ABSTRACT
Background
Race differences in body composition and fat distribution may in part explain the differences in insulin sensitivity and the disproportionate burden of type 2 diabetes in African Americans.
Objective
To determine if differences in body composition and fat distribution explain race differences in insulin sensitivity and identify obesity measures that were independently associated with insulin sensitivity.
Methods
Participants were 113 lean, overweight, and obese African-American and Caucasian-American adults without diabetes. Skeletal muscle insulin sensitivity was determined using a hyperinsulinemic-euglycemic clamp (SIClamp, insulin rate:120 mU/m2/min). Subcutaneous abdominal adipose tissue (SAAT), intra-abdominal adipose tissue (IAAT), and liver fat were measured by MRI; leg fat, total fat, and lean mass were measured by DXA.
Results
Race-by-adiposity interactions were significant in cross-sectional analyses utilizing multiple linear regression models for SIClamp (P < 0.05); higher BMI, fat mass, SAAT, leg fat, and liver fat were associated with lower SIClamp in Caucasian Americans but not African Americans. Race-by-IAAT interaction was not significant (P = 0.65). A central fat distribution (SAAT adjusted for leg fat) was associated with lower SIClamp in African Americans (β = −0.45, SE = 0.11, P < 0.001) but not Caucasian Americans (β = −0.42, SE = 0.30, P = 0.17). A peripheral fat distribution (leg fat adjusted for IAAT/SAAT) was associated with a higher SIClamp in African Americans (β = 0.11, SE = 0.05, P = 0.02) but lower SIClamp in Caucasian Americans (β = −0.28, SE = 0.14, P = 0.049). Lean mass was inversely associated with SIClamp in African Americans (β = −0.05, SE = 0.03, P = 0.04) but not Caucasian Americans (β = 0.08, SE = 0.05, P = 0.10) in the model for leg fat.
Conclusions
Measures of overall adiposity were more strongly associated with SIClamp in Caucasian Americans, whereas body fat distribution and lean mass showed stronger correlations with SIClamp in African Americans. Insulin sensitivity may have a genetic basis in African Americans that is reflected in the pattern of body fat distribution. These findings suggest a race-specific pathophysiology of insulin resistance, which has implications for the prevention of diabetes and related cardiometabolic diseases.
Keywords: race, adiposity, lean mass, leg fat, insulin sensitivity, hyperinsulinemic-euglycemic clamp
Introduction
In the USA, African Americans have the highest prevalence of obesity and are disproportionately affected by type 2 diabetes (T2D) (1). The underlying cause for this disparity is not known, but may relate to differences in insulin resistance or decreased insulin sensitivity, which is recognized as an important factor in the etiology of T2D (2). Previous studies using mainly indirect assessment methods report lower insulin sensitivity in African Americans compared with Caucasian Americans (3, 4). However, results have been inconsistent, differing with the method used to assess insulin sensitivity and the obesity status of the study population (3–7).
Furthermore, African Americans have a higher acute insulin response to glucose (3, 8), but lower fasting glucose (9), postchallenge glucose (5, 10), basal hepatic glucose production (11), and insulin clearance compared with Caucasian Americans (10, 12, 13). These racial differences in insulin-glucose homeostasis highlight the necessity of assessing race differences in insulin sensitivity using direct methods such as the reference standard hyperinsulinemic-euglycemicclamp, in both lean and obese individuals (14).
In addition, while obesity is a leading risk factor for T2D and insulin resistance (15), differences in adipose tissue distribution can influence the association between obesity and insulin resistance. Ectopic fat accumulation in insulin-sensitive tissues such as intrahepatic, intermuscular, or intramyocellular lipid is involved in the etiology of insulin resistance (16, 17). Higher levels of total, visceral, and subcutaneous abdominal adiposity have also been shown to be associated with increased insulin resistance in predominantly Caucasian-American populations (18, 19). Whether these associations extend to African Americans is less clear. Emerging evidence indicates that subcutaneous abdominal adipose tissue (SAAT) is a major determinant of insulin sensitivity in black women, who are more insulin resistant than BMI-matched white women despite having less visceral fat (20, 21). Collectively, these data suggest racial differences in the relation between regional fat distribution and insulin sensitivity.
Therefore, the primary objective of this study was to determine the extent that differences in obesity measures such as BMI, whole-body adiposity, as well as regional and ectopic fat distribution assessed using MRI explain the differences in skeletal muscle insulin sensitivity assessed by the hyperinsulinemic-euglycemic clamp (SIClamp) between African-American and Caucasian-American men and women without diabetes, across a wide range of adiposity. A secondary aim was to identify significant measures of body composition and regional fat distribution that are independently associated with SIClamp in lean, overweight, and obese African Americans and Caucasian Americans.
Methods
Study design, setting, and participants
This cross-sectional study was conducted at the University of Alabama at Birmingham (UAB), between 2013 and 2018. Lean, overweight, and obese African-American and Caucasian-American men and women, aged 19–45 y, who were sedentary to moderately active (<2 h/wk of moderate, intentional exercise) were recruited by public advertisement. Race was determined by self-report. Individuals with diabetes were excluded from participation following a screening 75 g oral-glucose-tolerance test (2-h glucose ≥200 mg/ dL). Other exclusion criteria included absence of a regular menstrual cycle; pregnant, lactating, or postmenopausal; smoking; not weight stable (change in weight >5 lbs or 2.3 kg) in the previous 6 mo; active engagement in unusual dietary practices (e.g. low-carbohydrate diets); taking oral contraceptives; use of any medication known to affect carbohydrate or lipid metabolism, or energy expenditure; and use of antihypertensive agents that affect glucose tolerance (e.g., thiazide diuretics at doses >25 mg/d, angiotensin-converting-enzyme inhibitors). Participants were instructed to maintain their usual activity level, to avoid strenuous physical activity the day prior to testing, and to avoid all physical activity on the morning of testing. Women were tested 3–7 d after cessation of menstruation, while in the follicular phase of the menstrual cycle. All study assessments were conducted at the core facilities of the Center for Clinical and Translational Science (CCTS), Nutrition Obesity Research Center (NORC), and Diabetes Research Center (DRC). The UAB Institutional Review Board approved the study and all participants provided written informed consent.
Insulin sensitivity assessed by SIClamp and the homeostasis model assessment of insulin resistance index 2
Skeletal muscle insulin sensitivity (SIClamp) was assessed using the hyperinsulinemic-euglycemic clamp. All SIClamp tests were performed in an outpatient setting in the Clinical Research Unit (CRU) at UAB's CCTS after a ≥10 h overnight fast. With the participant in a recumbent position, an intravenous catheter was placed in an antecubital vein for insulin and glucose infusion. The insulin solution (regular Humulin, Eli Lilly & Co.) was prepared with normal saline and infused at 120 mU/m2/min (individualized to the participant's body surface area) for 3 h using an Alaris point-of-care unit with Guardrails software (Carefusion Corp.). Blood glucose concentrations were measured at the bedside at 5-min intervals using a glucose analyzer (YSI 2300 STAT Plus, YSI, Inc.), and an infusion of 20% dextrose was adjusted to maintain the blood glucose concentration at the individual's fasting level. The steady-state period for each individual was defined as a ≥30 min period that occurred ≥1 h after initiation of the insulin infusion, during which the CVs for blood glucose, serum insulin, and glucose infusion rate were <5%. Another catheter was placed in the contralateral arm for sequential blood sampling every 10 min for the determination of glucose (SIRRUS analyzer, Stanbio Laboratories) and insulin (TOSOH AIA-II immunoassay analyzer, TOSCH Corp.) concentrations. The intra- and interassay CVs were 1.28% and 2.48% for serum glucose, and 1.49% and 3.95% for insulin, respectively.
