ABSTRACT
Background
Short chain fatty acids (SCFAs; e.g., acetate, propionate, and butyrate) are produced by microbial fermentation of fiber in the colon. Evidence is lacking on how high-fiber diets that differ in macronutrient composition affect circulating SCFAs.
Objectives
We aimed to compare the effects of 3 high-fiber isocaloric diets differing in %kcal of carbohydrate, protein, or unsaturated fat on circulating SCFAs. Based on previous literature, we hypothesized that serum acetate, the main SCFA in circulation, increases on all high-fiber diets, but differently by macronutrient composition of the diet.
Methods
OmniHeart is a randomized crossover trial of 164 men and women (≥30 y old); 163 participants with SCFA data were included in this analysis. We provided participants 3 isocaloric high-fiber (∼30 g/2100 kcal) diets, each for 6 wk, in random order: a carbohydrate-rich (Carb) diet, a protein-rich (Prot) diet (protein predominantly from plant sources), and an unsaturated fat–rich (Unsat) diet. We used LC-MS to quantify SCFA concentrations in fasting serum, collected at baseline and the end of each diet period. We fitted linear regression models with generalized estimating equations to examine change in ln-transformed SCFAs from baseline to the end of each diet; differences between diets; and associations of changes in SCFAs with cardiometabolic parameters.
Results
From baseline, serum acetate concentrations were increased by the Prot (β: 0.24; 95% CI: 0.12, 0.35), Unsat (β: 0.21; 95% CI: 0.10, 0.33), and Carb (β: 0.12; 95% CI: 0.01, 0.24) diets; between diets, only Prot compared with Carb was significant (P = 0.02). Propionate was decreased by the Carb (β: −0.10; 95% CI: −0.16, −0.03) and Unsat (β: −0.10; 95% CI: −0.16, −0.04) diets, not the Prot diet; between diet comparisons of Carb vs. Prot (P = 0.006) and Unsat vs. Prot (P = 0.002) were significant. The Prot diet increased butyrate (β: 0.05; 95% CI: 0.00, 0.09) compared with baseline, but not compared with the other diets. Increases in acetate were associated with decreases in insulin and glucose; increases in propionate with increases in leptin, LDL cholesterol, and blood pressure; and increases in butyrate with increases in insulin and glucose, and decreases in HDL cholesterol and ghrelin (Ps < 0.05).
Conclusions
Macronutrient composition of high-fiber diets affects circulating SCFAs, which are associated with measures of appetite and cardiometabolic health. This trial was registered at clinicaltrials.gov as NCT00051350.
Keywords: short-chain fatty acids, butyrate, acetate, propionate, microbiome, diet, fiber, macronutrient, protein, unsaturated fat
Introduction
There is a growing appreciation of the wide range of physiologic functions exerted by SCFAs (e.g., acetate, propionate, and butyrate). In the gut, SCFAs have long been recognized as an integral energy source for colonic epithelial cells and as signaling molecules, and there is evidence they may protect against colon cancer (1, 2). More recently, there has been increasing interest in SCFAs’ beneficial activity in the systemic circulation, participating in glucose and lipid metabolism (3, 4), blood pressure regulation (5, 6), and appetite control (7).
The majority of research on dietary determinants of SCFAs has focused on fiber, but other diet factors, such as macronutrient composition, might also affect SCFA concentrations. SCFAs are predominantly produced by microbial fermentation of indigestible carbohydrates, such as fiber, in the colon. A meta-analysis of trials found that dietary fiber increases fecal butyrate, more so than acetate and propionate (8). Yet, none of these trials examined SCFAs measured in the systemic circulation, which does not necessarily correlate with fecal SCFA concentrations (9, 10). This is important in light of recent literature suggesting circulating SCFAs, rather than fecal SCFAs, may better reflect the cardiometabolic protective properties of SCFAs (11,12). In addition, it is not known how substitution of protein or unsaturated fat for carbohydrate, independent of fiber and calories, affects SCFA concentrations in the systemic circulation.
Using data from the Optimal Macronutrient Intake Trial to Prevent Heart Disease (OmniHeart; NCT00051350), we examined the effects of 3 high-fiber diets that were equivalent in calories but varied in macronutrients (enriched with either carbohydrate, protein, or unsaturated fat) on circulating SCFAs in adults. We also evaluated how changes in circulating SCFAs were associated with measures of cardiometabolic health and appetite. We hypothesized that all high-fiber diets increase acetate, the main SCFA in circulation (10), and that there would be differences between diets due to their macronutrient composition.
Methods
The current study leverages biospecimens and data collected from the OmniHeart study. The rationale and the primary results of OmniHeart have been published previously (13). In brief, the OmniHeart trial compared the effects of macronutrients on blood pressure and lipids using a randomized, 3-period crossover design. Diets chosen for OmniHeart were derived from the successful Dietary Approaches to Stop Hypertension diet with varied percentages of calories from either carbohydrate [carbohydrate-rich diet (Carb diet)], mostly plant-based protein [protein-rich diet (Prot diet)], or unsaturated fats [unsaturated fat–rich diet (Unsat diet)]. We previously published a detailed description of the 3 diets (14). The primary outcomes for the original OmniHeart trial—blood pressure and lipids—have already been published (13).
Participants
Participants recruited from Baltimore, MD, and Boston, MA, were adult men and women aged ≥30 y with systolic blood pressure (SBP) 120–159 mm Hg, diastolic blood pressure (DBP) <100 mm Hg, fasting LDL cholesterol <220 mg/dL, and triglycerides <750 mg/dL. The recruitment goal of the OmniHeart trial was to recruit 50% black and 50% female participants. Eligibility was determined during a screening visit. Fasting blood samples taken at the screening visit were allowed to clot at room temperature for 15 min. and then centrifuged for 15 min. at 1500 RPM in a serum separator tube at 4°C to obtain serum, which was subsequently divided into aliquots and stored at −70°C. Participants then underwent a 6-d run-in period that included provision of meals on the menu of each of the 3 diet interventions. Participants who were not able to adhere to the protocol during the run-in period were excluded. More details on inclusion and exclusion criteria can be found in a previous publication (13) and in the participant flowchart (Supplemental Figure 1).
Controlled feeding
We randomly assigned participants to 1 of 6 sequences of the 3 diets (Carb, Prot, Unsat). The diets comprised foods and beverages commonly available in the US marketplace. We determined the initial calorie content of the meals individually, based on participant body size, sex, and physical activity level. This was a feeding study in which all diets were provided to the participants. By design, the targeted fiber amounts were set to be the same across all 3 diets. We assessed the menus using the Food Processor® software (version 7.9, ESHA Research, Salem, OR) and also performed chemical (nutrient) analyses of the composited 2100-kcal menus to make sure the actual nutrient (including fiber) amounts were consistent with our targets.
