ABSTRACT
Background
Aging appears to attenuate the response of skeletal muscle protein synthesis (MPS) to anabolic stimuli such as protein ingestion (and the ensuing hyperaminoacidemia) and resistance exercise (RE).
Objectives
The purpose of this study was to determine the effects of protein quality on feeding- and feeding plus RE–induced increases of acute and longer-term MPS after ingestion of whey protein (WP) and collagen protein (CP).
Methods
In a double-blind parallel-group design, 22 healthy older women (mean ± SD age: 69 ± 3 y, n = 11/group) were randomly assigned to consume a 30-g supplement of either WP or CP twice daily for 6 d. Participants performed unilateral RE twice during the 6-d period to determine the acute (via [13C6]-phenylalanine infusion) and longer-term (ingestion of deuterated water) MPS responses, the primary outcome measures.
Results
Acutely, WP increased MPS by a mean ± SD 0.017 ± 0.008%/h in the feeding-only leg (Rest) and 0.032 ± 0.012%/h in the feeding plus exercise leg (Exercise) (both P < 0.01), whereas CP increased MPS only in Exercise (0.012 ± 0.013%/h) (P < 0.01) and MPS was greater in WP than CP in both the Rest and Exercise legs (P = 0.02). Longer-term MPS increased by 0.063 ± 0.059%/d in Rest and 0.173 ± 0.104%/d in Exercise (P < 0.0001) with WP; however, MPS was not significantly elevated above baseline in Rest (0.011 ± 0.042%/d) or Exercise (0.020 ± 0.034%/d) with CP. Longer-term MPS was greater in WP than in CP in both Rest and Exercise (P < 0.001).
Conclusions
Supplementation with WP elicited greater increases in both acute and longer-term MPS than CP supplementation, which is suggestive that WP is a more effective supplement to support skeletal muscle retention in older women than CP.
This trial was registered at clinicaltrials.gov as NCT03281434.
Keywords: muscle protein synthesis, whey protein, collagen peptides, protein quality, resistance exercise, older women
Introduction
Sarcopenia is the loss of muscle mass and muscle strength with age, progressing at rates of ∼0.8%/y and 1–3%/y, respectively, and these are measurable in the sixth decade of life (1). Contributing to sarcopenic muscle decline is a decreased response of muscle protein synthesis (MPS) to normally robust anabolic stimuli such as protein ingestion (and the subsequent hyperaminoacidemia) and resistance exercise (RE). This age-related attenuation of MPS has been termed anabolic resistance (2, 3). Previously, we have shown that older men required ∼40% more isolated high quality (whey) protein per meal to stimulate rates of muscle protein synthesis (MPS) comparable with those of younger men (2). Given that many older adults, in particular older women (4), are not consuming recommended protein intakes for older persons of 1.0–1.3 g · kg−1 · d−1 (5) through their habitual diet, protein supplementation may be an effective strategy to augment total protein intake and combat anabolic resistance to maintain skeletal muscle health with aging (6).
RE improves muscle strength (7), increases skeletal muscle mass (7), and improves functional outcomes (8) in older adults. Further, RE induces marked increases in rates of MPS in both young and older men and women (9–11) and serves to sensitize skeletal muscle to the anabolic effects of protein ingestion (3,12). However, to date, few studies have examined the effects of protein supplements of varying quality on the stimulation of MPS in older adults.
Recently, we showed that recovery from inactivity was more effective with consumption of higher quality whey protein (WP), which increased rates of MPS in healthy older men and women (13) compared with an isonitrogenous quantity of collagen peptide (CP) placebo. Our findings highlight the important role that protein quality can play in recovery and retention of muscle mass. Nonetheless, 2 studies have reported positive effects of supplementation with low quality CP on lean body mass (LBM) gains with resistance training in sarcopenic older men (14) (which showed an extraordinary gain in LBM) and premenopausal women (15). Interestingly, a CP supplement improved nitrogen balance in older women (16); however, to our knowledge no study has examined the muscle protein synthetic response, and thus whether muscle is the tissue affected by CP supplementation, that may be underpinning the ostensibly favorable CP supplement–induced changes in body composition (14,15) or improved nitrogen balance (16). Thus, the primary outcome of this study was to compare the acute and longer-term effects of WP or CP supplementation on MPS alone and when combined with RE. As an exploratory, secondary outcome, and given that CP supplementation has been theorized to support greater rates of connective tissue synthesis/renewal (17), we determined if CP supplementation would facilitate increases in muscle-derived collagen protein (perimysium) synthesis [muscle collagen protein synthesis (MCPS)]. We hypothesized that both acute and longer-term MPS would be greater after consumption of WP than after CP and that RE would enhance the MPS response but more so in WP.
Methods
Ethical approval
The study (NCT03281434) was approved by the Hamilton Integrated Research Ethics Board and conformed to the standards for the use of human subjects in research as outlined by the Canadian Tri-Council Policy on the ethical use of human subjects in research (http://www.ethics.gc.ca/eng/documents/tcps2-2018-en-interactive-final.pdf). Each participant was informed of the purpose of the study, experimental procedures, and potential risks before written consent was obtained.
Participants
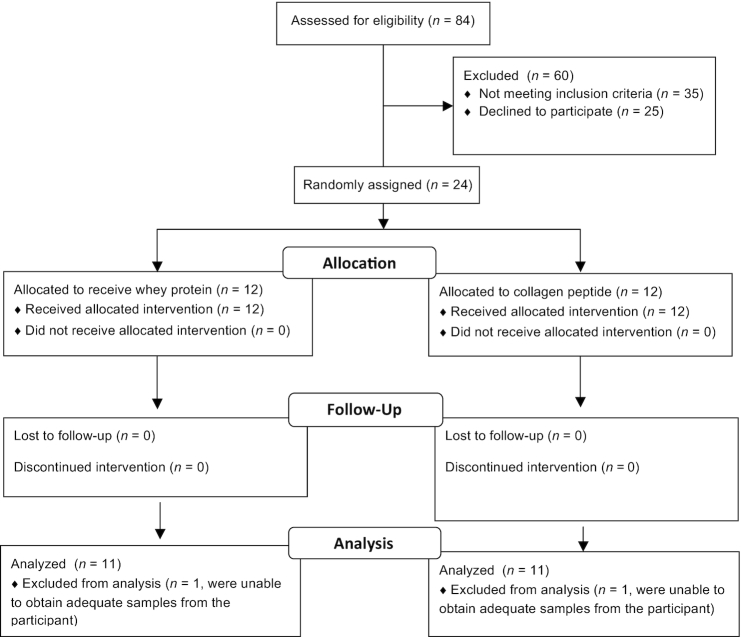
Twenty-two healthy older women were recruited from the greater Hamilton area in response to local advertisements to participate in this study. Sample size was determined with MPS as a primary outcome measure using variance from our previous data (18) and the likely chance we could detect a 20% difference between conditions with 80% power and α at 0.05%. Potential participants were screened first by telephone to ensure they were nonsmokers, nondiabetic, between the ages of 60 and 80 y, and taking between 3500 and 10,000 steps/d. Exclusion criteria included significant loss or gain of body mass in the past 6 mo (>2 kg); regular use of nonsteroidal anti-inflammatory drugs (with the exception of daily low-dose aspirin); consuming either simvastatin or atorvastatin; use of anticoagulants; the use of a walker, cane, or assistive walking device; current or recently remitted cancer; infectious disease; and/or gastrointestinal disease. See the CONSORT diagram in Figure 1 for subject flow through the protocol.
FIGURE 1.
CONSORT flow diagram.