SIClamp (10−4.dL.kg−1.min−1/[μU/mL]) was defined as M/(G × ΔI), where M is the steady-state glucose infusion rate (mg/kg body mass/min), G is the steady-state serum glucose concentration (mg/dL), and ΔI is the difference between basal and steady-state serum insulin concentrations (μU/mL).
The homeostasis model of assessment index 2- insulin resistance(HOMA2-IR) was also used to assess insulin resistance (22). HOMA2-IR is computed using fasting insulin and glucose and may primarily reflect hepatic insulin resistance.
Body composition and regional fat distribution determined by MRI and DXA
Total and regional body composition including total lean mass, fat mass, and leg fat were measured by DXA (iDXA instrument, GE Healthcare). Participants were scanned in light clothing while lying supine with arms at their sides.
SAAT, intra-abdominal adipose tissue (IAAT), and liver fat were determined by MRI (Ingenia 1.5T wide bore MRI system; Philips). Liver fat was assessed using the fast spin echo 2-point Dixon technique (23). Volumes of 2 abdominal adipose tissue compartments, SAAT and IAAT, were assessed via transverse abdominal images obtained using 3D volumetric T1-weighted magnetization-prepared rapid acquisition gradient echo (MPRAGE). The echo time, repetition time, and pulse flip angles were selected to optimize the signal-intensity contrast between adipose and nonadipose tissue. A series of 10 mm slices spaced at 5 mm intervals from the L1-L4/5 vertebrae was performed for each individual. Images were analyzed for IAAT and SAAT using Slice-O-Matic software (version 4.3, Tomovision) for abdominal fat volume.
Statistical analyses
Race comparisons of descriptive characteristics were made by chi-square tests (categorical variables) and independent samples t-test (continuous variables). Multiple linear regression models were used to assess the interaction between race and various measures of obesity, body composition, and regional fat distribution (e.g., BMI, fat mass, lean mass, SAAT, IAAT, liver fat, and leg fat) on SIClamp and HOMA2-IR. Independent variables were entered into the model by hierarchical regression. Age, sex, and total lean and total fat mass were included as covariates in the models, except when there was significant multicollinearity (e.g., between BMI/SAAT/leg fat and total fat). One outlier for SIClamp (>4 standardized residuals and high standardized DFFit statistic) was excluded from these analyses. Omission of the outlier improved the residuals of the models, and the homoscedasticity assumption was met. As residual values of HOMA2-IR obtained after adjustment for race, age, sex, liver fat, and total lean and total fat mass in multivariate analysis were not normally distributed, logarithmically transformed HOMA2-IR was used in these analyses.
ANCOVA was used to investigate whether differences in lean mass, or total and regional adiposity accounted for the racial difference in SIClamp. Comparisons of regression slopes (test of interaction between race and covariates) were conducted to ascertain whether the assumption of homogeneity of regression slopes was met. Where interaction terms were significant, the Johnson–Neyman (J–N) procedure was used to identify the regions of significance along the observed range of the covariate where the race difference in outcome measure occurred (e.g., range of BMI where African Americans and Caucasian Americans differed in SIClamp) (24). A total sample size of 60 individuals would provide >90% power at an α-level of 0.05 to detect the hypothesized marginal effect of BMI, hypothesized marginal effect of race, and the hypothesized interaction effect assuming an SD of glucose disposal of 40 mg/kg/min. All analyses were performed with SPSS 25.0 for Windows (SPSS Inc.). Statistical tests were 2-tailed with significance set at P < 0.05.
Results
Anthropometric and metabolic measurements
Table 1 presents the metabolic, anthropometric, and regional adiposity characteristics of study participants according to race. In total, 113 adults (49% African American, 49% males, aged 29 ± 8 y and BMI 27.5 ± 5.6 kg/m2) without diabetes participated in the study. Race groups were matched for age and gender distribution. Compared with Caucasian Americans, African Americans had a lower SIClamp, and higher fasting insulin and HOMA2-IR (P ≤ 0.03). African Americans had higher total lean mass, leg fat, and SAAT volume, corresponding to higher weight and BMI ( P < 0.03). Total fat also tended to be higher in African Americans (P = 0.06). IAAT and intrahepatic fat volumes were not significantly different between Caucasian Americans and African Americans (P ≥ 0.18).
TABLE 1.
Participant characteristics by race1
| African Americans (n = 55) | Caucasian Americans (n = 58) | P 2 | |
|---|---|---|---|
| Participant demographics | |||
| Age, y | 30 ± 1 | 28 ± 8 | 0.20 |
| Gender, males/females | 25/30 | 30/28 | 0.51 |
| Metabolic measurements | |||
| SIClamp, 10−4.dL.kg−1.min−1/(μU/mL) | 3.0 ± 1.2 | 5.2 ± 2.4 | <0.001 |
| Fasting glucose, mg/dL | 91.1 ± 8.4 | 88.6 ± 9.2 | 0.13 |
| Fasting insulin,3 μU/mL | 9.6 ± 5.2 | 7.5 ± 4.5 | 0.03 |
| HOMA2-IR3 | 1.1 ± 0.6 | 0.8 ± 0.5 | 0.03 |
| Body weight and composition | |||
| Weight, kg | 85.9 ± 19.1 | 76.5 ± 15.0 | 0.005 |
| BMI, kg/m2 | 29.3 ± 5.9 | 25.8 ± 4.7 | 0.001 |
| DXA measurements | |||
| Total fat mass, kg | 28.8 ± 12.1 | 24.8 ± 10.5 | 0.06 |
| Total fat mass, % | 32.9 ± 9.5 | 31.7 ± 8.9 | 0.49 |
| Total lean mass, kg | 53.4 ± 11.7 | 48.9 ± 9.2 | 0.02 |
| Leg fat, kg | 10.9 ± 5.0 | 8.8 ± 3.8 | 0.01 |
| MRI measurements | |||
| Subcutaneous abdominal adipose tissue, 4 L | 3.7 ± 2.2 | 2.9 ± 1.7 | 0.03 |
| Intra-abdominal adipose tissue, 4 L | 0.6 ± 0.5 | 0.8 ± 0.8 | 0.18 |
| Liver fat, 5 % | 0.4 ± 5.9 | 1.9 ± 7.6 | 0.26 |
Data are unadjusted mean ± SD, unless otherwise stated.
Total analyzed n = 113 (African Americans: 55, Caucasian Americans: 58) for all data unless otherwise stated.
P value for differences between races by independent samples t-test (continuous variables) or chi-square test (categorical variables).