During the trial we monitored body weight daily, and we adjusted calorie content to maintain initial body weight. We provided all meals, snacks, and beverages, except for discretionary calorie-free beverages, to the participants. In addition, we asked participants to maintain their usual intake of alcohol, not exceeding 2 drinks/d. We instructed participants to eat only foods provided and to maintain their usual physical activity levels. We monitored adherence through daily diet diaries and weekday visits to study centers. At the end of weeks 4 and 6 of each diet period, we drew fasting blood samples (8–12 h after a meal), centrifuged them to obtain serum, which was divided into aliquots and stored at −70°C until further laboratory analysis, according to the same protocol used for baseline samples. We then allowed participants to eat their usual free-living diet for ≥2 wk before beginning the next diet period. The controlled feeding interventions took place between April 2003 and June 2005.
Laboratory measurement
We analyzed stored fasting serum from 163 participants who successfully completed ≥2 of the 3 diet periods. Participants provided 4 samples for analysis: 1 at baseline and 1 after each of the three 6-wk diet intervention periods. Fasting blood samples were collected in tubes containing EDTA. We had samples pulled from freezers at BioLincc and shipped them to Metabolon on dry ice. All laboratory staff members were blinded to the sequences of the diet periods.
Measurement of SCFAs using LC-MS
Samples were spiked with a solution of 8 stable labelled internal standards and subjected to protein precipitation. After centrifugation (2500 RPM for 5 min. at room temperature), an aliquot of the supernatant was derivatized. The reaction mixture was analyzed by LC-tandem MS on an Agilent 1290/AB Sciex 5500 system. The peak areas of the respective analyte product ions were measured against the peak areas of the corresponding internal standard product ions. Measurement was performed using a weighted least-squares regression analysis generated from fortified calibration standards prepared immediately before each run. SCFA data below or above the limit of measurement were extrapolated beyond the lower or upper limit of measurement.
Measurement of cardiometabolic and appetite factors
We quantified concentrations of insulin, glucose, LDL cholesterol, and HDL cholesterol using fasting blood samples collected at baseline and at 4 and 6 wk of each feeding period, as previously described (13,15). We measured glucose using the enzymatic hexokinase kit from Roche on the Hitachi 917 and insulin using microparticle enzyme immunoassay technology on the Abbott IMx analyzer. We used enzymatic assays to measure HDL cholesterol concentrations, and we estimated LDL cholesterol concentrations by the Friedewald equation for specimens with a triglyceride concentration <400 mg/dL (16). We analyzed plasma for leptin (Linco Research, Inc.) and total ghrelin (Linco) (17). We measured SBP and DBP with trained staff according to standard protocols, as previously reported (13, 15). To enhance precision, we averaged the values taken at 4 and 6 wk to produce 1 intervention value for each diet in each participant.
Measurement of baseline covariates
We assessed participants’ eligibility and collected baseline data at screening visits when participants were eating their own diet. We also used FFQs to collect information on participants’ own diet and alcohol intake (servings/wk) during the screening visit. We used an interviewer-administered questionnaire to obtain information on self-reported race, education, annual household income, and smoking status. We measured height (m) and weight (kg) according to standard protocols and defined obesity as BMI (in kg/m2) ≥ 30.
Ethics
The study protocol was approved by the Institutional Review Boards at all affiliated institutions (Johns Hopkins University, Brigham & Women's Hospital, and the Harvard School of Public Health).
Statistical analysis
For this analysis, the predeclared primary endpoints were the individual SCFAs [acetic acid (acetate), propionic acid (propionate), butyric acid (butyrate), valeric acid (valerate), hexanoic acid (hexanoate), isobutyric acid (isobutyrate), isovaleric acid (isovalerate), and methylbutyric acid (methylbutyrate)] and total SCFA, which was the sum of all 8 SCFAs. For normalization purposes, we ln-transformed the concentrations of each SCFA and total SCFA. We used linear regression models with generalized estimating equation (GEE) estimates (exchangeable correlation structures with robust variance estimates) to estimate the mean change of each SCFA and total SCFA from baseline to the end of each 6-wk OmniHeart diet period, and the between-diet differences of these changes. We conducted subgroup analyses stratifying by race (black compared with nonblack) and sex (male compared with female). We included product terms (diet*race or diet*sex) to test for differences between strata.
Finally, we examined associations of circulating SCFAs with cardiometabolic and appetite parameters using linear regression models with GEE estimates. To assess if baseline covariates confounded the associations, we also adjusted for participants’ age (continuous), sex (male; female), race (black; nonblack), BMI (continuous), education (high school or below; some college; college graduate or above), and household income (<$30,000; $30,000–60,000; >$60,000). We considered a 2-sided P < 0.05 as statistically significant. We conducted all data analysis using Stata 15.1 (StataCorp) and R 3.6.1 (R Foundation for Statistical Computing).
Results
Baseline characteristics
Of the 164 participants who successfully completed ≥2 of the 3 diet periods, 1 did not have SCFA data at any diet period, leaving the final analytical data set with 163 participants. Attrition and diet adherence were evenly distributed across the 3 diets, as previously reported (13).
Table 1 shows the baseline characteristics of the 163 participants in the OmniHeart trial. All participants enrolled in OmniHeart had either prehypertension or hypertension (median SBP: 129 mm Hg; IQR: 124–137 mm Hg). The median age of participants was 52 y (IQR: 47–60 y), 45% were women, 55% black, 46% obese, 20% had a high school education or lower, 32% had an annual household income <$30,000, and 11% were current smokers. Adherence to all diets was high, with all study foods consumed on >95% of person-days, and weight decreased only minimally (1 kg) and equally across the 3 diets, as shown previously (13).
TABLE 1.
Baseline characteristics of participants in the OmniHeart trial1
| Variables | |
|---|---|
| Age, y | 52 (47–60) |
| Women | 73 (45) |
| Black | 90 (55) |
| BMI, kg/m2 | 28.8 (25.4–34.1) |
| Obesity status | |
| < Overweight or obese | 34 (21) |
| Overweight | 54 (33) |
| Obese | 75 (46) |
| Any alcohol intake | 72 (44) |
| Servings/wk in 72 drinkers | 3 (1–5) |
| Education | |
| High school or below | 33 (20) |
| Some college | 56 (35) |
| College graduate or above | 74 (45) |
| Annual household income, $ | |
| <30,000 | 52 (32) |
| 30,000–60,000 | 60 (37) |
| >60,000 | 44 (27) |
| Unknown, refused | 7 (4) |
| Smoking | |
| Current | 18 (11) |
| Former | 45 (28) |
| Never | 100 (61) |
| Systolic blood pressure, mm Hg | 129 (124–137) |
| Diastolic blood pressure, mm Hg | 77 (72–82) |
| Total SCFA, ng/mL (n = 162) | 3386 (2523–5858) |
| Acetic acid, ng/mL (n = 162) | 2230 (1200–4060) |
| Propionic acid, ng/mL (n = 162) | 195 (159–239) |
| Butyric acid, ng/mL (n = 162) | 85 (70–99) |
n = 163 unless otherwise indicated. Values are median (IQR) or n (%). Total SCFA is the sum of all 8 SCFAs (acetic acid, propionic acid, butyric acid, valeric acid, hexanoic acid, isobutyric acid, isovaleric acid, and methylbutyric acid).