Study overview
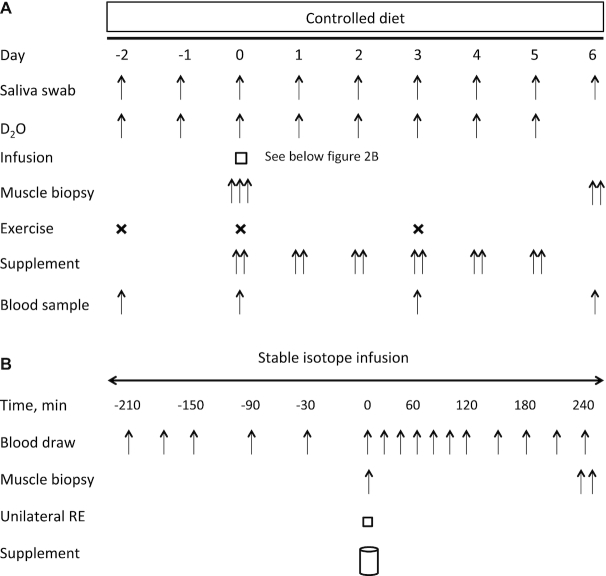
Figure 2 shows an overview of the study. The study was a double-blind, parallel-group, randomized controlled trial. Eligible participants were allocated to consume 1 of 2 types of protein supplement: 30 g amino acids (AAs) twice daily of WP or CP. Allocation was concealed from the participants and researchers for the duration of the study and until all analyses were complete. After baseline testing and familiarization with all study measures, participants commenced the 9-d protocol during which they consumed a controlled diet with all meals provided by the study investigators.
FIGURE 2.
Study schematic over the 9-d protocol (A) and infusion protocol on day 0 (B). RE, resistance exercise.
Baseline testing
Before commencement of the protocol, participants were asked to complete a physical activity and weighed food record (Nutribase version 11.5, Cybersoft Inc.) for 3 d (2 weekdays and 1 weekend day) to assess habitual physical activity levels and dietary intakes. Participants also underwent a DXA scan (GE-Lunar iDXA; Aymes Medical) for the determination of total fat- and bone-free (lean) body mass (LBM; all CVs < 1.2%). Participants were assessed for single leg muscle strength [one-repetition maximum (1RM)] on a manually loaded leg extension machine (Precor). After a 5-min cycling warm-up at a self-selected resistance, participants were familiarized with the knee extension machine by performing a single set of unloaded knee extensions for 10 repetitions. Participants rested for 2 min and then began testing for a 1RM. 1RM values were used to calculate the load, corresponding to ∼60% of these, to be used for the RE sessions throughout the study protocol. This study design employed the use of a within-subject design to enhance statistical power and to allow for the determination of the rest and exercise responses to a protein supplement within a person, a design that is unique to nutrition literature (19).
Diets
Each participant's energy requirement was determined with the use of the Oxford prediction equations for basal metabolic rate (20) using height and body mass for women over the age of 60 y. Activity factors were determined for each participant on the basis of their baseline physical activity records and the Physical Activity Scale for the Elderly questionnaire (21) for the determination of total energy intake throughout the study protocol. Participants were provided with a protein intake from food sources of 1.0 g · kg−1 · d−1, which reflects protein intakes consumed by adults aged 51 y and older in Canada (22), on days −2 and −1. Participants were then provided with a twice-daily protein supplement to increase total protein intake to 1.6 g · kg−1 · d−1 on days 0–6, achieved by reducing the proportion of food energy provided from carbohydrates while maintaining the proportion of energy from fat at ∼25% of total energy. Dietary protein came from a combination of plant- and animal-based protein sources. Participants were prescribed a customized meal plan according to food preferences and food was supplied at the beginning of each week. Food consisted of prepackaged frozen meals (Heart to Home Meals) and items that required minimal preparation. Participants were provided with a dietary log where they were to indicate the percentage of the provided food consumed during the day and were strongly encouraged to consume only the study diet. If food outside of the provided diet was consumed, additions were recorded in the dietary log. Overall, compliance with the prescribed diets and supplements was excellent, with subjects consuming 97% ± 2% (mean ± SD) of what was provided.
Supplementation
Supplements contained WP isolate (Whey Protein Isolate 895, Fonterra), or hydrolyzed CP (Gelita, Bodybalance®). Individual servings were identically flavored and packaged by Infinit Nutrition in powdered form. Participants were instructed to mix each package with 250 mL water before ingestion and were asked to consume the beverage in a single sitting within a 5-min period. Participants consumed the supplements twice daily, once in the morning before breakfast and once in the evening ∼1–2 h before sleep. Supplements were isonitrogenous and energy-matched. Their contents appear in Table 1 as per serving based on amino acid content provided by the manufacturer.
TABLE 1.
Amino acid composition of protein supplements1
| WP supplement | CP supplement | |
|---|---|---|
| Alanine | 1.7 | 3.1 |
| Arginine | 0.9 | 2.6 |
| Aspartic acid | 3.8 | 2.0 |
| Cysteine | 1.2 | 0 |
| Glutamic acid | 5.3 | 3 |
| Glycine | 0.5 | 8.0 |
| Histidine | 0.6 | 0.4 |
| Proline | 1.4 | 4.5 |
| Serine | 1.4 | 1.2 |
| Tyrosine | 1.3 | 0.3 |
| Tryptophan | 0.7 | 0 |
| Isoleucine | 1.9 | 0.5 |
| Leucine | 4.3 | 0.9 |
| Lysine | 3.4 | 1.3 |
| Methionine | 0.7 | 0.4 |
| Phenylalanine | 1.1 | 0.7 |
| Threonine | 1.6 | 0.6 |
| Valine | 1.7 | 0.8 |
| ΣEAAs | 15.4 | 5.6 |
| ΣNEAAs | 17.9 | 24.7 |
1Values are grams per serving. CP, collagen peptide; EAA, essential amino acid; NEAA, nonessential amino acid; WP, whey protein; Σ, sum of.
Acute and integrated muscle and collagen protein synthesis
Consumption of 2H2O (70 atom%; Cambridge Isotope Laboratories) was used to label newly synthesized myofibrillar proteins as previously described (23). Participants reported to the laboratory in the fasted state on day −2, and after the collection of a saliva sample (23) consumed 8 doses (0.625 mL/kg LBM) of 2H2O spread over 10.5 h (1 dose every 1.5 h). An additional dose (0.625 mL/kg LBM) of 2H2O was provided to participants to consume each morning after collection of a fasted saliva sample. Total body water deuterium enrichment was used as a surrogate of the precursor for plasma alanine labeling (23–25). Body water enrichment was determined from saliva swabs that were collected by participants between ∼07:00 and 09:00 each morning. Days −2 to 0 served for the determination of baseline acute MPS, whereas days 0–6 served as the exercise and nutritional supplement phases.
On day 0, participants reported to the laboratory in the fasted state, having restrained from strenuous exercise for 3 d, for the assessment of acute-phase MPS (Figure 2B). Catheters were placed in an antecubital vein of each arm: 1 for the sample of venous blood, and 1 for the infusion of L-[ring−13C6] phenylalanine. Baseline blood samples were drawn and then participants received a priming dose of the stable isotope (2 μmol/kg) before initiating a constant tracer infusion (0.05 μmol · kg−1 · min−1) (Cambridge Isotopes). Participants rested on a bed throughout the infusion and blood samples (8 mL) were taken every 20–30 min using evacuated heparinized tubes. After 210 min, a muscle biopsy was taken from the vastus lateralis of the nonexercising leg for the determination of fasted-state MPS. Upon completion of the biopsy, participants completed 4 sets of unilateral RE (∼60% 1RM) on their dominant leg. Participants rested for 2 min between each set. Loads were adjusted in order to maintain a repetition range between 8 and 10, then participants exercised until volitional fatigue in the final set. Upon completion of the exercise protocol, a blood sample was drawn and the participants immediately consumed their study beverage. Four hours after the consumption of the study beverage, participants had muscle biopsies taken from the vastus lateralis of both legs for the determination of feeding and feeding-plus-exercise effects.
