ABSTRACT
Background
Food advertising is a major contributor to obesity, and fast food (FF) restaurants are top advertisers. Research on the impact of food advertising in adolescents is lacking and no prior research has investigated neural predictors of food intake in adolescents. Neural systems implicated in reward could be key to understanding how food advertising drives food intake.
Objectives
To investigate how neural responses to both unhealthy and healthier FF commercials predict food intake in adolescents.
Methods
A cross-sectional sample of 171 adolescents (aged 13–16 y) who ranged from normal weight to obese completed an fMRI paradigm where they viewed unhealthy and healthier FF and nonfood commercials. Adolescents then consumed a meal in a simulated FF restaurant where foods of varying nutritional profiles (unhealthy compared with healthier) were available.
Results
Greater neural activation in reward-related regions (nucleus accumbens, r = 0.29; caudate nucleus, r = 0.27) to unhealthy FF commercials predicted greater total food intake. Greater responses to healthier FF relative to nonfood commercials in regions associated with reward (i.e., nucleus accumbens, r = 0.24), memory (i.e., hippocampus, r = 0.32), and sensorimotor processes (i.e., anterior cerebellum, r = 0.33) predicted greater total food and unhealthier food intake, but not healthier food intake. Lower activation in neural regions associated with visual attention and salience (e.g., precuneus, r = −0.35) to unhealthy relative to healthier FF commercials predicted healthier food intake.
Conclusions
These findings suggest that FF commercials contribute to overeating in adolescents through reward mechanisms. The addition of healthier commercials from FF restaurants is unlikely to encourage healthier food intake, but interventions that reduce the ability of unhealthy FF commercials to capture attention could be beneficial. However, an overall reduction in the amount of FF commercials exposure for adolescents is likely to be the most effective approach.
Keywords: food advertising, commercials, eating, fast food, adolescents, fMRI
Introduction
Food advertising to youth has been identified as a significant contributor to the growing global rates of obesity, and exposure to unhealthy food commercials is associated with increased consumption of calorie-dense, nutrient-poor foods (1, 2). Adolescents are a major target of food commercials, but only children aged ≤11 y receive any protections through industry self-regulation (3). Fast food (FF) restaurants are top advertisers to children and adolescents, and FF commercials typically feature unhealthy foods (4). Increasing commercials that feature healthier foods has been proposed as a strategy to encourage healthier food intake (2, 5), but little is known about the ability of healthier food commercials from FF restaurants to affect eating behavior (6, 7). Food commercials can influence behavior by priming automatic, sometimes unconscious, physiological and psychological responses that can be challenging to defend against (1). The ability of FF commercials to engage reward-related neural systems could be key to their effectiveness (1).
Neuroimaging studies have moved beyond identifying the basic patterns of neural activity and now use brain responses as predictors of important behaviors (8). The brain-as-predictor model is especially useful in capturing unconscious, biological processes that might not be captured by self-report (8), which makes it a useful approach for investigating how food commercials might be contributing to food intake. In adults, greater activation in the nucleus accumbens (NAcc), which is associated with reward motivation, in response to food images is uniquely predictive of increased food intake (9, 10). Adolescents could be particularly neurally vulnerable to the rewarding nature of food commercials, because systems associated with reward are heightened during this developmental stage (11). There has been no research investigating whether neural responses (to any type of stimuli) predict food intake in adolescents (8). Investigating whether neural responses to food commercials predict food intake in adolescents is an important step in identifying neural predictors of food intake and the mechanisms through which food commercials increase food intake in this age group.
The primary aim of the current study was to investigate in adolescents how neural responses to unhealthy and healthier FF commercials relative to each other and to nonfood (i.e., phone) commercials predicted postscan food intake. In the current study, 171 adolescents (aged 13–16 y) viewed unhealthy FF commercials (e.g., cheeseburgers, french fries), healthier FF commercials (e.g., salads, grilled chicken sandwiches), and nonfood commercials (i.e., phone commercials) in an fMRI paradigm. Then participants were able to consume foods featured in the FF commercials that varied in nutritional profile (e.g., cheeseburgers, salads) in a simulated FF restaurant. We hypothesized that greater reward-related neural response, particularly in the NAcc (9), to unhealthy FF commercials would predict greater total and unhealthy food intake. We hypothesized that greater reward-related neural response to healthier FF commercials would predict greater healthier food intake.
Methods
Participants
Participants who enrolled in the study were 193 adolescents [99 females, 94 males; mean age = 14.28 ± 1.03 y; range = 13–16 y; mean BMI (kg/m2) = 24.10 ± 5.35; BMI z-score = 0.87 ± 0.92; healthy weight: n = 103 (53.4%); overweight: n = 48 (24.9%); obese: n = 42 (21.2%)] recruited from southeast Michigan between 2015 and 2017. Participants reported the following racial and ethnic backgrounds: 8.9% Hispanic American, 2.6% American Indian/Alaska Native, 2.1% Asian American, 14.1% Black, 70.3% White, 2.6% other, 6.3% mixed, and 2.1% unknown. Participants were recruited to participate in a research study about how the brain responds to advertising. Inclusion criteria were English-speaking adolescents who were 13–16 y of age. Exclusion criteria were current use of psychotropic medications or illicit drugs, lifetime psychiatric disorder, a BMI percentile <5%, or fMRI contraindicators (e.g., presence of metal implants). In total, 186 adolescents completed the fMRI scan. Nine participants showed excessive movement during the scan. fMRI data of 2 participants were collected with an acquisition error resulting in altered deformation field output, resulting in unusable data. Two participants who completed the fMRI scan did not complete the food intake task, and food intake data of 2 participants were statistical outliers (>3 SDs from the mean) and were excluded from analyses. The final sample consisted of 171 participants (see Supplemental Figure 1 for a flowchart). Participants who were excluded from the final analyses did not significantly differ on age, BMI, sex or race/ethnicity (all P values >0.50).
Study procedures
All procedures were reviewed and approved by the University of Michigan Institutional Review Board. Participants came to the laboratory on 2 separate days. On the first day, participants provided written assent and legal guardians provided written informed consent according to the Declaration of Helsinki. At the first visit, participants completed a practice scan in a mock scanner. Participants returned for a second visit on average 4.14 ± 2.91 d after the baseline assessment in which they completed the fMRI commercial neuroimaging paradigm, rated liking for the commercials, and then had a meal in a simulated FF restaurant. The primary outcome variable was food intake in the simulated FF restaurant.