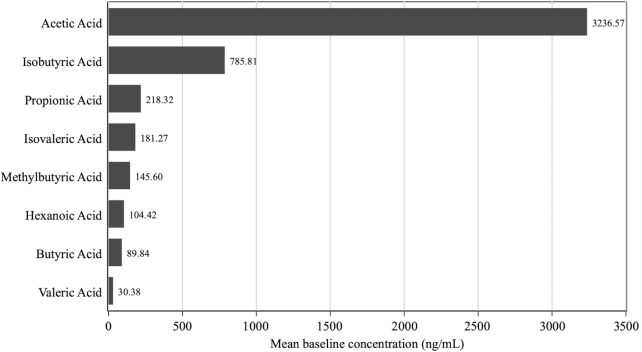
At baseline, the median absolute fasting serum concentration of total SCFA was 3386 ng/mL (IQR: 2523–5858 ng/mL), acetate 2230 ng/mL (IQR: 1200–4060 ng/mL), propionate 195 ng/mL (IQR: 159–239 ng/mL), and butyrate 85 ng/mL (IQR: 70–99 ng/mL) (Table 1). Figure 1 shows the mean concentration of each SCFA in fasting serum at baseline. Of all SCFAs, acetate was the most common (67.5%), followed by isobutyrate (16.4%) and propionate (4.6%). Other SCFAs contributed <4% each of the total. Correlations between each SCFA, which used data from all available SCFA samples (n = 646) from all participants while taking into account repeated measures for each participant, ranged from 0.09 to 0.91 (Table 2).
FIGURE 1.
Bar chart showing the mean concentration of each SCFA in baseline fasting serum (n = 162). SDs: acetic acid, 4224.10; isobutyric acid, 249.51; propionic acid, 101.47; isovaleric acid, 231.87; methylbutyric acid, 107.89; hexanoic acid, 25.38; butyric acid, 31.75; valeric acid, 11.69.
TABLE 2.
Correlation coefficients between fasting serum SCFAs1
| Acetic acid | Propionic acid | Isobutyric acid | Butyric acid | Methylbutyric acid | Isovaleric acid | Valeric acid | Hexanoic acid | Total SCFA | |
|---|---|---|---|---|---|---|---|---|---|
| Acetic acid | 1.00 | ||||||||
| Propionic acid | 0.24 | 1.00 | |||||||
| Isobutyric acid | 0.16 | 0.63 | 1.00 | ||||||
| Butyric acid | 0.25 | 0.48 | 0.28 | 1.00 | |||||
| Methylbutyric acid | 0.19 | 0.57 | 0.55 | 0.14 | 1.00 | ||||
| Isovaleric acid | 0.21 | 0.62 | 0.59 | 0.13 | 0.91 | 1.00 | |||
| Valeric acid | 0.09 | 0.56 | 0.36 | 0.60 | 0.27 | 0.26 | 1.00 | ||
| Hexanoic acid | 0.13 | 0.47 | 0.43 | 0.37 | 0.36 | 0.40 | 0.69 | 1.00 | |
| Total SCFA2 | 0.99 | 0.34 | 0.26 | 0.29 | 0.29 | 0.32 | 0.15 | 0.20 | 1.00 |
n = 163. Data from all available SCFA samples (n = 646) from all 163 participants were used when calculating the correlation coefficients. Repeated measures for each participant were taken into account when estimating the correlation coefficients.
Total SCFA is the sum of all 8 SCFAs (acetic acid, propionic acid, butyric acid, valeric acid, hexanoic acid, isobutyric acid, isovaleric acid, and methylbutyric acid).
Change in circulating SCFAs from baseline to end of diet period
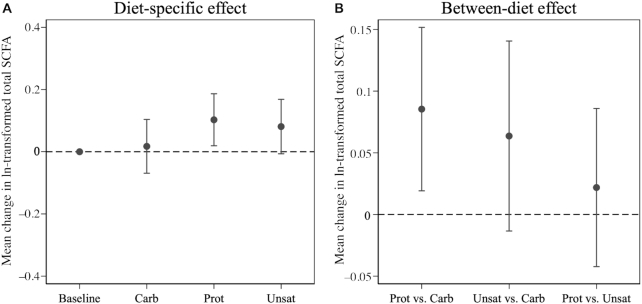
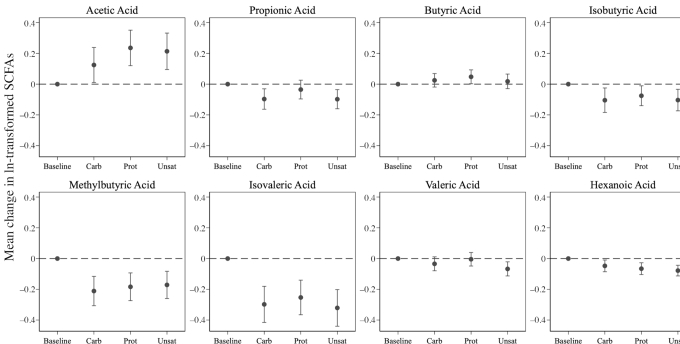
Of the 3 diets in this trial, only the Prot diet significantly increased serum total SCFA concentration from baseline (change in ln-transformed units = 0.10; 95% CI: 0.02, 0.19; Figure 2; Supplemental Table 1). Compared with baseline, all 3 diets were associated with increased serum concentration of acetate, and decreased concentrations of isobutyrate, methylbutyrate, isovalerate, hexanoate, and a tendency for lower propionate (Figure 3; Supplemental Table 1). Only the Prot diet was associated with increased serum butyrate concentration, and only the Unsat diet was associated with decreased valerate concentration (Figure 3; Supplemental Table 1).
FIGURE 2.
Mean change in ln-transformed fasting serum total SCFA from baseline to the end of each 6-wk OmniHeart diet period (A) and the between-diet effects of OmniHeart diets on change in ln-transformed fasting serum total SCFA from baseline to the end of the 6-wk diet period (B) (n = 163). Point estimates and corresponding 95% CIs are in Supplemental Tables 1 and 2. Carb, carbohydrate-rich diet; Prot, protein-rich diet; Unsat, unsaturated fat–rich diet.