Total analyzed n = 111 (African Americans: 54, Caucasian Americans: 57) for insulin and HOMA2-IR data; 2 outliers (values >3 SD over the group mean due to elevated fasting insulin) were excluded from analyses.
Total analyzed n = 112 (African Americans: 55, Caucasian Americans: 57) for SAAT and IAAT data; no abdominal MRI images were available for 1 Caucasian American.
Total analyzed n = 112 (African Americans: 54, Caucasian Americans: 58) for liver fat; data from 1 African American was excluded due to severe artifact. HOMA2-IR, homeostasis model of assessment index 2-insulin resistance; IAAT, intra-abdominal adipose tissue; SAAT, subcutaneous abdominal adipose tissue; SIClamp, skeletal muscle insulin sensitivity assessed by the hyperinsulinemic-euglycemic clamp.
Race differences in association between SIClamp and obesity measures
Race was independently associated with SIClamp (significantly lower in African Americans than Caucasian Americans, P < 0.001) in all multivariate models that examined the association between SIClamp and various obesity measures. Except for the model that included IAAT and leg fat, sex was independently associated with SIClamp (significantly lower in males than females, P < 0.05).
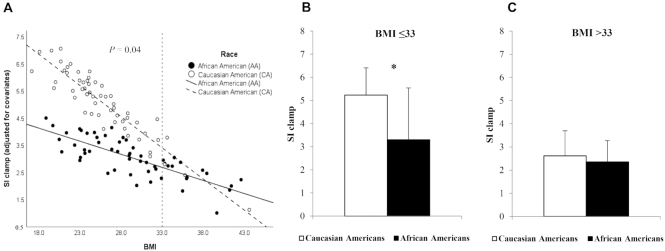
There was a significant race × BMI interaction (P = 0.04, R2 = 46%; Figure 1A). A higher BMI was more strongly associated with a lower SIClamp in Caucasian Americans (β = −0.33, SE = 0.06, P < 0.001, R2 = 37%) compared with African Americans (β = −0.08, SE = 0.03, P = 0.01, R2 = 30%). The J–N method was used to explore the association between race and SIClamp and identify the range of BMI where differences in SIClamp between African Americans and Caucasian Americans were statistically significant. The results showed SIClamp was lower in African Americans compared with Caucasian Americans for participants with BMI ≤ 33 (3.3 ± 1.2 versus 5.2 ± 2.2 [10−4.dL.kg−1.min−1/{μU/mL}]); Figure 1B). However, SIClamp was similar in both races for participants with BMI >33 (2.4 ± 1.1 versus 2.6 ± 1.0 [10−4.dL.kg−1.min−1/{μU/mL}]); Figure 1C).
FIGURE 1.
(A) Relation between skeletal muscle insulin sensitivity (SIClamp 10−4.dL.kg−1.min−1/[μU/mL]) assessed by the hyperinsulinemic-euglycemic clamp, and BMI, on the basis of a multiple linear regression model (n = 112) adjusted for the covariates sex, age, and total lean mass in African Americans (n = 55; black dots, bars, and line) and Caucasian Americans (n = 57; white dots, bars, and dashed line). P = 0.04 (race × BMI), P < 0.01 (overall model), R2 = 46%. Perforated line represents the critical point for the region of significance on BMI ≤ 33, SI Clamp significantly lower in African Americans (β = −0.08, SE = 0.03, P = 0.01, R2 = 30%) compared with Caucasian Americans (β = −0.33, SE = 0.06, P < 0.001, R2 = 37%). (B) *SIClamp significantly different between African Americans (n = 40) and Caucasian Americans (n = 53) with BMI ≤ 33. (C) SI Clamp not significantly different between African Americans (n = 15) and Caucasian Americans (n = 4) with BMI >33. Values are means; error bars in panels (B) and (C) are SD.
Similarly, the race × total fat interaction was significant (P = 0.01, R2 = 50%; Figure 2). A higher total fat mass was more strongly associated with a lower SIClamp in Caucasian Americans (β = −0.15, SE = 0.03, P < 0.001, R2 = 43%) than in African Americans (β = −0.04, SE = 0.02, P = 0.03, R2 = 28%).
FIGURE 2.
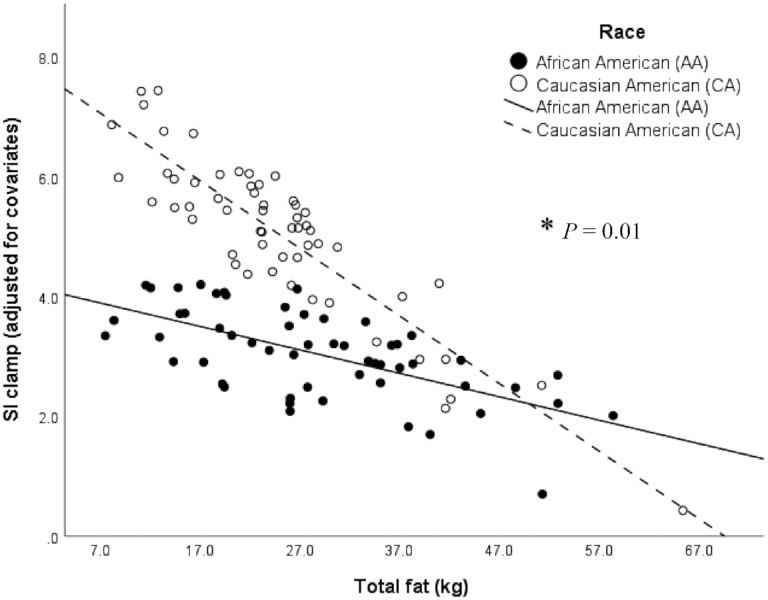
Relation between skeletal muscle insulin sensitivity (SI Clamp 10−4.dL.kg−1.min−1/[μU/mL]) assessed by the hyperinsulinemic-euglycemic clamp, and total fat mass (kg) measured by DXA, on the basis of a multiple linear regression model (n = 112) adjusted for the covariates sex, age, and total lean mass in African Americans (n = 55; black dots and line) and Caucasian Americans (n = 57; white dots and dashed line). *P = 0.01 significant race × total fat interaction, P < 0.01 (overall model), R2 = 50%. A higher total fat mass was more strongly associated with a lower SIClamp in Caucasian Americans (β = −0.15, SE = 0.03, P < 0.001, R2 = 43%) than in African Americans (β = −0.04, SE = 0.02, P = 0.03, R2 = 28%).
The race × SAAT interaction was significant (P = 0.046, R2 = 47%). A higher SAAT volume was more strongly associated with a lower SIClamp in Caucasian Americans (β = −0.82, SE = 0.17, P < 0.001, R2 = 33%) compared with African Americans (β = −0.27, SE = 0.07, P < 0.001, R2 = 38%). In assessing the effect of upper-body relative to lower-body fat distribution on SIClamp, leg fat was added as a covariate in the model for SAAT in addition to sex, age, and total lean mass (Table 2). The race × SAAT interaction remained significant (P = 0.047, R2 = 47%). The inverse association between SAAT and SIClamp was strengthened in African Americans (β = −0.45, SE = 0.11, P < 0.001; R2 = 44%), but was no longer significant in Caucasian Americans (β = −0.42, SE = 0.30, P = 0.17; R2 = 36%).
