease populations have elevated trimethylamine N-oxide levels associated with increased cardiovascular events. Kidney Int. 2016;89:1144–52. [DOI] [PubMed] [Google Scholar]
- 48. Mehedint MG, Zeisel SH. Choline's role in maintaining liver function: new evidence for epigenetic mechanisms. Curr Opin Clin Nutr Metab Care. 2013;16:339–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Villegas R, Yang G, Liu D, Xiang Y-B, Cai H, Zheng W, Shu XO. Validity and reproducibility of the food-frequency questionnaire used in the Shanghai Men's Health Study. Br J Nutr. 2007;97:993–1000. [DOI] [PubMed] [Google Scholar]
- 50. Shu XO, Yang G, Jin F, Liu D, Kushi L, Wen W, Gao Y-T, Zheng W. Validity and reproducibility of the food frequency questionnaire used in the Shanghai Women's Health Study. Eur J Clin Nutr. 2004;58:17–23. [DOI] [PubMed] [Google Scholar]
- 51. Hu FB, Satija A, Rimm EB, Spiegelman D, Sampson L, Rosner B, Camargo CA, Stampfer M, Willett WC. Diet assessment methods in the Nurses’ Health Studies and contribution to evidence-based nutritional policies and guidelines. Am J Public Health. 2016;106:1567–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.