FIGURE 3.
Mean change in ln-transformed fasting serum SCFAs from baseline to the end of each 6-wk OmniHeart diet period (n = 163). Point estimates and corresponding 95% CIs are provided in Supplemental Table 1. Carb, carbohydrate-rich diet; Prot, protein-rich diet; Unsat, unsaturated fat–rich diet.
Between-diet difference in circulating SCFA change from baseline to end of diet period
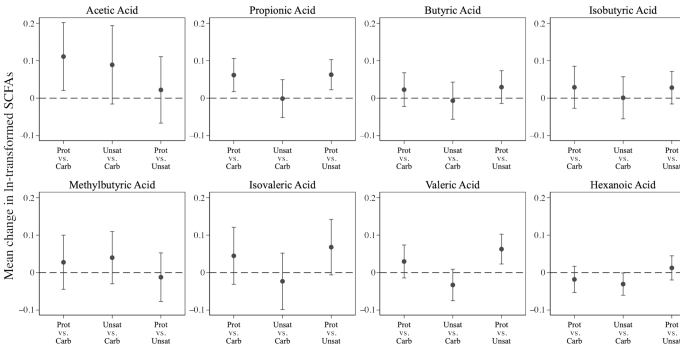
The Prot diet increased total SCFA by 0.09 ln-transformed units (95% CI: 0.02, 0.15) more than the Carb diet, but not significantly differently than the Unsat diet (between-diet difference in ln-transformed units = 0.02; 95% CI: −0.04, 0.09) (Figure 2; Supplemental Table 2). The Prot diet increased acetate by 0.11 ln-transformed units (95% CI: 0.02, 0.20) and propionate by 0.06 ln-transformed units (95% CI: 0.02, 0.11), compared with the Carb diet (Figure 4; Supplemental Table 2). The Unsat diet decreased hexanoate (difference in ln-transformed units = −0.03; 95% CI: −0.06, −0.001) compared with the Carb diet. Compared with the Unsat diet, the Prot diet increased propionate (difference in ln-transformed units = 0.06; 95% CI: 0.02, 0.10) and valerate (difference in ln-transformed units = 0.06; 95% CI: 0.02, 0.10), but no other SCFAs were significantly altered (Figure 4; Supplemental Table 2). We did not observe evidence of effect modification by race (P-interaction = 0.85) or sex (P-interaction = 0.58) for the effect of diet on total SCFA.
FIGURE 4.
Between-diet effect of OmniHeart diets on change in ln-transformed fasting serum SCFAs from baseline to the end of the 6-wk diet period (n = 163). Point estimates and corresponding 95% CIs are in Supplemental Table 2. Carb, carbohydrate-rich diet; Prot, protein-rich diet; Unsat, unsaturated fat–rich diet.
Associations of circulating SCFAs with cardiometabolic and appetite parameters
Higher concentrations of serum acetate were associated with lower fasting insulin (1-SD unit increment in acetate = −0.59-μIU/mL change in insulin; 95% CI: −1.17, −0.01 μIU/mL), lower fasting glucose (1-SD unit increment in acetate = −1.16-mg/dL change in glucose; 95% CI: −1.84, −0.49 mg/dL), and a tendency for lower LDL cholesterol (1-SD unit increment in acetate = −0.98-mg/dL change in LDL cholesterol; 95% CI: −1.98, 0.01 mg/dL). Acetate was not associated with blood pressure (Table 3). Propionate was associated with higher LDL cholesterol (2.74 mg/dL; 95% CI: 1.33, 4.15 mg/dL), lower HDL cholesterol (−0.72 mg/dL; 95% CI: −1.23, −0.21 mg/dL), and higher SBP (0.82 mm Hg; 95% CI: 0.19, 1.45 mm Hg) and DBP (0.50 mm Hg; 95% CI: 0.13, 0.87 mm Hg). Butyrate was associated with higher insulin (0.73 μIU/mL; 95% CI: 0.19, 1.28 μIU/mL) and glucose (1.59 mg/dL; 95% CI: 0.20, 2.98 mg/dL), and lower HDL cholesterol (−0.94 mg/dL; 95% CI: −1.65, −0.22 mg/dL).
TABLE 3.
Associations of circulating SCFAs with cardiometabolic and appetite parameters1
| Change (95% CI) in cardiometabolic parameters per SD increase in SCFAs | ||||||||
|---|---|---|---|---|---|---|---|---|
| Fasting insulin, μIU/mL | Fasting glucose, mg/dL | Fasting LDL cholesterol, mg/dL | Fasting HDL cholesterol, mg/dL | SBP, mm Hg | DBP, mm Hg | Ghrelin, ng/mL | Leptin, pg/mL | |
| Acetic acid (1 SD: 2959.48) | −0.59* (−1.17, −0.01) | −1.16* (−1.84, −0.49) | −0.98 (−1.98, 0.01) | −0.45 (−1.24, 0.35) | 0.14 (−0.58, 0.85) | 0.02 (−0.35, 0.40) | −25.57 (−51.47, 0.34) | −0.36 (−0.88, 0.15) |
| Propionic acid (1 SD: 68.30) | 0.37 (−0.22, 0.96) | 0.81 (−0.24, 1.86) | 2.74* (1.33, 4.15) | −0.72* (−1.23, −0.21) | 0.82* (0.19, 1.45) | 0.50* (0.13, 0.87) | −12.61 (−30.91, 5.69) | 0.72* (0.09, 1.36) |
| Butyric acid (1 SD: 32.03) | 0.73* (0.19, 1.28) | 1.59* (0.20, 2.98) | −1.11 (−2.85, 0.63) | −0.94* (−1.65, −0.22) | −0.14 (−0.85, 0.58) | 0.09 (−0.43, 0.61) | −21.86* (−42.79, −0.92) | −0.23 (−0.78, 0.31) |
| Isobutyric acid (1 SD: 185.85) | 0.09 (−0.29, 0.48) | 0.08 (−0.85, 1.02) | 2.79* (1.34, 4.24) | −0.25 (−0.66, 0.17) | 0.58 (−0.11, 1.27) | 0.33 (−0.05, 0.72) | 1.38 (−10.23, 12.99) | 0.77* (0.05, 1.50) |
| Methylbutyric acid (1 SD: 65.70) | 0.14 (−0.44, 0.72) | 0.87* (0.02, 1.72) | 3.40* (1.38, 5.42) | −0.27 (−0.66, 0.12) | 1.55* (1.00, 2.10) | 0.79* (0.50, 1.09) | −3.35 (−15.56, 8.86) | 1.01 (−0.05, 2.07) |
| Isovaleric acid (1 SD: 129.56) | 0.14 (−0.36, 0.64) | 0.50 (−0.13, 1.13) | 3.24* (0.73, 5.75) | −0.34 (−0.79, 0.11) | 1.74* (1.16, 2.32) | 0.85* (0.57, 1.14) | 2.63 (−7.37, 12.63) | 0.88 (−0.24, 2.00) |
| Valeric acid (1 SD: 8.94) | 0.65 (−0.03, 1.34) | 1.50 (−0.08, 3.09) | 1.90* (0.51, 3.28) | −0.36 (−0.94, 0.22) | 0.90* (0.24, 1.57) | 0.59* (0.17, 1.00) | −18.27 (−41.62, 5.09) | −0.04 (−0.50, 0.43) |
| Hexanoic acid (1 SD: 25.56) | 0.03 (−0.52, 0.58) | 1.04 (−0.24, 2.33) | 3.15* (1.67, 4.62) | 0.38 (−0.21, 0.96) | 1.37* (0.51, 2.24) | 0.81* (0.25, 1.37) | 18.44 (−7.05, 43.93) | 0.01 (−0.49, 0.50) |
| Total SCFA2 (1 SD: 3117.98) | −0.55 (−1.10, 0.01) | −1.05* (−1.73, −0.37) | −0.51 (−1.55, 0.52) | −0.49 (−1.28, 0.31) | 0.30 (−0.33, 0.93) | 0.11 (−0.22, 0.45) | −25.06 (−50.21, 0.09) | −0.24 (−0.76, 0.29) |