TABLE 2.
Multiple linear regression of subcutaneous abdominal adipose tissue on skeletal muscle insulin sensitivity by race
| R 2 | β | SE | P value for β | |
|---|---|---|---|---|
| Model I: African Americans (n = 55) | 44% | |||
| Sex | −0.009 | 0.54 | 0.99 | |
| Age, y | 0.001 | 0.02 | 0.97 | |
| Total lean mass, kg | −0.03 | 0.02 | 0.22 | |
| Leg fat, kg | 0.11 | 0.05 | 0.03 | |
| SAAT, L | −0.45 | 0.11 | <0.001 | |
| Model II: Caucasian Americans (n = 56) | 36% | |||
| Sex1 | 3.20 | 0.93 | 0.001 | |
| Age, y | 0.05 | 0.04 | 0.18 | |
| Total lean mass, kg | 0.09 | 0.05 | 0.06 | |
| Leg fat, kg | −0.23 | 0.14 | 0.12 | |
| SAAT, L | −0.42 | 0.30 | 0.17 |
Significant race × SAAT interaction (P = 0.047, R2 = 47%) in overall model. Boldface type indicates predictors with significant P values in race-specific models.
Males coded as 0, females coded as 1.
SIClamp, skeletal muscle insulin sensitivity assessed by the hyperinsulinemic-euglycemic clamp, SAAT, subcutaneous abdominal adipose tissue.
However, the race × IAAT interaction was not statistically significant (P = 0.65, R2 = 50%; Figure 3), and there was a similar association between IAAT and SIClamp in both African Americans (β = −0.66, SE = 0.47, P = 0.16, R2 = 31%) and Caucasian Americans (β = −0.54, SE = 0.53, P = 0.32, R2 = 44%). There was no significant difference between the intercepts (P = 0.34). Controlling for leg fat instead of total fat did not alter the results (race × IAAT, P = 0.68, R2 = 46%). The inverse association between IAAT and SIClamp reached statistical significance in both African Americans (β = −0.89, SE = 0.44, P = 0.048, R2 = 29%) and Caucasian Americans (β = −1.22, SE = 0.47, P = 0.01, R2 = 42%), and was strengthened in the latter model.
FIGURE 3.
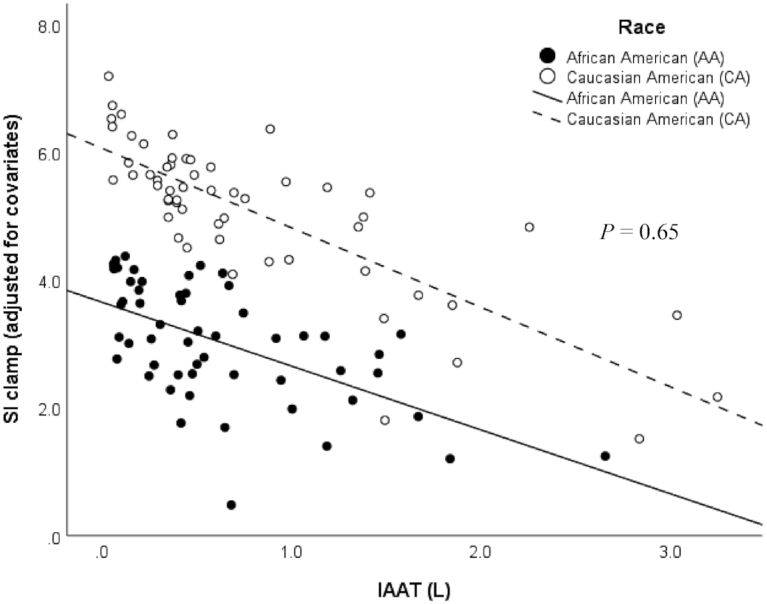
Relation between skeletal muscle insulin sensitivity (SIClamp 10−4.dL.kg−1.min−1[(μU/mL]) assessed by the hyperinsulinemic-euglycemic clamp, and intra-abdominal adipose tissue (IAAT [L]) measured by MRI, on the basis of a multiple linear regression model (n = 111) adjusted for the covariates sex, age, and total lean and total fat mass in African Americans (n = 55; black dots and line) and Caucasian Americans (n = 56; white dots and dashed line). P = 0.65 (race × IAAT interaction), P < 0.01 (overall model), R2 = 50%. IAAT, intra-abdominal adipose tissue.
There was also a significant race × liver fat interaction (P = 0.007, R2 = 52%; Figure 4). Liver fat was inversely associated with SIClamp in Caucasian Americans (β = −0.08, SE = 0.03, P = 0.02) but not in African Americans (β = 0.005, SE = 0.03, P = 0.87). The race-specific models that included liver fat explained 49% of the variance in SIClamp in Caucasian Americans, compared with 28% in African Americans.
FIGURE 4.
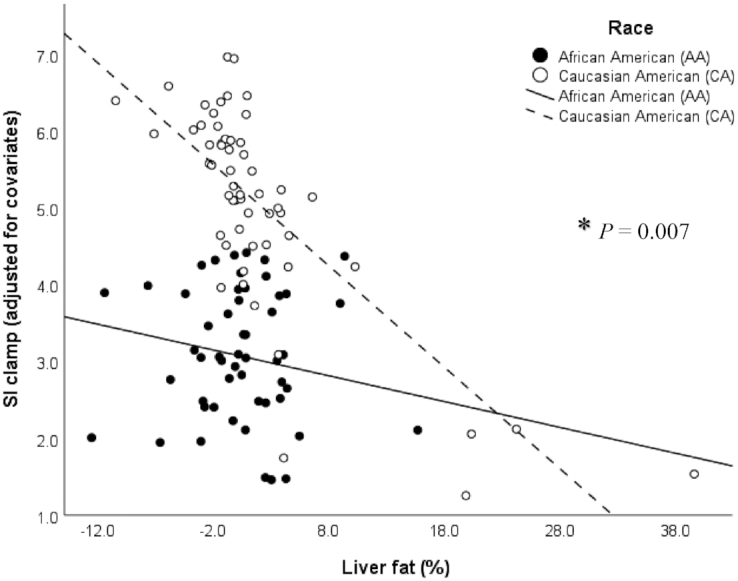
Relation between skeletal muscle insulin sensitivity (SIClamp 10−4.dL.kg−1.min−1/[μU/mL]) assessed by the hyperinsulinemic-euglycemic clamp, and liver fat (%) measured by MRI, on the basis of a multiple linear regression model (n = 111) adjusted for the covariates sex, age, and total fat and total lean mass in African Americans (n = 54; black dots and line) and Caucasian Americans (n = 57; white dots and dashed line). *P = 0.007 significant race × liver fat interaction, P < 0.01 (overall model), R2 = 52%. Liver fat was inversely associated with SIClamp in Caucasian Americans (β = −0.08, SE = 0.03, P = 0.02, R2 = 49%) but not in African Americans (β = 0.005, SE = 0.03, P = 0.87, R2 = 28%).