Scan procedures
Participants were asked to consume regular meals, but to refrain from eating or drinking (except water) following their last meal before the scan. Over 87% of the scans occurred between 15:00 and 18:00, whereas the remaining scans occurred between 10:30 and 14:00. Upon arrival, participants rated their hunger on a scale from 1 (not hungry at all) to 100 (extremely hungry); if a rating ≥70 was indicated, participants were offered a small snack to normalize their hunger to a neutral state. A total of 7% received a snack (n = 13) (e.g., crackers, fruit) that was not advertised in the fMRI commercials paradigm. Total scan duration was 45 min, including breaks and the necessary anatomical scan. Participants were instructed to watch the commercials. To motivate participants to attend to the clips, participants were told they would complete a commercial-recognition task after the scan. On average, participants correctly recognized 76.6% of the advertised FF items.
Measures
Anthropometrics
Participants’ height and weight were collected in light clothing without jackets, socks, or shoes, using an O'Leary Acrylic Stadiometer and Detecto Portable Scale in centimeters and kilograms (to the nearest tenth), respectively. BMI was calculated and z-scored (BMIz) for age and sex based on the CDC growth charts (12).
fMRI commercials paradigm
The fMRI commercials paradigm was developed to include commercials commonly viewed by adolescents. FF commercials are typically shown alongside nonfood commercials. Thus, to increase external validity and provide a nonfood comparison commercial, phone commercials were included in the fMRI commercials paradigm alongside the FF commercials. Phones were chosen because they are frequently advertised (13) and are a product that is relevant to adolescents. Based on Nielsen gross ratings point national data (13), McDonalds, Wendy's, AT&T, and Verizon ranked in the top 20 for companies with the most advertising viewed by young teens (ages 12–14 y) in 2012. Stimuli were 20 FF commercials promoting unhealthy food from McDonalds and Wendy's (e.g., Crispy Chicken Sandwich), 20 FF commercials for healthier food from McDonalds and Wendy's (e.g., Grilled Chicken Salad), and 20 phone commercials from AT&T and Verizon (e.g., iPhone). The healthfulness of food shown in the FF commercials was evaluated using the Nutritional Profiling Index (NPI), which is an objective measure of overall nutrition quality based on numerical nutrition information (e.g., calories, sugar, fat) adapted from the nutrient profiling system used in the United Kingdom to identify healthier food that can be advertised to children (14, 15). NPI scores range from 1 to 100, and lower scores indicate less-healthy items. Foods lose points for the inclusion of nutrients that should be limited (e.g., saturated fat, sugar, sodium) and receive points for nutrients that should be promoted (e.g., fiber, protein, inclusion of fruits/vegetables). Foods with an NPI score ≥64 qualify as healthier foods that can be advertised to children on television in the United Kingdom. Foods in the unhealthy FF commercials had an average NPI score of 44.05 ± 4.21, and food in the healthier FF commercials had an average NPI score of 70.20 ± 3.86 (see Supplemental Table 1 for a full list of commercials and corresponding NPI scores, and Supplemental Materials and Methods for more information on stimuli choice).
Each commercial in the paradigm lasted approximately 15 s and was shown only once. Between each commercial was a jittered fixation cross (4–8 s) (Figure 1). The paradigm consisted of four 7-min runs. The order of commercials (i.e., unhealthy FF, healthier FF, phone) was randomized in each of the runs and the order of the 4 runs was randomized over the participants.
FIGURE 1.
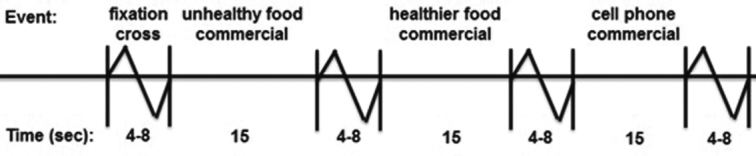
Example of timing and ordering of the presentation of the commercials during the commercials paradigm (n = 171).
Self-reported liking
Following the scan, participants were asked to report “How much do you LIKE the following products?” for each of the advertised items (i.e., unhealthy FF, healthier FF, phones). Responses were provided on a 5-point scale ranging from Dislike Extremely (1 point) to Like Extremely (5 points).
Food intake task
Food intake was assessed after the scan in an FF laboratory (16). The laboratory was designed to simulate an FF restaurant and included booths, menu boards, and a food preparation station (see Supplemental Figure 2 for images of the simulated FF restaurant). To provide an FF restaurant olfactory cue, french fries were cooked prior to the participants’ entry into the simulated FF restaurant. Menu options available to participants resembled those featured in the FF commercials: unhealthy foods (e.g., cheeseburgers, french fries) and healthier foods (e.g., grilled chicken sandwiches, salads) (see Supplemental Table 2 for full list of available foods and nutrition information). Participants were informed they could order as much as they wanted and had unlimited time to eat. Foods and beverages were weighed to the nearest tenth of a gram prior to serving and after the participant had finished eating. The food and beverages that remained were subtracted from the initial weight to quantify the amount consumed. This was used to compute the amount of kilocalories consumed of unhealthy and healthier foods based on each item's standard nutritional information. To assess participants’ total food intake (which was dependent on the combined amount of unhealthy and healthier food intake), the kilocalories of unhealthy and healthier food intake were summed.