n = 163. Baseline mean ± SD of the cardiometabolic parameters: fasting insulin: 10.08 ± 8.39 μIU/mL; fasting glucose: 92.75 ± 15.28 mg/dL; fasting LDL cholesterol: 128.94 ± 32.34 mg/dL; fasting HDL cholesterol: 50.02 ± 16.15 mg/dL; SBP: 131.22 ± 9.36 mm Hg; DBP: 77.05 ± 8.26 mm Hg; ghrelin: 817.00 ± 355.52 ng/mL; leptin: 17.67 ± 16.32 pg/mL. *Statistically significant, P < 0.05. DBP, diastolic blood pressure; SBP, systolic blood pressure.
Total SCFA is the sum of all 8 SCFAs (acetic acid, propionic acid, butyric acid, valeric acid, hexanoic acid, isobutyric acid, isovaleric acid, and methylbutyric acid).
In terms of appetite hormones, butyrate was inversely associated with ghrelin concentrations (change in ghrelin for 1-SD increment in butyrate: −21.86 ng/mL, 95% CI: −42.79, −0.92 ng/mL) and propionate and isobutyrate were positively associated with leptin concentrations (change in leptin for 1-SD increment in propionate: 0.72 pg/mL, 95% CI: 0.09, 1.36 pg/mL; and for 1-SD increment in isobutyrate: 0.77 pg/mL, 95% CI: 0.05, 1.50 pg/mL).
Associations of other SCFAs with selected cardiometabolic and appetite variables can be found in Table 3. The associations were not materially changed by multivariable adjustment for age, sex, race, and participant BMI (Supplemental Table 3).
Discussion
In this randomized, isocaloric, crossover feeding trial, all 3 high-fiber diets each with different macronutrient composition increased acetate, the predominant SCFA in circlulation, with the protein-rich diet increasing acetate more than the carbohydrate-rich diet. Propionate was significantly decreased in the carbohydrate-rich diet and in the unsaturated fat-rich diet compared with baseline, and butyrate was significantly increased in the protein-rich diet compared with baseline. Moreover, diet-induced changes in circulating SCFAs were associated with changes in measures of cardiometabolic health and appetite.
Our finding that all 3 high-fiber diets increased circulating acetate is consistent with the notion that increasing dietary fiber augments microbial production of SCFAs in the colon (18–21) from where acetate is most efficiently translocated into systemic circulation (10, 22). However, our findings on fiber cannot be directly compared with trials that used fecal SCFAs as an outcome. A recent meta-analysis on trials examining dietary fiber and fecal SCFAs found that dietary fiber increased butyrate, but not acetate (8), but this is not surprising given that SCFAs are rapidly absorbed by the colonic epithelium, and excreted acetate (measured in feces) is inversely correlated with circulating acetate (measured in blood) (9,10). Also, the effect of fiber-rich diet patterns on SCFA concentrations circulating after the postprandial period likely depends on the types of fibrous foods included in the diet. In a crossover trial, when (n = 5) participants consumed intact barley kernels (high content of nonstarch polysaccharides and resistant starch), compared with barley porridge (high content of nonstarch polysaccharides only), they had a greater increase in isotope-labeled serum acetate, but not butyrate or propionate, indicating that nonpostprandial serum acetate better reflects resistant starches in the diet (7).
Beyond dietary fiber, our study is the first randomized trial that we know of to isocalorically compare the effects of protein, unsaturated fat, and carbohydrate intake on circulating SCFAs. Other feeding studies have found dietary patterns affect SCFAs, measured in the stool. In a trial of 10 adults (6 men; 4 women), David et al. (23) showed that a plant-based diet compared with an animal-based diet, which did not differ significantly in calories, was associated with a significant increase in fecal butyrate and acetate over 5 d. In a trial of 91 overweight and obese adults randomly assigned to energy-restricted high-carbohydrate or low-carbohydrate diets for 8 wk, the low-carbohydrate diet reduced total fecal SCFAs and fecal butyrate (24). Similarly, in a study of 19 obese adults, total fecal SCFAs, driven by fecal butyrate, were reduced by a low-carbohydrate diet compared with a medium-carbohydrate version of a high-protein diet (25). A trial of 17 obese men found that reducing carbohydrates in a high-protein diet resulted in similar fecal butyrate reductions (26). In a more recent study of 217 participants from the Optimal Macronutrient Distribution Trial in China, which compared 3 isocaloric diets at different levels of dietary fat (low, medium, and high; achieved by replacing soybean oil for white rice and wheat flour), the high-fat diet decreased total fecal SCFAs, including butyrate, compared with the low-fat diet (27). Again, the effects of macronutrients on circulating SCFAs are not directly comparable with effects on fecal SCFAs, as SCFAs measured in the feces may reflect what is excreted whereas serum SCFAs reflect what is absorbed and bioactive in circulation (9). Our study also varies from previous trials by way of diet intervention, design, sample size, duration, and mix of participants.