The association between HOMA2-IR as a proxy for hepatic insulin resistance and liver fat was also investigated using multiple linear regression. There was a significant race × liver fat (P = 0.03, R2 = 46%; Figure 5) interaction. Greater liver fat was associated with higher HOMA2-IR in Caucasian Americans (β = 0.03, SE = 0.01, P < 0.001) but not in African Americans (β = −0.004, SE = 0.01, P = 0.80). The race-specific models that included liver fat explained 64% of the variance in HOMA2-IR in Caucasian Americans, compared with 25% in African Americans.
FIGURE 5.
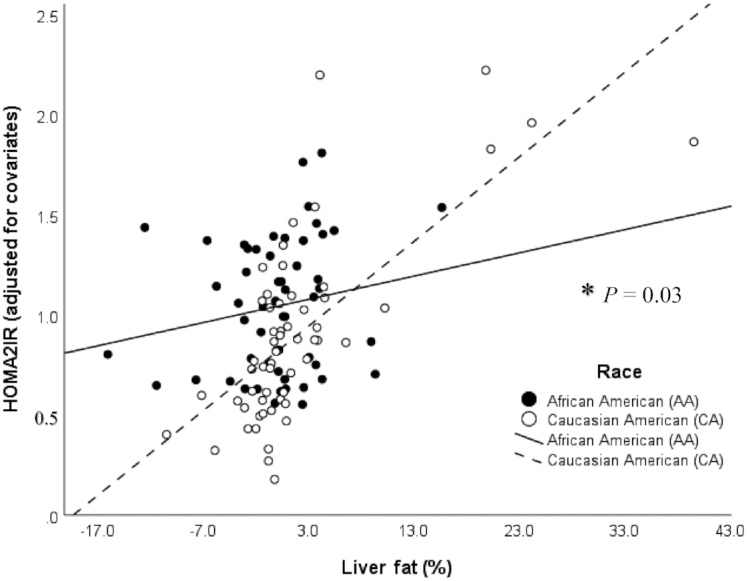
Relation between homeostasis model of assessment index 2-insulin resistance and liver fat (%) measured by MRI, on the basis of a multiple linear regression model (n = 111) adjusted for the covariates sex, age, and total fat and total lean mass in African Americans (n = 53; black dots and line) and Caucasian Americans (n = 58; white dots and dashed line). *P = 0.03 significant race × liver fat interaction, P < 0.01 (overall model), R2 = 46%. Greater liver fat was associated with higher HOMA2-IR in Caucasian Americans (β = 0.03, SE = 0.01, P < 0.001, R2 = 64%) but not in African Americans (β = −0.004, SE = 0.01, P = 0.80, R2 = 25%). HOMA2-IR, homeostasis model of assessment index 2-insulin resistance.
The race × leg fat interaction was significant (P = 0.009, R2 = 42%; Table 3). Higher leg fat was associated with a lower SIClamp (β = −0.39, SE = 0.08, P < 0.001, R2 = 34%) in Caucasian Americans, but not in African Americans (β = −0.05, SE = 0.04, P = 0.23, R2 = 23%). In these race-specific models, a higher total lean mass was associated with a lower SIClamp in African Americans (β = −0.05, SE = 0.03, P = 0.04) but not in Caucasian Americans (β = 0.08, SE = 0.05, P = 0.10). The race × leg fat interaction remained significant (P = 0.02, R2 = 51%) when SAAT and IAAT were added as covariates to the model to assess the effect of lower body relative to upper-body fat distribution on SIClamp (Table 4). In the models with SAAT and IAAT, higher leg fat was associated with a higher SIClamp in African Americans (β = 0.11, SE = 0.05, P = 0.02, R2 = 46%), but a lower SIClamp (β = −0.28, SE = 0.14, P = 0.049, R2 = 42%) in Caucasian Americans. Further, in these models, IAAT was inversely associated with SIClamp in Caucasian Americans (β = −1.16, SE = 0.53, P = 0.04), but not African Americans (β = −0.53, SE = 0.40, P = 0.19), and SAAT was inversely associated with SIClamp in African Americans (β = −0.41, SE = 0.11, P < 0.001) but not Caucasian Americans (β = −0.08, SE = 0.33, P = 0.81).
TABLE 3.
Multiple linear regression of leg fat (kg) on skeletal muscle insulin sensitivity by race
| R 2 | β | SE | P value for β | |
|---|---|---|---|---|
| Model I: African Americans (n = 55) | 23% | |||
| Sex | −0.23 | 0.62 | 0.71 | |
| Age, y | 0.11 | 0.02 | 0.59 | |
| Total lean mass, kg | −0.05 | 0.03 | 0.04 | |
| Leg fat, kg | −0.05 | 0.04 | 0.23 | |
| Model II: Caucasian Americans (n = 57) | 34% | |||
| Sex1 | 3.3 | 0.93 | 0.01 | |
| Age, y | 0.05 | 0.03 | 0.19 | |
| Total lean mass, kg | 0.08 | 0.05 | 0.10 | |
| Leg fat, kg | −0.39 | 0.08 | <0.001 |
Significant race × leg fat interaction (P = 0.009, R2 = 42%) in overall model. Boldface type indicates predictors with significant P values in race-specific models.
Males coded as 0, females coded as 1.
TABLE 4.
Multiple linear regression of leg fat (kg) on skeletal muscle insulin sensitivity by race, with subcutaneous abdominal adipose tissue and intra-abdominal adipose tissue as covariates
| R 2 | β | SE | P value for β | |
|---|---|---|---|---|
| Model I: African Americans (n = 55) | 46% | |||
| Sex | −0.19 | 0.55 | 0.73 | |
| Age, y | 0.02 | 0.02 | 0.44 | |
| Total lean mass, kg | −0.02 | 0.02 | 0.34 | |
| Leg fat, kg | 0.11 | 0.049 | 0.02 | |
| SAAT, L | −0.41 | 0.11 | <0.001 | |
| IAAT, L | −0.53 | 0.40 | 0.19 | |
| Model II: Caucasian Americans (n = 56) | 42% | |||
| Sex1 | 2.44 | 0.97 | 0.02 | |
| Age, y | 0.08 | 0.04 | 0.03 | |
| Total lean mass, kg | 0.09 | 0.05 | 0.05 | |
| Leg fat, kg | −0.28 | 0.14 | 0.049 | |
| SAAT, L | −0.08 | 0.33 | 0.81 | |
| IAAT, L | −1.16 | 0.53 | 0.04 |
Significant race × leg fat interaction (P = 0.02, R2 = 51%) in overall model. Boldface type indicates predictors with significant P values in race-specific models.
Males coded as 0, females coded as 1.
IAAT, intra-abdominal adipose tissue; SAAT, subcutaneous abdominal adipose tissue.
The race × total lean mass interaction was not significant (P = 0.54, R2 = 47%; Figure 6) and the difference between the intercepts was not statistically significant (P = 0.91).
FIGURE 6.