Upon completion of the acute infusion, participants were provided with the remainder of their study beverage packages to be consumed for the next 5 d. Participants returned to the laboratory in the fasted state on day 3 to perform the RE protocol performed on day 0. On the morning of day 6, participants returned to the laboratory after an overnight fast for the collection of a blood sample and bilateral muscle biopsies for the determination of integrated rates of MPS and collagen protein synthesis (CPS).
All biopsies were taken after administration of 1% xylocaine local anesthesia with the use of a 5-mm Bergström needle that was adapted for manual suction. Muscle tissue samples were freed from any visible connective and adipose tissue, rapidly frozen in liquid nitrogen for measurement of MPS, and stored at −80°C for further analysis.
Analytic methods
Muscle samples (∼30–50 mg) were homogenized to yield the myofibrillar and collagen fractions. Samples were homogenized on ice in buffer [10 μL/mg 25 mM Tris 0.5% vol:vol Triton X-100 and protease/phosphatase inhibitor cocktail tablets (Complete Protease inhibitor Mini-Tabs, Roche; and PhosSTOP, Roche Applied Science)] and centrifuged at 15,000 × g for 10 min at 4°C. The supernatant was removed for the protein expression analysis and the pellet was retained. For the measurement of MPS, the myofibrillar protein pellet was solubilized and centrifuged as previously described (25) and the supernatant containing the myofibrillar proteins was collected, leaving the collagen pellet. Myofibrillar proteins were precipitated in 1 mL 1 M perchloric acid, the supernatant was discarded, and the fraction was washed twice with 70% ethanol. The myofibrillar protein–enriched pellets were hydrolyzed in 0.5 M HCl at 110°C for 72 h to release their respective AAs.
The collagen protein–enriched pellet was washed with 0.5 M acetic acid and spun at 1600 × g for 30 min. The collagen protein fraction was extracted overnight in 1 mL 0.1% Pepsin using a vortex mixer at 4°C and collected by centrifugation. Protein-bound AAs were purified by ion exchange chromatography on Dowex H+ resin. Myofibrillar [13C6]-phenylalanine enrichment was determined using GC pyrolysis-isotope ratio MS (25). Myofibrillar and collagen 2H-alanine enrichment were determined using Thermo Finnigan Delta V isotope ratio MS coupled with Thermo Trace GC Ultra with GC pyrolysis interface III and Conflow IV as previously described (24).
Western blotting
Expression of intracellular signaling proteins was assessed by using western blotting. After homogenization for integrated myofibrillar muscle protein synthesis (MyoPS), total protein concentration of the sarcoplasmic fraction was determined by using a bicinchoninic acid assay (Thermo Fisher Scientific). Working samples of equal concentration were prepared in Laemmli buffer. Equal amounts of protein (10 mg) from each sample were run on 4–15% Criterion TGX Stain-Free protein gels (Bio-Rad) at 200 V for 45 min. A protein ladder (Fermentas PageRuler Prestained Ladder, Thermo Fisher Scientific) and a calibration curve were run on every gel. Proteins were then transferred to nitrocellulose membranes and were blocked for 1 h in 5% BSA. Transfer was visually checked with UV activation of the gel as well as the membrane pre- and posttransfer (ChemiDoc MP Imaging System, Bio-Rad). Membranes were then exposed for 12 h at 4°C to primary antibodies after which they were washed in tris-buffered saline and Tween 20 (MilliporeSigma) and incubated in anti-rabbit/anti-mouse IgG conjugates with horseradish peroxidase secondary antibodies (GE Healthcare Life Sciences) for 1 h at room temperature. Signals were detected by using chemiluminescence Super-SignalWest Dura Extended Duration Substrate (Thermo Fisher Scientific), and bands were quantified by using Image Lab Software for Mac version 6.0.1. Protein content was normalized using the calibration curve obtained from each gel (26, 27). The following antibodies used were purchased from Cell Signaling Technology: p-4E-BP1Thr37/46 (1:1000; 2855), mechanistic target of rapamycin (p-mTORSer2448; 1:1000; 2972S), protein kinase B (p-AKTSer473; 1:1000; 9271), ribosomal protein S6 (p-S6Ser235/236; 1:1000; 5364), and p70 S6 kinase 1 (p-p70S6K1Thr389; 1:750; 9202L). Analysis of candidate protein expressions was conducted at Baseline, the feeding leg (Rest), and the feeding plus exercise leg (Exercise) on day 0.
Plasma and saliva analysis
Saliva samples were analyzed for 2H enrichment by cavity ring-down spectroscopy using a liquid isotope analyzer (Picarro L2130-I analyzer, Picarro) with an automated injection system. The water phase of saliva was injected 6 times and the average of the last 3 measurements was used for data analysis (CV ≤ 0.5%). Standards were measured before and after each participant. The 2H isotopic enrichments for muscle and saliva initially expressed as δ2H ‰ were converted to atom percent excess using standard equations (24).
Plasma [13C6]-phenylalanine enrichment was determined by GC-MS (GC: 6890N; MS: 5973; Hewlett-Packard) as previously described (28).
Plasma AA concentrations were measured using an AA analysis kit for GC-MS (EZ:faastTM, Phenomenex). Samples were analyzed using an Agilent 5975C GC/MS (Source 240°C; Quad 180°C; MS Transfer Line 310°C). The instrument was configured to use electron impact ionization and was set to run in scan mode using the column supplied by Phenomenex (Phenom cgo-7169ZB-AAA; 325°C; 10 m × 250 µm × 0.25 µm). Aliquots of 1 µL of the derivatized sample/standard were injected into the mass spectrometer (MS) inlet set to run a 15:1 split mode injection at 250°C. The GC was operated in constant pressure mode at ∼20 kPa, producing a starting flow rate of 1.4 mL/min with an initial oven temperature of 110°C. The temperature ramp used was as follows: 110°C with no hold, followed by a ramp of 30°C/min to 320°C, followed by 1 min hold and 1 min postrun at 320°C for a total run time of 8 min. Chromatographs were quantified using the enhanced data analysis software provided in conjunction with the method instructions and EI ion database provided by the manufacturer. The CV for repeat injections for any given AA was <6%. AUC was calculated using the trapezoidal method (29).
Calculations
The fractional synthetic rate of myofibrillar proteins was determined as %/h and %/d for [13C6]- phenylalanine and [2H]-alanine, respectively, and for collagen proteins was determined as %/d for [2H]-alanine using the precursor-product equation as described previously (13, 30, 31).
Statistics
Baseline participant characteristics and AA concentrations [maximum concentration (Cmax), time to maximum concentration (Tmax), AUC] were compared using an independent-samples t test. MPS and western blotting analyses were compared using a 2-factor mixed ANOVA with between-persons (group) and within-person (conditions: Baseline, Rest, Exercise) factors. MCPS was compared using a 2-factor mixed ANOVA with between-persons (group) and within-person (conditions: Rest and Exercise) factors. All significant interaction terms for the ANOVA were further tested post hoc using Tukey's post hoc test. Significance was set at P < 0.05. All statistical analyses were completed using IBM SPSS Statistics for Mac version 21 (IBM Corp.). Graphical representations of the data are as box and whisker plots with the box representing the IQR, the line in each box indicating the median, the cross in each box indicating the mean, and the whiskers indicating the maximum and minimum values.
Results
Participants’ characteristics
Participants’ characteristics are presented in Table 2. There were no significant differences between groups for any variable. Twenty-two healthy older women were recruited for this study and were randomly assigned to each group. During processing of the MyoPS samples, a malfunction in a piece of laboratory equipment resulted in the loss of samples from 3 participants from WP and 4 participants from CP, thus analyses for these data are n = 8 and n = 7, respectively. Analyses for MCPS are n = 11 per group.
TABLE 2.