fMRI data acquisition, preprocessing, and analysis
Data were acquired with a GE Discovery MR750 3T scanner. An 8-channel head coil acquired data from the entire brain. In total, 156 scans were collected during each of 4 functional runs. Functional data were acquired using a spiral sequence with the following parameters: repetition time (TR) = 2000 ms; echo time (TE) = 30 ms; flip angle = 90°; field of view (FOV) = 22 × 22 cm2; acquisition matrix = 64 × 64; 3-mm slice thickness with no gap, 43 axial slices, voxel size = 3.44 × 3.44 × 3.0 mm. Anatomical scans were acquired using a high-resolution T1-weighted spoiled-gradient-recalled acquisition (TR = 12.3 ms; TE = 5.2 ms; inversion-time (TI) = 500 ms; flip angle = 15°; FOV = 22 × 22 cm2; slice thickness = 1.0 mm; voxel size = 1 × 1 × 1 mm). Prior to preprocessing, all images were manually realigned to the anterior commissure–posterior commissure line in statistical parametric mapping (SPM) and skullstripped using the Brain Extraction Tool in the Functional MRI of the Brain Software Library (FSL) (FMRIB Analysis Group). Neuroimaging data were preprocessed and analyzed primarily using SPM (SPM12; Wellcome Centre for Human Neuroimaging) in Matlab (Mathworks, Inc). Anatomical images were segmented and normalized to Montreal Neurological Institute (MNI) space with the use of the DARTEL (diffeomorphic anatomical registration through exponentiated lie algebra) toolbox (17). Anatomical data were coregistered to the mean functional image and segmented into 6 tissue types using a unified segmentation approach (18). Functional images were realigned to the mean, coregistered with the anatomical images, normalized to MNI space with the use of DARTEL, and smoothed with an 8-mm full-width at half-maximum isotropic Gaussian kernel. DARTEL was used to create a group anatomical template, transformations from which were applied to warp functional data to the International Consortium for Brain Mapping (ICBM)-152 template supplied with SPM12 (17). The Artifact Detection Tools (ART; http://www.nitrc.org/projects/artifact_detect/) software package was used for automatic detection of spike and motion in the functional data. Motion parameters were included as regressors in the design matrix at individual-level analysis. Additionally, image volumes where the z-normalized global brain activation was >3 SDs from the mean of the run or showed >1.5 mm of composite (linear plus rotational) movement were flagged as outliers and deweighted during individual-level model estimation. A general linear model was created with 3 regressors of interest, modeled as events: unhealthy FF commercials, healthier FF commercials, phone commercials. A high-pass filter of 128 s was applied to eliminate low-frequency fluctuations in the signal, and autoregressive(1) was used to correct for serial autocorrelations. The model was convolved with the canonical hemodynamic response function.
At the subject level, the blood oxygen level–dependent (BOLD) signal was modeled in a fixed effects analysis with separate regressors modeling each event of interest (i.e., unhealthy FF commercials, healthier FF commercials, phone commercials) (15 s). Individual SPM contrasts were constructed to compare the activations within each participant during unhealthy FF commercials compared with healthier FF commercials (unhealthy FF commercials > healthier FF commercials, and healthier FF commercials > unhealthy FF commercials), unhealthy FF commercials compared with phone commercials (unhealthy FF commercials > phone commercials, and phone commercials > unhealthy FF commercials), and healthier FF commercials compared with phone commercials (healthier FF commercials > phone commercials, and phone commercials > healthier FF commercials).
To test whether neural response to food commercials of differing nutritional profiles predicts unhealthy and healthier food intake in the FF laboratory, we entered the individual SPM contrasts (unhealthy FF commercials > healthier FF commercials; unhealthy FF commercials > phone commercials; healthier FF commercials > phone commercials) into second-level regression models with unhealthy food intake and healthier food intake as covariates. We conducted parallel regression models for total food intake (which is dependent on the combined amount of unhealthy and healthier food intake). Separate second-level regression models were conducted for each SPM contrast. All second-level regression models included hunger as a covariate of no interest because hunger modulates neural response to food stimuli (19) and hunger was a predictor of food intake in the current study. Sex, self-reported liking of unhealthy FF items, and self-reported liking of healthier FF items predicted food intake in the current study and were also included as covariates of no interest in all second-level regression models. BMIz was not a predictor of food intake in the current study and thus was not included as a covariate. We conducted exploratory analyses to test whether BMIz moderated the effects, but moderation analyses were also not significant (all P values >0.05).
Whole-brain analyses were conducted after the binarized DARTEL-derived sample-specific gray matter mask was applied. An overall significance level of P < 0.05, corrected for multiple comparisons across the gray matter–masked whole brain, was calculated. We employed the spatial autocorrelation option in Analysis of Functional Neuroimages (AFNI version_17.0.03) program 3dFWHMx (3dmerge full width at half maximum) to estimate intrinsic smoothness in the images (20) and the 3dClustSim program to estimate the probability of false positive clusters (21). Simulation results indicated activity surviving a threshold of P uncorrected <0.001 with k ≥ 52 being statistically significant corrected for multiple comparisons (see Supplemental Table 3 for main effects). Effect sizes (r) were derived from the Z-values (Z/√N). For a-priori tests seeking to replicate the previous effect of NAcc activation in response to food images predicting food intake in adults (9), we performed small volume correction (SVC) analyses. For these analyses, we used spherical regions-of-interest (ROIs; 6-mm–diameter spheres) that were built centered at MNI coordinates x = −9, y = 6, z = −4 (left NAcc), and x = 9, y = 6, z = −4 (right NAcc) (22, 23). For SVC analyses, peak activity with P values <0.05 corrected using voxel-level familywise error rate (pFWE) over the 6-mm sphere was considered significant (24). We used the MARSeille Boîte À Région d'Intérêt toolbox (MarsBaR; http://marsbar.sourceforge.net/) to extract parameter estimates from significant clusters (see Supplemental Table 4 for an all food commercials > phone commercials contrast results). Data were inspected for influential outliers. Two outliers in NAcc activation in response to the contrast unhealthy FF commercials > phone commercials, and 2 outliers in NAcc activation in response to the contrast healthy FF commercials > phone commercials were detected (parameter estimate exceeding 3 SDs from the mean parameter estimate). The removal of these outliers from analyses did not alter the significance of the findings (pFWE < 0.05) and they were therefore retained. An outlier in the thalamus was also detected and when it was removed results in this region became nonsignificant for some contrasts. Therefore, those findings are not reported below.
Total food intake was normally distributed, but unhealthy and healthier food intakes were zero-inflated (i.e., 15.2% of participants consumed no unhealthy foods and 31.6% of participants consumed no healthier foods). To account for these distributions, confirmatory zero-inflated negative binomial (ZINB) regression analyses were conducted in R (22). The extracted parameters from the significant SPM analyses were included as predictors of the unhealthy and healthier food intake in separate ZINB models with the same covariates as the SPM models. ZINB regression analyses predicted both the likelihood of consuming no unhealthy/healthier food (i.e., being a zero) and the quantity of food consumed for individuals who consumed any of the unhealthy/healthier foods. This distinction is indicated in Table 1 for whole-brain analyses and in the text for ROI analyses (see Supplemental Materials and Methods for more information on the ZINB analytic approach and Supplemental Table 5 for the ZINB results).
TABLE 1.