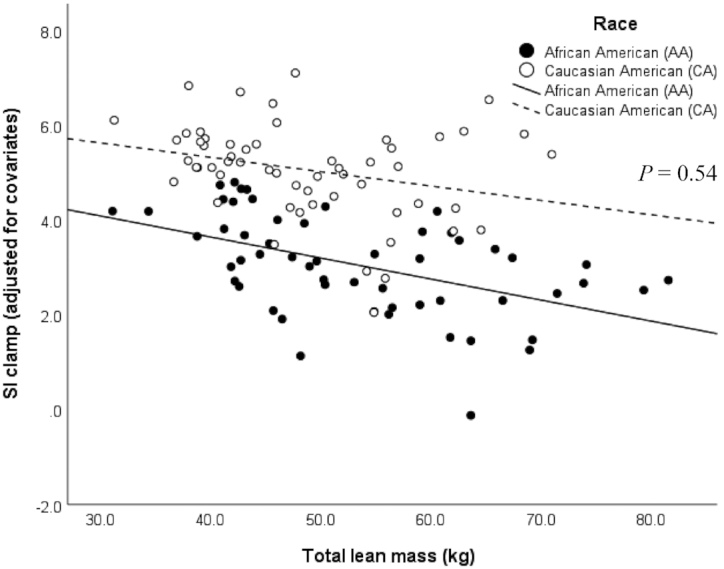
Relation between skeletal muscle insulin sensitivity (SIClamp 10−4.dL.kg−1.min−1/[μU/mL]) assessed by the hyperinsulinemic-euglycemic clamp, and total lean mass (kg) measured by DXA, on the basis of a multiple linear regression model (n = 112) adjusted for the covariates sex, age, and total fat mass in African Americans (n = 55; black dots and line) and Caucasian Americans (n = 57; white dots and dashed line). P = 0.54 (race × total lean mass interaction), P < 0.01 (overall model), R2 = 47%.
Discussion
The results of this study demonstrate that different metrics of adiposity and regional fat are differentially associated with insulin sensitivity in healthy African Americans and Caucasian Americans. Specifically, measures of overall adiposity were more strongly associated with SIClamp in Caucasian Americans, whereas body fat distribution and lean mass showed stronger correlations with SIClamp in African Americans. These observations resolve discrepancies in the literature regarding race differences in insulin sensitivity and suggest that development of race-specific preventive and therapeutic strategies may reduce the racial disparity in diabetes prevalence.
A key finding of this study was that higher total adiposity, reflected in total fat mass and BMI, was more strongly associated with a lower SIClamp in Caucasian Americans compared with African Americans. Examination of the significant race × BMI interaction showed that for participants with BMI ≤ 33, SIClamp was lower in African Americans. However, for participants with BMI > 33, SIClamp was similar in both races. Although a limited number of Caucasian Americans had BMI > 33, these results are corroborated by prospective data from a 16-wk controlled, weight loss intervention study that showed insulin sensitivity is similar between races among overweight individuals both before and after weight loss, but lower in African Americans compared with Caucasian Americans among never obese, lean individuals (6, 25). A positive association between adipocyte hypertrophy and insulin resistance has also been reported in insulin-sensitive but not insulin-resistant African American women (26). Collectively, the data imply the existence of a phenotype of insulin resistance in lean African Americans that protects against weight gain, and another of relative insulin sensitivity that predisposes to weight gain (25). Thus, the question of whether insulin sensitivity differs between African Americans and Caucasian Americans depends on the specific phenotype examined and may explain the inconsistent findings from previous research (3–5, 7, 21, 26).
Similar to measures of total adiposity, regional adipose depots explained more of the variance in SIClamp in Caucasian Americans than African Americans. A higher SAAT volume was more strongly associated with a lower SIClamp in Caucasian Americans compared with African Americans. This is consistent with reports that SAAT (especially deep SAAT) is strongly associated with insulin resistance in predominantly white populations (19, 21). However, controlling for leg fat mitigated the association in Caucasian Americans, suggesting that overall adiposity, whether in central or peripheral depots, is a more significant determinant of SIClamp in Caucasian Americans. In contrast, among African Americans, adjustment for leg fat strengthened the inverse association of SAAT with SIClamp, indicating that an upper-body fat distribution is a particularly relevant metabolic health measure in this racial group.
Likewise, high leg fat was associated with a lower SIClamp in Caucasian Americans but not African Americans. However, when SAAT and IAAT were included, the resulting model explained the most variance in SIClamp in African Americans. Leg fat was inversely associated with SIClamp in Caucasian Americans but positively associated with SIClamp in African Americans suggesting that a peripheral fat distribution is associated with a higher SIClamp in African Americans. In support of this concept, obese black women have lower expression of adipogenic and lipogenic genes in gluteal adipose tissue when compared with obese white women, and this lower gene expression is associated with lower insulin sensitivity (27). Evidence from integrative genetic analyses suggest that insulin resistance occurs as a result of genetically determined defects in adipose differentiation and expansion that manifest as an impaired capacity to store fat in the gluteofemoral region (28). Further, impaired peripheral adipose storage capacity predicts the development of cardiovascular disease and T2D (28). Taken together, these novel findings indicate that African Americans may be particularly sensitive to the development of insulin resistance in response to impaired gluteal adipogenic capacity and the inability to protect against ectopic fat accumulation. Whether this has a genetic basis is unclear and warrants investigation.
Both race groups showed similar inverse associations between IAAT and SIClamp, which agrees with the findings of previous studies (4, 26). However, liver fat was inversely associated with SIClamp only in Caucasian Americans. African Americans have less liver fat (29) due in part to variations in the PNPLA3 gene (30) and liver fat was not significantly associated with diabetes risk in African Americans (31). Nevertheless, a smaller study showed liver fat was associated with insulin resistance in both races (26). These discrepant findings may be explained by the higher clamp insulin infusion rate in the current study, which is expected to suppress hepatic glucose production (32). In this study, HOMA2-IR was associated with liver fat in Caucasian Americans but not African Americans and liver fat also explained more of the variance in SIClamp and HOMA2-IR in Caucasian Americans. Collectively, these findings suggest that liver fat may play a more important role in the development of insulin resistance in Caucasian Americans than African Americans.
Lean mass was inversely associated with SIClamp in African Americans but not Caucasian Americans in the model for leg fat. These results extend the findings of previous studies conducted in women that showed greater skeletal muscle volume in African Americans partially accounted for the race difference in insulin resistance (4). This paradoxical finding may be explained by physiological mechanisms intrinsic to skeletal muscle in African Americans. Impaired bioenergetic capacity of skeletal muscle mitochondria, which is linked to insulin resistance in T2D (33, 34), has been reported in African-American women (35, 36). African Americans have a higher proportion of glycolytic type II myofibers and a lower proportion of oxidative type I myofibers; this fiber composition may affect oxidative capacity and insulin sensitivity (37–42). Taken together, these differences in skeletal muscle physiology may predispose African Americans to the development of insulin resistance and T2D.