Participants’ characteristics and diet nutrition composition over the supplemental period1
| WP supplement (n = 11) | CP supplement (n = 11) | |
|---|---|---|
| Age, y | 67 ± 2 | 69 ± 4 |
| Height, m | 1.61 ± 0.06 | 1.60 ± 0.03 |
| Body mass, kg | 79.7 ± 13.2 | 70.6 ± 17.1 |
| BMI, kg/m2 | 30.6 ± 4.3 | 27.6 ± 5.8 |
| Body fat, % | 45.2 ± 5.7 | 40.5 ± 6.3 |
| LBM, kg | 41.7 ± 3.7 | 40.2 ± 6.1 |
| Knee extensor 1RM, kg | 11.6 ± 3.3 | 9.4 ± 2.8 |
| Steps/d | 9630 ± 3122 | 9062 ± 2564 |
| Daily PA > 3 METs, kcal/d | 151 ± 67 | 149 ± 65 |
| Average METs | 1.5 ± 0.3 | 1.5 ± 0.2 |
| Glucose, mM | 5.1 ± 0.6 | 5.1 ± 0.5 |
| Insulin, μIU/mL | 11.7 ± 2.1 | 10.0 ± 2.4 |
| TNF-α, ng/mL | 11.5 ± 5.7 | 8.0 ± 7.2 |
| IL-6, ng/mL | 8.2 ± 5.8 | 10.2 ± 6.0 |
| CRP, mg/L | 1.6 ± 0.6 | 2.0 ± 0.5 |
| Protein, g/kg | ||
| Baseline | 1.00 ± 0.03 | 0.98 ± 0.04 |
| Supplementation | 1.76 ± 0.12* | 1.87 ± 0.21* |
| Energy, kcal/kg | ||
| Baseline | 31 ± 2 | 35 ± 4 |
| Supplementation | 31 ± 3 | 34 ± 5 |
1Values are means ± SDs. *Significantly different from baseline, P < 0.0001. CP, collagen peptide; CRP, C-reactive protein; LBM, lean body mass; MET, metabolic equivalent; PA, physical activity; WP, whey protein; 1RM, one-repetition maximum.
Dietary intake
There were no differences between groups in any dietary variable (P > 0.05) (Table 2). Protein per kilogram of body mass and absolute protein intake were significantly greater during the supplementation phase than at baseline (P < 0.001).
Plasma AAs
Summed total AA concentration was increased in response to supplementation during the infusion trial in both groups (P = 0.006 and P = 0.05 for WP and CP, respectively) and returned to baseline by 240 min (Figure 3A). There were no differences in total AA AUC (P > 0.05), Cmax (P > 0.05), or Tmax (P > 0.05) between supplements (Table 3). Summed essential amino acid (EAA) AUC and Cmax were greater after WP ingestion than after CP ingestion (P = 0.003, P = 0.003, respectively). Summed EAA concentration increased in response to provision of both supplements (P = 0.01) but did not differ between supplements (P = 0.133) (Figure 3B). There was no difference in EAA Tmax between supplement types (P > 0.05). Plasma leucine concentrations increased above baseline in response to supplement provision in the WP group at 40 min (P = 0.001) and remained elevated above baseline at 240 min. Plasma leucine concentrations were not increased above baseline in CP (P > 0.05) (Figure 3C). Leucine AUC and Cmax were greater after WP ingestion than after CP ingestion (P = 0.001 and P < 0.001, respectively).
FIGURE 3.
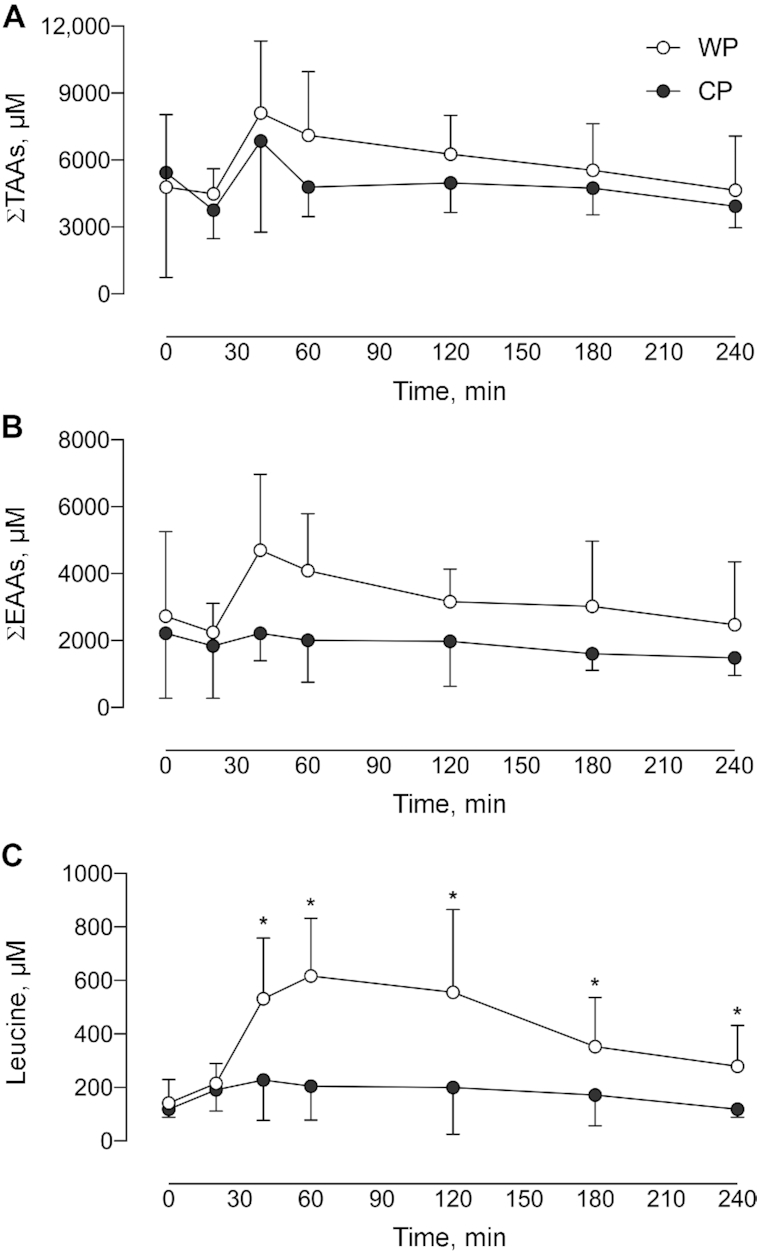
Summed concentrations of TAAs (A), summed concentrations of EAAs (B), and concentration of leucine (C) over 4 h after ingestion of either WP or CP. Data are reported for 11 participants/group. The group × time interaction for each of the panels was (A) P = 0.677; (B) P = 0.133; and (C) P < 0.0001. *Significant difference between supplement groups at that time, P < 0.0001. Values are means ± SDs. CP, collagen peptide; EAA, essential amino acid; TAA, total amino acid; WP, whey protein.
TABLE 3.
Amino acid-derived variables during the acute infusion trial1
| WP supplement (n = 11) | CP supplement (n = 11) | P | |
|---|---|---|---|
| ΣTotal amino acids | |||
| Cmax, μM | 10,041 ± 4793 | 8808 ± 5276 | 0.15 |
| Tmax, min | 64 ± 31 | 84 ± 37 | 0.10 |
| AUC, μmol · min/L | 1,431,600 ± 10,900 | 1,205,300± 16,900 | >0.05 |
| ΣEAAs | |||
| Cmax, μM | 5733 ± 2368 | 2869 ± 1604 | 0.003 |
| Tmax, min | 50 ± 10 | 46 ± 13 | 0.23 |
| AUC, μmol · min/L | 775,000 ± 12,800 | 442,900 ± 10,800 | 0.003 |
| Leucine | |||
| Cmax, μM | 645 ± 206 | 223 ± 117 | <0.001 |
| Tmax, min | 54 ± 9 | 62 ± 31 | 0.22 |
| AUC, μmol · min/L | 103,800 ± 17,700 | 43,600 ± 10,100 | 0.012 |
1Values are means ± SDs. AUC was calculated over the 240 min after study beverage ingestion. Analysis by nonpaired t test. Cmax, maximum concentration; CP, collagen peptide; EAA, essential amino acid; Tmax, time of maximum concentration; WP, whey protein; Σ, sum of.