The mechanism by which the protein-rich diet increased circulating acetate more than the carbohydrate-rich diet may relate to the trade-off between proteolytic and saccharolytic fermentation in the colon. Gut bacteria prefer carbohydrates over protein and thus protein fermentation disproportionately occurs in the distal colon (28). Macronutrient composition also influences which bacteria thrive with low-protein diets selecting for microbes able to use nitrogen derived from mucus (28). Bacteroides has been identified as the predominant proteolytic genus in the gut microbiota (29), followed by Parabacteroides and Alistipes (30), and as a major producer of SCFAs. However, the majority of previous studies have looked at animal-based protein; plant-based proteins, like those used in our high-protein diet, may have different effects on the gut microbiota and their SCFA-producing potential (31). Future research on how plant-protein rich diets affect gut microbiota composition and function could provide important mechanistic insight into our findings.
There is evidence that circulating SCFAs—particularly acetate—play a protective role in the etiology of various cardiometabolic health outcomes (3). Our finding that serum acetate was inversely associated with fasting insulin and glucose is consistent with literature on acetate and glucose metabolism (32–35). Acetate has been shown to improve glucose metabolism through effects on endogenous glucose production [in humans (36) and mice (37)], reducing glycolysis [in mice (38)], promoting glycogen synthesis [in mice (39)], and increasing insulin sensitivity [in humans (34, 35)]. Our finding of an inverse association of serum acetate with LDL cholesterol is consistent with evidence from animal studies that acetate may inhibit lipid synthesis (40, 41). We did not observe an association of acetate with blood pressure, which is in contrast to evidence from murine models showing acetate reduces blood pressure (5, 6) via action on SCFA receptors in the vascular endothelium or afferent arteriole (42–45), suggesting that, at least in our study, diet-induced changes in circulating fasting acetate may not be on the pathway to improved blood pressure.
With respect to other circulating SCFAs, propionate, valerate, hexanoate, and the branched SCFAs isobutyrate, isovalerate, and methylbutyrate were associated with higher blood pressure and LDL cholesterol. Circulating propionate and butyrate were also associated with lower HDL cholesterol, indicating that, unlike acetate, higher concentrations of these SCFAs in the fasting state may indicate worse cardiometabolic health. In a study of 30 adult males, the fecal branched SCFAs isobutyrate and isovalerate, which can be produced from valine and leucine fermentation (46), were associated with an unfavorable lipid profile (47). Valerate, which only represented 0.6% of circulating SCFAs in our study, is involved in cholesterol synthesis and may be a biomarker of hepatic fat (48). More studies are needed to evaluate the associations of circulating SCFAs with cardiometabolic health in comparison with fecal SCFAs, which have been associated with greater obesity (49–51) and worse cardiometabolic health (11).
Higher circulating SCFAs, particularly acetate (52), have also been associated with reduced appetite in animal studies and human studies (7). Our finding, that total SCFA was associated with lower concentrations of ghrelin, is largely consistent with a recent randomized trial of 25 adults in which 75 g inulin significantly increased postprandial SCFAs, and decreased circulating ghrelin, as compared with 75 g glucose (53). We also found that propionate was positively associated with leptin. Future studies are needed to better understand diet effects on the gut–brain axis and more specifically whether SCFAs may mediate the effects of diet composition on appetite signaling.
There are limitations to our study. Our study allowed for quantitative description of SCFAs reaching the circulation—the net result of production, absorption, and extraction by the small intestine, colon, and liver—and should not be considered a direct reflection of colonic SCFA generation. Nevertheless, Boets et al. (10) used a stable isotope MS to estimate the systemic availability of colonic SCFAs, and found acetate was the most systemically bioavailable SCFA (36%), followed by propionate (9%) and butyrate (2%). Acetate is also produced endogenously during fatty acid oxidation and glucose or amino acid metabolism, and our study design does not allow us to determine directionality with these or other metabolic changes. Another limitation was that we did not collect stool from our participants, which would have afforded insight into how the gut microbiome may have contributed to changes in SCFAs. To our knowledge there have not been studies to assess the impact of long-term storage on SCFAs, which are considered volatile compounds. However, if storage introduced measurement error in the quantification of SCFAs, we would expect this to be nondifferential, resulting in a conservative estimate of the association between diet periods and serum SCFAs. Although participants were randomly assigned to their diet patterns and total calories and major nutrients were held constant across the diets, unmeasured diet elements could have contributed to the effect of the OmniHeart diets on SCFAs. Finally, we cannot rule out the possibility that our findings were due to chance given the multiple statistical tests that were performed.
In conclusion, our findings indicate that all 3 high-fiber OmniHeart diets affected microbially derived circulating SCFAs, but results differed by type of diet, suggesting that macronutrients also affect SCFA concentrations. A plant protein-rich, high-fiber diet had the strongest effect on acetate, the most common SCFA in circulation and that which is most associated with improved glucose metabolism. Although animal experiments, cross-sectional studies, and a few small trials have provided important insights into the complex diet–microbiome relation to human health, this is the first feeding trial that we know of to assess the effects of whole-diet, high-fiber interventions on serum SCFAs. Our findings are consistent with the hypothesis that widescale adoption of a low-fiber American dietary pattern has resulted in a loss of SCFA-producing microbiota and an increase in cardiometabolic diseases (54, 55), and they support recent guidelines that emphasize the importance of high-fiber dietary patterns (56). Longitudinal studies are needed to examine the clinical significance of our findings and to test whether changes in circulating SCFAs mediate associations of high-fiber diets and macronutrient composition with health outcomes.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—NTM: designed the research (project conception, development of the overall research plan, and study oversight), wrote the first draft of the manuscript, and had primary responsibility for the final content; LJA and ERM: conducted research (hands-on conduct of the experiments and data collection); NTM and MZ: analyzed the data or performed statistical analysis; and all authors: made major contributions in revising the manuscript and read and approved the final manuscript. The authors report no conflicts of interest.
Notes
Supported by National Heart, Lung, and Blood Institute of the NIH grants K01HL141589 (to NTM) and K23HL135273 (to SPJ).
Supplemental Figure 1 and Supplemental Tables 1–3 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Data described in the article, code book, and analytic code will be made available upon request pending application and approval.
Abbreviations used: Carb diet, carbohydrate-rich diet; DBP, diastolic blood pressure; GEE, generalized estimating equation; OmniHeart, Optimal Macronutrient Intake Trial to Prevent Heart Disease; Prot diet, protein-rich diet; SBP, systolic blood pressure; SCFA, short-chain fatty acids; Unsat diet, unsaturated fat–rich diet.