Whole-brain analyses of correlations between BOLD activation to the contrasts of unhealthy FF commercials > healthier FF commercials, unhealthy FF commercials > phone commercials, and healthier FF commercials > phone commercials and subsequent total food intake, unhealthy food intake, and healthier food intake (n = 171)1
| Contrasts | k | Z-Value | MNI coordinates x, y, z | Effect size r (z/√n) |
|---|---|---|---|---|
| Total food intake | ||||
| Unhealthy FF commercials > phone commercials | ||||
| Caudate nucleus | 570 | 3.85 | 12, 8, 2 | 0.29 |
| Healthier FF commercials > phone commercials | ||||
| Hippocampus | 107 | 4.26 | 24, −31, −4 | 0.33 |
| Anterior cerebellum | — | 4.23 | 6, −40, −13 | 0.32 |
| Anterior cerebellum | — | 3.89 | 15, −37, −16 | 0.30 |
| Unhealthy food intake | ||||
| Unhealthy FF commercials > phone commercials | ||||
| Caudate nucleusb | 131 | 3.74 | 12, 5, 2 | 0.29 |
| Thalamusb | — | 3.66 | 6, −16, 5 | 0.28 |
| Thalamusb | — | 3.62 | −3, −16, 8 | 0.28 |
| Healthier FF commercials > phone commercials | ||||
| Anterior cerebellumb | 193 | 4.34 | 6, −40, −13 | 0.33 |
| Hippocampusb | — | 4.16 | 24, −31, −4 | 0.32 |
| Anterior cerebellumb | — | 4.10 | 15, −37, −16 | 0.31 |
| Healthier food intake | ||||
| Unhealthy FF commercials > healthier FF commercials | ||||
| Precuneusa,b | 119 | 4.61 | 12, −61, 56 | −0.35 |
| Precuneusa,b | — | 4.52 | 21, −52, 53 | −0.35 |
| Superior parietal lobulea | 87 | 3.68 | −21, −64, 53 | −0.28 |
| Superior parietal lobuleb | — | 3.52 | −24, −52, 56 | −0.27 |
1Second-level regression models were conducted in SPM12 (Wellcome Centre for Human Neuroimaging). All regression models included hunger, sex, self-reported liking of unhealthy FF items, and self-reported liking of healthier FF items as covariates of no interest. For all contrasts, activated regions, Z-values, and coordinates within the MNI coordinate system are displayed. Number of continuous voxels (k) are shown for peak coordinates. Peaks within the regions were considered significant at P < 0.001 and k ≥ 52, which corresponds to P < 0.05, corrected for multiple comparisons across the entire brain. For unhealthy and healthier kilocalorie intake, ZINB results are indicated by superscript letters: apredicted the likelihood of any intake (i.e., likelihood of not being zero); bpredicted the quantity of food consumed. BOLD, blood oxygen level–dependent; FF, fast food; MNI, Montreal Neurological Institute; SPM, statistical parametric mapping; ZINB, zero-inflated negative binomial.
Results
Self-reported liking by commercial type
Self-reported liking was higher for the unhealthy foods (mean = 2.85 ± 0.60 points) relative to healthier foods (mean = 2.68 ± 0.58 points) [t(170) = 3.975; P < 0.001] featured in the commercials. Self-reported liking for phones featured in the commercials was higher (mean = 3.05 ± 0.49 points) than both the unhealthy [t(170) = 3.98; P < 0.001] and healthier foods [t(170) = 7.03; P < 0.001].
Nonneural predictors of food intake
Participants consumed an average of 759.38 ± 423.57 total kcal, 568.58 ± 450.62 kcal of unhealthy food, and 190.80 ± 222.70 kcal of healthier food. Unhealthy food intake was negatively associated with healthier food intake (r = −0.37; P < 0.001). Self-reported liking for the unhealthy foods featured in the commercials predicted total (r = 0.28; P < 0.001) and unhealthy food intake (r = 0.27; P < 0.001), but not healthier food intake (r = −0.09; P = 0.23). Self-reported liking for the healthier foods featured in the commercials predicted total food intake (r = 0.17; P = 0.03), but not unhealthy or healthier food intake separately (P values >0.10). Hunger ratings were positively associated with greater total food intake (r = 0.32; P < 0.001) and unhealthy food intake (r = 0.26; P = 0.001), but not healthier food intake (r = 0.06; P = 0.46). Males had significantly more total food intake (mean = 909.39 ± 442.78 total kcal) and unhealthy food intake (mean = 704.14 ± 468.09 unhealthy kcal) than females (mean total food intake = 621.17 ± 354.74 total kcal; mean unhealthy food intake = 443.68 ± 397.10 unhealthy kcal): t(169) = 4.67 (P < 0.001) and t(169) = 3.93 (P < 0.001), respectively. There were no sex differences in healthier food intake [t(169) = 0.66; P = 0.51]. BMIz was not significantly correlated with total, unhealthy, or healthier food intake (P values ≥0.73) (see Supplemental Materials and Methods for more information on the analysis of other nonneural predictors of food intake).
Neural response to FF commercials as a predictor of total food intake
Whole-brain analyses showed that BOLD response in a cluster in the right caudate nucleus (MNI coordinates: 12, 8, 2, Z = 3.85; k = 70; r = 0.29) to the contrast unhealthy FF commercials > phone commercials was positively associated with total food intake (Figure 2A). This effect remained significant when excluding the statistical outlier (>3 SDs from the mean parameter estimate) from analyses (Z = 3.75; k = 60; r = 0.29). Further, BOLD responses in a cluster in the right hippocampus (r = 0.33) that extended into the right anterior cerebellum (r = 0.32 and r = 0.30) to the contrast healthier FF commercials > phone commercials were positively associated with total food intake (Table 1). ROI analyses found that an elevated BOLD response in the right NAcc (Figure 3A; MNI coordinates: 12, 11, −1; Z = 3.55; pFWE = 0.003; r = 0.27) in response to the contrast unhealthy FF commercials > phone commercials and in response to the contrast healthier FF commercials > phone commercials (Figure 3B; MNI coordinates: 9, 2, −1; Z = 3.17; pFWE = 0.01; r = 0.24) was associated with greater total food intake.
FIGURE 2.