Acute MyoPS
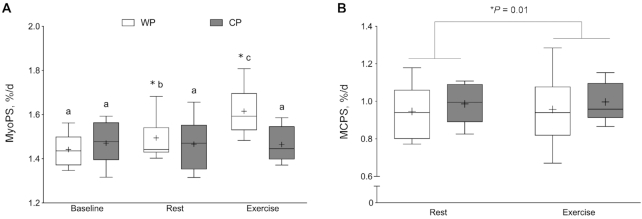
Figure 4 shows acute MyoPS responses. There were no differences in basal rates of MyoPS between WP and CP. In response to WP supplementation, MyoPS was increased significantly by 0.017 ± 0.008%/h in Rest and 0.032 ± 0.012%/h in Exercise (P < 0.001). CP supplementation did not significantly increase Rest MyoPS (0.009 ± 0.014%/h); however, Exercise MyoPS was increased by 0.012 ± 0.013%/h above Baseline rates (P < 0.001). Postprandial MyoPS was significantly greater in the WP group than in the CP group in both Rest and Exercise (P = 0.02). There was an association with the acute MyoPS response between Rest and Exercise for both WP (r = 0.74, P = 0.0229) and CP (r = 0.98, P = 0.0002).
FIGURE 4.
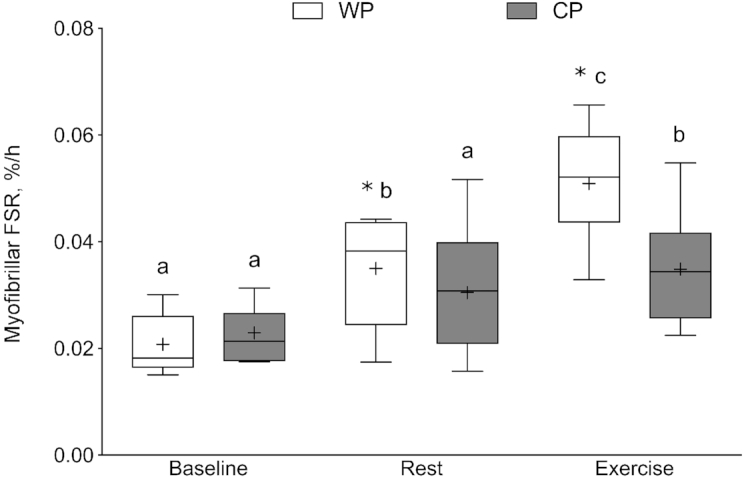
Acute myofibrillar muscle protein synthesis (%/h) in the fasted state (Baseline) and in response to feeding (Rest) and feeding with resistance exercise (Exercise). The box plot shows the median (line) and mean (+), with the box representing the IQR range and the whiskers representing the maximum and minimum values. Data are reported for 8 participants in the WP group and 7 participants in the CP group. Data were analyzed using a 2-factor mixed ANOVA with between-person (group) and within-person (condition: Baseline, Rest, Exercise) factors. Group × time interaction, P = 0.02. Means without a common letter are significantly different within the group, P < 0.05. *Significant difference between supplement groups within that condition, P < 0.0001. CP, collagen peptide; Exercise, feeding plus exercise leg; FSR, fractional synthetic rate; Rest, feeding leg; WP, whey protein.
Integrated MPS and MCPS
Figure 5A shows integrated MyoPS responses. There were no differences in baseline rates of MyoPS between WP and CP (P > 0.05) (days −2 to 0). In response to WP supplementation, MyoPS was increased by 0.063 ± 0.059%/d in Rest and by 0.173 ± 0.104%/d in Exercise (P < 0.001). With CP supplementation, rates of MyoPS were not significantly elevated above baseline in Rest (−0.011 ± 0.042%/d) or Exercise (0.020 ± 0.034%/d). Rates of integrated MyoPS were significantly greater in WP than in CP in both Rest and Exercise (P < 0.0001). There was an association with the integrated MyoPS response between Rest and Exercise for both WP (r = 0.93, P = 0.0034) and CP (r = 0.85, P = 0.003). There were no differences in MCPS between groups (P = 0.154); however, MCPS was increased in response to exercise (P = 0.01) (Figure 5B). There was also an association with the integrated MCPS response between Rest and Exercise for both WP (r = 0.97, P < 0.0001) and CP (r = 0.91, P < 0.001).
FIGURE 5.
Integrated MyoPS (%/d) in the fasted state (Baseline) and in response to feeding (Rest) and feeding plus resistance exercise (Exercise) (A). Integrated intramuscular collagen protein synthesis (%/d) in response to Rest and Exercise in the WP and CP groups (B). The box plot shows the median (line) and mean (+), with the box representing the IQR and the whiskers representing the maximum and minimum values. MyoPS and MCPS data were analyzed using a 2-factor mixed ANOVA with between-person (group) and within-person (condition: Baseline, Rest, Exercise) factors. (A) Data are reported for 8 participants in WP and 7 participants in CP; (B) data are reported for 11 participants/group. (A) Group × time interaction, P < 0.0001; means without a common letter are significantly different within the group, P < 0.05. *Significant difference between supplement groups under that condition, P ≤ 0.0001. (B) There was a significant main effect for time, P = 0.01, whereas the group × time condition was nonsignificant, P = 0.154. CP, collagen peptide; Exercise, feeding plus exercise leg; MCPS, muscle collagen protein synthesis; MyoPS, myofibrillar muscle protein synthesis; Rest, feeding leg; WP, whey protein.
Muscle anabolic signaling
There were no significant differences between supplemental groups in any target measured for changes in phosphorylation status (P > 0.05). In response to feeding, phosphorylation of p-4EBP1Thr37/46 was significantly reduced from Baseline but not different from Rest in Exercise (P = 0.027), and phosphorylation of p-AKTSer473 and p-mTORSer2448 were significantly reduced from Baseline in response to feeding but not different than Baseline with Exercise (P = 0.006 and P = 0.017, respectively). Phosphorylation of p-p70Thr389 and p-S6SER235/236 were unchanged with feeding and exercise (P > 0.05). Table 4 presents the anabolic signaling data.
TABLE 4.
Protein signaling at rest and 4 h after supplement ingestion1
| WP supplement | CP supplement | P | |
|---|---|---|---|
| Phospho-4EBP1Thr37/46 | |||
| Baselinea | 0.81 ± 0.19 | 1.00 ± 0.33 | Condition = 0.027 |
| 4-h Restb | 0.76 ± 0.17 | 0.81 ± 0.24 | Interaction = 0.123 |
| 4-h Exercisea,b | 0.79 ± 0.16 | 1.02 ± 0.43 | |
| Phospho-AKTSer 473 | |||
| Baselinea | 0.87 ± 0.19 | 1.00 ± 0.25 | Condition = 0.006 |
| 4-h Restb | 0.70 ± 0.18 | 0.83 ± 0.24 | Interaction = 0.835 |
| 4-h Exercisea | 0.83 ± 0.18 | 0.98 ± 0.32 | |
| Phospho-mTORSer 2448 | |||
| Baselinea | 0.70 ± 0.25 | 0.94 ± 0.67 | Condition = 0.017 |
| 4-h Restb | 0.61 ± 0.33 | 0.80 ± 0.58 | Interaction = 0.405 |
| 4-h Exercisea | 0.93 ± 0.49 | 1.01 ± 0.17 | |
| Phospho-p70Thr389 | |||
| Baseline | 1.00 ± 0.17 | 1.08 ± 0.21 | Condition = 0.798 |
| 4-h Rest | 0.91 ± 0.15 | 1.02 ± 0.41 | Interaction = 0.790 |
| 4-h Exercise | 0.99 ± 0.44 | 1.04 ± 0.31 | |
| Phospho-s6Ser235/236 | |||
| Baseline | 0.99 ± 0.63 | 0.95 ± 0.34 | Condition = 0.784 |
| 4-h Rest | 1.23 ± 0.89 | 0.87 ± 0.28 | Interaction = 0.342 |
| 4-h Exercise | 1.08 ± 0.68 | 1.02 ± 0.40 | |
1Values are means ± SDs, n = 22. Conditions within a protein without a common letter are significantly different, P < 0.05. There were no significant main effects of group, P > 0.05. AKT, protein kinase B; CP, collagen peptide; Exercise, feeding plus exercise leg; mTOR, mammalian target of rapamycin; Rest, feeding leg; WP, whey protein.