References
- 1. den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 2013;54(9):2325–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ríos-Covián D, Ruas-Madiedo P, Margolles A, Gueimonde M, de Los Reyes-Gavilán CG, Salazar N. Intestinal short chain fatty acids and their link with diet and human health. Front Microbiol. 2016;7:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Koh A, De Vadder F, Kovatcheva-Datchary P, Backhed F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. 2016;165(6):1332–45. [DOI] [PubMed] [Google Scholar]
- 4. Cummings JH, Pomare EW, Branch WJ, Naylor CP, Macfarlane GT. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987;28(10):1221–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kondo S, Tayama K, Tsukamoto Y, Ikeda K, Yamori Y. Antihypertensive effects of acetic acid and vinegar on spontaneously hypertensive rats. Biosci Biotechnol Biochem. 2001;65(12):2690–4. [DOI] [PubMed] [Google Scholar]
- 6. Na L, Chu X, Jiang S, Li C, Li G, He Y, Liu Y, Li Y, Sun C. Vinegar decreases blood pressure by down-regulating AT1R expression via the AMPK/PGC-1α/PPARγ pathway in spontaneously hypertensive rats. Eur J Nutr. 2016;55(3):1245–53. [DOI] [PubMed] [Google Scholar]
- 7. Verbeke K, Ferchaud-Roucher V, Preston T, Small AC, Henckaerts L, Krempf M, Wang H, Vonk RJ, Priebe MG. Influence of the type of indigestible carbohydrate on plasma and urine short-chain fatty acid profiles in healthy human volunteers. Eur J Clin Nutr. 2010;64(7):678–84. [DOI] [PubMed] [Google Scholar]
- 8. So D, Whelan K, Rossi M, Morrison M, Holtmann G, Kelly JT, Shanahan ER, Staudacher HM, Campbell KL. Dietary fiber intervention on gut microbiota composition in healthy adults: a systematic review and meta-analysis. Am J Clin Nutr. 2018;107(6):965–83. [DOI] [PubMed] [Google Scholar]
- 9. Vogt JA, Wolever TM. Fecal acetate is inversely related to acetate absorption from the human rectum and distal colon. J Nutr. 2003;133(10):3145–8. [DOI] [PubMed] [Google Scholar]
- 10. Boets E, Gomand SV, Deroover L, Preston T, Vermeulen K, De Preter V, Hamer HM, Van den Mooter G, De Vuyst L, Courtin CM et al.. Systemic availability and metabolism of colonic-derived short-chain fatty acids in healthy subjects: a stable isotope study. J Physiol. 2017;595(2):541–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de la Cuesta-Zuluaga J, Mueller NT, Álvarez-Quintero R, Velásquez-Mejía EP, Sierra JA, Corrales-Agudelo V, Carmona JA, Abad JM, Escobar JS. Higher fecal short-chain fatty acid levels are associated with gut microbiome dysbiosis, obesity, hypertension and cardiometabolic disease risk factors. Nutrients. 2018;11(1):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kurilshikov A, van den Munckhof ICL, Chen L, Bonder MJ, Schraa K, Rutten JHW, Riksen NP, de Graaf J, Oosting M, Sanna S et al.. Gut microbial associations to plasma metabolites linked to cardiovascular phenotypes and risk. Circ Res. 2019;124(12):1808–20. [DOI] [PubMed] [Google Scholar]
- 13. Appel LJ, Sacks FM, Carey VJ, Obarzanek E, Swain JF, Miller ER 3rd, Conlin PR, Erlinger TP, Rosner BA, Laranjo NM et al.. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: results of the OmniHeart randomized trial. JAMA. 2005;294(19):2455–64. [DOI] [PubMed] [Google Scholar]
- 14. Swain JF, McCarron PB, Hamilton EF, Sacks FM, Appel LJ. Characteristics of the diet patterns tested in the Optimal Macronutrient Intake Trial to Prevent Heart Disease (OmniHeart): options for a heart-healthy diet. J Am Diet Assoc. 2008;108(2):257–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gadgil MD, Appel LJ, Yeung E, Anderson CA, Sacks FM, Miller ER 3rd. The effects of carbohydrate, unsaturated fat, and protein intake on measures of insulin sensitivity: results from the OmniHeart trial. Diabetes Care. 2013;36(5):1132–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 17. Beasley JM, Ange BA, Anderson CA, Miller ER 3rd, Erlinger TP, Holbrook JT, Sacks FM, Appel LJ. Associations between macronutrient intake and self-reported appetite and fasting levels of appetite hormones: results from the Optimal Macronutrient Intake Trial to Prevent Heart Disease. Am J Epidemiol. 2009;169(7):893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, Collini S, Pieraccini G, Lionetti P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A. 2010;107(33):14691–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R et al.. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334(6052):105–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. De Filippis F, Pellegrini N, Vannini L, Jeffery IB, La Storia A, Laghi L, Serrazanetti DI, Di Cagno R, Ferrocino I, Lazzi C et al.. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut. 2016;65(11):1812–21. [DOI] [PubMed] [Google Scholar]
- 21. Wu GD, Compher C, Chen EZ, Smith SA, Shah RD, Bittinger K, Chehoud C, Albenberg LG, Nessel L, Gilroy E et al.. Comparative metabolomics in vegans and omnivores reveal constraints on diet-dependent gut microbiota metabolite production. Gut. 2016;65(1):63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Boets E, Deroover L, Houben E, Vermeulen K, Gomand SV, Delcour JA, Verbeke K. Quantification of in vivo colonic short chain fatty acid production from inulin. Nutrients. 2015;7(11):8916–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA et al.. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brinkworth GD, Noakes M, Clifton PM, Bird AR. Comparative effects of very low-carbohydrate, high-fat and high-carbohydrate, low-fat weight-loss diets on bowel habit and faecal short-chain fatty acids and bacterial populations. Br J Nutr. 2009;101(10):1493–502. [DOI] [PubMed] [Google Scholar]
- 25. Duncan SH, Belenguer A, Holtrop G, Johnstone AM, Flint HJ, Lobley GE. Reduced dietary intake of carbohydrates by obese subjects results in decreased concentrations of butyrate and butyrate-producing bacteria in feces. Appl Environ Microbiol. 2007;73(4):1073–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Russell WR, Gratz SW, Duncan SH, Holtrop G, Ince J, Scobbie L, Duncan G, Johnstone AM, Lobley GE, Wallace RJ et al.. High-protein, reduced-carbohydrate weight-loss diets promote metabolite profiles likely to be detrimental to colonic health. Am J Clin Nutr. 2011;93(5):1062–72. [DOI] [PubMed] [Google Scholar]
- 27. Wan Y, Wang F, Yuan J, Li J, Jiang D, Zhang J, Li H, Wang R, Tang J, Huang T et al.. Effects of dietary fat on gut microbiota and faecal metabolites, and their relationship with cardiometabolic risk factors: a 6-month randomised controlled-feeding trial. Gut. 2019;68(8):1417–29. [DOI] [PubMed] [Google Scholar]