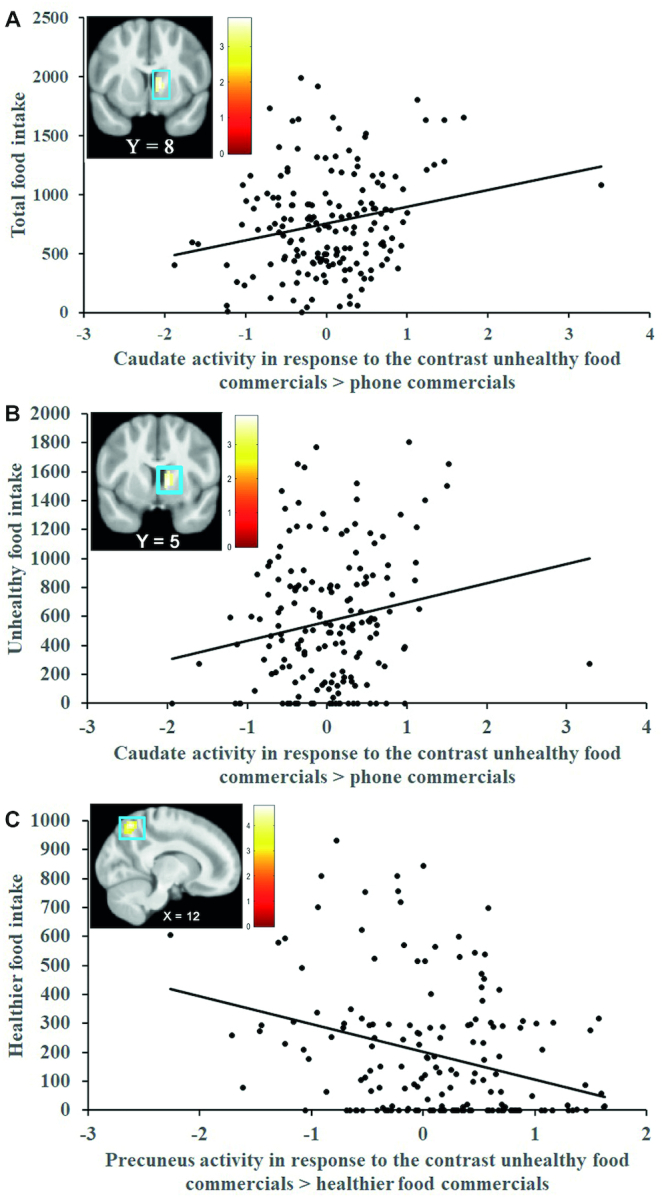
(A) BOLD activity in the right caudate nucleus (MNI coordinates: 12, 8, 2; r = 0.29; n = 171) in response to the contrast unhealthy FF commercials > phone commercials predicts total food intake. This effect remained significant when excluding the statistical outlier (>3 SDs from the mean parameter estimate) from analyses (Z = 3.75; k = 60; r = 0.29; n = 170). (B) BOLD activity in the right caudate nucleus (MNI coordinates: 12, 5, 2; r = 0.29; n = 171) in response to the contrast unhealthy FF commercials > phone commercials predicts unhealthy food intake. This effect remained significant when excluding the statistical outlier (>3 SDs from the mean parameter estimate) from analyses (Z = 3.75; k = 61; r = 0.29; n = 170). (C) BOLD activity in the right precuneus (MNI coordinates: 12, −61, 56; r = −0.35; n = 171) in response to the contrast unhealthy FF commercials > healthier FF commercials predicts healthier food intake. Scatterplots represent mean parameter estimates per subject extracted from the local peak response (i.e., right caudate nucleus, right thalamus, and right precuneus). The units on the y-axis refer to consumed kilocalories. The x-axis represent the range of mean parameter estimates from the local peak response. The color bars represent Z-values of the activation cluster. The X and Y values in the brain images reflect the MNI xand ycoordinates. All second-level regression models were conducted in SPM12 (Wellcome Centre for Human Neuroimaging) and included hunger, sex, self-reported liking of unhealthy FF items, and self-reported liking of healthier FF items as covariates of no interest (n = 171). BOLD, blood oxygen level–dependent; FF, fast food; MNI, Montreal Neurological Institute; SPM, statistical parametric mapping.
FIGURE 3.
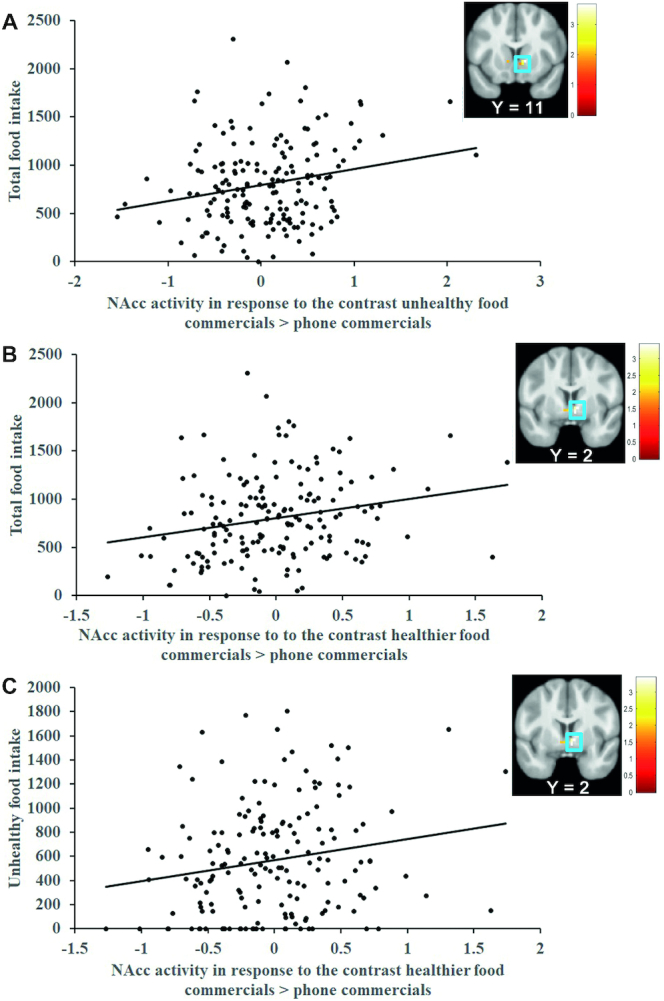
(A) BOLD activity in the right NAcc in response to the contrast unhealthy FF commercials > phone commercials (MNI coordinates: 12, 11, −1; r = 0.27; n = 171) and (B) in response to the contrast healthier FF commercials > phone commercials (MNI coordinates: 9, 2, −1; r = 0.24; n = 171) predicted total food intake. (C) BOLD activity in the right NAcc in response to the contrast healthier FF commercials > phone commercials (MNI: 9, 2, −1; r = 0.24; n = 171) predicted unhealthy food intake. The removal of the 2 outliers from analyses did not alter the significance of the findings (pFWE < 0.05; n = 169). Scatterplots represent mean parameter estimates per subject extracted from the right NAcc peak response (i.e., MNI coordinates: 12, 11, −1; MNI coordinates: 9, 2, −1; and MNI coordinates: 9, 2, −1). The units on the y-axis refer to consumed kilocalories. The x-axis represent the range of mean parameter estimates from the local peak response. The color bars represent Z-values of the activation cluster. The Y values in the brain images reflect the MNI y coordinate. All second-level regression models were conducted in SPM12 (Wellcome Centre for Human Neuroimaging) and included hunger, sex, self-reported liking of unhealthy FF items, and self-reported liking of healthier FF items as covariates of no interest (n = 171). BOLD, blood oxygen level–dependent; FF, fast food; MNI, Montreal Neurological Institute; NAcc, nucleus accumbens; pFWE, peak activity with P-values <0.05 corrected using voxel-level familywise error rate; SPM, statistical parametric mapping.