Discussion
The novel finding of our investigation was that supplementation with WP enhanced the MyoPS response to protein ingestion compared with the ingestion of an isonitrogenous and isoenergetic quantity of CP in healthy older women. We show here that ingestion of WP induced an acute increase in MyoPS above fasted rates that was further enhanced with RE; however, acute rates of MyoPS were increased only with exercise in the CP group and to a lesser extent than seen with WP. Importantly, we showed that supplementation with WP for 6 d resulted in an increased rate of integrated MyoPS above baseline in a rested leg and that 2 bouts of RE within the supplementation period further enhanced rates of integrated MyoPS, with no effect of daily protein supplementation or exercise in the CP group. These findings have important clinical implications and highlight the importance of protein quality for the maintenance of skeletal muscle mass in older women.
Increased availability of EAA, particularly leucine, has been shown to be a key stimulus to increase rates of MPS (12, 32, 33), particularly when combined with RE (3, 33). The contributions of EAAs to the WP and CP in the present study were 46% and 17%, respectively, with ∼5.5 times (3.5 g) more leucine provided per supplement dose in WP than in CP. Importantly, the WP and CP supplements provided in the current study were isonitrogenous yet the observed increases in MPS both acutely, and integrated over time, were significantly greater with the WP supplement with higher leucine and EAAs. Previous work from our laboratory, which used a similar experimental model to that employed in this study, compared a protein blend with a milk protein supplement containing ∼3.2 times (2.9 g) greater leucine. Consumption of the beverage containing more leucine resulted in greater acute and chronic elevations in MyoPS, despite the fact that, similarly to the current investigation, the supplements were isonitrogenous (∼15 g protein per drink), an effect that was also enhanced with RE (12).
Our findings are in accordance with previous work in which WP has been shown to be highly effective at stimulating MPS (18, 33, 26). A previous study from our laboratory showed no effect of CP supplementation on rates of MyoPS after 2 wk of reduced daily activity and 1 wk of recovery in healthy older adults (13). To date, 3 additional studies have looked at the efficacy of CP on body composition and showed that CP supplementation resulted in an extraordinary increase in LBM (not muscle) with resistance training in older men (14) and premenopausal women (15), and helped in maintaining nitrogen balance in older women (16). It should be noted that the effects of CP supplementation on LBM gains with RE shown by Zdzieblik et al. (14) were exceptional (∼5 kg) and have been questioned (27). This is particularly relevant given that several meta-analyses have concluded that protein supplementation during RE does not augment gains in LBM in older persons (34). Given that WP has a digestible indispensable AA score of 1.09 and CP has one of 0 because it lacks tryptophan. Even with supplemental tryptophan added, CP are low in methionine and leucine and thus the lack of stimulation of postprandial MyoPS acutely and after 6 d supplementation with CP is not surprising. Importantly, our data provides no support for the proposed AA- or peptide-based mechanisms that ostensibly underpinned the marked CP-induced increase in LBM reported previously (14) or for CP supplementation to support LBM in older persons in general.
Provision of an AA mixture (containing all AAs) has been shown to increase rates of MyoPS (35) largely through the activation of mTOR (complex-1) by the EAA leucine (36). Further, the administration of leucine has been repeatedly shown to independently stimulate MPS owing to its interaction directly with mTOR (37, 38), the primary signaling pathway affecting translation and initiation. Our finding of increased plasma leucine concentration after ingestion of WP in combination with the postprandial increase in acute and integrated MyoPS aligns with seminal dose-response data (39). Given that all EAAs are required in amounts sufficient to build muscle protein, it is not surprising that CP was unable to stimulate a robust longer-term MyoPS response either in the rested or in the exercised leg, particularly when observing the significantly lower concentrations of plasma leucine elicited by CP ingestion.
In order to determine the effects of protein quality on skeletal muscle protein kinetics, we examined the phosphorylation status of proteins involved in the mTOR pathway and its downstream effectors. Of note, we did not find significant increases in the phosphorylated targets of mTOR or its downstream targets in either group. Our data are in line with previous work that showed no changes in phosphorylated targets of the mTOR pathway 4 h after feeding and exercise (40). We hypothesize that because our muscle biopsy time points were chosen for measurements of MyoPS, the peak phosphorylation events for the proteins may have occurred earlier after protein ingestion and exercise.
Muscle-located (perimysium) collagen has been shown to be responsive to exercise stimuli but unresponsive to the nutritional provision of EAAs or a multinutrient, protein-based supplement in humans (41, 42). The CP supplement had a high content of glycine, proline, and arginine, AAs found in large quantities in collagen tissue (43). Thus, we hypothesized that supplementation with CP rather than EAAs would result in an increase in MCPS with feeding alone. However, we saw no effect of feeding on rates of MCPS. These data are in line with work examining the distribution of 14C-labeled gelatin in which the authors reported no change in the distribution of radioactivity to skeletal muscle, which was hypothesized to be due to a 90% removal of radioactivity from the gastrointestinal tract by excretion within the first 6 h of administration (44). We were not able to obtain a baseline biopsy in order to determine baseline MCPS and therefore were only able to compare CPS in protein supplementation phases with and without exercise.
In the current investigation we chose to examine the effects of protein ingestion and exercise in healthy older women owing in part to scant study of older women compared with men in these types of mechanistic studies. We believe that this is a major strength of the current investigation because older women are at an increased risk of falls, fractures, and mobility impairments in comparison with men (45). Unique aspects of male physiology, such as higher testosterone concentrations than in postmenopausal females, may interact with intracellular pathways related to MPS and contribute to this sex-based difference in muscle growth and MyoPS responses. Determining strategies to augment MPS in older women is imperative so that tailored nutritional interventions could be considered that are specific to women. Importantly, although protein ingestion and RE did not augment skeletal muscle mass (46), prolonged consumption of a diet high in protein resulted in maintenance of greater LBM than in individuals who consumed diets low in daily protein (47). Recently we showed that older women may not fully recover strength losses after acute inactivity (48). Further, given the increased risk of falls (45) and the greater life expectancy of women in comparison with men (49), strategies that promote retention of skeletal muscle may serve to prolong functional mobility and independence of the aging female population. We acknowledge that all participants in this small-scale study were healthy and free of chronic disease and thus our findings are limited to healthy older women.
In summary, our findings show that consumption of supplemental WP, but not CP, enhanced skeletal muscle protein anabolism both acutely and when measured over days. Corroborating previous work, we show that consumption of WP when consumed in conjunction with RE resulted in a further stimulation of MPS, reinforcing the importance of RE in the maintenance of skeletal muscle health. Importantly, we also show that RE in combination with low-quality CP protein was not sufficient to elevate rates of MyoPS above baseline in healthy older women indicating that the selection of protein sources for older women may be of great significance. Older women should aim to select high-quality dietary proteins and engage in regular RE in an effort to attenuate sarcopenic muscle declines.
ACKNOWLEDGEMENTS
We thank Dr. Stefan Gorrisen (McMaster University) for help in the preliminary phases of this trial and Noah Carr-Pries (McMaster University) for his assistance. We also thank Todd Prior and Fan Fei (McMaster University) for their technical assistance.