Collectively, these results have important implications for the prevention and treatment of insulin resistance and related metabolic conditions amongst individuals of different races. In contrast to measures that merely target overall adiposity, strategies that promote a redistribution of adipose by reducing ectopic fat and promoting peripheral fat deposition are likely to be more beneficial to African Americans. Emerging evidence shows a carbohydrate-restricted diet produced preferential loss of IAAT and intermuscular adipose tissue compared with a eucaloric low-fat diet in women with polycystic ovary syndrome (43). Future research should examine whether these improvements in body fat distribution occur to a greater extent in African Americans. Clinical studies should also assess the effects of thiazolidinediones on body fat distribution and insulin sensitivity in African Americans. These insulin-sensitizers affect fat metabolism by activating PPARγ, causing adipose tissue remodeling by promoting adipocyte differentiation and peripheral adipose expansion (44).
Important strengths of this study include the assessment of insulin sensitivity using the hyperinsulinemic-euglycemic clamp and assessment of fat distribution using MRI techniques in a biracial cohort of lean, overweight, and obese men and women. However, the cross-sectional nature of the study does not establish causality.
In conclusion, overall adiposity was significantly associated with SIClamp in Caucasian Americans, whereas body fat distribution and lean mass showed stronger associations with SIClamp in African Americans. Research is needed to determine if specific insulin resistance phenotypes have a genetic basis in both African Americans and Caucasian Americans, and how best to intervene in a phenotype-specific manner to minimize the development of metabolic disease.
ACKNOWLEDGEMENTS
We thank the volunteers for their participation and gratefully acknowledge the staff of the UAB Metabolism Core Laboratory (Nutrition Obesity Research Center, Diabetes Research and Training Center, Center for Clinical and Translational Science) for their involvement in this study.
The authors’ contributions were as follows—JT and BAG: had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis; JT and BAG: analysis and interpretation of data, drafting of the manuscript; all authors: study concept and design, critical revision of the manuscript for intellectual content, read and approved the final manuscript. Obtained funding: AMG, TWG, MEL, MJQ, GF, and BAG. None of the authors reported a conflict of interest related to this study.
Notes
Some preliminary data were presented at the American Diabetes Association's 77th scientific sessions, San Diego, CA, 9–13 June 2017.
This study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases at the NIH (R01DK096388), University of Alabama at Birmingham (UAB) Nutrition Obesity Research Center (P30DK56336), and Diabetes Research Center (P30DK079626). JT was supported by a postgraduate research scholarship from the Agency for Science, Technology and Research (A-STAR), Singapore. Funding sources had no role in the design, conduct, analysis, and interpretation of the data, reporting of the study, or the decision to submit the manuscript for publication.
Abbreviations used: HOMA2-IR, homeostasis model of assessment index 2-insulin resistance; IAAT, intra-abdominal adipose tissue; J–N, Johnson–Neyman procedure; SAAT, subcutaneous abdominal adipose tissue; SIClamp, skeletal muscle insulin sensitivity assessed by the hyperinsulinemic-euglycemic clamp; T2D, type 2 diabetes.
References
- 1. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Dept of Health and Human Services; 2017. [Google Scholar]
- 2. Reaven GM. Why syndrome X? From Harold Himsworth to the insulin resistance syndrome. Cell Metab. 2005;1:9–14. [DOI] [PubMed] [Google Scholar]
- 3. Haffner SM, D'Agostino R, Saad MF, Rewers M, Mykkanen L, Selby J, Howard G, Savage PJ, Hamman RF, Wagenknecht LE et al.. Increased insulin resistance and insulin secretion in nondiabetic African-Americans and Hispanics compared with non-Hispanic whites. The Insulin Resistance Atherosclerosis Study. Diabetes. 1996;45:742–8. [DOI] [PubMed] [Google Scholar]
- 4. Albu JB, Kovera AJ, Allen L, Wainwright M, Berk E, Raja-Khan N, Janumala I, Burkey B, Heshka S, Gallagher D. Independent association of insulin resistance with larger amounts of intermuscular adipose tissue and a greater acute insulin response to glucose in African American than in white nondiabetic women. Am J Clin Nutr. 2005;82:1210–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Weiss R, Dziura JD, Burgert TS, Taksali SE, Tamborlane WV, Caprio S. Ethnic differences in beta cell adaptation to insulin resistance in obese children and adolescents. Diabetologia. 2006;49:571–9. [DOI] [PubMed] [Google Scholar]
- 6. Gower BA, Weinsier RL, Jordan JM, Hunter GR, Desmond R. Effects of weight loss on changes in insulin sensitivity and lipid concentrations in premenopausal African American and white women. Am J Clin Nutr. 2002;76:923–7. [DOI] [PubMed] [Google Scholar]
- 7. Hannon TS, Bacha F, Lin Y, Arslanian SA. Hyperinsulinemia in African-American adolescents compared with their American white peers despite similar insulin sensitivity: a reflection of upregulated beta-cell function?. Diabetes Care. 2008;31:1445–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chandler-Laney PC, Phadke RP, Granger WM, Fernandez JR, Munoz JA, Man CD, Cobelli C, Ovalle F, Gower BA. Age-related changes in insulin sensitivity and beta-cell function among European-American and African-American women. Obesity (Silver Spring). 2011;19:528–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gower BA, Nagy TR, Trowbridge CA, Dezenberg C, Goran MI. Fat distribution and insulin response in prepubertal African American and white children. Am J Clin Nutr. 1998;67:821–7. [DOI] [PubMed] [Google Scholar]
- 10. Gower BA, Granger WM, Franklin F, Shewchuk RM, Goran MI. Contribution of insulin secretion and clearance to glucose-induced insulin concentration in African-American and Caucasian children. J Clin Endocrinol Metab. 2002;87:2218–24. [DOI] [PubMed] [Google Scholar]
- 11. Bacha F, Saad R, Gungor N, Janosky J, Arslanian SA. Obesity, regional fat distribution, and syndrome X in obese black versus white adolescents: race differential in diabetogenic and atherogenic risk factors. J Clin Endocrinol Metab. 2003;88:2534–40. [DOI] [PubMed] [Google Scholar]
- 12. Piccinini F, Polidori DC, Gower BA, Fernandez JR, Bergman RN. Dissection of hepatic versus extra-hepatic insulin clearance: ethnic differences in childhood. Diabetes Obes Metab. 2018;20:2869–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Piccinini F, Polidori DC, Gower BA, Bergman RN. Hepatic but not extrahepatic insulin clearance is lower in African American than in European American women. Diabetes. 2017;66:2564–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab. 2008;294:E15–26. [DOI] [PubMed] [Google Scholar]