Neural response to FF commercials as a predictor of unhealthy food intake
Whole-brain analyses showed that BOLD responses in a cluster in the right caudate nucleus (Figure 2B; r = 0.28; P < 0.001) that extended into the right thalamus (r = 0.28) to the contrast unhealthy FF commercials > phone commercials was positively associated with unhealthy food intake (Table 1). This effect remained significant when excluding the statistical outlier (>3 SDs from the mean parameter estimate) from analyses (Z = 3.75; k = 61; r = 0.29). BOLD responses in a cluster in the right anterior cerebellum (r = 0.33 and r = 0.31) that extended into the right hippocampus (r = 0.32) to the contrast healthier food commercials > phone commercials were positively associated with unhealthy food intake (Table 1). ROI analyses found that elevated BOLD responses in the right NAcc (MNI coordinates: 12, 11, −1; Z = 3.55; pFWE = 0.004; r = 0.27) in response to the contrast unhealthy FF commercials > phone commercials was associated with greater unhealthy food intake, but this effect was not significant (P = 0.06) in ZINB confirmatory analyses (see Supplemental Table 5). The ROI analysis for the contrast healthier FF commercials > phone commercials (Figure 3C; MNI coordinates: 9, 2, −1; Z = 3.10; pFWE = 0.02; r = 0.24) was significantly associated with greater unhealthy food intake and this was confirmed in the ZINB analyses (see Supplemental Table 5).
Neural response to FF commercials as a predictor of healthier food intake
BOLD responses in a cluster in the right precuneus (Figure 2C; r = −0.35) and a cluster in the left superior parietal lobe (SPL) (r = −0.28 and r = −0.27) to the contrast unhealthy FF commercials > healthier FF commercials were negatively associated with healthier food intake (Table 1). That is, participants who showed less activation during the unhealthy relative to healthier FF commercials in these regions had greater healthier food intake. NAcc ROI analyses did not significantly predict healthier food intake.
Discussion
In a sample of 171 adolescents, greater activation in key reward regions (i.e., NAcc, caudate nucleus) (25, 26) in response to unhealthy FF commercials relative to phone commercials predicted greater total food intake in a simulated FF restaurant. Unexpectedly, greater neural responses to healthier FF relative to phone commercials in regions associated with reward (i.e., NAcc) (25, 26), memory (i.e., hippocampus) (27), and sensorimotor processes (i.e., anterior cerebellum) (28, 29) predicted greater total food and unhealthy food intake, but not healthier food intake. Lower activation in neural regions associated with visual attention and salience (i.e., precuneus, SPL) (30, 31) to unhealthy relative to healthier FF commercials was the only predictor of healthier food intake. These findings are discussed in greater detail below.
Reward response to FF commercials
As with adults (9, 10), greater activation of the NAcc in adolescents in response to both unhealthy and healthier FF commercials predicted greater total food intake. The NAcc is a key region in reward-related neural systems and is associated with greater motivation and desire for a reinforcing substance, which can occur outside conscious awareness (26, 32). Greater activation in the caudate nucleus in response to unhealthy FF relative to phone commercials also predicted greater total food intake. The caudate nucleus is another key reward region associated with incentive motivation and reward valuation (26, 33). Prior work with younger children found that viewing food commercials increased the contribution of reward (compared with health) valuation in decisions about what to eat (34). The ability of FF commercials to engage reward-related neural circuitry appears to be key to their effectiveness. This is consistent with theories of obesity, such as the incentive sensitization theory, that predict that elevated reward response to food-related cues is a driver of food intake (26, 32). Adolescents who exhibit an elevated reward-related neural response to FF commercials also appear to be more likely to overeat, regardless of their current BMIz. Greater caudate nucleus activation in response to unhealthy FF commercials is predictive of future weight gain in adolescents (35). Thus, elevated reward-related neural response to FF commercials could be a risk factor for future weight gain and the development of obesity in adolescents. Longitudinal research will be needed to test this possibility. Despite higher self-reported liking ratings for the phone relative to FF items, neural activation in reward-related regions was not greater for the phone relative to the FF commercials (see Supplemental Table 3). This suggests a disconnect between self-report and neural responses, which highlights the utility of using neural responses to predict food intake (above and beyond self-report).
Impact of healthier FF commercials
Surprisingly, adolescents who exhibited greater activation in a number of neural regions (e.g., NAcc, hippocampus) when viewing healthier FF commercials did not have greater healthier food intake. Of note, these adolescents had greater total and unhealthy food intake. Healthier FF commercials from FF restaurants still include cues (e.g., logos, branding) associated with companies that sell predominantly unhealthy foods. Food logos alone can activate reward neural responses (36, 37). Adolescents who are more reactive to cues can still exhibit increased neural reactivity to these cues in healthier FF commercials, which can increase the likelihood of unhealthy food intake. This finding is consistent with the small behavioral literature on healthier FF commercials. In younger children, exposure to fast FF commercials for healthier foods did not lead to more nutritious food choices, but instead increased liking for FFs more generally (38). In the current study, participants who had greater perceptions of health for the foods featured in the healthier FF commercials had more total and unhealthy food intake, but not healthier food intake (see Supplemental Materials and Methods). The perception that FF restaurants are providing healthy items could provide a “health halo” (39) that increases overall FF intake (but not intake of healthier options). Thus, the addition of healthier FF commercials to the food advertising landscape is unlikely to encourage healthier food intake. An overall reduction in the amount of FF commercials (regardless of nutritional profile) would likely be more beneficial.