The authors’ responsibilities were as follows—SYO, CM, and SMP: designed the research; SYO, MJK, SKB, and CM: conducted the research; SYO and EKW: analyzed the data; SYO: performed statistical analysis and wrote the manuscript; SYO and SMP: had primary responsibility for the final content; and all authors: read and approved the final manuscript. SMP reports having received competitive research funding, travel expenses, and honoraria for speaking from the US National Dairy Council and the Dairy Farmers of Canada. All other authors report no conflicts of interest.
Notes
Supported by Canadian Institutes of Health Research grant 399384 (to SMP).
Data described in the article will be made available upon request pending filing of any IP and patent claims.
Abbreviations used: AA, amino acid; AKT, protein kinase B; Cmax, concentration max; CP, collagen peptide; CPS, collagen protein synthesis; EAA, essential amino acid; Exercise, feeding plus exercise leg; LBM, lean body mass; MCPS, muscle collagen protein synthesis; MPS, muscle protein synthesis; mTOR, mechanistic target of rapamycin; MyoPS, myofibrillar muscle protein synthesis; RE, resistance exercise; Rest, feeding leg; S6, ribosomal protein S6; Tmax, time to concentration maximum; WP, whey protein; 1RM, 1-repetition maximum.
References
- 1. English KL, Paddon-Jones D. Protecting muscle mass and function in older adults during bed rest. Curr Opin Clin Nutr Metab Care. 2010;13(1):34–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Moore DR, Churchward-Venne TA, Witard O, Breen L, Burd NA, Tipton KD, Phillips SM. Protein ingestion to stimulate myofibrillar protein synthesis requires greater relative protein intakes in healthy older versus younger men. J Gerontol A Biol Sci Med Sci. 2015;70(1):57–62. [DOI] [PubMed] [Google Scholar]
- 3. Yang Y, Breen L, Burd NA, Hector AJ, Churchward-Venne TA, Josse AR, Tarnopolsky MA, Phillips SM. Resistance exercise enhances myofibrillar protein synthesis with graded intakes of whey protein in older men. Br J Nutr. 2012;108(10):1780–8. [DOI] [PubMed] [Google Scholar]
- 4. Houston DK, Nicklas BJ, Ding Z, Harris TB, Tylavsky FA, Newman AB, Lee JS, Sahyoun NR, Visser M, Kritchevsky SB. Dietary protein intake is associated with lean mass change in older, community-dwelling adults: the Health, Aging, and Body Composition (Health ABC) Study. Am J Clin Nutr. 2008;87(1):150–5. [DOI] [PubMed] [Google Scholar]
- 5. Volpi E, Campbell WW, Dwyer JT, Johnson MA, Jensen GL, Morley JE, Wolfe RR. Is the optimal level of protein intake for older adults greater than the recommended dietary allowance?. J Gerontol A Biol Sci Med Sci. 2013;68(6):677–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Paddon-Jones D, Campbell WW, Jacques PF, Kritchevsky SB, Moore LL, Rodriguez NR, van Loon LJ. Protein and healthy aging. Am J Clin Nutr. 2015;101(6):1339S–45S. [DOI] [PubMed] [Google Scholar]
- 7. Häkkinen K, Kramer WJ, Newton RU, Alen M. Changes in electromyographic activity, muscle fibre and force production characteristics during heavy resistance/power strength training in middle-aged and older men and women. Acta Physiol Scand. 2001;171(1):51–62. [DOI] [PubMed] [Google Scholar]
- 8. Brandon LJ, Boyette LW, Gaash DA, Lloyd A. Effects of lower extremity strength training on functional mobility in older adults. J Aging Phys Act. 2000;8(3):214–27. [Google Scholar]
- 9. Bell KE, Séguin C, Parise G, Baker SK, Phillips SM. Day-to-day changes in muscle protein synthesis in recovery from resistance, aerobic, and high-intensity interval exercise in older men. J Gerontol A Biol Sci Med Sci. 2015;70(8):1024–9. [DOI] [PubMed] [Google Scholar]
- 10. Kumar V, Atherton P, Smith K, Rennie MJ. Human muscle protein synthesis and breakdown during and after exercise. J Appl Physiol (1985). 2009;106(6):2026–39. [DOI] [PubMed] [Google Scholar]
- 11. Hasten DL, Pak-Loduca J, Obert KA, Yarasheski KE. Resistance exercise acutely increases MHC and mixed muscle protein synthesis rates in 78–84 and 23–32 yr olds. Am J Physiol Endocrinol Metab. 2000;278(4):E620–E6. [DOI] [PubMed] [Google Scholar]
- 12. Devries MC, McGlory C, Bolster DR, Kamil A, Rahn M, Harkness L, Baker SK, Phillips SM. Protein leucine content is a determinant of shorter- and longer-term muscle protein synthetic responses at rest and following resistance exercise in healthy older women: a randomized, controlled trial. Am J Clin Nutr. 2018;107(2):217–26. [DOI] [PubMed] [Google Scholar]
- 13. Oikawa SY, McGlory C, D'Souza LK, Morgan AK, Saddler NI, Baker SK, Parise G, Phillips SM. A randomized controlled trial of the impact of protein supplementation on leg lean mass and integrated muscle protein synthesis during inactivity and energy restriction in older persons. Am J Clin Nutr. 2018;108(5):1060–8. [DOI] [PubMed] [Google Scholar]
- 14. Zdzieblik D, Oesser S, Baumstark MW, Gollhofer A, Konig D. Collagen peptide supplementation in combination with resistance training improves body composition and increases muscle strength in elderly sarcopenic men: a randomised controlled trial. Br J Nutr. 2015;114(8):1237–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jendricke P, Centner C, Zdzieblik D, Gollhofer A, Konig D. Specific collagen peptides in combination with resistance training improve body composition and regional muscle strength in premenopausal women: a randomized controlled trial. Nutrients. 2019;11(4):892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hays NP, Kim H, Wells AM, Kajkenova O, Evans WJ. Effects of whey and fortified collagen hydrolysate protein supplements on nitrogen balance and body composition in older women. J Am Diet Assoc. 2009;109(6):1082–7. [DOI] [PubMed] [Google Scholar]
- 17. Lis DM, Baar K. Effects of different vitamin-C enriched collagen derivatives on collagen synthesis. Int J Sport Nutr Exerc Metab. 2019;29(5):526–31. [DOI] [PubMed] [Google Scholar]
- 18. Devries MC, McGlory C, Bolster DR, Kamil A, Rahn M, Harkness L, Baker SK, Phillips SM. Leucine, not total protein, content of a supplement is the primary determinant of muscle protein anabolic responses in healthy older women. J Nutr. 2018;148(7):1088–95. [DOI] [PubMed] [Google Scholar]
- 19. MacInnis MJ, McGlory C, Gibala MJ, Phillips SM. Investigating human skeletal muscle physiology with unilateral exercise models: when one limb is more powerful than two. Appl Physiol Nutr Metab. 2017;42(6):563–70. [DOI] [PubMed] [Google Scholar]
- 20. Henry CJK. Basal metabolic rate studies in humans: measurement and development of new equations. Public Health Nutr. 2007;8(7a):1133–52. [DOI] [PubMed] [Google Scholar]
- 21. Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46(2):153–62. [DOI] [PubMed] [Google Scholar]
- 22. Statistics Canada. Table 13-10-0771-01: percentage of total energy intake from protein, by dietary reference intake age-sex group, household population aged 1 and over, Canadian Community Health Survey (CCHS) - nutrition, Canada and provinces. [Internet] Ottawa, Canada: Statistics Canada; 2019. Available from: https://www150.statcan.gc.ca/t1/tbl/en/tv.action?pid=1310077101.[Accessed 15 October, 2019]. [Google Scholar]
- 23. MacDonald AJ, Small AC, Greig CA, Husi H, Ross JA, Stephens NA, Fearon KC, Preston T. A novel oral tracer procedure for measurement of habitual myofibrillar protein synthesis. Rapid Commun Mass Spectrom. 2013;27(15):1769–77. [DOI] [PubMed] [Google Scholar]