- 28. Holmes AJ, Chew YV, Colakoglu F, Cliff JB, Klaassens E, Read MN, Solon-Biet SM, McMahon AC, Cogger VC, Ruohonen K et al.. Diet-microbiome interactions in health are controlled by intestinal nitrogen source constraints. Cell Metab. 2017;25(1):140–51. [DOI] [PubMed] [Google Scholar]
- 29. Macfarlane GT, Cummings JH, Allison C. Protein degradation by human intestinal bacteria. J Gen Microbiol. 1986;132(6):1647–56. [DOI] [PubMed] [Google Scholar]
- 30. Korpela K. Diet, microbiota, and metabolic health: trade-off between saccharolytic and proteolytic fermentation. Annu Rev Food Sci Technol. 2018;9:65–84. [DOI] [PubMed] [Google Scholar]
- 31. Zhernakova A, Kurilshikov A, Bonder MJ, Tigchelaar EF, Schirmer M, Vatanen T, Mujagic Z, Vila AV, Falony G, Vieira-Silva S et al.. Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science. 2016;352(6285):565–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Morrison DJ, Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. 2016;7(3):189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Layden BT, Yalamanchi SK, Wolever TM, Dunaif A, Lowe WL Jr. Negative association of acetate with visceral adipose tissue and insulin levels. Diabetes Metab Syndr Obes. 2012;5:49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mitrou P, Petsiou E, Papakonstantinou E, Maratou E, Lambadiari V, Dimitriadis P, Spanoudi F, Raptis SA, Dimitriadis G. Vinegar consumption increases insulin-stimulated glucose uptake by the forearm muscle in humans with type 2 diabetes. J Diabetes Res. 2015:175204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hernandez MAG, Canfora EE, Jocken JWE, Blaak EE. The short-chain fatty acid acetate in body weight control and insulin sensitivity. Nutrients. 2019;11(8):1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. White AM, Johnston CS. Vinegar ingestion at bedtime moderates waking glucose concentrations in adults with well-controlled type 2 diabetes. Diabetes Care. 2007;30(11):2814–15. [DOI] [PubMed] [Google Scholar]
- 37. Sakakibara S, Yamauchi T, Oshima Y, Tsukamoto Y, Kadowaki T. Acetic acid activates hepatic AMPK and reduces hyperglycemia in diabetic KK-A(y) mice. Biochem Biophys Res Commun. 2006;344(2):597–604. [DOI] [PubMed] [Google Scholar]
- 38. Fushimi T, Sato Y. Effect of acetic acid feeding on the circadian changes in glycogen and metabolites of glucose and lipid in liver and skeletal muscle of rats. Br J Nutr. 2005;94(5):714–19. [DOI] [PubMed] [Google Scholar]
- 39. Fushimi T, Tayama K, Fukaya M, Kitakoshi K, Nakai N, Tsukamoto Y, Sato Y. The efficacy of acetic acid for glycogen repletion in rat skeletal muscle after exercise. Int J Sports Med. 2002;23(3):218–22. [DOI] [PubMed] [Google Scholar]
- 40. Fushimi T, Suruga K, Oshima Y, Fukiharu M, Tsukamoto Y, Goda T. Dietary acetic acid reduces serum cholesterol and triacylglycerols in rats fed a cholesterol-rich diet. Br J Nutr. 2006;95(5):916–24. [DOI] [PubMed] [Google Scholar]
- 41. Li X, Chen H, Guan Y, Li X, Lei L, Liu J, Yin L, Liu G, Wang Z. Acetic acid activates the AMP-activated protein kinase signaling pathway to regulate lipid metabolism in bovine hepatocytes. PLoS One. 2013;8(7):e67880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Natarajan N, Hori D, Flavahan S, Steppan J, Flavahan NA, Berkowitz DE, Pluznick JL. Microbial short chain fatty acid metabolites lower blood pressure via endothelial G-protein coupled receptor 41. Physiol Genomics. 2016;48(11):826–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pluznick JL, Protzko RJ, Gevorgyan H, Peterlin Z, Sipos A, Han J, Brunet I, Wan LX, Rey F, Wang T et al.. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci U S A. 2013;110(11):4410–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pluznick J. A novel SCFA receptor, the microbiota, and blood pressure regulation. Gut Microbes. 2014;5(2):202–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jose PA, Raj D. Gut microbiota in hypertension. Curr Opin Nephrol Hypertens. 2015;24(5):403–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Smith EA, Macfarlane GT. Dissimilatory amino acid metabolism in human colonic bacteria. Anaerobe. 1997;3(5):327–37. [DOI] [PubMed] [Google Scholar]
- 47. Granado-Serrano AB, Martín-Garí M, Sánchez V, Riart Solans M, Berdún R, Ludwig IA, Rubió L, Vilaprinyó E, Portero-Otín M, Serrano JCE. Faecal bacterial and short-chain fatty acids signature in hypercholesterolemia. Sci Rep. 2019;9(1):1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. O'Sullivan JF, Morningstar JE, Yang Q, Zheng B, Gao Y, Jeanfavre S, Scott J, Fernandez C, Zheng H, O'Connor S et al.. Dimethylguanidino valeric acid is a marker of liver fat and predicts diabetes. J Clin Invest. 2017;127(12):4394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schwiertz A, Taras D, Schafer K, Beijer S, Bos NA, Donus C, Hardt PD. Microbiota and SCFA in lean and overweight healthy subjects. Obesity (Silver Spring). 2010;18(1):190–5. [DOI] [PubMed] [Google Scholar]
- 50. Rahat-Rozenbloom S, Fernandes J, Gloor GB, Wolever TM. Evidence for greater production of colonic short-chain fatty acids in overweight than lean humans. Int J Obes (Lond). 2014;38(12):1525–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fernandes J, Su W, Rahat-Rozenbloom S, Wolever TM, Comelli EM. Adiposity, gut microbiota and faecal short chain fatty acids are linked in adult humans. Nutr Diabetes. 2014;4:e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Frost G, Sleeth ML, Sahuri-Arisoylu M, Lizarbe B, Cerdan S, Brody L, Anastasovska J, Ghourab S, Hankir M, Zhang S et al.. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat Commun. 2014;5:3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rahat-Rozenbloom S, Fernandes J, Cheng J, Wolever TMS. Acute increases in serum colonic short-chain fatty acids elicited by inulin do not increase GLP-1 or PYY responses but may reduce ghrelin in lean and overweight humans. Eur J Clin Nutr. 2017;71(8):953–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sonnenburg ED, Sonnenburg JL. Starving our microbial self: the deleterious consequences of a diet deficient in microbiota-accessible carbohydrates. Cell Metab. 2014;20(5):779–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sonnenburg JL, Backhed F. Diet–microbiota interactions as moderators of human metabolism. Nature. 2016;535(7610):56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tagtow A, Rahavi E, Bard S, Stoody EE, Casavale K, Mosher A. Coming together to communicate the 2015–2020 Dietary Guidelines for Americans. J Acad Nutr Diet. 2016;116(2):209–12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.