Neural predictors of healthier food intake in adolescents
Healthier food intake was predicted by less activation in visual attention and salience regions to unhealthy relative to healthier FF commercials. The vast majority of adolescents are not consuming sufficient amounts of healthier foods (e.g., vegetables) (40); thus, it is essential that predictors of healthier food intake in adolescents are identified. The current findings suggest that reducing the attentional salience of unhealthy FF commercials could be important. Interventions that retrain attention away from unhealthy FF commercials (41) might be useful in encouraging healthier food intake. Restricting overall exposure to unhealthy FF commercials by reducing screen time, shifting to noncommercial content, or limiting commercials through policy is likely the most effective way to reduce the ability of these stimuli to capture attention.
Limitations and future directions
There are important limitations to consider. First, the current study is cross-sectional, thus causal relations cannot be determined. Second, all commercials were from FF restaurants, which limits the ability to investigate the neural correlates of commercials for companies that primarily market healthy foods. These companies occupy a small segment (<5%) of the current food advertising landscape (42), but it will be important for future studies to investigate how commercials from healthier companies might impact food intake. Third, all participants were exposed to unhealthy and healthier FF commercials. There is evidence that exposure to healthier food commercials on their own increases positive attitudes about healthier foods (e.g., vegetables), but this effect is attenuated when healthier food commercials are shown alongside unhealthy food commercials (43). Fourth, the foods that participants were provided with were unbranded. Research with younger children suggests there are different neural predictors for branded compared with unbranded foods, which also needs to be investigated in adolescents (44). Fifth, digital marketing (e.g., social media, apps) is a rising source of food advertising and an important area for future study (45). Finally, the current study was not sufficiently powered to investigate racial/ethnic differences. Black adolescents are exposed to higher levels of food commercials (3) and are at greater risk of obesity (particularly in underresourced settings). Thus, research on neural susceptibility to FF commercials in black youth is an important future direction.
Conclusions
The current study found that FF commercials can be implicated in greater food intake for adolescents by activating neurobiological systems associated with reward, memory, sensorimotor processing, and visual attention. The ability to prime these systems, potentially outside conscious awareness, can make it particularly challenging for adolescents to defend themselves against FF commercials. Greater neural reactivity to healthier FF commercials predicted unhealthy food intake, which suggests that adding healthier FF commercials to a food advertising landscape dominated by unhealthy FF commercials might not be beneficial in encouraging healthier eating. Reduced activation in visual salience neural regions to unhealthy FF commercials was the only predictor of healthier food intake. Thus, interventions that train attention away from unhealthy FF commercials could be beneficial in increasing healthier food intake. However, individual interventions are unlikely to provide population-level improvements without policy initiatives that substantially change the food advertising landscape (6).
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ contributions were as follows—ANG, SY: conceptualized the study, analyzed the data, and wrote the paper; ANG: conducted the research; JLH, LHE, JCL: read and edited the paper; and all authors: designed the study, and read and approved the final manuscript. The authors report no conflicts of interest.
Notes
This work was supported by a grant from the National Institute on Diabetes and Digestive and Kidney Diseases: R01 DK102532 (PI: ANG).
Data described in the manuscript, code book, and analytic code will be made available upon request pending application and approval.
Supplemental Materials and Methods, Supplemental Tables 1–5, and Supplemental Figures 1 and 2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: BMIz, body mass index z-scored; BOLD, blood oxygen level–dependent; DARTEL, diffeomorphic anatomical registration through exponentiated lie algebra; FF, fast food; FOV, field of view; MNI, Montreal Neurological Institute; NAcc, nucleus accumbens; NPI, nutritional profiling index; pFWE, peak activity with P-values <0.05 corrected using voxel-level familywise error rate; ROI, region of interest; SPL, superior parietal lobe; SPM, statistical parametric mapping; SVC, small volume correction; TE, echo time; TI, inversion-time, TR, repetition time; ZINB, zero-inflated negative binomial.
References
- 1. Folkvord F, Anschutz DJ, Boyland E, Kelly B, Buijzen M. Food advertising and eating behavior in children. Curr Opin Behav Sci. 2016;9:26–31. [Google Scholar]
- 2. White House Task Force. Solving the problem of childhood obesity within a generation: White House Task Force Report on Childhood Obesity Report to The President. Washington (DC): White House; 2010. [DOI] [PubMed] [Google Scholar]
- 3. Frazier WC, Harris JL. Trends in television food advertising to young people: 2017 update. [Internet]. Rudd Brief; 2018; [cited June 25, 2019]. Available from: http://www.uconnruddcenter.org/files/Pdfs/TVAdTrends2018_Final.pdf. [Google Scholar]
- 4. Harris JL, FACTS 2017 Food industry self-regulation after 10 years: progress and opportunities to improve food advertising to children. University of Connecticut, Rudd Center for Food Policy & Obesity; 2017. [Google Scholar]
- 5. Kraak VI, Gootman JA, McGinnis JM. Food marketing to children and youth: threat or opportunity?. Washington (DC): National Academies Press; 2006. [Google Scholar]
- 6. Harris JL, Brownell KD, Bargh JA. The food marketing defense model: integrating psychological research to protect youth and inform public policy. Soc Issues Policy Rev. 2009;3:211–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Harris JL, Bargh JA. Television viewing and unhealthy diet: implications for children and media interventions. Health Commun. 2009;24:660–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Giuliani NR, Merchant JS, Cosme D, Berkman ET. Neural predictors of eating behavior and dietary change. Ann N Y Acad Sci. 2018;1428:208–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lawrence NS, Hinton EC, Parkinson JA, Lawrence AD. Nucleus accumbens response to food cues predicts subsequent snack consumption in women and increased body mass index in those with reduced self-control. Neuroimage. 2012;63:415–22. [DOI] [PubMed] [Google Scholar]