- 24. Wilkinson DJ, Franchi MV, Brook MS, Narici MV, Williams JP, Mitchell WK, Szewczyk NJ, Greenhaff PL, Atherton PJ, Smith K. A validation of the application of D2O stable isotope tracer techniques for monitoring day-to-day changes in muscle protein subfraction synthesis in humans. Am J Physiol Endocrinol Metab. 2014;306(5):E571–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dufner DA, Bederman IR, Brunengraber DZ, Rachdaoui N, Ismail-Beigi F, Siegfried BA, Kimball SR, Previs SF. Using 2H2O to study the influence of feeding on protein synthesis: effect of isotope equilibration in vivo vs. in cell culture. Am J Physiol Endocrinol Metab. 2005;288(6):E1277–83. [DOI] [PubMed] [Google Scholar]
- 26. Tang JE, Moore DR, Kujbida GW, Tarnopolsky MA, Phillips SM. Ingestion of whey hydrolysate, casein, or soy protein isolate: effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J Appl Physiol (1985). 2009;107(3):987–92. [DOI] [PubMed] [Google Scholar]
- 27. Phillips SM, Tipton KD, van Loon LJ, Verdijk LB, Paddon-Jones D, Close GL. Exceptional body composition changes attributed to collagen peptide supplementation and resistance training in older sarcopenic men. Br J Nutr. 2016;116(3):569–70. [DOI] [PubMed] [Google Scholar]
- 28. Glover EI, Phillips SM, Oates BR, Tang JE, Tarnopolsky MA, Selby A, Smith K, Rennie MJ. Immobilization induces anabolic resistance in human myofibrillar protein synthesis with low and high dose amino acid infusion. J Physiol. 2008;586(Pt 24):6049–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Purves RD. Optimum numerical integration methods for estimation of area-under-the-curve (AUC) and area-under-the-moment-curve (AUMC). J Pharmacokinet Biopharm. 1992;20(3):211–26. [DOI] [PubMed] [Google Scholar]
- 30. Hector AJ, McGlory C, Damas F, Mazara N, Baker SK, Phillips SM. Pronounced energy restriction with elevated protein intake results in no change in proteolysis and reductions in skeletal muscle protein synthesis that are mitigated by resistance exercise. FASEB J. 2018;32(1):265–75. [DOI] [PubMed] [Google Scholar]
- 31. McGlory C, von Allmen MT, Stokes T, Morton RG, Hector AJ, Lago BA, Raphenya AR, Smith BK, McArthur AG, Steinberg GR et al.. Failed recovery of glycemic control and myofibrillar protein synthesis with two weeks of physical inactivity in overweight, pre-diabetic older adults. J Gerontol A Biol Sci Med Sci. 2017;73(8):1070–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Churchward-Venne TA, Breen L, Di Donato DM, Hector AJ, Mitchell CJ, Moore DR, Stellingwerff T, Breuille D, Offord EA, Baker SK et al.. Leucine supplementation of a low-protein mixed macronutrient beverage enhances myofibrillar protein synthesis in young men: a double-blind, randomized trial. Am J Clin Nutr. 2014;99(2):276–86. [DOI] [PubMed] [Google Scholar]
- 33. Churchward-Venne TA, Burd NA, Mitchell CJ, West DW, Philp A, Marcotte GR, Baker SK, Baar K, Phillips SM. Supplementation of a suboptimal protein dose with leucine or essential amino acids: effects on myofibrillar protein synthesis at rest and following resistance exercise in men. J Physiol. 2012;590(Pt 11):2751–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Thomas DK, Quinn MA, Saunders DH, Greig CA. Protein supplementation does not significantly augment the effects of resistance exercise training in older adults: a systematic review. J Am Med Dir Assoc. 2016;17(10):959.e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bennet WM, Connacher AA, Scrimgeour CM, Smith K, Rennie MJ. Increase in anterior tibialis muscle protein synthesis in healthy man during mixed amino acid infusion: studies of incorporation of [1–13C]leucine. Clin Sci. 1989;76(4):447–54. [DOI] [PubMed] [Google Scholar]
- 36. Dickinson JM, Fry CS, Drummond MJ, Gundermann DM, Walker DK, Glynn EL, Timmerman KL, Dhanani S, Volpi E, Rasmussen BB. Mammalian target of rapamycin complex 1 activation is required for the stimulation of human skeletal muscle protein synthesis by essential amino acids. J Nutr. 2011;141(5):856–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am J Physiol Endocrinol Metab. 2006;291(2):E381–7. [DOI] [PubMed] [Google Scholar]
- 38. Atherton PJ, Smith K, Etheridge T, Rankin D, Rennie MJ. Distinct anabolic signalling responses to amino acids in C2C12 skeletal muscle cells. Amino Acids. 2010;38(5):1533–9. [DOI] [PubMed] [Google Scholar]
- 39. Bohe J, Low A, Wolfe RR, Rennie MJ. Human muscle protein synthesis is modulated by extracellular, not intramuscular amino acid availability: a dose-response study. J Physiol. 2003;552(Pt 1):315–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wilkinson SB, Phillips SM, Atherton PJ, Patel R, Yarasheski KE, Tarnopolsky MA, Rennie MJ. Differential effects of resistance and endurance exercise in the fed state on signalling molecule phosphorylation and protein synthesis in human muscle. J Physiol. 2008;586(15):3701–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Babraj JA, Cuthbertson DJ, Smith K, Langberg H, Miller B, Krogsgaard MR, Kjaer M, Rennie MJ. Collagen synthesis in human musculoskeletal tissues and skin. Am J Physiol Endocrinol Metab. 2005;289(5):E864–9. [DOI] [PubMed] [Google Scholar]
- 42. Holm L, van Hall G, Rose AJ, Miller BF, Doessing S, Richter EA, Kjaer M. Contraction intensity and feeding affect collagen and myofibrillar protein synthesis rates differently in human skeletal muscle. Am J Physiol Endocrinol Metab. 2010;298(2):E257–69. [DOI] [PubMed] [Google Scholar]
- 43. Eastoe JE. The amino acid composition of mammalian collagen and gelatin. Biochem J. 1955;61(4):589–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Oesser S, Adam M, Babel W, Seifert J. Oral administration of 14C labeled gelatin hydrolysate leads to an accumulation of radioactivity in cartilage of mice (C57/BL). J Nutr. 1999;129(10):1891–5. [DOI] [PubMed] [Google Scholar]
- 45. Burlock A. Women with disabilities. [Internet] Ottawa, Canada: Statistics Canada; 2017. Available from: https://www150.statcan.gc.ca/n1/en/pub/89-503-x/2015001/article/14695-eng.pdf. [Accessed 15 October, 2019]. [Google Scholar]
- 46. Finger D, Goltz FR, Umpierre D, Meyer E, Rosa LH, Schneider CD. Effects of protein supplementation in older adults undergoing resistance training: a systematic review and meta-analysis. Sports Med. 2015;45(2):245–55. [DOI] [PubMed] [Google Scholar]
- 47. Farsijani S, Morais JA, Payette H, Gaudreau P, Shatenstein B, Gray-Donald K, Chevalier S. Relation between mealtime distribution of protein intake and lean mass loss in free-living older adults of the NuAge study. Am J Clin Nutr. 2016;104(3):694–703. [DOI] [PubMed] [Google Scholar]
- 48. Oikawa SY, Callahan DM, McGlory C, Toth MJ, Phillips SM. Maintenance of skeletal muscle function following reduced daily physical activity in healthy older adults: a pilot trial. Appl Physiol Nutr Metab. 2019;44(10):1052–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Statistics Canada. Health-adjusted life expectancy, by sex. [Internet]. Ottawa, Canada: Statistics Canada; 2018. Available from: https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1310037001. [Accessed 15 October, 2019]. [Google Scholar]