- 15. Bray GA, Jablonski KA, Fujimoto WY, Barrett-Connor E, Haffner S, Hanson RL, Hill JO, Hubbard V, Kriska A, Stamm E et al.. Relation of central adiposity and body mass index to the development of diabetes in the Diabetes Prevention Program. Am J Clin Nutr. 2008;87:1212–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goss AM, Gower BA.. Insulin sensitivity is associated with thigh adipose tissue distribution in healthy postmenopausal women. Metabolism. 2012;61:1817–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Virkamaki A, Korsheninnikova E, Seppala-Lindroos A, Vehkavaara S, Goto T, Halavaara J, Hakkinen AM, Yki-Jarvinen H. Intramyocellular lipid is associated with resistance to in vivo insulin actions on glucose uptake, antilipolysis, and early insulin signaling pathways in human skeletal muscle. Diabetes. 2001;50:2337–43. [DOI] [PubMed] [Google Scholar]
- 18. Karter AJ, Mayer-Davis EJ, Selby JV, D'Agostino RB Jr, Haffner SM, Sholinsky P, Bergman R, Saad MF, Hamman RF. Insulin sensitivity and abdominal obesity in African-American, Hispanic, and non-Hispanic white men and women. The Insulin Resistance and Atherosclerosis Study. Diabetes. 1996;45:1547–55. [DOI] [PubMed] [Google Scholar]
- 19. Kelley DE, Thaete FL, Troost F, Huwe T, Goodpaster BH. Subdivisions of subcutaneous abdominal adipose tissue and insulin resistance. Am J Physiol Endocrinol Metab. 2000;278:E941–8. [DOI] [PubMed] [Google Scholar]
- 20. Goedecke JH, Levitt NS, Lambert EV, Utzschneider KM, Faulenbach MV, Dave JA, West S, Victor H, Evans J, Olsson T et al.. Differential effects of abdominal adipose tissue distribution on insulin sensitivity in black and white South African women. Obesity (Silver Spring). 2009;17:1506–12. [DOI] [PubMed] [Google Scholar]
- 21. Lovejoy JC, de la Bretonne JA, Klemperer M, Tulley R. Abdominal fat distribution and metabolic risk factors: effects of race. Metabolism. 1996;45:1119–24. [DOI] [PubMed] [Google Scholar]
- 22. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487–95. [DOI] [PubMed] [Google Scholar]
- 23. Ma J, Son JB, Bankson JA, Stafford RJ, Choi H, Ragan D. A fast spin echo two-point Dixon technique and its combination with sensitivity encoding for efficient T2-weighted imaging. Magn Reson Imaging. 2005;23:977–82. [DOI] [PubMed] [Google Scholar]
- 24. Preacher KJ, Curran PJ, Bauer DJ. Computational tools for probing interaction effects in multiple linear regression, multilevel modeling, and latent curve analysis. J Educ Behav Stat. 2006;31:437–48. [Google Scholar]
- 25. Gower BA, Alvarez JA, Bush NC, Hunter GR. Insulin sensitivity affects propensity to obesity in an ethnic-specific manner: results from two controlled weight loss intervention studies. Nutr Metab (Lond). 2013;10:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Allister-Price C, Craig CM, Spielman D, Cushman SS, McLaughlin TL. Metabolic markers, regional adiposity, and adipose cell size: relationship to insulin resistance in African-American as compared with Caucasian women. Int J Obes (Lond). 2019;43(6):1164–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Goedecke JH, Evans J, Keswell D, Stimson RH, Livingstone DE, Hayes P, Adams K, Dave JA, Victor H, Levitt NS et al.. Reduced gluteal expression of adipogenic and lipogenic genes in Black South African women is associated with obesity-related insulin resistance. J Clin Endocrinol Metab. 2011;96:E2029–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lotta LA, Gulati P, Day FR, Payne F, Ongen H, van de Bunt M, Gaulton KJ, Eicher JD, Sharp SJ, Luan J et al.. Integrative genomic analysis implicates limited peripheral adipose storage capacity in the pathogenesis of human insulin resistance. Nat Genet. 2017;49:17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Guerrero R, Vega GL, Grundy SM, Browning JD. Ethnic differences in hepatic steatosis: an insulin resistance paradox?. Hepatology. 2009;49:791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, Boerwinkle E, Cohen JC, Hobbs HH. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Toledo-Corral CM, Alderete TL, Hu HH, Nayak K, Esplana S, Liu T, Goran MI, Weigensberg MJ. Ectopic fat deposition in prediabetic overweight and obese minority adolescents. J Clin Endocrinol Metab. 2013;98:1115–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Seppala-Lindroos A, Vehkavaara S, Hakkinen AM, Goto T, Westerbacka J, Sovijarvi A, Halavaara J, Yki-Jarvinen H. Fat accumulation in the liver is associated with defects in insulin suppression of glucose production and serum free fatty acids independent of obesity in normal men. J Clin Endocrinol Metab. 2002;87:3023–8. [DOI] [PubMed] [Google Scholar]
- 33. Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med. 2004;350:664–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51:2944–50. [DOI] [PubMed] [Google Scholar]
- 35. DeLany JP, Dube JJ, Standley RA, Distefano G, Goodpaster BH, Stefanovic-Racic M, Coen PM, Toledo FG. Racial differences in peripheral insulin sensitivity and mitochondrial capacity in the absence of obesity. J Clin Endocrinol Metab. 2014;99:4307–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sirikul B, Gower BA, Hunter GR, Larson-Meyer DE, Newcomer BR. Relationship between insulin sensitivity and in vivo mitochondrial function in skeletal muscle. Am J Physiol Endocrinol Metab. 2006;291:E724–8. [DOI] [PubMed] [Google Scholar]
- 37. Ceaser T, Hunter G.. Black and White race differences in aerobic capacity, muscle fiber type, and their influence on metabolic processes. Sports Med. 2015;45:615–23. [DOI] [PubMed] [Google Scholar]
- 38. Zierath JR, Hawley JA. Skeletal muscle fiber type: influence on contractile and metabolic properties. PLoS Biology. 2004;2:e348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stuart CA, McCurry MP, Marino A, South MA, Howell ME, Layne AS, Ramsey MW, Stone MH. Slow-twitch fiber proportion in skeletal muscle correlates with insulin responsiveness. J Clin Endocrinol Metab. 2013;98:2027–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Albers PH, Pedersen AJ, Birk JB, Kristensen DE, Vind BF, Baba O, Nohr J, Hojlund K, Wojtaszewski JF. Human muscle fiber type-specific insulin signaling: impact of obesity and type 2 diabetes. Diabetes. 2015;64:485–97. [DOI] [PubMed] [Google Scholar]
- 41. Oberbach A, Bossenz Y, Lehmann S, Niebauer J, Adams V, Paschke R, Schon MR, Bluher M, Punkt K. Altered fiber distribution and fiber-specific glycolytic and oxidative enzyme activity in skeletal muscle of patients with type 2 diabetes. Diabetes Care. 2006;29:895–900. [DOI] [PubMed] [Google Scholar]
- 42. Fisher G, Windham ST, Griffin P, Warren JL, Gower BA, Hunter GR. Associations of human skeletal muscle fiber type and insulin sensitivity, blood lipids, and vascular hemodynamics in a cohort of premenopausal women. Eur J Appl Physiol. 2017;117:1413–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Goss AM, Chandler-Laney PC, Ovalle F, Goree LL, Azziz R, Desmond RA, Wright Bates G, Gower BA. Effects of a eucaloric reduced-carbohydrate diet on body composition and fat distribution in women with PCOS. Metabolism. 2014;63:1257–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Spiegelman BM. PPAR-gamma: adipogenic regulator and thiazolidinedione receptor. Diabetes. 1998;47:507–14. [DOI] [PubMed] [Google Scholar]