- 10. Boswell RG, Kober H. Food cue reactivity and craving predict eating and weight gain: a meta‐analytic review. Obesity Rev. 2016;17:159–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Casey B, Jones RM. Neurobiology of the adolescent brain and behavior: implications for substance use disorders. J Am Acad Child Adolesc Psychiatry. 2010;49:1189–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, Mei Z, Curtin LR, Roche AF, Johnson CL. CDC growth charts: United States. Adv Data2000;(314):1–27. [PubMed] [Google Scholar]
- 13. Harris JL. Rudd Center Analysis of 2012 Nielsen Data. 2013. [Google Scholar]
- 14. Ofcom. Television advertising of food and drink products to children: final statement. [Internet]. Ofcom; 2007; [cited September 2019]. Available from: https://www.ofcom.org.uk/consultations-and-statements/category-2/foodads_new. [Google Scholar]
- 15. Rayner M, Scarborough P, Lobstein T. The UK Ofcom Nutrient Profiling Model. Defining ‘healthy’ and ‘unhealthy’ foods and drinks advertised to children. Oxford: Oxford University Press; 2009. [Google Scholar]
- 16. Joyner MA, Kim S, Gearhardt AN. Investigating an incentive-sensitization model of eating behavior: impact of a simulated fast-food laboratory. Clin Psychol Sci. 2017;5:1014–26. [Google Scholar]
- 17. Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. [DOI] [PubMed] [Google Scholar]
- 18. Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–51. [DOI] [PubMed] [Google Scholar]
- 19. Siep N, Roefs A, Roebroeck A, Havermans R, Bonte ML, Jansen A. Hunger is the best spice: an fMRI study of the effects of attention, hunger and calorie content on food reward processing in the amygdala and orbitofrontal cortex. Behav Brain Res. 2009;198:149–58. [DOI] [PubMed] [Google Scholar]
- 20. Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–73. [DOI] [PubMed] [Google Scholar]
- 21. Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA., Noll DC.. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–47. [DOI] [PubMed] [Google Scholar]
- 22. Neto LL, Oliveira E, Correia F, Ferreira AG. The human nucleus accumbens: where is it? A stereotactic, anatomical and magnetic resonance imaging study. Neuromodulation. 2008;11:13–22. [DOI] [PubMed] [Google Scholar]
- 23. Demos KE, Heatherton TF, Kelley WM. Individual differences in nucleus accumbens activity to food and sexual images predict weight gain and sexual behavior. J Neurosci. 2012;32(16):5549–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2013. [Google Scholar]
- 25. Berridge KC. ‘Liking'and ‘wanting’ food rewards: brain substrates and roles in eating disorders. Physiol Behav. 2009;9:537–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Berridge KC, Ho Cy, Richard JM, DiFeliceantonio AG. The tempted brain eats: pleasure and desire circuits in obesity and eating disorders. Brain Res. 2010;1350:43–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bannerman D, Rawlins JN, McHugh SB, Deacon RM, Yee BK, Bast T, Zhang WN, Pothuizen HH, Feldon J. Regional dissociations within the hippocampus—memory and anxiety. Neurosci Biobehav Rev. 2004;28:273–83. [DOI] [PubMed] [Google Scholar]
- 28. Zhu J-N, Wang J-J. The cerebellum in feeding control: possible function and mechanism. Cell Mol Neurobiol. 2008;28:469–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stoodley CJ, Schmahmann JD. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. Neuroimage. 2009;44:489–501. [DOI] [PubMed] [Google Scholar]
- 30. Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–83. [DOI] [PubMed] [Google Scholar]
- 31. Behrmann M, Geng JJ, Shomstein S. Parietal cortex and attention. Curr Opin Neurobiol. 2004;14:212–7. [DOI] [PubMed] [Google Scholar]
- 32. Devoto F, Zapparoli L, Bonandrini R, Berlingeri M, Ferrulli A, Luzi L, Banfi G, Paulesu E. Hungry brains: a meta-analytical review of brain activation imaging studies on food perception and appetite in obese individuals. Neurosci Biobehav Rev. 2018;94:271–85. [DOI] [PubMed] [Google Scholar]
- 33. Volkow ND, Wang GJ, Tomasi D, Baler RD. Obesity and addiction: neurobiological overlaps. Obes Rev. 2013;14:2–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bruce AS, Pruitt SW, Ha OR, Cherry JBC, Smith TR, Bruce JM, Lim SL. The influence of televised food commercials on children's food choices: evidence from ventromedial prefrontal cortex activations. J Pediatr. 2016;177:27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yokum S, Gearhardt AN, Harris JL, Brownell KD, Stice E. Individual differences in striatum activity to food commercials predict weight gain in adolescents. Obesity. 2014;22:2544–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bruce AS, Bruce JM, Black WR, Lepping J, Henry JM, Cherry JBC, Martin LE, Papa VB, Davis AM, Brooks WM et al.. Branding and a child's brain: an fMRI study of neural responses to logos. Soc Cogn Affect Neurosci. 2012;9(1):118–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bruce AS, Lepping RJ, Bruce JM, Cherry JB, Martin LE, Davis AM, Brooks WM, Savage CR. Brain responses to food logos in obese and healthy weight children. J Pediatr. 2013;162(4):759–64. [DOI] [PubMed] [Google Scholar]
- 38. Boyland EJ, Whalen R. Food advertising to children and its effects on diet: review of recent prevalence and impact data. Pediatr Diabetes. 2015;16:331–7. [DOI] [PubMed] [Google Scholar]
- 39. Chandon P, Wansink B. The biasing health halos of fast-food restaurant health claims: lower calorie estimates and higher side-dish consumption intentions. J Consum Res. 2007;34(3):301–14. [Google Scholar]
- 40. Kimmons J, Gillespie C, Seymour J, Serdula M, Blanck HM. Fruit and vegetable intake among adolescents and adults in the United States: percentage meeting individualized recommendations. Medscape J Med. 2009;11:26. [PMC free article] [PubMed] [Google Scholar]
- 41. Boutelle KN, Knatz S, Carlson J, Bergmann K, Peterson CB. An open trial targeting food cue reactivity and satiety sensitivity in overweight and obese binge eaters. Cogn Behav Pract. 2016;24:363–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. University of Conneticut Rudd Center; Council on Black Health; Salud America! Rudd Report: increasing disparities in unhealthy food advertising targeted to Hispanic and Black youth. [Internet]. UConn Rudd Center for Food Policy and Obesity; 2019; [cited September 2019]. Available from: http://uconnruddcenter.org/files/Pdfs/TargetedMarketingReport2019.pdf. [Google Scholar]
- 43. Dixon HG, Scully ML, Wakefield MA, White VM, Crawford DA. The effects of television advertisements for junk food versus nutritious food on children's food attitudes and preferences. Soc Sci Med. 2007;65:1311–23. [DOI] [PubMed] [Google Scholar]
- 44. Masterson TD, Stein WM, Beidler E, Bermudez M, English LK, Keller KL. Brain response to food brands correlates with increased intake from branded meals in children: an fMRI study. Brain Imaging Behav. 2018;13(4):1035–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Norman J, Kelly B, Boyland E, McMahon AT. The impact of marketing and advertising on food behaviours: evaluating the evidence for a causal relationship. Curr Nutr Rep. 2016;5:139–49. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


